Back to Journals » Drug Design, Development and Therapy » Volume 19
A Mechanistic Study of the Feasibility of Ursodeoxycholic Acid in the Treatment of Colon Adenocarcinoma
Authors Liu S, Zhou M, Huang X, Chen P, Li Q, Wang Y, Ge X, Wang F, Xu J, Gu J, Miao L, Deng X
Received 13 October 2024
Accepted for publication 1 March 2025
Published 12 March 2025 Volume 2025:19 Pages 1839—1852
DOI https://doi.org/10.2147/DDDT.S500721
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Shuyu Liu,1,2,* Mengyue Zhou,3,* Xiaoli Huang,2,* Peng Chen,2 Quanpeng Li,1 Yuting Wang,1 Xianxiu Ge,1 Fei Wang,1 Jianing Xu,1 Jiayi Gu,4 Lin Miao,1 Xueting Deng1
1Medical Center for Digestive Disease, The Second Affiliated Hospital of Nanjing Medical University, Nanjing, People’s Republic of China; 2Department of Gastroenterology, The Fourth Affiliated Hospital of Nanjing Medical University, Nanjing, People’s Republic of China; 3Department of Gastroenterology, Nanjing Pukou Hospital of Traditional Chinese Medicine, Nanjing, People’s Republic of China; 4Department of Neurology, The Fourth Affiliated Hospital of Nanjing Medical University, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lin Miao; Xueting Deng, Medical Center for Digestive Disease, The Second Affiliated Hospital of Nanjing Medical University, 121 Jiangjiayuan, Nanjing, Jiangsu, 210011, People’s Republic of China, Tel +86-13913862526, Email [email protected]; [email protected]
Purpose: Bile acids promote the progression of colon adenocarcinoma (COAD), and ursodeoxycholic acid (UDCA) is a key drug in promoting bile acid excretion, but its role in COAD unclear. Our study aims to investigate the relationship between COAD and bile acid metabolism and to assess the feasibility of UDCA for the treatment of COAD.
Methods: Firstly, biological targets closely related to COAD were identified: Based on the cancer genome atlas (TCGA) database, the core genes of COAD were obtained by differential expression analysis and weighted gene-coexpression network analysis (WGCNA), and subjected to gene ontology (GO) function annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. Secondly, finding a drug by target, after identifying UDCA as a candidate drug, the feasibility of UDCA in treating COAD was verified in reverse: Using databases to collect potential targets for COAD and UDCA, and the intersecting genes were the potential targets for UDCA to exert anti-tumor effects. Then Autodock was used for molecular docking to analyze the interaction between UDCA and core target proteins. Finally, experimental validation was performed: MTT assay, wound healing, transwell migration, and angiogenesis assays were used to detect the effects of UDCA on cell proliferation, migration, invasion, and neovascularization.
Results: 2064 differential genes were screened from TCGA. WGCNA obtained the module most relevant to CRC, containing 493 genes. KEGG analysis found that overlapping genes were mainly concentrated in bile acid metabolic pathways. A total of 26 UDCA anti-tumor targets were obtained in database, and 5 core targets were selected by STRING database and Cytoscape software: TNF, CYP27B1, MDM2, MMP2, CASP3. Molecular docking results showed that UDCA had good binding activity with the core targets. In vitro experiment showed UDCA effectively inhibited the proliferation, migration, invasion and neovascularization in colon cancer cells.
Conclusion: The antitumor activity of ursodeoxycholic acid may be related to cell apoptosis, proliferation, migration and vascular neogenesis.
Keywords: ursodeoxycholic acid, colon adenocarcinoma treatment, bile acid metabolism, cell death
Background
Colon adenocarcinoma is at the forefront of the incidence and mortality of malignant tumors. According to the 2023 Global Cancer Data report, the incidence of colon adenocarcinoma ranks the third among malignant tumors (10%) and the mortality ranks the second among malignant tumors (9.4%),1,2 which seriously endangers public life and health. The pathogenesis o f colon adenocarcinoma is complex, and the only way to treat the disease with drugs is intravenous infusion of fluorouracil drugs, such as the combination of 5-fluorouracil and folinic acid (5 Fu/LV), which has high toxicity, heavy adverse reactions and lack of specificity,3 and with the increase of treatment course, many patients are faced with the challenge of drug resistance. Although radiotherapy can accurately target the tumor, it inevitably causes damage to the surrounding normal tissue during irradiation, resulting in inflammatory reactions such as radiation enteritis. In addition, the high-energy radiation used in radiotherapy will increase the risk of recurrence of tumors, especially for young colorectal adenocarcinoma patients, pelvic radiotherapy may affect the function of the ovary or testis, causing long-term damage to the patient’s reproductive system.
