Back to Journals » Journal of Pain Research » Volume 18
A Multi-Site, Randomized, Parallel-Group, Controlled Trial of Virtually-Delivered Sahaj Samadhi Meditation for the Management of Moderate Depressive Symptoms in Chronic Pain
Authors Cheng DK, Simpson R, Moineddin R , Katz J, Mulsant BH, Vasudev A, Greiver M , Hosseiny F, Inzitari M, Newman RI, Rivlin L, Foat KD, Furlan AD, Flannery JF, Telner D, Bosma R, Naimer M, Chung C, Pinto AD, Nelson MLA, Upshur R, Sud A
Received 1 January 2025
Accepted for publication 5 June 2025
Published 13 June 2025 Volume 2025:18 Pages 2925—2946
DOI https://doi.org/10.2147/JPR.S515229
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jonathan Greenberg
Darren K Cheng,1 Robert Simpson,2– 5 Rahim Moineddin,6 Joel Katz,7– 9 Benoit H Mulsant,10,11 Akshya Vasudev,12 Michelle Greiver,6,13 Fardous Hosseiny,14 Marco Inzitari,15,16 Ronnie I Newman,17,18 Leon Rivlin,19 Kirk D Foat,1 Andrea D Furlan,2,4 John Francis Flannery,2,4 Deanna Telner,6,20 Rachael Bosma,21 Michelle Naimer,6,22 Chadwick Chung,23 Andrew D Pinto,6,24– 26 Michelle LA Nelson,1,26,27 Ross Upshur,1,6,26 Abhimanyu Sud6,28
1Lunenfeld-Tanenbaum Research Institute, Sinai Health, Toronto, ON, Canada; 2Toronto Rehabilitation Institute, University Health Network, Toronto, ON, Canada; 3Sunnybrook Research Institute, Toronto, ON, Canada; 4Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada; 5Institute of Health and Wellbeing, University of Glasgow, Glasgow, Scotland; 6Department of Family and Community Medicine, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada; 7Department of Psychology, York University, Toronto, ON, Canada; 8Department of Anesthesia and Pain Management, Toronto General Hospital, Toronto, ON, Canada; 9Department of Anesthesiology & Pain Medicine, University of Toronto, Toronto, ON, Canada; 10Department of Psychiatry, University of Toronto, Toronto, ON, Canada; 11Centre for Addiction and Mental Health, Toronto, ON, Canada; 12Medpoint Clinic, London, ON, Canada; 13Department of Family and Community Medicine, North York General Hospital, Toronto, ON, Canada; 14Atlas Institute for Veterans and Families, Ottawa, ON, Canada; 15REFiT Research Group, Parc Sanitari Pere Virgili and VHIR, Barcelona, Catalonia, Spain; 16Faculty of Health Sciences and eHealth Lab, Open University of Catalonia (UOC), Barcelona, Catalonia, Spain; 17Art of Living Foundation, Fort Lauderdale, FL, USA; 18Nova Southeastern University Lifelong Learning Institute, Fort Lauderdale, FL, USA; 19Emergency Medicine, Humber River Health, Toronto, ON, Canada; 20South East Toronto Family Health Team, Toronto, ON, Canada; 21Toronto Academic Pain Medicine Institute (TAPMI), Women’s College Hospital, Toronto, ON, Canada; 22Department of Family Medicine, Sinai Health, Toronto, ON, Canada; 23Canadian Memorial Chiropractic College, Toronto, ON, Canada; 24Upstream Lab, MAP Centre for Urban Health Solutions, Li Ka Shing Knowledge Institute, Unity Health, Toronto, ON, Canada; 25Department of Family and Community Medicine, St. Michael’s Hospital, Toronto, ON, Canada; 26Division of Clinical Public Health, Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada; 27Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada; 28Research Institute, Humber River Health, Toronto, ON, Canada
Correspondence: Abhimanyu Sud, Department of Family and Community Medicine, Temerty Faculty of Medicine, University of Toronto, 500 University Avenue, 5th Floor, Toronto, ON, M5G 1V7, Canada, Email [email protected]
Background: Chronic pain (CP) often co-occurs with depression, but promising scalable interventions have been under-investigated. We assessed the effectiveness of the virtually-delivered Sahaj Samadhi Meditation (SSM) program in reducing depressive symptoms in people with CP and moderate depressive symptoms.
Methods: We conducted a randomized controlled trial comparing SSM to the Health Enhancement Program (HEP), an active control. Participants were recruited from multiple sites in the Greater Toronto Area and virtually. Both 12-week programs were delivered virtually in groups by appropriately trained facilitators. Depressive symptoms were assessed using the Patient Health Questionnaire (PHQ-9) at baseline, 12 weeks, and 24 weeks. ClinicalTrials.gov registration number: NCT04039568.
Results: Of 108 participants enrolled, 89 were randomized to SSM (n=43) or HEP (n=46). Between-group differences for the PHQ-9 were not significant. Within-group mean differences for SSM were significant and greater than the minimal clinically important difference at both 12 weeks and 24 weeks (− 3.97 (95% CI − 6.69 to − 1.24) and − 4.96 (− 8.36 to − 1.56), respectively), while within-group mean differences were not significant for HEP.
Conclusion: This study suggests potential benefits of SSM for individuals with comorbid CP and depression. Future trials should include larger sample sizes in non-pandemic conditions to better evaluate the effectiveness of SSM. Further research should also explore pragmatic trial designs and the integration of mind-body interventions in clinical settings.
