Back to Journals » Drug Design, Development and Therapy » Volume 19
A Comparative Evaluation of the Safety and Efficacy of Oliceridine and Sufentanil in Gastrointestinal Endoscopy: A Single-Center, Randomized Controlled Trial
Authors Ma B , Li Y, Leng C, Ji A, Zhang N, Tao X, Cao Q, Wang S
Received 16 December 2024
Accepted for publication 5 June 2025
Published 17 June 2025 Volume 2025:19 Pages 5111—5121
DOI https://doi.org/10.2147/DDDT.S512529
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Mariana Carmen Chifiriuc
Baoyu Ma, Ying Li, Cuibo Leng, Aozhang Ji, Ning Zhang, Xinyi Tao, Qianqian Cao, Shoushi Wang
Department of Anesthesiology and Perioperative Medicine, Qingdao Central Hospital, University of Health and Rehabilitation Sciences (Qingdao Central Medical Group), Qingdao, 266042, People’s Republic of China
Correspondence: Shoushi Wang, Department of Anesthesiology and Perioperative Medicine, Qingdao Central Hospital, University of Health and Rehabilitation Sciences (Qingdao Central Medical Group), Qingdao, 266042, People’s Republic of China, Email [email protected]
Purpose: Gastrointestinal (GI) endoscopic sedation employs a range of medication regimens; however, safer and more effective sedation protocols must be identified. Oliceridine, a novel biased μ-opioid receptor agonist, can reduce opioid-related adverse events. However, compared to traditional opioids, data on its use in GI endoscopic sedation remain limited.
Patients and Methods: This single-center, randomized controlled clinical trial was conducted between April and July 2024. In total, 628 patients scheduled for GI endoscopy were randomly assigned to receive either remimazolam-etomidate-oliceridine or remimazolam-etomidate-sufentanil for sedation. The primary outcome was the incidence of respiratory depression, and the secondary outcomes included the incidence of hypoxemia, need for airway intervention, procedure- and sedation-related metrics, sedation success rate, and adverse events.
Results: Among the 628 patients, 305 and 307 were randomized in the oliceridine and sufentanil groups, respectively, and completed the trial. Respiratory depression occurred in 43 patients (14.1%) in the oliceridine group compared to 67 patients (21.8%) in the sufentanil group (odds ratio, 0.59; 95% CI, 0.39– 0.90; p=0.013). No significant differences were observed in the incidence of hypoxemia between the groups; however, the need for airway intervention was significantly higher in the sufentanil group (p< 0.001). The sedation success rates were 99.7% and 100% in the oliceridine and sufentanil groups, respectively. Additionally, the oliceridine group demonstrated lower incidence of hypotension (11.8% vs 18.2%, p=0.026), postoperative nausea and vomiting (4.6% vs 10.1%, p=0.009), and higher patient satisfaction scores (9 [9,9] vs 9 [8,9], p=0.003).
Conclusion: The sedation success rate for GI endoscopy using remimazolam and etomidate in combination with either oliceridine or sufentanil approaches 100%, with oliceridine demonstrating superior safety and enhanced patient satisfaction.
Keywords: oliceridine, sufentanil, procedural sedation, gastrointestinal endoscopy
Introduction
Gastrointestinal (GI) endoscopic procedures are widely used to diagnose and treat gastrointestinal diseases.1 Sedation during GI endoscopy can enhance the patient experience and typically involves the use of sedative hypnotics and opioid analgesics.2 Previous research evidence supports the effectiveness of propofol in GI endoscopy despite frequent adverse events.3 Compared with propofol, remimazolam, an ultrashort-acting sedative, demonstrates a superior safety profile. However, the sedation success rate of remimazolam was lower than that of propofol.4,5 Therefore, some researchers have started exploring the use of remimazolam in combination with propofol.6 They found that co-administration resulted in fewer adverse events than propofol monotherapy, and had a better sedative effect and higher endoscopist satisfaction than remimazolam monotherapy.6 Similarly, our previous study demonstrated that remimazolam combined with etomidate has a lower risk of respiratory depression (odds ratio 0.52; 95% CI 0.29–0.93) and more stable hemodynamics compared to its combination with propofol (Unpublished data, ChiCTR2400085904). Therefore, a combination of remimazolam and etomidate appears to be a suitable choice.
