Back to Journals » Cancer Management and Research » Volume 17
Activating Transcription Factor 5 Promotes Tumorigenic Capability in Cervical Cancer Through the Wnt/β-Catenin Signaling Pathway
Authors Shi F, Wei Y, Huang Y, Yao D
Received 20 September 2024
Accepted for publication 18 January 2025
Published 24 January 2025 Volume 2025:17 Pages 131—143
DOI https://doi.org/10.2147/CMAR.S496925
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Fengjuan Shi,1,2 Yumei Wei,2 Yingmei Huang,2 Desheng Yao1
1Department of Gynecologic Oncology, Guangxi Medical University Cancer Hospital, Nanning, Guangxi, People’s Republic of China; 2Department of Gynecology, the Fifth Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China
Correspondence: Desheng Yao, Email [email protected]
Purpose: Cervical cancer is the fourth leading cause of cancer-related death in women. Furthermore, owing to its significant risk of recurrence or metastasis, the overall prognosis of patients with cervical cancer remains poor. Activating transcription factor 5 (ATF5) plays a crucial role in cell proliferation, survival, and apoptosis, and has been implicated in the progression of various types of cancer. However, the biological function and precise mechanism of ATF5 in cervical cancer remain unclear. This study, aimed to explore the function of ATF5 and its potential mechanisms in cervical cancer.
Patients and Methods: Quantitative real-time PCR, Western blot and immunohistochemistry were used to detect the expression of ATF5 in cervical cancer tissues and cell lines. Knockdown ATF5 expression in cervical cancer cell lines was constructed using lentivirus-mediated shRNA to explore the role of ATF5 in cervical cancer through cell viability, transwell, and wound healing experiments. The expression of Wnt3a and β-catenin were investigated using quantitative real-time PCR and Western blot.
Results: ATF5 was overexpressed in cervical cancer, and upregulation of ATF5 expression was associated with a poor prognosis. ATF5 knockdown inhibited the proliferation, migration and invasion abilities of cervical cancer cells. Furthermore, the downregulation of ATF5 led to the suppression of Wnt3a and β-catenin expression, which are key molecules in the Wnt/β-catenin signaling pathway.
Conclusion: ATF5 promotes tumorigenic capability in cervical cancer through the Wnt/β-catenin signaling pathway. ATF5 may be a potential prognostic biomarker and therapeutic target in the management of cervical cancer.
Keywords: activating transcription factor 5, cervical cancer, Wnt/β-catenin signaling pathway, tumorigenicity, prognosis
Introduction
Cervical cancer is the fourth leading cause of cancer-related death in women, with approximately 604,000 new cases and 342,000 deaths worldwide in 2020.1 Most new cases and deaths due to cervical cancer occur in low-income countries and territories. Additionally, the disease also tends to occur in younger patients. In the USA, cervical cancer is the second leading cause of cancer-related death among women aged 20–39 years.2 In clinical practice, surgery, radiotherapy, and chemotherapy are the main treatment modalities for cervical cancer. However, the overall prognosis of patients with recurrent or metastatic disease remains poor.3 Despite advances in research, cervical cancer continues to pose a substantial burden and remains a serious threat to female health. Therefore, comprehensive studies of the molecular mechanism underlying cervical cancer tumorigenicity are imperative for the development of effective therapeutic approaches against this disease.
