Back to Journals » International Journal of Nanomedicine » Volume 19
Advanced Drug Delivery Technologies for Enhancing Bioavailability and Efficacy of Risperidone
Authors Rathi R , Mehetre NM, Goyal S , Singh I, Huanbutta K, Sangnim T
Received 23 August 2024
Accepted for publication 22 November 2024
Published 30 November 2024 Volume 2024:19 Pages 12871—12887
DOI https://doi.org/10.2147/IJN.S492684
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Ritu Rathi,1 Nitin Martandrao Mehetre,1 Shuchi Goyal,1 Inderbir Singh,1 Kampanart Huanbutta,2 Tanikan Sangnim3
1Chitkara College of Pharmacy, Chitkara University, Patiala, PB, India; 2Department of Manufacturing Pharmacy, College of Pharmacy, Rangsit University, Pathum Thani, Thailand; 3Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Burapha University, Chonburi, Thailand
Correspondence: Tanikan Sangnim, Department of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Burapha University, 169, Seansook, Muang, Chonburi, 20131, Thailand, Email [email protected]
Abstract: Multidisciplinary research has been conducted on novel drug delivery technologies to maximize therapeutic advantages while curtailing undesirable reactions. Drugs under BCS Class II often have a low bioavailability because the dissolution phase limits the absorption efficiency. In this review, risperidone was used as a pharmacological model to examine the impact of solubility enhancement at the primary administration site for such pharmaceuticals. For tackling drug-related pertains like disease diagnostics, therapy, and prophylactic measures at the cellular or molecular levels, implementing nanocarriers in therapeutics has significant potential. The comprehensive pharmaceutical compositions of risperidone nano-microparticles that have been developed to alleviate psychosis are highlighted in the study, which also illustrates potential future developments in such domains.
Keywords: nanoparticles, risperidone, psychosis, micro particulates, bioavailability
Introduction
Risperidone, an antipsychotic pharmaceutical ingredient, is administered to address psychotic conditions like schizophrenia and bipolar disorder (acute mania). Yet, it has also been given approval to be employed to cure autism-related hyperactivity.1 Considering it has less extrapyramidal adverse consequences than traditional antipsychotics, the medication was recognized by the US FDA as a second-generation antipsychotic (SGA) medicine in 1993.2 It was reported to be the first medication that the FDA accepted for autism. It was introduced to the market at the beginning of the 1990s, many years after the advent of the prototype of the SGA, clozapine, which was licensed in Europe in the 1970s. Majorly, it is well managed with dose-dependency but infrequent extrapyramidal deleterious reactions.3
Risperidone has already been marketed under various brand names. Among these, risperdal and resomer tablets are widely used for the treatment of schizophrenia and bipolar disorders. Oral solution of risperidone under the brand name Risperdal and Risdone, which is easy to administer. Risperdal Costa is a long-acting injectable for patients who cannot adhere to a daily dose regimen. Risperdal M-Tab is an oral disintegrating tablet, that is convenient and quick to use for patients having swallowing difficulty. Other than these, risperidone is available in a generic version as well under the name risperidone. Microparticle formulations- This includes examples like Perseris.
Risperidone is a benzisoxazole derivative. The chemical entity of risperidone is “3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one”4 scaffold as the core linked via piperidine to a benzisoxazole moiety (Figure 1).
