Back to Journals » Neuropsychiatric Disease and Treatment » Volume 20
Abnormal Serum BDNF and p-mTOR in MDD in Adolescents with Childhood Trauma
Authors Zhao X, Jie H, Wang J, Liu Y, Liu Y, Qin F, Long Q, Hou X, Zhang XW, Wu W, Wu X, Li J, Zeng Y
Received 13 December 2023
Accepted for publication 15 July 2024
Published 2 August 2024 Volume 2024:20 Pages 1513—1522
DOI https://doi.org/10.2147/NDT.S454370
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yu-Ping Ning
Xinling Zhao,1,2,* Huijin Jie,3,* Jun Wang,1 Yu Liu,4 Yilin Liu,2 Fuyi Qin,2 Qing Long,2 Xi Hou,5 Xin-Wen Zhang,6 Wenzhi Wu,7 Xiaoqin Wu,2 Jing Li,8 Yong Zeng2
1Department of Clinical Psychology, People’s Hospital of Chongqing Liang Jiang New Area, Chongqing, People’s Republic of China; 2Department of Psychiatry, the Second Affiliated Hospital of Kunming Medical University, Kunming, People’s Republic of China; 3Haining Fourth People’s Hospital, Haining, People’s Republic of China; 4Department of Psychiatry, the First Affiliated Hospital of Kunming Medical University, Kunming, People’s Republic of China; 5Department of Clinical Psychology, The Sixth Affiliated Hospital of Kunming Medical University, Yuxi, People’s Republic of China; 6Department of Psychiatry, Hong He Second People’s Hospital, Jian Shui, People’s Republic of China; 7Faculty of psychology, Beijing Normal University, Beijing, People’s Republic of China; 8Sichuan Provincial Center for Mental Health, Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jing Li; Yong Zeng, Email [email protected]; [email protected]
Background: Adolescents with major depressive (MDD) episodes associated with childhood trauma have a poorer response to treatment and a higher risk of suicide. The underlying etiology is unclear. Brain-derived neurotrophic factor (BDNF) could improve depressive symptoms by down-regulating mammalian target of rapamycin (mTOR) signaling pathways, which was involved in adverse environmental stimuli during neurodevelopment. BDNF and mTOR have not been reported simultaneously in adolescents with major depressive episodes associated with childhood trauma.
Methods: Childhood Trauma Questionnaire-Short Form (CTQ-SF), Children’s Depression Inventory (CDI) and Children’s Depression Rating Scale-Revised (CDRS-R) were used to evaluate the recruited adolescents with major depression episodes. Serum BDNF and p-mTOR levels were measured by ELISA in 31 adolescents with major depression episodes with childhood trauma and 18 matched healthy control.
Results: The serum levels of BDNF were significantly lower (p< 0.001); and the serum levels of p-mTOR were high (p=0.003) in the adolescents with the first episode of major depressive episode accompanied by childhood trauma. Of the 31 adolescents with major depressive episodes, 17 had suicide or self-injury. Compared with the healthy control group, the serum levels of BDNF in patients with suicide or self-injury were lower than those without suicide or self-injury(p< 0.001); the serum levels of p-mTOR were higher than those without suicide or self-injury (p=0.01). While in patients without suicide or self-injury, only serum p-mTOR was significantly higher than that in healthy group (p=0.028). BDNF was negatively correlated with CDRS-R (r=− 0.427, p=0.006), p-mTOR was positively correlated with CDI (r=0.364, p=0.048). According to Receiver Operating Characteristic Curve (ROC), the combination of serum BDNF and p-mTOR levels have better diagnostic value.
Conclusion: Neurotrophic and signaling pathways, involving BDNF and p-mTOR, may play a role in adolescent MDD with a history of childhood trauma, especially patients with suicide and self-injury tendencies.