Keywords: chronic pain, depressive disorder, mind-body therapies, meditation, clinical trial
Introduction
Chronic pain (CP) is a common and disabling condition affecting about 20% of North American adults.1–3 CP, including conditions such as fibromyalgia, arthritis, and chronic low back pain, is associated with a number of psychological comorbidities such as major depression.4 While the prevalence of major depression in the general population is estimated at 8–10%,5,6 people living with CP are disproportionately affected, with comorbid prevalence rates ranging from 23% in primary care to 85% in other specialty settings.7–9 The two conditions can also exacerbate one another, with evidence of poorer well-being and functionality in those with both conditions compared to CP alone,10,11 and CP increasing the frequency and duration of depressive episodes.12,13
Conventional interventions that are effective in treating each condition alone may be less effective in the presence of both; for example, a recent study identified fewer functional benefits from antidepressant use in people with both depression and CP, compared to those with depression alone.12 A recent trial identified that virtually-delivery of Acceptance and Commitment Therapy and Behavioral Activation Therapy for Depression for chronic low back pain and comorbid depressive symptoms was effective in improving pain interference and pain catastrophizing, but did not show improvements for depressive symptoms compared to treatment as usual.14 Furthermore, when examining the broader evidence base, CP clinical trials that report depressive symptom outcomes often include people with CP that do not meet criteria for major depression,15 which may substantially limit the real-world applicability of this evidence. Likewise, few clinical practice guidelines for CP make recommendations for the management of comorbid depression and, when such recommendations are made, they tend to draw from a very limited evidence base.16
Mind-body interventions such as yoga, tai chi and meditation have been increasingly investigated as treatments for both CP and major depression, though separately. A recent guideline for CP management in primary care recommended yoga and tai chi for the management of chronic low back pain and osteoarthritis, citing multiple meta-analyses.17 Meditation practices have shown positive effects for depression,18 yoga has been recommended as adjunctive treatment for depression management in a national clinical guideline,19 and a recent network meta-analysis demonstrated yoga as having clinically important effects in improving depression, superior to that of SSRIs.20 Despite evidence and guidelines recommending mind-body interventions for CP and depression separately, when considering comorbid depression in people with CP, current evidence is limited and suggests small to moderate effects on depression symptoms.21 Thus, there is a need for further research into effective interventions, including psychological, behavioral, and mind-body interventions, that purposefully include those with comorbid CP and depression.
Virtually-delivered mind-body interventions have shown positive effects for improving CP,22 as well as depression in older adults.23 Virtual delivery allows for safe provision within the context of physical distancing and other pandemic response measures, and may also address accessibility issues and provide opportunities for scale-up outside of such contexts. However, digital access and digital literacy may present new barriers.24,25 The implementation of programs such as meditation, which can leverage expert instructors who are not part of the formal healthcare system, may also help alleviate the existing strains on healthcare services. Specifically, the Sahaj Samadhi Meditation program was selected for this trial because this program is already widely delivered globally by a single organization, following consistent protocols across jurisdictions and delivered by expert certified instructors required to maintain teaching competency. Thus, this program provides a unique opportunity, should it demonstrate effectiveness in this context, for scale and spread.
The purpose of this study was to investigate the effectiveness of a virtually-delivered meditation program for people living with CP and moderate depressive symptoms. We hypothesized that the 12-week group-based virtually-delivered Sahaj Samadhi Meditation program would be more effective in reducing depressive symptoms compared to an education-based active control.
Methods
Study Design
A parallel randomized controlled trial (RCT) with 1:1 allocation ratio was conducted to evaluate the effectiveness of virtually-delivered Sahaj Samadhi Meditation, compared to the Health Enhancement Program as an active control, for improving depressive symptoms in people with chronic pain and significant depressive symptoms. All study participants were blinded to study hypotheses to prevent expectancy bias. Given the nature of the interventions, participants could not be blinded to intervention allocation. The study biostatistician was blinded to participant allocation. All data collection after study screening was completed by participants through online surveys without involvement of study staff. Author KF was a patient advisor for this study and was involved in the study’s conceptualization, design, and validation. The study was conducted in accordance with the Declaration of Helsinki, registered at ClinicalTrials.gov (NCT04039568), approved by the Mount Sinai Hospital research ethics board (MSH REB), Toronto, Canada, and follows the Consolidated Standards of Reporting Trials (CONSORT) statement to report study findings.
Modifications to Original Trial Protocol
Modifications were made to the original study protocol26 in December 2019 to accommodate the rapidly decreasing frequency of opioid prescribing for chronic pain in the communities in which the trial was being conducted. Specifically, the inclusion criterion of being prescribed an opioid was removed, and the minimum age was reduced from 45 to 18 years. Additional major modifications were also made after March 2020 in response to the COVID-19 pandemic. The original trial was planned for in-person delivery for both arms and so activities were paused due to the pandemic and related local public health restrictions. In December 2020, all study activities and programs were revised for the interventions to be delivered virtually through video calls and support additional communication through email. While there was not an opportunity to formally conduct feasibility testing of virtual-delivery, the Sahaj Samadhi Meditation program was being widely delivered in the community virtually during the pandemic, and continued to be delivered in this way to promote accessibility after public health restrictions were lifted. Eligibility criteria were revised to include access to the internet and ability to participate in video calls, and additional sites were also added to support recruitment which was made feasible by virtual delivery. All trial participants participated in the same, modified protocol. Modifications were approved by MSH REB, and captured in the protocol registration and the CONSERVE-CONSORT extension guideline (Supplemental file, Part 1).
Study Participants
Study recruitment took place from April 2021 to November 2022 using the following inclusion criteria: 1) 18 years of age or older; 2) CP defined as self-reported pain in any body region for at least 3 months with no specification for severity;27 3) moderate depressive symptoms (Patient Health Questionnaire-9 (PHQ-9) score ≥ 10, <20); 4) ability to sit for 20–25 minutes without significant discomfort; 5) understanding of English language; 6) regular access to internet and video calling.
Individuals were excluded for: 1) major mental health condition including history of psychosis or bipolar disorder, current severe depression (PHQ-9 ≥ 20), risk of imminent suicide, or active substance use disorder using the Mini International Neuropsychiatric Interview (MINI); 2) current participation in another mind-body intervention; and 3) inability to provide informed consent. Individuals with current severe depression were excluded so as to not expose them to potentially ineffective interventions in a context where well-established treatments for the management of depression are available.
Study Procedures and Randomization
Participant recruitment was supported by three community pain clinics; three academic primary care teams; and three chiropractic college sites all in the Greater Toronto Area, Ontario, Canada. Frontline administrative, nursing and medical staff within patients’ circle of care referred potential participants to the study at three of the sites. At all sites, study promotional materials (through websites and emails for virtual visits and the posters and brochures for in person visits) were made available to potential participants. These physical promotional materials were also distributed to over 30 independent community sites that provide care for people with chronic pain such as pharmacies, health centers, and rehabilitation clinics. A website with study information was also created and shared in virtual spaces, including online peer-support groups for people with chronic pain in Canada. All study materials emphasized that both study arms might be useful for improving mood in order to blind participants to trial hypotheses. Research ethics board approvals were obtained from all applicable institutions for respective recruitment methods.
