Back to Journals » Drug Design, Development and Therapy » Volume 19
A Bibliometric and Visual Analysis of Oliceridine Research (2013–2024)
Authors Song C, Huang X, Chen N, Song Q, Qiu Y
Received 26 October 2024
Accepted for publication 17 February 2025
Published 24 February 2025 Volume 2025:19 Pages 1305—1321
DOI https://doi.org/10.2147/DDDT.S497186
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Tamer Ibrahim
Cheng Song,1,* Xinxing Huang,2,* Nianping Chen,1 Qiliang Song,3 Yuanli Qiu1
1Department of Anesthesiology, Affiliated Hospital of Shaoxing University, Zhejiang, 312000, People’s Republic of China; 2Department of Anesthesiology, Jinhua Maternal and Child Health Hospital, Zhejiang, 321000, People’s Republic of China; 3Department of Anesthesiology, Shaoxing People’s Hospital, Shaoxing, Zhejiang, 312000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuanli Qiu, Department of Anesthesiology, Affiliated Hospital of Shaoxing University, Shaoxing, Zhejiang, 312000, People’s Republic of China, Email [email protected]
Purpose: To explore and analyze the current research progress, hotspots, and future trends in oliceridine research using bibliometric methods.
Patients and Methods: We searched the Web of Science (WOS) database utilizing the keywords TS = (“oliceridine*” OR “TRV 130*” OR “TRV-130*” OR “olinvyk*” OR “TRV130*” OR “C22H31CIN2O2S*”) for relevant research literature on oliceridine from its inception to June 16, 2024. Bibliometric methods were applied, and analysis software such as VOSviewer and CiteSpace were used to visualize the publication timeline, authors, countries and regions, keywords, sources of literature, research hotspots, and co-cited documents related to oliceridine. Co-occurrence and aggregation analyses were conducted, and maps relevant to institutional cooperation were generated.
Results: A total of 151 relevant articles were retrieved and included in the final analysis. Most articles were published between 2020 and 2021. The United States has the highest number of publications and citations in this field. Molecular structure development is a pivotal point in this field. Research hotspots were diverse, including acute pain, opioid receptors, β-arrestin, postoperative pain, therapeutic window, respiratory depression, clinical trials, and chronic pain.
Conclusion: Oliceridine, a newly developed analgesic, has garnered global interest. The USA is a leading contributor to this field. Recent research has shifted from basic studies to clinical practice.
Keywords: oliceridine, bibliometrics, opioid drugs, research trends
Introduction
Opioid analgesics have revolutionized pain management and surgical procedures, yet opioid-related adverse events (ORAEs) remain a major concern. The search for an ideal opioid agent that provides effective pain relief while minimizing side effects continues.1,2 Oliceridine, a novel opioid analgesic, was first introduced in 2013 as TRV130 by DeWire et al.3 It was approved by the Food and Drug Administration (FDA) in 2020 — as the first drug that selectively activates the G protein-coupled opioid mu-type opioid receptor (MOR), while limiting β-arrestin recruitment. It has shown promising analgesic efficacy in preclinical studies.4 Given its potent painkilling effects, reduced respiratory depression, and fewer gastrointestinal adverse effects, oliceridine is increasingly used for postoperative analgesia and pain management outside the perioperative period.5 Over the past decade, research on oliceridine has steadily increased, shifting from basic pharmacological to clinical studies. However, the overall oliceridine research landscape remains unclear. This study used bibliometric techniques to systematically synthesize and visually analyze current research on oliceridine, aiming to provide a comprehensive overview, highlight key research, and offer insights for future studies.
Materials and Methods
Materials
A search was conducted in the Web of Science (WOS) core database using the search terms TS = (“oliceridine*” OR “TRV 130*” OR “TRV-130*” OR “olinvyk*” OR “TRV130*” OR “C22H31CIN2O2S*”) from the inception of data up to June 16, 2024, yielding 187 results. The relevant literature was exported in a pure text format (Figure 1).
 |
Figure 1 Detailed process for literature screening. |
The Web of Science Core Collection (WOScc) is a high-quality digital bibliographic database considered most suitable for bibliometric analysis.6 Thus, WOScc was the data source for this study.
Data Sources
The final dataset was curated meticulously to ensure consistency and accuracy involving the following:
- Synonym Consolidation: Terms that are semantically equivalent but differ in case or form were unified. For instance, “ligand” and “ligands” were standardized to avoid ambiguity.
- Terminological Uniformity: Compound terms, such as “beta-arrestin-2 recruitment” and “beta-arrestin2 recruitment”, were standardized to maintain consistency across the dataset.
- Author Name Expansion: Abbreviated author names were expanded to their full forms to prevent confusion and ensure proper attribution.
Extensive standardization of synonyms was critical for accurate data analysis and interpretation, as it eliminates ambiguity and inconsistencies in the dataset, enabling more reliable identification of research trends and collaborations. Two authors independently reviewed the data. All disagreements were discussed among all authors, and the final decision was made by the corresponding author.
Analytical Tools
Bibliometrics is the use of statistical tools to uncover patterns, trends, and influential factors within academic publications. This study utilized data exported from the Web of Science Core Collection (WOScc) and employed software such as VOSviewer (1.6.20), CiteSpace (6.3.R2), Excel (2016), Pajek (1.0.0), and Scimago Graphica (1.0.43) to analyze various aspects of oliceridine-related literature. VOSviewer was chosen for its ability to create clear and intuitive network visualizations,7 and CiteSpace was selected for its advanced clustering and trend analysis capabilities.8 Specifically, publication time, authors, countries and regions, keywords, sources of literature, research hotspots, and co-cited documents were analyzed. The relevant data underwent co-occurrence and aggregation operations, and visual maps were created. Among CiteSpace’s clustering capabilities, the average silhouette value (S value) and modularity (Q value) were used to assess clustering effectiveness. An S value > 0.5 indicates well-separated clusters, while a Q value > 0.3 signifies substantial configuration.