Keywords: brain-derived neurotrophic factor, p-mTOR, major depressive disorder, suicide, adolescent, childhood trauma
Introduction
Major depression is one of the leading causes of death and disease burden in adolescents aged 10–24.1 Childhood trauma is an adverse environmental factor during the neurodevelopment and could affect the emotional circuit.2 Childhood maltreatment could result in earlier onset, chronic and severe course of major depression and impaired cognitive function.3,4 The patients who experienced emotional abuse in childhood had more serious negative cognition and stronger suicide; Both antidepressants and non-pharmacological treatments have poor efficacy.5 In addition, adolescents’ internal perception of emotions is often at odds with their parents’ external observation of their emotional behavior.6 This makes it difficult to seek early help to adolescents. It is a challenge to treat major depressive episodes in an adolescent who experienced a childhood trauma timely.
mTOR could integrate intracellular and extracellular signals and regulate nerve growth and protein synthesis. In a maternal separation (MS) model in mice, the mTOR signaling pathway is reported to be abnormal;7,8 p-mTOR(p-mTOR) indicates the active mTOR. The amazing thing is the p-mTOR levels of mice separated at birth is significantly lower than that of chronic stress group. mTOR signaling pathway abnormalities have also been reported in MDD patients.7,9 Increased mTOR activity was revealed in Prefrontal Cortex (PFC) / Brodmann Area 9 (BA9) in MDD patients who died by suicide, regardless of drug use.10 Interestingly, when ketamine rapidly improves treatment-resistant depressive disorder, the expressions of mTOR and brain-derived neurotrophic factor (BDNF) are up-regulated.11 BDNF is the most prevalent neurotrophic factor in human, with widespread present throughout the nervous system. Its highest concentration is found in the hippocampus and cortex. The down-regulation of BDNF, which plays an important role in neuronal plasticity, has also been reported in many animal models of MDD.12–14 Low levels of BDNF have been reported in peripheral blood and postmortem brain studies of suicidal individuals.15,16 BDNF has also been suggested as a suicide risk predictor possibly independent of MDD17,18.19 In previous study, BDNF could improve depressive symptoms by down-regulating the PI3K-AKT-mTOR pathway and up-regulating autophagy.20 It is therefore clear that BDNF and the mTOR signaling pathway in major depressive episodes seem to be closely linked and complex.
Whether BDNF and mTOR are involved in major depressive episodes in adolescents with childhood trauma and whether there is a regulatory relationship between BDNF and mTOR is worth exploring. Serum BDNF and p-mTOR levels have not been reported in adolescents with major depressive disorder associated with childhood trauma. We detected the levels of serum BDNF and p-mTOR in adolescents with major depressive episodes associated with childhood trauma to explore the specific markers in peripheral blood for major depressive episodes in adolescents with childhood trauma, and predict the change and outcome of the disease.
Materials and Methods
Subjects
Adolescents diagnosed with major depressive episode for the first time without medication were recruited from inpatient department from august 2022 to June 2023. Entry criteria: (1) Young people aged between 10 and 19 years (including 10 and 19 years); (2) Comply with the Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition) (DSM-V) for major depressive episodes; (3) Two attending psychiatrists assessed the total CDRS-R score ≥40 points;(4) Adolescent patients with major depressive episode did not take any antidepressant drugs before enrollment;(6) Patients or family members sign informed consent. Exclusion criteria: (1) Patients with a history of mental retardation, epilepsy, encephalitis or other brain organic diseases, other neurological diseases. (2) Patients with diseases such as immune, or endocrine or metabolic disorders. (3) History of acute respiratory tract infection or chronic infection in the last half month. (4) People with a history of drug or psychoactive substance abuse. (5) Those who have experienced a major adverse life event in the last 6 months after age 16. (6) There is a clear risk of suicide, harm to oneself or others. Healthy controls aged 11–19 were recruited at physical examination center. Exclusion criteria are the same as above. All adolescent patients who met the aforementioned criteria and were hospitalized were recruited for the study. Children’s Depression Inventory (CDI) and CTQ scales were assessed on the same day, followed by fasting blood collection on the subsequent day. The physician responsible for diagnosing the disease was different from the one in charge of scale assessment. All subjects completed fasting blood collection the next day after admission. A total of 31 individuals participated in this study, with only two not meeting the childhood trauma criteria. We did not include 1 adolescent with depression who were not associated with childhood trauma, and only 30 MDD participants and 18 matched healthy controls were statistically analyzed.
