Back to Journals » Journal of Asthma and Allergy » Volume 17
A Case Series of Patients Undergoing Bronchial Thermoplasty a Second Time for Severe Asthma
Authors Foo CT, Langton D, Thien F
Received 23 August 2024
Accepted for publication 16 November 2024
Published 30 November 2024 Volume 2024:17 Pages 1239—1245
DOI https://doi.org/10.2147/JAA.S492730
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Chuan T Foo,1,2 David Langton,2,3 Francis Thien1,2
1Department of Respiratory Medicine, Eastern Health, Melbourne, VIC, Australia; 2Faculty of Medicine, Nursing and Health Sciences, Monash University, Melbourne, VIC, Australia; 3Department of Thoracic Medicine, Peninsula Health, Frankston, VIC, Australia
Correspondence: Chuan T Foo, Department of Respiratory Medicine, Eastern Health, Melbourne, VIC, Australia, Tel +613 9095 2414, Email [email protected]
Abstract: Bronchial thermoplasty is a treatment option for patients with severe asthma. We report a case series of 6 patients who underwent bronchial thermoplasty on two separate occasions for poorly controlled asthma. The repeat procedures were well tolerated with no unexpected complications. One patient developed a focal area of mild bronchiectasis on imaging 6-months after repeat treatment, but this was not felt to be clinically relevant. Individual responses to repeat bronchial thermoplasty were varied, with some patients showing great improvement after treatment, whereas others did not. This series highlights the safety and feasibility of performing repeat ablation on previously ablated airways, as well as the potential clinical benefit in a select group of patients.
Keywords: Asthma, mechanism of action, pathophysiology, treatment failure
Introduction
Asthma is a common disease characterized pathologically by the increased thickness of the airway smooth muscle layer (ASM). ASM plays a crucial role in bronchoconstriction, and correlates with worsening asthma severity.1
Bronchial thermoplasty (BT) is a treatment option for severe asthma and has been shown to improve clinical outcomes for at least 10 years.2,3 BT utilizes radiofrequency ablation to shrink the ASM, reducing airway hyperresponsiveness4–6 and the response to bronchodilators,7–9 with histological changes shown to persist for up to 2.5 years10 and perhaps even longer.11
About 20–40% of the patients fail to respond to BT, although the mechanism remains unknown.2,8,12–14 Given that the ASM is the primary target of BT, one possible explanation is that BT failed to reduce the ASM. Mathematical modelling and a small number of studies suggest a causal relationship between the degree of ASM ablation and clinical improvements.12,15,16 If this is true, there might be a potential role for re-treatment in patients who fail to respond to BT.
To our knowledge, there are no reports of repeat BT in the published literature. In this series, we document our initial experience of 6 patients with severe asthma who underwent repeat BT. We highlight the safety and feasibility of repeat BT, and describe three different outcomes using the following 3 cases (from the 6 listed in Tables 1 and 2). Repeat BT was approved by the Peninsula Health Human Research Ethics Committee and included permission to publish the cases. All patients have provided written informed consent for their data to be published.
 |
Table 1 Demographic and Clinical Characteristics for Patients N1-3 at Baseline, After Initial BT and After Repeat BT |
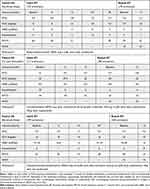 |
Table 2 Demographic and Clinical Characteristics for Patients N4-6 at Baseline, After Initial BT and After Repeat BT |
Cases
Case N2
A 62-year-old male had poorly controlled atopic asthma despite being treated with fluticasone propionate/formoterol 250/10 mcg 2 puffs twice daily, ciclesonide 160 mcg 2 puffs daily, glycopyrronium 50mcg daily, prednisolone 10 mg daily, and omalizumab. Pre BT, Asthma Control Questionnaire (ACQ) was 2.6, with an average reliever use of 5 nebulized salbutamol (5 mg)/day, and 12 exacerbations in the last 12 months. Forced expiratory volume in 1 second (FEV1) was 46% predicted, with a 75% bronchodilator response (BDR). BT was undertaken with 238 activations and resulted in a significant reduction in ACQ (defined as a ≥0.5 point reduction17), exacerbation frequency, and a 50% decrease in maintenance oral corticosteroid (OCS). FEV1 fluctuated over time, with ongoing large BDR. Three years later, the patient reported increasing asthma symptoms and more frequent exacerbations. Re-endotyping showed no change and the patient requested for repeat BT as he felt it had the greatest impact on his asthma control in his entire asthma history. Assessments before repeat BT showed an ACQ of 2.4, with an average reliever use of 6 nebulized salbutamol (5 mg) a day, and 12 exacerbations in the last 12 months. FEV1 was 32% predicted with a 166% BDR. Repeat BT was performed with 363 activations and resulted in a substantial decrease in exacerbation frequency and reliever use, as well as significant improvements in ACQ and FEV1 at 12-month follow-up. A summary of his clinical course can be found in Table 1.