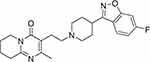 |
Figure 1 Chemical structure of Risperidone. |
The chemical composition is C23H27FN4O2 with a molecular weight of 410.49 g/mol. Nearly incompatible in water, risperidone is a white to almost white powder that is soluble in methylene chloride, methanol, and 0.1 N HCl.5
Risperidone has documented low oral bioavailability and a 3-hour half-life due to first-pass metabolism. Additionally, risperidone’s unintended administration has an assortment of undesirable outcomes.6 Risperidone, like many other drugs, needs to cross the blood–brain barrier (BBB) to employ its impact on the central nervous system, where it primarily acts on dopamine and serotonin receptors.7 The BBB is a highly selective and protective obstacle that separates the bloodstream from the brain’s extracellular fluid. It regulates the movement of components, including drugs, from the blood into the brain.8
Risperidone-loaded Nano-Micromaterials were investigated to prevent these pharmaceutical-delivering complications. Risperidone’s biodistribution and bioavailability can potentially be increased for better pharmacotherapy through the intravenous delivery of nanoparticles (Nps) to maintain plasma levels.9
The use of nano/microcarriers in medicine holds considerable prospects for addressing drug-related issues like disease diagnosis, therapy, and preventive strategies at the cellular and molecular divisions. The spectrum of nanoformulations particle sizes is 1–1000 nm (1 m). For diagnostics and/or therapy, chemicals are typically adsorbed, entrapped, conjugated, or encapsulated in nanosciences.10 The nanotechnology’s selective ligand(s) in the nanotechnology may interact with distinct targets or receptors on the cell surface to produce targeted or synergistic consequences. Currently, sophisticated nanoformulations have been created to approach particular cellular compartments.11
To attain desirable or beneficial in vitro/in vivo characteristics, such as bioavailability advancement, toxicity elimination, dose diminution, solubility improvements, and drug targeting, along with product stability, surface-modified nanotechnologies (ie, hydrophilic surfaces) via PEG or its analogs in a spectrum of 100–200 nm are typically favored.12
Benefits of Nano-Micro carriers for risperidone include improved bioavailability, sustained release formulations, targeted delivery, reduced side effects, and improved patient compliance (Figure 2).13
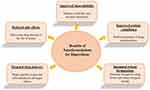 |
Figure 2 Representation focusing on the benefits of nanoformulations. |
Mechanism of Action
Risperidone inhibits the brain’s D2 dopaminergic and 5-HT2A serotonergic receptors. Schizophrenia is hypothesized to be caused by an overabundance of serotonergic 5-HT2A and dopaminergic D2 action, accordingly, which activates the central mesolimbic and mesocortical networks. Risperidone temporarily inhibits D2 dopaminergic receptors, which lowers dopaminergic neurotransmission and lessens the favorable signs of schizophrenia, including delusions and hallucinations.14 Risperidone interacts with the dopaminergic D2 receptors momentarily and with low potency; the receptor’s activation should be between 60% and 70% for best results.15 Risperidone’s quick dissociation from D2 receptors helps to reduce the likelihood of extrapyramidal symptoms (EPS), which are brought on by the persistent and intense blocking of D2 dopaminergic receptors. Risperidone differs from other antipsychotic medications because it binds to the D2 receptor with low potency and dissociates quickly. There is a reduction in serotonergic reactivity as a result of risperidone’s strong propensity for adhering to 5-HT2A receptors. Additionally, blocking the 5-HT2A receptor lowers the incidence of EPS, perhaps by enhancing dopamine production from the frontal cortex rather than the nigrostriatal tract.16
The pharmacological development of risperidone was based on its predecessor ritanserin, a 5-HT2A blocker that was able to demonstrate effectiveness in managing adverse sensations, but not sufficiently in treating positive symptoms of schizophrenia. It also reduced neuroleptic-induced extrapyramidal symptoms (EPS).17 A drug that possesses the primary pharmacologic activity of D2-/5-HT2A receptor antagonism, with a greater impact on 5-HT2A receptors than on D2 receptors, was created by altering the compound. Other serotonin-dopamine antagonists were developed using these pharmacologic characteristics as a framework. A significant improvement in therapeutic options for schizophrenia individuals is provided by risperidone and the related sdas.18
Medication with risperidone may cause neurological adverse impacts such as akathisia, rigidity, or tremor. Nevertheless, several research initiatives looking at the connection between risperidone or 9-hydroxy risperidone concentrations and neurological impairment came up with inconsistent conclusions. Another frequent problem for risperidone individuals is hyperprolactinemia. In addition to potentially causing sexual dysfunction, it may also pose an extended risk for cardiovascular disease and a decline in bone mineral density.19
The illustration representing the mechanism of action of risperidone is shown in Figure 3.