Results
Basic Information
This study included 151 relevant studies authored by 572 researchers from 209 institutions across 25 countries and regions, published in 89 journals. Figure 2 shows a fluctuating increase in the number of studies, indicating sustained research interest within the academic community and suggesting that future publications will continue to rise.
 |
Figure 2 Distribution of annual publication volume. |
Analysis of Authors and Institutions
In alignment with Price’s Law, authors who have published more than 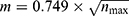 papers within a specific domain are considered core contributors. In this context,
papers within a specific domain are considered core contributors. In this context,  represents the total publications by the most prolific author, which is 26. Therefore, those with a publication count of four or more are categorized as the core author group, totaling 19 individuals. Visual analytics Using VOS software reveal extensive collaboration among these core authors.
represents the total publications by the most prolific author, which is 26. Therefore, those with a publication count of four or more are categorized as the core author group, totaling 19 individuals. Visual analytics Using VOS software reveal extensive collaboration among these core authors.
Soergel and Skobieranda are identified as the most prolific authors in this field (Figure 3A and B) (Table 1), each with 26 publications since 2013.
 |
Figure 3 Visualization map of authors. (A) Cooperation networks between different authors. (B) Density visualization map of authors. (C) Timeline visualization map of authors. |
 |
Table 1 Most Influential Authors in Oliceridine Research |
The VOS software analysis indicated that the research of Michalsky and Bergese is relatively new, represented by the color yellow in the diagram (Figure 3C). Their current focus includes tolerability,9 respiratory depression,10 and nausea and vomiting11 associated with oliceridine, as well as its therapeutic application in geriatric patients.12
The examination of institutions involved in this field highlights a collaborative network. VOS software delineates 13 distinct clusters, with Trevena, Inc. at the core. As the originator of oliceridine, Trevena Inc. has been crucial in advancing the compound from preclinical to clinical stages through both in vitro and in vivo research. The company has been instrumental in landmark trials such as the APPOLO-1, APPOLO-2, and ATHENA.
Prominent institutions such as SUNY Stony Brook, Medical University of South Carolina, Harvard Medical School, Stanford University, and Duke University have also made significant contributions to oliceridine research. The Medical University of South Carolina has partnered with Trevena Inc. to research oliceridine’s pharmacology and clinical use, promoting its application in clinical practice. SUNY Stony Brook and Harvard Medical School have also published research on oliceridine, exploring its mechanisms, evidence, and potential for postoperative pain management. Their involvement has enriched the academic and clinical exploration of this innovative opioid analgesic (Figure 4).
 |
Figure 4 Institutional cooperation. |
Analysis of Journals in the Field
According to Bradford’s law, journals were categorized by publication volume into three groups: those with more than three papers, with 2–3 papers, and with one paper, with a ratio of 8:26:55, closely aligning with Bradford’s law. Eight journals with more than three publications are considered core journals in this field. The “Journal of Pain” leads in publication volume on oliceridine, although its citation counts are notably lower. In stark contrast, the “Journal of Pain Research” not only has a high publication output but also boasts the highest citation count, with an impressive total of 921 citations and an average citation rate of 184.2 per article, highlighting significant recognition of its content by the academic community. Furthermore, classical journals, such as “Pain” and “Trends in Pharmacological Sciences”, while not publishing a large volume of articles on this topic, are highly regarded, with average citation counts of 125.33 and 131.67, respectively (Table 2).
 |
Table 2 Top 10 Journals for Oliceridine Research |
Dual-Map Overlay of Journals
The dual-map overlay visually represents citation connections between citing and cited journals, with the left side showing citing journal clusters and the right side depicting cited journal clusters. The yellow paths indicate that journals in Molecular Biology and Genetics are most often cited by journals in Molecular Biology and Immunology. Similarly, the green path shows that journals in molecular biology, genetics, and nursing medicine are most likely to be cited by journals in the medical, clinical, and healthcare sectors (Figure 5).
 |
Figure 5 Dual-Map overlay of journals. The dual-map layout displays journal citations, with citing journal on the left and cited journals on the right. |
Analysis of Country/Region Distribution
There is a notable disparity in publication output among countries in oliceridine research. The United States leads with 100 publications and has accumulated 3569 citations, establishing its central role in this field (Figure 6A and B). China ranks second in publication volume, but has a low average citation count of only 11.87. Australia has the highest average citation rate at 73.4 (Table 3).
 |
Table 3 Top 5 Countries Pioneering Oliceridine Research |
Keywords Analysis
Using CiteSpace 6.3, R2 was set to slice =1 and g-index k=30 to ensure consistent node information in each time slice. Larger circles represent higher frequencies of occurrence, with purple circles indicating pivotal nodes with a betweenness centrality greater than 0.1.
Co-Occurrence of Keywords
The term with the highest centrality is “analgesia” at 0.29, followed by “double blind” at 0.20. Morphine and the mu opioid receptor lead in citation count, each with 40 citations. Table 4 presents the top ten keywords ranked by citation counts and centrality.
 |
Table 4 Top 10 Citation Counts and Centrality Nodes in Keywords |
Cluster of Keywords
Keywords are classified into 11 main clusters (Q value = 0.5156, S value = 0.8037): #0 acute pain, #1 TRV130, #2 opioid receptor, #3 beta-arrestin, #4 postoperative pain, #5 therapeutic window, #6 respiratory depression, #7 heroin, #8 clinical trial, #9 chronic pain, and #10 protein-coupled receptors. A high silhouette value of 0.8037 indicates clear cluster delineation, and a modularity value of 0.5156 supports the existence of substantial clustering (Figure 7A and B) (Table 4).
