Back to Journals » Infection and Drug Resistance » Volume 18
A Rare Fatal Case of COVID-19 Co-Infection with Community-Acquired Methicillin-Resistant Staphylococcus Aureus in a Diabetic Patient
Authors Meng Z, Wuxiuer R, Zhao F, Yang Q
Received 17 December 2024
Accepted for publication 1 April 2025
Published 17 April 2025 Volume 2025:18 Pages 1935—1939
DOI https://doi.org/10.2147/IDR.S512885
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Zhaolu Meng,1 Rehanguli Wuxiuer,1 Feng Zhao,2 Qiao Yang1,3
1Department of Infectious Diseases, Sir Run Run Shaw Hospital, Alaer Hospital, Zhejiang University School of Medicine, Alaer, 843300, People’s Republic of China; 2Department of Clinical Laboratory, Sir Run Run Shaw Hospital, Alaer Hospital, Zhejiang University School of Medicine, Alaer, 843300, People’s Republic of China; 3Department of Infectious Diseases, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, 310016, People’s Republic of China
Correspondence: Qiao Yang, Email [email protected]
Background: The co-infection of COVID-19 with community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) is rare but can lead to severe outcomes, especially in diabetic patients with impaired immune function. CA-MRSA, especially PVL-positive strains, can exacerbate COVID-19 symptoms and drive rapid disease progression.
Patient and Methods: We present a case of a 53-year-old diabetic female patient with poor glycemic control, who was hospitalized with COVID-19 and rapidly progressed to severe pneumonia. Despite antiviral treatment with Paxlovid and dexamethasone, as well as antimicrobial therapy with piperacillin-tazobactam, her condition deteriorated, leading to respiratory failure requiring extracorporeal membrane oxygenation. A post-mortem sputum culture confirmed MRSA on the day following the patient’s death.
Conclusion: This case underscores the critical need for screening and treating bacterial co-infections in COVID-19 management, particularly in immunocompromised hosts, due to the enhanced pathogenicity of CA-MRSA strains. Early recognition and appropriate antimicrobial therapy are essential in reducing the impact of such co-infections.
Keywords: COVID-19, methicillin-resistant Staphylococcus aureus, co-infection, diabetes mellitus, pneumonia
Introduction
The global spread of COVID-19 has escalated the incidence of pulmonary co-infections.1 Notably among bacterial co-infections, community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) stands out due to its resistance to conventional antimicrobial agents. The virulence of CA-MRSA, particularly strains carrying the Pantón-Valentine leukocidin (PVL) gene, can significantly exacerbate COVID-19 symptoms and contribute to rapid disease progression.2 Patients with diabetes are at higher risk for infections, including MRSA, attributed to their impaired immune function and propensity for poor wound healing.3 The co-infection of COVID-19 with CA-MRSA in diabetic patients is infrequently reported and can lead to severe clinical outcomes due to the synergistic effects on the immune system. We report the case of a diabetic female patient in China who was co-infected with COVID-19 and CA-MRSA, resulting in death.
Case Report
On Sep 17, 2023, a 53-year-old female patient presented to the infectious disease department at Alaer Hospital with a 3-day history of fever up to 40.3°C, chills, myalgia, and a 1-day history of productive cough with hemoptysis and chest pain. Upon symptom onset, she was treated with oseltamivir and ibuprofen at a local clinic, but without symptomatic relief. The patient with underlying type 2 diabetes mellitus presented with poor glycemic control despite concurrent treatment with insulin and oral hypoglycemic agents. The patient had no previous COVID-19 infections or vaccinations.
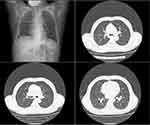
On admission, a physical examination showed coarse breath sounds without rales. Peripheral leukocyte count was 3.75×109 cells/L (86.3% neutrophils, 9.9% lymphocyte, 3.7% monocytes), platelet count was 93 × 109/L and C-reactive protein (CRP) level was 5.3 mg/L. Glycemic assessment showed a random blood glucose level of 17.8 mmol/L and a glycated hemoglobin levels of 13.4%. Transaminase and creatinine level were within normal limits. A nasopharyngeal swab for COVID-19 antigen tested positive. The patient’s COVID-19 nucleic acid assay was positive, with a Ct value of 27.5 for the ORF gene and 26.55 for the N gene of SARS-CoV-2 RNA. Results were negative for influenza A/B virus antigen, Mycoplasma pneumoniae IgM, Chlamydia pneumoniae IgM, cytomegalovirus IgM, and adenovirus IgM. Arterial blood gas analysis revealed a pH of 7.44, partial pressure of CO2 38.3 mmHg, partial pressure of O2 59.5 mmHg (fraction of inspired O2 21%) and lactate 0.5 mmol/L. The electrocardiogram (ECG) and cardiac auscultation were normal. Chest computed tomography (CT) scan showed findings indicative of viral infection (Figure 1).