Formulation Development Strategies for Risperidone
The main factor attributing to the reduced therapeutic efficacy of nano-formulations is the capacity to develop self-aggregation when exposed to low concentrations of drugs, hence compromising the stability of the formulation. There are various major factors to consider in formulating drugs in nano-form to improve uptake and efficiency20–22 as shown in (Figure 4), Stabilization criteria, Process elements, Constituent attributes, and Characterization methodology are the four main divisions of the Ishikawa flowchart.23 All aspects were represented under each section. From these, the following significant elements were chosen and addressed: Instrumental, Temperature, stirring time, and speed of stirring higher stirring rates could result in better interaction, which may produce nanoparticles that are smaller and more homogeneous.24 Fortunately, overly rapid spinning rates can also lead to the aggregation or fragmentation of nanoparticles. Environmental- temperature, pH, as well as chemical interactions normally proceed swiftly at elevated temperatures, and pH affects the surface charge of nanoparticles. Temperature also affects the reaction rates. Regardless of the pH level, nanomaterials can have positive, negative, or neutral charged potentials. It has been demonstrated in the characterization methodology that energy attributes, particle size, PDI (Polydispersity index), interface charge, %EE (Entrapment efficiency), %DL (Drug loading), chemical content, and coating layers exhibit a substantial influence on the density and packing characteristics of nanoparticle aggregates Transmission electron microscopy (TEM), dynamic light scattering (DLS), and laser diffraction are all displayed using PDI.25 The manner of specimen setup, microscopy technique, and data interpretation can impact the precision of coating thickness estimates. Other factors, such as surfactants and stabilizers, may also influence the electrostatic associations between nanoparticles and their persistence in suspension. Lipophilic and hydrophilic drugs can be incorporated. Hydrophilic drugs may have faster distribution patterns than lipophilic drugs due to changes in kinetics. While shortest sequences may have a higher drug loading capability, longer ones may have a higher degree of steric integrity. The extruder covering pore size also significantly impacts the ultimate product size.26
 |
Figure 4 Ishikawa diagram representing various critical parameters for the preparation of nanoparticles. |
Risperidone Liposome
Liposomes are micro or colloidal carriers, generally ranging from 0.05 to 5.0 µm in diameter, that develop when certain lipids are hydrated in aqueous solutions. The size of liposomes is an essential variable that influences the amount of liposomes removed by the reticuloendothelial system risperidone (RES).27 Liposomes with a diameter smaller than 0.1 µm show a less severe opsonization process compared to liposomes with a diameter larger than 0.1 µm. Therefore, the rates at which liposomes are taken up by the RES are directly related to the size of the vesicle.28 Liposomes can be characterized based on their lamellarity, including uni-, oligo-, and multi-lamellar vesicles. Also, liposomes can be classified according to their size, so they can be small, intermediate, or large. Moreover, liposomes may be classified based on the method used for their production, such as reverse phase evaporation vesicles (REVs) or various other methods. Unilamellar vesicles have a single lipid bilayer and usually have sizes ranging from 50 to 250 nm.29 Microspheres are small, offer a substantial aqueous core, and are typically used for the encapsulation of medicines that are soluble in water. Multilamellar vesicles consist of many annular lipid bilayers grouped in a manner like the layers of an onion skin. Such vesicles generally have sizes ranging from 1 to 5 μm. Passive entrapment of lipid-soluble drugs is helped by the increased lipid content shown within these multilamellar vesicles.30
Liposomes have been utilized as delivery methods for multiple chemicals caused of their special biocompatibility. NPs give significant improvements in the therapeutic indices of the therapeutic molecules enclosed in them.31 Liposomes have a biphasic nature, allowing them to act as carriers for drugs that are both lipophilic and hydrophilic. The distribution and behavior of drug molecules within a liposomal environment vary according to their solubility and partition characteristics, which lead to distinct entrapment and release characteristics.32
Liposomes are widely acknowledged as effective for drug delivery due to their structural responsiveness, as well as their natural properties like biocompatibility, biodegradability, non-toxicity, and non-immunogenicity.33 The application of liposomes as a drug delivery system has significantly enhanced treatments in various biomedical areas. The following works through the stabilization of therapeutic compounds, overcoming barriers related to cellular and tissue absorption, as well as the targeted delivery and distribution of compounds to specific areas in cells.34 Liposomes offer two significant advantages concerning drug administration in organisms, specifically biocompatibility, and biodegradability, respectively, both of which can be related to the natural properties of lipids.35
The In vitro dissolution studies showed that Narayan et al. These liposomes were generated using the thin-film hydration approach, where usual liposomes made from soya phosphatidylcholine (SPC) and cholesterol were loaded. The lipid-based film hydration method has been used with some modifications. To achieve better brain penetration. The enhanced formulation was assessed based on its physicochemical characteristics. The liposomes revealed distinct spherical vesicular structures, such as a smooth bilayered surface, with a size range that extends from 90 to 100 nm. A sustained high encapsulation efficiency, ranging from 91% to 94%, was observed.36
Risperidone Nanoparticles
Nanotechnology is a field of study and innovation that focuses on research and development performed at the atomic, molecular, or macromolecular scales. Nanoparticles can be readily identified as the fundamental components for the study of nanotechnology.37 Nanoparticles can be identified as dispersions of solid particles with diameters usually lying within the range of 10–1000nm. Pharmaceutical drugs are solubilized, immobilized, encapsulated, or linked onto a matrix that includes nanoparticles. Nanoparticles, nanospheres, and nanocapsules can be obtained via the chosen method of preparation.38,39 R Rukmangathen.et al formulated nanoparticles for the management of schizophrenia through intranasal administration. Using Chitosan, tripolyphosphate, and tween 80/poloxamer 188, Nps were formulated by ionic gelation. It was observed that Nps were shown controlled drug release via Fickian diffusion. As risperidone is classified as part of BCS class II, it has a high hydrophobicity and is extensively metabolized by the liver, as a result, its bioavailability is reported to be variable (70%). L. Lugasi et al, reported that encapsulation of RSP into nanoparticles was done to stabilize it by enhancing tolerability and adherence and thus increasing the bioavailability, thereby improving the antipsychotic activity and reducing the side effects.
