Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
A Review of Datasets, Optimization Strategies, and Learning Algorithms for Analyzing Alzheimer’s Dementia Detection
Authors Thulasimani V, Shanmugavadivel K, Cho J , Veerappampalayam Easwaramoorthy S
Received 14 September 2024
Accepted for publication 14 November 2024
Published 20 November 2024 Volume 2024:20 Pages 2203—2225
DOI https://doi.org/10.2147/NDT.S496307
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Taro Kishi
Vanaja Thulasimani,1 Kogilavani Shanmugavadivel,1 Jaehyuk Cho,2 Sathishkumar Veerappampalayam Easwaramoorthy3
1Department of Artificial Intelligence, Kongu Engineering College, Perundurai, Tamilnadu, India; 2Department of Software Engineering and Division of Electronics and Information Engineering, Jeonbuk National University, Jeonju-Si, Republic of Korea; 3School of Engineering and Technology, Sunway University, Selangor, Darul Ehsan, Malaysia
Correspondence: Jaehyuk Cho, Email [email protected]
Abstract: Alzheimer’s Dementia (AD) is a progressive neurological disorder that affects memory and cognitive function, necessitating early detection for its effective management. This poses a significant challenge to global public health. The early and accurate detection of dementia is crucial for several reasons. First, timely detection facilitates early intervention and planning of treatment. Second, precise diagnostic methods are essential for distinguishing dementia from other cognitive disorders and medical conditions that may present with similar symptoms. Continuous analysis and improvements in detection methods have contributed to advancements in medical research. It helps to identify new biomarkers, refine existing diagnostic tools, and foster the development of innovative technologies, ultimately leading to more accurate and efficient diagnostic approaches for dementia. This paper presents a critical analysis of multimodal imaging datasets, learning algorithms, and optimisation techniques utilised in the context of Alzheimer’s dementia detection. The focus is on understanding the advancements and challenges in employing diverse imaging modalities, such as MRI (Magnetic Resonance Imaging), PET (Positron Emission Tomography), and EEG (ElectroEncephaloGram). This study evaluated various machine learning algorithms, deep learning models, transfer learning techniques, and generative adversarial networks for the effective analysis of multi-modality imaging data for dementia detection. In addition, a critical examination of optimisation techniques encompassing optimisation algorithms and hyperparameter tuning strategies for processing and analysing images is presented in this study to discern their influence on model performance and generalisation. Thorough examination and enhancement of methods for dementia detection are fundamental for addressing the healthcare challenges posed by dementia, facilitating timely interventions, improving diagnostic accuracy, and advancing research in neurodegenerative diseases.
Keywords: Alzheimer’s Dementia, Machine learning, Deep Learning, Transfer Learning and Generative Adversarial Network
Introduction
Alzheimer’s Dementia (AD) is a common and debilitating neurodegenerative disorder that impacts a substantial part of the population.1 AD is a progressive neurological disorder that primarily affects memory, thinking skills, and ability to perform daily activities.2 It is the most common cause of dementia and a general term for severe memory loss and cognitive decline. AD typically slows, gradually worsens over several years, and eventually becomes severe enough to interfere significantly with daily tasks. Alzheimer’s dementia research originated in 1906 with Dr. Alois Alzheimer’s identification of the disorder. The main feature of Alzheimer’s Dementia is the presence of plaques and tangles in the brain. Typically, Alzheimer’s Dementia is typically divided into three primary phases: mild, moderate, and severe. In the early stages, individuals may experience subtle memory lapses, making it challenging to remember names, places, or recent events. As AD progresses to the moderate stage, memory loss and confusion intensify, impacting language, reasoning abilities, and everyday activities such as dressing and eating. In the severe stage, individuals lose the ability to communicate coherently, recognise loved ones, or perform basic tasks.
Early identification of AD is crucial for effective management and intervention. Although there is no cure for AD, early diagnosis allows for the initiation of treatment to alleviate symptoms and slow down the progression of the disease. Various diagnostic methods, including cognitive assessment, neuroimaging, and genetic testing, can aid early detection. Additionally, a mentally and physically active lifestyle, maintaining a healthy diet, and social engagement are believed to contribute to reducing the risk of developing AD and delaying its onset. AD symptoms differ from person to person, making early diagnosis challenging because these initial symptoms are typically mild and can be easily mistaken for normal aging or other benign conditions, thus delaying the identification of the disease. Indeed, early detection significantly enhances the effectiveness of Alzheimer’s dementia treatment and positively impacts the overall quality of life of patients. The ability to identify the disease in its early stages allows for timely intervention, enabling the initiation of appropriate treatments and strategies to effectively manage AD symptoms.3
Early interventions can help slow the progression of the disease, manage cognitive decline, and maintain functional abilities for a longer duration. Moreover, it provides patients and their families with an opportunity to plan and make informed decisions about their care, fostering a sense of control and understanding of what can be challenging. Ultimately, emphasis on early detection amplifies the potential for a better and more dignified life for individuals with this formidable disease. Artificial Intelligence (AI) has significantly impacted the medical field, especially in neuroimaging and the diagnosis of diseases such as Alzheimer’s disease. AI is revolutionising healthcare by leveraging data-driven insights to enhance diagnostics, personalised treatment plans, and drug discovery. AI algorithms analyse vast amounts of medical data, aiding in the early detection of diseases through image interpretation and predictive analytics.4 This technology not only improves diagnostic accuracy but also optimises treatment strategies based on individual patient profiles.
The present study endeavors to investigate these research concerns by locating all pertinent research findings from prior investigations. The research questions addressed in this analysis are as follows:
- Which medical imaging modalities exist to detect dementia?
- Which image pre-processing methods are most commonly used to process multimodal images?
- What type of segmentation techniques have been adopted for medical image processing?
- Is it possible to apply optimization techniques for pre-processing?
- What are the different learning algorithms available for analysing multimodality image datasets to detect Alzheimer’s dementia?
These research questions are crucial for advancing AI-based Alzheimer’s Dementia detection (ADD). Understanding the available imaging modalities aids in selecting appropriate techniques, whereas identifying prevalent pre-processing methods ensures data quality. Segmentation techniques pinpoint pathological regions for precise analysis, and optimisation techniques refine the image quality. Knowledge of diverse learning algorithms enhances AI models for accurate AD detection, and offers promise for early diagnosis and management. The fields of medical imaging and neuroimaging are rapidly evolving, driven by advancements in technology, computational methods, and artificial intelligence.5 By concentrating on studies published within the last five years, we can incorporate the latest techniques and tools that may not have been available or widely adopted in earlier years.
Our systematic literature review focused on studies related to our research topic between 2018 and 2023, sourced from prominent databases such as Elsevier, IEEE Xplore, Springer, and Google Scholar. We utilised various keyword combinations such as “Medical Neuro Image Analysis”, “Medical Image Processing”, “Alzheimer’s Dementia Diagnosis”, and “Diagnostic Techniques”. The search yielded 155 articles relevant to the present study.
After removing duplicate articles and filtering based on publication year, we proceeded to the next step of the review. We carefully evaluated the titles and abstracts of these studies and identified a new set of keywords, including “Alzheimer’s dementia Magnetic Resonance Imaging (MRI) Image Classification”, “Alzheimer’s dementia using EEG signals”, “Bio-inspired algorithms”, “AD Machine Learning”, “AD Deep Learning”, “AD Transfer Learning”, and “AD GAN (Generative Adversarial Network)”. These refined keywords guided us to articles in the fourth stage of our systematic exclusion technique, covering diverse topics and methods related to Machine Learning (ML) and Deep Learning (DL) strategies. Specifically, ML methods were found in,2,3,5–9,11, 12–22 DL strategies in23−41, Transfer Learning approaches in,4,42–60 and GAN approaches in.61–80
Ultimately, we selected 105 studies that met our research criteria for in-depth analysis. The final selection not only resulted from automated keyword-based selection, but also aligned with our research questions. In this systematic literature review, we categorised articles into Machine Learning (ML), Deep Learning (DL), and Generative Adversarial Networks to highlight the different methodologies used in medical neuroimaging analysis and Alzheimer’s Dementia diagnosis. ML encompasses various computational techniques, whereas DL methods represent a specific category of ML techniques characterised by their deep architectures and ability to handle large volumes of high-dimensional data effectively. Transfer Learning involves transferring knowledge between tasks, which is useful when labelled data are limited. DL methods represent a specific category of ML techniques characterised by their deep architectures and ability to effectively handle large volumes of high-dimensional data.42
In this paper, ADD Figure Analysis concentrates on Alzheimer’s Dementia Detection (ADD) through neuro image analysis, focusing mainly on MRI, Positron Emission Tomography (PET), and electroencephalography (EEG). Pre-processing steps, segmentation, and feature extraction techniques are detailed to enhance the image quality and extract relevant features crucial for subsequent analysis, all of which are facilitated by optimised algorithms. In Materials and Methods, diverse AI methods are explored, including machine learning, deep learning utilising neural networks, transfer learning for leveraging prior knowledge, and GAN for data generation and evaluation, all of which are significant for advancing Alzheimer’s detection and analysis in neuroimaging.
