Back to Journals » International Journal of General Medicine » Volume 17
A Novel Scoring System to Predict Acute Radiation Enteritis Recovery in Cervical Cancer Patients Undergoing Concurrent Chemoradiotherapy: A Southwest China Cohort Study
Authors Zeng C, Ji J, Huang Y, Peng Y, Zhang X, Yang Z, Guo Z
Received 18 August 2024
Accepted for publication 26 November 2024
Published 9 December 2024 Volume 2024:17 Pages 5907—5919
DOI https://doi.org/10.2147/IJGM.S485087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kenneth Adler
Chuan Zeng,1,2 Jia Ji,3 Yusheng Huang,1,2 Yuan Peng,1,2 Xiaoyue Zhang,1,2 Zhenzhou Yang,1,2 Zhengjun Guo1,2,4
1Department of Cancer Center, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 2Chongqing Key Laboratory of Immunotherapy, Chongqing, People’s Republic of China; 3Department of Neuro-Oncology, Chongqing University Cancer Hospital, Chongqing, People’s Republic of China; 4Department of Biomedical Sciences, City University of Hong Kong, Hong Kong, People’s Republic of China
Correspondence: Zhengjun Guo, Email [email protected]
Purpose: To establish a pragmatic and effective predictive model for monitoring the recovery of radiation enteritis (RE) in cervical cancer patients undergoing concurrent chemoradiotherapy (CCRT).
Methods: This study included 105 cervical cancer patients undergoing CCRT. We assessed baseline clinicopathologic characteristics, evaluated the effects of CCRT on circulating immune cells, tumor biomarkers, and inflammatory cytokines, and developed a predictive scoring system, the Immune-Tumor-Score (ITS), using the LASSO-Cox regression model. The model performance of LASSO-Cox and nomogram was compared via ROC curve and calibration curve.
Results: The median age of the patients was 55 years, with 53.3% having a normal BMI and 46.7% having positive lymph nodes. Post-CCRT, significant decreases were observed in lymphocyte counts, T-cell subpopulations, and tumor markers (CA125, TPA, SCCA, CYFRA21). The CD4/CD8 ratio and IL10 levels were significantly higher post-CCRT, while inflammation indexes (NLR, ELR) increased, and LMR decreased. The ITS, derived from 11 significant parameters, effectively predicted RE recovery, outperforming a traditional nomogram. Higher ITS scores correlated with shorter RE recovery times, as validated by Kaplan–Meier analyses and ROC curves (AUC = 0.822).
Conclusion: The ITS system provides a robust and reliable tool for predicting RE recovery in cervical cancer patients undergoing CCRT, surpassing traditional models in accuracy and reliability. This tool enables better patient management by allowing for timely interventions and personalized treatment strategies. Future research should focus on validating these findings in larger cohorts and integrating additional clinical parameters to enhance the predictive power of the ITS.
Keywords: radiation enteritis, cervical cancer, concurrent chemoradiotherapy, inflammatory-tumor score, LASSO-Cox regression model
Introduction
Cervical cancer is a major global health concern, ranking as the fourth most prevalent cancer and the fourth leading cause of cancer-related death in females worldwide.1 As per the 2020 estimates from the Global Cancer Observatory, China, is expected to account for over 18% of all cervical cancer cases and approximately 17% of estimated global deaths from this disease.2 Although several Asian countries have reported a decline in cervical cancer incidence, the rate in China has remained relatively stable, even showing a slight increase between 2007 and 2017.2
Radiotherapy is a cornerstone in the treatment of cervical cancer, especially for patients with advanced disease or those unsuitable for surgery.3 However, radiation enteritis (RE), a common side effect of pelvic radiotherapy, can significantly impact patients’ quality of life, treatment compliance, and overall treatment duration.4,5
Previous studies have identified the factors that contribute to RE, such as the small bowel radiotherapy and the interruptions during radiotherapy.5,6 In 2023, Zhou’s group highlighted that the anal bulge rating and disease activity index (DAI) score were independent predictors of severe acute RE.7 While, the factors that contribute to the DAI score, such as weight loss, stool viscosity, and stool bleeding, may not comprehensively represent the spectrum of symptoms and side effects experienced by cervical cancer patients undergoing radiotherapy.7 This underscores the need for further research to establish more accurate and comprehensive prediction system for RE in cervical cancer patients.8
To address this issue, our study explored a novel prognostic scoring system, the Inflammatory-Tumor marker Score (ITS). This system leverages peripheral blood count, inflammatory, and tumor markers before and after CCRT to predict the RE recovery status in cervical cancer patients. We propose that the ITS scoring system can enhance clinicians’ strategic decision-making and patient management, offering a more accurate prediction of RE recovery. Our study aims to contribute to the development of more precise and comprehensive prediction systems for radiation enteritis in cervical cancer patients, ultimately improving patient outcomes.