Timeline View Keyword Clusters and Keyword Bursts
The top-ranked item by bursts is “discovery” with bursts of 3.26, followed by “healthy volunteers” with bursts of 3.04, and “knockout mice” with bursts of 2.61. Early oliceridine research focused on “knockout mice”, “functional selectivity” and “beta arrestin 2” from 2013 to 2018. Current keywords include “moderate”, “receptor”, and “protein biased ligand” (Figure 7C). The clusters that have persisted to the present are 0 # acute pain, 1 # TRV130, 4 # postoperative pain, and 5 # therapeutic window (Figure 7D).
Co-Citations Analysis
Co-Occurrence of Co-Citations
The top-ranked item by citation counts is Viscusi ER, 2019, J Pain Res, with 48 citations. The top-ranked item by centrality is Váradi A, 2016, J Med Chem, with a centrality of 0.15.
Cluster of Co-Citations
The diagram shows that co-cited literature is divided into several distinct clusters (Q=0.7824, S=0.9094). Cluster #1, focusing on the conformational state, is central to the research continuum. The article “Manglik A, 2016, Nature” is significant within this cluster. Cluster #5 functional selectivity, #7 promise, and #9 morphine have progressively refined Cluster #1. Research advances from Cluster #1 to Cluster #2, novel synthetic opioids; #15, morphine-induced side effects; #4, pain management; and ultimately, to Cluster 0, oliceridine injection (Figure 8A and B) (Table 5).
 |
Table 5 Top 10 Citation Counts and Centrality Nodes in Co-Citations |
Timeline View Co-Citation Clusters and Co-Citation Bursts
The most intense bibliographic burst occurred in DeWire (2013), published in the Journal of Pharmacology and Experimental Therapeutics, from 2013 to 2018. Ongoing burst literature includes studies by Gillis A (2020) in Science Signaling, Bergese S (2020) in Pain Research and Management, Beard TL (2021) and Brzezinski M (2021) in Pain Therapy (Figure 8C and D).
Discussion
Our bibliometric analysis of oliceridines research, based on data from the WOScc database, revealed a progression from basic molecular studies to clinical applications over recent decades. The study illustrated trends in publication volume, authorship distribution of authorship, geographic connections, co-citation, and aggregation, despite including only 151 articles on oliceridine published between March 2013 and June 2024. The analysis highlights current research hotspots and future prospects.
Basic Information Analysis
The publication volume on oliceridine has fluctuated, Showing a notable increase in publication from 2016 to 2021, likely due to advancements in molecular studies and clinical research.
The United States leads in oliceridines research and development, with the highest impact in publications and citations. The United States’ leadership in oliceridine research can be attributed to its strong pharmaceutical industry, robust funding for biomedical research, and a culture that encourages innovation and collaboration between academia, industry, and regulatory agencies. Oliceridine was originally developed by Trevena, Inc., a U.S.-based company, in 2013 and remains the most productive contributor in this field, solidifying the United States’ leading position in this area. In August 2020, the US Food and Drug Administration (FDA) approved oliceridine for market use. By May 2023, oliceridine has also achieved market certification and passed audits in China, facilitating further research in this populous country. In this specialized field, 19 core authors have been identified, with two—Soergel DG and Skobieranda F—being particularly productive. These authors played pivotal roles in oliceridine research, contributing to major studies like APPOLO-1, APPOLO-2, and ATHENA. Both Soergel DG and Skobieranda F are affiliated with Trevena Inc. (Chesterbrook, PA, USA) and have collaborated closely in pharmaceutical development. However, their affiliation with Trevena Inc. raises potential conflicts of interest. The “Journal of Pain Research” has a high publication output and the highest citation count, largely driven by a single high-impact review article that garnered 654 citations. This article addressed clinical challenges and therapeutic strategies for inadequate postoperative pain management, highlighting oliceridine’s potential to provide rapid analgesic effects and reduce adverse events. This prominence may be attributed to the journal’s open-access status, which makes its research findings more accessible and easily citable However, this accessibility could also introduce biases in citation metrics, as articles from open-access journals might be cited more frequently due to their availability rather than solely based on their scientific quality. Despite ongoing debates about open-access journals, they have facilitated the dissemination and advancement of research on this medication. Renowned journals such as “Pain” and “Trends in Pharmacological Sciences” have fewer articles on oliceridine but maintain high-quality standards and produce frequently cited articles, reinforcing their impact.