Potential participants were screened for eligibility by study staff via video call, and then given at least 24 hours to review study documents before consenting to participate in the trial. Participants were randomized to the intervention or active control group by simple 1:1 random allocation. A randomization sequence was generated with Microsoft Excel and was managed and controlled by study staff that were not involved in other procedures of the trial. These study staff assigned a specified unique code to each participant to enable tracking. When a participant was successfully enrolled into the study, the next allocation in the sequence was revealed to the study coordinator to allow for scheduling. The study coordinator provided as needed logistical and technical support for delivery of the cohorts, but did not have any direct role during outcome assessment or other data acquisition.
Interventions
Participants were asked to attend a 12-week meditation or education program. There was no interaction between participants from different arms as part of the trial, reducing the possibility of intervention diffusion. As described below, the Health Enhancement Program (HEP) has been developed as an active control in research settings for mind-body interventions, so both programs included a training week with 4 sessions in the first week (2 hours/day), then weekly reinforcement sessions for 11 weeks (75 minutes/week) as well as expected daily practice of the meditation technique or other activities for the control arm. Thus, total contact time for both arms was 8 hours for week one and 13 hours and 45 minutes through the follow-up periods. Home practice without facilitator contact was encouraged for both groups, suggesting up to a total of 40 minutes per day through the study period. Both program sessions were conducted virtually through video calls in small groups with 1–2 program facilitators (2 facilitators if there were more than 5 participants in a program cohort). No equipment or materials were required to participate beyond access to video calling from a secure and private location. Participants were reminded of program sessions and home practice by email, text message, or phone call by the study coordinator. Program facilitators reported any modifications made to program delivery.
Intervention: Virtually-Delivered Sahaj Samadhi Meditation (SSM)
Participants attended the standardized Sahaj Samadhi Meditation program for depressive populations. This protocol includes a longer version of the program taught in the community, in order to accommodate the comparably greater illness burden in the study population.26 SSM is a form of meditation that requires neither concentration nor vigilant attention, but involves relaxed attention to a particular sound (mantra). The program was delivered by certified meditation teachers from the non-profit Art of Living Foundation. On day one, participants learned about the nature of meditation and then learned meditation through individual guidance from the instructor. Training on days two to four included understanding the nature of the mind and the thoughts arising from it, meditation guided by the teacher, and discussions of what is correct and incorrect meditation practice. Participants learned how to respond to experiences that arise in meditation and discussed what enhances or detracts from effective meditation. Instructions for an independent home practice of meditation were also provided and reviewed. Participants were encouraged to practice Sahaj Samadhi Meditation for twenty minutes twice daily at home. In addition, weekly 75-minute reinforcement sessions included 20 minutes of meditation practice guided by the instructor, and then focused on participants’ experiences with independent home practice of meditation during the week, additional observations, and a review of relevant knowledge to support their practice at home.
Control: Virtually-Delivered Health Enhancement Program (HEP)
The HEP has been designed and used as a manualized active control in other meditation-based intervention trials.28,29 The program was delivered by regulated healthcare providers with formal training by an experienced HEP facilitator. HEP is an education program that aims to control for several non-specific factors found in meditation programs, including: group support and morale, behavioral activation, reduction of stigma, facilitator attention, treatment duration, and time spent on at-home practice (adapted from a published manual30 to match the schedule of SSM). HEP was tailored to be structurally equivalent to SSM with similar-sized groups, meeting schedule, total contact hours, amount of home practice and encouragement to keep practice logs. Instructions for home practice were provided weekly and included a variety of activities such as keeping a hydration log, journaling, and poetry writing. Participants were taught about health promotion, healthy diet, music, and exercise, but did not learn meditation. In HEP, participants received the support of a group and facilitator, and talked through and attempted to implement positive health-enhancing life changes.
Measures
Demographics
Participants were asked to provide demographic information at baseline, which included age, gender, ethnic background, employment status, housing type, highest level of education achieved, smoking status, caffeine intake, drug and alcohol use, duration of pain, and pain diagnosis. Participants were also asked about psychiatric history including age of first contact with mental health services for mental disorder (and which disorder) if any, history of hospital admissions, and the number of previous episodes of depression.
Outcomes
Outcome measures were completed at baseline, 12 weeks (primary endpoint), and 24 weeks (secondary exploratory endpoint). At each timepoint, participants completed self-reported questionnaires that measured depressive symptoms, pain severity, health-related quality of life, and a medication log.
Primary Outcome
The primary outcome was change in depressive symptoms from baseline to 12 weeks. Depressive symptoms were measured at each time point using the PHQ-9.31,32 The PHQ-9 is a well-validated and widely used self-report scale in depression and chronic pain clinical care and research, with studies using both continuous and percent changes as outcome measures.33–35 A self-report scale was more feasible than an assessor-rated scale, given the virtual nature of the study and multiple research sites. PHQ-9 scores range from 0 to 27, and scores from 0–4 suggest no depression, 5–9 mild depression, 10–19 moderate depression, and 20–27 severe depression.32 We used estimates for minimum clinically important difference (MCID) for the PHQ-9 of 2 units36 or 20%37 change from baseline score.
Secondary outcomes
Pain severity and interference with function was measured using the Brief Pain Inventory (BPI) at each time point, with 12 weeks as the primary endpoint. The BPI is a validated self-report scale used in pain trials and clinical pain practice and is a core outcome measure per the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) recommendations.38 The BPI ranges from 0–10, with higher scores indicating worse pain, and an MCID of 1 unit or 15–20% improvement from baseline.39
Health-related quality of life was measured at each time point using the 36-Item Short Form Survey Instrument (SF-36), which has been validated as a measure for quality of life for people with chronic pain10,40,41 and is recommended by IMMPACT.38 This measure includes 36 items that evaluate 8 health domains which are scored on a range of 0 to 100, with a higher score indicating a more favorable health state. Domain scores are calculated from averaging relevant item scores.42 These eight domains are often grouped into two health dimension summary scores: the physical composite score (role limitations due to physical problems, physical function, bodily pain, and general health perception) and the mental composite score (role limitation due to emotional problems, social functioning, energy/fatigue, and emotional well-being). Composite scores were calculated using a validated simple unweighted method which has been correlated with other methods that use US norm means.43 The MCID of 10 points was used for the individual domains (ie, role functioning and social functioning), and of 5 points for the composite scores (ie, physical functioning and emotional functioning).44
Participants were also asked to complete a medication log at each timepoint to record any changes in medication use and any participant-reported adverse events were monitored using a participant log, reporting directly to study staff, or to study staff via reporting by the participants to the interventionists.