The Wnt signaling pathway is one of the most studied signaling pathways and has been implicated in multiple biological processes, including cell differentiation, proliferation, survival and migration.4,5 The Wnt signaling pathways are broadly categorized into two types: canonical pathway and non-canonical pathways. The Wnt/β-catenin signaling is classified as a canonical pathway,6 and plays a crucial role in the development, tissue homeostasis, angiogenesis, and development of cancer.7 In recent studies, there is growing evidence that the Wnt//β-catenin signaling pathway affects the tumorigenicity and metastasis in cervical cancer.8 Excessive activation of the Wnt//β-catenin signaling pathway leads to cervical cancer progression and chemoresistance.9–11 Hyperactivated Wnt//β-catenin signaling induces EMT and angiogenesis in cervical cancer.12,13 The deregulation of Wnt/β-catenin signaling is associated with worse outcomes in patients with cervical cancer.7
Activating transcription factor 5 (ATF5) also known as ATFx, is a member of the activating transcription factor/cAMP response element binding protein (ATF/CREB) family.14 ATF5, which has a basic-region leucine zipper (bZIP), binds the cAMP-responsive element (CRE) that forms homodimers.15 Based on the dimerization properties of its leucine zipper, ATF5 is classified as a subfamily of ATF4.16 The human ATF5 gene is located on band q13.33 of chromosome 19, and the ATF5 protein consists of 282 amino acids.17 ATF5 plays important roles in cell proliferation, survival, apoptosis and inhibition of neural differentiation.18–21 Its expression is highly upregulated in various types of cancer, such as glioblastoma, lung cancer, breast cancer, rectal cancer, and ovarian cancer, among others.22 This suggests that ATF5 may have an oncogenic role in these cancer types. Numerous studies have examined the effectiveness of anti-ATF5 in cancer treatment.22 ATF4 promotes the Wnt//β-catenin signaling in lung cancer and bone disorder.23,24 ATF5 activates the Wnt//β-catenin signaling in bladder cancer and gastric cancer.25,26 However, little is known about the role and biological function of ATF5 in the treatment of cervical cancer. Furthermore, its molecular mechanism is also largely unclear.
In this study, we investigated the biological function and underlying molecular mechanism of ATF5 against cervical cancer in vitro. This research seeks to establish a theoretical foundation and identify a novel therapeutic target for the treatment of cervical cancer.
Materials and Methods
Clinical Data and Specimens
A total of 83 paraffin-embedded cervical cancer tissues were obtained from Guangxi Medical University Cancer Hospital and the Fifth Affiliated Hospital of Guangxi Medical University in Nanning, China. Clinical data were also collected, including age, clinic stage, pathological types, tumor grades, tumor sizes, lymph node metastasis, and lymphovascular space invasion. All patients were followed-up until death or until May 2024. Cancerous tissues and adjacent normal tissues were obtained from 12 patients with cervical cancer who underwent radical hysterectomies. None of the patients underwent any treatment preoperatively. The study was approved by the Ethics Committee of the Fifth Affiliated Hospital of Guangxi Medical University.
Cell Culture
Cell lines, including SiHa, C-33A, HeLa and Ect1/E6E7, were purchased from the American Type Culture Collection (ATCC, USA). The cells were cultured in Dulbecco’s modified Eagle medium (DMEM; VivaCell, Shanghai, China) supplemented with 10% fetal bovine serum (FBS; HyCyte, Suzhou, China) and penicillin/streptomycin (100 U/mL). The cells were incubated at 37 °C and 5% carbon dioxide (CO2) environment.
RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated from cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. Afterwards, cDNA was synthesized using Hifair® III 1st Strand cDNA Synthesis SuperMix for qPCR (gDNA digester plus) (Yeasen, Shanghai, China). RT-PCR was then performed using Hieff UNICON® Universal Blue qPCR SYBR Green Master Mix (Yeasen, Shanghai, China) and primers to amplify the cDNA. To normalize the samples, β-actin was selected as an internal reference. Expressions were calculated using the 2^−ΔΔCt method, whereby Ct denotes each transcript’s threshold cycles, ΔCt = Ct (target gene) –Ct (reference gene), ΔΔCt = ΔCt (test sample) –ΔCt (control sample). The primer sequences were as follows:
ATF5 Forward primer: 5′-AAGTCGGCGGCTCTGAGGTA- 3′
ATF5 Reverse primer: 5′-GGACTCTGCCCGTTCCTTCA- 3′
β-actin Forward primer: 5′- CCTGGCACCCAGCACAAT- 3′
β-actin Reverse primer: 5′- GGGCCGGACTCGTCATAC- 3′
Wnt3a Forward primer: 5′-CCCCACTCGGATACTTCTT- 3′
Wnt3a Reverse primer: 5′-GCCAATCTTGATGCCCT- 3′
β-catenin Forward primer: 5′-CAAGTGGGTGGTATAGAGGCT- 3′
β-catenin Reverse primer: 5′-TGGGATGGTGGGTGTAAGAG- 3′
Western Blot (WB) Assay
Protein was extracted using RIPA buffer (Solarbio, Beijing, China) and plus 1% protease inhibitor (Solarbio, Beijing, China). Protein concentration was quantified using the BCA assay kit (Solarbio, Beijing, China). Proteins from cell lysates were denatured at 100 °C for 5–10 min. Afterwards, the proteins were separated by SDS-PAGE and then transferred to a PVDF membrane. The membranes were incubated with identified primary antibodies at 4 °C overnight, followed by incubation with HRP-conjugated secondary antibodies. Immunoreactive bands were imaged using ECL reagents (Beyotime, Shanghai, China). The antibodies used were as follows: anti-ATF5 (ab184923, Abcam, UK, 1:2000), anti-β-actin (A2228, Sigma-Aldrich, USA, 1:3000), anti-β-catenin (51067-2-AP, Proteintech, Wuhan, China, 1:5000) and anti-Wnt-3a (822111, ZenBio, Chengdu, China, 1:500).