Recently, some studies have combined bioinformatics methods to clarify the key roles of metabolism, inflammation, and bile acid secretion in colon adenocarcinoma.4,5 However, none of these studies applied weighted gene co-expression network analysis (WGCNA) to look for Hub genes in colon adenocarcinoma. At present, researchers have developed drugs targeting metabolism and inflammation, such as STF-31, V-9302, and achieved good clinical benefits. However, there are few studies on the application of drugs targeting bile acid metabolism in the treatment of colon adenocarcinoma.6,7 Therefore, in order to deeply understand the molecular mechanism of colon adenocarcinoma, In addition, to verify whether improving bile acid metabolism can alleviate colon adenocarcinoma (CRC) progression, we innovatively combined WGCNA and network pharmacology in this study to explore the feasibility of ursodeoxycholic acid in the treatment of colon adenocarcinoma. Network pharmacology is an emerging research method to analyze the mechanism of drug multi-target treatment of diseases from the system level, which can effectively locate drug targets and establish a “drug-target-disease” biological network to reveal the regulation principle of small molecules in a high-throughput manner.8,9 Network pharmacology explores the mechanism and pathway of drug action, which helps to improve the therapeutic effect of drugs, reduce toxic side effects, and improve the success rate of clinical trials of new drugs.
Ursodeoxycholic acid (UDCA) is a natural hydrophilic bile acid that is actively used in the treatment and prevention of cholelithiasis by inhibiting the activity of cholesterol 7A-hydroxylase and cholesterol esterase in bile, reducing cholesterol synthesis and esterification, and thus reducing cholesterol saturation.10 In addition, UDCA can improve the function and expression of Cl-/HCO3- cotransporter AE2 (Anion2), which is a key transporter for bile outflow,11 and the expression of bile acid transporters in the capillary bile ducts of hepatocytes is upregulated,12 such as BSEP, MRP2, thereby promoting bile, bile acid outflow, widely used clinically in primary biliary cholangitis (PBC) and primary sclerosing cholangitis (PSC). In recent years, the potential of UDCA in cancer adjuvant therapy has attracted much attention. Narisawa et al13 found that ursodeoxycholic acid (UDCA) could reduce the incidence of colon adenocarcinoma in animal models of colon adenocarcinoma induced by N-methylnitrosourea, possibly because the concentration and composition of bile acids in feces affected the abundance and distribution of intestinal flora, thus affecting the occurrence and development of colon adenocarcinoma. This study investigated the mechanism of ursodeoxycholic acid in reducing the occurrence of colon adenocarcinoma through network pharmacological research, and provided a new treatment plan for colon adenocarcinoma patients, so as to reduce the incidence and mortality of colon adenocarcinoma.
Methods and Materials
Data Source and Pre-Processing
In this study, the cancer type was selected as Colon adenocarcinoma (TCGA-COAD) and the file data type was selected as RNA-seq in the Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/). Directly download the expression data and patient-related clinical data, and use Perl script and python script to integrate the data extraction. RNA sequencing data corresponded to 41 normal tissue samples and 455 tumor samples from 456 colon cancer patients. If the first two principal components determined by principal component analysis could not distinguish tumor tissue from normal tissue, we exclude the sample. Patient samples with incomplete clinical data should also be excluded. The research workflow is shown in Figure 1.
 |
Figure 1 Study flow chart. |
Ethics Approval
This study involving human data, including publicly available data, was reviewed and approved by The Second Affiliated Hospital of Nanjing Medical University Ethics Committee (KY-2020-092).
DEGs Screening
Software (edgeR package.) was used to analyze the COUNT data of mRNA in tumor tissue and normal tissue to obtain cancer-related differentially expressed genes (DEGs) in COAD. The thresholds for differential expression screening were |logFC|>2 and FDR<0.05. The DEGs volcano map was drawn using “ggplo2” package in the R.
Weighted Gene Co-Expression Network Analysis
R-Software (WGCNA package) was used to perform gene module clustering for all the genes obtained in 1.1, and the correlation between module eigengene (ME) and clinical characterization was evaluated. The soft threshold β was set to 7 to ensure a scale-free network, and the blockwiseModules function was used to construct a clustering dendrogram, and the branches of the clustering tree were merged into different gene modules by the dynamic tree clipping method, with the minimum number of genes set to 30 and the heterogeneity threshold set to 0.25. We evaluated the association of different modules with the onset of colon adenocarcinoma and selected the most relevant modules as WGCNA-derived hub genes, and visualized the association of clinical characterization data with gene modules using R-package “pheatmap”.
Go/KEGG Analysis
Gene ontology (GO) and KEGG enrichment analysis were performed using DAVID (https://david.ncifcrf.gov/)14 in order to facilitate our comprehension of the potential mechanism of COAD.
Prediction of Ursodeoxycholic Acid and Its Targets
Ursodeoxycholic acid related targets were obtained through the search of Drugbank database. In order to increase the reliability of the data, it is necessary to consider the combination of multiple databases and text mining tools to supplement the collection of other potential targets. Firstly, the SMILE and MOL format files of drugs were searched through Drugbank, and then imported into Swiss Target Prediction (STITCH) (http://www.swisstargetprediction.ch/) and Similarity ensemble approach (SEA) to obtain the potential targets of the active ingredients. At the same time, using PharmMapper (http://www.lilab-ecust.Cn/PharmMapper/submitfile.html) to further supplement the potential targets of ursodeoxycholic acid using the reverse pharmacophore matching method. Subsequently, the predicted targets mentioned above were merged and imported into the UniProt database (http://www.Uniprot.org/), limiting the species to Homo Sapiens, and corrected for the official name of the gene.
