Back to Journals » International Journal of Nanomedicine » Volume 19
Adeno-Associated Virus Engineering and Load Strategy for Tropism Modification, Immune Evasion and Enhanced Transgene Expression
Authors Zhou X, Liu J, Xiao S, Liang X, Li Y , Mo F , Xin X, Yang Y, Gao C
Received 16 January 2024
Accepted for publication 21 June 2024
Published 29 July 2024 Volume 2024:19 Pages 7691—7708
DOI https://doi.org/10.2147/IJN.S459905
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Eng San Thian
Xun Zhou,1,2,* Jingzhou Liu,2,* Shuang Xiao,2,3,* Xiaoqing Liang,1,2 Yi Li,2 Fengzhen Mo,3 Xin Xin,2 Yang Yang,2 Chunsheng Gao1,2
1School of Pharmacy, Henan University, Kaifeng, People’s Republic of China; 2State Key Laboratory of Toxicology and Medical Countermeasures, Beijing Institute of Pharmacology and Toxicology, Beijing, People’s Republic of China; 3School of Pharmacy, Guangxi Medical University, Nanning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fengzhen Mo; Xin Xin, Email [email protected]; [email protected]
Abstract: Gene therapy aims to add, replace or turn off genes to help treat disease. To date, the US Food and Drug Administration (FDA) has approved 14 gene therapy products. With the increasing interest in gene therapy, feasible gene delivery vectors are necessary for inserting new genes into cells. There are different kinds of gene delivery vectors including viral vectors like lentivirus, adenovirus, retrovirus, adeno-associated virus et al, and non-viral vectors like naked DNA, lipid vectors, polymer nanoparticles, exosomes et al, with viruses being the most commonly used. Among them, the most concerned vector is adeno-associated virus (AAV) because of its safety, natural ability to efficiently deliver gene into cells and sustained transgene expression in multiple tissues. In addition, the AAV genome can be engineered to generate recombinant AAV (rAAV) containing transgene sequences of interest and has been proven to be a safe gene vector. Recently, rAAV vectors have been approved for the treatment of various rare diseases. Despite these approvals, some major limitations of rAAV remain, namely nonspecific tissue targeting and host immune response. Additional problems include neutralizing antibodies that block transgene delivery, a finite transgene packaging capacity, high viral titer used for per dose and high cost. To deal with these challenges, several techniques have been developed. Based on differences in engineering methods, this review proposes three strategies: gene engineering-based capsid modification (capsid modification), capsid surface tethering through chemical conjugation (surface tethering), and other formulations loaded with AAV (virus load). In addition, the major advantages and limitations encountered in rAAV engineering strategies are summarized.
Keywords: AAV engineering, capsid modification, surface tethering, virus load, rational design, directed evolution, machine learning
Introduction
Gene therapy refers to the introduction of exogenous normal genes into target cells to correct or compensate for diseases caused by defective and abnormal genes in order to achieve therapeutic purposes and has gained widespread attention because of its ability to treat allopathic causes.1,2 Various genetic materials, such as DNA, mRNA, small interfering RNA (siRNA), anti-sense oligonucleotide (ASO), and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (CRISPR-Cas9) systems, have been used to treat diseases. However, the clinical application of gene therapy is hindered by its immunogenicity, off-target effects, and carcinogenicity. Moreover, because genetic materials are hydrophilic, negatively charged, and easily degraded by enzymes, there is a need for delivery vehicles that effectively load gene drugs and deliver them to the site of interest. Existing gene delivery vectors can be divided into viral and non-viral delivery vectors. For example, non-viral lipid nanoparticles (LNPs), which have gained interest, exhibit good safety, biocompatibility, and high delivery efficiency. In addition, LNPs have several advantages, including the transient expression of LNP-loaded gene agents, chemical synthetic components and feasible large-scale production.3 However, LNPs have the drawbacks of limited delivery to extrahepatic tissues, temporary expression of gene agents, and passive transgene expression, which limits the application of LNPs in situations where non-liver delivery and long-term expression are required. Viral vectors have broader tissue tropism, including the brain, eyes and muscles, than nonviral vectors,4,5 and they can transduce specific cell populations by optimizing cell-specific promoters,5 as well as inducing long-term gene expression in vivo. Therefore, viral vectors are promising gene delivery vehicles. Viral vectors currently in use include lentiviruses, adenoviruses, and adeno-associated viruses (AAVs), among others.6 AAV is particularly suitable for in vivo gene delivery because of its safety, low immunogenicity, long-lasting expression, broad tropism, and ease of production.7,8 Since AAVs are non-integrating, they can only be expressed permanently in non-dividing cells over long periods of time.
AAV belongs to the genus Dependoparvovirus within the family Parvoviridae,9 and its life cycle depends on the participation of replication viruses, such as adenovirus and other auxiliary viruses. AAV can be divided into wild-type AAV and recombinant AAV according to their sources. Wild-type AAV can be integrated into AAVS1 site of human chromosome 19 at a fixed point, while recombinant AAV cannot be integrated due to the lack of two genes necessary for integration and replication. Therefore, recombinant AAV mainly exists in the form of appendages free from chromosomes and can exist in non-splinter cell for a long time. Table 1 lists the characteristics of natural AAV serotypes at present. AAV is a single-stranded DNA virus with a ~4.7 kb genome that comprises four known open reading frames, rep, cap, MAAP, and AAP, flanked by two 145 bp inverted terminal repeats (ITRs).10 The rep gene encodes for Rep78, Rep68, Rep52, and Rep40, which support viral genome replication, transcriptional regulation, and virion assembly. The cap gene encodes three viral proteins, VP1, VP2, and VP3.11 The 60 subunits of VP at a 1:1:10 ratio of VP1:VP2:VP3 form an AAV capsid with a size of 23–28 nm.5,12 The common VP3 protein forms the actual capsid and displays a core eight-stranded antiparallel β-barrel motif.13,14 A highly conserved α-helix is present between the βC and βD strands.15 The surface-exposed loops between β strands feature nine variable regions (VRI-Ⅸ), which vary among AAV serotypes. The VRs are mainly found at capsid protrusions, which are crucial for the interaction between AAVs and host cells, determining immunogenicity and receptor binding.15 Some VRs, such as VR-IV, V and VII;, are exposed on the surface of the protrusions, making them favored site for capsid engineering.16 Capsid proteins function in endosomal escape and nuclear localization13 and are responsible for tissue tropism.17,18
 |
Table 1 Properties of AAV Serotypes |
The various AAV serotypes show different tissue tropisms that involve primary receptors and co-receptors on the cell surface.5,11 AAV is endocytosed into endosomes by binding these receptors.42 The additional sequences at the N-termini of VP1 and VP2 are buried within the capsid.13,14 Once in the endosome, AAV undergoes a structural change that results in the exposure of phospholipase A2 (PLA2) domain located in the N-terminus of VP1. The activity of PLA2 is required for endosomal release and nuclear location.43,44 After AAV enters the nucleus, it releases its single-stranded genome, which is then duplicated into a double-stranded genome from which transgenes can be expressed.45 Table 1 lists the properties of current natural AAV serotypes.
Recombinant AAV (rAAV), an AAV with a genome engineered for gene therapy (Figure 1), which has been widely used in clinical trials. In 2012, the first AAV product, Glybera, which expresses lipoprotein lipase in muscle to treat lipoprotein lipase deficiency, was approved by the European Medicines Agency.46 Six AAV drugs, namely Luxturna, Zolgensma, Hemgenix, Roctavain, Elevidys and Beqvez, have been approved by the United States Food and Drug Administration (US FDA). In 2017, Luxturna, which uses AAV2 encoding RPE65 as gene therapy for Leber congenital amaurosis, was the first US FDA-approved AAV bioproduct.47 Before the approval of Luxturna, no drugs were available for the treatment of retinal dystrophy.8 In 2019 Zolgensma, an AAV9 vector from Novartis Gene Therapies, was approved by the US FDA for the treatment of type I spinal muscular atrophy in children, which is the most common single-gene disease that causes infant death.48 Hemgenix is an AAV5 vector that expresses highly active factor IX (FIX) protein variant Padua for the treatment of haemophilia B. This CSL Behring gene therapy product, originally developed by UniQure, was approved by the FDA in 2022 based on safety and efficacy studies in adult male patients with severe or moderately severe disease.49,50 Roctavian is an AAV5 vector-based gene therapy for the treatment of patients with severe hemophilia A who do not have a history of coagulation factor VIII inhibitors or detectable antibodies to the AAV5 type. On August 24, 2022, this therapy was conditionally approved by the European Medicines Agency, and on June 29, 2023, it was approved by the US FDA.51 On June 22, 2023, Sarepta announced that Elevidys, an AAVrh74 vector isolated from non-human primates, became the world’s first single-use gene therapy for Duchenne muscular dystrophy (DMD) and received FDA approval to be marketed in the United States for the treatment of independently ambulatory children aged 4–5 years old. DMD is a commonly observed- X-linked recessive hereditary muscle degenerative disease in which most affected children are unable to walk by the age of 12 years and die at approximately the age of 30 years.52 Furthermore, Beqvez also used AAVrh74 as vectors for the treatment of adults with moderate-to-severe hemophilia B (congenital factor IX deficiency). Many rAAV-based clinical trials involve neurological diseases, eye diseases and muscular disorders.5,53
The commonly used AAV-mediated gene therapies involve gene replacement, addition, and gene silencing.1 Gene replacement can treat single-gene diseases by introducing functional gene copies. This approach, such as the US FDA-approved Luxturna gene therapies aforementioned, is promising for the treatment of rare, otherwise untreatable diseases.46,48 Gene addition has even greater clinical potential than gene replacement because it can be used to treat non-single-gene diseases such as common chronic or infectious diseases. For example, arthrogen, currently in clinical trials (NCT02727764), encodes an anti-inflammatory IFN-β transgene to treat rheumatoid arthritis.54 Gene silencing typically involves the use of RNA interference (RNAi) to silence specific functional genes. AAV takes advantage of the RNAi process in target cells to generate siRNA by encoding short-hairpin RNA (shRNA). siRNA is a double stranded RNA; of the two strands, one can bind to mRNA by complementary base pairing to suppress gene expression. However, AAV-mediated gene silencing remains predominantly used in preclinical research because of off-target effects and competition with endogenous RNAi constructs.55,56 The AAV-CRISPR/Cas system has also been used for gene silencing to address the limitations of RNAi.57
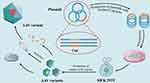
Although different AAV serotypes have different tissue tropisms, there are still limited cell types that native AAV can target. Currently, commercially available AAV drugs are based on the natural tropism of their serotypes, which limits widening their clinical application. In addition to limited tropism, AAV has been shown to induce immune responses, including neutralizing antibodies (NAbs) and cytotoxic T lymphocyte (CTL) responses. Notably, approximately 70% of the population have NAbs for AAV1 and AAV2, 45% for AAV9 and AAV6, 38% for AAV8.58 These NAbs limit AAV uptake by target cells and intracellular transport and uncoating in the nucleus, preventing transduction and limiting systemic delivery. Concerns regarding the transduction efficiency of AAV have also been raised. AAV engineering is one method used to address the limitations. Strategies to modify AAV can be divided into three categories, capsid modification, surface tethering, and virus load (Figure 2). Table 2 lists the advantages and limitations of the three AAV engineering strategies. The following sections address these strategies in detail.