Risperidone Nanoemulsions
Nanoemulsions are liquid-in-liquid dispersions characterized as having kinetic stability having droplet sizes usually in the range of 100 nm.40,41 The narrow sizes of such particles give rise to beneficial features, including a significant surface area corresponding to their volume, strong strength, clearly transparent attributes, and customized rheological characteristics.42 A standard nanoemulsion is composed of three primary components: oil, water, and an emulsifier. The usage of an emulsifier is essential for promoting the development of small droplets since it decreases the interfacial tension, which refers to the energy per unit area at the interface between both the water and oil phases of the emulsion. The emulsifier also fulfills an objective in the stabilization of nanoemulsions by using repulsive electrostatic interactions and steric hindrance.43 The small dimensions of these particles allow them to effectively bypass tissues at a profound level, hence extending their presence inside the bloodstream and allowing separate bio-nano interactions. On average, a surfactant acts as an emulsifier, however, proteins and lipids have also proven efficacy in the development of nanoemulsions. They have kinetic stability, indicating that with time, a phase separation occurs in nanoemulsions. To create nanoemulsions, a process that involves two stages is necessary. Initially, coarse emulsions develop, and they are subsequently treated with high-pressure homogenization or ultrasonication to minimize the size of the larger droplets to the nano-size, resulting in the formation of nanoemulsions.44,45
Đorđević et al proposed that a possible enhancement of brain drug availability in P80- and PL188-containing preparations of risperidone could be attributed, in part, to the restriction of the P-gp efflux system at the blood–brain barrier46
In vivo studies carried out with risperidone nanoemulsion (RSP-nes) stabilized with P80 (RSP-nes) showed a 1.2–1.5-fold increase in relative bioavailability, a 1.1–1.8-fold decrease in liver accumulation, and around 1.3-fold higher drug entry to the brain after the intraperitoneal injection of RSP-nes compared to the administration of the free drug solution in a rat model.47 Also, according to behavioral research, it was observed that rats treated with RSP-nes had decreased in both initial and amphetamine-induced locomotor activity. The animals that received RSP-NE revealed an early initiation of antipsychotic symptoms that were maintained for around 90 minutes after delivery.48
The investigators conducted a study into a nanoemulsion formulation containing risperidone (RSP) to enhance medication delivery to the brain via intranasal administration. The study’s investigation of both risperidone nanoemulsion (RNE) and mucoadhesive nanoemulsion (RMNE) encompassed the assessment of drug concentration, globule size, pH, percentage transmittance, and zeta potential. The study primarily examined the biodistribution of RMNE, RNE, and risperidone solution (RS) in the brain and blood of Swiss albino rats. The drugs were administered using intravenous and intranasal ways to accomplish this.49 The biodistribution study of risperidone formulation was conducted using technetium-labeled substances, with suitable optimization techniques. The localization of the medication in the rat brain was determined using gamma scintigraphy imaging, followed by intravenous and intranasal administrations.50 The results of the study demonstrated that mucoadhesive nanoemulsions showed higher drug transport efficiency (DTE %) and direct nose-to-brain drug transport (direct transport percentage, DTP%) compared to other produced nanoemulsions.51 These findings suggest that mucoadhesive nanoemulsions are more effective and offer better brain targeting for the delivery of RSP. Multiple studies have provided solid evidence of the swift and substantial transportation of the respiratory syncytial virus (RSV) surface protein (RSP) via the intranasal route using respiratory mucosal nanotechnology enhancers (RMNE). This transport efficiency was found to be much higher compared to the intranasal administration of RSV alone (RS), as well as intranasal and intravenous administration of RSV surface protein encapsulated in nanocarriers (RNE). Moreover, these investigations specifically focused on the transportation of RSP into the brain of rats.52
Risperidone Nanosuspensions
Nanosuspension technology is currently seeing significant growth and advancement in the field of pharmaceutical science research and development. The utilization of the nanosuspension method is a prevalent and widely applicable technique in the field of nanotechnology.53 This is mainly because newly developed chemical entities (NCEs) show practical insoluble in aqueous environments and offer difficulties for formulation using traditional methods. Pharmaceutical nanosuspensions consist of aqueous dispersions of insoluble drug particles that can be nanosized and exhibit variability. Those nanosuspensions are stabilized by the presence of surfactants.54 Nanosuspensions are now showing potential in both in vivo and in vitro conditions for the delivery of water-insoluble drugs. This can be related to their small size at the nanoscale, thus great specific surface area, and distinct physicochemical characteristics. The achievement of a high drug loading capacity (100%) in nanosuspensions can lead to the efficient delivery of drugs into cells, resulting in achieving therapeutic concentrations that are sufficiently high and optimizing the pharmacological effects.55
Risperidone Niosomes