ADD Image Analysis
Analysing biomarkers associated with Alzheimer’s dementia using imaging techniques such as Magnetic Resonance Imaging, Positron Emission Tomography, and electroencephalography are essential for the early detection and understanding of disease progression. MRI is instrumental in capturing structural changes in the brain, such as atrophy and alterations in specific regions, aiding in the identification of potential biomarkers. Key modalities employed in the early detection of Alzheimer’s Dementia: EEG, MRI, and PET. Each of these diagnostic tools plays a crucial role in the assessment and diagnosis of AD during the initial stages.
PET scans, which focus on molecular changes, are pivotal in detecting abnormal protein deposits such as beta-amyloid plaques and tau tangles, which are hallmark indicators of Alzheimer’s disease. Additionally, EEG offers valuable functional insights by measuring the electrical activity in the brain, detecting abnormalities, and connectivity disruptions. Figure 1 shows a diagrammatic view of modalities such as EEG, MRI, and PET images of data used for ADD diagnosis, whereas Figure 2 illustrates the ADD diagnosis process for neuroimaging encompassing MRI, PET, and EEG scans. The process involves crucial stages such as pre-processing, segmentation, and feature extraction from the neuro images.
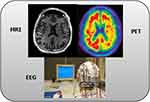 |
Figure 1 Modalities used for Alzheimer’s Dementia Detection. |
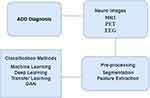 |
Figure 2 Neuro Image ADD diagnosis process. |
These extracted features are then utilised for classification and prediction, aiding in early ADD diagnosis and showcasing a comprehensive pathway towards effective analysis and potential clinical insights. Integration of these diverse imaging biomarkers provides a comprehensive view of both the structural and functional changes associated with Alzheimer’s, enabling better diagnostic accuracy and potentially facilitating early interventions.
This study provides an extensive overview of datasets frequently employed in Alzheimer’s disease research. The Alzheimer’s Disease Neuroimaging Initiative (ADNI) datasets spanning ADNI-1, ADNI-2, and ADNI-3 are notable for their longitudinal collection of clinical, imaging, genetic, and biochemical data from individuals across various stages of Alzheimer’s disease progression, including those with mild cognitive impairment and cognitively normal controls. These datasets provide valuable insights into disease trajectory, biomarker identification, and treatment response. Additionally, the Open Access Series of Imaging Studies (OASIS) dataset contains cross-sectional MRI data from individuals with Alzheimer’s disease, mild cognitive impairment, and healthy controls, making it a valuable resource for investigating structural brain changes associated with neurodegeneration.
Image Pre-Processing
Brain image pre-processing methods in Alzheimer’s dementia research are crucial for extracting meaningful information from complex neuroimaging datasets. The common pre-processing steps include intensity normalization for consistent brightness, noise reduction to improve clarity, bias correction to address scanner artifacts, skull stripping to isolate brain structures, and feature extraction to identify biomarkers relevant to dementia. These techniques enhance image quality and analytic accuracy in MRI-based studies. In,81 anatomical data were obtained using a Brain Extraction Tool to remove non-brain tissue from T1 anatomical images. This process enhances the accuracy of the subsequent analyses by eliminating unwanted elements. Another vital step is image registration, which involves aligning the brain images to a standardised anatomical space. This alignment allows for a comparative analysis across different subjects and modalities, aiding in understanding the structural and functional changes associated with Alzheimer’s dementia.
Furthermore, the intensity normalisation method utilised in82 ensures uniform intensity levels across images, mitigating variations due to diverse acquisition parameters and improving the consistency of the dataset. Voxel-Based Morphometry (VBM) is a crucial pre-processing technique that involves segmenting brain images into distinct tissue types, such as gray matter and white matter) to quantitatively analyse structural differences. VBM facilitates the detection of atrophy and other anatomical alterations characteristic of Alzheimer’s Dementia Further, Region of Interest (RoI) process the extraction focus on specific brain regions relevant to Alzheimer’s pathology, like the hippocampus.
Extracting features from the defined RoIs used in39,83 enables targeted analysis and biomarker quantification, aiding disease progression. The batch normalisation method is a technique used to improve the training of neural networks by normalising the activations of each layer within a mini-batch, aiding in faster convergence, and better generalisation was examined in.84 The smoothing technique applied in85 helps reduce noise and enhance image features, whereas the data augmentation technique utilised in19 generates additional training samples by applying transformations such as rotations or scaling to enhance the robustness of the model. In,86 ZCA whitening was proposed, which is a pre-processing step that decorrelates features in images, promotes better representation, and simplifies subsequent learning tasks.
Image Segmentation Methods for ADD
Segmentation is a fundamental concept in digital image processing, and serves as a crucial step in the analysis of images by dividing them into distinct regions based on their unique features and properties. The primary objective of segmentation is to enhance the clarity and understanding of an image, thus facilitating further analysis and interpretation. Among the various segmentation techniques, thresholding is a popular and efficient method, owing to its simplicity and effectiveness. In thresholding, an image is separated into different objects or regions by utilising a specific gray value, referred to as the threshold. However, applying thresholding to brain images can be particularly challenging, because these images often exhibit complex intensity-level distributions. The intricate distribution of intensities in brain images makes selecting an appropriate threshold value a nontrivial task, which requires careful consideration and potentially more advanced methods to achieve accurate segmentation results.
Kapur and Otsu are thresholding techniques that are used in image processing. Otsu enhances in-class differences, whereas Kapur maximises the entropy to identify homogeneous data. Both are suitable for binary segmentation; however, when applied to complex intensity distribution images that are better segmented using Multilevel Thresholding (MT), they require multiple thresholds, leading to computational complexity. To reduce the computation time, methods such as stimulating the activation function computation through iterative mechanisms, including metaheuristic optimisation, have been devised. Multi-level thresholding offers benefits, such as avoiding local optima, finding global optimal solutions, and maintaining simplicity and versatility in the segmentation process. Various bioinspired optimisation methods are pivotal for AD detection, including Genetic Algorithms (GA) inspired by natural selection. Particle swarm optimisation (PSO) simulating bird flocking or fish schooling efficiently selects the relevant features. Ant colony optimisation (ACO), which mimics ant foraging behaviour, aids in feature selection and image processing optimisation.
Artificial Bee Colony (ABC) algorithms, inspired by honeybee foraging, optimise the selection of critical features or weights in machine learning models and refine AD classification and prediction. In,87 brain image segmentation was performed using a multi-level thresholding approach, initially using PSO and then enhancing it with a Markov Random Field model. In,88 the segmentation of the hippocampal region from the brain subregions was examined using various optimisation techniques, including the lion optimisation algorithm (LOA), genetic algorithm, BAT algorithm, particle swarm optimisation, and ABC optimisation. The LOA outperformed the others because of its ability to avoid local optima. In,89 the authors proposed a hybrid approach for combining GA and PSO along with a deep neural network for efficient disease classification using brain MRI images.
In a previous study,90 the author focused on segmenting the corpus callosum and ventricle regions by utilising multi-level thresholding methods such as ACO and ABC optimisation techniques. ABC optimisation achieved a higher accuracy (93%) than ACO. In,91 multilevel Tsallis-based grey wolf optimisation (GWO) was used to segment brain tissues, followed by feature extraction from white matter, grey matter, and cerebrospinal fluid using a Convolutional Neural Network. In,92 the Patch Image Differential Clustering (PIDC) principle was employed to initialise cluster centres, and PSO was used to enhance the segmentation accuracy. These bioinspired techniques significantly contribute to the advancement of Alzheimer’s detection by leveraging natural principles to enhance the computational efficiency and accuracy.
In Table 1, researchers have employed bioinspired optimisation algorithms such as particle swarm optimisation, ant colony optimisation, artificial bee colony, grey wolf optimisation, and lion optimisation algorithm (LOA) for image segmentation. These algorithms have been applied to various datasets, including the ADNI dataset, real-time brain MR images, and Open Access Series of Imaging Studies dataset. The modality mainly involved MRI, and segmentation was performed using deep-learning techniques and PSO-Segmentation. The accuracy achieved in these studies varied, with results ranging from 78% to 97.5%, demonstrating the effectiveness of bioinspired optimisation algorithms in the context of medical image segmentation for different datasets and years.
 |
Table 1 Bioinspired Optimization Algorithms for ADD Image Segmentation |
Feature Extraction Approaches for ADD
In brain image datasets related to Alzheimer’s, the feature extraction process involves identifying and quantifying significant patterns and characteristics within the images. This transforms the raw image data into informative features that aid in the analysis and diagnosis of the disease. Key aspects include structural features (identification of brain regions and atrophy), molecular features (quantification of abnormal proteins), and functional features (capturing changes in electrical activity). These extracted features formed the basis for further analysis and diagnosis. In the feature extraction process of image datasets, leveraging transfer learning models involves the utilisation of pre-trained neural networks. The pre-trained model layers were adapted and fine-tuned to extract the relevant features specific to Alzheimer’s. However, the optimisation algorithm, inspired by natural processes such as GA, PSO, and GWO, as mentioned in detail in the previous section, extracts distinctive features by biological behaviours. Extracting the features using the CBGWO model used in93 reduces the number of features without the loss of significant information and is classified by the Adaptive Neuro-Fuzzy Inference System (ANFIS). Detrended Cross-Correlation Analysis (DCCA) of the EEG signals was used94 for AD detection.