Collection of Targets Related to Colon Adenocarcinoma
First, the intersection of DEGs and WGCNA-derived hub genes was obtained. Then enter the keyword “Colon adenocarcinoma” and select “Homo sapiens” as the species at GeneCards (http://www.genecards.Org/),15 Drugbank (https://go.drugbank.com/),16 DisGenet (https//:www.disgenet.org/),17 OMIM18 (https://omim./) and TDD (https://db.idrblab.net/ttd/) data set, searching the disease-related genes. Finally, the above two parts are merged.
Drawing Wayne Diagram
By introducing compound targets and disease targets into R-software, overlapping genes are potential targets for bioactive compounds that interact with the body in patients.
Constructing Protein-Protein Interaction Network (PPI)
The STRING (https://stringdb.org) database collects a large number of known or predicted results of protein-protein interactions (PPI).19 The overlapping genes were uploaded to the database, the species selected “Homo sapiens”, set the confidence level (0.40) as the minimum interaction score requirement, and removed the discrete targets. Plot a network of interactions between targets. The evaluation index of network node importance is expressed in terms of betweenness centrality, closeness centrality and degree. Cytoscape 3.8.0 software was used to analyze the PPI network.
Molecular Docking Results
Open the ursodeoxycholic acid 3D structure file downloaded by PubChem (https://pubchem.ncbi.nlm.nih.gov/). Download the human target protein structure corresponding to ursodeoxycholic acid and key target gene of COAD from RCSB protein database (https://www.rcsb.org/). PyMOL1.0, AutoDockTools1.5.6 and other software were used for docking and visualization of key target molecules. Before docking, root mean square deviation (RMSD) of ligand molecules was calculated to evaluate the rationality of docking parameter setting, and RMSD<2 was selected as the optimal conformation ligand molecule. Binding energy less than −5.0kcal/mol indicates good binding activity. The smaller the binding energy, the more stable the molecular docking.
Cell Culture
Human colorectal cancer cell lines HCT116 and SW620 were purchased from the Chinese Academy of Sciences (Shanghai) Cell Bank. Human umbilical vascular endothelial cells were purchased from Beijing Huizhiheyuan Biotechnology Co. HCT116, SW620 and HUVEC cells were cultured in DMEM medium (Gibco, USA) with 10% fetal bovine serum (FBS) (Gibco, USA) and 1% penicillin/streptomycin (Thermo, USA). All cell lines were incubated in a 37°C incubator with 5% CO2 according to the manufacturer’s instructions. Cell line morphology was evaluated regularly to ensure cell viability and quality.
Drug
Ursodeoxycholic acid (UDCA), specification: 1 g/ bottle (Quality standard: >98%, BR.), manufactured by Nanjing Best Biotechnology Co., LTD., Lot No.: A04088.0.1g of UDCA powder was dissolved in 2mL ethanol, which was stock1. 400ul stock1 and 1ul sterile dimethyl sulfoxide (DMSO, Sigma-Aldrich, USA) were added to 600ul PBS to form stock2. stock2 was diluted with DMEM to different concentrations (0, 0.00625, 0.0125, 0.025, 0.05, 0.1mg/mL) so that the final concentration of DMSO in the medium did not exceed 0.1% in all treatments.
Cell Viability Assay
Cell proliferation and cytotoxicity were detected by MTT colorimetry. SW620 and HCT116 cells of logarithmic growth stage were inoculated in 96-well plates with a density of 1×104 cells/well and cultured in an incubator at 5%CO2 and 37°C for 24h. After the cells grew to 80% under a microscope, the supernatant was discarded. Media containing different concentrations of UDCA (0,0.00625,0.0125,0.025,0.05,0.1mg/mL) were added to each well (each group has 5 multiple holes). After 24h culture, 10μL solution containing 10% MTT was added to each well and cultured for 4h away from light. The supernatant was discarded and I00μL DMSO was added to each well for 15 minutes. The absorbance was read at 490 nm on the enzyme-labeler of Bio-Rad Laboratories (United States).
Flatbed Cloning Experiment
SW620 cells of logarithmic growth stage were inoculated in 6-well plates with a density of 1000 cells/well, cultured in an incubator at 5%CO2 and 37°C for 24h, then the supernatant was discarded, cleaned with lxPBS, and DMEM and culture medium containing 0.02mg/mL UDCA were added. Two weeks later, the supernatant was discarded, cleaned with 1xP BS, and 1mL of pre-cooled paraformaldehyde was added to fix it for 15min. After cleaning with ultra-pure water, lmL of crystal violet dyeing solution containing 0.1% was added to stain for 15min. Then, it was gently rinsed and dried for photo taking and analysis.
Cell Migration and Invasion Assays
Both migration and invasion tests were performed using 24-well plates. Serum-free culture-supplemented cells were inoculated in the upper chamber of Transwell.
The subchamber of Transwell was filled with 600 μL medium containing different concentrations (0.02mg/mL) of UDCA and 10% fetal bovine serum. After incubation at 37°C for 24h, the cells were fixed with cold methanol for 20 minutes and then stained with 0.1% crystal violet for 20 minutes. The cells were cleaned with lxPBS and fixed with pre-cooled paraformaldehyde for 15min. After washing, 1mL of 0.1% crystal violet staining solution was added to the chamber for 15min. The cells were washed again and dried to capture cell images in the field of view of each filter under the microscope. The only difference between migration and invasion tests was whether the upper surface of the transwell membrane was pre-coated with Matrigel matrix (BD, USA).