 |
Table 2 Advantages and Limitations of AAV Engineering Strategies |
Capsid Modification Strategy
AAV capsids can be engineered to evade immune responses and enhance cellular targeting. The three forms of capsid modification are rational design, directed evolution, and in silico design.
Rational Design
The amino acid sequence and structure of the capsid protein on the surface of AAV determine its affinity for different primary receptors and co-receptors located on the cell surface, providing key information that can be used to enhance or improve cell targeting via rational design. The rational design strategy, which includes point mutations and peptide insertion, attempts to alter the amino acid residues of the AAV capsid or insert targeting peptides into the capsid structure via genetic modifications to improve the tropism or evasion of NAbs and CTLs.
This strategy has been used to develop gene therapies for DMD, a life-threatening disease caused by mutations in the dystrophin gene. Achieving these therapeutic goals, a chimeric AAV capsid variant (AAV2.5) was designed by engrafting five mutations from AAV1 onto the AAV2 capsid.81 These five amino acid residues are responsible for the high muscle tropism. As shown in a clinical trial, compared with AAV2, AAV2.5 reduced cross reactivity against NAbs and enhanced skeletal muscle transduction.81 This technique utilizes the knowledge of AAV’s receptor-binding domains.
Site-directed mutagenesis technique is one way to improve brain tropism and reduce the liver targeting of AAV. Wang et al hypothesized that even a few changes in the capsid residues would alter the tissue tropism of AAV. Among the AAV9 variants, the single mutant vector rAAV9.R533S (which indicates that position 533 of the R residue becomes the S residue) and the double mutant vector AAV9.HR containing H527Y and R533S changes through rational design demonstrated significantly reduced liver targeting following intravascular (IV) and intramuscular (IM) administration. Quantitative polymerase chain reaction (qPCR) analysis showed that genome copy number of that of AAV9.HR in the liver was only 0.5% or 8% of wild-type (WT) AAV9 after IV or IM injection, respectively. Moreover, AAV9.HR retained its ability to cross the blood–brain barrier (BBB), resulting in widespread neuronal transduction.82 Seo et al constructed two engineered rAAVs, namely PHP.eB and CAP-B10. The PHP.eB vectors contained a modification at position 588 relative to AAV9, and the CAP-B10 vectors had further residue changes in the position 455 relative to PHP.eB. Although AAV9, PHP.eB, and CAP-B10 showed similar liver accumulation, the two engineered AAVs were mainly internalized by Kupffer cells and thus showed reduced hepatocytes transduction compared with AAV9. In addition, PHP.eB and CAP-B10 displayed enhanced brain accumulation in comparison to AAV9.83 In addition, PHP.eB variants show high levels of transduction into the retina in mice.84 Gilkes et al constructed two AAV8 variants: a double mutant (Y447 + 733F) and a triple-mutant (Y447 + 733F + T494V) and showed the triple-mutant AAV8 variant enhanced brain transduction compared with AAV8 and double-mutant AAV8 variant.85 With this approach, it is possible to increase bioavailability, which will maximize the therapeutic potential.
The peptide insertion strategy ligates degenerate oligonucleotides into the cap ORF in order to insert peptide sequences into defined sites of capsid.86 Several studies have attempted to insert specific nucleic acid sequences encoding targeting peptides into appropriate sites of the capsid genome. In 1998, Yang et al fused the single-chain fragment variable region (sFv) of an antibody against CD34 to the N-terminus of VP by linking it to the capsid gene. Of the three sFv-VP containing AAV2s, only sFv-VP2 containing AAV2 showed increased transduction in CD34-expressing KG-1 cells, a human acute myeloid leukemia cell line with a positive surface CD34 molecule. However, further improvements are required for in vivo application.59 Wu et al performed mutational analysis of the AAV2 capsid gene and constructed 93 mutants by inserting peptides at the N-termini of VP1 and VP2. Insertion of the serpin receptor ligand at the N-termini of VP2 resulted in increased targeting of IB3 cells,87 a lung epithelial cell line believed to express the serpin receptor. They also showed that fusion of the serpin receptor ligand into the N-termini of VP1 resulted in increased infectivity compared with that of wild-type AAV2.87 Residue 587 (I587) is also an appropriate insertion site for targeting peptides.60,88–92 For example, Girod et al constructed six AAV2 mutants by inserting an L14 targeting peptide into I-261, I-381, I-447, I-534, I-573, and I-587. Of the mutants, only I-587-L14 containing rAAV2 re-targeted viral tropism and increased transduction in AAV2-resistant Co-115 and B16F10 cells.60 Nicklin et al used the same position to insert the SIGYPLP peptide, which efficiently targets endothelial cells (ECs) that are otherwise resistant to AAV vectors. Their results showed that SIGYPLP mediated AAV2 enhanced reporter gene expression in various ECs lines, including human umbilical vein endothelial cells and primary ECs.88
Recently, this peptide insertion strategy has been used for other capsid proteins, serotypes, and targeting cell types. For example, in a recent study, the P1 peptide (RGDLGLS) was introduced into the VP3 of AAV9 to form a high astrocyte-targeting AAV variant (rAAV9P1).93,94 The rAAV9P1 showed >90% astrocyte transduction in intact cerebral cortex and efficiently transduced reactive astrocytes in a mouse model of brain injury after intravenous injection.94 The rAAV9P1 vectors also transduced human astrocytic cell lines more efficiently (>75%) than the wild-type rAAV9 (<20%) did.94 Cell-penetrating peptides (CPPs) can also cross biological membranes.95 Several studies had shown that the complexes of AAV and CPP can enhance the transduction of AAV2, AAV8 and AAV9.96–98 Furthermore, some studies attempted to insert CPP into AAV capsids generate an AAV variant library. Yao et al identified an optimized CPP sequence (TVSALK) with increased brain penetration in mice through iterative screening and optimization of AAV variants. The obtained AAV.CPP.16 displays enhanced transcytosis at the BBB as well as increased efficiency of cellular transduction relative to AAV9, and that it can be used to deliver antitumour payloads in a mouse model of glioblastoma.99 For transducing cardiomyocytes, AAV2-THGTPAD and AAV2-NLPGSGD variants were identified using an in vivo peptide display library and showed significantly enhanced efficacy in cardiomyocyte transduction compared with the AAV2 serotype.100 For achieving high-level retina transduction, an AAV2-based peptide display library was used to select AAV2 variants.101 For lung delivery, the peptide THALWHT was inserted into the AAV2 capsid to obtain an AAV2 variant with enhanced affinity and high transduction efficiency to human airway epithelium.61,102,103
Rational design strategies have also been employed for immune evasion. For example, owing to the compatibility of different AAV serotypes, Chai et al generated haploid AAV vectors by co-infecting AAV2 and AAV8 helper plasmids at ratios of 3:1, 1:1 and 1:3. These haploid viruses, such as AAV2/8 3:1, obtained higher transduction than their parental AAV2 and AAV8 after muscular administration. Moreover, they also produced the triploid virus AAV2/8/9 to further improve NAb evasion compared with haploid serotypes.104 Inspired by the self-peptide (SP), a bioactive region of CD47, that acts as a “don’t-eat-me” signal for macrophages, Robinson et al inserted SP onto the capsid of AAV2 to obtain AAV2 mutants with decreased phagocytic susceptibility in vitro.105 In another study, after injecting the AAV capsid fused to Tregitopes, the human MHC class II epitopes, CD8+ T in splenocytes of mice displayed reduced responses to AAV capsid.104,106
In addition, several studies have leveraged point mutations and peptide insertions to achieve a more delicate function than that reported in the literature. For example, the rAAV-retro vectors, which include point mutation of N382D and V708I, as well as LADQDYTKTA peptide insertion at position 587 of AAV2 capsid, were constructed for achieving specific neural circuits targeting and retrograde tracing.107 Further study of rAAV-retro displayed that the 10-mer peptide insertion is mainly responsible for the retrograde transport of neurons projecting to striatum and the restoration of V708I enhances the retrograde functionality in the amygdala-striatum pathway; nevertheless, the change in N382D has little effect on the retrograde ability.108
In summary, these results suggest that a rational design strategy is able to efficiently generate mutants and alter tissue tropism, reduce the capture of immune system, and improve the transduction efficiency and specificity of AAV. However, because this strategy is based on an adequate understanding of AAV biology and the diversity of mutations is relatively low, its clinical use may be limited. With the in-depth understanding of AAV capsid protein and biological structure, through the comprehensive application of site-directed mutagenesis technique and peptide insertion strategy, rational design can greatly enhance the application potential of AAV in gene therapy.