Case N5
A 37-year-old male with eosinophilic asthma remained highly symptomatic despite receiving treatment with fluticasone furoate/vilanterol 200/25 mcg daily, umeclidinium 62.5 mcg daily, ciclesonide 160 mcg 2 puffs twice daily, prednisolone 50 mg daily, and mepolizumab. Prior to BT, he had an ACQ of 6, average reliever >16 puffs/day, 20 exacerbations in the last 12 months, and FEV1 34% predicted with no BDR. BT was performed with 117 activations. Although improvements in ACQ, maintenance OCS, and exacerbation frequency were observed, the patient continued to be highly symptomatic with an ACQ of 4.2, maintenance prednisolone of 25 mg, and an average of 1 exacerbation per month at 12-month follow-up. Six months later, the FEV1 had fallen to 23.4% predicted and prednisolone was at 50 mg daily. Due to the ongoing high symptom burden, excessive prednisolone requirement, and low number of activations during the initial BT, the patient underwent repeat BT with 180 activations. Follow-up 12-months post showed a non-significant reduction in ACQ from 4.2 to 3.8, with accompanying decreases in exacerbations, maintenance prednisolone, and an improvement in FEV1. A summary of his clinical course can be found in Table 2.
Case N6
A 38-year-old female with severe atopic asthma was being treated with fluticasone propionate/salmeterol 250/25 mcg two puffs twice daily, tiotropium 2.5mcg two puffs daily, prednisolone 10 mg daily, and omalizumab. Despite this, the patient was highly symptomatic with an ACQ of 4.4, average reliever 14–16 puffs/day, and 6 exacerbations in the preceding 12 months. FEV1 was 63.4% predicted with 27% BDR. BT was undertaken with 190 activations. This led to a 6–12-month improvement in asthma control (reduced ACQ, reliever use, and exacerbation frequency), which gradually worsened by 24-months. This prompted a change in the patient medications — fluticasone propionate/salmeterol was replaced with fluticasone furoate/vilanterol 200/25 mcg twice daily, and omalizumab was switched to mepolizumab (after re-endotyping). Nonetheless, the patient suffered from ongoing poor asthma control over the next 12 months with an ACQ of 4.2, an average reliever of 16–20 puffs/day, and 6 exacerbations in the intervening period. FEV1 was 57.7% predicted with 12.4% BDR. Repeat BT was undertaken with 220 activations. No change in asthma control was observed at 12-months. A summary of her clinical course can be found in Table 2.
Discussion
This is the first series describing repeat BT in patients with severe asthma, and highlights the safety and feasibility of performing further ablation on previously ablated airways. We also describe positive outcomes in a subset of patients who have regressed after initial treatment.
All patients in this series were non-smokers and had a diagnosis of severe asthma as defined by the European Respiratory Society/American Thoracic Society,18 and underwent BT as part of their clinical care. All patients were severely affected by their disease, as evidenced by high symptoms burden, frequent exacerbations, and persistent lung function impairment despite being on GINA step 4–5 treatment, with 4 out of 6 patients requiring maintenance OCS.
After the initial BT, each patient experienced a different degree of improvement, but ultimately, all of them deteriorated albeit at varying timepoints. Individual response following repeat BT was also highly variable, with patients N1-3 clearly deriving benefit, while improvements were marginal (N4,5) or absent in the others (N6).