Scratch Experiment
SW620 and HCT116 cells of logarithmic growth stage were inoculated into 6-well plates and incubated until the cell density was about 90–95%. A vertical line was drawn vertically in the center of each well with a 10μL gun tip, and washed twice with IxPBS. Culture medium with different concentrations (0, 0.01, 0.02, 0.04mg/mL) of UDCA was added to each well. They were observed and photographed under microscope at 0 and 24h, respectively.
Angiogenesis Experiment
HUVEC cells were treated with different concentrations of UDCA (0, 0.01, 0.02, 0.04 mg/mL) for 24 h. After adding 30 μL of Matrigel Matrix Gel to a 24-well plate and incubating in a 37°C incubator for half an hour, each group of HUVEC cells was made into a cell suspension at a cell density of 3×105 cells/mL, and then inoculated in 24-well plates and continued to be incubated in the incubator. Photos were taken at 2h, 6h and 12 h, respectively.
Results and Analysis
DEGs Screening
We first obtained the data of colon adenocarcinoma from the TCGA database. Based on principal component analysis, it was found that tumors and normal samples could be well distinguished. Then, differential expression threshold =|logFC|>2 and FDR<0.05 were used as screening criteria to screen differentially expressed genes between tumor samples and near-normal tissue samples. A total of 2064 differential genes (1000 up-regulated and 1064 down-regulated) were identified (Figure 2A and B).
Construction of WGCNA Network and Identification of Colon Adenocarcinoma Related Modules
To identify the potential gene modules with the highest association with colon adenocarcinoma, we performed WGCNA analysis of all candidate genes from the TCGA colon adenocarcinoma data set (Figure 3A) and obtained correlation coefficients. Finally, the Brown module was the most associated with tumor among all modules (p=0.91) (Figure 3B).
Go/KEGG Analysis to Find Critical Paths
We ultimately screened 128 overlapping genes as candidate hub genes that may play an important role in the development and progression of colon adenocarcinoma (Figure 4A). GO and KEGG analyses were then performed to further explore the potential role of these 128 overlapping genes (Figure 4B and C). GO enrichment analysis showed that overlapping genes mainly affect biological functions such as metabolism, exosomes, and various hydrolase activities. KEGG enrichment analysis showed that overlapping genes mainly affected metabolism and bile acid secretion pathways.
Prediction of Ursodeoxycholic Acid and Its Targets
Through database analysis, 164 ursodeoxycholic acid-related target genes and 1202 colon adenocarcinoma targets were identified. At the intersection of the two types of targets, 26 potential intersection targets of ursodeoxycholic acid anti-tumor were obtained (Figure 5A).
Construction of Ursodeoxycholic Acid-Colon Adenocarcinoma Related PPI Network and Screening of Core Targets
The String database was applied to mine ursodeoxycholic acid-colon adenocarcinoma related PPI network (Figure 5B).
Subsequently, secondary screening was performed using the CytoNCA plug-in in Cytoscape, and the core network diagram was finally obtained (Figure 5C), and TNF, CYP27B1, CASP3, MDM2, MMP2 may have a vital function in the treatment of this disease.
Molecular Docking
Molecular docking of five representative core targets: TNF, CYP27B1, MDM2, MMP2 and CASP3 obtained in the previous step were performed with ursodeoxycholic acid respectively. Before molecular docking, the root mean squared deviation (RMSD) was evaluated, molecules with RMSD<2 were selected for docking and the key target docking information table was drawn. After molecular docking, the binding mode diagram of ursodeoxycholic acid and core target protein receptors was drawn (Figure 6). The results showed that the binding energy of ursodeoxycholic acid and 5 key target proteins were all <-5.0kcal/mol, indicating that it had good binding activity with all 5 key target proteins (Table 1).
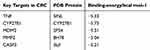 |
Table 1 Results of Ursodeoxycholic Acid Docking Scores with Key Targets in CRC |
UDCA Can Inhibit the Proliferation of Colorectal Cancer Cells in vitro
In order to evaluate the effect of UDCA on the proliferation of SW620 and HCT116 cells, SW620 and HCT116 cells were treated with different concentrations of UDCA (0, 0.00625, 0.0125, 0.025, 0.05, 0.1mg/mL), respectively, and the cell growth was detected by MTT assay. In Figure 7A, UDCA significantly inhibited cell proliferation, indicating that UDCA had potential anti-proliferation effects. IC50 calculations after 24 h of treatment showed that the IC50 values of SW620 and HCT116 cells were 0.02mg/mL and 0.016mg/mL, respectively. The clonogenesis experiment results showed that compared with the control group, the clonogenesis ability of UDCA treated cells was significantly decreased (Figure 7B).
UDCA Inhibits the Migration and Invasion of Colorectal Cancer in vitro
We studied the effect of UDCA on colorectal cancer cell migration in vitro by using scratch assay. As shown in Figure 8A and B, in SW620 and HCT116 cells, the degree of wound healing was gradually reduced after 0, 12, 24 and 36 hours of UDCA treatment compared with the control group. The cell migration and matrix gel invasion tests were also performed. The results in Figure 8C and D showed that the number of UDCA-treated colorectal cancer cells crossing the membrane was significantly reduced compared with the control group. The results showed that UDCA could effectively inhibit cell migration and invasion.