Scale Evaluation
The Childhood Depression Scale (CDI) is specifically designed to evaluate the level of depression in children and adolescents aged 7–17. Participants were requested to select the most appropriate way to articulate their emotions experienced within the past two weeks. It comprises a total of 27 items categorized into five dimensions: anhedonia, negative affect, negative self-perception, inefficacy, and interpersonal difficulties. Responses are rated on a 54-point scale ranging from 0 to 2, representing “infrequently”, “frequently”, and “consistently” respectively denoting frequency.21
The Childhood Trauma Questionnaire (CTQ), developed by Bernstein in 1998, is globally recognized as one of the most widely utilized assessment tools for measuring childhood abuse. The Chinese version of the Childhood Abuse Questionnaire was revised in 2004 by Xing-Fu Zhao, Ya-Lin Zhang, and Long-Fei Li. It consists of a total of 28 items categorized into five subscales: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect. Scoring is based on a 5-level scale where higher scores indicate greater levels of abuse.22
Sample Collection
About 5 mL of fasting venous blood was taken with a coagulant tube on the day of admission or in the morning of the next day. The samples were left at room temperature for several hours, centrifuged at 4000 rpm for 10 min, serum was obtained, and stored at −80 °C until testing. The control group was enrolled with the same method of blood collection and storage.
BDNF, p-mTOR Concentration Measurement
The serum concentrations of BDNF and p-mTOR were determined by Human BDNF Enzyme-Linked Immunosorbent Assay (ELISA) KIT (mlbio, ml98839-J) and Human p-mTOR ELISA KIT(ST-H10642). Samples were analyzed in duplicate, and the results were averaged. One extreme value of serum BDNF and p-mTOR were excluded (they are more than 2.5 times the mean). Serum BDNF and p-mTOR were quantified by 752 Ultraviolet Spectrophotometer at 450nm.
Statistical Analysis
SPSS 21.0 was used for statistical analysis. To get better statistical power, the serum BDNF and p-mTOR levels were transformed by square root in the case and the control group. The comparison of transformed BDNF and p-mTOR between the case and control group was performed by t test, including comparison of major depressive adolescents with suicide or self-injury or without suicide or self-injury with the control group. Education represents the years of study. χ2 test was used for sex and t test was used for age and education. According to whether they are continuous variable, whether they are normal distribution and homoscedasticity, Pearson correlation analysis was used to determine the relationship between serum BDNF and p-mTOR, and their correlation with emotional abuse, emotional neglect respectively in the patient group. The relationship between serum BDNF, p-mTOR and somatic abuse, sexual abuse, somatic neglect was analyzed by Spearman Correlation Analysis. A receiver operating characteristic curve was performed to confirm the diagnostic value of BDNF, p-mTOR. The test level α=0.05 was used for bilateral test.
Results
Characteristics of the Case Group and the Control Group
There were significant differences in education (F=0.109, p=0.01) between the MDD and healthy control group. There was no significant difference in age and gender between the two groups. See Table 1.
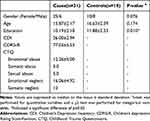 |
Table 1 Demographic Data for the Cases and Healthy Controls |
Biochemical Parameters
Serum level of BDNF were significantly lower (Transformed, F=0.416, p < 0.001) and p-mTOR level (Transformed, F=0.521, p =0.013) were significantly higher in adolescents with MDD compared to healthy controls [Figure 1A and Table 2]. Of the 31 adolescents with major depressive episodes, 17 had suicide or self-injury behaviors. Of the 31 enrolled patients, 13 had attempted suicide. There were 13 individuals who had self-inflicted injuries. Seven had both suicide attempts and self-harm. Compared with healthy people, there were more lower levels in serum BDNF levels (F=1.452, p<0.001), and there were more higher levels in serum p-mTOR levels (F=0.072, p=0.010) in patients with suicide or self-injury. See Figure 1B and Table 3. While compared with healthy people, those with no suicide or self-injury only showed significant difference in serum p-mTOR level (F=0.997, p=0.028). See Figure 1C and Table 4. There were no significant differences in serum BDNF or p-mTOR levels between adolescents with suicide or self-injury and those with no suicide or self-injury. See Figure 1D.