Materials and Methods
Inclusion Criteria and Exclusion Criteria
Patients and Study Design
We enrolled 105 cervical cancer patients treated with CCRT at the Second Affiliated Hospital of Chongqing Medical University from January 2019 to January 2022. Patients received standard treatment using a Vitalbeam linear accelerator (Varian, USA) and 3D conformal radiation technique. Eligibility criteria includes: 1) stage IB2 or above (FIGO 2018) cervical cancers; 2) standard CCRT; 3) unsuitable for resection according to guideline; 4) no preoperative parenteral nutrition, acute inflammation, immune diseases, COVID-19 infection or other malignancies; and 5) available clinicopathological and follow-up data. Patients with emergency surgery due to complications or death within 30 days post-CCRT were excluded. Inclusion required meeting the RE diagnostic criteria per the Common Terminology Criteria for Adverse Events (CTCAE).9
This study was approved by the Institutional Review Board and in accordance with the Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from each patient before enrollment. They were fully informed about the study’s purpose, procedures, potential risks, and benefits. The patients were assured that their participation was voluntary and that they could withdraw from the study at any time without any repercussions. Confidentiality of personal data was strictly maintained throughout the study.
Data Collection and Definition of Variables
Clinicopathological information was collected, including age at diagnosis, tumor sites, pathological tumor type, and stage (FIGO, 2018). Patients underwent routine procedures, and clinical and pathological data were retrospectively reviewed. The routine procedure encompasses hematological analysis, assessment of hepatic and renal functionality, as well as screening for tumor markers, among others. A total of 33 continuous variables were included as follows: RBC, hemoglobin (Hb), white blood cell count (WBC), neutrophil count (NC), lymphocyte count (LC), monocyte count (MC), eosinophil count (EC), BC, PLT, CA125, CA50, CEA, CYFRA21, SCCA, TPA, CD3+ T lymphocyte count, CD4+ T lymphocyte count, CD4+/CD8+ lymphocyte ratio, CD8+ T lymphocyte count, IL2, IL4, IL6, IL10, TNF-α, and IFN-γ. All variables were determined using routine laboratory technique and performed in accordance with the manufacturer’s instructions. The NLR, lymphocyte-to-monocyte ratio (LMR), eosinophil-to-lymphocyte ratio (ELR), and platelet-to-lymphocyte ratio (PLR) were calculated as follows: NLR = N/L, LMR = L/M, ELR = E/L, PLR = P/L (N: neutrophil count, L: lymphocyte count, M: monocyte count, E: Eosinophil count, and P: platelet count). SII, was calculated as follows: SII = P × N/L. The formula for calculating the SII was based on previously published research by Hu et al.10 Prefixes “pre” and “po” denote pre- and post-CCRT parameters, respectively. The pre-CCRT parameters are measured within 7 days before the start of CCRT, while the post-CCRT parameters are obtained within 7 days after the completion of CCRT.
Cutoff Values of Inflammatory Indexes and Patient Follow-Up
The cutoff values of NLR, LMR, ELR, PLR, and SII were determined via X-tile software (Yale University).11 After analyses, NLR = 9.68, LMR = 2.56, ELR = 0.22, PLR = 413.7, and SII = 1284 were the most accurate cut-off values for RE patients. The RE-free time (REFT) was defined as the time from the incidence of RE until recovery from RE. The median follow-up period is 15 days.
Statistical Analysis
Continuous variables were compared with Student’s t-test or Chi-squared test. LASSO-Cox regression model was employed to select the most useful prognostic variables from all available hematological, inflammatory, and tumor biomarkers. This model utilizes an L1 penalty to shrink the regression coefficients towards zero, thereby reducing the risk of overfitting. The optimal penalty parameter, lambda, was determined through a 10-fold cross-validation process with minimum criteria. Features retained with nonzero coefficients were utilized to construct the ITS. Kaplan–Meier method generated RE recovery curves, and Cox proportional hazards models performed univariate and multivariate analyses to identify independent prognostic factors. Integrating variables and utilizing Cox proportional hazards model, we constructed a nomogram to evaluate the prognostic significance of these features on a website platform, Sangerbox 3.0 (http://vip.sangerbox.com/). To compare the predictive accuracy of the LASSO-Cox model and the nomogram, we performed ROC curves and calibration curves. ROC curves were used to assess the sensitivity and specificity of the models, while calibration curves compared the predicted survival probabilities with the actual observed outcomes. This dual evaluation allowed for a comprehensive comparison of the models’ performance in terms of both discrimination and calibration. Analyses were conducted using SPSS 24.0 and R 4.0.2 (http://www.R-project.org), with p < 0.05 considered statistically significant.