Implementation Evaluation
To inform future implementation of SSM in various contexts, participants were asked to report the days that they completed their daily home SSM practice between weekly program sessions (ie as a measure of “dose”), their perspectives on accessing virtual programs (at 12 weeks), and their practice at home by the time of last data collection (24 weeks). Focus groups were also completed with the meditation teachers before and after the study to understand (anticipated) barriers and enablers of the SSM program. This parallel implementation evaluation will be analyzed and reported in further depth in a companion manuscript to this current evaluation of intervention effectiveness.
Sample Size Calculation and Statistical Analysis
The target sample size was calculated based on the primary outcome of change in depressive symptoms. The standard deviation of the PHQ-9 is 5 units and we anticipated a 2.5-unit difference at 12 weeks between the intervention and control groups based on available pilot data.26 With a two-arm RCT, a two-sample t-test was used to calculate the sample size to have at least 80% power to detect differences in PHQ-9 scores of 2.5 or larger at 5% type 1 error (alpha = 0.05). The resulting estimated sample size was 64 participants in each arm. We increased the sample size by 25% to account for dropouts, which is reasonable given an attrition rate of 13% seen in pilot data. Therefore, the target sample size for recruitment and randomization was 160 participants, with 80 participants in each arm.
Data Analysis
Descriptive statistics were used for demographic characteristics and outcome measures. The distributions of primary and secondary outcomes were also examined for normality. A longitudinal data analysis approach was used to assess between-group changes (difference between two groups at each time point) and within-group changes (change over time within each arm), while these comparisons were adjusted for the correlation among the repeated measures and potential confounders (such as age, gender, ethnicity, and employment status). We used a Random Effect linear regression model (random intercept) with unstructured covariance structure to account for the repeated measures within participants.45 Time was coded as class/categorical variable in the model. For the primary analysis, time has only two values baseline and 12 weeks. A post-hoc responder analysis was conducted to examine the clinical significance of changes in PHQ-9 scores and Fisher’s exact test was used to examine associations between PHQ-9 responders and non-responders and participant demographic characteristics.
We conducted both intention-to-treat (ITT) and per protocol analyses. Since results did not differ across analyses, we report the ITT analysis below and include the per-protocol analyses results tables in Supplemental file, Part 2. There were no pre-specified subgroup analyses.
Results
Participants
From April 2021 to November 2022, 510 potential participants were referred to the study, of which 147 were consented and screened for eligibility. A total of 108 participants met criteria and were enrolled in the study. Because of significant trial changes due to the COVID-19 pandemic, and resulting trial resource limitation, recruitment was stopped before reaching the target sample size. Of the 108 participants, 52 were randomly allocated to SSM, and 56 to HEP (Figure 1). Nineteen participants (9 in SSM and 10 in HEP) did not begin the trial programs, so in total, 43 and 46 participants participated in SSM and HEP, respectively. Cohorts for both arms were run between June 2021 and February 2023, with final data collection completed in May 2023.
 |
Figure 1 Participant flow diagram. Abbreviations: HEP, Health Enhancement Program; n, sample size; PHQ-9, patient health questionnaire; SSM, Sahaj Samadhi Meditation. |
At baseline, the mean participant age was 50.8 years (SD=13.3), with the majority of study participants being women (84%). Mean duration with chronic pain was 11.9 years (SD=11.6). The most common primary chronic pain conditions were general chronic pain (23.2%), axial pain such as chronic low back pain (17.6%), and arthritis (14.8%) (Table 1). The follow-up rate for outcomes were 90.9% and 86.8% at 12 weeks, and 66.7% and 55.3% at 24 weeks, for SSM and HEP groups, respectively.
 |
Table 1 Demographic and Baseline Characteristics of Study Participants (n = 89) |
Program Modifications
In total, 27 program cohorts were carried out (14 SSM, 13 hEP), with 16 that completed programs within the standard 12-week duration. Seven cohorts were extended by 1 week and 4 cohorts were extended by 2 weeks in order to accommodate holidays and absences due to illness. Two cohorts experienced participant dropout to the point where one participant remained; these cohorts continued so as to not create undue burden for the remaining participant to restart in another cohort.
Primary Outcome: Depressive Symptoms
PHQ-9 scores improved for participants in both arms and there were no significant between-group differences at either 12 weeks (mean difference (MD) adjusted for repeated measures of −0.70, 95% Confidence Interval (CI) −4.53 to 3.13) or 24 weeks (MD adjusted for repeated measures of −2.28, 95% CI −6.81 to 2.25) for the PHQ-9 (Table 2, Figure 2). There were no important changes in outcomes with adjustment for potential confounders. Reliability of the PHQ-9 was acceptable for both arms at all study points (Supplemental file, Part 3).
