Back to Journals » Infection and Drug Resistance » Volume 18
A Case of Severe Community-Acquired Pneumonia Caused by Coinfection of Five Pathogens
Received 19 July 2024
Accepted for publication 6 March 2025
Published 19 March 2025 Volume 2025:18 Pages 1515—1519
DOI https://doi.org/10.2147/IDR.S483156
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Sha Min, Qingqing Lu, Yiling Zhang
Department of Pneumology, Zunyi Medical University, Zunyi, People’s Republic of China
Correspondence: Yiling Zhang, Zunyi Medical University and Guizhou Provincial People’s Hospital, Zunyi and Guiyang, Guizhou, 563006, People’s Republic of China, Tel +8618786775713, Email [email protected]
Introduction: Severe pneumonia is a serious pulmonary infection, and its high morbidity and mortality are associated with underlying diseases, treatment-induced immunodeficiency, co-infection of multiple pathogens, and increase of multi-resistant pathogens; For severe community-acquired pneumonia (SCAP) in immunocompromised patients, most of which are infected with rare atypical pathogens, mNGS as an unbiased and hypothesis-free approach to rapidly detect potential infectious agents in pulmonary mixed infections. The cases of simultaneous co-infection of five non-respiratory core pathogens represented by Nocardia farcinica have not been reported.
Case Presentation: This article will elaborate on a case of immunocompromised patient with nephrotic syndrome after corticosteroid treatment, who was diagnosed as SCAP after hospital admission and relevant laboratory examination. Bronchoalveolar lavage fluid (BALF) metagenome next-generation sequencing (mNGS) method identified as Nocardia farcinica, Aspergillus fumigatus, Pneumocystis jirovecii, cytomegalovirus and human coronavirus OC43 five pathogens co-infection, the patient improved and he was discharged after receiving the combination treatment of imipenem, ganciclovir, compound sulfamethoxazole, and fluconazole.
Conclusion: For SCAP patients with immunocompromised, there may be possible co-infection of multiple rare pathogens, low positive rate of conventional laboratory tests, mNGS can quickly and accurately identify pathogens, which can be used for targeted drug treatment, promote the early recovery of patients and reduce the abuse of broad-spectrum antibiotics.
Keywords: co-infection, SCAP, immunocompromised, mNGS
Introduction
Severe pneumonia is a serious lung infection that may present as community-acquired pneumonia (CAP), hospital-acquired pneumonia (HAP), or ventilator-associated pneumonia (VAP). Streptococcus pneumoniae is the most common bacterial pathogen in SCAP, and other pathogens include viruses, atypical bacteria, enteric gram-negative bacteria as well as rarely anaerobes.1–3 Among pathogens not covered by standard CAP therapy, immunocompromised patients are more likely to be infected with Nocardia farcinica, Aspergillus fumigatus, pneumocystis jirovecii, and viruses other than influenza, according to an international multicentre study analysis.4 The morbidity of SCAP is increasing year by year, the infection pathogen is more complex and may be more than one, the etiological diagnosis is difficult and the treatment is extremely challenging.
The etiologies of up to 62% of CAP remain undiagnosed despite comprehensive diagnostic work-up, possible reasons for such few detections include an inability to obtain lower respiratory tract specimens, antibiotic use before specimen collection, insensitive diagnostic tests for known pathogens.5 mNGS may serve as a new technique to overcome the shortcomings of conventional diagnostic methods. The chief advantage of mNGS lies in its unbiased sampling, high sensitivity and less affected by previous antibiotic exposure, which enables the simultaneous identification of all potentially infectious agents in samples.6
We report a case of SCAP caused by simultaneous pulmonary infection with Nocardia farcinica, Aspergillus fumigatus, Aspergillus fumigatus, cytomegalovirus and human coronavirus OC43, and have successfully treated one patient. No cases of concurrent pulmonary infection caused by these five pathogens have been reported.