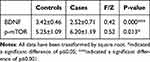 |
Table 2 Comparison of p-mTOR, BDNF in Peripheral Blood Between the Controls and the Cases |
 |
Table 3 Comparison of p-mTOR, BDNF in Peripheral Blood Between the Control Group and the Cases with Suicide or Self-Injury |
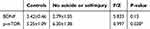 |
Table 4 Comparison of p-mTOR, BDNF in Peripheral Blood Between the Control Group and the Cases with No Suicide or Self-Injury |
Correlation Analysis
We analyzed the correlation between the levels of serum BDNF, p-mTOR and CDI, CDRS-R, CTQ and sub-scores respectively. By Pearson Correlation Analysis, p-mTOR was positively correlated with CDI (r=0.364, P=0.048); BDNF was negatively correlated with CDRS-R (r=−0.427, p=0.006). According to Bonferroni correction, p< 0.0025. There was no statistical correlation between serum BDNF, p-mTOR levels and CDI or CDRS-R scores; there was no statistical correlation between serum BDNF, p-mTOR levels and the sub-scores of physical abuse, emotional abuse, somatic neglect, and emotional neglect of CTQ-SF. See Figure 2.
Receiver Operating Characteristic (ROC) Curve Analysis
ROC curve analysis was performed to detect whether serum BDNF and p-mTOR levels could be effective as biomarkers for distinguishing major depressive episodes in adolescents with childhood trauma from healthy controls. As shown in Figure 3, the AUC of BDNF was 0.883 (95% CI, 0.777–0.989; P = 0.000; Figure 3A), the best cutoff value was 0.354(specificity was 0.889; sensitivity,0.857). The AUC of p-mTOR was 0.773 (95% CI, 0.620–0.925; P =0.002; Figure 3B), the best cutoff value was 0.392(specificity was 0.882; sensitivity,0.800). The combined AUC clearly distinguished patients from healthy controls, with an AUC of 0.887 (95% CI, 0.783–0.993; P = 0.000; Figure 3C), the best cut-off value was 0.512(specificity was 0.765; sensitivity,0.926).
Discussion
We detected significantly lower serum BDNF and higher serum p-mTOR levels in treatment naive adolescents during a major depressive episode and with a history of childhood trauma compared to healthy controls. In the ROC curve analysis, serum BDNF and p-mTOR levels could be effective as biomarkers for distinguishing major depressive episodes in adolescents with childhood trauma from healthy controls. These could reveal an association between BDNF and p-mTOR in adolescents with major depression associated with childhood trauma. Although BDNF, mTOR have been respectively revealed in MDD, as a key signaling pathway, mTOR has never been validated in adolescents with major depressive episodes associated with childhood trauma.
Before, low BDNF expression in MDD peripheral blood has been repeatedly shown, which even was expected to predict the recurrence of MDD.23,24 In addition, single nucleotide polymorphism of BDNF is associated with treatment-resistant depression (TRD).25 So, it is clear that BDNF is involved in the pathogenesis of MDD. Intriguingly, BDNF Val66Met polymorphism is also thought as an independent risk factor for MDD suicide attempts.15,16 Early Cognitive behavioral therapy (CBT) intervention is revealed to reduce suicidal thoughts and self-injury in young people;26,27 Serum BDNF levels increased after long-term CBT intervention.28 The effect of CBT was thought to be depend on the BDNF Val66Met polymorphism.29 It is worth noting that improvements in BDNF levels and working memory may be identified as markers of ketamine’s anti-suicide effects.30,31 Increased mTOR activity has also been reported in PFC/BA9 in MDD deaths from suicide.10 In animal models, rapamycin, an inhibitor of the mTOR pathway, has been reported to have antidepressant activity.32 Our study also confirmed high p-mTOR and low BDNF levels, especially in adolescents with suicidal behavior or self-injury.