Immunohistochemistry (IHC)
The sections were deparaffinized by heating at 60 °C for 2 h, followed by incubation in xylene for 3 min each. Subsequent incubations were performed in 95% and 85% ethanol for 2 min each. The specimens were then microwaved in sodium citrate buffer (pH 9) for heat-mediated antigen retrieval. A 3% hydrogen peroxide in methanol solution was used to block endogenous peroxidase activity. The sections were then transferred into phosphate-buffered saline. The slides were incubated at 37 °C for 70 min with a primary antibody (anti-ATF5: R23549, ZenBio, Chengdu, China, 1:600), followed by six washes in phosphate-buffered saline (PBS). After 20 min of incubating the dilute secondary antibody at 37 °C, visualization was achieved using DAB reagent according to the manufacturer’s protocol. Hematoxylin was used for counterstaining.
ATF5 immunostaining was classified into high and low expression based on staining indices (SI). SI = staining intensity score × staining cell proportions. The staining intensity score was assessed as follows: 0, negative; 1, weak; 2, moderate; 3, strong. The staining cell proportions was assessed as follows: 0, < 5%; 1, 5–25%, 2, 25–50%; 3, > 50%. Samples with SI ≥4 were defined as having high expression, while those with SI <4 were regarded as having low expression. Two pathologists who had been blinded to the patients’ information independently evaluated the immunostaining results.
Retroviral Infection and Transfection
Lentiviruses expressing short hairpin RNA targeting ATF5 (shATF5) and scrambled shRNA (shNC) were purchased from GenePharma (Suzhou, China). SiHa and C-33A cells were infected with shATF5 and shNC lentiviral particles, mixed with 1 μg/mL polybrene, to increase the infection efficiency. Stable cell lines were selected with 2 μg/mL puromycin and confirmed using qRT-PCR and WB. The sequences of shRNA were as follows:
shNC: 5′-TTCTCCGAACGTGTCACGT- 3′
shATF5: 5′-GCTGGGATGGCTCGTAGACTA- 3′
Cell Viability Assay
Cell viability was investigated using Cell Count Kit-8 (CCK-8) analysis. Cells were seeded into 96-well plates (3 x 103 cells/well). Afterwards, 10 μL of CCK-8 (Vazyme, Nanjing, China) solution was added to each well after the cells had been grown for 0, 24, 48, and 72 h, then incubated at 37 °C and 5% CO2 environment for 2 h. Finally, optical density (OD) was measured using a microplate reader at a wavelength of 450 nm.
Transwell Assay
The cells were collected and resuspended in a serum-free media. Afterwards, 5×104 cells (migration), and 8×104 cells (invasion) were seeded into the upper chamber. The lower chamber was filled with 500-μL medium containing 10% FBS. After incubation at 37 °C in a 5% CO2 environment for 12 h (SiHa cells) or 24 h (C-33A cells), cells were washed with PBS, and fixed with 4% formaldehyde for 20 min. The cells remaining on the upper membrane were then removed. Afterwards, 0.1% crystal violet solution was used to stain cells remaining on the lower membrane. Colonies were visualized using a microscope. The number of stained cells was counted using Image J 1.8.0 software (https://imagej.nih.gov/ij/).