Case Presentation
The patient was a 54-year-old male, developed cough, expectoration and chest pain without obvious inducement one week ago, and attended the local doctor, the treatment effect of the hospital was not satisfactory, so he was transferred to Guizhou Provincial People’s Hospital for treatment on September 06, 2023. History of hypertension for over 7 years; Nephrotic syndrome was diagnosed for 6 months, and 48 mg corticosteroids were taken regularly for treatment. After admission for physical examination: respiratory sounds in both lungs were reduced, wet rales were audible in both lungs, no positive signs in the heart and abdomen, and no swelling in both lower limbs. The patient initially had repeated hyperthermia, with a maximum body temperature of 39.2°C and 30 breaths per minute. The laboratory test results (Table 1) were as follows: Arterial blood gas analysis showed PH 7.45, Arterial partial pressure of oxygen (PaO2) 52mmHg, Arterial partial pressure of carbon dioxide (PaCO2) 32mmHg, oxygenation index 179mmHg; Inflammatory indicators were significantly increased, erythrocyte sedimentation rate (ESR) was rapid, cytomegalovirus DNA quantification was high, albumin, CD series, tuberculosis T Cell Spot Test (T-SPOT) number were low, and other examinations including sputum culture, blood culture, novel coronavirus nucleic acid detection, antibody detection of eleven respiratory pathogens, 1.3-β-d-glucan, EB virus DNA quantification, anti-neutrophil cytoplasmic antibody were negative. Chest computed tomography (CT) showed diffuse multiple lung lesions with partial fusion, cavity and peripheral halo signs. Mediastinal lymph node enlargement and necrosis; Bilateral pleural effusion (Figure 1). Echocardiography showed a small amount of pericardial effusion, and abdominal ultrasound showed no abdominal effusion.
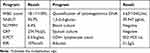 |
Table 1 Patient’s Laboratory Indicators |
 |
Figure 1 CT images of the patient’s lung and mediastinum at three stages: day 1 (A and B), day 16 (C and D), and day 47 (E and F). |
The initial diagnosis was SCAP with respiratory failure and cytomegalovirus infection. In view of the patient’s long-term corticosteroids treatment, immunocompromised and repeated high fever, the possibility of infection by opportunistic pathogens such as fungi should be considered, empirical antibiotics cefoperazone sodium and sulbactam sodium, ganciclovir, sulfamethoxazole, voriconazole anti-infection and thymalfasin should be used to enhance immunity. After the above treatment, the peak temperature of the patient was lower than before, but moderate fever still appeared repeatedly. On the sixth day, the patient developed mild edema of both lower limbs and B-ultrasound of blood vessels of the extremities showed deep venous blood flow, which was considered to be related to nephrotic syndrome after consultation by nephrologist of our hospital. And the level of D-dimer was high, so heparin preventive anticoagulation therapy was given.
On the tenth day, bacterial culture in BALF was negative, but mNGS results showed the following five pathogens: Nocardia farcinica, Aspergillus fumigatus, Pneumocystis jirovecii, cytomegalovirus, Human coronavirus OC43, the sequences of each pathogen are shown in the Table 2. After 3 days of the antibiotic change to imipenem treatment, the body temperature dropped to normal, inflammation index decreased significantly, quantification of cytomegalovirus DNA returned to normal; Pulmonary CT showed less absorption and more fluid in the right pleural cavity and interlobar fissure. Mediastinal lymph nodes enlarged as before (Figure 1).
 |
Table 2 mNGS Results in This Patient |
On the 21st day, the patient’s cough and sputum were significantly improved, and he was not bothered by cold, fever, chest pain and other discomfort, inflammatory indicators and CD series basically returned to normal, D-dimer decreased compared with the previous level. Imipenem and ganciclovir were stopped, and the patient was discharged from the hospital to continue oral treatment with compound sulfamethoxazole and voriconazole. One month later, the patient was followed up by telephone, CT examination of the lungs in another hospital showed that the two lungs were scattered in the ground glass shadow and the cord-like shadow, no obvious swelling of lymph nodes in the mediastinum, no obvious effusion in the chest (Figure 1), no cough, sputum and chest pain symptoms, and the clinical recovery was basically complete.
Discussion
As far as we know, this is the first case report of simultaneous detection of Nocardia farcinica, Aspergillus fumigatus, Pneumocystis jirovecii, cytomegalovirus and human coronavirus OC43 in bronchoalveolar lavage fluid of patients with severe community-acquired pneumonia with immunocompromised, which has important learning significance. First, the patient receiving long-term (>3 months) treatment with steroids because of nephrotic syndrome, belonging to the immunocompromised population;7 Secondly, the patient had an out-of-hospital onset, which was consistent with the diagnosis of severe community-acquired pneumonia combined with the patient’s clinical manifestations, laboratory examination and lung CT results.8
Immunocompromised patients with SCAP are more likely to be infected with Nocardia farcinica, Aspergillus fumigatus, Pneumocystis jirovecii, cytomegalovirus and viruses other than influenza, and have a higher incidence of co-infection with multiple pathogens.4,9 It is a great challenge for clinicians to identify the pathogens, conventional etiological detection methods such as sputum culture and blood culture were all negative for the above pathogens, with a low positive rate and samples easily contaminated, mNGS was used to detect the above pathogens. In recent years, mNGS has been gradually known and increasingly used for clinical assistance in diagnosis and treatment. It can better identify rare, novel, difficult-to-detect and coinfected pathogens,10,11 and for pneumonia patients, mNGS in BALF is more sensitive to the detection of bacteria and fungi than mNGS in blood.12 In the case of multiple non-respiratory core pathogens co-infection, mNGS once again shows its unique advantages.