Directed Evolution
Directed evolution is another capsid modification strategy that targets viral vectors via mutagenesis, including error-prone PCR, gene shuffling, Cre recombination-based AAV targeted evolution (CREATE), and a combination of above strategy and rational design. This strategy is useful when information on the structure or properties is insufficient.
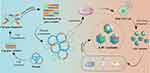
Error-prone PCR is low fidelity PCR which incorporates random point mutations into the AAV cap ORF at a pre-set and adjustable rate, which leads to the novel variants library. Subsequently, selection can be performed by binding affinity columns, in vitro cell culture models or in vivo model.86 Maheshri et al first used error-prone PCR and a staggered extension process to generate AAV2 mutants,62 generating random point mutations in the cap gene throughout VP1-3. Subsequently, a large AAV2 mutant library was obtained, and high-throughput selection processes were used to isolate mutants with high expression of primary receptor HSPG and low NAbs-binding.62 This process is time-consuming because of many variants. Structural analyses combined with error-prone PCR may narrow the size of the AAV variant library. For instance, Pulicherla et al obtained 43 viable variants using only mutations in the GH loop at amino acids 390–627 (VP1 numbering) on the outer surface of VP3. AAV9.45 and AAV9.61 showed liver-detargeted functions, and the number of vectors was reduced by approximately 10- to 25-fold. Furthermore, AAV9.45 displayed higher than AAV9.61 transduction in the cardiac and musculoskeletal tissues and decreased liver transduction.109 A diagram of the error-prone PCR is shown in Figure 3.
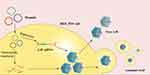
Gene shuffling strategy shuffles short gene fragments obtained by a modified PCR with other strands to produce new genes library, which leads to the production of novel AAV capsid variants (Figure 4).14,62,110–113 The idea behind gene shuffling libraries is to combine multiple advantageous features of different AAV serotypes, for example, to randomly reassemble the cap genome fragments from AAV1-9. The obtained chimeric-1829, with its genome composed of fragments from AAV1, 2, 8, and 9, showed improved tropism in AAV-resistant integrin minus hamster melanoma cells by binding to heparan sulfate receptors on the cell surface.112 Compared with AAV1 and 2, chimeric-1829 displayed a three-fold and five-fold increase, respectively, in transduction efficiency in the tumor model; a lower level of transgene expression was observed in the liver and skeletal muscle.112 Liu et al combined DNA shuffling and peptide display to generate AAV libraries, followed by cardiovascular EC-enriched variant isolation in a mouse model using flow cytometry and PCR. EC71 and EC73 variants were enriched in cardiovascular cells and transgene expression increased 8.8-fold and 6.3-fold, respectively, compared with AAV1 in vivo. By contrast, the vector genome (vg) copy numbers in the liver were reduced by 23.3-fold for EC71 and 28.5-fold for EC73, compared with AAV1. EC71 and EC73 also mediated long-term transgene expression in ECs for up to 4 months and show an approximately four-fold higher expression level in the heart than AAV1 dose.114 Moreover, progress has also been made for developing the AAV variants to effectively transduce brain,115–118 spinal cord,119 muscle,115,120,121 myocardium,110,114 pancreas,115 liver122 and lung.115
CREATE can be used to develop brain-targeting AAVs because the BBB hinders the entry of delivery vehicles into the CNS.39 In this approach, AAV9 is used as a template to generate an AAV library. Subsequently, seven randomized amino acids are inserted at position 588–589 (VP1 numbering). AAV libraries are then administered intravenously to transgenic animals with specific Cre-expressing cells. The most abundant variant in the brain and spinal cord, AAV-PHP.B (containing the TLAVPFK peptide), was selected in vivo and showed over 40-fold greater transduction efficiency in the CNS than AAV9 did.39 By using this approach, multiple variants with high permeability across the BBB were also obtained.123–127
A directed evolution strategy has also been used to create novel immune evading AAV. In 2008, Kay et al used a gene shuffling strategy to construct a library of hybrid capsids from 8 WT viruses. By iteratively amplifying this library in human liver cells, the obtained single chimeric AAV variant, AAV-DJ, resisted NAb neutralization better than AAV2 did.113 Tse et al developed a structure-guided evolutionary approach based on the hypothesis that antibody recognition sites are evolutionarily conserved. An evolved capsid antigenic motif (CAM), CAM130, was able to effectively evade NAb while maintaining tissue tropism in non-human primate and human serum compared with the parent AAV.128 Likewise, Havlik et al evolved a humanized AAV8 capsid, AAVhum.8, which displayed human hepatocytes tropism and NAb evasion.129
Directed evolution can also generate highly diverse mutants and expand the pool of rAAV. Unlike rational design strategies, directed evolution strategies do not require understanding AAV biology. However, this approach is time-consuming because of the many variants and the in vivo selection process. Among diverse mutants, AAV with targeting specificity, safety and durability may be included. In combination with a rational design, it could have wide applications and reduce the workload of future clinical trials and achieve the goal of safe and lasting gene therapy.
In silico Design
Some studies have also adopted in silico designs to discover novel AAV variants, such as ancestral reconstruction and machine learning.
Ancestral reconstruction is a computational design approach that uses phylogenetic data and gene sequences to generate new capsids.63,130 Unlike modern serotypes, ancestral variants exhibit unique cellular entry mechanisms. For example, Ortiz et al used 32 variable sites to obtain an ancestral AAV library. These variants had similar infection efficiencies as the most efficient modern serotypes in various cell lines, such as C2C12 mouse myoblast and glioblastoma tumor, but showed 19–31-fold higher muscle tropism than AAV1 did. The ancestral variants also displayed a NAb-binding ability similar to that of AAV1. Furthermore, these ancestral capsids show higher thermostability and do not bind to the usual receptors of modern AAV serotypes, such as HSPG, sialic acid, and galactose.63 In another study, the Anc80 ancestral AAV vector was selected from 75 AAV capsids via in silico ancestral sequence reconstruction and tested in mice and non-human primates. This Anc80 vector is considered the ancestor of AAV1, 2, 8, and 9 and was able to transduce multiple tissues more efficiently, including the muscle, retina, and liver, than AAV2 and AAV8 can.130 Anc80 was also used in a study leveraging the Anc80L65 clone as a vehicle to deliver the USH1C gene encoding harmonin to the cochlea and vestibular sensory organs in a murine model of Usher syndrome to improve hearing loss. The results showed that Anc80L65-mediated harmonin expression rescued sensory transduction in Ush1c hair cells, auditory brainstem responses, and auditory and vestibular behaviors in an Ush1c mouse model.131
Machine learning (ML) is a promising strategy for variants AAVs designs. Ogden et al replaced random mutagenesis with an algorithmic approach. In this study, the AAV2 mutation landscape was restricted to capsid positions 561–588.132 Bryant et al leveraged deep learning to construct an AAV2 variants library. The authors tested three training data libraries designed using complete, random, or additive sampling strategies. The three datasets contained (i) a complete single variant set plus randomly chosen variants with double mutants, (ii) complete single mutation variants plus, and (iii) randomly chosen variants with multiple-mutants between 2 and 10 mutations plus the additive model-designed variants. Three machine algorithms were trained using the three datasets and used to generate the AAV2 variants.133 Nevertheless, these starting libraries often consist of a substantial number of variants that are incapable of assembling properly or packaging their genomes, which leads to a significant waste of library. Therefore, Zhu et al developed ML-guided AAV peptide insertion libraries to balance the diversity and packaging fitness. In a selection of primary human brain tissue, the ML-guided library produced a 10-fold increase in the number of infectious variants in comparison to standard NNK library, with minimal impact on diversity.134 The data-driven approach displays significant viability compared with random mutagenesis and allows deeper research on unexplored AAV sequences, transforming future capsid engineering strategies. However, the predictive power of machine learning depends heavily on model design, data representation and training data generation.64
Surface Tethering Strategy
In contrast with gene engineering, surface tethering strategies use chemical modifications to evade NAbs or achieve tissue targeting. Thus, it provides a simple, multi-functional, and universal approach to extending the reach of AAV.
