Back to Journals » Journal of Pain Research » Volume 18
A Real-World Study of Cetylated Fatty Acids Food Supplement Administration in Italian Adults for Sub-Acute or Chronic Musculoskeletal Pain
Authors Lanzisera R, Gervasoni F , Rossato MS , Tarantino G, Lo Mauro A, Geri E
Received 30 December 2024
Accepted for publication 9 May 2025
Published 30 May 2025 Volume 2025:18 Pages 2751—2760
DOI https://doi.org/10.2147/JPR.S511708
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor King Hei Stanley Lam
Rosaria Lanzisera,1 Fabrizio Gervasoni,2 Maria Sole Rossato,3 Germano Tarantino,3 Antonella Lo Mauro,4 Elisabetta Geri1
1UOC Recupero E Riabilitazione Funzionale, Ambito Territoriale Pisano, ASL Toscana Nordovest, Pisa, Italy; 2ASST Fatebenefratelli Sacco, Milan, Italy; 3PharmaNutra S.p.A., Pisa, Italy; 4Dipartimento di Elettronica, Informazione e Bioingegneria, Politecnico di Milano, Milan, Italy
Correspondence: Maria Sole Rossato, Email [email protected]
Purpose: This real-world observational study was conducted to evaluate decrease in pain after 30-day administration of a new oral cetylated fatty acids (CFA) food supplement, assess if decreased pain resulted in a lower consumption of oral non-steroidal anti-inflammatory drugs (NSAIDs), and improvement in related gastric side effects. It was the first study of this food supplement in a real-world setting.
Patients and Methods: A hundred and twenty Italian primary care physicians recruited 562 patients who were prescribed oral CFA. Patients completed the Brief Pain Inventory (BPI) questionnaire at baseline and after 30 days of dosing. Their CFA intake adherence and use of NSAIDs were recorded. All analyses were done using R statistical software; p-value ≤ 0.005 was considered statistically significant.
Results: We analyzed data of 196 males and 366 females aged in an average 49.2 years. After 30 days of CFA intake, we observed a statistically significant reduction (38.84%) in the overall pain score, 44.53% reduction in the interference score in daily activities, relief from pain within the previous 24 hours 47.16 (± 11.44%) at baseline and 62.14 (± 27.93%) after 30 days, and progressive reduction in NSAID intake frequency and total dose over time. More than half of participants (55.2%) reported improvement in gastric side effects typically associated with NSAID use.
Conclusion: Data analyses indicated that using the new oral CFA food supplement decreased pain, which helped improve the quality of life, better perform daily activities (interference reduced by 44.5%), and reduce painkiller consumption by 19.7% in terms of both dose and frequency. Half of participants (55.2%) rated NSAID-related hyperchlorhydria and heartburn as improved. Although placebo effect might have contributed, the results suggest that CFA may have a positive effect in patients with sub-acute and chronic musculoskeletal pain and can enhance therapies typically used in this population.
Keywords: pain, cetylated fatty acids, quality of life
Introduction
Chronic musculoskeletal pain in the back, neck, shoulder, knee, and generalized pain is prevalent in the elderly (>65 years), but increasingly affects younger adults due to sedentary lifestyle or injuries sustained during exercising and sports. This pain is associated with a significant disability as it reduces mobility and is often accompanied by depression, anxiety, family and social dysfunction. Therapeutic options include non-pharmacological treatments (e.g., self-management counseling and education, physical therapy, manual therapy, and psychosocial interventions), complementary therapies (e.g. acupuncture) and pharmacological interventions (e.g. analgesics, non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections).1 If symptoms persist despite therapy, surgical interventions are considered.
In the elderly, complex medical conditions present a high risk of being poly-treated and possible multiple drug-drug interactions. General practitioners, geriatricians, and pain specialists need to work together and develop a patient-specific health plan to improve quality of life while minimizing the risk of adverse events and side effects.2 Data collected in the US in 2015–20183 showed that almost 15.1% of adults older than 60 years were using one or more prescribed painkillers, compared to only 5.4% of adults aged 20–39. These drugs can result in comorbidities due to adverse drug reactions (ADRs), often related to impaired renal function in the elderly.