The patient began treatment with Paxlovid (nirmatrelvir/ritonavir 300mg/100mg orally every 12 hours) and intravenous dexamethasone (5mg twice daily). On the second hospital day, her condition worsened, marked by high fever (up to 39.4°C), fatigue, increasing dyspnea, and severe chest pain. Laboratory results showed a leukocyte count 2.51×109 cells/L (40.0% neutrophils, 49.6% lymphocyte, 10.0% monocytes), platelet count of 93 × 109/L, CRP level rising to 167.1 mg/L, and procalcitonin level of 1.19 ng/mL. Cardiac enzyme panel, troponin, and N-terminal pro b-type natriuretic peptide were all within normal limits. Owing to clinical progression and suspicion of secondary bacterial infection, the patient was given piperacillin-tazobactam, a β-lactam antibiotic, in addition to the existing anti-COVID-19 treatment. Repeat arterial blood gas analysis revealed a pH of 6.87, partial pressure of CO2 83.8 mmHg, partial pressure of O2 80.5 mmHg (fraction of inspired O2 100%) and lactate level of 18 mmol/L. Chest CT scan demonstrated significant progression of lung infiltrates (Figure 2). The patient was diagnosed with severe pneumonia caused by COVID-19 and needed advanced life support, including intensive care unit transfer and endotracheal intubation. Despite initiating extracorporeal membrane oxygenation (ECMO) for severe respiratory failure due to COVID-19, the patient could not sustain adequate oxygenation and, sadly, died on the third hospital day (Sep 19, 2023). A sputum culture taken on admission day yielded a positive result for MRSA one day following the patient’s death. The MRSA isolate, genotyped as ST338, exhibited resistance to antibiotics from multiple classes, including β-lactams (cefoxitin), tetracyclines (tetracycline), macrolides (erythromycin), and lincosamides (clindamycin), while remaining susceptible to vancomycin, gentamicin, trimethoprim-sulfamethoxazole, levofloxacin, ciprofloxacin, moxifloxacin, nitrofurantoin, rifampicin, linezolid, and tigecycline. The blood culture was unremarkable. The final diagnosis was COVID-19 co-infected with severe CA-MRSA pneumonia, resulting in the patient’s death.
Discussion
Numerous studies have concentrated on the incidence and microbial spectrum of secondary pulmonary infections among hospitalized COVID-19 patients.4,5 A collective analysis of 14 observational studies reported a 16% prevalence of secondary bacterial pulmonary infections in hospitalized COVID-19 patients, ranging from 4.8% to 42.8% across studies, based on 580 cases out of 3633 patients.4 Staphylococcus aureus was the most frequent Gram-positive bacterium causing secondary infections, responsible for 13% of cases. MRSA pneumonia, in the setting of COVID-19, typically occurred in critically ill patients needing intensive care, including mechanical ventilation, and was frequently linked to increased mortality and morbidity.6 However, reports specifically addressing COVID-19 co-infections are relatively few. In a cohort of 989 patients, only 2.5% had community-acquired bacterial co-infections at COVID-19 diagnosis. Among these, patients with community-acquired co-infections, especially those with comorbidities like diabetes, were more likely to be admitted to the ICU than those without such infections.6 Additionally, CA-MRSA infections were exceptionally rare in this cohort, with only two cases identified among 989 patients. The rarity of such co-infections often leads to a failure in initial empirical treatment to provide targeted antimicrobial therapies, resulting in high mortality without early diagnosis and treatment. A study published in August 2019 found the prevalence of MRSA colonization to be approximately 1.67 times higher in diabetics than in non-diabetics.7 Patients with diabetes have compromised immune function due to hyperglycemia and metabolic disorders, increasing the local MRSA bacterial load in the respiratory tract and thus raising the risk of infection.8