The administration of sedatives can lead to a state of unconsciousness in patients during GI endoscopy; however, their bodies may still react to painful stimuli. Consequently, the use of analgesics during the diagnostic procedures is imperative to ensure patient comfort. The use of opioid analgesics during GI endoscopy improves patient tolerance; however, it increases adverse events, including respiratory depression, postoperative nausea, and vomiting. Oliceridine is a novel μ-opioid receptor agonist with a unique mechanism of action as a G protein-biased ligand.7 Unlike traditional opioids (eg, morphine, sufentanil), which activate both G protein-coupled signaling and β-arrestins pathways, oliceridine preferentially engages G protein signaling while minimizing β-arrestins recruitment. This biased agonism is hypothesized to preserve analgesic efficacy while reducing β-arrestins-mediated adverse effects, such as respiratory depression, gastrointestinal dysfunction, and tolerance development.8,9 Pharmacokinetically, oliceridine exhibits rapid onset (2–5 minutes) and short duration of action (half-life: 1.3–3.7 hours), making it well-suited for procedural sedation requiring rapid titration and recovery.10,11 Currently, several studies have highlighted the potential of remimazolam and oliceridine in the field of procedural sedation.12–14 However, no existing research has compared the safety and efficacy of oliceridine with those of traditional opioids for procedural sedation. Therefore, we designed a randomized controlled trial to compare the sedation efficacy and safety of remimazolam combined with etomidate, along with either oliceridine or sufentanil.
Materials and Methods
This trial was conducted at the Endoscopic Center between April 2024 and July 2024 and conformed with the principles of the Declaration of Helsinki. Patients scheduled to undergo elective GI endoscopy received either a remimazolam-etomidate-oliceridine or a remimazolam-etomidate-sufentanil regimen of sedation regimen. Written informed consent was obtained from all participants. The study was approved by the Medical Ethics Committee of Qingdao Central Medical Group on March 22, 2024, and was registered in the Chinese Clinical Trial Registry (ChiCTR2400082452).
Participants
The inclusion criterion for the trial was as follows: American Society of Anesthesiologists (ASA) physical status classification of I–III for adults undergoing elective gastroscopy combined with colonoscopy examination under procedural sedation. The exclusion criteria were severe respiratory depression; acute or severe bronchial asthma; known or suspected gastrointestinal obstruction; known allergy to the medications used in this study; severe hepatic, renal, or adrenal insufficiency; long-term use of opioids or benzodiazepines; abnormal QT interval; and anticipation of a difficult airway. Patients who initially underwent colonoscopy were excluded to maintain consistency in our outcome assessments.
Randomization and Blinding
The research staff generated a block randomization scheme. For sedation, the participants were randomly assigned to receive either oliceridine or sufentanil in a 1:1 ratio. Group allocation was kept secret from the participants and staff who administered procedural sedation, and the outcomes were evaluated. The unblinded staff members were the nursing staff who prepared oliceridine (Nhwa Pharma. Corporation, Jiangsu, China) and sufentanil (Humanwell Healthcare, Wuhan, China).
Intervention
Upon admission to the outpatient operating room, monitoring equipment was connected to detect vital signs, including blood oxygen saturation (SPO2), automated non-invasive blood pressure (NIBP), electrocardiography (ECG), and partial pressure of end-tidal carbon dioxide (PETCO2). Adequate oxygenation was provided with an endoscopic mask under spontaneous respiration (8–10 L/min for 3–5 min). Oxygen (6 L/min) was administered during the endoscopic procedure until the patient was fully awake.
Equal volumes of either oliceridine (1 or 1.5 mg, 1 mg/mL) or sufentanil (5 or 7.5 µg, 5 µg/mL) were prepared by nursing staff. Oliceridine or sufentanil was administered 3 min prior to remimazolam administration. Remimazolam was administered intravenously using a microinjection pump (0.15 mg/kg over 1 min), followed by slow injections of etomidate (0.1 mg/kg) and lidocaine (1 mg/kg). Subsequently, GI endoscopy was initiated when the patient was adequately sedated (Modified Observer’s Assessment of Alertness/Sedation [MOAA/S] ≤3). The GI endoscopies were performed by experienced attending physicians or higher. Supplemental doses of remimazolam (2.5 mg bolus) were administered after a 1-min interval of the etomidate dose if sedation was not considered adequate or gastroscopic entry failed. A maximum of five supplemental doses were administered within 15 min of sedation maintenance; otherwise, sedation was judged to have failed. If the initial and supplemental doses were insufficient to achieve adequate sedation during endoscopy, a remedial dose of etomidate (0.075 mg/kg) was administered. GI endoscopy may be performed under general anesthesia with tracheal intubation if the use of a remedial dose raises safety concerns. Anesthesiologists who had completed at least one training course were responsible for assessing the sedation levels. Sedation levels were assessed at 3-min intervals throughout the procedure, maintaining MOAA/S ≤3. Flumazenil (0.2–0.5 mg) was administered after withdrawal from the colonoscopy, after which the level of sedation was assessed at 1-min intervals until the patients were fully awakened (three consecutive MOAA/S scores of 5).