In study,95 Genetic Algorithms were employed to identify the most relevant features with the smallest possible set of features for automated assessment of brain PET images. In,96 the authors combined Recursive Feature Elimination (RFE) and Genetic Algorithms (GA) with logistic regression and linear support vector machine classifiers using a wrapper technique. This combination is used to select highly relevant features from a large dataset. In a previous study,97 the group grey wolf optimisation (GGWO) technique was utilised to enhance detection performance. Decision Tree, K-Nearest Neighbour, and Convolutional Neural Network classifiers were employed to identify a reduced set of useful features without compromising the performance. In,98 the authors utilised binary particle swarm optimisation (BPSO), binary grey wolf optimisation (BGWO), and Binary Differential Evolution (BDE) for feature selection. These algorithms were compared, and three classifiers, K-Nearest Neighbour, Random Forest, and Support Vector Machine, were used. The comparison results showed that BGWO outperformed BABC, which is a competitive method for this purpose.
The study99 introduced the Harris hawk optimisation (HHO) algorithm, referred to as ILHHO, with Kernel Extreme Learning Machine (KELM)- ILHHO-KELM model for AD diagnosis, enhancing optimisation, and classification. The Multimodal fusion of MRI, PET, and CSF biomarkers achieved superior accuracy, highlighting the importance of combining heterogeneous data. A recent study introduced an Enhanced Dementia Detection and Classification Model (EDCM), which significantly improved classification accuracy by incorporating a gray wolf optimisation-driven approach for feature selection and hyperparameter tuning, achieving up to 97% accuracy post-optimisation.100
Table 2 provides a comprehensive overview of the applications of natural bio-inspired algorithms for feature extraction in the context of ADD images. The table includes the reference number, publication year, specific feature extraction method used, algorithm employed, imaging modality (eg MRI and PET), dataset used for analysis, and accuracy obtained in each study. These bio-inspired algorithms are instrumental in extracting meaningful features from medical images, contributing to the accuracy of Alzheimer’s dementia detection, as indicated by reported accuracy percentages. In addition, another method called the integrated multiple signal classification and empirical wavelet transform (MUSIC-EWT) was used in101 to predict AD. Extraction of features for the Eth EEG epoch using Continuous Wavelet Transform (CWT), BiSpectrum (BiS), and multi-modal of (CWT+BiS) features are used in13 as input for the classifications of machine learning models. A hybrid EEG-fNIRS model was used for AD classification.102 These features were extracted using the Pearson correlation coefficient-based feature-selection (PCCFS) model. In,103 we employed brain subregions and four optimisation algorithms, GA, PSO, GWO, and Cuckoo Search (CS), and (COA, to diagnose Alzheimer’s dementia. GWO yields promising results by selecting the global optimum solution, and deep learning classifiers are used for the classification.
 |
Table 2 Bio-Inspired Algorithms for Feature Extraction Process in ADD Images |
Materials and Methods
Machine Learning Approaches for ADD
Machine learning is a branch of artificial intelligence that focuses on creating algorithms and models that enable computers to learn and improve their performance on a specific task without explicit programming. This involves the use of statistical techniques to enable machines to recognise patterns, make predictions, or optimise decision-making based on data. Machine-learning algorithms are designed to learn from data, identify patterns, and generalise to make accurate predictions or decisions when faced with new, unseen data. Several types of machine-learning algorithms are suited to specific tasks. Supervised learning algorithms learn from labelled data, in which the input data are paired with the corresponding output labels to accurately predict future outputs. In machine learning, finding the best model typically requires a process of experimentation and refinement.106 Figure 3 shows the framework of unsupervised learning algorithms that work with unlabelled data to identify patterns and structures within the data, such as clustering similar data points or reducing the dimensionality of the data.
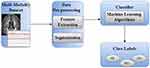 |
Figure 3 Architectural Framework for Early-Stage ADD using Machine Learning. |
Table 3 summarises the different types of machine learning algorithms used in medical diagnosis. The table includes details, such as the reference, publication year, methods or algorithms applied, modality of medical data, dataset source, and accuracy achieved in the respective studies. These algorithms play a significant role in medical diagnosis across different datasets using MRI, PET, and EEG modalities, and demonstrate varying levels of accuracy for different medical conditions and tasks. The Support Vector Machine (SVM) used in research works17 and18 is commonly used for classification tasks, aiming to find the optimal hyperplane that separates the data into distinct classes. K-Nearest Neighbours (KNN) is a simple and effective algorithm for classification and regression that identifies the nearest data points and determines the outcomes based on their majority.
 |
Table 3 Machine Learning Algorithms for the Analysis of ADD |
Naive Bayes (NB) a probabilistic algorithm widely used for text classification and spam filtering that assumes independence between features. Random Forest (RF) and Decision Trees (DT) are ensemble methods, in which RF combines multiple decision trees to improve accuracy and robustness, whereas DT splits data based on features to reach a decision. Linear Discriminant Analysis (LDA) was used in,21 and Quadratic Discriminant Analysis (QDA) was used for classification tasks by finding linear and quadratic decision boundaries, respectively. K-means clustering, a popular unsupervised learning algorithm used to group data points into clusters based on their similarities, was utilized in.9 In,3 the author used Hjorth parameters and three different classifiers (SVM, KNN, and regularised linear discriminant analysis (RLDA)) to classify healthy subjects, mild Alzheimer’s, and moderate Alzheimer’s cases.
The research work,15 the proposed SVM and KNN were employed for classification using spectral and wavelet features, respectively. In,8 the author utilised decision trees and random forests, with DT (C4.5) achieving a high accuracy. In,10 various machine-learning algorithms for FFT and CWT features were compared, and KNN consistently showed the best classification accuracy. Examining the study,13 the combination of CWT and BiS features improved classification, with the multi-layer perceptron (MLP) classifier outperforming the other models. A previous study12 explored multiclass classification approaches (LDA, QDA, and MLP) to classify data based on trials and subjects. Complexity-based features, such as Spectral Entropy and Zero Crossing Rate, classifying data using the K-nearest neighbour, were examined in.16 In study,20 various classifiers, including a multi-layer perceptron, KNN, support vector machines, naïve Bayes, and decision trees, were evaluated for whole-brain dynamics. Studies17 and18 used an SVM classifier for their research work.
Study94 introduced the DCCA cross-correlation coefficient was introduced as a measure of the cross-correlation between EEG electrodes in patients with AD. In a previous study,2 the K-NN was found to be the most effective classification algorithm when compared with the SVM and DT classifiers. In study102 employed feature selection was employed based on the Pearson correlation coefficient and LDA for classification using EEG- and fNIRS-derived features. A previous study14 used KNN, NB, and CART decision tree methods for classification based on specific brain connections. For early AD detection, these machine-learning algorithms demonstrate accurate predictions and aid in decision accuracy with the broad availability of datasets. Examining the study,13 the combination of CWT and BiS features improved classification, with the multi-layer perceptron classifier outperforming other models. The Multi-layer Perceptron used in7,19 represents a neural network with multiple layers that is widely used for complex tasks such as image recognition through deep learning.
ADD Deep Learning Models
Deep learning is a subfield of artificial intelligence that focuses on creating sophisticated models inspired by the neural networks of the human brain. At its core, deep learning employs complex architectures comprising of multiple interconnected layers of nodes or neurons. These layers form a neural network with each layer, extracting and transforming the features from the data input. The initial layers identify simple features, whereas subsequent layers build upon them to discern more intricate patterns. Deep-learning architectures, such as Convolutional Neural Networks (CNNs) for image processing and Recurrent Neural Networks (RNNs) for sequential data, excel in learning representations directly from raw data. Through the iterative process of forward and backward propagation, these architectures optimise the model parameters to minimise the difference between the predicted and actual outcomes, thereby allowing for highly accurate predictions and complex task executions. Figure 4 illustrates a deep learning-based structural framework designed for early-stage Alzheimer’s Dementia Detection. Convolutional neural networks (CNNs), such as MRI and PET scans, are commonly employed for AD detection.
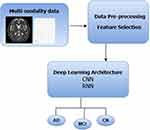 |
Figure 4 Deep Learning-Based Structural Framework for Early-Stage ADD. |
Table 4 provides an overview of the deep learning architectures used for early stage ADD with different datasets, including MRI, PET, and EEG. The table includes reference numbers, publication years, methods used (eg CNN, RNN), modality of medical data, specific datasets employed, and the reported accuracy achieved in each study. These deep-learning architectures play a critical role in the early detection of Alzheimer’s Dementia using various imaging modalities and datasets. The initial layers in these architectures detect basic features such as edges or textures, whereas deeper layers progressively combine these features to recognise more complex patterns indicative of AD. By leveraging the depth and computational power of the deep learning model, it is possible to identify subtle biomarkers and anomalies that are often early signs of the disease. This early detection potential holds immense promise for timely intervention and improved AD management.
 |
Table 4 Deep Learning Architectures for Analysis of ADD |
Transfer Learning Approaches for ADD
Transfer learning, a vital concept in machine learning, has been instrumental in advancing the detection of AD. This approach involves utilising pretrained deep learning models to enhance the performance of a model on a specific task, making it particularly effective in the medical image analysis of MRI, PET, and EEG for AD detection. Notable architectures like ResNet, VGG16 used in49, VGG19 was used in42, AlexNet, MobileNet, Inception V3, Inception V4, DenseNet, and LeNet have been widely used for this purpose. Research works45,48 and residual networks (ResNet) are known for their ability to train very deep networks using residual learning, addressing the AD classification problem. VGG16 and VGG19, which are characterised by their simplicity and depth, have shown remarkable performance in various image-related tasks including AD detection. AlexNet, a pioneering deep learning model, has demonstrated significant advancements in image-classification tasks4,59.