Results
Baseline Clinicopathologic Characteristics
The baseline clinical characteristics of the 105 patients are shown in Table 1. Participants had a median age of 55 years, ranging from 24 to 75. Approximately half of the group (53.3%) had a normal body mass index, while the rest (46.7%) were classified outside the normal range (Table 1). In terms of menopausal status, 38 patients (36.2%) were premenopausal, while the remaining 67 (63.8%) were postmenopausal. The majority of patients were diagnosed with squamous cell carcinoma, which represented 71.4% of cases. Adenocarcinoma was less common, accounting for 16.2% of cases. Most patients (71.4%) received Cisplatin at a dose of 40 mg/m2 (QW), while others (21.9%) received a higher dose of Cisplatin at 100 mg/m2 every 3 weeks (Q3W). A smaller subset (6.7%) was treated with Carboplatin (AUC 5) every 3 weeks (Q3W). Lymph node status varied among the participants, with 46.7% showing positive lymph nodes, indicating metastasis, while the remaining 53.3% had negative lymph nodes. The mean radiation doses received by critical organs at risk were as follows: small bowel (32.56 ± 8.52 Gy), rectum (44.55 ± 4.54 Gy), and bladder (40.47 ± 5.31 Gy). Radiation toxicity, based on RTOG criteria, was primarily mild to moderate. Degree I toxicity was noted in 41% of patients, and degree II in 36.2%. A smaller portion of patients (20%) experienced degree III toxicity, and a rare 2.9% encountered severe, life-threatening symptoms due to radiation enteritis (degree IV).
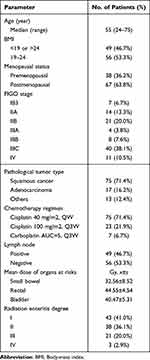 |
Table 1 Patients’ Characteristics |
CCRT Has a Profound Effect on Circulating Immune Cells, Tumor Biomarkers, and Inflammation Cytokines
After comparing blood parameters before CCRT (pre-CCRT) and after CCRT (post-CCRT) using a paired Student’s t-test, several key changes were observed. White blood cell (WBC) and NC remained stable after CCRT, showing no significant difference between pre- and post-CCRT levels (p > 0.05, Figure 1A). In contrast, eosinophil counts (EC) increased notably from an average of 0.16 × 109/L (range 0–0.63) pre-CCRT to 0.25 × 109/L (range 0–1.44) post-CCRT (p = 0.002, Figure 1A). BC decreased slightly, with post-CCRT levels at 0.01 × 109/L (range 0–0.08) compared to 0.02 × 109/L pre-treatment (p = 0.012, Figure 1A). Lymphocytes, which are markers of inflammation, also declined, with counts dropping from 1.10 × 109/L (range 0.12–3.18) pre-CCRT to 0.83 × 109/L (mean, range 0.09–1.93) post-CCRT (p = 0.012, Figure 1A). Similarly, T cell subpopulations, including naive T cells (CD3+ T), helper T cells (CD4+ T), and cytotoxic T cells (CD8+ T), all showed substantial reductions post-CCRT (p < 0.001 for all; Figure 1B). The CD4/CD8 ratio, however, was significantly higher after CCRT than before (p = 0.005, Figure 1D).
Conventional tumor biomarkers, such as CA125, CA50, CEA, CYFRA21, SCCA, and TPA, were also detected.12,13 All markers showed significant decreases post-treatment except CA50 and CEA, which remained stable (Figure 1B). Notable reductions included CA125, which dropped from 27.79 U/mL to 19.14 U/mL (p = 0.006, Figure 1C). TPA is a pan-cancer serum factor,14 which declined by 53%, from 83.05 U/L to 38.95 U/L (p < 0.0001, Figure 1C). SCCA levels also fell, from an average of 3.73 ng/mL (range 0.22–46.96) to 2.35 ng/mL (range 0.20–22.36) (p = 0.007, Figure 1C). The serum concentration of CYFRA21 also dropped from 2.37 ng/mL (range 0.28–28.18) to 1.70 ng/mL (range 0.15–12.66) (p = 0.031, Figure 1C).