To reduce medication intake, various food supplements have been proposed to decrease musculoskeletal pain in osteoarthritis, rheumatic diseases, and spine degeneration, such as avocado and soybean unsaponifiables, capsaicin, turmeric, glucosamine, melatonin, polyunsaturated fatty acids, and vitamin D. While the risks are rather low, the true benefit of these substances remains uncertain,4 which warrants further research of food supplement intake in patients with musculoskeletal pain.
Cetylated fatty acids (CFAs) are a group of compounds based on fatty acids derived from plants or animals esterified with cetyl alcohol. Different CFA formulations have been shown to reduce inflammation in vitro by significantly decreasing the expression of IL-6, monocyte chemoattractant protein-1 (MCP-1), and TNF-α in stimulated RAW264.7 mouse macrophage cells. Additionally, these compounds promote the chondrogenic differentiation of human adipose-derived stem cells by enhancing the expression of chondrogenic markers under chondrogenic induction conditions.5
A new food supplement containing plant-based cetylated fatty acids (CFAs) has been marketed in Italy since 2022 and appears to be a promising nutritional strategy for managing musculoskeletal pain of various origin. The same active substance has already been successfully applied topically in many conditions, including knee arthrosis,6 athletic pubalgia,7 shoulder tendinopathies,8 and myofascial pain syndrome of the neck.9 In all these cases, the administration of CFA improved muscle strength and mobility and decreased pain. A recently published study showed that a four-week oral administration of CFA in patients with axial discogenic lumbar pain decreased disability and pain with minimal adverse effects.10
Our study was the first one using the new oral CFA supplement in a real-world setting. We aimed to collect real-world data (RWD) related to patient outcomes after using this CFA formulation in normal clinical practice. We hypothesized that it may reduce musculoskeletal pain, potentially decrease consumption of NSAIDs, and improve related gastric side effects.
Population, Materials, and Methods
Study Design
This was a prospective observational single-arm non-controlled study conducted in 120 sites in Italy in 2022. The protocol did not define a CFA intake compliance threshold for continuing the study and did not require exclusion of non-compliant participants.
Study Objectives
The primary objective was to evaluate pain reduction after 30 days of CFA intake compared to baseline.
The secondary objective was to evaluate if pain decrease resulted in a lower consumption of oral NSAIDs.
The exploratory objective was to evaluate whether the CFA intake influenced NSAID gastric side effects.
Population
To be eligible for the study, participants had to be older than 18 years, experience sub-acute or chronic muscular, osteo-articular, or tendinous pain, willing to complete the Brief Pain Inventory (BPI) questionnaire before administration of the CFA and after 30 days of intake, and willing to sign informed consent.
Potential participants who used corticosteroids chronically, cancer patients undergoing active treatment or whose pain was related to their cancer therapy, and participants who had undergone surgery (osteo-articular and non-articular) in the 3 months preceding the study as well as participants not willing to sign the informed consent were to be excluded.
Data Collection
We planned to collect data from patients prescribed the oral CFA supplement by their primary care physicians. Before collecting their data, all patients were to be duly informed about and consent to their personal data processing. All procedures were to be performed per normal clinical practice. The study duration for eligible participants was planned to last 30 days. The choice of follow-up after 30 days was based on clinical practice as Italian clinicians typically check on patients after 1 month. Based on the study of Pelak et al,10 we considered 30 days a sufficient time to observe results with oral intake of CFA. The average use of systemic painkillers over the previous 30 days was to be recorded by investigators who asked the participants about painkiller name, dose, and time of intake as well as any gastric complaints at the baseline and at the 30-day follow-up. Adverse events and CFA intake compliance were to be recorded as well.
Treatment
Participants were to be instructed to take 1.6 g CFA a day equivalent to 2 sachets of Cetilar® ORO for 10 days and 0.8 g CFA a day equivalent to 1 sachet of Cetilar® ORO (Pharmanutra SPA, Italy) for 20 consecutive days. This dosing corresponds to Cetilar® ORO label approved by the Italian regulatory authority. All participants were to complete a short version of the Brief Pain Inventory questionnaire (BPI) at baseline before starting the CFA intake (T0) and after 30 days of intake (T1). The question related to pain relief assessment within the previous 24 hours was formulated to prompt a “yes” or “no” answer.
Brief Pain Inventory Questionnaire
The BPI (a sample BPI questionnaire in English, The University of Texas, MD Anderson Cancer Center®) is a brief and easy-to-use tool for assessment of pain in both clinical and research settings. It uses easy-to-understand, simple numeric rating scales (NRS) from 0 to 10. The Italian version was validated by Bonezzi et al.11
The BPI scale defines pain as follows:
- Worst Pain Score: 1–4, Mild Pain.