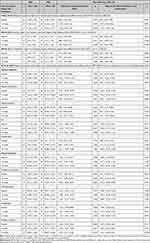 |
Table 2 Results for Primary and Secondary Outcomes with Adjusted Between-Group Mean Differences (n = 89) |
For SSM, mean PHQ-9 scores decreased from 15.67 at baseline to 11.76 at 12 weeks, a significant adjusted within-group mean difference of −3.92 (95% CI −6.65 to −1.20), and was also significantly reduced at 24 weeks with an adjusted within-group mean difference from baseline of −4.75 (95% CI −8.15 to −1.30) (Table 3). For HEP, PHQ-9 scores decreased from 14.83 to 12.40 from baseline to 12 weeks, a non-significant within-group mean difference of −2.38 (95% CI −5.21 to 0.46) and subsequently increased at 24 weeks to 13.04, a non-significant mean difference from baseline of −1.63 (95% CI −4.76 to 1.51).
 |
Table 3 Within-Group Mean Difference From Baseline (n=64) |
Post-Hoc PHQ-9 Responder Analysis
While the between-group mean difference from baseline to 24 weeks was greater than the MCID of 2 in favor of the SSM group, when examining changes in PHQ-9 score of 2 points or greater from baseline to 24 weeks, differences in proportion between SSM and HEP was not significant (p=0.263) (Figure 3). Twelve of 19 (63.2%) SSM participants reported a reduction of 2 points or greater compared to 9 (39.1%) of HEP participants. Only 3 of 19 (15.8%) SSM participants showed an increase in symptoms by 2 points or greater compared to 8 (34.8%) HEP participants. Four (21.0%) SSM participants and 6 (26.1%) HEP participants showed less than a 2-point change from baseline.
Likewise, analyzing for a change of 20% or greater at 24 weeks, differences between SSM and HEP were marginally significant (p=0.076). Ten (52.6%) SSM participants reported at least a 20% decrease in symptoms, compared to 8 (34.8%) HEP participants. Only 1 (5.3%) SSM participant reported a greater than 20% increase in PHQ-9 scores compared to 8 (34.78%) HEP participants.
At 24 weeks, HEP responders were more likely to be employed than non-responders (p=0.008, response ≥2-points; p=0.003, response ≥20%), and we did not identify any differences between responders and non-responders in the SSM group. At 12 weeks, we did not identify any differences between responders and non-responders in the HEP group. In the SSM group, responders were more likely to be employed (p=0.034, response ≥20%) and not use recreational drugs (p=0.016, response ≥2-points) (Supplemental file, Part 3).
Secondary Outcomes
At both outcome timeframes, for pain severity there were minimal changes all below MCID in both groups and no significant between-group differences (Table 2). Effects for pain interference were slightly larger for both groups, with some within-group effects approaching the MCID for both groups (Table 3).
For quality of life, there were no significant between-group differences for the physical or mental composite scores (Table 2). Both groups showed significant within-group improvements for the mental composite scores at both time points, approximating the MCID of 10. Within-group physical composite score changes were not as large.
Medication data were incompletely reported across the trial. Complete medication log data was available at baseline and 12 weeks for 60 participants, and 44 (73.3%) did not report changes in medications, 21 (70.0%) from SSM and 23 (76.7%) from HEP.
No adverse events were reported for either SSM or HEP groups.
Discussion
Summary and Interpretation of Findings
This RCT is one of the first to examine the effect of a virtually-delivered mind-body intervention on depressive symptoms as a primary outcome among people living with chronic pain and significant depressive symptoms. Both arms demonstrated improvement for depressive symptoms, and this trial did not demonstrate effectiveness of SSM over HEP. The primary outcome at 12 weeks showed a mean difference of −0.70 (95% CI −4.53 to 3.13). This change in depressive symptoms was also reflected in improvements for both arms in the composite mental quality of life scores.
HEP, as an active control, includes elements such as journaling, nutrition education, and group interaction that may all improve depression and pain symptomatology. HEP has previously shown evidence of improving depressive symptoms and psychological distress in patients with CP.28 The attenuated between-group findings from our study are similar to other trials of mind-body interventions in this population which have used active controls.46 The non-significant differences between groups are likely further explained by the trial’s small sample size. Due primarily to major adjustments and disruptions to the trial due to the COVID-19 pandemic, we were unable to reach our recruitment target of 160 participants. Finally, SSM was not designed for virtual delivery and was adapted for this purpose mid-trial. The impact of this adaptation on the effectiveness of SSM is not yet known. Specifically, participants in the SSM arm may have received sub-therapeutic doses of the intervention with few reporting practicing meditation at the expected frequency to affect depressive symptomatology. This speculation will be further assessed through a comprehensive companion implementation evaluation. While the exclusion of people with more severe depression (PHQ ≥ 20) limits the generalizability of the findings to this important population, if anything, this decision would have biased the trial results towards underestimating intervention effectiveness.
While the difference between groups was not statistically significant for the primary outcome, a possible superior effect of SSM compared to HEP is suggested by significant within-group differences larger than the MCID for PHQ-9 at both timepoints for SSM compared to non-significant within-group changes for HEP. This possible relative effect of SSM is further supported by the post-hoc responder analysis which demonstrated that more than half of SSM participants had greater than 20% improvement in the PHQ-9 compared to about one third in the HEP group. A sufficiently powered study will be required to refine the precision of these effect estimates.
Generally, these results compare similarly to other interventions available for this population. Various systematic reviews have consistently estimated that mind-body interventions such as yoga, tai chi, and meditation have small to medium effects (standardized mean difference (SMD) range 0.05 to 0.63) on depressive symptoms in people living with CP.21 However, the trials included in these reviews often did not include people with significant depressive symptoms, so these effect sizes may in fact be under-estimates of potential effectiveness in those with suprathreshold symptoms.15
Besides mind-body interventions, effect sizes for changes in depressive scores have been estimated for a variety of other kinds of interventions for people with CP. One umbrella review identified fluoxetine, web-based psychotherapies, and Acceptance and Commitment Therapy as the only interventions with at least a medium effect size (SMD >0.5) from systematic reviews and meta-analyses of at least moderate quality.46 Effect size estimates for these interventions were often determined against placebo, usual care, or no control and ranged from short to medium outcome timeframes. Thus, the mean difference for SSM from this trial, measured over a long outcome timeframe and against an active control, may compare well to these other promising interventions for this population. Previous systematic reviews and meta-analyses have suggested comparable small effect sizes of other common and important lifestyle measures such as exercise for the improvement of depressive symptoms in chronic pain.