To assess changes in the immune microenvironment, we examined immune cytokines (IL2, IL4, IL6, IL10, TNF-α, and IFN-γ).15 Among these, only IL10 showed a significant increase post-CCRT (p = 0.008, Figure 1D), with other cytokines showing no significant change (p > 0.05, Figure 1D). We also analyzed immune inflammation indexes (NLR, LMR, ELR, PLR, and SII) as dynamic indicators of immune changes.16–18 Post-CCRT levels of NLR and ELR were elevated compared to pre-treatment (p = 0.01 and p = 0.02, respectively, Figure 1E). However, the LMR significantly decreased after CCRT (p = 0.003, Figure 1E). The SII and PLR did not show significant changes pre- and post-CCRT.
Chi-Square Test, Univariate and Multivariate Analysis
Chi-square test results, summarized in Table 2, revealed several significant differences between pre- and post-CCRT patient groups. Significantly lower LC (p = 0.026) and BC (p < 0.001) were observed in patients post-CCRT patients. Similarly, post-CCRT patients showed reduced levels of CD3+ T, CD4+ T, and CD8+ T lymphocytes (all p < 0.001). In contrast, WBC (p = 0.053), NC (p = 0.138) and the CD4/CD8 lymphocyte ratio (p = 0.069) remained stable (Table 2). Tumor biomarkers also showed significant changes after CCRT. Patients with higher CEA levels had notable reductions (p = 0.037), and TPA decreased significantly as well (p < 0.001). In terms of immune inflammation indexes, a lower LMR was associated with patients after CCRT (p < 0.001), whereas NLR, ELR and SII were significantly higher after CCRT (p = 0.016, p < 0.001, and p < 0.001, respectively).
 |
Table 2 The Blood Features Before and After CCRT of Cervical Cancer Patients with RE |
Univariate and multivariate Cox regression analyses, shown in Table 3, identified several factors associated with recovery from radiation enteritis (RE). In univariate analysis, increased MC (p = 0.015), PLT (p = 0.006), NLR (p = 0.015), ELR (p = 0.026), and PLR (p = 0.007) emerged as favorable factors for RE recovery. Conversely, elevated CA125 (p = 0.016), SSCA (p = 0.035), CD3+ T lymphocytes (p = 0.011), CD4+ T lymphocytes (p = 0.03), CD4/CD8 ratio (p = 0.037), and SII (p = 0.001) were identified as unfavorable factors. The multivariate Cox model identified 13 independent predictors of RE recovery. Among the favorable factors were MC, LC, NC, EC, IL6, ELR, IFNγ, and TPA, each significantly promoting RE recovery (p < 0.05). In contrast, elevated levels of CD3+ T lymphocytes, WBC, CA125, SCCA, and CA50 were significantly associated with poorer recovery outcomes (p < 0.05).
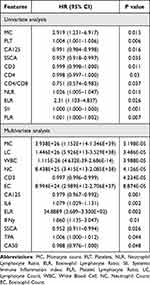 |
Table 3 Univariate, Multivariate and LASSO-Cox Analyses of Clinical Features for Patients with aAE |
LASSO-Cox Regression Analysis and ITS Groups
We aimed to develop and validate a scoring system, the ITS, to predict the risk of RE in patients undergoing radiotherapy. To achieve this, we employed the LASSO-Cox model. A total of 105 patients with RE with 33 parameters were included in this study, and R package glmnet was deployed to integrate time to RE recovery, event of RE recovery and the dynamic changes of parameters. To avoid the influence of multicollinearity factors, the LASSO-Cox model was performed with nonzero coefficients according to the minimum criteria with λ=0.071 (Figure 2A). Furthermore, the formula of the ITS scoring system was 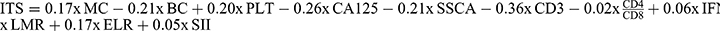 . The paired likelihood deviance was minimized when λ = 0.071 (Figure 2B), ensuring the robustness of this scoring model. A forest plot analysis identified significant predictors of RE recovery, CD3+ T lymphocytes (p < 0.001), BC (p = 0.011), CA125 (p = 0.001) and SSCA (p = 0.002) were unfavorable factors, while PLT (p = 0.005), ELR (p = 0.006) and MC (p = 0.018) promoted recovery (Figure 2C). RE recovery time curves via Kaplan–Meier analyses and Log rank tests of ITS are presented in Figure 2D (p < 0.001). The best cutoff value of ITS was −0.278, which was calculated using the R package “maxstat”.19 The overall significance was p = 9.771 × 10−14 (Log rank test) and the C-index was 0.822.