- Worst Pain Score: 5–6, Moderate Pain.
- Worst Pain Score: 7–10, Severe Pain.
Since pain can vary considerably over a day, the BPI asks respondents to rate their pain at the time of completing the questionnaire. In addition, the questionnaire also asks them to specify the pain at its worst, least, and average over the previous 24 hours. A score of 0 means no pain, while a score of 10 indicates the worst pain imaginable. A total Pain Severity Score is calculated as the average of these four ratings. Question 8 assesses the percentage of pain relief from medications in the past 24 hours. The last question aims to assess how much pain has interfered with seven daily activities, including general activity, mood, walking ability, normal work, relation with other people, and sleep. A score of 0 indicates that the pain did not interfere, while a score of 10 indicates that it completely interfered with the indicated activity. A total Interference BPI Score is calculated as the average of these seven ratings. The short form of this assessment tool was used which generally takes about five minutes to complete.
Statistical Analysis
Five hundred (500) participants were expected to be enrolled in the study. No sample size and power calculations were done, the number was estimated based on the number of study sites and their expected recruitment.
Depending on their distributions, the variables were to be represented as mean ± standard deviation, median and interquartile interval, or count and percentage. The normality of distributions was to be assessed by evaluating the Q-Q plots. Paired comparisons were to be performed with paired sample t-test, Wilcoxon test, or McNemar’s test, as appropriate. The linear trend of proportions was to be analyzed using the chi-square test for trend, and 2×2 contingency tables were to be analyzed by chi-square test with continuity correction. Principal component analysis was to be applied to the 11 items of BPI,11 extracting a Pain Score and an Interference Score;12 the number of principal components to be extracted was to be determined applying the Kaiser-Guttman rule to the screen plot; correlation circles were to be plotted, displaying how much the original variables correlated with the first two principal components; the goodness of representation of the original variables on the extracted principal components was to be represented by cos2 plots, and the loading coefficients were to interpret the meaning of principal components. Repeated measures two-way analysis of variance was to be performed to assess the impact of time (ie, CFA intake) and gender (or age) on the BPI Pain and Interference Scores. The Pearson correlation coefficient was used to assess how compliance and adverse events are related. All analyses were to be done using R statistical software.13 Any p-value ≤ 0.05 was to be considered statistically significant.
Results
Demographic Characteristics at Baseline
After screening and eligibility assessment, 562 participants were enrolled, their average age was 49.2 (± 15.7) years. Potential participants, who were screened but found non-eligible, were not recorded. More women (366, 65.24%) were recruited than men (196, 34.88%). The age distribution was even between older than 60 years (274, 48.75%) and younger than 60 years (288, 51.25%). Main causes of pain included arthrosis (244, 41.36%), tendinopathies (92, 15.59%), and postural overload (74, 12.54%). Other causes (63, 10.68%) included those not listed in the data collection form, such as fibromyalgia and discopathies. Forty-two (7.5%) participants indicated more than one cause; however, most reported a single cause. Participants were also asked about the main location of pain, and each participant could specify more than one area. Most frequently reported areas were the spine (302, 33.26%), namely, cervical (103, 11.34%) and lumbar (199, 21.92%), followed by the knee (128, 14.1%) and the shoulder (103, 11.34%) (Supplementary Table 1).
Pain Analyses
We analyzed data reported by participants in BPI questionnaires at T0 and T1. We observed a statistically significant reduction of 38.84% (from 5.69 ± 1.56 to 3.48 ± 1.79 points, p < 0.001) in the overall Pain BPI Score after 30 days of CFA intake; a reduction of 44.53% (from 5.48 ± 2.01 to 3.04 ± 1.99, p <0.001) was also observed in the Interference BPI Score (Figure 1).
Analyses of Subgroups
We analyzed subgroups to assess whether gender, age, or presence/absence of arthrosis-influenced pain and interference scores. We observed that more females experienced improvement compared to males; however, the improvement over time was comparable between males and females. This indicated that the higher percentage of females reporting improvement was merely due to their higher proportion in recruited participants. Based on reported increased in painkiller consumption after 60 years of age by Kakatkar et al,3 we decided to divide the sample into under 60s and over 60s to explore the effect between the younger and older patients. We did not observe any statistically significant difference related to age, both younger and older patients showed the same trend. The subgroup of patients with arthrosis (n = 318, 41.36%) showed higher baseline values regarding both pain and interference in daily activities; however, the two groups improved equally over the time (Figure 2A–C).