UDCA Inhibits the Formation of Blood Vessels in Colorectal Cancer in vitro
Angiogenesis experiment was used to study the effect of UDCA on the new blood vessels of colorectal cancer cells. As shown in Figure 9, in HUVEC cells, compared with the control group, new vessels were significantly reduced in the UDCA-treated group after 2h, 6h, 12h, among which the inhibition effect was most obvious in the 0.02mg/mL and 0.04mg/mL treatment groups.
Discussion
Colon adenocarcinoma is a malignant tumor occurring in the mucosal epithelium of the colon. At present, surgery combined with chemotherapy is a comprehensive treatment method that is widely used, but it is not suitable for advanced and older patients. Targeted therapeutic drugs such as cetuximab and bevacizumab are expensive, so this paper aims to find an adjuvant drug that can effectively prolong the survival of cancer patients.
In this work, we used a public database to obtain the differential genes between colon adenocarcinoma and normal tissue samples, and then used WGCNA to identify the modules most associated with colon adenocarcinoma, where the two intersected to get the core genes, which greatly narrowed the range of genes to screen for. Further enrichment analysis showed that differential genes were mainly enriched in metabolic pathways and bile acid metabolic pathways. Firstly, the genes enriched in the metabolic pathway include AHCYL2, ADH1C, LDHD, PDE9A, AKR1B10, PLAAT2, etc. In previous studies, cyclin-dependent kinase 7 (CDK7) phosphorylated nuclear Yes-associated protein 1 (YAP) at S127 and S397, enhancing its transcriptional function and promoting the expression of LDHD protein. CDK7-YAP-LDHD enables cancer cells to escape from iron death induced by D-lactic acid, and targets LDHD to inhibit cancer cell proliferation and renewal.19 PDE9A encodes phosphodiesterase 9A, a family of enzymes that catalyze the hydrolysis of cyclic nucleotides, hydrolyzing cyclic nucleotides, thereby reducing intracellular cAMP (cyclic adenosine monophosphate) and cGMP (cyclic guanosine monophosphate) levels. PDE9A specifically targets cGMP. Phosphodiesterase inhibitors activate caspase, inhibit Wnt/b-catenin pathway and TCF transcription through the increase of cGMP, thereby inhibiting CDK and survivin’s anticancer effects.20,21 In addition, inhibiting the expression of AKR1B10 and PLAAT2 can affect the production of lipid signaling molecules, which contribute to the proliferation, survival and invasion of cancer cells.22,23,24 Inhibitors targeting LDHD, PDE9A, and AKR have been shown to negatively regulate cancer cells and are being explored as potential therapeutics. In addition, many drugs targeting tumor metabolism have been applied in clinical practice and achieved good survival benefits.25 The authors’ KEGG results showed that, in addition to the enrichment of metabolic pathways, differential genes were the next most enriched in bile acid metabolic pathways. However, there are few reports on the relationship between drugs targeting bile acid metabolism and colon adenocarcinoma. We found that many UGT family molecules were enriched in the core genes of bile acid metabolism pathway: UGT2B15, UGT2A3, UGT2B17, etc. By searching the literature, we found that ursodeoxycholic acid can regulate bile acid metabolism through UGT3A1 and UGT2B7,26–28 and bile acid metabolism has a regulatory effect on the composition and abundance of intestinal flora.29,30 The role of intestinal flora and its metabolites in colon cancer and other cancers has been widely confirmed.31,32 So we hypothesized that ursodeoxycholic acid could alleviate colon adenocarcinoma progression. Next, we explored the feasibility of ursodeoxycholic acid as an adjuvant therapy for colon adenocarcinoma based on network pharmacology.
According to the database, 164 ursodeoxychacid-related target genes and 1202 colon adenocarcinoma targets were identified, including 26 common targets. Five representative core targets including TNF, CASP3, CYP27B1, MDM2 and MMP2 were further selected by String and Cytoscape software. The TNF gene, which encodes tumor necrosis factor α (TNF-α), is a cytokine involved in inflammation and immune system regulation. The inflammatory microenvironment is closely related to tumor invasion and metastasis, and chronic inflammation may be one of the important reasons leading to tumor invasion and metastasis. TNF-αwas found to activate MAPKKKs through TRAF2 aggregation and JNK and p38 through MKK4 and MKK7 (MAP ERK kinases). Min-Kyung Choo et al found that TNF-α can enhance the lung metastasis ability of colon adenocarcinoma cells by activating the ERK signaling pathway.33 Simone et al found that TNF can promote the growth of colon cancer cells by activating STAT3 and NF-kB.34 Caspase3 encoded by CASP3 plays a role as a core molecule in cell apoptosis. Previous studies have believed that caspase-3 can eliminate cancerous cells and is a widespread tumor suppressor.35 Surprisingly, Liu et al found that Caspase-3 can promote the genetic instability of cells during radiation and chemotherapy. It plays an important role in tumor transformation.36,37 The CYP27B1 encoded hydroxylase CYP27B1 is primarily responsible for converting 25-hydroxyvitamin D (calcifediol) to its active form 1, 25-dihydroxyvitamin D (calcitriol), After calcitriol binds to vitamin D receptor (VDR) or retinoid-related orphan receptors α or γ (RORα and RORγ), it binds to Vitamin D response elements (VDRE) located in the promoter region to regulate vitamin D related genes. A