. The paired likelihood deviance was minimized when λ = 0.071 (Figure 2B), ensuring the robustness of this scoring model. A forest plot analysis identified significant predictors of RE recovery, CD3+ T lymphocytes (p < 0.001), BC (p = 0.011), CA125 (p = 0.001) and SSCA (p = 0.002) were unfavorable factors, while PLT (p = 0.005), ELR (p = 0.006) and MC (p = 0.018) promoted recovery (Figure 2C). RE recovery time curves via Kaplan–Meier analyses and Log rank tests of ITS are presented in Figure 2D (p < 0.001). The best cutoff value of ITS was −0.278, which was calculated using the R package “maxstat”.19 The overall significance was p = 9.771 × 10−14 (Log rank test) and the C-index was 0.822.
Model Comparison
Parallel to the LASSO-Cox model, we constructed a nomogram using the same dataset and features. Based on the independent prognostic features identified via the Cox proportional hazards model, we constructed a nomogram to predict the recovery time for patients with radical enteritis (Figure 3B). The performance of both models was compared and evaluated via ROC curve. The AUC showed that the LASSO-Cox model was better than the Nomogram model (Figure 3C). The calibration curves for the LASSO-Cox model and the nomogram model probabilities showed a safe conclusion. For model of LASSO-Cox, both the MAE and MSE were lower than those in the model of Nomogram, suggesting better average accuracy in prediction and in squared differences. Also, the LASSO-Cox model showed a lower value for the 0.9 quantile (0.045 vs 0.062), indicating that a higher percentage of predictions are closer to the actual values (Figure 3E and F). In summary, the LASSO-Cox model’s predictions are more accurate and have smaller errors on average and at the 0.9 quantile level.
Discussion
The management of RE in patients with cervical cancer underwent pelvic CCRT for cervical cancer is a significant clinical challenge. Over 80% of these patients experience radiation-induced toxicity, with RE being a prevalent complication.7 RE not only affects the quality of life but also leads to radiotherapy intolerance, prolongation or termination of radiotherapy, which negatively impacts the therapeutic outcome.20
Previous studies have identified risk factors for RE onset but lacked predictive models for RE recovery.21 The incidence of RE escalates with CCRT in locally advanced cervical cancer, especially in patients who have undergone post-pelvic lymph node dissection.22 Prof. Liu’s study highlighted the volume of irradiated small bowel as an independent risk factor for diarrhea in patients undergoing conventional RT.23 Similarly, Wang et al reported that interruptions in radiotherapy, prolonging total irradiation time, heighten the incidence of RE.5 However, these studies did not provide a combined model for predicting RE recovery in cervical cancer patients undergoing radiotherapy or CCRT. Ma et al established a visual nomogram prediction model for cervical cancer radiotherapy patients, showing a strong correlation between certain clinical parameters and severe acute RE.7 However, their study had limitations, including a small sample size and a lack of individual clinical parameters.7
In this study, we innovatively developed a prediction model for RE recovery based on individual clinical parameters before CCRT. By identifying 20 significant variables and categorizing patients into different RE recovery statuses, the ITS system offers a nuanced understanding of individual patient trajectories. Our study’s innovation lies in the comprehensive assessment of dynamic biomarkers and the development of a predictive model for RE recovery, which fulfilled the gap in this field.
The use of a LASSO-Cox regression model addresses the issue of multicollinearity, which is often encountered in traditional univariate and multivariate analysis methods. This approach minimizes variable interference, leading to improved performance and precise prediction. While the nomogram offers good predictive performance, the ITS system outperforms it, providing a more accurate and reliable tool for predicting RE recovery. The comparison of these two models underscores the importance of choosing the right predictive tool in clinical practice and highlights the potential of the ITS system as a superior predictive tool for RE recovery.