Analysis of Pain Assessment Within Previous 24 hours
The percentage of pain relief achieved within the previous 24 hours was 47.16% ± 11.44% at the first interview (T0) and 62.14% ± 27.93% at the end of CFA administration (T1); the observed difference was statistically significant (p <0.001).
Consumption of NSAIDs
The number of participants taking NSAIDs during the previous month was 419 (74.55%) at T0 and 308 (54.80%) at T1, the difference was statistically significant (p <0.001). Participants who used NSAIDs during the previous month reported when and how often they used them. We observed a progressive reduction in intake frequency and a statistically significant reduction in intake over time (p <0.001) (Figure 3, Table 1).
 |
Table 1 Duration of NSAID intake within the previous month |
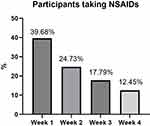 |
Figure 3 Percentage of participants taking NSAIDs each week. |
NSAID Gastric Side Effects
At the end of the study (T1), 290 (51.60%) participants reported typical NSAID-related gastrointestinal side effects, such as hyperchlorhydria or heartburn. When they were asked if they experienced any change in symptoms during CFA consumption, 55.2% of participants reported improvement with 14% of participants reporting their condition as much improved, 21% of participants as improved, and 21% of participants as slightly improved. Forty-three percent (43%) of participants reported no change, 1% of participants rated their condition as slightly worse, and 1% of participants as worse (Figure 4).
Compliance and Side Effects
We observed good adherence to CFA intake during the study period; 336 (61.8%) participants reported 100% adherence, 134 (24.6%) more than 90%, 54 (9.9%) 75%-90%, 14 (2.6%) 50%-75%; only 6 (1.1%) reported adherence less than 50%.
As for side effects attributed to CFA intake, 31 (5.5%) participants reported gastrointestinal disorder and 5 (0.9%) other types of disorders. The correlation between side effects and compliance was analyzed and none was found (r = −0.046; CI −0.13-0.04; p = 0.3207).
Discussion
We aimed to evaluate if CFA can benefit patients with chronic musculoskeletal pain who take systemic painkillers for pain relief. The results of analyses suggest positive effects, including reduction in pain, lower interference with daily activities, and a reduced consumption of NSAIDs. These outcomes did not depend on age. We observed a higher percentage of females who reported improvements; however, this was attributed to the double number of females than males participating in the study. Participants reported improvement in NSAID-related gastric side effects; however, we could not determine if this was due to the CFA or solely due to reduction of NSAID intake. The tolerability was good with minimal adverse events the participants attributed to CFA.
Publications about CFA are generally sparse. CFA for topical or systemic use have been studied to investigate their activity.14 In 2020, Hudita et al published an article about a different CFA formulation than ours. The authors concluded that CFAs facilitated the chondrogenic differentiation process of human adipose-derived stem cells by stimulating the expression of chondrogenic markers under chondrogenic induction conditions.15 In 2022, Izzo et al published results of topical CFA in breast cancer survivors with chronic neck pain and reported a statistically significant decrease in pain as well as improved mobility.8 In 2023, Pelak et al published results of oral CFA effects on axial discogenic low back pain leading to reduced pain and disability.9 The precise mechanism of action of oral CFA has not yet been identified. The main mechanism of action likely involves cell membrane fluidity and function; CFA are known to incorporate into the phospholipid bilayer of cell membranes. This incorporation can modify the physical and functional properties of cell membranes, potentially enhancing membrane fluidity, which is altered in case of inflammation as reported by Calder.16 This mechanism might help improve the body’s endogenous response to osteoarthritis, joint or tendon disease, or muscular injuries, leading to a rapid improvement in pain and a reduced use of systemic painkillers. Our results related to observed decrease in pain and reduced painkiller consumption concur with these publications.
Mohebi at al. studied a different CFA-based oral formulation administered for 30 days. It was compared to a 10-day administration of NSAID (meloxicam) in patients with knee osteoarthritis.17 Results demonstrated no significant differences in functionality index and an improvement in pain in the CFA group. Rescue pain medication was used by patients in CFA group during the first two weeks only. Although the study was conducted with a different CFA formulation used in a different dosage and in patients with knee osteoarthritis only, there are some similarities with our study. In both studies, more females were recruited than males, patients using CFA reported decrease in pain, reduction in NSAIDs consumption, and good tolerability.