Outcomes
Primary Outcome
The primary outcome was the incidence of respiratory depression, defined as a respiratory rate of <8 breaths/min or SPO2<90%.15 The respiratory rate was determined by testing PETCO2. In case of a decrease in the respiratory rate or SPO2, the anesthesiologist must assess the accuracy of the values to ensure that the readings are normal. If a patient’s ineffective breathing due to airway obstruction is relieved by intervention, a respiratory rate above the threshold is not considered respiratory depression. The type and frequency of interventions must be documented. Specific interventions include repositioning, jaw thrust, and mask ventilation.
Other Outcomes
Other outcomes included the incidence of hypoxemia (SPO2 <90%, >10 s), number of airway interventions (eg, jaw lift and mask ventilation), number of endoscopes removed due to hypoxemia, and success rate of sedation (I. completion of the entire GI endoscopy procedure; II. no need for remedial sedation; and III. up to five supplemental doses of remimazolam were administered within 15 min of the initial dose). The procedure time (placement of gastroscope to withdrawal of colonoscopy), sedation time (administration of analgesics to complete awakening), and awakening time (administration of flumazenil to complete awakening) were recorded. The doses of administered sedatives and analgesics were recorded. The vital signs (mean arterial pressure [MAP], heart rate [HR], and SPO2) were recorded at different time points. Postoperative nausea and vomiting (PONV) were recorded in the post-anesthesia care unit (PACU). Endoscopist satisfaction was recorded (a score of 0–10 was assigned, with 0–3 categorized as unsatisfactory, 4–7 categorized as relatively satisfactory, and 8–10 categorized as satisfactory). On the first postoperative day (POD1), patient satisfaction (based on a score of 10 points, 0–3 was unsatisfactory, 4–7 was relatively satisfactory, and 8–10 was satisfactory), nausea, and vomiting was recorded.
Finally, adverse events were noted during the procedure, including hypotension (20% decrease in SBP from baseline or MAP < 60 mmHg), hypotension requiring treatment (hypotension and use of vasoactive agents), bradycardia (HR < 60 beats/min, >10 s), painful injections, and muscular tremors.
Sample Size
Incorporating the findings from our previous study and other published studies,6,16 the incidence of respiratory depression when using a combination of hypnotic drugs and opioids for GI endoscopy ranged from 10% to 20%. Based on a reference respiratory depression incidence of 15%, we anticipated that oliceridine would reduce the incidence of depression by 50%. We estimated a sample size of 278 patients, which had an 80% power to detect a significant difference level of 0.05. Considering a dropout rate of 10%, 306 patients were recruited in each group.
Statistical Analyses
All statistical analyses were performed using R software (version 4.2.2, R Foundation for Statistical Computing, Vienna, Austria), and MSTATA software (https://www.mstata.com/). For normally distributed data, continuous variables were analyzed using the Welch’s two-sample t-test and are presented as mean ± standard deviation. Non-normally distributed data were compared using the Wilcoxon rank-sum test, wherever appropriate, and the data are presented as the median and interquartile range. Categorical variables are expressed as numbers and frequencies and were analyzed using the χ2 test or Fisher’s exact test. Statistical significance was set at p<0.05. Furthermore, a subgroup analysis was performed to identify differences in the primary outcome according to age, sex, body mass index, ASA, Mallampati score, comorbidities, and PRODIGY score.15
Results
Figure 1 depicts an overview of the study schedule following the Consolidated Standards of Reporting Trials (CONSORT) guidelines.17 We screened 640 patients who scheduled to undergo elective GI endoscopy between April 2024 and July 2024. Among them, 12 patients were excluded for the following reasons: eight patients were on long-term benzodiazepines for insomnia, one patient had an abnormal QT interval on ECG, and three patients had poor communication. In total, 628 eligible patients were randomized to receive oliceridine (n=314) or sufentanil analgesia (n=314). In the oliceridine group, nine patients were unable to complete the trial: one patient opted for a gastroscopy only because of inadequate bowel preparation, one patient was found to have refluxed food in the esophagus, and for seven patients, their endoscopists decided to perform a colonoscopy first. In the sufentanil group, the endoscopists of five patients chose to perform colonoscopy first, and two patients chose to perform gastroscopy only because of poor intestinal preparation. Finally, 305 and 307 patients in the oliceridine and sufentanil groups, respectively, completed the study and follow-up.