Trauma exposure is a key risk factor for recurrent MDD.33 In multiracial American adults, suicidal ideation is associated with interpersonal violence and childhood abuse.34 Childhood trauma may lead to significant clinical heterogeneity of MDD.35 Childhood trauma and cortisol levels may be mediators of antidepressant response at different treatment stages.36 Astonishingly, the mTOR signaling pathway has also been shown to be involved in stress-related disorders. Low p-mTOR level was reported in animal model of maternal separation; while RNA and Assay for Transposase Accessible Chromatin (ATAC) sequencing revealed that monocytes from stressed mice and humans showed activation of mTOR.7,37 BDNF was also associated with stress-related disorders. Under long-term stress, increased glucocorticoids enter the hippocampus and activate the Mitogen-Activated Protein Kinase (MAPK) pathway through Tropomyosin receptor kinase B (TrkB) phosphorylation, enhancing the formation of negative memories.38,39 Glucocorticoid stress hormone acts on Glucocorticoid Receptor (GR) and interacts with BDNF Val66Met polymorphism to determine hippocampal gated fear and spatial memory.40 Previous studies have shown that childhood trauma is more likely to lead to negative attention bias.41 This suggests that BDNF may be involved in negative attention bias caused by trauma in childhood. While the mTOR inhibitor rapamycin, acting alone or in combination with the cannabinoid CB1/2 receptor, may diminish reactivated emotional memories, which have been thought a potential therapeutic strategy for traumatic memory-related disorders.42
In addition, as the most prominent neurotrophic factor, BDNF could activate mTORC1 and enhance protein synthesis involving in dendrite formation, axon extension during brain development.43 Interestingly, Ketamine could directly and rapidly activate mammalian target protein complex of rapamycin 1 (mTORC1), enhance brain-derived neurotrophic factor (BDNF) -mTOR pathway, and promote the rapid release of BDNF in the brain.44,45 In previous studies, BDNF could improve depressive symptoms by down-regulating the PI3K-AKT-mTOR pathway and up-regulating autophagy.20 BDNF could mediate autophagy through the PI3K-Akt-mTOR pathway.20 Pharmacological enhancement of autophagy may have a stabilizing effect on mood.46 In the study, the expression of serum BDNF was significantly lower, while p-mTOR was significantly increased, the correlation between BDNF, p-mTOR and autophagy cannot be excluded.
This study only detected the levels of serum BDNF and p-mTOR, which not fully explain the central system, and we only focus on serum BDNF and p-mTOR, which could not further elucidate the regulatory relationship and the signal pathways involved. Secondly, this study only included adolescents with MDD who experienced childhood trauma, and healthy controls, we did not include adolescents with MDD without childhood trauma. What’ more, we have not completed the follow-up of the MDD group, then the changes of serum BDNF and mTOR after treatment can be detected to better explain the relationship between the two. In the future, whether BDNF, p-mTOR may be key molecule in autophagy involving in adolescent MDD, as well as the latter in traumatic memory, should be further explore. Lastly, due to illness, these adolescents are unable to learn normally, resulting in different years of education than healthy controls. Obviously, our sample size is small. Next, we would continue to complete the enrollment of the study and healthy group, the therapeutic follow-up of the MDD group, and the retest of BDNF and p-mTOR levels after treatment.
Serum BDNF and p-mTOR may be involved in major depressive disorder in adolescents with childhood trauma, especially those with suicide and self-injury.
Abbreviations
MDD, Major depressive disorder; BDNF, Brain-derived neurotrophic factor; mTOR, mammalian target of rapamycin; MS, maternal separation; CDI, Children’s Depression Inventory; CTQ-SF, Childhood Trauma Questionnaire-Short Form; CDRS-R, Children’s depression Rating Scale-Revision; GR. Glucocorticoid receptor; MAPK. Mitogen activated protein kinase; PFC, Prefrontal cortex; BA9, Brodmann area 9; CBT, Cognitive behavioral therapy; CB1, Cannabinoid receptor 1.
Data Sharing Statement
All data have been attached to the below link: https://data.mendeley.com/datasets/x8h8zzvcb2/1.
Ethics Approval and Consent to Participate
The experimental protocol was developed in accordance with the ethical guidelines of the Declaration of Helsinki and has been approved by the Ethics Committee of the People’s Hospital of Chongqing Liang jiang New Area ((2023) No. 22). Informed consent was signed by all participants and signed by the minor’s guardian.