Limitations
This was a single-arm study; hence, we did not make any comparisons with active control or placebo and any potential placebo effect could not be accounted for. Since pain assessment is inherently subjective, it could have been affected by unconscious bias at the time the BPI questionnaire completion. The study could have included functional tests to assess improvement more objectively. The sample size was relatively small precluding generalization of results. Some data related to adherence and side effects were missing, limiting the reliability of safety results and precluding analysis of compliance effect. Safety data collected during the study could have been influenced by asking about participants’ opinion regarding relatedness to CFA, while no evaluation by physicians was performed. Larger randomized clinical trials are needed to generate data and provide insights regarding the CFA effectiveness as well as to determine if hyperchlorhydria and heartburn reduction is an effect of CFA intake or merely results from reduced NSAID consumption.
Conclusions
Data analyses indicated that using the new oral CFA food supplement resulted in an average pain decrease of 38.8%. Decreased pain helped improve the quality of life, better perform daily activities (interference reduced by 44.5%), and reduce painkiller consumption by 19.75% in terms of both dose and frequency. Half of participants (55.2%) rated NSAID-related hyperchlorhydria and heartburn as improved.
Although placebo effect might have contributed, the results suggest that CFA may have a positive effect in patients with sub-acute and chronic musculoskeletal pain and can enhance therapies typically used in this population.
Data Sharing Statement
A unique participant identification code was used that allowed identification of all data reported for each participant. Study data may be made available to third parties (eg, in case of request by journal reviewers) provided the data are treated confidentially and the participants’ privacy and confidentiality is guaranteed. Data were obtained and preserved according to current local regulations to ensure all data protection requirements are satisfied. Documentation that identifies the study participants has been kept confidential and will not be publicly available according to current local regulations.
Ethical Statement
The study was conducted in compliance with the Declaration of Helsinki.
Ethical aspects were carefully considered. Since this study was observational, performed by primary care physicians all over Italy, thus, in different regions, and, since the CFA had already been approved by the Italian Ministry of Health, the authors, after discussions with bioethicists, decided to have the study evaluated post hoc. The study was assessed and retrospectively approved by the ethics committee at Politecnico Milano with registration number 50/2024.
Acknowledgments
The authors thank Kamila Novak, MSc, of KAN Consulting MON. I.K.E., Athens, Greece, for providing medical writing support, which was funded by PharmaNutra S.p.A., Italy, in accordance with Good Publication Practice (GPP3) guidelines and position of the European Medical Writers Association. http://www.ismpp.org/gpp3.
The authors also thank Andrea Ripoli, for statistical analysis, which was funded by PharmaNutra S.p.A., Italy.