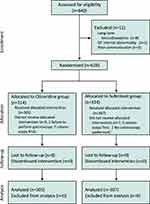 |
Figure 1 The flowchart of the participants in the study. |
The patients in both groups were comparable in terms of sex, age, body mass index, ASA, smoking, drinking, surgery history, PRODIGY score, STOP-Bang score, comorbiditie (Table 1).
 |
Table 1 Patient Demographics and Baseline Characteristics |
Procedure and Sedation-Related Outcomes
No statistically significant differences were observed in the procedure (31.5 ± 4.9 vs 32.1 ± 5.2), sedation (41.2 ± 5.2 vs 41.7 ± 5.3), and awake times (4[4,4] vs 4[4,4]) between the two groups (Table 2). In the oliceridine group, an increase in the use of remimazolam ([27.7 ± 3.5 vs 24.6 ± 4.5], p<0.001) was observed; conversely, in the sufentanil group, the infusion of crystalloid fluid was higher (500 [500,500] vs 500[500, 750], p=0.008). No statistical differences were observed in the amount of etomidate (6.99 ± 1.21 vs. 6.90 ± 1.27), lidocaine (70 ± 13 vs 69 ± 13), and flumazenil (0.3[0.3,0.3] vs 0.3[0.3,0.3]) used between the two groups. Figure 2 shows the documented changes in vital signs with respect to the elapsed sedation time.
 |
Table 2 Procedure and Sedation-Related Outcomes |
Primary and Secondary Outcomes
The primary outcome, respiratory depression, occurred in 43 patients (14.1%) in the oliceridine group and 67 patients (21.8%) in the sufentanil group (odds ratio, 0.59; 95% CI, 0.39–0.90; p=0.013) (Table 3). For respiratory depression, subgroup analyses were performed for predefined subgroups. Age, sex, body mass index, ASA, Mallampati score, comorbidities, and PRODIGY score were among the predetermined stratification factors (Figure S1). Oliceridine showed no benefits compared with sufentanil in an exploratory analysis of patients aged 18–45 years. A possible reason for this could be that younger individuals have a higher tolerance to low-dose opioids than older individuals. Conversely, the primary outcome demonstrated robustness in the analyses of other subgroups.
 |
Table 3 Primary and Secondary Outcomes |
Regarding secondary outcomes, hypoxemia occurred in six patients (2.0%) in the oliceridine group and in 13 patients (4.2%) in the sufentanil group, whereas endoscopes were removed due to hypoxemia in six patients (2.0%) in the oliceridine group and in 13 patients (4.2%) in the sufentanil group. Airway intervention was performed in 172 patients (56.4%) in the oliceridine group and 231 patients (75.2%) in the sufentanil group, with a statistically significant difference (p<0.001). A statistically significant difference was observed in the distribution of the number of airway interventions between the two groups (p<0.001), with 93 patients (30.3%) in the sufentanil group requiring 2–3 airway interventions and only 43 patients (14.1%) in the oliceridine group requiring airway interventions. One patient in the oliceridine group underwent remedial sedation, with a sedation success rate of 99.7%, whereas the patients in the sufentanil group did not undergo remedial sedation, with a sedation success rate of 100%.
Adverse Events and Postoperative Follow up
Table 4 lists the other adverse events during the GI endoscopy besides respiratory depression. Hypotension was observed in 11.8% of patients sedated with oliceridine versus 18.2% of patients sedated with sufentanil (p=0.026). Among those who developed hypotension, four patients (1.3%) in the oliceridine group and six patients (2.0%) in the sufentanil group required treatment, a difference that was not statistically significant (p=0.752). Bradycardia occurred in 40 patients (13.1%) in the oliceridine group and 45 patients (14.7%) in the sufentanil group. Bradycardia was treated aggressively in 11 patients (3.6%) in the oliceridine group and 18 patients (5.9%) in the sufentanil group. No patients experienced injection pain; however, muscle tremor was recorded in three patients, with one (0.3%) in the oliceridine group and two (0.7%) in the sufentanil group.
 |
Table 4 Adverse Events and Post-Operative Follow up |
In the PACU, PONV occurred in 14 patients (4.6%) in the oliceridine group and in 31 patients (10.1%) in the sufentanil group (p=0.009). Among them, six patients (2.0%) within the oliceridine group underwent the treatment, whereas 12 patients (3.9%) in the sufentanil group were treated accordingly. On the first postoperative day, PONV manifested in four patients (1.3%) in the sufentanil group and none in the oliceridine group. No significant differences were observed in endoscopist satisfaction between the two sedation regimens (9[9,9] vs 9[9,9.5]). Conversely, patients were more satisfied with the oliceridine group (9[9,9] vs 9[8,9], p=0.003).