Participants in the SSM arm demonstrated some improvements for the secondary outcomes of pain severity, pain interference, and quality of life measures, though there were no significant differences for these measures when compared to HEP. Two previous reviews have indicated mindfulness-based interventions to have small to medium effects on pain-related symptomatology and physical health-related quality of life.22,47 Compared to cognitive behavioral therapy (CBT), meditation interventions showed no significant difference (SMD 0.02, 95% CI −0.43 to 0.48), but a small effect when compared to various other controls such as usual care or waitlist controls (SMD −0.34, 95% CI −0.79 to 0.03) for pain intensity. This was very comparable to previous reports on the effects of meditation on pain intensity in the context of CP compared to treatment as usual, passive controls, or support groups (SMD 0.32, 95% CI 0.09 to 0.54).22 This current study compared SSM to an active control which may explain why the primary estimation of effect size for SSM on pain was comparatively modest and similar to the comparison of meditation interventions to CBT, while within-group estimations of effects more comparable to estimations of meditation against passive controls.
Taken together, these findings suggest that SSM may be most effective for improving mental health compared to physical health of people living with both depression and CP. Likewise, this trial emphasizes the multiple challenges of assessing the effects of mind-body and behavioral interventions.48 This includes the challenges of determining appropriate controls for mind-body interventions that satisfy competing pulls of maximizing rigor and internal validity versus applicability and external validity. Future trials of SSM for this population that emphasize pragmatism and implementation considerations may, for example, consider waitlist control designs which are increasingly being deployed for such purposes, including to support recruitment.22,49,50 Despite evidence for the effectiveness of meditation programs for CP and depression, and high informal utilization of these interventions by patients, we have yet to see wide formal implementation in North American health systems. A further implementation evaluation of virtually-delivered SSM within the specific context of clinical primary and specialized pain care will be reported separately.
Trial Adaptations and COVID-19 Effects
It is important to acknowledge the context of conducting this trial during the COVID-19 pandemic, which impacted every aspect of the trial. Due to the pandemic, changes were made to conduct the trial remotely, which may have introduced barriers to effective learning of and incorporation of SSM, thus affecting the effectiveness of this intervention for all outcomes. Likewise, virtual delivery may have impacted initial and ongoing participation for some individuals, such as those that are less familiar with remote devices or those with limited access to technology and internet. Such trial changes may have contributed to recruitment challenges, despite efforts to improve recruitment through the addition of multiple sites and methods for promoting the study. Our experience aligns with the findings on a global scale, where recruitment for clinical trials was significantly affected by the pandemic.51
The COVID-19 pandemic also had effects on people’s health, including increased rates of depressive symptoms, anxiety and psychological distress.52–54 Multiple participants became ill with COVID during program participation, and multiple participants lost family members during the pandemic. These larger trends may have blunted improvement in depression and pain symptoms from the interventions and thus resulted in under-estimates of the effects of the interventions. While we did not collect data specific to such changes in this study, due to the random assignment of participants, the adverse psychological impacts of the pandemic should have been distributed equally between the two groups.
Strengths and Limitations
Important strengths of this trial include the use of randomization and allocation concealment, as well as adjustment for confounders. Importantly, 72% of participants who started the trial interventions remained committed to the trial until the end of the 12-week treatment period, suggesting the feasibility of delivering SSM virtually even during the challenging pandemic context.
Some important limitations are the non-blinded outcome assessment, due to the nature of the interventions and the feasible means for reporting outcomes. Likewise, the majority of participants in this trial were women and were of higher socioeconomic status. This gender disparity is reflective of the larger literature of clinical trials examining depression outcomes in CP as well as mind-body research.15,55 The skewing of participation towards those of higher socioeconomic status may be reflective of requirements for access to technology and technological literacy. These issues will be explored further in the implementation evaluation which will facilitate an analysis of barriers and facilitators to trial participation. Likewise, this was the first time these interventions were adapted and studied for virtual delivery, as necessitated by the pandemic. Future studies comparing in-person to virtually-delivered SSM may be valuable to better understand impacts of approach to delivery on both intervention effectiveness and accessibility.
Conclusion
Due in part to multiple trial disruptions from the COVID-19 pandemic which affected modes of delivery and recruitment, this RCT in people living with comorbid pain and depression was unable to detect any significant differences in between SSM and HEP for depressive symptomatology. Overall, the effect estimates for SSM from this trial are promising and concordant with the effects of other interventions that have been studied for this population. Future trials outside of the pandemic context that are sufficiently powered and that examine in-person delivery methods, consider appropriate controls, and include participants more representative of the population will allow us to better refine estimates of effectiveness of SSM as an intervention for the chronic pain and depression comorbidity.
Data Sharing Statement
All data sets analyzed in this study are available in the main text or upon reasonable request. Further information and requests for de-identified participant data should be directed to the corresponding author Abhimanyu Sud at [email protected].
Acknowledgments
The authors thank Vahid Ashoorion, Sarah Selvadurai and Kawsari Abdullah for helpful comments on previous versions of this manuscript; the Art of Living Foundation for supporting intervention delivery; Victoria Mills, Irum Atta, and Misbah Majeed for supporting HEP delivery; and the staff and clinicians from our recruitment sites.
This work was supported by the Canadian Institutes of Health Research, Project Operating Grant—Evaluation of Interventions to Address the Opioid Crisis: non-pharmacological interventions for pain—grant number EO1–162072. The funders had no role in study design, data collection, data analysis, or decision to publish.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr Benoit Mulsant is part of the Labatt Family Chair in Depression Biology in Late-Life Adults and the Department of Psychiatry at the University of Toronto; reports compensation from Centre for Addiction and Mental Health, grants from Brain Canada, Canadian Institutes of Health Research, CAMH Foundation, Patient-Centered Outcomes Research (PCORI), US National Institutes of Health, other from The Czarina Santos-Borja, MD Memorial Lecture (Sheppard-Pratt), honorarium from UMASS Psychiatry Grand Rounds, AAGP Invited Plenary Speaker, University of Arizona, University of Washington, board of trustees of CAMH, non-financial support for use of software funded by CAMH Foundation from Capital Solutions Design LLC, non-financial support for use of software funded by Brain Canada from HAPYneuron, outside the submitted work. Andrea D. Furlan reports authoring the My Opioid Manager book and app owned by the University Health Network, receiving an unrestricted educational grant from the Canadian Generics Pharmaceutical Association to maintain an online self-assessment opioid course for health care professionals in Canada, and receiving payments from Google Inc. as a member of the YouTube partner program. Ronnie Newman is an employee of the Art of Living (United States) which delivers the Sahaj Samadhi Meditation program. She did not receive any compensation for involvement in this study. Andrew Pinto is supported as a clinician scientist by the Department of Family and Community Medicine, Faculty of Medicine at the University of Toronto and at St. Michael’s Hospital. Andrew Pinto is additionally supported by the Li Ka Shing Knowledge Institute, St. Michael’s Hospital, and a Canadian Institutes of Health Research Applied Public Health Chair in Upstream Prevention. Abhimanyu Sud is supported as a Clincian Investigator by the Department of Family and Community Medicine, Faculty of Medicine at the University of Toronto and as a Research Chair in Primary Care and Population Health Systems at Humber River Health. The opinions, results, and conclusions reported in this article are those of the authors and are independent from any funding sources. All remaining authors have no conflicts of interest to report for this work.