Several studies have used the polyethylene glycol (PEG) strain to attach polypeptides, nanoparticles, and biomedical molecules.65–67 PEGylation is the process by which PEG tethering or coating endows molecules or nanoparticles with the ability to evade the immune response, prolonging their circulation time. PEGylation has also been used to modify AAV surfaces. Lee et al first investigated whether PEG length and the PEG:lysine ratio affect serum neutralization and viral infectivity.68 PEG with succinimidyl propionic acid groups reacts with the abundant lysine residues on the viral capsids. However, an excessive PEG coating ratio may compromise the interactions between AAV and its receptors, impairing viral infectivity. A small PEG coating range showed improved infectivity and NAbs escape, and the width varied with PEG size.68 Another study examined the covalent conjugations of PEG to AAV.135 Succinimidyl succinate and tresyl chloride linkers improved lung transduction efficiency despite similar liver and muscle targeting. The use of tresyl chloride for PEG attachment provided the best protection from NAbs, whereas the cyanuric chloride linker induced AAV aggregation, compromising transduction.135 Other conjugation methods have been used. For example, an azide moiety has been incorporated into the AAV capsid to achieve precise site-specific PEGylation using click chemistry.136
In addition to PEGylation to protect against neutralization, the use of a targeting peptide or protein incorporation strategy can improve or alter the tropism of AAV capsids. Owing to the high affinity of streptavidin for biotin, proteins genetically fused with core streptavidin have been used to conjugate biotinylated AAV2 capsid.70 In a study using the epidermal growth factor (EGF) or fibroblast growth factor 1α (FGF1α) as a targeting ligand, EGF-AAV2 and FGF1α-AAV2 showed increased infectivity in EGFR-positive SKOV3.ip1 cells or FGFR-1α-positive M07e cells. Nevertheless, the capsid-modified AAV2 vectors retained their natural tropism.70 Liu et al developed a site-specific modification strategy that involved the genetic inserting of a 13-amino acid peptide (LCTPSRAALLTGR) at amino acid position 587, in which the cysteine in the peptides was converted to an aldehyde-containing formylglycine residue in the presence of a formylglycine generating enzyme. The aldehyde group showed good reactivity toward hydrazide- or aminooxy-functionalized molecules such as targeting ligands or fluorophores.69 In this case, incorporation of the anti-HLA antibody into AAV2 significantly increased 293T cell transduction, whereas AAV2 with a chemically modified arginine-glycine-aspartate (RGD) motif increased HeLa cell transduction compared with wild-type AAV2.69 Zdechlik et al genetically introduced an HUH tag into the AAV capsid. Because the HUH tag can covalently link to sequence-specific single-stranded DNA (ssDNA), an ssDNA-conjugated antibody was incorporated into the viral capsid. The AAV-HUH-antibody composites displayed increased transduction in HEK293 cells compared with wild-type AAV. Furthermore, anti-L1CAM-AAV displayed significantly increased transduction efficiency toward neurons but not glial cells, which do not express L1CAM.137
In addition to the biochemical materials aforementioned, nanoparticles can be linked to AAV to enhance transduction. Magnetic nanoparticles have been widely used in nanomedicine because of their unique magnetic tropism.138 Several attempts have been made to combine magnetic nanoparticles with AAV. Viral vectors were linked with heparin-coated superparamagnetic iron oxide nanoparticles (HpNPs), which magnetize in a magnetic field.71 Magnetically guided AAV was shown to enhance delivery efficiency in PC12 and HEK293T cell lines. Moreover, HpNP-mediated AAV delivery has a shorter transduction time (<180 min) than but a similar transduction efficiency to the conventional protocol which typically requires 24 h of incubation.71 In another study, streptavidin-coated superparamagnetic iron oxide nanoparticles (SPIONs) were linked to biotin-nitrilotriacetic acid (NTA), which can chelate Ni ions. For further conjugation with AAV, hexa-histidine was inserted into AAV capsids. The acquired complexes (AAV/NiStNPs) displayed enhanced infection and rapid internalization by non-permissive human neural stem cells.139 Although magnetic nanoparticle-mediated AAV delivery shows rapid, efficient transduction in various cell types, in vivo magnetic delivery of AAV remains a challenge. Recently, Mukherjee et al explored local magnetic targeting of AAV in vivo. The treatment of noise-induced hearing loss is limited by the absence of an effective drug delivery strategy. Mukherjee et al used SPION-AAV complexes for local magnetic delivery of brain-derived neurotrophic factor. AAV2 (quad Y-F), in which the four capsid tyrosine residues were replaced with four phenylalanine residues, was conjugated with commercial Mag4C-Ad SPIONs. Through magnetic targeting of delivery to the inner ear in a noise-induced hearing loss rat model, SPIONs-AAV2 (quad Y-F) carriers increased brain-derived neurotrophic factor gene expression, improving hearing ability.140
The scope of surface tethering strategies continues to expand, because this strategy can be used to attach various materials (eg, polymers, antibodies, peptides, fluorescent probes, nanoparticles) via diverse conjugation methods especially chemical conjugation. Surface tethering strategies can affect circulation and clearance, accumulation and retention in tissues, and interaction and internalization of cells, so it has a wider application range than capsid modification does. However, complex protocols may limit their clinical applications and compromise their clinical value.
Virus Load Strategy
The load strategy is another efficient method for protecting AAVs from neutralization and changing the natural targets. Most importantly, this strategy provides a protocol for the co-delivery of AAVs and other drugs and endows rAAVs with the additional benefit of responsive or controllable release.
Liposomes have a bilayer membrane structure and can load various substances such as plasmids, proteins, and small-molecule drugs. Since 1984, liposomes consisting of cholesterol, phosphatidylcholine, and phosphatidylglycerol prepared using the reverse-phase evaporation method have been used to encapsulate retroviruses, and have shown improved transduction efficiency in resistant cell lines.141 The encapsulation of rAAV into liposomes also yielded exciting results. For example, rAAV-associated liposomes, which comprise N-(α-trimethylammonio-acetyl)didodecyl-D-glutamate chloride, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), and dilauroyl phosphatidylcholine, exhibited more than six-fold targeting efficiency compared with rAAV alone in three glioma cell lines.73 However, in vivo viral delivery involves a more complex physiological environment; thus, the requirements for rAAV-associated liposomes are higher. Fiandaca et al used cationic lipids containing gadolinium and rAAV to achieve real-time magnetic resonance imaging (MRI) visualization of rAAV in non-human primate brain. Liposomes containing gadolinium were prepared via a thin-film hydration method using 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), cholesterol, and 1,2-distearoyl-sn-glycero-3-methoxy (polyethylene glycol)-2000 (PEG-DSG). Gadolinium-loaded liposomes were monitored for rAAV distribution. The distribution of rAAV shown using immunohistochemistry (IHC) was consistent with that of the gadolinium-loaded liposomes on MRI scans.142 In another study, two cationic lipids were synthesized to encapsulate AAV2/9 and AAV2/6.2, which showed unique tropism for the alveolar epithelium and conducting airway/alveolar epithelium, respectively. After encapsulation, the complexes exhibited positive zeta potentials and increased infection efficiency in A549 cells. In the analysis of β-galactosidase (β-gal) expression by AAV, AAV-encapsulating liposomes showed a four-fold increase in β-gal expression in vivo compared with control AAV2/9 or AAV2/6.2 after intranasal administration. Moreover, AAV2/9-encapsulating liposomes change the viral tropism and increased the conducting airway transduction efficiency of AAV2/9.143 AAV vectors were entrapped in the liposome, hindering the AAV capsid-cell membrane interaction. The altered tropism might result from the uptake of liposomes into the cells. These cationic lipids are also responsible for retargeting AAV vectors. Moreover, cell targeting can be conferred on rAAV-associated liposomes via conjugation with specific antibodies or targeting peptides.
Exosomes, lipid membrane vesicles derived from cells (40–160 nm in the diameter), contain multiple components including DNA, RNA, lipids, and cell-surface proteins and function as transit systems between cells.144 With the advantages of non-immunogenicity, natural tropism and non-toxicity, exosomes have attracted increasing attention as natural drug delivery systems, especially for gene delivery. Notably, exosomes can protect against NAbs and target various tissues because of their natural tropism. Some AAVs are associated with exosomes in viral production and are termed vexosomes or exosome-AAV (Figure 5). Vexosomes were shown to display enhanced transduction in 293T and U87 cell lines and improve NAbs evasion compared with standard AAV.74 Moreover, their tropism of vexosomes can be modified by expressing transmembrane receptors on the surface. In one study, the biotin acceptor peptide-transmembrane domain (BAP-TM) and biotin ligase BirA were introduced into 293T cells using lentivirus transduction to produce BAP-TM vexosomes. After incubating BAP-TM vexosomes with magnetic beads containing streptavidin, BAP-TM vexosomes showed two-fold enhanced transduction compared with that of vexosomes within magnetic fields.74 Vexosomes are also suitable gene delivery tools for treating CNS diseases. Maguire et al isolated vexosomes from conditioned media of 293T producer cells via ultracentrifugation. The exosome associated AAV vector effectively targeted the CNS after systemic administration and crossed the BBB in vitro, which may be explained by the ability of exosomes to cross the BBB. Furthermore, intravenously injected exosomes-AAV9 showed higher transduction of neurons and astrocytes than with AAV9 alone.145 Other studies have reported that exosome-AAVs were able to improve gene therapy in various tissues, including the liver,146 lung,147 muscle,148 ear,149 and retina.150 These findings highlight the obvious benefits of exosome-AAV, which can be summarized as (i) natural or modified tropism, (ii) NAbs evasion, and (iii) the ability to cross biological barriers. Unlike synthetic liposomes, which are mainly composed of natural or synthetic lipids, multiple transmembrane receptors are naturally expressed in exosomes derived from parent cells.151 The cell source influences exosome membrane protein types, which further influences the biodistribution and cell tropism of exosomes. The heterogeneity and endogeneity of exosomes provide unique advantages over liposomes regarding targeted delivery and immune evasion. However, despite exosome-AAV, there are multiple benefits of exosome-AAV compared to AAV or liposome-AAV, a manufacturing process of exosome-AAV is challenging. For example, the extraction and purification of therapeutic exosome-AAV in a homogeneous manner to meet both scale and clinical requirements present great difficulties.75
Hydrogels can also endow AAV with unique pharmacological characteristics such as sustained release, controlled release, and local administration. Hydrogels are widely used to deliver biomacromolecules. Thus, PEG-based hydrogels can serve as biocompatible scaffolds for AAV release. By incorporating polyhistidine into PEG hydrogels, pH-sensitive hydrogels were synthesized for AAV2 encapsulation. These hydrogels released AAV2 at different rates under different pH conditions. Owing to the more acidic physiological environment of injured tissues, PEG-polyhistidine hydrogels have faster viral release.76 Self-assembled peptide-based hydrogels have also shown ideal biocompatibility. Cucchiarini et al constructed pure RAD peptide hydrogels or RAD hydrogels combined with hyaluronic acid to achieve sustained release of AAV into human mesenchymal stem cells, providing effective delivery vehicles for tissue lesions.77 In another study, AAV9SLR vectors were embedded in alginate hydrogels for targeted delivery into the abdominal aorta of mice.78 These studies demonstrate that hydrogels are safe, effective biomaterials for coating viral vectors and improving in vivo kinetics and targeting abilities.