In this case, the patient, who had type 2 diabetes mellitus for 20 years and was on insulin and oral hypoglycemics, showed poor glycemic control, with a random blood glucose level of 17.8 mmol/L and an HbA1c of 13.4% at admission. Elevated HbA1c levels have also been identified as a significant risk factor for MRSA-induced pulmonary infections in patients with COVID-19, including those with co-infections.9 Although diabetes does not seem to raise the risk of COVID-19 infection, poorly managed diabetes, lack of vaccination, and the co-infection with COVID-19 and CA-MRSA likely contributed to the rapid progression of the disease. It is well-established that diabetes can worsen COVID-19 outcomes, possibly by increasing viral tropism and cell penetration due to hyperglycemia, resulting in greater virulence and vulnerability to severe infections.10 In this case, post-COVID-19 infection, the patient showed a decrease in lymphocyte counts, indicating lymphopenia. This immunosuppression, marked by lowered CD4+ and CD8+ T-cell counts, is typical in severe COVID-19 cases and is associated with worse clinical outcomes.11 Additionally, the initial blood routine at admission showed a reduced platelet count, which is atypical for mild to moderate COVID-19.12 This concurrent thrombocytopenia should alert clinicians to the possibility of an underlying bacterial co-infection or severe COVID-19.
The MRSA strain identified in this case was ST338, which harbors a smaller SCCmec V type and the PVL gene. PVL is a leukocidin that can lyses neutrophils, leading to a decrease in peripheral blood leukocyte counts. This not only impairs the innate immune response to bacterial infections but also triggers the release of reactive oxygen species and inflammatory mediators, causing further local tissue damage and contributing to necrotizing pneumonia.13 In severe Staphylococcus aureus infections, leukopenia may result from direct cytotoxicity, immune-mediated damage, bone marrow suppression, or consumptive coagulopathy, adversely affecting immune status and prognosis. On the second day, further declines in all three blood cell lines were noted, strongly suggesting disease progression. This genotype is recognized for its heightened invasiveness, causing rapid and severe pulmonary lesions that evolved over a 24-hour period (Figures 1 and 2). Despite advanced life support measures, including ECMO and escalated antibiotics, the patient died from COVID-19 complications, exacerbated by CA-MRSA co-infection. The patient’s rapid disease progression is multifactorial, underscoring the necessity of screening for CA-MRSA in community settings to facilitate early detection and treatment of infections. Given this multidrug-resistant CA-MRSA isolate’s potential resistance to multiple antibiotics (including β-lactams, tetracyclines, macrolides, and lincosamides), treatment may require glycopeptide antibiotics or other novel antimicrobials, posing challenges for empirical clinical therapy.14
This case highlights the significant challenges in managing severe COVID-19, especially when complicated by MRSA co-infections. The unfortunate outcome underscores the necessity of screening for bacterial co-infections in COVID-19 patients to facilitate early detection and targeted treatment.15
Abbreviations
CA-MRSA, Community-acquired methicillin-resistant staphylococcus aureus; COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; CT, Computed tomography; ECG, Electrocardiogram; ECMO, Extracorporeal membrane oxygenation; PVL, Pantón-Valentine leukocidin.
Acknowledgments
The authors would like to thank the team of Dr. Chen Yan for sequencing the CA-MRSA strain in this case. We would like to acknowledge the patient’s daughter who gave informed consent for this publication.
Ethics Statement
The study (Ethics Code: No. Lw2025001) was approved by the Ethics Review and Scientific Investigation Board of Sir Run Run Shaw Hospital, Alaer Hospital, Zhejiang University School of Medicine, Alaer 843300, China.