Discussion
This randomized controlled trial compared the efficacy and safety of oliceridine and sufentanil in combination with remimazolam and etomidate during gastrointestinal endoscopy. The results showed that oliceridine provided comparable efficacy with a superior safety profile than sufentanil.
A survey found that outside operating theatres and intensive care units, endoscopy was the most frequent location where procedural sedation was used.18 The purpose of procedural sedation is to facilitate a diagnostic or therapeutic procedure with a target state in which airway patency, spontaneous respiration, protective airway reflexes, and hemodynamic stability are preserved, while alleviating anxiety and pain.19 The efficacy of sedation has garnered widespread recognition, and the ongoing pursuit of safety enhancement remains a focal point of current research efforts.12 Optimizing medication regimens and standardizing vital sign monitoring have been recognized as rational approaches.12 Researchers have made significant efforts to optimize medications for procedural sedation, leading to the continuous emergence of new drugs offering a wider range of options. Remimazolam and oliceridine are viewed as having significant potential for future applications due to their safety profiles.10–12 The safety of remimazolam for gastrointestinal endoscopy has been previously established.4–6 The integration of oliceridine into sedation protocols for GI endoscopy has yielded encouraging results. In this study, we observed a reduction in respiratory depression (14.1% vs 21.8%, p=0.013), hypotension (11.8% vs 18.2%, p=0.026), need for airway intervention (56.4% vs 75.2%, p<0.001), and PONV (4.6% vs 10.1%, p=0.009) when remimazolam and etomidate were combined with oliceridine than with sufentanil. These findings are noteworthy, as they demonstrate that oliceridine exhibits superior safety in GI endoscopic sedation relative to sufentanil, a benefit likely attributable to its unique pharmacological properties as a G protein-biased µ-opioid receptor agonist. However, other factors influencing outcome assessment must also be considered, including patients’ baseline physical condition and the generalizability of the outcome measures. Given the evidence obtained from this trial, a comprehensive evaluation and cautious interpretation are essential to effectively translate these findings into clinical practice.
The purpose of using analgesics in procedural sedation is to reduce noxious stimuli, requiring strong analgesic effects and rapid onset. Opioids are one of the best choices, despite their significant side effects. Both the analgesic and adverse effects of conventional opioids are mediated by the µ-opioid receptor.20,21 Activation of µ-opioid receptors engages analgesic signaling through G protein coupling to inhibit nociception by neuronal hyperpolarization but also engages β-arrestins to the same receptor, which promotes hypoventilation and gastrointestinal dysfunction.22 Oliceridine engages G protein coupling but with less β-arrestins recruitment, thus showing equivalent analgesic effects and fewer adverse events than morphine.8,9 Associated with lower side effects, oliceridine generates lower postoperative care costs compared to morphine when used for postoperative pain relief.23 And, pharmacological experiments have shown that dose adjustment is not required when oliceridine is used in patients with end-stage renal disease or mild to moderate hepatic impairment.24 A bibliometric analysis of oliceridine research from 2013 to 2024 reveals that scholars have shown significant interest in whether oliceridine could serve as a candidate to address the current challenges associated with opioid outcomes.25 For instance, researchers are investigating the optimal analgesic comparison between oliceridine and remifentanil for mechanically ventilated patients (NCT06454292), with the hope of obtaining noteworthy results.26
These studies, alongside our own, suggest promising application prospects; however, each drug has its limitations. The limitation of oliceridine lies in the ongoing controversy among scholars regarding its biased agonism capability. A recent study suggested that oliceridine does not display biased agonism, but rather weak intrinsic efficacy for G protein and β-arrestins activation.27 Consistent with these findings, our study demonstrated a higher remimazolam requirement in the oliceridine group compared to the sufentanil group, potentially attributable to oliceridine’s weaker intrinsic efficacy in providing adjunctive sedation. Irrespective of whether the low adverse event profile of oliceridine is attributed to biased agonism or weak intrinsic efficacy, as demonstrated in this study, compared with sufentanil, the use of oliceridine in GI endoscopy is advantageous for patients. Although clinical data indicate that higher doses of oliceridine and morphine can lead to respiratory depression,22 the duration of adverse events with oliceridine is significantly shorter than that with morphine,22,28 supporting the suitability of oliceridine for use in endoscopic procedures.