Inception V3 used in48 and Inception V4 developed in51,57 are architectures that utilise inception modules and optimise the computational resources. By leveraging these pre-trained deep learning models and adapting them to AD-specific datasets, transfer learning significantly enhances the accuracy and efficiency of AD detection. This approach holds immense promise in aiding the early diagnosis and intervention of AD. A detailed examination of the ResNet-18 architecture has been conducted59. In54, the DenseNet-121 model demonstrated a superior performance for AD diagnosis. In57, the InceptionV3 neural network was used with an international AD dataset comprising brain MRI scans. In110, transfer learning was employed and a pre-trained AlexNet convolutional network was fine-tuned to classify AD images.
In a research work50, a probability-based fusion method was used to combine 3D-DenseNets with various architectures. Extensive experiments were conducted to assess the performance of the 3D-DenseNet with different hyperparameters and architectural configurations. A deep multitask multichannel Learning (DM2L) framework was proposed in52 for simultaneous brain disease classification and clinical score regression using MRI data and demographic information. In a study55, a novel approach for hippocampal analysis was proposed, integrating global and local features through three-dimensional densely connected convolutional networks and shape analysis, with a focus on Alzheimer’s disease diagnosis. In the research work56, the authors combined features learned from multi-task CNN and DenseNet models to classify the disease status more effectively.
In45, AlexNet, a pre-trained CNN from ImageNet, was employed to address complex classification tasks. In44, the authors introduced ResNet29, an end-to-end 3D-CNN, and utilised transfer learning on sMRI scans to train the model. In43, the study focused on enhancing Alzheimer’s disease image classification by employing Deep Convolutional Neural Networks (DCNN), which included convolutional neural networks such as VGG16 and VGG19, combined with transfer learning, using MRI data. In47, VoxCNNs and a random forest classifier were applied separately to address a four-class classification problem. In a previous study53, a hybrid model combining convolutional and recurrent neural networks was introduced for a more comprehensive analysis of the hippocampus using structural MR images in Alzheimer’s disease research. In111, an efficient approach utilising transfer learning fine-tuned a pretrained convolutional network, AlexNet, for image classification.
An ensemble learner combining two deep learning networks to evaluate volumetric and grid-based brain scan features achieved an average diagnostic accuracy of 91.83% for Alzheimer’s disease detection.112,113 Proposed multi-modal imaging approaches for dementia diagnosis utilising deep neural networks to extract structural and functional features from both MRI and FDG-PET scans, demonstrating the superiority of multi-modal models over single-modal solutions in focusing on diverse features114. The paper presents a deep ensemble learning framework that integrates multisource data using deep learning algorithms to achieve improved classification accuracy, outperforming six established ensemble approaches by 4% based on experiments conducted with a clinical dataset from the National Alzheimer’s Coordinating Centre. The study combined EfficientNetV2S-based transfer learning with densely learned features and achieved significant improvements in classification accuracy, demonstrating up to 91.54% accuracy on the OASIS dataset and outperforming other methods in validation115. In116, the authors introduced MultiAz-Net, a novel ensemble-based deep neural network learning model that integrates diverse information from PET and MRI images to enhance Alzheimer’s disease diagnosis.
In study117, focused on developing a simple, low-capacity, high-performance model for Alzheimer’s disease classification by evaluating 14 commonly used transfer learning models, such as InceptionV3, ResNet101, ResNet101V2, ResNet152V2, ResNetRS50, DenseNet121, DenseNet169, DenseNet201, InceptionResNetV2, RegNetX002, RegNetX320, MobileNetV2, MobileNetV3Large, EfficientNetB0, EfficientNetB7, and NASNetLarge. The study118, developed the transfer learning approach using variance-based pruning and Avg-TopK pooling to optimize models like InceptionV3 and DenseNet201 for improved efficiency and accuracy in specific tasks. The MTAP model used in the paper119 enhances diagnosis accuracy to 99.69% using ADASYN, pruning, and Avg-TopK pooling for efficient feature extraction. The RGB-Angle-Wheel data augmentation technique enhances deep learning model performance by rotating color channels, improving accuracy and generalizability in image processing tasks120.
Table 5 provides an overview of the transfer-learning architectures employed in ADD. Finally, in60, the VGG architecture was employed with pretrained weights. It includes details such as the author, publication year, methods or architectures used, imaging modality, specific datasets utilised, and the reported accuracy obtained in each study. Transfer learning techniques play a crucial role in leveraging pre-trained models to improve the accuracy and efficiency of ADD systems, as indicated by the varied accuracy percentages across different studies and datasets. The schematic overview in Figure 5 provides insights into the application of transfer learning approaches in ADD, offering a comprehensive understanding of how pretrained models can enhance the diagnostic system.
 |
Table 5 Transfer Learning Architectures for Analysing ADD |
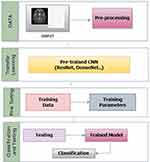 |
Figure 5 A Schematic Overview of Transfer Learning Approaches for ADD. |
GAN Model for ADD
A GAN, or Generative Adversarial Network, is a machine learning framework involving two neural networks, the generator and discriminator, engaged in a competitive process to generate and evaluate data. GANs are useful for AD detection in data augmentation, improving model generalisation, and simulating disease progression. They generate synthetic data to enrich training datasets, enhance model performance across different datasets, and created realistic data to train models for early diagnosis. By creating realistic imaging data, GANs improve data diversity and model generalization, making them a valuable tool for addressing the challenges of limited dataset size and demographic imbalance. An architectural framework was presented for the early detection of ADD using GAN-based methods, offering a novel approach for improving diagnostic accuracy.
The GAN method employed in65 was utilised to enhance image quality and improve image-generation performance. To predict AD in67, the data were pre-processed by removing noise, and used extraction method was used. The cycle GAN model proposed in68 achieved a high Structural Similarity Index Measure (SSIM) and peak signal-to-noise ratio (PSNR) when compared with the pix2pix model of PET-FBB images generated by PET-FDG images66. Used MR images of second-order statistics by extracting features with tensor-trained high-order pooling and Semi-supervised GAN (THS-GAN). A novel fusion of rs-fMRI and DTI data to obtain results with high accuracy of AD prediction using the CT-GAN method was examined in63. Deep Convolutional GAN and Super-resolution GAN were used in80 to enhance the resolution of MRI scans by using deep learning classifiers to predict AD.
Table 6 shows the utilisation of GAN methods for ADD using various imaging modalities and datasets. GAN-based techniques have shown promise in improving the accuracy and quality of medical image processing for ADD tasks. Notable highlights include the DCGAN-based augmentation in the study72, which enhanced the MRI data using CNN and VGG16. In71, the authors proposed an innovative Deep Convolutional GAN (DCGAN-ADD) approach for MRI data from ADNI and OASIS. A study74 applied data augmentation using a GAN to MRI data. Other approaches, such as Multi-Purpose GAN (MP-GAN), medical anomaly detection GAN (MADGAN), and multi-information GAN (mi-GAN) in research works,69,75,76 respectively, demonstrate the potential of GANs in advancing MRI data analysis for Alzheimer’s disease.
 |
Table 6 Application of GAN Methods in Analysing ADD |
In Figure 6, a framework for early stage ADD is shown, prominently featuring the integration of GAN-based methods. This approach allows the GAN to generate synthetic data, thereby enhancing the diagnostic accuracy and enabling more effective interventions in the early stages of ADD. Moreover, study70 utilized GAN on MRI data with UNet. In79, the author introduced Rev GAN by utilising MRI and PET data from ADNI with CNN. In contrast73 applied a GAN to PET data from ADNI using a CNN. In61, a GAN was applied to MRI data by using a CNN. The THS-GAN model in66 demonstrated a versatile performance for MRI data. Finally, another study62 adopted GAN-based augmentation on MRI data from the AIBL, ADNI, and NACC datasets. A novel approach, hypergraph generative adversarial network (HGGAN), was introduced in77, incorporating the interactive hyperedge neuron module (IHEN) and Optimal Hypergraph Homomorphism algorithm (OHGH) to synthesise multimodal connectivity of the Brain Network from combined rs-fMRI and DTI data.