Acknowledgments
The authors thank all of the participants to this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by Chongqing Science and Health Joint Medical Research Project (Grant No. 2022QNXM028), China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Danese A, Smith P, Chitsabesan P, Dubicka B. Child and adolescent mental health amidst emergencies and disasters. Br J Psychiatry. 2020;216(3):159–162. doi:10.1192/bjp.2019.244
2. Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–191. doi:10.1016/j.tins.2008.01.004
3. Vares EA, Salum GA, Spanemberg L, et al. Childhood trauma and dimensions of depression: a specific association with the cognitive domain. Braz J Psychiatry. 2015;38(2):127–134. doi:10.1590/1516-4446-2015-1764
4. Tunnard C, Rane LJ, Wooderson SC, et al. The impact of childhood adversity on suicidality and clinical course in treatment-resistant depression. J Affect Disord. 2014;152-154:122–130. doi:10.1016/j.jad.2013.06.037
5. Williams LM, Debattista C, Duchemin AM, Schatzberg AF, Nemeroff CB. Childhood trauma predicts antidepressant response in adults with major depression: data from the randomized international study to predict optimized treatment for depression. Transl Psychiatry. 2016;6(5):e799. doi:10.1038/tp.2016.61
6. Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J. Childhood and adolescent depression: a review of the past 10 years. Part II. J Am Acad Child Adolesc Psychiatry. 1996;35(12):1575–1583. doi:10.1097/00004583-199612000-00008
7. Wang A, Zou X, Wu J, et al. Early-life stress alters synaptic plasticity and mtor signaling: correlation with anxiety-like and cognition-related behavior. Front Genet. 2020;11:590068. doi:10.3389/fgene.2020.590068
8. Kambali MY, Anshu K, Kutty BM, Muddashetty RS, Laxmi TR. Effect of early maternal separation stress on attention, spatial learning and social interaction behaviour. Exp Brain Res. 2019;237(8):1993–2010. doi:10.1007/s00221-019-05567-2
9. Jernigan CS, Goswami DB, Austin MC, et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(7):1774–1779. doi:10.1016/j.pnpbp.2011.05.010
10. Salort G, Hernández-Hernández E, García-Fuster MJ, García-Sevilla JA. Regulation of cannabinoid CB(1) and CB(2) receptors, neuroprotective mTOR and pro-apoptotic JNK1/2 kinases in postmortem prefrontal cortex of subjects with major depressive disorder. J Affect Disord. 2020;276:626–635. doi:10.1016/j.jad.2020.07.074
11. Abelaira HM, Réus GZ, Ignácio ZM, et al. Ketamine Exhibits Different Neuroanatomical Profile After Mammalian Target of Rapamycin Inhibition in the Prefrontal Cortex: the Role of Inflammation and Oxidative Stress Mol Neurobiol. 2017;54(7):5335–5346.
12. Numakawa T, Richards M, Nakajima S, et al. The role of brain-derived neurotrophic factor in comorbid depression: possible linkage with steroid hormones, cytokines, and nutrition. Front Psychiatry. 2014;5:136. doi:10.3389/fpsyt.2014.00136
13. Zhang JC, Wu J, Fujita Y, et al. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol. 2014;18(4);1.
14. Jia J, Le W. Molecular network of neuronal autophagy in the pathophysiology and treatment of depression. Neurosci Bull. 2015;31(4):427–434. doi:10.1007/s12264-015-1548-2
15. Youssef MM, Underwood MD, Huang YY, et al. Association of BDNF val66Met polymorphism and brain BDNF levels with major depression and suicide. Int J Neuropsychopharmacol. 2018;21(6):528–538. doi:10.1093/ijnp/pyy008
16. Schenkel LC, Segal J, Becker JA, Manfro GG, Bianchin MM, Leistner-Segal S. The BDNF Val66Met polymorphism is an independent risk factor for high lethality in suicide attempts of depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):940–944. doi:10.1016/j.pnpbp.2010.04.023
17. Lee BH, Kim YK. Potential peripheral biological predictors of suicidal behavior in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):842–847. doi:10.1016/j.pnpbp.2010.08.001
18. Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60(8):804–815. doi:10.1001/archpsyc.60.8.804
19. Pandey GN, Ren X, Rizavi HS, Conley RR, Roberts RC, Dwivedi Y. Brain-derived neurotrophic factor and tyrosine kinase B receptor signalling in post-mortem brain of teenage suicide victims. Int J Neuropsychopharmacol. 2008;11(8):1047–1061. doi:10.1017/S1461145708009000
20. Chen A, Xiong LJ, Tong Y, Mao M. Neuroprotective effect of brain-derived neurotrophic factor mediated by autophagy through the PI3K/Akt/mTOR pathway. Mol Med Rep. 2013;8(4):1011–1016. doi:10.3892/mmr.2013.1628
21. Kovacs M. The Children’s Depression, Inventory (CDI). Psychopharmacol Bull. 1985;21(4):995–998.
22. Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry. 1994;151(8):1132–1136.