Among the myriad factors that influence research outcomes, the general physical condition of the patients and the criteria for outcome assessment are of paramount significance. In our subgroup analysis data, it appears that oliceridine may offer greater advantages in patients who are older and have higher PRODIGY scores. This phenomenon warrants further exploration in subsequent studies. Compared with previous study, our findings revealed a higher incidence of respiratory depression, likely because of the differing criteria for the duration of respiratory depression.15,29 However, our study demonstrated a significantly lower occurrence of hypoxemia (sufentanil group, 4.2% vs oliceridine group, 2.0%) than in previous studies (propofol group, 14.7% vs remimazolam group, 9.3%),30 which may be attributed to the use of endoscopy masks (enhancing oxygen concentration and providing mild positive pressure). This suggests that endoscopy-specific masks should be recommended during endoscopic procedures, when feasible. Another significant factor contributing to a lower incidence of hypoxemia was proactive airway intervention. The rate of airway intervention was 75.2% in the sufentanil group and 56.4% in the oliceridine group. This suggests that proactive airway interventions should be performed before hypoxemia occurs. Additionally, the rate and frequency of airway interventions were lower in the oliceridine group than those in the sufentanil group, further underscoring the safety of oliceridine. Another concern, prevalent in almost 40% of cases,4,5 is hypotension. Prolonged fasting and bowel preparation lead to insufficient fluid volume, and the use of sedatives and analgesics can suppress the vascular tone, especially in older individuals and patients with hypertension, where the decrease in blood pressure is more pronounced. Remimazolam and etomidate cause less cardiovascular depression than propofol.4,5,31 In our study, the incidence of hypotension in the oliceridine group was 11.8% compared to 18.2% in the sufentanil group, which is generally in line with previous studies.32,33 The hemodynamic stability demonstrated by these results is likely to encourage healthcare providers to prefer a combination of remimazolam, etomidate, and oliceridine. Our study revealed a higher incidence of bradycardia (13.1% in the oliceridine group vs 14.7% in the sufentanil group), especially during colonoscopy, during which prolonged intestinal stimulation triggers a strong vagal reflex. Proactive management, including the use of cardiovascular medications and fluid infusions, is essential. During etomidate administration, anesthesiologists primarily focus on two issues: muscle tremors and the suppression of adrenal cortical function. In this study, muscle tremors occurred in three patients (0.5%), with two in the sufentanil group and one in the oliceridine group; such a low incidence did not appear to be clinically significant. Regarding adrenal cortical function suppression, the etomidate dosage used in this study was minimal, and previous studies have indicated that total intravenous anesthesia with etomidate only temporarily suppresses adrenal cortical function.34 Beyond safety, the primary tenets of sedation during endoscopic procedures include patient comfort and satisfaction. Our study found that patients in the oliceridine group exhibited higher satisfaction scores, which were likely associated with a lower incidence of PONV. Patient satisfaction is predominantly derived from reduced anxiety and expedited postoperative recovery, with PONV significantly impairing comfort during medical care. Oliceridine has been previously demonstrated to have fewer gastrointestinal side effects than morphine,22 and the findings of this study corroborate this observation.
Overall, the focus of research on oliceridine has primarily centered on its potential to reduce adverse effects. Our study demonstrated that oliceridine, when used for sedation during gastrointestinal endoscopy, is associated with fewer side effects compared to sufentanil, thereby providing positive evidence for its efficacy. Based on these findings, we recommend the use of oliceridine, a novel opioid, in sedation protocols for gastrointestinal endoscopy. Additionally, our study advocates the use of specialized endoscopic masks during sedation and emphasizes proactive management of adverse reactions, including airway interventions, aggressive fluid resuscitation, and the administration of cardiovascular medications.
However, our findings should be interpreted in light of several limitations. First, as the trial was a single-center study, further multi-center prospective studies are necessary. Second, when assessing respiratory depression and postoperative nausea and vomiting, the assessment metrics could be more refined and improved, such as changes in ventilation and nausea and vomiting scores. Third, the trial was not able to properly assess the comparative sedation effects, such as somatic movements and choking responses that affect endoscopist manipulation. Future studies should include multi-center, large-sample studies to clarify the efficacy and safety of oliceridine for endoscopy, which needs to be evaluated in different populations, including older individuals and high-risk groups. Moreover, the risks of possible complications must be further explored.