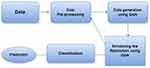 |
Figure 6 Framework for Early-Stage ADD Using GAN-Based Methods. |
Discussion
Answer for the research questions that are addressed in this analysis are,
“Q1. Which medical imaging modalities exist for detecting dementia?” Several medical imaging modalities have been employed to detect dementia, including magnetic resonance imaging, positron emission tomography, electroencephalography, and diffusion-tensor imaging are being analysed in this work. MRI was used in 75 articles that provided detailed visualisation of the brain structure and stands as the leading imaging modality in Alzheimer’s research, primarily due to its widespread availability in public datasets. PET data were utilized in ten articles to observe the metabolic activity and protein distribution. EEG was used in 20 articles that measured electrical brain activity. These modalities play an essential role in the diagnosis and treatment of dementia. MRI is the most widely used imaging technique in Alzheimer’s research primarily due to its widespread availability in public datasets, which has contributed to its extensive use in this domain.
“Q2 Which image pre-processing methods are most used to process multi-modality images?” Image pre-processing methods commonly employed for multimodal images include image registration to align different modalities into a common coordinate system, intensity normalisation for consistent brightness, noise reduction for improved quality, bias correction to address intensity variations, skull stripping for isolating brain structures, and feature extraction to identify relevant information. These methods are crucial for enhancing the image quality and ensuring accurate analysis when dealing with multiple imaging modalities in medical applications.
“Q3. Which segmentation techniques have been adopted for medical image processing?” Various segmentation techniques have been employed for medical image processing in the context of AD detection. These techniques often include region-based, edge-based, or deep learning-based segmentation methods, and some studies have integrated bio-inspired optimisation algorithms such as genetic algorithms, particle swarm optimisation, and ant colony optimisation. These bio-inspired optimisation approaches enhance the accuracy and efficiency of segmenting brain structures and pathological regions in AD-related medical images, thereby contributing to a more effective diagnosis and research in the field.
“Q4. Is it possible to apply optimisation techniques for pre-processing?” Yes, it is possible to apply bio-inspired optimisation techniques in the pre-processing stage of medical image analysis. In some of the studies in this survey, bio-inspired optimisation algorithms such as genetic algorithms, particle swarm optimisation, and ant colony optimisation were used. are used not only for segmentation and feature extraction but also for optimising the pre-processing steps, which can include tasks such as image registration, normalisation, and noise reduction. This approach enhances the overall image quality and aids in more accurate segmentation and feature extraction for ADD medical imaging applications.
“Q5. What are the different learning algorithms available for analysing multimodality image datasets to detect Alzheimer’s dementia?” To analyse multimodality image datasets for Alzheimer’s dementia detection, ML, DL, TL, and GAN learning algorithms were applied in this survey. Machine Learning techniques were employed in 20 articles, including traditional methods, such as SVM, KNN, and Random Forest. Deep Learning approaches, such as CNNs and RNNs, were utilised in another 20 articles, enabling automated extraction of complex features from images. Transfer Learning (TL) was a prevalent choice in 20 articles, allowing the adaptation of pre-trained models to medical image analysis tasks. Additionally, Generative Adversarial Networks (GANs) have been employed in 20 studies, often for data augmentation and improving the quality of multi-modal data. These diverse learning algorithms play a crucial role in enhancing the accuracy and efficiency of Alzheimer’s Dementia detection using multimodality image datasets.
This discussion highlights the importance of utilising various imaging modalities and advanced techniques, such as image pre-processing, segmentation, and optimisation algorithms, for dementia diagnosis. Continued exploration of innovative approaches and collaboration across interdisciplinary domains will be crucial for further advancements in dementia detection and understanding of neurodegenerative diseases. While various imaging modalities, such as MRI, PET, and EEG, have proven effective in detecting Alzheimer’s dementia, each has inherent limitations. For example, MRI provides detailed structural information but lacks the real-time functional insights offered by EEG. PET, on the other hand, is excellent for metabolic activity observation but is expensive and less accessible. Moreover, image pre-processing techniques such as intensity normalization and noise reduction improve image quality but can introduce variability when applied to datasets from different imaging centres. Segmentation techniques, particularly deep learning-based methods, show high accuracy but are computationally expensive and require large datasets for training.
Bio-inspired optimization algorithms have been helpful in improving segmentation and feature extraction; however, their computational intensity limits their practical application. Current Alzheimer’s datasets often lack demographic diversity, which limits the generalization of ML and DL models across diverse populations. Future datasets should aim to include broader demographic representation to improve model accuracy and reliability. Additionally, data augmentation or synthetic data could help address these gaps temporarily. Future research can focus on integrating multimodal data to offset the limitations of single modalities and standardizing pre-processing techniques across centers. Additionally, developing efficient segmentation methods and explainable AI models will improve clinical applicability.
Conclusion
In conclusion, the field of Alzheimer’s dementia detection has seen remarkable advancements in recent years, largely driven by the integration of multimodal imaging datasets, sophisticated learning algorithms, and optimisation techniques. Various algorithms and methods have been applied to Alzheimer’s dementia detection using different modalities such as MRI, EEG, and PET. In this comprehensive examination of Alzheimer’s Dementia detection, we observed that optimisation techniques are integral to the success of the various learning algorithms. Feature selection, hyperparameter tuning, and cross-validation are pivotal components that help fine-tune models and reduce the risk of overfitting, thereby ensuring the generalisation of algorithms to unseen data. It is worth noting that the selection of optimisation methods should be well-matched to the specific characteristics of the dataset and the chosen learning algorithm. Machine learning methods have shown notable success in AD detection, with SVM and KNN yielding particularly good results, particularly in the context of EEG data. However, deep learning methods, which prominently feature CNN-based models, consistently deliver high accuracy, making them strong contenders in the field. Several studies have demonstrated their efficiency in AD detection, underlining their potential in advanced diagnostics and research.
Transfer learning, which specifically leverages pre-trained models, such as DenseNet, VGG, and CNN, has shown significant promise when applied to MRI data analysis. Researchers have improved the accuracy and efficiency of Alzheimer’s Dementia detection by leveraging the knowledge encoded by these models. In addition, the relatively new domain of Generative Adversarial Networks has shown potential, particularly for data augmentation. The primary contributions of this paper include a comprehensive review of existing methodologies for Alzheimer’s dementia detection, critical evaluation of imaging techniques and algorithms, and the identification of current gaps in the literature. Our work advances the field by highlighting the need for more efficient, standardized approaches and the potential for multimodal data integration to improve diagnostic outcomes. The importance of future research focusing on explainable AI, including the need for standardized pre-processing protocols, more efficient segmentation algorithms, and further exploration of transfer learning and explainable AI to ensure the clinical applicability of AI-driven dementia diagnostics.
Funding
This work was supported by the Korea Environmental Industry & Technology Institute (KEITI) with a grant funded by the Korean government, Ministry of Environment (Development of IoT-based technology for collecting and managing big data on environmental hazards and health effects) under Grant RE202101551.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this study.
References
1. Kumar R, Azad C. Comprehensive overview of Alzheimer’s disease utilizing Machine Learning approaches. Multimedia Tools Appl. 2024;1–53.
2. Pirrone D, Weitschek E, Di Paolo P, De Salvo S, Cristina De Cola M. EEG signal processing and supervised machine learning to early diagnose Alzheimer’s disease. Appl Sci. 2022;11:5413. doi:10.3390/app12115413
3. Safi MS, Mohammad Mehdi Safi S. Early detection of Alzheimer’s disease from EEG signals using Hjorth parameters. Biomed Signal Proce Cont. 2021;65:102338. doi:10.1016/j.bspc.2020.102338
4. Hazarika RA, Kumar Maji A, Kandar D, et al. An approach for classification of Alzheimer’s disease using deep neural network and brain magnetic resonance imaging(MRI). Electronics. 2023;12(3):676. doi:10.3390/electronics12030676
5. AlSaeed D, Fouad Omar S. Brain MRI analysis for Alzheimer’s disease diagnosis using CNN-based feature extraction and machine learning. Sensors. 2022;22(8):2911. doi:10.3390/s22082911
6. Silva IRR, Silva GSL, de Souza RG, Dos Santos WP, de A. Fagundes RA. Model based on deep feature extraction for diagnosis of Alzheimer’s disease. In:
7. Lee E, Choi J-S, Kim M, Suk H-I; Alzheimer’s Disease Neuroimaging Initiative. Toward an interpretable Alzheimer’s disease diagnostic model with regional abnormality representation via deep learning. Neuroimage. 2019;202:116113. doi:10.1016/j.neuroimage.2019.116113
8. Miltiadous A, Tzimourta KD, Giannakeas N, et al. Alzheimer’s disease and frontotemporal dementia: a robust classification method of EEG signals and a comparison of validation methods. Diagnostics. 11(2021):1437. doi:10.3390/diagnostics11081437
9. Janghel RR, Rathore YK. Deep convolution neural network based system for early diagnosis of Alzheimer’s disease. Irbm. 2021;42:258–267. doi:10.1016/j.irbm.2020.06.006
10. Durongbhan P, Zhao Y, Chen L, et al. A dementia classification framework using frequency and time-frequency features based on EEG signals. IEEE Trans Neural Syst Rehabil Eng. 2019;27(5):826–835. doi:10.1109/TNSRE.2019.2909100
11. Sharma N, Kolekar MH, Jha K, Kumar Y. EEG and cognitive biomarkers based mild cognitive impairment diagnosis. Irbm. 2019;40(2):113–121. doi:10.1016/j.irbm.2018.11.007
12. Ruiz-Gómez SJ, Gómez C, Poza J, et al. Automated multiclass classification of spontaneous EEG activity in Alzheimer’s disease and mild cognitive impairment. Entropy. 20(2018):35. doi:10.3390/e20010035
13. Ieracitano C, Mammone N, Hussain A, Carlo Morabito F. A Convolutional Neural Network based self-learning approach for classifying neurodegenerative states from EEG signals in dementia. In:
14. Song Z, Deng B, Wang J, Wang R. Biomarkers for Alzheimer’s disease defined by a novel brain functional network measure IEEE. Trans Biomed Engin. 2018;66(1):41–49. doi:10.1109/TBME.2018.2834546
15. Bairagi V. EEG signal analysis for early diagnosis of Alzheimer disease using spectral and wavelet based features.. Inter J Infor Tech. 2018;10:403–412.