large number of cell and animal experiments have confirmed that vitamin D can inhibit colon cancer through a variety of ways. Kaler et al found that vitamin D can inhibit the growth of colon adenocarcinoma cells by inhibiting the production of IL-I-β by macrophages and thus blocking the Wnt signaling pathway.38 It was subsequently found that IL-IB could also protect colon adenocarcinoma cells from apoptosis mediated by tumor necrosis factor-associated apoptosis-inducing ligand (TRAIL).39 A recent study also found that vitamin D may promote iron death in colon adenocarcinoma stem cells by regulating SLC7A11 expression.40 MMP2 is a widely studied gene closely related to cell migration.41 MDM2 is an E3 ligase, and studies have found that MDM2 interacts with p53 and inhibits the transcriptional activity of p5342 P53 is not only a tumor suppressor gene, but also a key gene in the regulation of cellular radiosensitivity and chemotherapy sensitivity. Overexpression of MDM2 can inhibit p53-mediated ability to detect DNA damage in G1 phase and block the cell cycle.43 Inhibition of MDM2 expression in glioma cells can also play an important role in resistance to temozolomide through regulation of p53/MDM2 signaling pathway.44 Therefore, it is speculated that ursodeoxycholic acid acts on the above target proteins, which may play a role in adjuvant colon adenocarcinoma therapy through the regulation of cell apoptosis, iron death, cell proliferation and migration and other multicellular biological processes. The results of molecular docking showed that the binding energy of the above target proteins to ursodeoxycholic acid was less than −5 kcal/mol-1, indicating stable ligand-receptor binding. This result further verifies the reliability of network pharmacological predictive analysis.
Goossens et al found that UDCA can protect epithelial cells from injury and apoptosis, while inducing inhibition of cancer cell proliferation and apoptosis and/or autophagy death.45 Similar results were obtained in our study, and the clonogenesis experiment showed that the clonogenesis ability of UDCA-treated cells was significantly reduced compared with the control group. In addition to the optimal inhibitory concentration (0.02mg/mL), we also set low concentration (0.01mg/mL) and high concentration group (0.04mg/mL) for scratch test, which results showed that the healing ability of UDCA treated cells was significantly weakened compared with the control group. After 36 hours, the inhibitory effect of 0.01mg/mL and 0.02mg/mL groups was still good, but the inhibitory effect of 0.04mg/mlUDCA group was worse, which may be because the cells were treated with higher concentration of drugs for a long time, and the sensitivity of cells to drugs changed to a certain extent. The Transwell chamber experiment showed that the number of UDCA-treated colorectal cancer cells passing through the chamber was significantly reduced compared to the control group, and consistent results were obtained with the addition of matrix glue. The angiogenesis experiment showed that compared with the control group, the angiogenesis ability of HUVEC cells after UDCA treatment was significantly inhibited, especially in the medium concentration and high concentration treatment groups. These results confirm that UDCA has an inhibitory effect on the proliferation, migration and invasion of colorectal cancer.
In summary, this study was conducted based on the bile acid pathway enriched by differential genes between colon adenocarcinoma and normal people. It was found that this pathway enriched a large number of UGT family molecules, and a drug targeting this molecule, namely ursodeoxycholic acid, was found. Subsequently, network pharmacology and molecular docking technology were used to verify its feasibility in the treatment of colon adenocarcinoma at the molecular level, in vitro experiments were performed to validate its inhibitory effect on proliferation, migration and invasion of colorectal cancer cells at the cellular level. However, this study has some limitations. First, data on drug and disease targets are obtained from literature and databases, so the reliability and accuracy of the method’s predictions depend on the quality of the data. Secondly, this study is based on data mining and bioinformatics analysis, and further clinical trials and animal experiments need to be verified.
Conclusion
Based on the sequencing data, this study innovatively searched for COAD enrichment pathways and key targets, identified ursodeoxycholic acid as the target drug, and then used network pharmacology methods to explore the molecular mechanism and feasibility of its treatment of COAD. Through the screening and re-study of known drugs, new uses of the drug were realized, and it was found that it may have effects on the key targets of apoptosis, proliferation and migration, and has good anti-tumor activity, providing new ideas and new hopes for anti-tumor drug research and development. To clarify the anti-tumor potential of UDCA, more animal and human studies are needed in the future.
Abbreviations
CRC, Colorectal cancer; COAD, Colon adenocarcinoma; DEGs, differentially expressed genes; DMSO, dimethyl sulfoxide; GO, gene ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; PPI, Protein-Protein Interaction Network; RMSD, root mean squared deviation; SEA, Similarity ensemble approach; TCGA, the cancer genome atlas; TOM, topological overlap matrix; UDCA, ursodeoxycholic acid; WGCNA, weighted gene co-expression network analysis.