Analyses Based on Co-Citations
Cited references form the foundation of research by indicating key works, with highly central articles representing pivotal nodes in the academic domain.8 In 2016, Váradi A was identified as the most significant article with a betweenness centrality of 0.15 from a co-citation perspective. This study examined mitragynine pseudoindoxyl (similar to TRV130), which exhibited fewer side effects compared to morphine, such as tolerance, dependence, and respiratory depression.14 As for total citations, Viscusi ER’s ranked highest with 48 citations. This randomized, placebo, and active-controlled Phase III trial demonstrated the clear efficacy of oliceridine for postoperative analgesia, with reduced respiratory depression and gastrointestinal side effects.13 Timeline and burst literature analyses revealed that DeWire SM’s 2013 publication had the highest burst strength from 2013 to 2018. This seminal article, the first to introduce TRV130 into human embryonic kidney cells and mice, demonstrated that oliceridine had superior analgesic efficacy compared to morphine.3 The citation burst period, which ended in 2024, focused primarily on comparative analyses between oliceridine and morphine, highlighting the evaluation of benefits and risks regarding respiratory depression and analgesic potency,29,30 regulatory mechanisms and signal transduction pathways of opioid receptors,26 and reducing nausea and vomiting as adverse effects.11 In sub-group patients, such as those over 65 years old or those with a higher Body Mass Index (BMI), the administration of oliceridine post-surgery does not increase the risk of Opioid-Induced Respiratory Depression (OIRD).10
Cluster dependencies help identify inter-cluster dependencies and the continuity of transitions from one cluster to another. Examination of the cluster graph showed that research in cluster #5 (functional selectivity), cluster #7 (promise), and cluster #9 (morphine) progressively deepened and refined, leading to the formation of cluster #1 (conformational state). Using Cluster #1 as a pivotal node, research advanced to cluster #2 (novel synthetic opioids), cluster #15 (morphine-induced side effects), cluster #4 (pain management), and ultimately, to cluster #0 (oliceridine injection). This aligns with the dual-map overlay analysis of the journals. Analysis of the literature within clusters, particularly articles with high citation counts and centrality, revealed that breakthroughs in conformational analysis of the mu-opioid receptor (MOR) were key nodes. This result also indicates that such breakthroughs are crucial for the research of novel opioids, significantly advancing their clinical studies. Research on molecular conformation is a key focus for future exploration of new opioid drugs. These breakthroughs, combined with the refinement of animal experiments and clinical trials, have created a knowledge map in this field.
Analysis of Molecular Identification Based on Co-Citations
In the 1990s, the MOR was established as a crucial role for opioid analgesia.31 Common opioid drugs such as morphine and fentanyl exert their analgesic effects primarily through the G protein signaling pathway, but they also trigger β-arrestin, recruitment, leading to opioid-related adverse events. Fenalti G, in 2014 (centrality 0.11), first provided the high-resolution crystal structure of the human δ-opioid receptor at 1.8 Å, revealing the specific location and mechanism of action of sodium ions within the receptor. This discovery provides a theoretical foundation for understanding the novel regulatory mechanisms of G Protein-Coupled Receptors (GPCRs).16 Subsequently, the binding process of TRV130 with activated MOR was analyzed using multi-microsecond timescale all-atom molecular dynamics (MD) simulations, which revealed a stable conformation and energetically favorable binding pathway of TRV130 at the orthogonal site of MOR.32 Manglik A, in 2016 (46 citations),19 used computational methods to screen and optimize the molecular scaffold, leading to the discovery and synthesis of PZM21— a G protein-biased MOR agonist with reduced β-arrestin-2 recruitment, similar to oliceridine. In 2018, Kennedy et al further elucidated the synthesis and optimization of G protein-biased MOR agonists, specifying essential molecular structural characteristics for binding to MOR and eliciting biological effect, including the central ring structure, its dimensions, the N-benzyl substituent, and the substituents on the benzimidazole ring. Additionally, they identified that certain hydrophobic substituents, such as halogen atoms, play critical roles in achieving extreme G protein coupling bias.33 Researchers employed MD simulations to suggest that TRV130’s interaction with specific MOR residues downregulates β-arrestin signaling, while hydrophobic ring interactions bias the receptor towards G protein activation.34 Recent research as of 2022 utilized cryo-electron microscopy to determine the structures of the MOR-G protein complex bound to various opioid drugs, providing detailed views of the interactions between these drugs and the receptor.35 This study showed that TRV130’s pyridine ring is tilted 35 degrees relative to fentanyl’s N-phenylamine group, resulting in weaker hydrophobic interactions with GPCR’s TM6 and TM7, which may explain its diminished β-arrestin signaling.35
Analysis of Animal Testing Based on Co-Citations
Previous studies have indicated that β-arrestin2 knockout in mice can reduce side effects such as respiratory depression and constipation and also decrease the tolerance to morphine.36 In 2013, Dewire et al found that TRV130 showed less respiratory depression and better gastrointestinal tolerance than morphine and fentanyl in mice, but also had lower oral bioavailability.3 Using cellular research andβ-arrestin-2 gene-knockout mice, Chen et al confirmed that G protein-biased MOR agonists can enhance analgesic effects while reducing adverse reactions.4 Schmid CL, in 2017 (citations 40), suggested that TRV130 emerged as a G protein-biased MOR agonist, displaying a larger therapeutic window compared to β-arrestin-biased agonists, such as fentanyl, indicating that TRV130 exerts analgesic effects and reduces the incidence of respiratory depression.23
However, animal studies have indicated that TRV130 can produce adverse effects similar to traditional opioids with varying administration routes and dosages. In a study by Altarifi AA, in 2017 (34 citations), acute TRV130 administration demonstrated significant analgesic effects with minimal gastrointestinal suppression and low abuse potential in rodents. However, chronic treatment did not lead to antinociceptive tolerance but resulted in gastrointestinal effects similar to morphine and increased the risk of abuse. Naltrexone pretreatment significantly inhibited intracranial self-stimulation behavior in rats.25 Additionally, administration of TRV130 at a lower dose, specifically 100 ng applied to the dorsal surface of the rat’s hind paw, resulted in hyperalgesia.37 Algera MH, in 2019 (centrality 0.11), indicated that naloxone is preferred for reversing TRV130-induced respiratory depression, though it might precipitate pain and withdrawal. Non-opioid antagonists such as ketamine, GAL021, and doxapram are alternative reversal agents.24 Concurrently, research is underway on other MOR agonists similar to oliceridine that do not recruit β-arrestin-2. Váradi A, in 2016 (centrality 0.15), discovered that mitragynine pseudoindoxyl exhibits strong analgesic effects, with slower development of tolerance, milder respiratory and gastrointestinal suppression, and lower addictive potential.14,20 Manglik (2016) reported that TRV130 still caused respiratory depression in mice, whereas PZM21, at equianalgesic doses, hardly induced respiratory suppression or addictive behaviors, with its analgesic effect lasting up to 180 minutes, significantly longer than morphine and TRV130. Gillis A, in 2020 (26 citations), evaluated the profiles of new MOR-biased agonists, revealing that oliceridine, PZM21, and SR-17018 had lower intrinsic efficacy than morphine in all G protein signaling pathways. This led to the proposal of the “low intrinsic efficacy” hypothesis, suggesting that agonists with low intrinsic efficacy, despite limited receptor activation, can produce sufficient analgesia without strong side effects, potentially reducing complications.26 This theory explains explain the advantages of TRV130.