16. Kulkarni N. Use of complexity based features in diagnosis of mild Alzheimer disease using EEG signals. Int J Infor Tech. 2018;10:59–64. doi:10.1007/s41870-017-0057-0
17. Nobukawa S, Yamanishi T, Kasakawa S, Nishimura H, Kikuchi M, Takahashi T. Classification methods based on complexity and synchronization of electroencephalography signals in Alzheimer’s disease. Fronti Psychi. 2020;11:255. doi:10.3389/fpsyt.2020.00255
18. Vecchio F, Francesca M, Francesca A, et al. Classification of Alzheimer’s disease with respect to physiological aging with innovative EEG biomarkers in a machine learning implementation. J Alzheimer’sdise. 2020;4:1253–1261.
19. Albright J, Disease Neuroimaging Initiative A. Forecasting the progression of Alzheimer’s disease using neural networks and a novel preprocessing algorithm. Alzheim Dement Transl Res Cli Interve. 2019;483–491.
20. Tzimourta KD, Giannakeas N, Tzallas AT, et al. EEG window length evaluation for the detection of Alzheimer’s disease over different brain regions. Brain Scie. 4(2019):81.
21. Jiao B, Rihui L, Zhou H, et al. Neural biomarker diagnosis and prediction to mild cognitive impairment and Alzheimer’s disease using EEG technology. Alzheim Rese ther. 2023;15:32. doi:10.1186/s13195-023-01181-1
22. Zhang Y, Wang S, Xia K, Jiang Y; Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s disease multiclass diagnosis via multimodal neuroimaging embedding feature selection and fusion. Infor Fusion. 2021;66:170–183. doi:10.1016/j.inffus.2020.09.002
23. Amini M, Mohsen Pedram M, Moradi A, Ouchani M. Diagnosis of Alzheimer’s Disease by Time‐Dependent Power Spectrum Descriptors and Convolutional Neural Network Using EEG Signal. Comput Math Meth Medi. 2021;(1):5511922.
24. Ferri R, Babiloni C, Karami V, et al. Stacked autoencoders as new models for an accurate Alzheimer’s disease classification support using resting-state EEG and MRI measurements. Clin Neurophysiol. 2021;132:232–245. doi:10.1016/j.clinph.2020.09.015
25. Cosimo I, Mammone N, Hussain A, Morabito FC. A novel multi-modal machine learning based approach for automatic classification of EEG recordings in dementia. Neural Networks. 2020;123:176–190. doi:10.1016/j.neunet.2019.12.006
26. Hongming L, Habes M, Wolk DA; Alzheimer’s Disease Neuroimaging Initiative. A deep learning model for early prediction of Alzheimer’s disease dementia based on hippocampal magnetic resonance imaging data. Alzheim &dementia. 2019;15(8):1059–1070.
27. Suriya M, Chandran Venkatesan MGS, Manoharan S. DEMNET: a deep learning model for early diagnosis of Alzheimer diseases and dementia from MR images. Ieee Access. 2021;9:90319–90329. doi:10.1109/ACCESS.2021.3090474
28. Choi JY, Lee B. Combining of multiple deep networks via ensemble generalization loss, based on MRI images, for Alzheimer’s disease classification. IEEE Signal Process Lett. 2020;27:206–210. doi:10.1109/LSP.2020.2964161
29. Spasov SE, Passamonti L, Duggento A, Lio P, Toschi N. A multi-modal convolutional neural network framework for the prediction of Alzheimer’s disease. In:
30. Yan W, Yang Y, Guo X, et al. A novel multimodal MRI analysis for Alzheimer’s disease based on convolutional neural network. In:
31. Ekin Y, Citi L, Diciotti S, Marzi C, Workalemahu Atnafu S, Seco De Herrera AG. 3d Convolutional neural networks for diagnosis of Alzheimer’s disease via structural mri. In:
32. Duc NT, Ryu S, Naveed Iqbal Qureshi M, Choi M, Ho Lee K, Lee B. 3D-deep learning based automatic diagnosis of Alzheimer’s disease with joint MMSE prediction using resting-state fMRI. Neuroinformatics. 2020;18:71–86. doi:10.1007/s12021-019-09419-w
33. Basaia S, Agosta F, Wagner L, Canu E, Magnani G, Santangelo R; Alzheimer’s Disease Neuroimaging Initiative. Automated classification of Alzheimer’s disease and mild cognitive impairment using a single MRI and deep neural networks. NeuroImage Clin. 2019;21:101645. doi:10.1016/j.nicl.2018.101645
34. Kim HW, Eun Lee H, Kyeong Taek O, Yun M, Kook Yoo S, Yoo SK. Slice-selective learning for Alzheimer’s disease classification using a generative adversarial network: a feasibility study of external validation. Eur J Nucl Med Mol Imag. 2020;47:2197–2206. doi:10.1007/s00259-019-04676-y
35. Ayub N, Zubair Ahmad Shah S, Assad A, Mohi Ud Din N. Deep 3D-CNN using Resonance Imaging for Diagnosing Alzheimer’s. In:
36. Ahmed S, Yeong Choi K, Jae Lee J, et al. Ensembles of patch-based classifiers for diagnosis of Alzheimer diseases. IEEE Access. 2019;7:73373–73383. doi:10.1109/ACCESS.2019.2920011
37. Cui R, Liu M; Alzheimer’s Disease Neuroimaging Initiative. RNN-based longitudinal analysis for diagnosis of Alzheimer’s disease. Comp Med Imag Grap. 2019;73(73):1–10. doi:10.1016/j.compmedimag.2019.01.005
38. Basher A, Kim BC, Ho Lee K, Yub Jung H. Volumetric feature-based Alzheimer’s disease diagnosis from sMRI data using a convolutional neural network and a deep neural network. IEEE Access. 2021;9:29870–29882. doi:10.1109/ACCESS.2021.3059658
39. Spasov S, Passamonti L, Duggento A, Lio P, Toschi N; Alzheimer’s Disease Neuroimaging Initiative. A parameter-efficient deep learning approach to predict conversion from mild cognitive impairment to Alzheimer’s disease. Neuroimage. 2019;189:276–287. doi:10.1016/j.neuroimage.2019.01.031
40. Lee G, Nho K, Kang B, Sohn K-A, Kim D. Predicting Alzheimer’s disease progression using multi-modal deep learning approach. Sci Rep. 2019;9(1):1952. doi:10.1038/s41598-018-37769-z
41. Liu C-F, Padhy S, Ramachandran S, et al. Using deep Siamese neural networks for detection of brain asymmetries associated with Alzheimer’s disease and mild cognitive impairment. Magnetic Resonance Imag. 2019;64:190–199. doi:10.1016/j.mri.2019.07.003
42. Deepanshi IB, Garg D. Alzheimer’s disease classification using transfer learning. In:
43. Ajagbe SA, Amuda KA, Oladipupo MA, Oluwaseyi FAFE, Okesola KI. Multi-classification of Alzheimer disease on magnetic resonance images (MRI) using deep convolutional neural network (DCNN)approaches. Int J Adv Comput Res. 2021;53:51. doi:10.19101/IJACR.2021.1152001
44. Bae J, Stocks J, Heywood A, et al. Transfer learning for predicting conversion from mild cognitive impairment to dementia of Alzheimer’s type based on a three-dimensional convolutional neural network. Neurobiol Aging. 2021;99:53–64. doi:10.1016/j.neurobiolaging.2020.12.005
45. Afzal S, Maqsood M, Nazir F, et al. A data augmentation-based framework to handle class imbalance problem for Alzheimer’s stage detection IEEE access 7 (2019): 115528–115539.