23. Teng Z, Wang L, Li S, et al. Low BDNF levels in serum are associated with cognitive impairments in medication-naïve patients with current depressive episode in BD II and MDD. J Affect Disord. 2021;293:90–96. doi:10.1016/j.jad.2021.06.018
24. Liu X, Li P, Ma X, Zhang J, Zhang Y. Association between plasma levels of BDNF and GDNF and the diagnosis, treatment response in first-episode MDD. J Affect Disord. 2022;315:190–197. doi:10.1016/j.jad.2022.07.041
25. Santos M, Lima L, Carvalho S, et al. The impact of BDNF, NTRK2, NGFR, CREB1, GSK3B, AKT, MAPK1, MTOR, PTEN, ARC, and SYN1 genetic polymorphisms in antidepressant treatment response phenotypes. Int J Mol Sci. 2023;24(7):6758. doi:10.3390/ijms24076758
26. Asarnow JR, Hughes JL, Babeva KN, Sugar CA. Cognitive-Behavioral Family Treatment for Suicide Attempt Prevention: a Randomized Controlled Trial. J Am Acad Child Adolesc Psychiatry. 2017;56(6):506–514. doi:10.1016/j.jaac.2017.03.015
27. Weinstein SM, Cruz RA, Isaia AR, Peters AT, West AE. Child- and family-focused cognitive behavioral therapy for pediatric bipolar disorder: applications for suicide prevention. Suicide Life Threat Behav. 2018;48(6):797–811. doi:10.1111/sltb.12416
28. Kobayashi K, Shimizu E, Hashimoto K, et al. Serum brain-derived neurotrophic factor (BDNF) levels in patients with panic disorder: as a biological predictor of response to group cognitive behavioral therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(5):658–663. doi:10.1016/j.pnpbp.2005.04.010
29. Peters RB, Xavier J, Mondin TC, et al. BDNF Val66Met polymorphism and resilience in major depressive disorder: the impact of cognitive psychotherapy. Braz J Psychiatry. 2020;43(1):22–28. doi:10.1590/1516-4446-2019-0726
30. Chen X, Wang M, Hu Y, et al. Working memory associated with anti-suicidal ideation effect of repeated-dose intravenous ketamine in depressed patients. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):431–438. doi:10.1007/s00406-020-01221-z
31. Grunebaum MF, Ellis SP, Keilp JG, et al. Ketamine versus midazolam in bipolar depression with suicidal thoughts: a pilot midazolam-controlled randomized clinical trial. Bipolar Disord. 2017;19(3):176–183. doi:10.1111/bdi.12487
32. De Berardis D, Fornaro M, Valchera A, et al. Eradicating suicide at its roots: preclinical bases and clinical evidence of the efficacy of ketamine in the treatment of suicidal behaviors. Int J Mol Sci. 2018;19(10):2888. doi:10.3390/ijms19102888
33. Mundy J, Hübel C, Gelernter J, et al. Psychological trauma and the genetic overlap between posttraumatic stress disorder and major depressive disorder. Psychol Med. 2021;52(16):1–10. doi:10.1017/S0033291721000830
34. Beristianos MH, Maguen S, Neylan TC, Byers AL. TRAUMA EXPOSURE AND RISK OF SUICIDAL IDEATION AMONG ETHNICALLY DIVERSE ADULTS. Depress Anxiety. 2016;33(6):495–501. doi:10.1002/da.22485
35. Thorp JG, Gerring ZF, Colodro-Conde L, et al. The association between trauma exposure, polygenic risk and individual depression symptoms. Psychiatry Res. 2023;321:115101. doi:10.1016/j.psychres.2023.115101
36. Ju Y, Wang M, Lu X, et al. The effects of childhood trauma on the onset, severity and improvement of depression: the role of dysfunctional attitudes and cortisol levels. J Affect Disord. 2020;276:402–410. doi:10.1016/j.jad.2020.07.023