Conclusion
In conclusion, our study found that oliceridine demonstrated superior safety compared with sufentanil in gastrointestinal endoscopic procedures, offering an additional analgesic option for gastrointestinal endoscopy.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to the privacy policy but are available from the corresponding authors on reasonable requests.
Ethical Approval
All procedures performed on the patients were in accordance with the 1964 helsinki declaration and its later amendments. The study was approved by the Medical Ethics Committee of Qingdao Central Medical Group (KY202404702).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Wu Jieping Medical Foundation (320.6750.2024-05-44).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Yao K, Uedo N, Kamada T, et al. Guidelines for endoscopic diagnosis of early gastric cancer. Dig Endosc. 2020;32(5):663–698. doi:10.1111/den.13684
2. Gotoda T, Akamatsu T, Abe S, et al. Guidelines for sedation in gastroenterological endoscopy (second edition). Dig Endosc. 2021;33(1):21–53. doi:10.1111/den.13882
3. Dossa F, Megetto O, Yakubu M, Zhang DDQ, Baxter NN. Sedation practices for routine gastrointestinal endoscopy: a systematic review of recommendations. BMC Gastroenterol. 2021;21(1):22. doi:10.1186/s12876-020-01561-z
4. Barbosa EC, Espírito Santo PA, Baraldo S, Meine GC. Remimazolam versus propofol for sedation in gastrointestinal endoscopic procedures: a systematic review and meta-analysis. Br J Anaesth. 2024;132(6):1219–1229. doi:10.1016/j.bja.2024.02.005
5. Zhao MJ, Hu HF, Li XL, Li XM, Wang DC, Kuang MJ. The safety and efficacy between remimazolam and propofol in intravenous anesthesia of endoscopy operation: a systematic review and meta-analysis. Int J Surg. 2023;109(11):3566–3577. doi:10.1097/JS9.0000000000000638
6. Wei A, Ma S, Dou Y, et al. The safety and efficacy of remimazolam tosylate combined with propofol in upper gastrointestinal endoscopy: a multicenter, randomized clinical trial. PLoS One. 2023;18(8):e0282930. doi:10.1371/journal.pone.0282930
7. Chen XT, Pitis P, Liu G, et al. Structure-activity relationships and discovery of a G protein biased μ opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan-9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J Med Chem. 2013;56(20):8019–8031. doi:10.1021/jm4010829
8. Bergese S, Berkowitz R, Rider P, et al. Low incidence of postoperative respiratory depression with oliceridine compared to morphine: a retrospective chart analysis. Pain Res Manag. 2020;2020:7492865. doi:10.1155/2020/7492865
9. Niu J, Hu W, Lu Y, Tang H. Efficacy and safety of oliceridine treatment in patients with postoperative pain: a systematic review and meta-analysis of randomized controlled trials. Expert Rev Clin Pharmacol. 2023;16(6):589–599. doi:10.1080/17512433.2023.2213889
10. Simons P, van der Schrier R, van Lemmen M, et al. Respiratory effects of biased ligand oliceridine in older volunteers: a pharmacokinetic-pharmacodynamic comparison with morphine. Anesthesiology. 2023;138(3):249–263. doi:10.1097/ALN.0000000000004473
11. Soergel DG, Subach RA, Sadler B, et al. First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J Clin Pharmacol. 2014;54(3):351–357. doi:10.1002/jcph.207
12. Sneyd JR. Developments in procedural sedation for adults. BJA Educ. 2022;22(7):258–264. doi:10.1016/j.bjae.2022.02.006
13. Finlay JE, Leslie K. Sedation/analgesia techniques for nonoperating room anesthesia: new drugs and devices. Curr Opin Anaesthesiol. 2021;34(6):678–682. doi:10.1097/ACO.0000000000001057
14. Goudra B, Singh PM. Oliceridine and its potential to revolutionize GI endoscopy sedation. Saudi J Anaesth. 2020;14(3):349–354. doi:10.4103/sja.SJA_813_19
15. Khanna AK, Bergese SD, Jungquist CR, et al. Prediction of opioid-induced respiratory depression on inpatient wards using continuous capnography and oximetry: an international prospective, observational trial. Anesth Analg. 2020;131(4):1012–1024. doi:10.1213/ANE.0000000000004788