Our study also highlights the immunosuppressive effects of CCRT, which pose challenges in patient management.24 While CCRT is effective in treating the cancer, it unfortunately also suppresses the immune system, potentially leading to a lower surveillance status and creating a favorable environment for cancer recurrence.25 Although tumor-specific markers, such as CA125, SSCA, and TPA decreased significantly, the systemic immune status remained in a low immune surveillance status after CCRT. Increased WBC, NC, and MC after CCRT might create a systemic inflammatory activation microenvironment and was unfavorable for the recovery of mucosal injury.25 The decline in LC and all LC subpopulations directly represents a low anti-tumor response.26 NLR is regarded as an evaluation indicator for the systemic balance of pro-tumor inflammation response and anti-tumor immune response.27 An increase of NLR suggests an escalated pro-tumor inflammation status and a decreased anti-tumor immune response.28 A decreased LMR is perceived as an impaired immunologic reaction against cancer and immunosuppressive conditions.29 IL10 suppressed the Th1-type immune response and fosters a tumor-favorable environment.30 The CCRT-induced low immune response status might be regarded as a risk factor for the recurrence of cervical cancer. To address this, future research could focus on strategies to correct this immunosuppressive status. This could involve the use of immunomodulatory agents or therapies designed to boost the immune response. Additionally, personalized treatment plans that consider the patient’s immune status could be beneficial. Regular monitoring of immune markers could help tailor treatments to individual patients, potentially improving outcomes.
Also, these findings suggest a complex interplay between immune cells, inflammatory markers, and specific biomarkers in the recovery process. The identification of MC, PLT, NLR, ELR, and PLR as recovery-promoting factors underscores the importance of certain immune cells and inflammatory markers in the recovery process. Monocytes and platelets are known for their roles in immune response and tissue repair, respectively, which may explain their positive association with recovery.31–33 The inflammatory markers NLR, ELR, and PLR indicate that controlled inflammatory responses might be beneficial for recovery, possibly through the promotion of tissue repair mechanisms.34 Conversely, CA125, SSCA, CD3+, CD4+, CD4/CD8 ratio, and SII emerged as significant unfavorable features for RE recovery. CA125 and SSCA are biomarkers traditionally associated with cancer prognosis, and their negative association with RE recovery suggests potential underlying pathophysiological mechanisms that warrant further investigation. The role of CD3+ and CD4+ T lymphocytes, as well as the CD4/CD8 ratio, in recovery is particularly intriguing. While these cells are crucial for immune responses, their negative impact on recovery could be attributed to immune dysregulation or an overactive immune response that might hinder the healing process.33
Several clinical trials have demonstrated that combining radiotherapy with immunotherapy can significantly improve cure rates for patients.35 The rationale for this combination is that it may counteract immunosuppression and stimulate robust antitumor T cell responses.36 However, the overlapping toxicities from such a combined approach pose a substantial challenge, as radiotherapy and immunotherapy impact different stages of the immune response, which may heighten the risk of adverse events.36,37 Nevertheless, Prof. Wu suggests that combining immunotherapy with radiotherapy might not necessarily lead to increased toxicity.38,39 To clarify these safety and efficacy concerns, additional prospective clinical trials are needed to provide comprehensive data on potential toxicities and therapeutic outcomes.
This study, however, is not without limitations. First, the retrospective nature of the study may have introduced sample selection bias. Second, the sample size was not sufficiently large. Third, we did not consider the degree of RE in patients or symptomatic treatment approaches. Therefore, expanding the sample size, conducting multicenter research, incorporating more parameters into the prediction model and upgrading the algorithm of the model are necessary for the development of a novel and more comprehensive model for RE patients.
Conclusion
In conclusion, our study demonstrates that the LASSO-Cox model, with the derived ITS, provides a superior predictive tool compared to the traditional nomogram. These findings highlight the potential of the LASSO-Cox model in improving the prediction and management of radiation enteritis. Future research should focus on validating these results in larger cohorts and exploring additional predictive features.
Funding
This study was supported by the fund of Natural Science Foundation of Chongqing (CSTB2023NSCQ-MSX0059), Science and Technology Innovation Medical Development Foundation of Beijing (Grant No. KC2021-JX-0186-11), the Chongqing Municipal Health Commission (Grant No. 2023WSJK043), as well as the Program for Youth Innovation in Future Medicine, Chongqing Medical University (Grant No. W0172).
Disclosure
The authors claim no conflicts of interest.