The multi-lamellar vesicular complexes known as niosomes are made of non-ionic surfactants. They resemble liposomes but are made of non-ionic surfactant rather than the phospholipids found in liposomes.56,57
A widely recognized antipsychotic medication, risperidone is frequently used for the management of schizophrenia alongside other psychotic diseases. Risperidone taken orally is converted by the cytochrome P-450 enzymes into the equipotent 9-hydroxy risperidone, which has a narrow window of entry into the BBB. To solve this problem, risperidone-containing niosomes were developed, improved, and tested under the presumption that non-ionic surfactants inhibit cytochrome P-450 enzymes from metabolizing risperidone into its derivatives. Following testing of various levels and a span of 60 compositions, which had the finest entrapment efficacy (92.83%), niosomes were made using the sonication process. Vesicle sizes between 180 nm and 388.9 nm had higher zeta potential ranges and lower PDI (0.171 to 0.437) (−20.4 mv to −50.6 mv). Vesicles appeared to be spherical by TEM, and no probable incompatibilities among the formulation constituents were found, according to FTIR and DSC analyses. The in-vitro prolonged release profiles and Fickian diffusing mechanisms of niosomes were both present. For the compositions maintained at ambient temperatures (25±2°C) and under refrigeration (4±1°C) for 90 days, no appreciable changes in fundamental attributes, vesicle size, or entrapment effectiveness were found. The niosomes were resistant to the bile salts’ (sodium desoxycholate) solubilizing effects. Niosomes provide higher risperidone bioavailability and so can be exploited for efficient drug administration. Niosomes are self-assembled vesicles synthesized from non-ionic amphiphilic surfactants. Cholesterol and the charged compounds are introduced to the solution to increase stabilization and give the bilayers more stiffness. Niosomes were developed as a substitute for liposomes since they have advantages over them in terms of stability, sterilization, and mass synthesis. They are structurally identical to liposomes. Like liposomes, niosomes have hydrophilic and bilayer chambers that can, correspondingly, contain hydrophilic and hydrophobic pharmaceuticals. Additionally, they might augment the biodistribution along with the absorption of medicines, facilitate drug targeting, and ameliorate pharmacokinetics.58–62 Risperidone-containing niosomes were created by Sambhakar S. et al. Additionally, it was shown that the flow and permeability coefficient improved despite the usage of bile salts, resulting in a bioavailability of 111%. An additional group of investigators generated proniosomes of RIS and upon executing the assessed study prolonged releasing drugs with improved bioavailability was detected. The augmentation proportion was discovered to be approximately 2-fold for niosomes without bile salts and 1.33-fold for niosomes having bile salts. They also concluded that more potent medications may be provisioned further.63,64
Risperidone Microparticles/Microspheres
Since they exhibit better pharmacological and diagnostic effectiveness than traditional drug delivery forms (DDS), microparticulate technologies (typically of 1–1000 m) are frequently used as DDS. It is possible to achieve consistent and prolonged blood levels with successive injections by using microparticle technologies that encase risperidone in a biodegradable polymer and sequential hydrolysis of the microspheres.65,66 First and subsequent levels of risperidone and its active component could very well be provided by extended-release microparticles of the drug. Individuals who are diagnosed with schizophrenia will adhere to treatment more readily, experience fewer negative consequences, and have a better quality of life if long-acting dosage forms made with atypical antipsychotics are prepared properly.67 If the medicine is adjusted, systemic regulated release microspheres are a dependable way for delivering it to the target site with precision and keeping the appropriate concentrations at the region of concern without triggering any negative side implications. Due to its many potential uses, the biodegradable microspheric DDS has attracted a lot of consideration. Compared to other dose forms, it offers more benefits.68 Additionally, because the microspheres are micron-sized, they can conveniently fit into numerous parenteral regions and capillary beds. Although an array of polymers can be used to create these microspheres, biodegradable polymers like polylactide-co-glycolide (PLGA) and polycaprolactone (PCL) have become increasingly popular due to their useful and commercially feasible DDS.69 Due to their simplicity in planning, feasible commercial accessibility, adaptability, biological compatibility, hydrolytic deterioration into innocuous services, and potential for regulated release uses, the aforementioned polymers have drawn attention as suitable matrices for drug delivery microspheres. It is also biocompatible with a wide range of other polymers.70 Medical professionals using TDM for risperidone LAI microsphere formulations should: 1) examine steady state to be attained 6 weeks shortly after the first injection; 2) be cognizant of co-medications with inducers/inhibitors; 3) serious inflammations/infections; and 4) hepatic/renal impairment; and 3) apply Castberg’s suggestions to estimate risperidone dosage, who allocated the implemented LAI dose by the quantity of days.71,72. Table 1 below summarizes various formulation development strategies of risperidone for improving solubility and bioavailability.