16. Dong SA, Guo Y, Liu SS, et al. A randomized controlled clinical trial comparing remimazolam to propofol when combined with alfentanil for sedation during ERCP procedures. J Clin Anesth. 2023;86:111077. doi:10.1016/j.jclinane.2023.111077
17. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732. doi:10.7326/0003-4819-152-11-201006010-00232
18. SWARM. Sedation practice in six acute hospitals-a snapshot survey. Anaesthesia. 2015;70:407–415. doi:10.1111/anae.12940
19. Green SM, Irwin MG, Mason KP; International Committee for the Advancement of Procedural Sedation. Procedural sedation: providing the missing definition. Anaesthesia. 2021;76(5):598–601. doi:10.1111/anae.15213
20. Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3(3):159–180. doi:10.1054/jpai.2002.123652
21. Kieffer BL. Opioids: first lessons from knockout mice. Trends Pharmacol Sci. 1999;20:19–26. doi:10.1016/S0165-6147(98)01279-6
22. Soergel DG, Subach RA, Burnham N, et al. Biased agonism of the μ-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain. 2014;155(9):1829–1835. doi:10.1016/j.pain.2014.06.011
23. Simpson KN, Fossler MJ, Wase L, Demitrack MA. Cost-effectiveness and cost-benefit analysis of oliceridine in the treatment of acute pain. J Comp Eff Res. 2021;10(15):1107–1119. doi:10.2217/cer-2021-0107
24. Nafziger AN, Arscott KA, Cochrane K, Skobieranda F, Burt DA, Fossler MJ. The influence of renal or hepatic impairment on the pharmacokinetics, safety, and tolerability of oliceridine. Clin Pharmacol Drug Dev. 2020;9(5):639–650. doi:10.1002/cpdd.750
25. Wang C, Liu L, Bai X. Global Trends in Oliceridine (TRV130) research from 2013 to 2024: a bibliometrics and knowledge graph analysis. Drug Des Devel Ther. 2024;18:4681–4692. doi:10.2147/DDDT.S475205
26. Luo JC, Lu S, Fu XL, et al. Comparison of Oliceridine to Remifentanil for Optimal Analgesia in Mechanical Ventilation (CO-ROAM): study protocol for a multicenter randomized controlled trial. Pain Ther. 2024;13(6):1695–1704. doi:10.1007/s40122-024-00669-4
27. Ventriglia E, Rizzo A, Gomez JL, et al. Essential role of P-glycoprotein in the mechanism of action of oliceridine. Neuropsychopharmacology. 2023;48(5):831–842. doi:10.1038/s41386-022-01507-x
28. Hill R, Sanchez J, Lemel L, et al. Assessment of the potential of novel and classical opioids to induce respiratory depression in mice. Br J Pharmacol. 2023;180(24):3160–3174. doi:10.1111/bph.16199
29. Hu B, Jiang K, Shi W, et al. Effect of remimazolam tosilate on respiratory depression in elderly patients undergoing gastroscopy: a multicentered, prospective, and randomized study. Drug Des Devel Ther. 2022;16:4151–4159. doi:10.2147/DDDT.S391147
30. Liu F, Cheng X, Wang Y, et al. Effect of remimazolam tosilate on the incidence of hypoxemia in elderly patients undergoing gastrointestinal endoscopy: a bi-center, prospective, randomized controlled study. Front Pharmacol. 2023;14:1131391. doi:10.3389/fphar.2023.1131391
31. Goudra B. Big sleep: beyond propofol sedation during GI endoscopy. Dig Dis Sci. 2019;64(1):1–3. doi:10.1007/s10620-018-5287-x
32. Liu X, Ding B, Shi F, et al. The efficacy and safety of remimazolam tosilate versus etomidate-propofol in elderly outpatients undergoing colonoscopy: a prospective, randomized, single-blind, non-inferiority trial. Drug Des Devel Ther. 2021;15:4675–4685. doi:10.2147/DDDT.S339535
33. Zhu H, Su Z, Zhou H, et al. Remimazolam dosing for gastroscopy: a randomized noninferiority trial. Anesthesiology. 2024;140(3):409–416. doi:10.1097/ALN.0000000000004851
34. Lu Z, Zheng H, Chen Z, et al. Effect of etomidate vs propofol for total intravenous anesthesia on major postoperative complications in older patients: a randomized clinical trial. JAMA Surg. 2022;157(10):888–895. doi:10.1001/jamasurg.2022.3338
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.