The reasons for these observations remain unclear. Low tissue activations may have contributed to treatment failure, eg in patient N5 whom only had 117 activations on initial BT. This is much lower than the 140 activations recommended in one study,19 and less than the 151 activations (average) in the AIR2 trial.2 Given repeat BT with 180 activations only resulted in a marginal improvement, low tissue activations may not be the sole reason for his treatment failure.
Another explanation is that in standard BT, airways are treated at 65°C, the temperature shown to be the most effective in reducing ASM whilst minimizing collateral damage.20
Subsequent real-world data showed an approximately 50% reduction in ASM in patients post BT, a finding that correlates with clinical outcomes.10 Whether this magnitude of reduction is sufficient to elicit a beneficial response in patients with a thicker ASM remains unclear. Additionally, impaired reduction in ASM has been found in some patients after routine BT, raising the possibility of ASM resistance to thermal ablation, and the need for higher temperatures to achieve successful ablation.12,16 In addition to shrinking the ASM, BT has also been shown to cause sloughing of the airway epithelium on optical coherence tomography immediately after treatment.21 This recovers by 6-weeks, with evidence of increased epithelial integrity on histological biopsies at 3 months.22 Concerns over airway wall scarring have not been substantiated,23 and was not observed in any of our patients on serial chest computed tomography (CT).
Given that the ASM plays a crucial role in bronchoconstriction, it follows that a reduction in ASM post BT should accompany a decrease in bronchial reactivity. This, however, has not been consistently demonstrated in clinical studies. For example, the response to methacholine was mitigated by BT in,4–6 but not in.8,24 Similarly, the response to bronchodilator therapy after BT was reduced in,7–9 but unaffected in.12,13,25 In this case series, individual bronchodilator responsiveness was not observed to decrease after initial or repeat BT.
The role of the airway epithelium must also be considered. Epithelial damage from external insults (viruses, allergens, pollutants, BT etc.) stimulates the release of epithelial cytokines such as thymic stromal lymphopoietin, interleukin (IL)-33 and IL-25. These cytokines trigger a downstream inflammatory cascade that is abnormally perpetuated in asthmatics, ultimately leading to epithelial dysfunction, mucus hypersecretion and plugging, ASM hypertrophy and hyperplasia, and airway hyperresponsiveness (collectively known as airway remodelling).26,27 While none of our six patients showed evidence of mucus hypersecretion or plugging on serial chest CT, ASM regrowth from persistent airway inflammation and remodelling may explain the clinical trajectory in patients N1-N3 ie regrowth of the ASM after initial BT (treatment failure), and its successful re-ablation during repeat BT (restoration of asthma control). This phenomenon has previously been described in a single case report.28
The downregulation of the airway inflammatory cascade has recently been associated with clinical improvements post BT.29 In a small study involving 23 patients with severe asthma, BT responders, defined as those with <3 exacerbations in the 12 months post BT, were found to display a significantly different immunological profile than partial responders. In brief, responders had a higher incidence of atopy, blood eosinophilia, and serum IgE levels at baseline compared to partial responders. Twelve months post BT, blood eosinophil and IgE levels were exclusively reduced in responders, and associated with significant improvements in asthma control and quality of life. Importantly, 93% of the responders had previously failed to improve with omalizumab, suggesting a role for BT in this group of patients. Interestingly, both responders and partial responders had similar reductions in ASM, highlighting the impact of BT in attenuating the T2-high response. Of note, several other studies have also found significant correlations between patients with a T2-high endotype and clinical improvements post BT.8,30
All patients in this series had a T2-high endotype and were receiving asthma biologics throughout their initial and repeat BT with the exception of patients N1 and N3 (Patient N1 commenced asthma biologic 42 months after initial BT and was switched 12 months later; Patient N3 was receiving treatment with an asthma biologic prior to initial BT but ceased 6-months later due to lack of perceived efficacy). Despite this, all patients had poorly controlled asthma and a variable response to BT. Whether BT was successful in reducing the T2-high response in these patients remains uncertain.
Given the lack of airway biopsies and immune-profiling, these mechanisms remain speculative and require confirmation in future studies. Moreover, it is likely that a combination of factors, including some yet to be identified, are ultimately involved in determining treatment response/failure in BT.