Microneedles (MNs) are small needles that can overcome biological barriers and deliver biomacromolecules to specific regions. Ye et al used AAV-harboring MNs to target rat hearts and burst-release agents, thereby improving heart function.79 These MNs were prepared using polyvinyl alcohol (PVA) and did not degrade. The AAV was dispensed onto the surface of the MNs. The MNs displayed good drug-loading capacity, and over 4.93 × 1010 vg of virus could be loaded in the MN array. MNs showed a burst release, releasing 90.93% of AAV within 2 s and 92.42% of AAV within 5 s. Twenty-eight days after the administration of MN-AAV loaded with the vascular endothelial growth factor (VEGF) gene, myocardial infarction model rats showed improved cardiac function, reduced infarct size, and improved adverse remodeling.79 Liu et al used MN-AAV-VEGF to treat ischemic stroke. MNs were prepared using gelatin methacryloyl (GelMA), which is biocompatible, biodegradable, and photocrosslinkable via photoinitiators. In contrast with PVA, the GelMA MNs released AAV in a sustained and controlled manner, avoiding potential burst release-induced neurotoxicity. MN-AAV-VEGF delivery promoted angiogenesis and neurogenesis and reduced modified neurological severity scores (mNSS) in a stroke rat model.80 These findings support the value of MN-AAV as a minimally invasive and locally distributed strategy.
The load strategy regards AAV as a biomacromolecule drug and takes advantage of pharmaceutical methods, offering more possibilities than the capsid modification or surface tethering strategies. The loading strategy, as a noncovalent surface modification strategy, can increase AAV transduction efficiency and change the tropism, in addition to endowing AAV with responsive and controlled release characteristics, which will be beneficial for clinical translation.
Conclusions
Gene therapy has substantial potential for the treatment of various diseases. Thus, the attention to developing safe, effective gene delivery vectors is increasing. Owing to its well-tolerated properties and effective mechanism of entry into the nucleus, AAV is a useful delivery vector for gene therapy and has been used in many clinical trials. However, the use of AAV is limited by its low transduction efficiency in the human body, limited tissue tropism, low payload, immune responses against viral capsids or transgenes, and insufficient viral production. AAV engineering strategies have attempted to improve tissue or cell tropism and evade immune responses. Modern technologies, including bioinformatics, structural analysis, and proteomics, provide useful information on capsid modification and surface tethering. Various pharmaceutical methods have been applied to AAV load strategies to provide additional advantages. Despite numerous preclinical studies suggesting that these novel rAAV forms have obvious advantages over standard AAV in animal models, additional evidence is required to confirm whether these benefits extend to humans. Moreover, the challenges are encountered to make these novel rAAV forms to effectively manufacture. For example, the AAV engineering strategies result in lower yield and higher cost and increase the risk of high variability of product quality. Furthermore, limited understanding on the AAV engineering also hinders the manufacture. The above has posed challenges to regulatory. Therefore, further studies are warranted in this regard. Engineering of rAAV should consider various parameters, such as serotypes, target cells, administration routes, and dosage forms, all of which influence the overall curative effect. Improving the design and AAV manufacturing will benefit clinical outcomes and facilitate US FDA approval. Besides, the novel clinical frameworks are required for the assessment and management of these novel AAV therapies.
Abbreviations
AAV, adeno-associated virus; ASO, anti-sense oligonucleotide; CRISPR-Cas9, (CRISPR)/CRISPR-associated protein 9; LNPs, lipid nanoparticles; ITRs, inverted terminal repeats; VP1, viz virion protein 1; rAAV, recombinant AAV; US FDA, the United States Food and Drug Administration; DMD, Duchenne muscular dystrophy; RNAi, RNA interference; shRNA, short-hairpin RNA; NAbs, neutralizing antibodies; CTL, cytotoxic T lymphocyte; IV, intravascular; IM, intramuscular; qPCR, quantitative polymerase chain reaction; WT, wild-type; BBB, blood–brain barrier; CPPs, Cell-penetrating peptides; SP, self-peptide; CREATE, Cre recombination-based AAV targeted evolution; CAM, capsid antigenic motifs; PEG, polyethylene glycol; EGF, epidermal growth factor; FGF1α, fibroblast growth factor 1α; ssDNA, single-stranded DNA; HpNPs, heparin-coated superparamagnetic iron oxide nanoparticles; SPIONs, superparamagnetic iron oxide nanoparticles; BNTA, biotin-nitrilotriacetic acid; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; MRI, magnetic resonance imaging; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; IHC, immunohistochemistry; PVA, polyvinyl alcohol; VEGF, vascular endothelial growth factor; GelMA, gelatin methacryloyl; mNSS, modified neurological severity scores; SYFP2, Super Yellow Fluorescent Protein-2.
Acknowledgments
This work was supported by Beijing Natural Science Foundation (Grant No. 7234405) and National Natural Science Foundation of China (Grant No. 82073783).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Landhuis E. The definition of gene therapy has changed. Nature. 2021. doi:10.1038/d41586-021-02736-8
2. Zu H, Gao D. Non-viral vectors in gene therapy: recent development, challenges, and prospects. Aaps J. 2021;23(4):78. doi:10.1208/s12248-021-00608-7
3. Raguram A, Banskota S, Liu DR. Therapeutic in vivo delivery of gene editing agents. Cell. 2022;185(15):2806–2827. doi:10.1016/j.cell.2022.03.045
4. Lugin ML, Lee RT, Kwon YJ. Synthetically engineered adeno-associated virus for efficient, safe, and versatile gene therapy applications. ACS Nano. 2020;14(11):14262–14283. doi:10.1021/acsnano.0c03850
5. Li C, Samulski RJ. Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet. 2020;21(4):255–272. doi:10.1038/s41576-019-0205-4
6. Lundstrom K. Viral vectors in gene therapy. Diseases. 2018;6(2):42. doi:10.3390/diseases6020042
7. Kotterman MA, Chalberg TW, Schaffer DV. Viral vectors for gene therapy: translational and clinical outlook. Annu Rev Biomed Eng. 2015;17(1):63–89. doi:10.1146/annurev-bioeng-071813-104938
8. Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018;359(6372). doi:10.1126/science.aan4672
9. Mori S, Wang L, Takeuchi T, Kanda T. Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology. 2004;330(2):375–383. doi:10.1016/j.virol.2004.10.012
10. Hastie E, Samulski RJ. Adeno-associated virus at 50: a golden anniversary of discovery, research, and gene therapy success--a personal perspective. Hum Gene Ther. 2015;26(5):257–265. doi:10.1089/hum.2015.025
11. Korneyenkov MA, Zamyatnin AA. Next step in gene delivery: modern approaches and further perspectives of AAV tropism modification. Pharmaceutics. 2021;13(5):750. doi:10.3390/pharmaceutics13050750
12. Xie Q, Bu W, Bhatia S, et al. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(16):10405–10410. doi:10.1073/pnas.162250899
13. Salganik M, Aydemir F, Nam HJ, McKenna R, Agbandje-McKenna M, Muzyczka N. Adeno-associated virus capsid proteins may play a role in transcription and second-strand synthesis of recombinant genomes. J Virol. 2014;88(2):1071–1079. doi:10.1128/JVI.02093-13
14. Büning H, Huber A, Zhang L, Meumann N, Hacker U. Engineering the AAV capsid to optimize vector-host-interactions. Curr Opin Pharmacol. 2015;24:94–104. doi:10.1016/j.coph.2015.08.002
15. Macdonald J, Marx J, Büning H. Capsid-engineering for central nervous system-directed gene therapy with adeno-associated virus vectors. Hum Gene Ther. 2021;32(19–20):1096–1119. doi:10.1089/hum.2021.169
16. Tseng YS, Agbandje-McKenna M. Mapping the AAV capsid host antibody response toward the development of second generation gene delivery vectors. Front Immunol. 2014;5:9. doi:10.3389/fimmu.2014.00009
17. Rabinowitz JE, Rolling F, Li C, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002;76(2):791–801. doi:10.1128/JVI.76.2.791-801.2002
18. Grimm D, Pandey K, Nakai H, Storm TA, Kay MA. Liver transduction with recombinant adeno-associated virus is primarily restricted by capsid serotype not vector genotype. J Virol. 2006;80(1):426–439. doi:10.1128/JVI.80.1.426-439.2006
19. Arnett AL, Beutler LR, Quintana A, et al. Heparin-binding correlates with increased efficiency of AAV1- and AAV6-mediated transduction of striated muscle, but negatively impacts CNS transduction. Genet Ther. 2013;20(5):497–503. doi:10.1038/gt.2012.60
20. Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ, Walsh CE. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther. 2000;2(6):619–623. doi:10.1006/mthe.2000.0219
21. Du L, Kido M, Lee DV, et al. Differential myocardial gene delivery by recombinant serotype-specific adeno-associated viral vectors. Mol Ther. 2004;10(3):604–608. doi:10.1016/j.ymthe.2004.06.110
22. Passini MA, Watson DJ, Vite CH, Landsburg DJ, Feigenbaum AL, Wolfe JH. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J Virol. 2003;77(12):7034–7040. doi:10.1128/jvi.77.12.7034-7040.2003
23. Burger C, Gorbatyuk OS, Velardo MJ, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10(2):302–317. doi:10.1016/j.ymthe.2004.05.024
24. Nakai H, Fuess S, Storm TA, Muramatsu S, Nara Y, Kay MA. Unrestricted hepatocyte transduction with adeno-associated virus serotype 8 vectors in mice. J Virol. 2005;79(1):214–224. doi:10.1128/JVI.79.1.214-224.2005
25. Taymans JM, Vandenberghe LH, Haute CV, et al. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther. 2007;18(3):195–206. doi:10.1089/hum.2006.178
26. Pacak CA, Mah CS, Thattaliyath BD, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006;99(4):e3–e9. doi:10.1161/01.RES.0000237661.18885.f6
27. Shen S, Troupes AN, Pulicherla N, Asokan A. Multiple roles for sialylated glycans in determining the cardiopulmonary tropism of adeno-associated virus 4. J Virol. 2013;87(24):13206–13213. doi:10.1128/JVI.02109-13
28. Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16(6):1073–1080. doi:10.1038/mt.2008.76
29. Liu G, Martins IH, Chiorini JA, Davidson BL. Adeno-associated virus type 4 (AAV4) targets ependyma and astrocytes in the subventricular zone and RMS. Genet Ther. 2005;12(20):1503–1508. doi:10.1038/sj.gt.3302554
30. Zabner J, Seiler M, Walters R, et al. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol. 2000;74(8):3852–3858. doi:10.1128/JVI.74.8.3852-3858.2000
31. Quinn PM, Buck TM, Mulder AA, et al. Human iPSC-derived retinas recapitulate the fetal CRB1 CRB2 complex formation and demonstrate that photoreceptors and Müller glia are targets of AAV5. Stem Cell Rep. 2019;12(5):906–919. doi:10.1016/j.stemcr.2019.03.002
32. Zincarelli C, Soltys S, Rengo G, Koch WJ, Rabinowitz JE. Comparative cardiac gene delivery of adeno-associated virus serotypes 1–9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin Transl Sci. 2010;3(3):81–89. doi:10.1111/j.1752-8062.2010.00190.x
33. Gregorevic P, Blankinship MJ, Allen JM, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10(8):828–834. doi:10.1038/nm1085
34. Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(18):11854–11859. doi:10.1073/pnas.182412299
35. Wang Z, Zhu T, Qiao C, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23(3):321–328. doi:10.1038/nbt1073
36. Inagaki K, Fuess S, Storm TA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14(1):45–53. doi:10.1016/j.ymthe.2006.03.014
37. Chen M, Maeng K, Nawab A, et al. Efficient gene delivery and expression in pancreas and pancreatic tumors by capsid-optimized AAV8 vectors. Hum Gene Ther Methods. 2017;28(1):49–59. doi:10.1089/hgtb.2016.089
38. Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27(1):59–65. doi:10.1038/nbt.1515
39. Deverman BE, Pravdo PL, Simpson BP, et al. Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol. 2016;34(2):204–209. doi:10.1038/nbt.3440
40. Quinn K, Quirion MR, Lo CY, Misplon JA, Epstein SL, Chiorini JA. Intranasal administration of adeno-associated virus type 12 (AAV12) leads to transduction of the nasal epithelia and can initiate transgene-specific immune response. Mol Ther. 2011;19(11):1990–1998. doi:10.1038/mt.2011.146
41. Schmidt M, Voutetakis A, Afione S, Zheng C, Mandikian D, Chiorini JA. Adeno-associated virus type 12 (AAV12): a novel AAV serotype with sialic acid- and heparan sulfate proteoglycan-independent transduction activity. J Virol. 2008;82(3):1399–1406. doi:10.1128/JVI.02012-07
42. Pillay S, Carette JE. Host determinants of adeno-associated viral vector entry. Curr Opin Virol. 2017;24:124–131. doi:10.1016/j.coviro.2017.06.003
43. Kronenberg S, Böttcher B, von der Lieth CW, Bleker S, Kleinschmidt JA. A conformational change in the adeno-associated virus type 2 capsid leads to the exposure of hidden VP1 N termini. J Virol. 2005;79(9):5296–5303. doi:10.1128/JVI.79.9.5296-5303.2005
44. Bleker S, Sonntag F, Kleinschmidt JA. Mutational analysis of narrow pores at the fivefold symmetry axes of adeno-associated virus type 2 capsids reveals a dual role in genome packaging and activation of phospholipase A2 activity. J Virol. 2005;79(4):2528–2540. doi:10.1128/JVI.79.4.2528-2540.2005
45. Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14(3):316–327. doi:10.1016/j.ymthe.2006.05.009
46. Ylä-Herttuala S. Endgame: glybera finally recommended for approval as the first gene therapy drug in the European Union. Mol Ther. 2012;20(10):1831–1832. doi:10.1038/mt.2012.194
47. Li X, Le Y, Zhang Z, Nian X, Liu B, Yang X. Viral vector-based gene therapy. Int J Mol Sci. 2023;24(9):7736. doi:10.3390/ijms24097736
48. Keeler AM, Flotte TR. Recombinant adeno-associated virus gene therapy in light of luxturna (and Zolgensma and Glybera): where are we, and how did we get here? Annu Rev Virol. 2019;6(1):601–621. doi:10.1146/annurev-virology-092818-015530
49. Herzog RW, VandenDriessche T, Ozelo MC. First hemophilia B gene therapy approved: more than two decades in the making. Mol Ther. 2023;31(1):1–2. doi:10.1016/j.ymthe.2022.12.001
50. Dhungel BP, Winburn I, Pereira CDF, Huang K, Chhabra A, Rasko JEJ. Understanding AAV vector immunogenicity: from particle to patient. Theranostics. 2024;14(3):1260–1288. doi:10.7150/thno.89380
51. VandenDriessche T, Pipe SW, Pierce GF, Kaczmarek R. First conditional marketing authorization approval in the European Union for hemophilia ”A” gene therapy. Mol Ther. 2022;30(11):3335–3336. doi:10.1016/j.ymthe.2022.09.020
52. Mullard A. FDA approves first gene therapy for Duchenne muscular dystrophy, despite internal objections. Nat Rev Drug Discov. 2023;22(8):610. doi:10.1038/d41573-023-00103-y
53. Escandell JM, Pais DA, Carvalho SB, Vincent K, Gomes-Alves P, Alves PM. Leveraging rAAV bioprocess understanding and next generation bioanalytics development. Curr Opin Biotechnol. 2022;74:271–277. doi:10.1016/j.copbio.2021.12.009
54. Vrouwe JPM, Meulenberg JJM, Klarenbeek NB, et al. Administration of an adeno-associated viral vector expressing interferon-β in patients with inflammatory hand arthritis, results of a Phase I/II study. Osteoarthr Cartil. 2022;30(1):52–60. doi:10.1016/j.joca.2021.09.013
55. Borel F, Kay MA, Mueller C. Recombinant AAV as a platform for translating the therapeutic potential of RNA interference. Mol Ther. 2014;22(4):692–701. doi:10.1038/mt.2013.285
56. Chen S, Luo M, Kou H, Shang G, Ji Y, Liu H. A review of gene therapy delivery systems for intervertebral disc degeneration. Curr Pharm Biotechnol. 2020;21(3):194–205. doi:10.2174/1389201020666191024171618
57. Wang D, Zhang F, Gao G. CRISPR-based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell. 2020;181(1):136–150. doi:10.1016/j.cell.2020.03.023
58. Vandamme C, Adjali O, Mingozzi F. Unraveling the complex story of immune responses to AAV vectors trial after trial. Hum Gene Ther. 2017;28(11):1061–1074. doi:10.1089/hum.2017.150
59. Yang Q, Mamounas M, Yu G, et al. Development of novel cell surface CD34-targeted recombinant adenoassociated virus vectors for gene therapy. Hum Gene Ther. 1998;9(13):1929–1937. doi:10.1089/hum.1998.9.13-1929
60. Girod A, Ried M, Wobus C, et al. Genetic capsid modifications allow efficient re-targeting of adeno-associated virus type 2. Nat Med. 1999;5(9):1052–1056. doi:10.1038/12491
61. Liqun Wang R, McLaughlin T, Cossette T, et al. Recombinant AAV serotype and capsid mutant comparison for pulmonary gene transfer of alpha-1-antitrypsin using invasive and noninvasive delivery. Mol Ther. 2009;17(1):81–87. doi:10.1038/mt.2008.217
62. Maheshri N, Koerber JT, Kaspar BK, Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat Biotechnol. 2006;24(2):198–204. doi:10.1038/nbt1182
63. Santiago-Ortiz J, Ojala DS, Westesson O, et al. AAV ancestral reconstruction library enables selection of broadly infectious viral variants. Gene Ther. 2015;22(12):934–946. doi:10.1038/gt.2015.74
64. Becker J, Fakhiri J, Grimm D. Fantastic AAV gene therapy vectors and how to find them-random diversification, rational design and machine learning. Pathogens. 2022;11(7):756. doi:10.3390/pathogens11070756
65. Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–221. doi:10.1038/nrd1033
66. Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51. doi:10.1016/j.addr.2015.09.012
67. Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs. 2008;22(5):315–329. doi:10.2165/00063030-200822050-00004
68. Lee GK, Maheshri N, Kaspar B, Schaffer DV. PEG conjugation moderately protects adeno-associated viral vectors against antibody neutralization. Biotechnol Bioeng. 2005;92(1):24–34. doi:10.1002/bit.20562
69. Liu Y, Fang Y, Zhou Y, et al. Site-specific modification of adeno-associated viruses via a genetically engineered aldehyde tag. Small. 2012;9(3):421–429. doi:10.1002/smll.201201661
70. Ponnazhagan S, Mahendra G, Kumar S, Thompson JA, Castillas M. Conjugate-based targeting of recombinant adeno-associated virus type 2 vectors by using avidin-linked ligands. J Virol. 2002;76(24):12900–12907. doi:10.1128/JVI.76.24.12900-12907.2002
71. Hwang J-H, Lee S, Kim E, et al. Heparin-coated superparamagnetic nanoparticle-mediated adeno-associated virus delivery for enhancing cellular transduction. Int J Pharm. 2011;421(2):397–404. doi:10.1016/j.ijpharm.2011.10.019
72. Araujo EV, Carneiro SV, Neto DMA, et al. Advances in surface design and biomedical applications of magnetic nanoparticles. Adv Colloid Interface Sci. 2024;328:103166. doi:10.1016/j.cis.2024.103166
73. Mizuno M, Yoshida J. Improvement of transduction efficiency of recombinant adeno-associated virus vector by entrapment in multilamellar liposomes. Jpn J Cancer Res. 1998;89(4):352–354. doi:10.1111/j.1349-7006.1998.tb00570.x
74. Maguire CA, Balaj L, Sivaraman S, et al. Microvesicle-associated AAV vector as a novel gene delivery system. Mol Ther. 2012;20(5):960–971. doi:10.1038/mt.2011.303
75. Yom-Tov N, Guy R, Offen D. Extracellular vesicles over adeno-associated viruses: advantages and limitations as drug delivery platforms in precision medicine. Adv Drug Deliv Rev. 2022;190:114535. doi:10.1016/j.addr.2022.114535
76. Zeng Y-F, Tseng SJ, Kempson IM, Peng S-F, W-T W, Liu J-R. Controlled delivery of recombinant adeno-associated virus serotype 2 using pH-sensitive poly(ethylene glycol)-poly-l-histidine hydrogels. Biomaterials. 2012;33(36):9239–9245. doi:10.1016/j.biomaterials.2012.09.018
77. Rey-Rico A, Venkatesan JK, Frisch J, et al. Effective and durable genetic modification of human mesenchymal stem cells via controlled release of rAAV vectors from self-assembling peptide hydrogels with a maintained differentiation potency. Acta Biomater. 2015;18:118–127. doi:10.1016/j.actbio.2015.02.013
78. Remes A, Basha DI, Puehler T, et al. Alginate hydrogel polymers enable efficient delivery of a vascular-targeted AAV vector into aortic tissue. Mol Ther. 2021;21:83–93. doi:10.1016/j.omtm.2021.02.017
79. Shi H, Xue T, Yang Y, et al. Microneedle-mediated gene delivery for the treatment of ischemic myocardial disease. Sci Adv. 2020;6(25). doi:10.1126/sciadv.aaz3621
80. Liu Y, Long L, Zhang F, et al. Microneedle-mediated vascular endothelial growth factor delivery promotes angiogenesis and functional recovery after stroke. J Control Release. 2021;338:610–622. doi:10.1016/j.jconrel.2021.08.057
81. Bowles DE, McPhee SW, Li C, et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther. 2012;20(2):443–455. doi:10.1038/mt.2011.237
82. Wang D, Li S, Gessler DJ, et al. A rationally engineered capsid variant of AAV9 for systemic CNS-directed and peripheral tissue-detargeted gene delivery in neonates. Mol Ther Methods Clin Dev. 2018;9:234–246. doi:10.1016/j.omtm.2018.03.004
83. Seo JW, Ajenjo J, Wu B, et al. Multimodal imaging of capsid and cargo reveals differential brain targeting and liver detargeting of systemically-administered AAVs. Biomaterials. 2022;288:121701. doi:10.1016/j.biomaterials.2022.121701
84. Palfi A, Chadderton N, Millington-Ward S, et al. AAV-PHP.eB transduces both the inner and outer retina with high efficacy in mice. Mol Ther Methods Clin Dev. 2022;25:236–249. doi:10.1016/j.omtm.2022.03.016
85. Gilkes JA, Judkins BL, Herrera BN, et al. Site-specific modifications to AAV8 capsid yields enhanced brain transduction in the neonatal MPS IIIB mouse. Gene Ther. 2021;28(7–8):447–455. doi:10.1038/s41434-020-00206-w
86. Kotterman MA, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet. 2014;15(7):445–451. doi:10.1038/nrg3742
87. Wu P, Xiao W, Conlon T, et al. Mutational analysis of the adeno-associated virus type 2 (AAV2) capsid gene and construction of AAV2 vectors with altered tropism. J Virol. 2000;74(18):8635–8647. doi:10.1128/JVI.74.18.8635-8647.2000
88. Nicklin SA, Buening H, Dishart KL, et al. Efficient and selective AAV2-mediated gene transfer directed to human vascular endothelial cells. Mol Ther. 2001;4(3):174–181. doi:10.1006/mthe.2001.0424
89. Yu CY, Yuan Z, Cao Z, et al. A muscle-targeting peptide displayed on AAV2 improves muscle tropism on systemic delivery. Gene Ther. 2009;16(8):953–962. doi:10.1038/gt.2009.59
90. Uhrig S, Coutelle O, Wiehe T, Perabo L, Hallek M, Büning H. Successful target cell transduction of capsid-engineered rAAV vectors requires clathrin-dependent endocytosis. Gene Ther. 2012;19(2):210–218. doi:10.1038/gt.2011.78
91. Lee NC, Falk DJ, Byrne BJ, et al. An acidic oligopeptide displayed on AAV2 improves axial muscle tropism after systemic delivery. Genet Vaccines Ther. 2012;10(1):3. doi:10.1186/1479-0556-10-3
92. Ried MU, Girod A, Leike K, Büning H, Hallek M. Adeno-associated virus capsids displaying immunoglobulin-binding domains permit antibody-mediated vector retargeting to specific cell surface receptors. J Virol. 2002;76(9):4559–4566. doi:10.1128/JVI.76.9.4559-4566.2002
93. Kunze C, Börner K, Kienle E, et al. Synthetic AAV/CRISPR vectors for blocking HIV-1 expression in persistently infected astrocytes. Glia. 2018;66(2):413–427. doi:10.1002/glia.23254
94. Bauer A, Puglisi M, Nagl D, et al. Molecular signature of astrocytes for gene delivery by the synthetic adeno-associated viral vector rAAV9P1. Adv Sci. 2022;9(16):e2104979. doi:10.1002/advs.202104979
95. Kardani K, Milani A, H. Shabani S, Bolhassani A. Cell penetrating peptides: the potent multi-cargo intracellular carriers. Expert Opin Drug Deliv. 2019;16(11):1227–1258. doi:10.1080/17425247.2019.1676720
96. Meng Y, Sun D, Qin Y, Dong X, Luo G, Liu Y. Cell-penetrating peptides enhance the transduction of adeno-associated virus serotype 9 in the central nervous system. Mol Ther Methods Clin Dev. 2021;21:28–41. doi:10.1016/j.omtm.2021.02.019
97. Zhang X, He T, Chai Z, Samulski RJ, Li C. Blood-brain barrier shuttle peptides enhance AAV transduction in the brain after systemic administration. Biomaterials. 2018;176:71–83. doi:10.1016/j.biomaterials.2018.05.041
98. Liu Y, Kim YJ, Ji M, et al. Enhancing gene delivery of adeno-associated viruses by cell-permeable peptides. Mol Ther Methods Clin Dev. 2014;1:12. doi:10.1038/mtm.2013.12
99. Yao Y, Wang J, Liu Y, et al. Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood-brain barrier in rodents and primates. Nat Biomed Eng. 2022;6(11):1257–1271. doi:10.1038/s41551-022-00938-7
100. Rode L, Bär C, Groß S, et al. AAV capsid engineering identified two novel variants with improved in vivo tropism for cardiomyocytes. Mol Ther. 2022;30(12):3601–3618. doi:10.1016/j.ymthe.2022.07.003
101. Pavlou M, Schön C, Occelli LM, et al. Novel AAV capsids for intravitreal gene therapy of photoreceptor disorders. EMBO Mol Med. 2021;13(4):e13392. doi:10.15252/emmm.202013392
102. Jost PJ, Harbottle RP, Knight A, Miller AD, Coutelle C, Schneider H. A novel peptide, THALWHT, for the targeting of human airway epithelia. FEBS Lett. 2001;489(2–3):263–269. doi:10.1016/S0014-5793(00)02236-5
103. White AF, Mazur M, Sorscher EJ, Zinn KR, Ponnazhagan S. Genetic modification of adeno-associated viral vector type 2 capsid enhances gene transfer efficiency in polarized human airway epithelial cells. Hum Gene Ther. 2008;19(12):1407–1414. doi:10.1089/hum.2008.117
104. Chai Z, Sun J, Rigsbee KM, Wang M, Samulski RJ, Li C. Application of polyploid adeno-associated virus vectors for transduction enhancement and neutralizing antibody evasion. J Control Release. 2017;262:348–356. doi:10.1016/j.jconrel.2017.08.005
105. Robinson TM, Chen MY, Lam MT, Ykema MR, Suh J. Display of self-peptide on adeno-associated virus capsid decreases phagocytic uptake in vitro. ACS Synth Biol. 2020;9(9):2246–2251. doi:10.1021/acssynbio.0c00203
106. Hui DJ, Basner-Tschakarjan E, Chen Y, et al. Modulation of CD8+ T cell responses to AAV vectors with IgG-derived MHC class II epitopes. Mol Ther. 2013;21(9):1727–1737. doi:10.1038/mt.2013.166
107. Tervo DG, Hwang BY, Viswanathan S, et al. A designer AAV variant permits efficient retrograde access to projection neurons. Neuron. 2016;92(2):372–382. doi:10.1016/j.neuron.2016.09.021