Wound Healing Assay
A linear wound was made using a 200-μm sterile plastic pipette tip when the cells had grown to 95% confluence. The cells were then washed three times with PBS. After adding serum-free media, the cells were placed in an incubator at 37 °C with 5% CO2 environment for 48 h. Images were then taken at time 0, 24, and 48 h. Scratch areas were measured using Image J 1.8.0 software and presented as a percentage of scratch area at 0 h.
Bioinformatics Analysis
Cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC) RNA sequencing transcriptome was downloaded from The Cancer Genome Atlas database (TCGA: https://portal.gdc.cancer.gov/). Cervix RNA sequencing transcriptome was downloaded from Genotype-Tissue Expression databases (GTEx: http://commonfund.nih.gov/GTEx/). The R DESeq2 package was used for differential analysis, and the clusterProfiler package was used for Gene set enrichment analysis (GSEA). The median ATF5 gene expression was considered as the cut-off point to divide the cervical cancer samples into the ATF5 high and low groups.
Statistical Analysis
All experiments were repeated three times. Data analyses were performed using GraphPad Prism 8.0.1 (GraphPad Software, La Jolla, CA, USA) and R 4.3.2 (http://www.r-project.org/). Comparison of means between the two groups was performed using unpaired Student’s t-test. One-way ANOVA was used to compare the differences between multiple groups. The chi-square test was used to compare differences between the classification data groups. Survival curves were generated using the Kaplan-Meier method and compared using the long-rank test. P-value < 0.05 was considered statistically significant.
Results
ATF5 Is Overexpressed in Cervical Cancer
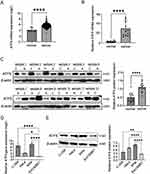
To investigate the expression of ATF5 in cervical cancer, we obtained RNA sequence transcriptome data from TCGA and GTEx databases, comprising 306 cases of cervical cancer and 13 cases of normal cervix. After performing data normalization, we extracted the expression of matrix of ATF5. ATF5 mRNA expression was overexpressed in cervical cancer (Figure 1A). Subsequently, we detected ATF5 mRNA and ATF5 protein levels in 12 cases of cervical cancer. Compared to adjacent normal tissues, ATF5 mRNA and ATF5 protein expression in cancer tissues were significantly increased (Figure 1B and C, Figure S1A). In cell lines, cervical cancer cell lines C-33A, HeLa, and SiHa showed higher expression compared to normal cervix cell line Ect1/E6E7 (Figure 1D and E, Figure S1B). These data indicated that ATF5 is overexpressed in cervical cancer compared to that in the normal cervix.
ATF5 Overexpression Correlates With Poor Prognosis in Cervical Cancer
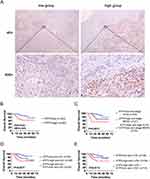
To assess the correlation between ATF5 expression and clinicopathological characteristics of cervical cancer, 83 specimens from patients with cervical cancer were stained using IHC. The samples were classified into low (n=43) and high (n=40) groups based on their ATF5 expression levels (Figure 2A). Statistical analysis revealed that ATF5 expression was associated with clinical stage, lymph node metastasis (LN) and lymphovascular space invasion (LVSI). However, ATF5 expression was not correlated with age, pathological types, tumor grade, or tumor size (Table 1). Survival analysis demonstrated that patients with higher ATF5 expression had a lower overall survival time (Figure 2B). Additionally, compared to other patients with cervical cancer, patients with high ATF5 expression and advanced stage, LN positive, or LVSI positive had a worse prognosis (Figure 2C-E). These results indicated that ATF5 overexpression was associated with a poor prognosis in cervical cancer.