References
1. Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. 2022;163(2):e328–e332. doi:10.1097/j.pain.0000000000002291
2. Schopflocher D, Taenzer P, Jovey R. The prevalence of chronic pain in Canada. Pain Res Manag. 2011;16(6):445–450. doi:10.1155/2011/876306
3. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–1006. doi:10.15585/mmwr.mm6736a2
4. Tunks ER, Crook J, Weir R. Epidemiology of chronic pain with psychological comorbidity: prevalence, risk, course, and prognosis. Can J Psychiatry. 2008;53(4):224–234. doi:10.1177/070674370805300403
5. Brody DJ, Pratt LA, Hughes JP. Prevalence of depression among adults aged 20 and over: United States, 2013–2016. NCHS Data Brief. 2018;(303):1–8.
6. Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336–346. doi:10.1001/jamapsychiatry.2017.4602
7. Orhurhu V, Olusunmade M, Akinola Y, et al. Depression trends in patients with chronic pain: an analysis of the nationwide inpatient sample. Pain Physician. 2019;22(5):E487–E494. doi:10.36076/ppj/2019.22.E487
8. Poole H, White S, Blake C, Murphy P, Bramwell R. Depression in chronic pain patients: prevalence and measurement. Pain Pract. 2009;9(3):173–180. doi:10.1111/j.1533-2500.2009.00274.x
9. Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–2445. doi:10.1001/archinte.163.20.2433
10. Elliott TE, Renier CM, Palcher JA. Chronic pain, depression, and quality of life: correlations and predictive value of the SF-36. Pain Med. 2003;4(4):331–339. doi:10.1111/j.1526-4637.2003.03040.x
11. Munce SEP, Weller I, Robertson Blackmore EK, Heinmaa M, Katz J, Stewart DE. The role of work stress as a moderating variable in the chronic pain and depression association. J Psychosom Res. 2006;61(5):653–660. doi:10.1016/j.jpsychores.2006.03.048
12. Roughan WH, Campos AI, García-Marín LM, et al. Comorbid chronic pain and depression: shared risk factors and differential antidepressant effectiveness. Front Psychiatry. 2021;12:643609. doi:10.3389/fpsyt.2021.643609
13. Ohayon MM. Specific characteristics of the pain/depression association in the general population. J Clin Psychiatry. 2004;65(Suppl 12):5–9.
14. Sanabria-Mazo JP, Colomer-Carbonell A, Borràs X, et al. Efficacy of videoconference group acceptance and commitment therapy (ACT) and behavioral activation therapy for depression (BATD) for chronic low back pain (CLBP) plus comorbid depressive symptoms: a randomized controlled trial (IMPACT study). J Pain. 2023;24(8):1522–1540. doi:10.1016/j.jpain.2023.04.008
15. Cheng DK, Ullah MH, Gage H, Moineddin R, Sud A. Chronic pain trials often exclude people with comorbid depressive symptoms: a secondary analysis of 346 randomized controlled trials. Clin Trials. 2023;20(6):632–641. doi:10.1177/17407745231182010
16. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020;72(2):149–162. doi:10.1002/acr.24131
17. Korownyk CS, Montgomery L, Young J, et al. PEER simplified chronic pain guideline: management of chronic low back, osteoarthritic, and neuropathic pain in primary care. Can Fam Physician. 2022;68(3):179–190. doi:10.46747/cfp.6803179
18. Saeed SA, Cunningham K, Bloch RM. Depression and anxiety disorders: benefits of exercise, yoga, and meditation. Am Fam Physician. 2019;99(10):620–627.
19. Ravindran AV, Balneaves LG, Faulkner G, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 5. complementary and alternative medicine treatments. Can J Psychiatry. 2016;61(9):576–587. doi:10.1177/0706743716660290
20. Noetel M, Sanders T, Gallardo-Gómez D, et al. Effect of exercise for depression: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2024;384:e075847. doi:10.1136/bmj-2023-075847
21. Sud A, Lai KSP, Cheng DKY, Chung C, Pico-Espinosa OJ, Rice DB. Mind-body interventions for depressive symptoms in chronic pain: a systematic review of meta-analyses. Pain Physician. 2021;24(1):61–72.
22. Hilton L, Hempel S, Ewing BA, et al. Mindfulness meditation for chronic pain: systematic review and meta-analysis. Ann Behav Med. 2017;51(2):199–213. doi:10.1007/s12160-016-9844-2
23. Wahbeh H. Internet Mindfulness Meditation Intervention (IMMI) Improves Depression Symptoms in Older Adults. Medicines. 2018;5(4):119. doi:10.3390/medicines5040119
24. Simpson R, Simpson S, Wood K, Mercer SW, Mair FS. Using normalisation process theory to understand barriers and facilitators to implementing mindfulness-based stress reduction for people with multiple sclerosis. Chronic Illn. 2019;15(4):306–318. doi:10.1177/1742395318769354
25. Simpson R, Simpson S, Wasilewski M, Mercer S, Lawrence M. Mindfulness-based interventions for people with multiple sclerosis: a systematic review and meta-aggregation of qualitative research studies. Disabil Rehabil. 2022;44(21):6179–6193. doi:10.1080/09638288.2021.1964622
26. Sud A, Nelson MLA, Cheng DK, et al. Sahaj Samadhi meditation versus a health enhancement program for depression in chronic pain: protocol for a randomized controlled trial and implementation evaluation. Trials. 2020;21(1):319. doi:10.1186/s13063-020-04243-z
27. Merskey H, Bogduk N. Classification of Chronic Pain.
28. MacCoon DG, Imel ZE, Rosenkranz MA, et al. The validation of an active control intervention for Mindfulness Based Stress Reduction (MBSR). Behav Res Ther. 2012;50(1):3–12. doi:10.1016/j.brat.2011.10.011
29. MacCoon DG, MacLean KA, Davidson RJ, Saron CD, Lutz A. No sustained attention differences in a longitudinal randomized trial comparing mindfulness based stress reduction versus active control. PLoS One. 2014;9(6):e97551. doi:10.1371/journal.pone.0097551
30. MacCoon DG, Sullivan J, Thurlow J, et al. Health Enhancement Program (HEP) guidelines; 2011. Available from: http://digital.library.wisc.edu/1793/28198.
31. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. primary care evaluation of mental disorders. Patient health questionnaire. JAMA. 1999;282(18):1737–1744. doi:10.1001/jama.282.18.1737
32. Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatric Annals. 2002;32(9):509–515. doi:10.3928/0048-5713-20020901-06
33. McMillan D, Gilbody S, Richards D. Defining successful treatment outcome in depression using the PHQ-9: a comparison of methods. J Affect Disord. 2010;127(1–3):122–129. doi:10.1016/j.jad.2010.04.030
34. Rayner L, Hotopf M, Petkova H, Matcham F, Simpson A, McCracken LM. Depression in patients with chronic pain attending a specialised pain treatment centre: prevalence and impact on health care costs. Pain. 2016;157(7):1472–1479. doi:10.1097/j.pain.0000000000000542
35. Meader N, Mitchell AJ, Chew-Graham C, et al. Case identification of depression in patients with chronic physical health problems: a diagnostic accuracy meta-analysis of 113 studies. Br J Gen Pract. 2011;61(593):e808–e820. doi:10.3399/bjgp11X613151
36. Bauer-Staeb C, Kounali DZ, Welton NJ, et al. Effective dose 50 method as the minimal clinically important difference: evidence from depression trials. J Clin Epidemiol. 2021;137:200–208. doi:10.1016/j.jclinepi.2021.04.002
37. Kounali D, Button KS, Lewis G, et al. How much change is enough? Evidence from a longitudinal study on depression in UK primary care. Psychol Med. 2022;52(10):1875–1882. doi:10.1017/S0033291720003700
38. Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi:10.1016/j.pain.2004.09.012
39. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi:10.1016/j.jpain.2007.09.005
40. Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33(5):350–357. doi:10.3109/07853890109002089
41. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. doi:10.1097/00005650-199206000-00002
42. RAND Health Care Communications. 36-item short form survey (SF-36) scoring instructions. Available from: https://www.rand.org/health-care/surveys_tools/mos/36-item-short-form/scoring.html.
43. Andersen JR, Breivik K, Engelund IE, et al. Correlated physical and mental health composite scores for the RAND-36 and RAND-12 health surveys: can we keep them simple? Health Qual Life Outcomes. 2022;20(1):89. doi:10.1186/s12955-022-01992-0
44. Ward MM, Guthrie LC, Alba MI. Clinically important changes in short form 36 health survey scales for use in rheumatoid arthritis clinical trials: the impact of low responsiveness. Arthritis Care Res. 2014;66(12):1783–1789. doi:10.1002/acr.22392
45. Schober P, Vetter TR. Repeated measures designs and analysis of longitudinal data: if at first you do not succeed-try, try again. Anesth Analg. 2018;127(2):569–575. doi:10.1213/ANE.0000000000003511
46. Cheng DK, Lai KSP, Pico-Espinosa OJ, et al. Interventions for depressive symptoms in people living with chronic pain: a systematic review of meta-analyses. Pain Med. 2021. doi:10.1093/pm/pnab248
47. Khoo EL, Small R, Cheng W, et al. Comparative evaluation of group-based mindfulness-based stress reduction and cognitive behavioural therapy for the treatment and management of chronic pain: a systematic review and network meta-analysis. Evid Based Ment Health. 2019;22(1):26–35. doi:10.1136/ebmental-2018-300062
48. Kinser PA, Robins JL. Control group design: enhancing rigor in research of mind-body therapies for depression. Evid Based Complement Alternat Med. 2013;2013:140467. doi:10.1155/2013/140467
49. Ryk J, Simpson R, Hosseiny F, et al. Virtually-delivered Sudarshan Kriya Yoga (SKY) for Canadian veterans with PTSD: a study protocol for a nation-wide effectiveness and implementation evaluation. PLoS One. 2022;17(10):e0275774. doi:10.1371/journal.pone.0275774
50. Querstret D, Cropley M, Fife-Schaw C. The effects of an online mindfulness intervention on perceived stress, depression and anxiety in a non-clinical sample: a randomised Waitlist control trial. Mindfulness. 2018;9(6):1825–1836. doi:10.1007/s12671-018-0925-0
51. McDonald K, Seltzer E, Lu M, et al. Quantifying the impact of the COVID-19 pandemic on clinical trial screening rates over time in 37 countries. Trials. 2023;24(1):254. doi:10.1186/s13063-023-07277-1
52. Vahratian A, Blumberg SJ, Terlizzi EP, Schiller JS. Symptoms of anxiety or depressive disorder and use of mental health care among adults during the COVID-19 pandemic - United States, August 2020-February 2021. MMWR Morb Mortal Wkly Rep. 2021;70(13):490–494. doi:10.15585/mmwr.mm7013e2
53. Wu T, Jia X, Shi H, et al. Prevalence of mental health problems during the COVID-19 pandemic: a systematic review and meta-analysis. J Affect Disord. 2021;281:91–98. doi:10.1016/j.jad.2020.11.117
54. Xiong J, Lipsitz O, Nasri F, et al. Impact of COVID-19 pandemic on mental health in the general population: a systematic review. J Affect Disord. 2020;277:55–64. doi:10.1016/j.jad.2020.08.001
55. Upchurch DM, Johnson PJ. Gender differences in prevalence, patterns, purposes, and perceived benefits of meditation practices in the United States. J Womens Health. 2019;28(2):135–142. doi:10.1089/jwh.2018.7178
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