108. Zhang Y, Wang J, Li J, et al. Functional analysis of mutations endowing rAAV2-retro with retrograde tracing capacity. Neurosci Lett. 2022;784:136746. doi:10.1016/j.neulet.2022.136746
109. Pulicherla N, Shen S, Yadav S, et al. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol Ther. 2011;19(6):1070–1078. doi:10.1038/mt.2011.22
110. Yang L, Jiang J, Drouin LM, et al. A myocardium tropic adeno-associated virus (AAV) evolved by DNA shuffling and in vivo selection. Proc Natl Acad Sci U S A. 2009;106(10):3946–3951. doi:10.1073/pnas.0813207106
111. Koerber JT, Jang JH, Schaffer DV. DNA shuffling of adeno-associated virus yields functionally diverse viral progeny. Mol Ther. 2008;16(10):1703–1709. doi:10.1038/mt.2008.167
112. Li W, Asokan A, Wu Z, et al. Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles. Mol Ther. 2008;16(7):1252–1260. doi:10.1038/mt.2008.100
113. Grimm D, Lee JS, Wang L, et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol. 2008;82(12):5887–5911. doi:10.1128/JVI.00254-08
114. Liu YB, Xu BC, Chen YT, et al. Directed evolution of AAV accounting for long-term and enhanced transduction of cardiovascular endothelial cells in vivo. Mol Ther Methods Clin Dev. 2021;22:148–161. doi:10.1016/j.omtm.2021.05.015
115. Choudhury SR, Fitzpatrick Z, Harris AF, et al. In vivo selection yields AAV-B1 capsid for central nervous system and muscle gene therapy. Mol Ther. 2016;24(7):1247–1257. doi:10.1038/mt.2016.84
116. Lin R, Zhou Y, Yan T, et al. Directed evolution of adeno-associated virus for efficient gene delivery to microglia. Nat Methods. 2022;19(8):976–985. doi:10.1038/s41592-022-01547-7
117. Nonnenmacher M, Wang W, Child MA, et al. Rapid evolution of blood-brain-barrier-penetrating AAV capsids by RNA-driven biopanning. Mol Ther Methods Clin Dev. 2021;20:366–378. doi:10.1016/j.omtm.2020.12.006
118. Chan KY, Jang MJ, Yoo BB, et al. Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci. 2017;20(8):1172–1179. doi:10.1038/nn.4593
119. Siu JJ, Queen NJ, Huang W, et al. Improved gene delivery to adult mouse spinal cord through the use of engineered hybrid adeno-associated viral serotypes. Gene Ther. 2017;24(6):361–369. doi:10.1038/gt.2017.27
120. Tabebordbar M, Lagerborg KA, Stanton A, et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell. 2021;184(19):4919–4938.e4922. doi:10.1016/j.cell.2021.08.028
121. Weinmann J, Weis S, Sippel J, et al. Identification of a myotropic AAV by massively parallel in vivo evaluation of barcoded capsid variants. Nat Commun. 2020;11(1):5432. doi:10.1038/s41467-020-19230-w
122. Wang Y, Yang C, Hu H, et al. Directed evolution of adeno-associated virus 5 capsid enables specific liver tropism. Mol Ther Nucleic Acids. 2022;28:293–306. doi:10.1016/j.omtn.2022.03.017
123. Zhang S, Hu ZW, Luo HY, et al. AAV/BBB-mediated gene transfer of CHIP attenuates brain injury following experimental intracerebral hemorrhage. Transl Stroke Res. 2020;11(2):296–309. doi:10.1007/s12975-019-00715-w
124. Hanlon KS, Meltzer JC, Buzhdygan T, et al. Selection of an efficient AAV vector for robust CNS transgene expression. Mol Ther Methods Clin Dev. 2019;15:320–332. doi:10.1016/j.omtm.2019.10.007
125. Chen X, Ravindra Kumar S, Adams CD, et al. Engineered AAVs for non-invasive gene delivery to rodent and non-human primate nervous systems. Neuron. 2022;110(14):2242–2257.e2246. doi:10.1016/j.neuron.2022.05.003
126. Goertsen D, Flytzanis NC, Goeden N, et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat Neurosci. 2022;25(1):106–115. doi:10.1038/s41593-021-00969-4
127. Ravindra Kumar S, Miles TF, Chen X, et al. Multiplexed Cre-dependent selection yields systemic AAVs for targeting distinct brain cell types. Nat Methods. 2020;17(5):541–550. doi:10.1038/s41592-020-0799-7
128. Tse LV, Klinc KA, Madigan VJ, et al. Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion. Proc Natl Acad Sci U S A. 2017;114(24):E4812–E4821. doi:10.1073/pnas.1704766114
129. Havlik LP, Simon KE, Smith JK, et al. Coevolution of Adeno-associated Virus Capsid Antigenicity and Tropism through a Structure-Guided Approach. J Virol. 2020;94(19). doi:10.1128/JVI.00976-20
130. Zinn E, Pacouret S, Khaychuk V, et al. In silico reconstruction of the viral evolutionary lineage yields a potent gene therapy vector. Cell Rep. 2015;12(6):1056–1068. doi:10.1016/j.celrep.2015.07.019
131. Pan B, Askew C, Galvin A, et al. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nat Biotechnol. 2017;35(3):264–272. doi:10.1038/nbt.3801
132. Ogden PJ, Kelsic ED, Sinai S, Church GM. Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science. 2019;366(6469):1139–1143. doi:10.1126/science.aaw2900
133. Bryant DH, Bashir A, Sinai S, et al. Deep diversification of an AAV capsid protein by machine learning. Nat Biotechnol. 2021;39(6):691–696. doi:10.1038/s41587-020-00793-4
134. Zhu D, Brookes DH, Busia A, et al. Optimal trade-off control in machine learning-based library design, with application to adeno-associated virus (AAV) for gene therapy. Sci Adv. 2024;10(4):eadj3786. doi:10.1126/sciadv.adj3786
135. Le HT, Yu QC, Wilson JM, Croyle MA. Utility of PEGylated recombinant adeno-associated viruses for gene transfer. J Control Release. 2005;108(1):161–177. doi:10.1016/j.jconrel.2005.07.019
136. Yao T, Zhou X, Zhang C, et al. Site-specific PEGylated adeno-associated viruses with increased serum stability and reduced immunogenicity. Molecules. 2017;22(7):1155. doi:10.3390/molecules22071155
137. Zdechlik AC, He Y, Aird EJ, Gordon WR, Schmidt D. Programmable assembly of adeno-associated virus–antibody composites for receptor-mediated gene delivery. Bioconjugate Chem. 2019;31(4):1093–1106. doi:10.1021/acs.bioconjchem.9b00790
138. Wu K, Su D, Liu J, Saha R, Wang J-P. Magnetic nanoparticles in nanomedicine: a review of recent advances. Nanotechnology. 2019;30(50):502003. doi:10.1088/1361-6528/ab4241
139. Kim E, Oh J-S, Ahn I-S, Park KI, Jang J-H. Magnetically enhanced adeno-associated viral vector delivery for human neural stem cell infection. Biomaterials. 2011;32(33):8654–8662. doi:10.1016/j.biomaterials.2011.07.075
140. Mukherjee S, Kuroiwa M, Oakden W, et al. Local magnetic delivery of adeno-associated virus AAV2(quad Y-F)-mediated BDNF gene therapy restores hearing after noise injury. Mol Ther. 2022;30(2):519–533. doi:10.1016/j.ymthe.2021.07.013
141. Faller DV, Baltimore D. Liposome encapsulation of retrovirus allows efficient superinfection of resistant cell lines. J Virol. 1984;49(1):269–272. doi:10.1128/jvi.49.1.269-272.1984
142. Fiandaca MS, Varenika V, Eberling J, et al. Real-time MR imaging of adeno-associated viral vector delivery to the primate brain. NeuroImage. 2009;47:T27–T35. doi:10.1016/j.neuroimage.2008.11.012
143. Fein DE, Limberis MP, Maloney SF, Heath JM, Wilson JM, Diamond SL. Cationic lipid formulations alter the in vivo tropism of AAV2/9 vector in lung. Mol Ther. 2009;17(12):2078–2087. doi:10.1038/mt.2009.173
144. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
145. Hudry E, Martin C, Gandhi S, et al. Exosome-associated AAV vector as a robust and convenient neuroscience tool. Genet Ther. 2016;23(4):380–392. doi:10.1038/gt.2016.11
146. Meliani A, Boisgerault F, Fitzpatrick Z, et al. Enhanced liver gene transfer and evasion of preexisting humoral immunity with exosome-enveloped AAV vectors. Blood Adv. 2017;1(23):2019–2031. doi:10.1182/bloodadvances.2017010181
147. Liu B, Li Z, Huang S, et al. AAV-containing exosomes as a novel vector for improved gene delivery to lung cancer cells. Front Cell Develop Biol. 2021;9:707607. doi:10.3389/fcell.2021.707607
148. Matsuzaka Y, Hirai Y, Hashido K, Okada T. Therapeutic application of extracellular vesicles-capsulated adeno-associated virus vector via nSMase2/Smpd3, satellite, and immune cells in Duchenne muscular dystrophy. Int J Mol Sci. 2022;23(3):1551. doi:10.3390/ijms23031551
149. György B, Sage C, Indzhykulian AA, et al. Rescue of hearing by gene delivery to inner-ear hair cells using exosome-associated AAV. Mol Ther. 2017;25(2):379–391. doi:10.1016/j.ymthe.2016.12.010
150. Wassmer SJ, Carvalho LS, György B, Vandenberghe LH, Maguire CA. Exosome-associated AAV2 vector mediates robust gene delivery into the murine retina upon intravitreal injection. Sci Rep. 2017;7(1). doi:10.1038/srep45329
151. György B, Maguire CA. Extracellular vesicles: nature’s nanoparticles for improving gene transfer with adeno-associated virus vectors. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018;10(3):e1488. doi:10.1002/wnan.1488
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.