 |
Table 1 Tabular Representation Emphasizing Formulation Development Strategies of Risperidone Employed for Improving the Solubility and Bioavailability |
Toxicological Studies
When atypical antipsychotic medications are used therapeutically, undesirable adverse reactions commonly emerge. These side events might happen either early or late in the duration of the treatment and can be idiopathic or dose-dependent.95 Numerous neurological disorders, including schizophrenia, bipolar disorder, and depressive symptoms, are treated with drugs like risperidone. Risperidone’s acute and long-term toxicities provide the most threat.96
These drugs have a complicated pharmacology and serious adverse interactions. Risperidone’s negative consequences, which include immune system modifications, have been extensively researched. According to clinical reports, individuals on risperidone are more vulnerable to infections.97 Risperidone and its active derivative, paliperidone, appear to have a primary impact on the bone marrow compartment, causing immunosuppression, myeloid dysplasia, leukopenia, neutropenia, lymphopenia, and thrombocytopenia.
Risperidone is linked to a reduction in platelet-associated antibody titers, a block in phagocytosis, and the emergence of intermittent eosinophilic pneumonia. The strongest evidence points to decreased blood levels of several cytokines and immune regulators as the rationale for the risperidone-induced immunosuppression. Immune function networks connected to T cell maturation/differentiation were among the more severely damaged by risperidone therapy. The vitality of human blood lymphocytes is decreased by risperidone.98
In lymphocytes that were administered with risperidone, the lysosome membrane was harmed. ROS, particularly superoxide radicals and hydrogen peroxide, are linked to lysosomal destruction.99 The lysosomes’ high iron (ferruginous) component enables interactions that result in potent oxidative species, including the production of an exceedingly reactive hydroxyl radical via a Fenton-type response, which causes membrane LPO and lysosome leakiness and the ensuing dissolution of its digesting proteases. The oxidative stress produced by mitochondria and redox-active, iron-rich lysosomes is increased as a result of this damaging mechanism.100 Risperidone excess dosage was typically accompanied by brief, typically mild neuromuscular (lethargy, muscle spasms/dystonia) and cardiovascular (tachycardia, hypotension, alterations in the electrocardiogram) symptoms. The hazardous consequences of an excessive intake of risperidone are mostly an amplification of its pharmacological actions. CNS and respiratory depression, miosis or mydriasis, hypertension or orthostatic hypotension, sinus tachycardia, agitation, psychosis, anticholinergic stigmata, and, less frequently, seizures, cardiac conduction abnormalities, atrial and ventricular dysrhythmia fibrillation are all signs of toxic effect.101
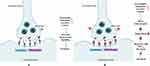
The more prevalent symptoms are CNS repercussions, which may vary from moderate poisoning indications like ataxia and fatigue to serious symptoms like significant unconsciousness and decline in neurological responses. Upon overdosing with any atypical drug, sinus tachycardia, and orthostatic hypotension are usually observed.102 Other adverse events included anxiety, droopy eyes, early menstrual flow, fainting spell, headache, heavy tongue, hot flashes, hyperthermia, multiple bruises, musculoskeletal pain, nervousness, oculogyric crisis, premature adrenarche, restlessness, tachycardia, thirst, worsened behavioral problem, and worsened muscle cramp as well as extrapyramidal manifestations.103 Risperidone induces oxidative stress and damages lysosomes and mitochondria in normal blood cells. Figure 5 shows risperidone mainly inhibits certain receptors responsible for its therapeutic action and adverse reaction. Some of the receptors promoting therapeutic action are D2 dopamine and 5-HT2A serotonin and the other reliable for adverse reactions are Muscarinic M3, Histamine H1, Adrenergic receptors.