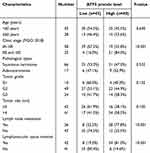 |
Table 1 Correlation Analysis of ATF5 Expression With Clinicopathological Features of Patients With Cervical Cancer |
ATF5 Enhances the Proliferation of Cervical Cancer Cells
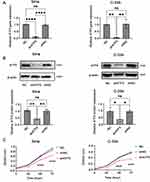
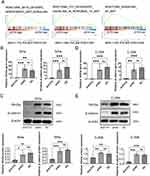
To further validate the biological function of ATF5, ATF5 expression was knocked down in SiHa and C-33A cells using lentivirus-mediated shRNA and confirmed using qRT-PCR (Figure 3A) and WB (Figure 3B, Figure S2A). Afterwards, we performed CCK-8 assays to analyze the influence of ATF5 on cervical cancer cell growth. At each time point, the OD values of the shATF5 group were lower than those of the shNC and NC groups in SiHa and C-33A cells (Figure 3C). The results showed that ATF5 knockdown inhibited the proliferation of cervical cancer cells.
ATF5 Promotes the Migration and Invasion of Cervical Cancer Cells
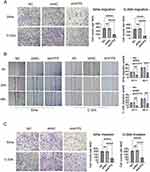
Transwell and wound healing experiments were performed to investigate the migration and invasion abilities of cervical cancer cells following ATF5 knockdown. The transwell migration assay indicated that the migration rates of the shATF5 group were lower than those of the shNC and NC groups in SiHa and C-33A cells (Figure 4A). The observation was further confirmed by the wound healing assay (Figure 4B). In the transwell invasion assay, the shATF5 group exhibited a reduced number of invasive cells compared to the shNC and NC groups in both cell lines (Figure 4C). Our findings suggest that ATF5 enhanced the migration and invasion ability of cervical cancer cells.
ATF5 Activates the Wnt/β-Catenin Signaling Pathway in Cervical Cancer Cells
To explore the mechanisms of ATF5 in cervical cancer, GSEA analysis was performed using the TCGA-CESC datasets. We chose the median ATF5 gnen expression as the cut-off point to divide the samples into the ATF5 high group and low group. The results showed that ATF5 expression was positively correlated with Wnt-related gene signatures (Figure 5A), implying that ATF5 stimulated the Wnt/β-catenin signaling pathway in cervical cancer. Furthermore, we detected the expression of Wnt3a and β-catenin, which are key molecules in the Wnt/β-catenin signaling pathway. QRT-PCR and WB analyses confirmed that the downregulation of ATF5 reduced the expression of Wnt3a and β-catenin both in SiHa cells (Figure 5B and C, Figure S2B) and C-33A cells (Figure 5D and E, Figure S2C). These data confirmed that the upregulation of ATF5 could activate the Wnt/β-catenin signaling pathway.
Discussion
ATF5 is a cellular pro-survival transcription factor that is well-known for its role in transcriptional activation of various genes, which in turn promotes the progression of various types of cancer.22 Available evidence suggests that ATF5 is upregulated in esophageal cancer and can facilitate the proliferation, migration, and invasion abilities of esophageal cancer cell lines.27 ATF5 has also been found to be highly expressed in malignant glioma; moreover, the inhibition of ATF5 expression induces apoptosis in rats and human malignant glioma cells.28,29 The role of ATF5 in breast cancer involves enhancing mammary tumor cell proliferation, migration, and overall aggressiveness, thereby promoting mammary tumor growth.30 Additionally, previous studies have shown that ATF5 enhanced the invasiveness of various cancer cell lines, including HeLa cell.31 However, its precise role in cervical cancer is unclear. In our study, we confirmed that ATF5 was upregulated in cervical cancer tissues and cell lines compared to normal cervix. The expression of ATF5 was positively related to clinical stage, LN, and LVSI. High expression of ATF5 was associated with poor prognosis in patients with cervical cancer. The combination of ATF5 with clinical stage, LN, and LVSI, may enhance the accuracy of prognostic evaluation in cervical cancer. These findings suggest that ATF5 could be a potential prognostic biomarker of cervical cancer. Furthermore, we investigated the role of ATF5 in cervical cancer. Functional studies have shown that ATF5 knockdown inhibited proliferation, migration and invasion of cervical cancer cells. These findings suggest that ATF5 plays a tumorigenic role in cervical cancer pathogenesis. Thus, ATF5 may serve as a promising therapeutic target for the management of cervical cancer.