37. Barrett TJ, Corr EM, Van Solingen C, et al. Chronic stress primes innate immune responses in mice and humans. Cell Rep. 2021;36(10):109595. doi:10.1016/j.celrep.2021.109595
38. Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry. 2014;19(7):791–800. doi:10.1038/mp.2013.105
39. Notaras M, van den Buuse M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol Psychiatry. 2020;25(10):2251–2274. doi:10.1038/s41380-019-0639-2
40. Notaras M, Hill R, Gogos JA, van den Buuse M. BDNF Val66Met genotype determines hippocampus-dependent behavior via sensitivity to glucocorticoid signaling. Mol Psychiatry. 2016;21(6):730–732. doi:10.1038/mp.2015.152
41. Flechsenhar A, Seitz KI, Bertsch K, Herpertz SC. The association between psychopathology, childhood trauma, and emotion processing. Psychol Trauma. 2022;16(Suppl 1):S190–S203. doi:10.1037/tra0001261
42. Zubedat S, Akirav I. The involvement of cannabinoids and mTOR in the reconsolidation of an emotional memory in the hippocampal-amygdala-insular circuit. Eur Neuropsychopharmacol. 2017;27(4):336–349. doi:10.1016/j.euroneuro.2017.01.011
43. Moya-Alvarado G, Tiburcio-Felix R, Ibáñez MR, et al. BDNF/TrkB signaling endosomes in axons coordinate CREB/mTOR activation and protein synthesis in the cell body to induce dendritic growth in cortical neurons. Elife. 2023;1:12.
44. Li YF. A hypothesis of monoamine (5-HT) - glutamate/GABA long neural circuit: aiming for fast-onset antidepressant discovery. Pharmacol Ther. 2020;208:107494. doi:10.1016/j.pharmthera.2020.107494
45. Xu S, Yao X, Li B, et al. Uncovering the underlying mechanisms of ketamine as a novel antidepressant. Front Pharmacol. 2021;12:740996. doi:10.3389/fphar.2021.740996
46. Kara NZ, Flaisher-Grinberg S, Anderson GW, Agam G, Einat H. Mood-stabilizing effects of rapamycin and its analog temsirolimus: relevance to autophagy. Behav Pharmacol. 2018;29(4):379–384. doi:10.1097/FBP.0000000000000334
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Alleviating Excessive Worries Improves Co-Occurring Depression and Pain in Adolescent and Young Adult Cancer Patients: A Network Approach
Li W, Xu Y, Luo X, Wen Y, Ding K, Xu W, Garg S, Yang Y, Sun H
Neuropsychiatric Disease and Treatment 2022, 18:1843-1854
Published Date: 25 August 2022
Erroneous Thought in Inpatients with Major Depressive Disorder: The Role of Psychological Trauma During Childhood and Adulthood
Chiu CD, Chou LS, Hsieh YC, Lin CH, Li DJ
Neuropsychiatric Disease and Treatment 2023, 19:337-348
Published Date: 5 February 2023
Health-Related Behaviors and Psychological Status of Adolescent Patients with Atopic Dermatitis: The 2019 Korea Youth Risk Behavior Web-Based Survey
Park JH, Prochnow T, Chang J, Kim SJ
Patient Preference and Adherence 2023, 17:739-747
Published Date: 18 March 2023
The Effect of Mental Health Status and Family Function on Nonsuicidal Self-Injury: A Longitudinal Analysis of Chinese Children and Adolescents
Chen Y, Hu R, Xu X, Hong B, Zhang J, Jia P, Zhao L
Psychology Research and Behavior Management 2023, 16:4491-4500
Published Date: 2 November 2023
Synapse-Related Serum and P300 Biomarkers Predict the Occurrence of Mild Cognitive Impairment in Depression
Xue Z, Zhu X, Wu W, Zhu Y, Xu Y, Yu M
Neuropsychiatric Disease and Treatment 2024, 20:493-503
Published Date: 5 March 2024