Data Sharing Statement
The original contributions presented in the study are included in the article. The dataset used in this study is available in an online database and the name and registration number of the dataset can be found in the article. Further inquiries can be directed to the corresponding authors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
All authors declare that there exists no potential competing interest. No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. doi:10.3322/caac.21565
3. Morris VK, Kennedy EB, Baxter NN, et al. Treatment of metastatic colorectal cancer: ASCO guideline. J Clin Oncol. 2023;41(3):678–700. doi:10.1200/JCO.22.01690
4. Tang Q, Huang H, Xu H, et al. Endogenous Coriobacteriaceae enriched by a high-fat diet promotes colorectal tumorigenesis through the CPT1A-ERK axis. NPJ Biofilms Microbiomes. 2024;10(1):5. doi:10.1038/s41522-023-00472-7
5. Yang J, Wang X, Hu T, et al. Entero-toxigenic Bacteroides fragilis contributes to intestinal barrier injury and colorectal cancer progression by mediating the BFT/STAT3/ZEB2 pathway. Cell Cycle. 2024;23(1):70–82. doi:10.1080/15384101.2024.2309005
6. Kraus D, Reckenbeil J, Veit N, et al. Targeting glucose transport and the NAD pathway in tumor cells with STF-31: a re-evaluation. Cell Oncol. 2018;41(5):485–494. doi:10.1007/s13402-018-0385-5
7. Schulte ML, Fu A, Zhao P, et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat Med. 2018;24(2):194–202. doi:10.1038/nm.4464
8. Nogales C, Mamdouh ZM, List M, Kiel C, Casas AI, Schmidt HHHW. Network pharmacology: curing causal mechanisms instead of treating symptoms. Trends Pharmacol Sci. 2022;43(2):136–150. doi:10.1016/j.tips.2021.11.004
9. Zhao L, Zhang H, Li N, et al. Network pharmacology, a promising approach to reveal the pharmacology mechanism of Chinese medicine formula. J Ethnopharmacol. 2023;309:116306. doi:10.1016/j.jep.2023.116306
10. Fan N, Meng K, Zhang Y, et al. The effect of ursodeoxycholic acid on the relative expression of the lipid metabolism genes in mouse cholesterol gallstone models. Lipids Health Dis. 2020;19(1):158. doi:10.1186/s12944-020-01334-3
11. Arenas F, Hervias I, Uriz M, Joplin R, Prieto J, Medina JF. Combination of ursodeoxycholic acid and glucocorticoids upregulates the AE2 alternate promoter in human liver cells. J Clin Invest. 2008;118(2):695–709. doi:10.1172/JCI33156
12. Zhang L, Su H, Li Y, et al. Different effects of ursodeoxycholic acid on intrahepatic cholestasis in acute and recovery stages induced by alpha-naphthylisothiocyanate in mice. Toxicol Appl Pharmacol. 2018;342:69–78. doi:10.1016/j.taap.2018.01.019
13. Narisawa T, Fukaura Y, Terada K, Sekiguchi H. Prevention of N-methylnitrosourea-induced colon tumorigenesis by ursodeoxycholic acid in F344 rats. Jpn J Cancer Res. 1998;89(10):1009–1013. doi:10.1111/j.1349-7006.1998.tb00489.x
14. Sherman BT, Hao M, Qiu J, et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022;50(W1):W216–W221. doi:10.1093/nar/gkac194
15. Safran M, Dalah I, Alexander J, et al. GeneCards Version 3: the human gene integrator. Database. 2010;2010:baq020. doi:10.1093/database/baq020
16. Wishart DS, Feunang YD, Guo AC, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46(D1):D1074–D1082. doi:10.1093/nar/gkx1037
17. Piñero J, Saüch J, Sanz F, Furlong LI. The DisGeNET Cytoscape app: exploring and visualizing disease genomics data. Comput Struct Biotechnol J. 2021;19:2960–2967. doi:10.1016/j.csbj.2021.05.015
18. Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A. OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43(Database issue):D789–D798. doi:10.1093/nar/gku1205
19. Lv M, Gong Y, Liu X, et al. CDK7-YAP-LDHD axis promotes D-lactate elimination and ferroptosis defense to support cancer stem cell-like properties. Signal Transduct Target Ther. 2023;8(1):302. doi:10.1038/s41392-023-01555-9
20. Mehta A, Patel BM. Therapeutic opportunities in colon cancer: focus on phosphodiesterase inhibitors. Life Sci. 2019;230:150–161. doi:10.1016/j.lfs.2019.05.043
21. Peng T, Gong J, Jin Y, et al. Inhibitors of phosphodiesterase as cancer therapeutics. Eur J Med Chem. 2018;150:742–756. doi:10.1016/j.ejmech.2018.03.046
22. Qu J, Li J, Zhang Y, et al. AKR1B10 promotes breast cancer cell proliferation and migration via the PI3K/AKT/NF-κB signaling pathway. Cell Biosci. 2021;11(1):163. doi:10.1186/s13578-021-00677-3
23. Chung YT, Matkowskyj KA, Li H, et al. Overexpression and oncogenic function of aldo-keto reductase family 1B10 (AKR1B10) in pancreatic carcinoma. Mod Pathol. 2012;25(5):758–766. doi:10.1038/modpathol.2011.191