46. Nigri E, Ziviani N, Cappabianco F, Antunes A, Veloso A. Explainable deep CNNs for MRI-based diagnosis of Alzheimer’s disease. In:
47. Arijit D, Chowdhury AS. DTI based Alzheimer’s disease classification with rank modulated fusion of CNNs and random forest. Expert Syst Appl. 2021;169:114338. doi:10.1016/j.eswa.2020.114338
48. Islam J, Zhang Y. Brain MRI analysis for Alzheimer’s disease diagnosis using an ensemble system of deep convolutional neural networks. Brain Informatics. 2018;5:1–14. doi:10.1186/s40708-018-0080-3
49. Sato R, Iwamoto Y, Cho K, Kang D-Y, Chen Y-W. Comparison of CNN models with different plane images and their combinations for classification of Alzheimer’s disease using PET images. In:
50. Wang H, Shen Y, Wang S, et al. Ensemble of 3D densely connected convolutional network for diagnosis of mild cognitive impairment and Alzheimer’s disease. Neurocomputing. 2019;333:145–156. doi:10.1016/j.neucom.2018.12.018
51. Ding Y, Ho Sohn J, Kawczynski MG, et al. A deep learning model to predict a diagnosis of Alzheimer disease by using 18F-FDG PET of the brain. Radiology. 2019;290(2):456–464. doi:10.1148/radiol.2018180958
52. Liu M, Zhang J, Adeli E, Shen D. Joint classification and regression via deep multi-task multi-channel learning for Alzheimer’s disease diagnosis IEEE. Trans Biomed Engin. 2018;66(5):1195–1206. doi:10.1109/TBME.2018.2869989
53. Li F, Liu M. Alzheimer’s Disease Neuroimaging Initiative A hybrid convolutional and recurrent neural network for hippocampus analysis in Alzheimer’s disease. J Neuroscien Meth. 323:108–118. doi:10.1016/j.jneumeth.2019.05.006
54. Hazarika RA, Kandar D, Kumar Maji A. An experimental analysis of different deep learning based models for Alzheimer’s disease classification using brain magnetic resonance images. J King Saud Univ Comp Infor Sci. 2022;34(10):8576–8598. doi:10.1016/j.jksuci.2021.09.003
55. Cui R, Liu M. Hippocampus analysis by combination of 3-D DenseNet and shapes for Alzheimer’s disease. Diagnosis IEEE Journal of Biomedical and Health Informatics. 2018;23(5):2099–2107. doi:10.1109/JBHI.2018.2882392
56. Liu M, Fan L, Yan H, Wang K, Yixin M, Shen L; Alzheimer’s Disease Neuroimaging Initiative. A multi-model deep convolutional neural network for automatic hippocampus segmentation and classification in Alzheimer’s disease. Neuroimage. 2020;208:116459. doi:10.1016/j.neuroimage.2019.116459
57. Cui Z, Gao Z, Leng J, Zhang T, Quan P, Zhao W. Alzheimer’s disease diagnosis using enhanced inception network based on brain magnetic resonance image. In:
58. Helaly HA, Badawy M, Haikal AY. Deep learning approach for early detection of Alzheimer’s disease. Cogni Comput. 2022;14(5):1711–1727. doi:10.1007/s12559-021-09946-2
59. Ramzan F, Usman Ghani Khan M, Rehmat A, et al. A deep learning approach for automated diagnosis and multi-class classification of Alzheimer’s disease stages using resting-state fMRI and residual neural networks. Journal of Medical Systems. 2020;44:1–16. doi:10.1007/s10916-019-1475-2
60. Mehmood A, Yang S, Feng Z, et al. A transfer learning approach for early diagnosis of Alzheimer’s disease on MRI images. Neuroscience. 2021;460:43–52. doi:10.1016/j.neuroscience.2021.01.002
61. Vashisht S, Sharma B, Lamba S. Alzheimer detection using CNN and GAN augmentation. In:
62. Zhou X, Qiu S, Joshi PS, et al. Enhancing magnetic resonance imaging-driven Alzheimer’s disease classification performance using generative adversarial learning. Alzheimer’s Res Ther. 2021;13:1–11.
63. Pan J, Jing C, Zuo Q, Nieuwoudt M, Wang S. Cross-modal transformer GAN: a brain structure-function deep fusing framework for Alzheimer’s disease. In:
64. Nguyen H-D, Clément M, Planche V, Mansencal B, Coupé P. Deep grading for MRI-based differential diagnosis of Alzheimer’s disease and Frontotemporal dementia. Artif. Intell. Med. 2023;144:102636. doi:10.1016/j.artmed.2023.102636
65. Jung E, Luna M, Hyun Park S. Conditional GAN with 3D discriminator for MRI generation of Alzheimer’s disease progression. Pattern Recogn. 2023;133:109061. doi:10.1016/j.patcog.2022.109061
66. Wen Y, Lei B, Ng MK, Cheung AC, Shen Y, Wang S. Tensorizing GAN with high-order pooling for Alzheimer’s disease assessment. IEEE Trans Neural Net Learn Syst. 2021;33(9):4945–4959.
67. Baskaran KR, Sanjay V. Deep learning based early Diagnosis of Alzheimer’s disease using Semi Supervised GAN. Ann Roman Soc Cell Biol. 2021;7391–7400.
68. Choi HJ, Seo M, Kim A, Hoon Park S. Generation of Conventional 18F-FDG PET Images from 18F-Florbetaben PET Images Using Generative Adversarial Network: a Preliminary Study Using ADNI Dataset. Medicina. 2023;59(7):1281. doi:10.3390/medicina59071281
69. Han C, Rundo L, Murao K, et al. MADGAN: unsupervised medical anomaly detection GAN using multiple adjacent brain MRI slice reconstruction. BMC Bioinf. 2021;22:1–20. doi:10.1186/s12859-020-03936-1
70. Han C, Rundo L, Murao K, et al. GAN-based multiple adjacent brain MRI slice reconstruction for unsupervised Alzheimer’s disease diagnosis. In: International Meeting on Computational Intelligence Methods for Bioinformatics and Biostatistics. Cham: Springer International Publishing; 2019:44–54.
71. Tian B, Mingyu D, Zhang L, et al. A novel Alzheimer’s disease detection approach using GAN-based brain slice image enhancement. Neurocomputing. 2022;492:353–369. doi:10.1016/j.neucom.2022.04.012
72. Jain V, Nankar O, Jacob Jerrish D, Gite S, Patil S, Kotecha K. A novel AI-based system for detection and severity prediction of dementia using MRI. IEEE Access. 2021;9:154324–154346. doi:10.1109/ACCESS.2021.3127394
73. Islam J, Zhang Y. GAN-based synthetic brain PET image generation. Brain Informatics. 2020;7(1):3. doi:10.1186/s40708-020-00104-2
74. Ma D, Donghuan L, Popuri K, Faisal Beg M. Differential diagnosis of frontotemporal dementia and Alzheimer’s disease using generative adversarial network. arXiv preprint arXiv. 2021.
75. Wen Y, Lei B, Wang S, et al. Morphological feature visualization of Alzheimer’s disease via multidirectional perception GAN IEEE. Trans Neural Netwo Learni Syst. 2022;34(8):4401–4415.
76. Zhao Y, Baoqiang M, Jiang P, Zeng D, Wang X, Shuyu L. Prediction of Alzheimer’s disease progression with multi-information generative adversarial network. IEEE J Biomed Health Inform. 2020;25(3):711–719. doi:10.1109/JBHI.2020.3006925
77. Pan J, Lei B, Shen Y, Liu Y, Feng Z, Wang S. Characterization multimodal connectivity of brain network by hypergraph GAN for Alzheimer’s disease analysis. In:
78. Wang Sunny MRI-Based Diagnosis Of Alzheimers Disease Using Deep Learning With Cyclegan For Data Augmentation; 2022.
79. Lin W, Lin W, Chen G, Zhang H, Gao Q, Huang Y; ; Alzheimer’s Disease Neuroimaging Initiative. Bidirectional mapping of brain MRI and PET with 3D reversible GAN for the diagnosis of Alzheimer’s disease. Front Neurosci. 2021;15:646013. doi:10.3389/fnins.2021.646013
80. SinhaRoy R, Sen A. A Hybrid Deep Learning Framework to Predict Alzheimer’s Disease Progression using generative adversarial networks and deep convolutional neural networks. Arab J Sci Eng. 2024;49(3):3267–3284. doi:10.1007/s13369-023-07973-9
81. Sarraf S, Tofighi G. Classification of Alzheimer’s disease using fmri data and deep learning convolutional neural networks. arXiv preprint, arXiv. 2016;1603.
82. Rachna J, Jain N, Aggarwal A, Jude Hemanth D. Convolutional neural network based Alzheimer’s disease classification from magnetic resonance brain images. Cognit Syst Res. 2019;57:147–159. doi:10.1016/j.cogsys.2018.12.015
83. Zaabi M, Smaoui N, Derbel H, Hariri W. Alzheimer’s disease detection using convolutional neural networks and transfer learning based methods. In:
84. Hongming L, Habes M, Wolk DA, Fan Y; Alzheimer’s Disease Neuroimaging Initiative. A deep learning model for early prediction of Alzheimer’s disease dementia based on hippocampal magnetic resonance imaging data. Alzheimer’s Dementia. 2019;15(8):1059–1070. doi:10.1016/j.jalz.2019.02.007
85. Anees A, Zening F, Yuhui D, Calhoun VD. Multimodal data fusion of deep learning and dynamic functional connectivity features to predict Alzheimer’s disease progression. In:
86. Xiaojun B, Wang H. Early Alzheimer’s disease diagnosis based on EEG spectral images using deep learning. Neural Networks. 2019;114:119–135. doi:10.1016/j.neunet.2019.02.005
87. Raju M, Sudila TV, Varun P. Classification of mild cognitive impairment and Alzheimer’s disease from magnetic resonance images using deep learning. In:
88. Chitradevi D, Prabha S, Daniel Prabhu A. Diagnosis of Alzheimer disease in MR brain images using optimization techniques. Neural Comput Appl. 2021;33:223–237. doi:10.1007/s00521-020-04984-7
89. Kaur M, Singh R. Recognition, Analysis and classification of Alzheimer ailment using Hybrid Genetic and Particle Swarm with Deep Learning Technique. Inter J Computer Applic Infor Techn. 2022;13:428–438.