Our thanks go to the general practitioners for their valuable contribution to the recruitment of patients, as well as to the collection of informed consents and the questionnaires completed by the participants. In alphabetical order: Dr.ssa Abodi Maria Vittoria, Dr. Albano Giuseppe, Dr.ssa Alfero Patrizia, Dr. Alise Marco, Dr.ssa Ammendola Annamaria, Dr. Arcone Gianpaolo, Dr. Ardito Antonello, Dr.ssa Alucino Caterina, Dr.ssa Baronio Cristina, Dr.ssa Bisogno Cristina, Dr.ssa Bisson Tatiana, Dr.ssa Bombonati Gaia, Dr. Bonfirraro Saverio, Dr. Borghesi Pierfrancesco, Dr. Cacciotella Luca, Dr.ssa Carloni Maria Cristina, Dr.ssa Casella Concetta, Dr.ssa Cerasa Marzia, Dr. Cerrato Mario, Dr. Colaci Andrea, Dr. Conte Raffaele, Dr. Conti Marco, Dr. Conti Alessandro, Dr.ssa Crotti Silvia, Dr.ssa Curti Emanuela, Dr D’Amato Gilberto, Dr. D’Amato Matteo, Dr.ssa D’Ascanio Paola, Dr.ssa D’Odorico Beatrice, Dr. D’Onofrio Domenico, Dr. D’Ursi Dario, Dr.ssa De Leonardis Sonia, Dr.ssa Di Perna Valentina, Dr.ssa Di Mauro Antonia, Dr.ssa Dirocco Alessia, Dr.ssa Divietri Simona, Dr. Eleonori Stefano, Dr.ssa Fancello Paola, Dr. Farese Almerico, Dr. Filippetto Matteo, Dr. Fossati Lorenzo, Dr.ssa Gennaro Paola, Dr. Geraci Carmelo, Dr. Giambanco Salvatore, Dr.ssa Giampaolo Cinzia Franca, Dr. Giangrande Marco, Dr. Giordano Angelo, Dr.ssa Giozani Giulia, Dr. Gnesi Giorgio, Dr. Greco Marcello, Dr. Grimaldi Manlio, Dr.ssa Guarino Angiolina, Dr.ssa Jager Annalisa, Dr.ssa Lanni Annamaria, Dr. Ledda Franco, Dr. Lenzo Giovanni, Dr. Lo Surgo Giuseppe, Dr. Losavio Francesco Gregorio, Dr.ssa Magrì Lina, Dr. Mancino Giuseppe, Dr.ssa Mandolesi Elisa, Dr.ssa Mandrile Carla, Dr. Marrocco Giovanni, Dr. Martines Francesco, Dr. Martino Giuseppe, Dr. Martucci Angelo, Dr.ssa Marulli Chiara, Dr. Mauro Nicola, Dr. Meloni Daniele, Dr.ssa Messina Clelia, Dr.ssa Mezzadri Romina, Dr. Monaco Salvatore, Dr Monzer Naji, Dr.ssa Morena Silvia, Dr Morini Daniele, Dr. Nirta Aurelio, Dr. Palma Giuseppe, Dr.ssa Paradiso Francesca, Dr.ssa Parente Annalisa, Dr. Parisi Salvatore, Dr. Parola Matteo, Dr.ssa Pastò Elena, Dr. Pattavina Marco Rosario, Dr. Pecoraro Alfonso, Dr. Pensabene Paolo, Dr.ssa Persegani Cristina, Dr. Piacenti Gaetano Sergio, Dr. Piazza Calogero, Dr. Piccinocchi Gaetano, Dr. Pili Marcello, Dr.ssa Pizzi Vittoria, Dr.ssa Podda Angelica, Dr.ssa Prosco Rosanna, D. Putigliano Rosario, Dr.ssa Pulvirenti Tiziana, Dr. Rolli Mauro, Dr. Romualdo Mauro, Dr. Rosa Michele, Dr. Rosino Stefano, Dr Rucco Maurizio, Dr. Saitta Luigi, Dr. Scardicchio Alfredo, Dr.ssa Siani Fabrizia, Dr.ssa Silecchia Ombretta, Dr.ssa Spezzani Valentina, Dr.ssa Stellini Giulia, Dr. Tarakji Abdul Latif, Dr. Tarquinio Mauro, Dr.ssa Testa Lucia, Dr Testi Gianluca, Dr. Tosatto Danilo, Dr.ssa Vaglio Chiara, Dr. Valvo Andrea, Dr.ssa Venco Elena, Dr. Visconti Vincenzo, Dr. Vitalone Rosario Renato.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Statistical analysis and medical writing were funded by PharmaNutra S.p.A.; also, it provided the investigational product as free samples.
Disclosure
Dr Fabrizio Gervasoni reports personal fees from PharmaNutra SpA, personal fees from Chiesi Italia SpA, personal fees from Guna SpA, personal fees, non-financial support from IACER - ITECH Medical Division, personal fees from Abiogen, personal fees from Italfarmaco, personal fees from Angelini Holding S.p.A., personal fees from Uriach - Laborest, grants from Società Aziende Chimiche Riunite Angelini Francesco A.C.R.A.F. S.p.A., personal fees from GlaxoSmithKline Consumer Healthcare S.r.l. Unipersonale, personal fees from Saluber MD, personal fees from Gruppo Humantech, personal fees from IBSA, non-financial support from Podartis, personal fees from Haleon Italy S.r.l., personal fees, non-financial support from Ortopedia Castagna, outside the submitted work; Dr Germano Tarantino has a patent 102019000007311 pending to Pharmanutra Spa. The authors Maria Sole Rossato and Germano Tarantino are Pharmanutra S.p.A. employees. Other Authors declare that they have no conflict of interest.