However, some studies have reported contradictory findings. Kliewer A, in 2019 (23 citations, centrality 0.11), showed that in MOR phosphorylation-deficient mice, despite a significant reduction in β-arrestin recruitment, side effects such as respiratory depression and constipation were not reduced but rather worsened, indicating that these effects might be mediated through β-arrestin-independent mechanisms.22 Further studies are required to clarify the causes of respiratory depression and constipation in mice with defective MOR phosphorylation.
Analyses of Clinical Trials Based on Co-Citations
In the first clinical trial with healthy volunteers, Soergel DG (2014) (centrality 0.11) demonstrated that a single dose of TRV130 (3.0 mg or 4.5 mg) provided better pain relief than a single 10 mg dose of morphine while reducing gastrointestinal and respiratory side effect. Lower doses of TRV130 (1.5 mg and 3.0 mg) also showed a reduced incidence of nausea and vomiting.38 In a Phase 2 randomized controlled trial conducted by Viscusi in 2016 (citations 43), TRV130 (2 mg/3 mg every 3 h) provided effective pain relief within 5 minutes of administration, significantly better than morphine (4 mg every 4 h), with a greater reduction in average pain intensity compared to placebo over 48-hour.21 In a 2017 Phase IIb study by Singla N (citations 47), patients received intravenous oliceridine PCA (Patient-Controlled Analgesia) postoperatively. The oliceridine regimen (loading dose 1.5 mg/patient-controlled dose 0.35 mg) provided the shortest median time to pain relief (0.3 hours), superior to both a lower-dose oliceridine regimen (1.5 mg/0.10 mg) and morphine (4.0 mg/1.0 mg).15 Subsequent analysis of Phase I and II studies led to the development of a population PK/PD model, considering body weight, gender, and CYP2D6 metabolism, and identifying the EC50 of Oliceridine for pain relief as 10.1 ng/mL (95% CI, 8.4–12.1 ng/mL).39 The frequently cited APOLLO-1 trial by Viscusi ER, 2019 (citations 48), a Phase 3 randomized, controlled, double-blind, placebo, and multicenter study, of oliceridine in postoperative pain management following bunionectomy. Patients receiving oliceridine (1.5 mg loading dose) followed by patient-controlled analgesia with (0.1 mg, 0.35 mg, or 0.5 mg demand doses) had faster and more effective relief from moderate-to-severe pain compared to morphine (4 mg), with improved safety and tolerability, particularly regarding respiratory and gastrointestinal side effects, The incidence of nausea and vomiting was dose-dependent.13 In the APPOLO-2 phase 3 study, Singla NK in 2019 (citations 46) various doses of Oliceridine were assessed, revealing that Oliceridine had a lower respiratory safety burden than morphine, with the 0.35 mg demand resulting in fewer nausea and vomiting incidents than morphine, and the 0.5 mg dose showing similar incidences.17 In the ATHENA phase 3 trial by Bergese SD, 2019 (citation 23), involving 41 centers, demonstrated that intravenous oliceridine or PCA significantly reduced pain scores within 30 minutes. Common side effects are mild to moderate nausea (31%), constipation (11%), and vomiting (10%), with 2.9% of patients experiencing prolonged ECG QTc intervals, potentially linked to electrolyte imbalances or QTc-prolonging medications. No deaths or severe complications were reported.5
While these studies closely mimicked real-world conditions and focused on the safety and efficacy during the acute treatment phase, they did not assess the long-term safety of oliceridine. Additionally, certain populations, such as patients with chronic pain or cancer, were underrepresented. Future studies should include more diverse patient populations to evaluate oliceridine’s applicability in a wider range of clinical scenarios.
Analyses of Theme Trends and Hot Topics Based on Keywords
The keywords highlight current research hotspots and frontiers. We conducted cluster, frequency, and centrality analyses for these keywords. The specific categorization of keyword clustering into 11 classifications was as follows: 0: acute pain, 4: postoperative pain, 5: therapeutic window, 6: respiratory depression, 8: clinical trial, and 9: chronic pain. This indicates that the research emphasis is centered on the mechanism of action of oliceridine, side effects such as respiratory depression and addiction, management of acute and chronic pain, and clinical trials. These findings align with the results of the co-citation analysis.