References
1. Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409–436. doi:10.3322/caac.21731
2. Singh D, Vignat J, Lorenzoni V, et al. Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO global cervical cancer elimination initiative. Lancet Glob Health. 2022;11:e197–e206. doi:10.1016/S2214-109X(22)00501-0
3. Kokka F, Bryant A, Olaitan A, et al. Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer. Cochrane Database Syst Rev. 2022;8:CD010260. doi:10.1002/14651858.CD010260.pub3
4. Gandle C, Dhingra S, Agarwal S. Radiation-induced enteritis. Clin Gastroenterol Hepatol. 2020;18:A39–A40. doi:10.1016/j.cgh.2018.11.060
5. Wang Y, Kong W, Lv N, et al. Incidence of radiation enteritis in cervical cancer patients treated with definitive radiotherapy versus adjuvant radiotherapy. J Cancer Res Ther. 2018;14:S120–S4. doi:10.4103/0973-1482.163762
6. Abayomi J, Kirwan J, Hackett A. The prevalence of chronic radiation enteritis following radiotherapy for cervical or endometrial cancer and its impact on quality of life. Eur J Oncol Nurs. 2009;13:262–267. doi:10.1016/j.ejon.2009.02.007
7. Ma CY, Zhao J, Gan GH, et al. Establishment of a prediction model for severe acute radiation enteritis associated with cervical cancer radiotherapy. World J Gastroenterol. 2023;29:1344–1358. doi:10.3748/wjg.v29.i8.1344
8. Chen SW, Liang JA, Yang SN, et al. Radiation injury to intestine following hysterectomy and adjuvant radiotherapy for cervical cancer. Gynecol Oncol. 2004;95:208–214. doi:10.1016/j.ygyno.2004.07.003
9. Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). JAMA Oncol. 2015;1:1051–1059. doi:10.1001/jamaoncol.2015.2639
10. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212–6222. doi:10.1158/1078-0432.CCR-14-0442
11. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi:10.1158/1078-0432.CCR-04-0713
12. Diamandis EP. Cancer biomarkers: can we turn recent failures into success? J Natl Cancer Inst. 2010;102:1462–1467. doi:10.1093/jnci/djq306
13. Zhi W, Ferris D, Sharma A, et al. Twelve serum proteins progressively increase with disease stage in squamous cell cervical cancer patients. Int J Gynecol Cancer. 2014;24:1085–1092. doi:10.1097/IGC.0000000000000153
14. Sundstrom BE, Stigbrand TI. Cytokeratins and tissue polypeptide antigen. Int J Biol Markers. 1994;9:102–108. doi:10.1177/172460089400900207
15. Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. 2013;14:e218–28. doi:10.1016/S1470-2045(12)70582-X
16. Cupp MA, Cariolou M, Tzoulaki I, et al. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18:360. doi:10.1186/s12916-020-01817-1
17. Lin ZQ, Ma C, Cao WZ, et al. Prognostic significance of NLR, PLR, LMR and tumor infiltrating T lymphocytes in patients undergoing surgical resection for hilar cholangiocarcinoma. Front Oncol. 2022;12:908907. doi:10.3389/fonc.2022.908907
18. Nost TH, Alcala K, Urbarova I, et al. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol. 2021;36:841–848. doi:10.1007/s10654-021-00752-6
19. Wright MN, Dankowski T, Ziegler A. Unbiased split variable selection for random survival forests using maximally selected rank statistics. Stat Med. 2017;36:1272–1284. doi:10.1002/sim.7212
20. Loge L, Florescu C, Alves A, Menahem B. Radiation enteritis: diagnostic and therapeutic issues. J Visc Surg. 2020;157:475–485. doi:10.1016/j.jviscsurg.2020.08.012
21. Wang W, Zhang F, Hu K, Hou X. Image-guided, intensity-modulated radiation therapy in definitive radiotherapy for 1433 patients with cervical cancer. Gynecol Oncol. 2018;151:444–448. doi:10.1016/j.ygyno.2018.09.024
22. Sun Myint A, Mukhopadhyay T, Ramani VS, et al. Can increasing the dose of radiation by HDR brachytherapy boost following pre operative chemoradiotherapy for advanced rectal cancer improve surgical outcomes? Colorectal Dis. 2010;12(Suppl 2):30–36. doi:10.1111/j.1463-1318.2010.02322.x
23. Li Q, Chen J, Zhu B, et al. Dose volume effect of acute diarrhea in post-operative radiation for gynecologic cancer. Rev Invest Clin. 2017;69:329–335. doi:10.24875/RIC.17002373
24. Gjyshi O, Grippin A, Andring L, et al. Circulating neutrophils and tumor-associated myeloid cells function as a powerful biomarker for response to chemoradiation in locally advanced cervical cancer. Clin Transl Radiat Oncol. 2023;39:100578. doi:10.1016/j.ctro.2023.100578
25. Zhang Z, Liu X, Chen D, Yu J. Radiotherapy combined with immunotherapy: the Dawn of cancer treatment. Signal Transduct Target Ther. 2022;7:258.10.1038/s41392–022–01102–y.