mNGS as a culture-independent detection method the average typical turnaround times for most mNGS platforms from specimen receipt to the final results is 48 h,13 the duration of microbiological diagnosis is significantly shortened and accelerates clinical decision-making process. Miao et al investigated a cohort of 561 patients with acute or chronic infections demonstrated that mNGS has 50.7% sensitivity and 85.7% specificity for diagnosing infectious diseases. Moreover, the analytical performance of mNGS outperformed that of the culture, especially for fastidious organisms, such as Mycobacterium tuberculosis, viruses, anaerobes, and fungi14 In a meta-analysis about the diagnostic performance of metagenomic next-generation sequencing for the detection of pathogens in BALF results are shown as follows: the pooled sensitivity was 78% and the pooled specificity was 77% for mNGS. The positive detection rate of mNGS for pathogens in BALF of severely or immunocompromised pulmonary-infected patients was 92%.15 mNGS enables a broad range of pathogens to be identified from culture or directly from clinical samples on the basis of uniquely identifiable DNA and/or RNA sequences,16 the clinical utility of NGS in diagnosis may be in the most difficult-to-diagnose cases or for immunocompromised patients, in whom the spectrum of potential pathogens is greater.
Early antibiotic treatment is very beneficial to improve the survival rate of severe CAP patients, because the pathogenic pathogen cannot be identified when patients are first admitted to the hospital, it is necessary to ensure that the most popular pathogens are covered in the initial empirical antibiotic treatment.17 However, for patients with severe CAP with immunocompromised, there is no clear and unified standard for initial empirical treatment. The preliminary strategic consensus statement reached by Ramirez et al18 points out that in the absence of any other risk factors for drug-resistant bacteria, initial empirical treatment is only targeted at core respiratory pathogens. If there are risk factors for infection by resistant bacteria or opportunistic pathogens, empirical therapy should be extended beyond core respiratory pathogens; Combined treatment with cefoperazone sodium, sulbactam sodium, ganciclovir, sulfamethoxazole and voriconazole was initially used as an experience. Although the peak temperature of the patient decreased, the fever was still repeated, and the patient’s condition was completely controlled after the combination treatment with imipenem was replaced with antibiotics after the mNGS results were returned. Therefore, mNGS improves the detection conditions for fastidious organisms and promotes rational antibiotic therapy.
Nocardia farcinica infection can cause suppurative lesions such as pituitary abscess, brain abscess and empyema.19–21 In this case, enhanced chest CT showed enlargement and necrosis of mediastinum lymph nodes, and a significant increase in neutrophil percentage was found in a laboratory examination, so it is considered a suppurative infection. However, lymph node puncture could not be performed to determine the nature of the lesions due to the patient’s serious condition at that time. All in all, this case can provide some reference value for related clinical cases in diagnosis and treatment.
Abbreviations
SCAP, severe community-acquired pneumonia; mNGS, metagenome next-generation sequencing; CAP, community-acquired pneumonia; HAP, hospital-acquired pneumonia; VAP, ventilator-associated pneumonia; BALF, Bronchoalveolar lavage fluid; ESR, erythrocyte sedimentation rate; PaO2, Arterial partial pressure of oxygen; PaCO2, Arterial partial pressure of carbon dioxide; T-SPOT, T Cell Spot Test.
Acknowledgments
We would like to thank the patient for their approval of this paper. The patient provided written informed consent for the publication of case details and any accompanying images. Institutional approval was not required for case details to be published.
Funding
This study was supported by Department of Science and Technology of Guizhou Province Qiankehe basic project (ZK [2021] General No.344), the National Natural Science Foundation of China (Nos. No. 81860008).
Disclosure
The author(s) report no conflicts of interest in this work.