Recent studies have confirmed that ATF5 expression is related to the progression of various types of cancers; however, the underlying mechanism is largely unknown, especially in cervical cancer. In bladder cancer, ATF5 promotes tumorigenic capability by activating the Wnt/β-catenin signaling pathway.25 In addition, ATF5 promotes cancer stemness by enhancing β-catenin nuclear translocation in gastric cancer.26 The Wnt/β-catenin signaling pathway is essential for the development of embryonic and adult tissue homeostasis.32 Abnormal activations of this pathway can facilitate cancer stem cell renewal, cell proliferation, and differentiation.33 This pathway is often abnormally stimulated in various types of malignancies, including cervical cancer.34 In our study, GSEA analysis revealed that ATF5 stimulated the Wnt/β-catenin signaling pathway. Wnt3a and β-catenin are key molecules in this pathway. Wnt3a is a Wnt ligand known to be the strongest stimulator of the Wnt/β-catenin signaling pathway. Upon binding to cell membrane receptors, the Wnt/β-catenin signaling pathway is activated.35 β-catenin is a pivotal component of the Wnt/β-catenin signaling pathway. Once activated, intracellular β-catenin is rapidly enriched and then translocated into the nucleus to regulate the expression of downstream target genes involved in cell proliferation, survival, differentiation, and migration.36 Our findings indicated that knockdown of ATF5 reduced the expression of Wnt3a and β-catenin, thereby inhibiting the transcription of downstream target genes. Thus, we conclude that ATF5 could promote tumorigenic capability partially by activating the Wnt/β-catenin pathway in cervical cancer. However, we did not conduct in vivo experiments and the number of clinical samples obtained in our study was small. Additionally, the targets that directly connect between ATF5 and the Wnt/β-catenin pathway and the detailed mechanisms thereof need to be clarified. We hope that future research will address this limitation and explore the mechanisms involved at a deeper level.
Conclusion
In summary, we found that ATF5 was overexpressed in cervical cancer, and a high level of ATF5 expression was associated with a poor prognosis. A series of vitro experiments showed that ATF5 played a tumorigenic role in the pathogenesis of cervical cancer. Additionally, we preliminarily verified the mechanism of ATF5 in cervical cancer and observed that it was closely related to the Wnt/β-catenin signaling pathway. Our findings revealed new insights into the functional role and mechanism of ATF5 in cervical cancer, which might be a potential prognostic biomarker and therapeutic target for cervical cancer.
Data Sharing Statement
Part of the data described in this manuscript can be obtained from public databases, and others are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
Studies involving human participants were reviewed and approved by the Fifth Affiliated Hospital of Guangxi Medical University (Medical Ethics No. LW2024-05). The studies complied with the Declaration of Helsinki, and were conducted in accordance with the local legislation and institutional requirements. All participants provided written informed consent to participate in this study.