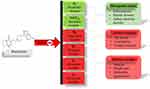 |
Figure 5 Receptors responsible for the therapeutic and adverse reactions of risperidone. |
Clinical Trials of Risperidone and Risperidone Related Formulations
Risperidone is an efficient antipsychotic that alleviates both unfavorable and favorable aspects of schizophrenia, according to scientific research. Extrapyramidal adverse reactions are not more frequent at acceptable dosage regimens than they are with a placebo. The medication seems to be a step forward in the management of psychosis.104 The outcomes of additional controlled trials assessing this medication’s tolerability and efficiency with other antipsychotic medications, as well as clinical evidence, will determine the level to which it will be used as a first-line therapy for the management of schizophrenia patients.105
Experimental trials were found evaluating the effectiveness and acceptability of risperidone. Table 2 below summarizes the findings of the identified risperidone for various neurological disorders depending on the stability, safety, and efficacy profiles.
 |
Table 2 Clinical Trials Conducted for risperidone106 |
Patents Related to and/or Risperidone-Based Formulations
Risperidone continues to be the focus of multiple patents and compositions, which exemplify the continuous endeavors in pharmaceutical studies to improve its therapeutic effectiveness, distribution methods, and patient results. Patents of risperidone frequently encompass original formulations, modes of delivery, and inventive amalgamations with other substances. For example, pharmaceutical formulations may prioritize enhancing the bioavailability of drugs, prolonging the release patterns of active compounds, or mitigating the adverse effects linked to medication. The aforementioned patents not only represent the continued commitment to enhancing the pharmacological features of risperidone but also highlight the broader industry advances regarding personalized treatment and optimal drug delivery methods. The ever-changing intellectual rights landscape in the pharmaceutical sector is a testament to the industry’s commitment to tackling the complex difficulties related to antipsychotic medications, with the eventual goal of enhancing the well-being of persons suffering from psychiatric diseases.107–112 The patents associated with risperidone-based formulations are listed below in Table 3.
 |
Table 3 Tabular Insights of Patents Associated with Risperidone-Based Formulations |
Future Perspectives
Nano and microparticulate systems hold great potential for research in advancing drug delivery systems and improving the therapeutic efficacy of drugs. Risperidone, an antipsychotic drug used for treating schizophrenia and bipolar disorder, has limited therapeutic efficacy due to poor physicochemical properties. The nanocarriers of risperidone present exciting possibilities for future research. Nanocarriers have the potential to improve the solubility and bioavailability of risperidone, resulting in better drug absorption and distribution. Future research could explore more strategies that could improve the solubility and bioavailability of risperidone. Future research could look at the importance of controlled sustained drug release formulations, which could result in prolonged therapeutic action, reduced administration frequency, and improved patient compliance. Targeted drug delivery systems aim to target specific tissues or organs, especially in the central nervous system, which is another exciting possibility that can improve the therapeutic outcome and minimize side effects. Efforts can be made to improve the stability and shelf life of these formulations, for improving long-term viability. Combination therapies, involving a combination of risperidone with another therapeutic agent, and personalized therapies could be a potential research area for effective management of diseases/disorders. If common challenges viz. commercial production, economy, toxicity, stability, and regulatory approval associated with nanocarriers are adequately addressed, their application in drug delivery could be more viable and justified. Leading technologies such as 3D printing, nanometers, nanotubes, and microneedles can also be investigated for the delivery and therapeutic effectiveness of risperidone.
Conclusion
Nanopsychiatry is a novel term that is used to emphasize the importance of combining psychiatry with nanocarriers. Nano/microcarriers are used for curing psychosis, illustrating the clinical success development for the treatment of psychotic disorders. A recent review shows the promising results of antipsychotic nano/microcarriers for bioavailability enhancement, dose reduction, extended and controlled drug release, and toxicity reduction. The development of risperidone nano and microparticles represents a significant advancement in pharmaceutical technology. These innovative drug formulations have the potential to address key challenges associated with the delivery and efficacy of risperidone. Researchers, pharmaceutical companies, and regulatory agencies continue to explore and evaluate the potential benefits and challenges associated with risperidone nano and microparticles. As this field of research evolves, it may lead to improved treatment options for individuals with psychiatric disorders and pave the way for similar innovations in drug delivery for other therapeutic areas.