The burst analysis of keywords reveals that “Discovery” (strength 3.26), “healthy volunteers” (strength 3.02), and “knockout mice” (strength 2.58) exhibit the highest burst strength. Early research focused on mechanism studies and animal experiments, with keywords such as “functional selectivity” and “knockout mice” persisting from 2013 to 2019. The midterm focus shifted to clinical trials involving “healthy volunteers” from 2016 to 2018. The current focus is on studies related to “moderate” and “receptor”. “Analgesia” (centrality 0.29) and “double blind” (centrality 0.20) have the highest betweenness centrality, indicating that research related to analgesia and double-blind methodology is a key point and turning point in the current research field of oliceridine. The keyword “Discovery” (strength 3.26) indicated that the main research objective was to explore this new drug, the first biased agonist opioid. Around the keyword “knockout mice” (strength 2.58), the efficacy, safety, dosage, antagonists, adverse reactions,4,19,25,40 and addiction potential of oliceridine in mice have been gradually explored, clarifying that the β-arrestin2 pathway is responsible for morphine tolerance, while the Gi signaling pathway is associated with respiratory depression and constipation, significantly related to phosphorylation sites S363 and T370.41 Focusing on the keyword “healthy volunteers” (strength 3.02), the exploration revealed that oliceridine exhibits stronger analgesic effects, rapid onset, and milder opioid-related side effects than morphine; however, it still presents adverse reactions such as respiratory depression, nausea, dizziness, constipation, itching, and prolonged QT interval.
Analysis of the timeline indicated that clusters 0 # acute pain, 1# TRV130, 4 # postoperative pain, and 5 # therapeutic window have persisted to the present. This suggests that the current research is primarily focused on the precise application of oliceridine in managing acute pain and postoperative analgesia.42 Rat self-administration experiments and hot-plate analgesia tests suggest that G protein-biased MOR agonists do not offer an advantage in expanding the therapeutic window.43 However, the current body of research suggests that oliceridine has a broader therapeutic window than morphine or fentanyl in the management of acute pain and postoperative analgesia compared to morphine or fentanyl.15,21,23 This is believed to be related to its low intrinsic agonist efficacy.26 Phase I clinical trials have established that a single intravenous dose of 3 mg of oliceridine in healthy volunteers is effective, providing analgesia with lower respiratory depression, nausea, and vomiting.38 In patient-controlled analgesia (PCA) treatment, patients aged 18–65 using a loading dose of 1.5 mg and a self-controlled demand dose of 0.35 mg can rapidly and effectively achieve potent analgesia with minimal side effects.33 The therapeutic window of oliceridine in specific patient populations is also the focus of current research.10 For example, elderly patients exhibit increased sensitivity to opioids. Recent studies have shown that in older adults (ages 55–89), oliceridine administered intravenously over more than 60 seconds at a low dose of 0.5 mg is safe, while a dose of 2 mg can cause respiratory depression. The peak effect occurs within 0.5 to 1 hour after administration, but the onset and offset of respiratory depression are significantly faster than those of morphine, approximately five times faster, with less intensity of respiratory depression.12 According to the ATHENA trial data,5 oliceridine demonstrates a broader therapeutic window for elderly patients (aged ≥65 years) and obese patients (BMI ≥30 kg/m²) compared to commonly used opioids such as morphine and fentanyl.10 However, the study had a relatively small sample size and insufficient coverage. Further research is required to confirm the safety of oliceridine in these patients. Additionally, the therapeutic window for special patient groups, including children, pregnant women, patients with chronic pain, patients at high risk of postoperative vomiting and nausea (POVN), and those with sleep apnea, requires further exploration. Concurrently, similarly biased agonists like PMZ21 have shown lower respiratory depression and addiction potential than oliceridine,19 suggesting a potentially broader therapeutic window. Rigorous randomized controlled trials (RCTs) comparing oliceridines with these agents are necessary in the future.
Limitations
The current study had several limitations. First, we only selected the WOScc database for enrolling English-language literature, potentially omitting some studies. However, as WOScc is widely used in bibliometric analysis, we considered it comprehensive enough to depict the current research status of oliceridine. Second, as a newly developed analgesic, only 151 articles about oliceridine were included due to its recent introduction into clinical practice, and the ongoing nature of bibliometric analysis meant that recent publications were not included. Additionally, the limited number of human studies on oliceridine necessitates further research to clarify its clinical role and characteristics. Future studies should use larger sample sizes, more comprehensive databases, and include unpublished research data to ensure that the conclusions more accurately reflect real-world scenarios. Despite these limitations, this study provides a valuable overview of the current status and development trends in the field, offering crucial references and information for future oliceridine research and the development of new opioid drugs.
Conclusion
Oliceridine, an innovative opioid analgesic, has garnered significant research interest, especially for its clinical applications. We utilized visualization tools like VOS Viewer and CiteSpace to conduct a visual analysis of oliceridine research trends, providing pain management professionals with insights into its pharmacological properties and key aspects. CiteSpace’s Cluster Dependencies feature provides a comprehensive view of the oliceridine research landscape, highlighting its pivotal points. Future studies should focus on large-scale, randomized trials to expand oliceridine’s therapeutic profiles and assess benefits and risks in diverse populations, beyond surgical settings. Emphasis should be placed on long-term and patient-reported outcomes, personalized treatment protocols, and the development of molecular structures. Additionally, international collaboration, particularly between the US and China, is crucial for advancing the global research agenda on oliceridine.
Abbreviations
FDA, food and drug administration; MOR, mu opioid receptor; β-A, beta-arrestin; WOS, web of science; VOSviewer, visualization of similarities viewer; CiteSpace, citation space; WOScc, web of science core collection; S, silhouette Value; Q, modularity value; J Pain Res, journal of pain research; J Med Chem, journal of medicinal chemistry; J Pharmacol Exp Ther, journal of pharmacology and experimental therapeutics; SI, standard international (Units); TRV, Trevena (TRV); APPOLO, a phase 3 study of oliceridine in acute pain; BMI, body mass index; OIRD, opioid-induced respiratory depression; MD, molecular dynamics; GPCRs, g protein-coupled receptors; TRV130, brand name of a specific drug, PZM21, brand name of a specific drug; PCA, patient-controlled analgesia; PK/PD, pharmacokinetics/pharmacodynamics; EC50, half-maximal effective concentration; QTc, corrected qt interval; and CI, confidence interval.