90. Chitradevi D, Prabha S. Analysis of Alzheimer disease using optimization techniques. In:
91. Kavitha G Study of tissue variation and analysis of MR brain images using optimized multilevel threshold and deep CNN features in neurodegenerative disorders. In:
92. Arunprasath T, Pallikonda Rajasekaran M, Vishnuvarathanan G. MR Brain image segmentation for the volumetric measurement of tissues to differentiate Alzheimer’s disease using hybrid algorithm. In:
93. Anter AM, Wei Y, Jiahui S, et al. A robust swarm intelligence-based feature selection model for neuro-fuzzy recognition of mild cognitive impairment from resting-state fMRI. Inf Sci. 2019;503:670–687. doi:10.1016/j.ins.2019.07.026
94. Chen Y, Cai L, Wang R, et al. DCCA cross-correlation coefficients reveals the change of both synchronization and oscillation in EEG of Alzheimer disease patients Physica a Stat Mech Appl. 490 (2018): 171–184.
95. Díaz-álvarez J, Matias-Guiu JA, Nieves Cabrera-Martín M, et al. Genetic algorithms for optimized diagnosis of Alzheimer’s disease and Frontotemporal dementia using Fluorodeoxyglucose positron emission tomography imaging. Front Aging Neurosci. 2022;13:708932. doi:10.3389/fnagi.2021.708932
96. Divya R, Shantha Selva Kumari R. Genetic algorithm with logistic regression feature selection for Alzheimer’s disease classification. Neural Comput Appl. 2021;33(14):8435–8444. doi:10.1007/s00521-020-05596-x
97. Shankar K, Lakshmanaprabu SK, Tanwar S, Rodrigues JJPC, Ranjan Roy N, Roy NR. Alzheimer detection using Group Grey Wolf Optimization based features with convolutional classifier. Comput Electr Eng. 2019;77:230–243. doi:10.1016/j.compeleceng.2019.06.001
98. Keleş MK, Kiliç Ü. Classification of brain volumetric data to determine Alzheimer’s disease using artificial bee colony algorithm as feature selector. IEEE Access. 2022;10:82989–83001. doi:10.1109/ACCESS.2022.3196649
99. Sheng J, Zhang Q, Wang L, Yang Z, Xin Y, Wang B. A hybrid multimodal machine learning model for Detecting Alzheimer’s disease. Compu Biol Med. 2024;170:108035. doi:10.1016/j.compbiomed.2024.108035
100. Talaat FM, Ramadan Ibraheem M. Dementia diagnosis in young adults: a machine learning and optimization approach. Neural Comput Appl. 2024;1–14.
101. Amezquita-Sanchez JP, Mammone N, Morabito FC, Marino S, Adeli H. A novel methodology for automated differential diagnosis of mild cognitive impairment and the Alzheimer’s disease using EEG signals. J Neuroscie Meth. 2019;322:88–95. doi:10.1016/j.jneumeth.2019.04.013
102. Cicalese PA, Rihui L, Ahmadi MB, et al. An EEG-fNIRS hybridization technique in the four-class classification of Alzheimer’s disease. Journal of Neuroscience Methods. 2020;336:108618. doi:10.1016/j.jneumeth.2020.108618
103. Chitradevi D, Prabha S. Analysis of brain sub regions using optimization techniques and deep learning method in Alzheimer disease. Appl Soft Compu. 2020;86:105857. doi:10.1016/j.asoc.2019.105857
104. Sharma Moolchand S, Pradhyumna P, Shubham G. Machine learning and evolutionary algorithms for the diagnosis and detection of Alzheimer’s disease. In:
105. Divager B, Azura Husin N. Analysing Brain images for detecting AD disease using natured inspired cuckoo optimized recurrent networks.
106. Othmani A, Brahem B, Haddou Y. Machine learning-based approaches for post-traumatic stress disorder diagnosis using video and EEG sensors: a review. IEEE Sensors Journal. 2023. doi:10.1109/JSEN.2023.3312172
107. Gupta Y, Ho Lee K, Yeong Choi K, Jae Lee J, Chae Kim B, Rak Kwon G; National Research Center for Dementia, and Alzheimer’s Disease Neuroimaging Initiative. Early diagnosis of Alzheimer’s disease using combined features from voxel-based morphometry and cortical, subcortical, and hippocampus regions of MRI T1 brain images. PLoS One. 2019;14(10):e0222446. doi:10.1371/journal.pone.0222446
108. Hao X, Bao Y, Guo Y, et al. Multi-modal neuroimaging feature selection with consistent metric constraint for diagnosis of Alzheimer’s disease. Med Image Anal. 2020;60:101625. doi:10.1016/j.media.2019.101625
109. Kim J, Lee B. Identification of Alzheimer’s disease and mild cognitive impairment using multimodal sparse hierarchical extreme learning machine. Human Brain Mapp. 2018;39(9):3728–3741. doi:10.1002/hbm.24207
110. Sistaninejhad B, Rasi H, Nayeri P. A review paper about deep learning for medical image analysis. Comput Math Met Med. 2023;2023(1):7091301. doi:10.1155/2023/7091301
111. Maqsood M, Nazir F, Khan U, et al. Transfer learning assisted classification and detection of Alzheimer’s disease stages using 3D MRI scans. Sensors. 2019;19(11):2645. doi:10.3390/s19112645
112. Yiğit A, Baştanlar Y, Işık Z. Dementia diagnosis by ensemble deep neural networks using FDG-PET scans. Signal Image Video Process. 2022;16(8):2203–2210. doi:10.1007/s11760-022-02185-4
113. Yiğit A, Işık Z, Baştanlar Y. Dementia Detection with Deep Networks Using Multi-Modal Image Data. In: Diagnosis of Neurological Disorders Based on Deep Learning Techniques. CRC Press; 2023:185–203.
114. Ning A, Ding H, Yang J, Rhoda A, Ang TFA. Deep ensemble learning for Alzheimer’s disease classification. J biomed informat. 105(2020):103411.
115. Muhammad Sakib Khan I, Sabrin Sworna N, Muzahidul Islam AKM; Alzheimer’s Disease Neuroimaging Initiative. A slice selection guided deep integrated pipeline for Alzheimer’s prediction from Structural Brain MRI. Biomed. Signal Process. Control. 2024;89:105773. doi:10.1016/j.bspc.2023.105773
116. Ismail WN, Fathimathul Rajeena PP, Ali MAS. A meta-heuristic multi-objective optimization method for Alzheimer’s disease detection based on multi-modal data. Mathematics. 2023;11:957. doi:10.3390/math11040957
117. Ozdemir C, Dogan Y. Advancing early diagnosis of Alzheimer’s disease with next-generation deep learning methods. Biomed. Signal Process. Control. 2024;96:106614. doi:10.1016/j.bspc.2024.106614
118. Ozdemir C. Adapting transfer learning models to dataset through pruning and Avg-TopK pooling. Neural Comput Appl. 2024;36(11):6257–6270. doi:10.1007/s00521-024-09484-6
119. Ozdemir C, Dogan Y. Advancing brain tumor classification through MTAP model: an innovative approach in medical diagnostics. Med Biol Eng Comput. 2024;1–12.
120. Ozdemir C, Dogan Y, Kaya Y. RGB-Angle-Wheel: a new data augmentation method for deep learning models. Knowledge-Based Syst. 2024;291:111615. doi:10.1016/j.knosys.2024.111615
121. Sharma MK, Shamim Kaiser M, Ray K. Deep convolutional neural network framework with multi-modal fusion for Alzheimer’s detection. Inter J Reconfig Embed Sys. 2024;13(1):179–191.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Nomogram Based on Super-Resolution Ultrasound Images Outperforms in Predicting Benign and Malignant Breast Lesions
Yang L, Ma Z
Breast Cancer: Targets and Therapy 2023, 15:867-878
Published Date: 2 December 2023
A Systematic Review of Real-Time Deep Learning Methods for Image-Based Cancer Diagnostics
Sriraman H, Badarudeen S, Vats S, Balasubramanian P
Journal of Multidisciplinary Healthcare 2024, 17:4411-4425
Published Date: 9 September 2024
An Artificial Intelligence Pipeline for Hepatocellular Carcinoma: From Data to Treatment Recommendations
Zhang X, Yang L, Liu C, Yuan X, Zhang Y
International Journal of General Medicine 2025, 18:3581-3595
Published Date: 2 July 2025