References
1. Cillóniz C, Torres A, Niederman MS. Management of pneumonia in critically ill patients. BMJ. 2021;375:e065871. doi:10.1136/bmj-2021-065871
2. Nair GB, Niederman MS. Updates on community acquired pneumonia management in the ICU. Pharmacol Ther. 2021;217:107663. doi:10.1016/j.pharmthera.2020.107663
3. Vallés J, Diaz E, Martín-Loeches I, et al. Evolution over a 15-year period of the clinical characteristics and outcomes of critically ill patients with severe community-acquired pneumonia. Med Intensiva. 2016;40(4):238–245. doi:10.1016/j.medin.2015.07.005
4. Di Pasquale MF, Sotgiu G, Gramegna A, et al, GLIMP Investigators. Prevalence and etiology of community-acquired pneumonia in immunocompromised patients. Clin Infect Dis. 2019;68(9):1482–1493. doi:10.1093/cid/ciy723
5. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. New Engl J Med. 2015;373(5):415–427. doi:10.1056/NEJMoa1500245
6. Wang J, Han Y, Feng J. Metagenomic next-generation sequencing for mixed pulmonary infection diagnosis. BMC Pulm Med. 2019;19(1):252. doi:10.1186/s12890-019-1022-4
7. Azoulay E, Russell L, Van de Louw A, et al, Nine-i Investigators. Diagnosis of severe respiratory infections in immunocompromised patients. Intensive Care Med. 2020;46(2):298–314. doi:10.1007/s00134-019-05906-5
8. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious diseases society of America; American thoracic society. infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44 Suppl 2(Suppl 2):S27–72. doi:10.1086/511159
9. Wu X, Sun T, Cai Y, et al. Clinical characteristics and outcomes of immunocompromised patients with severe community-acquired pneumonia: a single-center retrospective cohort study. Front Public Health. 2023;11:1070581. doi:10.3389/fpubh.2023.1070581
10. Han D, Li Z, Li R, et al. mNGS in clinical microbiology laboratories: on the road to maturity. Crit Rev Microbiol. 2019;45(5–6):668–685. doi:10.1080/1040841X.2019.1681933
11. Sun T, Wu X, Cai Y, et al. Metagenomic next-generation sequencing for pathogenic diagnosis and antibiotic management of severe community-acquired pneumonia in immunocompromised adults. Front Cell Infect Microbiol. 2021;11:661589. doi:10.3389/fcimb.2021.661589
12. Chen X, Ding S, Lei C, et al. Blood and bronchoalveolar lavage fluid metagenomic next-generation sequencing in pneumonia. Can J Infect Dis Med Microbiol. 2020;2020:6839103. doi:10.1155/2020/6839103
13. Diao Z, Han D, Zhang R, et al. Metagenomics next-generation sequencing tests take the stage in the diagnosis of lower respiratory tract infections. J Adv Res. 2021;38:201–212. doi:10.1016/j.jare.2021.09.012
14. Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl_2):S231–S240. doi:10.1093/cid/ciy693
15. Chen S, Kang Y, Li D, et al. Diagnostic performance of metagenomic next-generation sequencing for the detection of pathogens in bronchoalveolar lavage fluid in patients with pulmonary infections: systematic review and meta-analysis. Int J Infect Dis. 2022;122:867–873. doi:10.1016/j.ijid.2022.07.054
16. Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20(6):341–355. doi:10.1038/s41576-019-0113-7
17. Rello J, Perez A. Precision medicine for the treatment of severe pneumonia in intensive care. Expert Rev Respir Med. 2016;10(3):297–316. doi:10.1586/17476348.2016.1144477
18. Ramirez JA, Musher DM, Evans SE. Treatment of community-acquired pneumonia in immunocompromised adults: a consensus statement regarding initial strategies. Chest. 2020;158(5):1896–1911. doi:10.1016/j.chest.2020.05.598
19. Scheitler KM, Bauman MMJ, Carlstrom LP, Graffeo CS, Meyer FB. Nocardia farcinica pituitary abscess in an immunocompetent patient: illustrative case. J Neurosurg Case Lessons. 2022;4(18):CASE22266. doi:10.3171/CASE22266
20. Zayet S, Lang S, Ben Abdallah Y, Klopfenstein T, Gendrin V. Asymptomatic cerebral abscesses after pleuropulmonary Nocardia farcinica infection. New Microbes New Infect. 2020;38:100808. doi:10.1016/j.nmni.2020.100808
21. Ishikawa K, Mori N. Empyema necessitans due to Nocardia farcinica. IDCases. 2022;29:e01545. doi:10.1016/j.idcr.2022.e01545
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