24. Vecchi L, Araújo TG, Azevedo FVPV, et al. Phospholipase A2 drives tumorigenesis and cancer aggressiveness through its interaction with annexin A1. Cells. 2021;10(6):1472. doi:10.3390/cells10061472
25. Wang Y, Huang T, Gu J, Lu L. Targeting the metabolism of tumor-infiltrating regulatory T cells. Trends Immunol. 2023;44(8):598–612. doi:10.1016/j.it.2023.06.001
26. Mackenzie PI, Rogers A, Treloar J, Jorgensen BR, Miners JO, Meech R. Identification of UDP glycosyltransferase 3A1 as a UDP N-acetylglucosaminyltransferase. J Biol Chem. 2008;283(52):36205–36210. doi:10.1074/jbc.M807961200
27. Zhou D, Kong L, Jiang Y, et al. UGT-dependent regioselective glucuronidation of ursodeoxycholic acid and obeticholic acid and selective transport of the consequent acyl glucuronides by OATP1B1 and 1B3. Chem Biol Interact. 2019;310:108745. doi:10.1016/j.cbi.2019.108745
28. Erichsen TJ, Aehlen A, Ehmer U, Kalthoff S, Manns MP, Strassburg CP. Regulation of the human bile acid UDP-glucuronosyltransferase 1A3 by the farnesoid X receptor and bile acids. J Hepatol. 2010;52(4):570–578. doi:10.1016/j.jhep.2010.01.010
29. Guo X, Okpara ES, Hu W, et al. Interactive relationships between intestinal flora and bile acids. Int J Mol Sci. 2022;23(15):8343. doi:10.3390/ijms23158343
30. Wang Z, Chen J, Chen Z, Xie L, Wang W. Clinical effects of ursodeoxycholic acid on patients with ulcerative colitis may improve via the regulation of IL-23-IL-17 axis and the changes of the proportion of intestinal microflora. Saudi J Gastroenterol. 2021;27(3):149–157. doi:10.4103/sjg.SJG_462_20
31. Qu R, Zhang Y, Ma Y, et al. Role of the gut microbiota and its metabolites in tumorigenesis or development of colorectal cancer. Adv Sci. 2023;10(23):e2205563. doi:10.1002/advs.202205563
32. Chen W, Wen L, Bao Y, et al. Gut flora disequilibrium promotes the initiation of liver cancer by modulating tryptophan metabolism and up-regulating SREBP2. Proc Natl Acad Sci U S A. 2022;119(52):e2203894119. doi:10.1073/pnas.2203894119
33. Choo MK, Sakurai H, Koizumi K, Saiki I. Stimulation of cultured colon 26 cells with TNF-alpha promotes lung metastasis through the extracellular signal-regulated kinase pathway. Cancer Lett. 2005;230(1):47–56. doi:10.1016/j.canlet.2004.12.027
34. De Simone V, Franzè E, Ronchetti G, et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34(27):3493–3503. doi:10.1038/onc.2014.286
35. Zhou M, Liu X, Li Z, Huang Q, Li F, Li CY. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int J Cancer. 2018;143(4):921–930. doi:10.1002/ijc.31374
36. Cartwright IM, Liu X, Zhou M, Li F, Li CY. Essential roles of Caspase-3 in facilitating Myc-induced genetic instability and carcinogenesis. Elife. 2017;6:e26371. doi:10.7554/eLife.26371
37. Liu X, He Y, Li F, et al. Caspase-3 promotes genetic instability and carcinogenesis. Mol Cell. 2015;58(2):284–296. doi:10.1016/j.molcel.2015.03.003
38. Kaler P, Augenlicht L, Klampfer L. Macrophage-derived IL-1beta stimulates Wnt signaling and growth of colon cancer cells: a crosstalk interrupted by vitamin D3. Oncogene. 2009;28(44):3892–3902. doi:10.1038/onc.2009.247
39. Kaler P, Galea V, Augenlicht L, Klampfer L. Tumor associated macrophages protect colon cancer cells from TRAIL-induced apoptosis through IL-1beta-dependent stabilization of Snail in tumor cells. PLoS One. 2010;5(7):e11700. doi:10.1371/journal.pone.0011700
40. Guo S, Zhao W, Zhang W, Li S, Teng G, Liu L. Vitamin D promotes ferroptosis in colorectal cancer stem cells via SLC7A11 downregulation. Oxid Med Cell Longev. 2023;2023:4772134. doi:10.1155/2023/4772134
41. Wang J, Cai H, Liu Q, et al. Cinobufacini inhibits colon cancer invasion and metastasis via suppressing Wnt/β-catenin signaling pathway and EMT. Am J Chin Med. 2020;48(3):703–718. doi:10.1142/S0192415X20500354
42. Ingelshed K, Spiegelberg D, Kannan P, et al. The MDM2 inhibitor navtemadlin arrests mouse melanoma growth in vivo and potentiates radiotherapy. Cancer Res Commun. 2022;2(9):1075–1088. doi:10.1158/2767-9764.CRC-22-0053
43. Chen CY, Oliner JD, Zhan Q, Fornace AJ, Vogelstein B, Kastan MB. Interactions between p53 and MDM2 in a mammalian cell cycle checkpoint pathway. Proc Natl Acad Sci U S A. 1994;91(7):2684–2688. doi:10.1073/pnas.91.7.2684
44. Chen Q, Wang W, Chen S, Chen X, Lin Y. miR-29a sensitizes the response of glioma cells to temozolomide by modulating the P53/MDM2 feedback loop. Cell Mol Biol Lett. 2021;26(1):21. doi:10.1186/s11658-021-00266-9
45. Goossens JF, Bailly C. Ursodeoxycholic acid and cancer: from chemoprevention to chemotherapy. Pharmacol Ther. 2019;203:107396. doi:10.1016/j.pharmthera.2019.107396
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.