Data Sharing Statement
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.
Consent for Publication
All authors have read and approved the final version of the manuscript.
Acknowledgments
We are grateful to Professor Zongming Jiang, a senior anesthesiologist employed at Shaoxing People’s Hospital, for his help in conception, writing, and critical review during the preparation of the manuscript.
Funding
This study was supported by the Medical and Health Technology Plan of Zhejiang Province (No:2024KY1727) and Zhejiang Provincial Natural Science Foundation of China (No: LY24H090002).
Disclosure
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Coussens NP, Sittampalam GS, Jonson SG, et al. The opioid crisis and the future of addiction and pain therapeutics. J Pharmacol Exp Ther. 2019;371(2):396–408. doi:10.1124/jpet.119.259408
2. Viscusi ER. Improving the therapeutic window of conventional opioids: novel differential signaling modulators. Reg Anesth Pain Med. 2019;44(1):32–37. doi:10.1136/rapm-2018-000010
3. DeWire SM, Yamashita DS, Rominger DH, et al. A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344(3):708–717. doi:10.1124/jpet.112.201616
4. Chen XT, Pitis P, Liu G, et al. Structure activity relationships and discovery of a G protein biased μ opioid receptor ligand, [(3-Methoxythiophen-2-yl)methyl]2[(9R)-9-(pyridin-2-y1)-6-oxaspiro-[4.5]decan-9-yl]ethylamine (TRV130), for the treatment of acute severe pain. J Med Chem. 2013;56(20):8019–8031. doi:10.1021/jm4010829
5. Bergese SD, Brzezinski M, Hammer GB, et al. ATHENA: a phase 3, open-label study of the safety and effectiveness of oliceridine (TRV130), A G-protein selective agonist at the μ-opioid receptor, in patients with moderate to severe acute pain requiring parenteral opioid therapy. J Pain Res. 2019;12:3113–3126. doi:10.2147/JPR.S217563
6. Thelwall M. Bibliometrics to webometrics. J Inf Sci. 2008;34(4):605–621. doi:10.1177/0165551507087238
7. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi:10.1007/s11192-009-0146-3
8. Chen C, Chen Y. Searching for clinical evidence in CiteSpace. AMIA Annu Symp Proc AMIA Symp. 2005;2005:121–125.
9. Hammer GB, Khanna AK, Michalsky C, et al. Oliceridine exhibits improved tolerability compared to morphine at equianalgesic conditions: exploratory analysis from two phase 3 randomized placebo and active controlled trials. Pain Ther. 2021;10(2):1343–1353. doi:10.1007/s40122-021-00299-0
10. Brzezinski M, Hammer GB, Candiotti KA, et al. Low incidence of opioid-induced respiratory depression observed with oliceridine regardless of age or body mass index: exploratory analysis from a Phase 3 open-label trial in postsurgical pain. Pain Ther. 2021;10(1):457–473. doi:10.1007/s40122-020-00232-x
11. Beard TL, Michalsky C, Candiotti KA, et al. Oliceridine is associated with reduced risk of vomiting and need for rescue antiemetics compared to morphine: exploratory analysis from two Phase 3 randomized placebo and active controlled trials. Pain Ther. 2021;10(1):401–413. doi:10.1007/s40122-020-00216-x
12. Simons P, van der Schrier R, van Lemmen M, et al. Respiratory effects of biased ligand oliceridine in older volunteers: a pharmacokinetic-pharmacodynamic comparison with morphine. Anesthesiology. 2023;138(3):249–263. doi:10.1097/ALN.0000000000004473
13. Viscusi ER, Skobieranda F, Soergel DG, Cook E, Burt DA, Singla N. APOLLO-1: a randomized placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the μ-opioid receptor, for management of moderate-to-severe acute pain following bunionectomy. J Pain Res. 2019;12:927–943. doi:10.2147/JPR.S171013
14. Váradi A, Marrone GF, Palmer TC, et al. Mitragynine/corynantheidine pseudoindoxyls as opioid analgesics with Mu agonism and delta antagonism, which do not recruit β-Arrestin-2. J Med Chem. 2016;59(18):8381–8397. doi:10.1021/acs.jmedchem.6b00748
15. Singla N, Minkowitz HS, Soergel DG, et al. A randomized, Phase IIb study investigating oliceridine (TRV130), a novel μ-receptor G-protein pathway selective (μ-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J PAIN Res. 2017;10:2413–2424. doi:10.2147/JPR.S137952
16. Fenalti G, Giguere PM, Katritch V, et al. Molecular control of δ-opioid receptor signalling. Nature. 2014;506(7487):191–196. doi:10.1038/nature12944
17. Singla NK, Skobieranda F, Soergel DG, et al. APOLLO-2: a randomized, placebo and active-controlled phase III study investigating oliceridine (TRV130), a G protein-biased ligand at the μ-opioid receptor, for management of moderate to severe acute pain following abdominoplasty. Pain Pract. 2019;19(7):715–731. doi:10.1111/papr.12801
18. Burford NT, Livingston KE, Canals M, et al. Discovery, synthesis, and molecular pharmacology of selective positive allosteric modulators of the δ-opioid receptor. J Med Chem. 2015;58(10):4220–4229. doi:10.1021/acs.jmedchem.5b00007
19. Manglik A, Lin H, Aryal DK, et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature. 2016;537(7619):185–190. doi:10.1038/nature19112
20. Kruegel AC, Gassaway MM, Kapoor A, et al. Synthetic and receptor signaling explorations of the Mitragyna Alkaloids: Mitragynine as an atypical molecular framework for opioid receptor modulators. J Am Chem Soc. 2016;138(21):6754–6764. doi:10.1021/jacs.6b00360