The real-world observational study described in this paper was not registered in any publicly accessible database or trial registry.
References
1. Hashem M, AlMohaini RA, Alharbi TM, Aljurfi MM, Alzmamy SA, Alhussainan FS. Impact of musculoskeletal pain on health-related quality of life among adults in Saudi Arabia. Cureus. 2024;16(3):e57053. PMID: 38681335; PMCID: PMC11051673. doi:10.7759/cureus.57053
2. Schwan J, Sclafani J, Tawfik VL. Chronic pain management in the elderly. Anesthesiol Clin. 2019;37(3):547–560. doi:10.1016/j.anclin.2019.04.012
3. Kakatkar S, Narayan A, Balkrishnan R. Prescription analgesic overuse in older adults: can we mitigate this growing problem? Aging Med. 2022;5:294–296. doi:10.1002/agm2.12228
4. Crawford C, Boyd C, Paat CF, et al. Dietary ingredients as an alternative approach for mitigating chronic musculoskeletal pain: evidence-based recommendations for practice and research in the military. Pain Med. 2019;20(6):1236–1247. doi:10.1093/pm/pnz040
5. Hudita A, Galateanu B, Dinescu S, et al. In vitro effects of cetylated fatty acids mixture from celadrin on chondrogenesis and inflammation with impact on osteoarthritis. Cartilage. 2020;11(1):88–97. doi:10.1177/1947603518775798
6. Ariani A, Parisi S, Guidelli GM, Bardelli M, Bertini A, Fusaro E. Short-term effect of topical cetylated fatty acid on early and advanced knee osteoarthritis: a multi-center study. Arch Rheumatol. 2018;33(4):438–442. doi:10.5606/ArchRheumatol.2018.6711
7. Pampaloni E, Pera E, Maggi D, et al. Association of cetylated fatty acid treatment with physical therapy improves athletic pubalgia symptoms in professional roller hockey players. Heliyon. 2020;6(7):e04526. doi:10.1016/j.heliyon.2020.e04526
8. Lanzisera R, Baroni A, Lenti G, Geri E. A prospective observational study on the beneficial effects and tolerability of a cetylated fatty acids (CFA) complex in a patch formulation for shoulder tendon disorders. BMC Musculoskelet Disord. 2022;23(1):352. doi:10.1186/s12891-022-05304-x
9. Izzo R, Rossato M, Tarantino G, Mascolo N, Puleio M. Effects of esters’ cetylated fatty acids taping for chronic neck pain with mobility deficit in patients with breast cancer. Support Care Cancer. 2022;31(1):20. doi:10.1007/s00520-022-07497-2
10. Pelak A, Barve A, Carroll K, Madrazo-Ibarra A, Vad V. Effect of cetylated fatty acid supplementation on axial discogenic low back pain. Int J Phys Med Rehabil. 2023;11:662.
11. Bonezzi C, Nava A, Barbieri M, et al. Validazione della versione italiana del Brief Pain Inventory nei pazienti con dolore cronico [Validazione della versione italiana del Brief Pain Inventory nei pazienti con dolore cronico]. Minerva Anestesiol. 2002;68(7–8):607–611. Italian. PMID: 12244292.
12. Cararceni A, Mendoza TR, Mencaglia E, et al. A validation study of an Italian version of the brief pain inventory. Pain. 1996;65:87–92. doi:10.1016/0304-3959(95)00156-5
13. Cleeland CS. The brief pain inventory. User Guide. 2009. Available from: https://www.mdanderson.org/documents/Departments-and-Divisions/Symptom-Research/BPI_UserGuide.pdf.
14. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2023.
15. Welsh TP, Yang AE, Makris UE. Musculoskeletal pain in older adults: a clinical review. Med Clin North Am. 2020;104(5):855–872. PMID: 32773050; PMCID: PMC8034863. doi:10.1016/j.mcna.2020.05.002
16. Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. 2010;2(3):355–374. PMID: 22254027; PMCID: PMC3257651. doi:10.3390/nu2030355
17. Mohebi S, Farpour HR, Dehghanian KS, Khoshnazar SS. An oral form of cetylated fatty acids versus meloxicam for knee osteoarthritis: a randomised clinical trial. Mediterr J Rheumatol. 2023;34(4):460–468. PMID: 38282946; PMCID: PMC10815532. doi:10.31138/mjr.220823.aof
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