Acknowledgments
We thank the laboratory of Guangxi Medical University Cancer Hospital and Mengjie Chen for their assistance with the experiments.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Disclosure
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
3. Cohen PA, Jhingran A, Oaknin A, et al. Cervical cancer. Lancet. 2019;393(10167):169–182. doi:10.1016/S0140-6736(18)32470-X
4. Jung Y, Park J. Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp. Mol. Med. 2020;52(2):183–191. doi:10.1038/s12276-020-0380-6
5. Wiese K, Nusse R, van Amerongen R. Wnt signalling: conquering complexity. Development. 2018;145(12). doi:10.1242/dev.165902
6. Grumolato L, Liu G, Mong P, et al. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24(22):2517–2530. doi:10.1101/gad.1957710
7. Zhang X, Dong N, Hu X. Wnt/β-catenin signaling inhibitors. Curr Top Med Chem. 2023;23(10):880–896. doi:10.2174/1568026623666230303101810
8. McMellen A, Woodruff ER, Corr BR, et al. Wnt signaling in gynecologic malignancies. Int J mol Sci. 2020;21(12):4272. doi:10.3390/ijms21124272
9. Zhang C, Liu L, Li W, et al. Upregulation of FAM83F by c-Myc promotes cervical cancer growth and aerobic glycolysis via Wnt/β-catenin signaling activation. Cell Death Dis. 2023;14(12):837. doi:10.1038/s41419-023-06377-9
10. Su M, Shan S, Gao Y, et al. 2-Deoxy-D-glucose simultaneously targets glycolysis and Wnt/β-catenin signaling to inhibit cervical cancer progression. IUBMB Life. 2023;75(7):609–623. doi:10.1002/iub.2706
11. Lin X, Wang F, Chen J, et al. N(6)-methyladenosine modification of CENPK mRNA by ZC3H13 promotes cervical cancer stemness and chemoresistance. Mil Med Res. 2022;9(1):19. doi:10.1186/s40779-022-00378-z
12. Wang T, Li W, Ye B, et al. FTO-stabilized lncRNA HOXC13-AS epigenetically upregulated FZD6 and activated Wnt/β-catenin signaling to drive cervical cancer proliferation, invasion, and EMT. J buon. 2021;26(4):1279–1291.
13. Prasad CB, Singh D, Pandey LK, et al. VEGFa/VEGFR2 autocrine and paracrine signaling promotes cervical carcinogenesis via β-catenin and snail. Int J Biochem Cell Biol. 2022;142:106122. doi:10.1016/j.biocel.2021.106122
14. Angelastro J, Ignatova T, Kukekov V, et al. Regulated expression of ATF5 is required for the progression of neural progenitor cells to neurons. J Neurosci. 2003;23(11):4590–4600. doi:10.1523/JNEUROSCI.23-11-04590.2003
15. Cui A, Ding D, Li Y. Regulation of hepatic metabolism and cell growth by the ATF/CREB family of transcription factors. Diabetes. 2021;70(3):653–664. doi:10.2337/dbi20-0006
16. Vinson C, Myakishev M, Acharya A, et al. Classification of human B-ZIP proteins based on dimerization properties. mol Cell Biol. 2002;22(18):6321–6335. doi:10.1128/MCB.22.18.6321-6335.2002
17. Hai T, Hartman MG. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene. 2001;273(1):1–11. doi:10.1016/S0378-1119(01)00551-0
18. Sun X, Jefferson P, Zhou Q, et al. Dominant-negative ATF5 compromises cancer cell survival by targeting CEBPB and CEBPD. mol Cancer Res. 2020;18(2):216–228. doi:10.1158/1541-7786.MCR-19-0631
19. Juliana CA, Yang J, Rozo AV, et al. ATF5 regulates beta-cell survival during stress. Proc Natl Acad Sci U S A. 2017;114(6):1341–1346. doi:10.1073/pnas.1620705114
20. Angelastro JM. Targeting ATF5 in Cancer. Trends Cancer. 2017;3(7):471–474. doi:10.1016/j.trecan.2017.05.004
21. Teske BF, Fusakio ME, Zhou D, et al. CHOP induces activating transcription factor 5 (ATF5) to trigger apoptosis in response to perturbations in protein homeostasis. mol Biol Cell. 2013;24(15):2477–2490. doi:10.1091/mbc.e13-01-0067
22. Sears TK, Angelastro JM. The transcription factor ATF5: role in cellular differentiation, stress responses, and cancer. Oncotarget. 2017;8(48):84595–84609. doi:10.18632/oncotarget.21102
23. Du J, Liu H, Mao X, et al. ATF4 promotes lung cancer cell proliferation and invasion partially through regulating Wnt/β-catenin signaling. Int J Med Sci. 2021;18(6):1442–1448. doi:10.7150/ijms.43167
24. Xu J, Hu P, Zhang X, et al. Magnesium implantation or supplementation ameliorates bone disorder in CFTR-mutant mice through an ATF4-dependent Wnt/β-catenin signaling. Bioact Mater. 2022;8:95–108. doi:10.1016/j.bioactmat.2021.06.034
25. Zhou J, Tian H, Zhi X, et al. Activating transcription factor 5 (ATF5) promotes tumorigenic capability and activates the Wnt/b-catenin pathway in bladder cancer. Cancer Cell Int. 2021;21(1):660. doi:10.1186/s12935-021-02315-x
26. Zhang P, Xiang H, Peng Q, et al. METTL14 attenuates cancer stemness by suppressing ATF5/WDR74/β-catenin axis in gastric cancer. Cancer Sci. 2024;116:112–127.