21. Viscusi ER, Webster L, Kuss M, et al. A randomized, phase 2 study investigating TRV130, a biased ligand of the μ-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157(1):264–272. doi:10.1097/j.pain.0000000000000363
22. Kliewer A, Schmiedel F, Sianati S, et al. Phosphorylation-deficient G-protein-biased μ-opioid receptors improve analgesia and diminish tolerance but worsen opioid side effects. Nat Commun. 2019;10(1):367. doi:10.1038/s41467-018-08162-1
23. Schmid CL, Kennedy NM, Ross NC, et al. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell. 2017;171(5):1165–1175.e13. doi:10.1016/j.cell.2017.10.035
24. Algera MH, Kamp J, Van Der Schrier R, et al. Opioid-induced respiratory depression in humans: a review of pharmacokinetic–pharmacodynamic modelling of reversal. Br J Anaesth. 2019;122(6):e168–e179. doi:10.1016/j.bja.2018.12.023
25. Altarifi AA, David B, Muchhala KH, Blough BE, Akbarali H, Negus SS. Effects of acute and repeated treatment with the biased mu opioid receptor agonist TRV130 (oliceridine) on measures of antinociception, gastrointestinal function, and abuse liability in rodents. J Psychopharmacol. 2017;31(6):730–739. doi:10.1177/0269881116689257
26. Gillis A, Gondin AB, Kliewer A, et al. Low intrinsic efficacy for G protein activation can explain the improved side effect profiles of new opioid agonists. Sci Signal. 2020;13(625):eaaz3140. doi:10.1126/scisignal.aaz3140
27. Soergel DG, Subach RA, Sadler B, et al. First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol. 2014;54(3):351–357. doi:10.1002/jcph.207
28. Hill R, Disney A, Conibear A, et al. The novel μ-opioid receptor agonist PZM21 depresses respiration and induces tolerance to antinociception. Br J Pharmacol. 2018;175(13):2653–2661. doi:10.1111/bph.14224
29. Dahan A, van Dam CJ, Niesters M, et al. Benefit and risk evaluation of biased μ-receptor agonist oliceridine versus morphine. Anesthesiology. 2020;133(3):559–568. doi:10.1097/ALN.0000000000003441
30. Bergese S, Berkowitz R, Rider P, et al. Low incidence of postoperative respiratory depression with oliceridine compared to morphine: a retrospective chart analysis. Pain Res Manag. 2020;2020:7492865. doi:10.1155/2020/7492865
31. Matthes HWD, Maldonado R, Simonin F, et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the µ-opioid-receptor gene. Nature. 1996;383(6603):819–823. doi:10.1038/383819a0
32. Schneider S, Provasi D, Filizola M. How oliceridine (TRV-130) binds and stabilizes a μ-opioid receptor conformational state that selectively Triggers G protein signaling pathways. Biochemistry. 2016;55(46):6456–6466. doi:10.1021/acs.biochem.6b00948
33. Kennedy NM, Schmid CL, Ross NC, et al. Optimization of a series of Mu Opioid Receptor (MOR) agonists with high G protein signaling bias. J Med Chem. 2018;61(19):8895–8907. doi:10.1021/acs.jmedchem.8b01136
34. Cheng J, Cheng T, Li W, et al. Computational insights into the G-protein-biased activation and inactivation mechanisms of the μ opioid receptor. ACTA Pharmacol Sin. 2018;39(1):154–164. doi:10.1038/aps.2017.158
35. Zhuang Y, Wang Y, He B, et al. Molecular recognition of morphine and fentanyl by the human μ-opioid receptor. Cell. 2022;185(23):4361. doi:10.1016/j.cell.2022.09.041
36. Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408(6813):720–723. doi:10.1038/35047086
37. Araldi D, Ferrari LF, Levine JD. Mu-opioid Receptor (MOR) biased agonists induce biphasic dose-dependent hyperalgesia and analgesia, and hyperalgesic priming in the rat. Neuroscience. 2018;394:60–71. doi:10.1016/j.neuroscience.2018.10.015
38. Soergel DG, Subach RA, Burnham N, et al. Biased agonism of the μ-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain. 2014;155(9):1829–1835. doi:10.1016/j.pain.2014.06.011
39. Fossler MJ, Sadler BM, Farrell C, et al. Oliceridine (TRV130), a novel G protein-biased ligand at the μ-opioid receptor, demonstrates a predictable relationship between plasma concentrations and pain relief. I: development of a pharmacokinetic/pharmacodynamic model. J Clin Pharmacol. 2018;58(6):750–761. doi:10.1002/jcph.1076
40. Schwienteck KL, Faunce KE, Rice KC, et al. Effectiveness comparisons of G-protein biased and unbiased mu opioid receptor ligands in warm water tail-withdrawal and drug discrimination in male and female rats. Neuropharmacology. 2019;150:200–209. doi:10.1016/j.neuropharm.2019.01.020
41. Xia J, Li X, Zhu H, et al. The μ-opioid receptor-mediated Gi/o protein and β-arrestin2 signaling pathways both contribute to morphine-induced side effects. Eur J Pharmacol. 2024;966:176333. doi:10.1016/j.ejphar.2024.176333
42. Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287–2298. doi:10.2147/JPR.S144066
43. Zamarripa CA, Edwards SR, Qureshi HN, Yi JN, Blough BE, Freeman KB. The G-protein biased mu-opioid agonist, TRV130, produces reinforcing and antinociceptive effects that are comparable to oxycodone in rats. Drug Alcohol Depend. 2018;192:158–162. doi:10.1016/j.drugalcdep.2018.08.002
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