Funding
This study was supported by the State Key Laboratory of Pathogenesis, Prevention, Treatment of Central Asian High Incidence Diseases Fund, SKL-HIDCA-2023-ALE2.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Lehmann CJ, Pho MT, Pitrak D, Ridgway JP, Pettit NN. Community-acquired coinfection in coronavirus disease 2019: a retrospective observational experience. Clin Infect Dis. 2021;72(8):1450–1452. doi:10.1093/cid/ciaa902
2. Garcia-Vidal C, Sanjuan G, Moreno-García E, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83–88. doi:10.1016/j.cmi.2020.07.041
3. Tomic D, Shaw JE, Magliano DJ. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 2022;18(9):525–539. doi:10.1038/s41574-022-00690-7
4. Chong WH, Saha BK, Ramani A, Chopra A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection. 2021;49(4):591–605. doi:10.1007/s15010-021-01602-z
5. Grasselli G, Cattaneo E, Florio G. Secondary infections in critically ill patients with COVID-19. Crit Care. 2021;25(1):317. doi:10.1186/s13054-021-03672-9
6. Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS One. 2021;16(5):e0251170. doi:10.1371/journal.pone.0251170
7. Stacey HJ, Clements CS, Welburn SC, Jones JD. The prevalence of methicillin-resistant Staphylococcus aureus among diabetic patients: a meta-analysis. Acta Diabetol. 2019;56(8):907–921. doi:10.1007/s00592-019-01301-0
8. Holt RIG, Cockram CS, RCW M, Luk AOY. Diabetes and infection: review of the epidemiology, mechanisms and principles of treatment. Diabetologia. 2024;67(7):1168–1180. doi:10.1007/s00125-024-06102-x
9. Adalbert JR, Varshney K, Tobin R, Pajaro R. Clinical outcomes in patients co-infected with COVID-19 and Staphylococcus aureus: a scoping review. BMC Infect Dis. 2021;21(1):985. doi:10.1186/s12879-021-06616-4
10. Herder C, Roden M, Venteclef N. Diabetes and pulmonary infection: how hyperglycaemia shapes the immune system. Signal Transduct Target Ther. 2024;9(1):67. doi:10.1038/s41392-024-01784-6
11. Diao B, Wang C, Tan Y, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827. doi:10.3389/fimmu.2020.00827
12. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
13. Jin Y, Zhou W, Yin Z, et al. The genetic feature and virulence determinant of highly virulent community-associated MRSA ST338-SCCmec Vb in China. Emerg Microbes Infect. 2021;10(1):1052–1064. doi:10.1080/22221751.2021.1914516
14. Mahjabeen F, Saha U, Mostafa MN, et al. An update on treatment options for Methicillin-Resistant Staphylococcus aureus (MRSA) bacteremia: a systematic review. Cureus. 2022;14(11):e31486. doi:10.7759/cureus.31486
15. Aggarwal NR, Nordwall J, Braun DL, et al. Viral and host factors are associated with mortality in hospitalized patients with COVID-19. Clin Infect Dis. 2024;78(6):1490–1503. doi:10.1093/cid/ciad780
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Association Between Obesity and COVID-19 Disease Severity in Saudi Population
Alqahtani FY, Aleanizy FS, Mohamed RAEH, Al-Maflehi N, Alrfaei BM, Almangour TA, Alkhudair N, Bawazeer G, Shamlan G, Alanazi MS
Diabetes, Metabolic Syndrome and Obesity 2022, 15:1527-1535
Published Date: 16 May 2022
Old Age is an Independent Risk Factor for Pneumonia Development in Patients with SARS-CoV-2 Omicron Variant Infection and a History of Inactivated Vaccine Injection
Tong X, Huang Z, Zhang X, Si G, Lu H, Zhang W, Xue Y, Xie W
Infection and Drug Resistance 2022, 15:5567-5573
Published Date: 21 September 2022
Platelet-to-White Blood Cell Ratio as a Predictor of Mortality in Patients with Severe COVID-19 Pneumonia: A Retrospective Cohort Study
Thungthienthong M, Vattanavanit V
Infection and Drug Resistance 2023, 16:445-455
Published Date: 24 January 2023
Application, Benefits, and Limitations of Telepharmacy for Patients with Diabetes in the Outpatient Setting
Iftinan GN, Elamin KM, Rahayu SA, Lestari K, Wathoni N
Journal of Multidisciplinary Healthcare 2023, 16:451-459
Published Date: 19 February 2023
Value of Laboratory Indicators in Predicting Pneumonia in Symptomatic COVID-19 Patients Infected with the SARS-CoV-2 Omicron Variant
Zhu K, Ma S, Chen H, Xie J, Huang D, Fu C, Ma G, Huang Y
Infection and Drug Resistance 2023, 16:1159-1170
Published Date: 28 February 2023