All repeat BT procedures were generally well tolerated, with no unexpected periprocedural complications. Additionally, no major CT abnormalities were identified on serial scans taken before initial and repeat BT, and 6-months after repeat BT. Although patient N2 develop developed a localized area of mild bronchiectasis after repeat BT, this was not felt to be clinically relevant, as similar findings have been reported after standard BT.3
Conclusion
In conclusion, we present the first series describing the use of repeat BT in severe asthma, highlighting the safety and feasibility of such an approach, as well as the potential clinical benefit in a select group of patients. Further studies are needed to elucidate the mechanism of treatment response/failure and long-term safety of this approach.
Acknowledgments
The authors would like to thank Peninsula Health, Eastern Health and Monash University for supporting this research work. C.F is the recipient of a Monash University post-graduate scholarship.
Disclosure
C.F is the recipient of a Monash University post-graduate scholarship. D.L and F.T have no conflict of interest to report.
References
1. James AL, Bai TR, Mauad T, et al. Airway smooth muscle thickness in asthma is related to severity but not duration of asthma. Eur Respir J. 2009;34(5):1040–1045. doi:10.1183/09031936.00181608
2. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181(2):116–124. doi:10.1164/rccm.200903-0354OC
3. Chaudhuri R, Rubin A, Sumino K, et al. Safety and effectiveness of bronchial thermoplasty after 10 years in patients with persistent asthma (BT10+): a follow-up of three randomised controlled trials. Lancet Respir Med. 2021;9(5):457–466. doi:10.1016/S2213-2600(20)30408-2
4. Brown R, Wizeman W, Danek C, Mitzner W. Effect of bronchial thermoplasty on airway closure. Clin Med Circ Respirat Pulm Med. 2007;1:1–6. doi:10.4137/ccrpm.s365
5. Danek CJ, Lombard CM, Dungworth DL, et al. Reduction in airway hyperresponsiveness to methacholine by the application of RF energy in dogs. J Appl Physiol. 2004;97(5):1946–1953. doi:10.1152/japplphysiol.01282.2003
6. Cox G, Miller JD, McWilliams A, Fitzgerald JM, Lam S. Bronchial thermoplasty for asthma. Am J Respir Crit Care Med. 2006;173(9):965–969. doi:10.1164/rccm.200507-1162OC
7. Henry C, Biardel S, Boucher M, et al. Bronchial thermoplasty attenuates bronchodilator responsiveness. Respir Med. 2023;217:107340. doi:10.1016/j.rmed.2023.107340
8. Goorsenberg AWM, d’Hooghe JNS, Srikanthan K, et al. Bronchial thermoplasty induced airway smooth muscle reduction and clinical response in severe asthma. The TASMA randomized trial. Am J Respir Crit Care Med. 2021;203(2):175–184. doi:10.1164/rccm.201911-2298OC
9. Madsen H, Henriksen DP, Backer V, Siersted HC, Bjerring N, Ulrik CS. Efficacy of bronchial thermoplasty in patients with severe asthma. J Asthma. 2021;58(2):216–222. doi:10.1080/02770903.2019.1678636
10. Wijsman PC, Goorsenberg AWM, d’Hooghe JNS, et al. Airway smooth muscle and long-term clinical efficacy following bronchial thermoplasty in severe asthma. Thorax. 2024;79(4):359–362. doi:10.1136/thorax-2023-220967
11. Gagnon PA, Cote A, Klein M, et al. The reduction of airway smooth muscle by bronchial thermoplasty stands the test of time. ERJ Open Res. 2023;9(4):00024–2023. doi:10.1183/23120541.00024-2023
12. Pretolani M, Bergqvist A, Thabut G, et al. Effectiveness of bronchial thermoplasty in patients with severe refractory asthma: clinical and histopathologic correlations. J Allergy Clin Immunol. 2017;139(4):1176–1185. doi:10.1016/j.jaci.2016.08.009