27. He F, Xiao H, Cai Y, et al. ATF5 and HIF1alpha cooperatively activate HIF1 signaling pathway in esophageal cancer. Cell Commun Signal. 2021;19(1):53. doi:10.1186/s12964-021-00734-x
28. Feldheim J, Kessler AF, Schmitt D, et al. Expression of activating transcription factor 5 (ATF5) is increased in astrocytomas of different WHO grades and correlates with survival of glioblastoma patients. Onco Targets Ther. 2018;11:8673–8684. doi:10.2147/OTT.S176549
29. Hu M, Li H, Xie H, et al. ELF1 transcription factor enhances the progression of glioma via ATF5 promoter. ACS Chem Neurosci. 2021;12(7):1252–1261. doi:10.1021/acschemneuro.1c00070
30. Ben-Shmuel S, Rashed R, Rostoker R, et al. Activating transcription factor-5 knockdown reduces aggressiveness of mammary tumor cells and attenuates mammary tumor growth. Front Endocrinol. 2017;8:173. doi:10.3389/fendo.2017.00173
31. Nukuda A, Endoh H, Yasuda M, et al. Role of ATF5 in the invasive potential of diverse human cancer cell lines. Biochem Biophys Res Commun. 2016;474(3):509–514. doi:10.1016/j.bbrc.2016.04.131
32. Liu J, Xiao Q, Xiao J, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7(1):3. doi:10.1038/s41392-021-00762-6
33. Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13(1):165. doi:10.1186/s13045-020-00990-3
34. Aghbash PS, Hemmat N, Baradaran B, et al. The effect of Wnt/β-catenin signaling on PD-1/PDL-1 axis in HPV-related cervical cancer. Oncol Res. 2022;30(3):99–116. doi:10.32604/or.2022.026776
35. He S, Lu Y, Liu X, et al. Wnt3a: functions and implications in cancer. Chin J Cancer. 2015;34(12):554–562. doi:10.1186/s40880-015-0052-4
36. Yu F, Yu C, Li F, et al. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct Target Ther. 2021;6(1):307. doi:10.1038/s41392-021-00701-5
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Low Level FLT3LG is a Novel Poor Prognostic Biomarker for Cervical Cancer with Immune Infiltration
chen L, Huang Y, Dong B, Gu Y, Li Y, Cang W, Sun P, Xiang Y
Journal of Inflammation Research 2022, 15:5889-5904
Published Date: 19 October 2022
Significance of Immunogenic Cell Death-Related Prognostic Gene Signature in Cervical Cancer Prognosis and Anti-Tumor Immunity
Jiang S, Cui Z, Zheng J, Wu Q, Yu H, You Y, Zheng C, Sun Y
Journal of Inflammation Research 2023, 16:2189-2207
Published Date: 22 May 2023
An Energy-Efficient Test and Predictive Model for Recurrence After Radiotherapy in Localized Intermediate and Advanced Cervical Cancer Were Created Using Thymidine Kinase 1 in Conjunction with Inflammatory Markers and Tumor Markers
Luo Y, Ma X
International Journal of General Medicine 2023, 16:5789-5797
Published Date: 8 December 2023
Predictive Value of Advanced Lung Cancer Inflammation Index and Development of a Nomogram for Prognosis in Patients with Cervical Cancer Treated with Radiotherapy
Yu Q, Sun Y, Zhang S, Xu X, Pi G, Jin X
Journal of Inflammation Research 2025, 18:5371-5382
Published Date: 21 April 2025