26. Lakomy DS, Wu J, Lombe D, et al. Immune correlates of therapy outcomes in women with cervical cancer treated with chemoradiotherapy: a systematic review. Cancer Med. 2021;10:4206–4220. doi:10.1002/cam4.4017
27. Jonska-Gmyrek J, Gmyrek L, Zolciak-Siwinska A, et al. Pretreatment neutrophil to lymphocyte and platelet to lymphocyte ratios as predictive factors for the survival of cervical adenocarcinoma patients. Cancer Manag Res. 2018;10:6029–6038. doi:10.2147/CMAR.S178745
28. Moses K, Brandau S. Human neutrophils: their role in cancer and relation to myeloid-derived suppressor cells. Semin Immunol. 2016;28:187–196. doi:10.1016/j.smim.2016.03.018
29. Kim D, Bae SJ, Ahn SG, et al. RT-induced dynamic changes in the lymphocyte-to-monocyte ratio in patients with breast cancer indicate poor prognosis. Breast Cancer Res Treat. 2022;193:637–647. doi:10.1007/s10549-022-06601-8
30. Ji H, Ba Y, Ma S, et al. Construction of interferon-gamma-related gene signature to characterize the immune-inflamed phenotype of glioblastoma and predict prognosis, efficacy of immunotherapy and radiotherapy. Front Immunol. 2021;12:729359. doi:10.3389/fimmu.2021.729359
31. Gentile P, Garcovich S. Systematic review-the potential implications of different Platelet-Rich Plasma (PRP) concentrations in regenerative medicine for tissue repair. Int J Mol Sci. 2020;21. doi:10.3390/ijms21165702
32. Ochando J, Mulder WJM, Madsen JC, et al. Trained immunity - basic concepts and contributions to immunopathology. Nat Rev Nephrol. 2023;19:23–37. doi:10.1038/s41581-022-00633-5
33. Foy BH, Sundt TM, Carlson JCT, et al. Human acute inflammatory recovery is defined by co-regulatory dynamics of white blood cell and platelet populations. Nat Commun. 2022;13:4705. doi:10.1038/s41467-022-32222-2
34. Eming SA, Wynn TA, Martin P. Inflammation and metabolism in tissue repair and regeneration. Science. 2017;356:1026–1030. doi:10.1126/science.aam7928
35. Yang X, Ren H, Fu J. Combinations of radiotherapy with immunotherapy in cervical cancer. J Cancer. 2022;13:1480–1489. doi:10.7150/jca.65074
36. Ngwa W, Irabor OC, Schoenfeld JD, et al. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18:313–322. doi:10.1038/nrc.2018.6
37. Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12:237–251. doi:10.1038/nrc3237
38. Ferrall L, Lin KY, Roden RBS, et al. Cervical cancer immunotherapy: facts and hopes. Clin Cancer Res. 2021;27:4953–4973. doi:10.1158/1078-0432.CCR-20-2833
39. Sha CM, Lehrer EJ, Hwang C, et al. Toxicity in combination immune checkpoint inhibitor and radiation therapy: a systematic review and meta-analysis. Radiother Oncol. 2020;151:141–148. doi:10.1016/j.radonc.2020.07.035
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Nomogram to Predict Radiation Enteritis in Cervical Squamous Cell Carcinoma
Wang J, Hu G
Cancer Management and Research 2022, 14:3303-3311
Published Date: 25 November 2022
Dynamic Nomogram Based on the Metastatic Number and Sites and Therapy Strategies Predicting the Prognosis of Patients with Metastatic Cervical Cancer
Ma Y, Li J, Tan X, Cai M, Zhang X, Ma J
International Journal of Women's Health 2022, 14:1807-1819
Published Date: 22 December 2022
Prognostic Value of Body Composition and Systemic Inflammatory Markers in Patients with Locally Advanced Cervical Cancer Following Chemoradiotherapy
Guo H, Feng S, Li Z, Yin Y, Lin X, Yuan L, Sheng X, Li D
Journal of Inflammation Research 2023, 16:5145-5156
Published Date: 10 November 2023
An Energy-Efficient Test and Predictive Model for Recurrence After Radiotherapy in Localized Intermediate and Advanced Cervical Cancer Were Created Using Thymidine Kinase 1 in Conjunction with Inflammatory Markers and Tumor Markers
Luo Y, Ma X
International Journal of General Medicine 2023, 16:5789-5797
Published Date: 8 December 2023
Disparities in Survival Outcomes Between Locally Advanced Cervical Squamous Cell Carcinoma and Adenocarcinoma Treated with Chemoradiotherapy
Hong SS, Li Y, Lin YY, Wu SG, Chen LY, Zhou J
International Journal of Women's Health 2024, 16:401-410
Published Date: 6 March 2024