13. Langton D, Ing A, Bennetts K, et al. Bronchial thermoplasty reduces gas trapping in severe asthma. BMC Pulm Med. 2018;18(1):155. doi:10.1186/s12890-018-0721-6
14. Foo CT, Donovan GM, Thien F, Langton D, Noble PB. Bronchial thermoplasty improves ventilation heterogeneity measured by functional respiratory imaging in severe Asthma. J Asthma Allergy. 2024;17:399–409. doi:10.2147/JAA.S454951
15. Donovan GM, Elliot JG, Green FHY, James AL, Noble PB. Unraveling a clinical paradox: why does bronchial thermoplasty work in Asthma? Am J Respir Cell Mol Biol. 2018;59(3):355–362. doi:10.1165/rcmb.2018-0011OC
16. Doeing DC, Husain AN, Naureckas ET, White SR, Hogarth DK. Bronchial thermoplasty failure in severe persistent asthma: a case report. J Asthma. 2013;50(7):799–801. doi:10.3109/02770903.2013.796974
17. Juniper EF, Bousquet J, Abetz L, Bateman ED, Committee G. Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the Asthma control questionnaire. Respir Med. 2006;100(4):616–621. doi:10.1016/j.rmed.2005.08.012
18. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
19. Langton D, Sha J, Ing A, Fielding D, Thien F, Plummer V. Bronchial thermoplasty: activations predict response. Respir Res. 2017;18(1):134. doi:10.1186/s12931-017-0617-7
20. Miller JD, Cox G, Vincic L, Lombard CM, Loomas BE, Danek CJ. A prospective feasibility study of bronchial thermoplasty in the human airway. Chest. 2005;127(6):1999–2006. doi:10.1378/chest.127.6.1999
21. Goorsenberg AWM, d’Hooghe JNS, de Bruin DM, van den Berk IAH, Annema JT, Bonta PI. Bronchial thermoplasty-induced acute airway effects assessed with optical coherence tomography in severe Asthma. Respiration. 2018;96(6):564–570. doi:10.1159/000491676
22. Chernyavsky IL, Russell RJ, Saunders RM, et al. In vitro, in silico and in vivo study challenges the impact of bronchial thermoplasty on acute airway smooth muscle mass loss. Eur Respir J. 2018;51(5):1701680. doi:10.1183/13993003.01680-2017
23. Jendzjowsky N, Laing A, Malig M, et al. Long-term modulation of airway remodelling in severe asthma following bronchial thermoplasty. Eur Respir J. 2022;59(1):2100622. doi:10.1183/13993003.00622-2021
24. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327–1337. doi:10.1056/NEJMoa064707
25. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132(6):1295–1302. doi:10.1016/j.jaci.2013.08.009
26. Varricchi G, Brightling CE, Grainge C, Lambrecht BN, Chanez P. Airway remodelling in asthma and the epithelium: on the edge of a new era. Eur Respir J. 2024;63(4):2301619. doi:10.1183/13993003.01619-2023
27. Russell RJ, Boulet LP, Brightling CE, et al. The airway epithelium: an orchestrator of inflammation, a key structural barrier and a therapeutic target in severe asthma. Eur Respir J. 2024;63(4):2301397. doi:10.1183/13993003.01397-2023
28. Kirby M, Ohtani K, Lopez Lisbona RM, et al. Bronchial thermoplasty in asthma: 2-year follow-up using optical coherence tomography. Eur Respir J. 2015;46(3):859–862. doi:10.1183/09031936.00016815
29. Ladjemi MZ, Di Candia L, Heddebaut N, et al. Clinical and histopathologic predictors of therapeutic response to bronchial thermoplasty in severe refractory asthma. J Allergy Clin Immunol. 2021;148(5):1227–35e6. doi:10.1016/j.jaci.2020.12.642
30. Sierra M, Fernandez-Bussy S, Mehta H, et al. Bronchial thermoplasty in severe uncontrolled Asthma with different phenotypes. Chest. 2017;152(4):A29. doi:10.1016/j.chest.2017.08.059
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Bronchial Thermoplasty Improves Ventilation Heterogeneity Measured by Functional Respiratory Imaging in Severe Asthma
Foo CT, Donovan GM, Thien F, Langton D, Noble PB
Journal of Asthma and Allergy 2024, 17:399-409
Published Date: 22 April 2024