Acknowledgments
The authors are thankful to Chitkara University and Burapha University for providing library and infrastructure facilities.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chopko TC, Lindsley CW. Classics in chemical neuroscience: risperidone. ACS Chem Neurosci. 2018;9(7):1520–1529. doi:10.1021/acschemneuro.8b00159
2. Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first-and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;29:757–777. doi:10.2147/TCRM.S117321
3. Nagaraj R, Singhi P, Malhi P. Risperidone in children with autism: randomized, placebo-controlled, double-blind study. J Child Neurol. 2006;21(6):450–455. doi:10.1177/08830738060210060801
4. Fodor AA, Pascu AM, Poroch V, et al. Long Term Efficacy of the Treatment with Olanzapine Pamoate, Risperidone and Aripiprazole Monohydrate. Rev Chim. 2018;69(3):650–653. doi:10.37358/RC.18.3.6168
5. Arowona IT, Sonibare MA, Yeye EO, Rauf K, Iqbal FJ. Isolation and structure elucidation of cyclohexanepentol, an anti-psychotic agent from Cissampelos owarensis (P. Beauv.) leaves. Niger J Pharm. 2022;18(2):101–112. doi:10.4314/njpr.v18i2.2
6. Rajeev MR, Manjusha V, Anirudhan TS. Transdermal delivery of doxorubicin and methotrexate from polyelectrolyte three layer nanoparticle of graphene oxide/polyethyleneimine/dextran sulphate for chemotherapy: in vitro and in vivo studies. J Chem Eng. 2023;466:143244. doi:10.1016/j.cej.2023.143244
7. Tao X, Zhu X, Liu Y, et al. Gas therapy strategies for depression and schizophrenia: a review. Medicine. 2023;102(46):e36156. doi:10.1097/MD.0000000000036156
8. Zhang S, Gan L, Cao F, et al. The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res Bull. 2022;190:69–83. doi:10.1016/j.brainresbull.2022.09.017
9. Kumari Y, Singh SK, Kumar R, et al. Modified apple polysaccharide capped gold nanoparticles for oral delivery of insulin. Int J Biol Macromol. 2020;149:976–988. doi:10.1016/j.ijbiomac.2020.01.302
10. Muthu MS, Leong DT, Mei L, Feng SS. Nanotheranostics˗ application and further development of nanomedicine strategies for advanced theranostics. Theranostics. 2014;4(6):660. doi:10.7150/thno.8698
11. Muthu MS, Agrawal P, Singh RP. Antipsychotic nanomedicine: a successful platform for clinical use. Nanomedicine. 2014;9(14):2071–2074. doi:10.2217/nnm.14.164
12. Bhattacharya T, Soares GA, Chopra H, et al. Applications of phyto-nanotechnology for the treatment of neurodegenerative disorders. Materials. 2022;15(3):804. doi:10.3390/ma15030804
13. Gupta H, Panchal R, Acharya N, Mehta PJ. Controlled parenteral formulations: an efficacious and favourable way to deliver the anti-psychotic drugs. Curr Psychiatry Rev. 2020;16(1):42–59. doi:10.2174/2666082216666191226143446
14. Begum AU, Fathima J. Huntington’s disease: a rare neurodegerative disorder. World J Pharm Res. 2020;9:353–435.
15. Servonnet A, Samaha AN. Antipsychotic-evoked dopamine supersensitivity. Neuropharmacology. 2020;163:107630. doi:10.1016/j.neuropharm.2019.05.007
16. Galimi R. Interaction Between anti-Alzheimer’s Disease Drugs and Antipsychotic Agents in the Treatment of Behavioral and Psychological Symptoms: extrapyramidal Side Effects. Adv Neur Neur Sci. 2022;5(2):119.
17. Matuszewska A, Kowalski K, Jawień P, et al. The Hypothalamic-Pituitary-Gonadal Axis in Men with Schizophrenia. Int J Mol Sci. 2023;24(7):6492. doi:10.3390/ijms24076492
18. Achtyes ED, Hopkins SC, Dedic N, et al. Ulotaront: review of preliminary evidence for the efficacy and safety of a TAAR1 agonist in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2023;10:1–4.
19. Locatelli I, Kastelic M, Koprivšek J, et al. A population pharmacokinetic evaluation of the influence of CYP2D6 genotype on risperidone metabolism in patients with acute episode of schizophrenia. Eur J Pharm Sci. 2010;41(2):289–298. doi:10.1016/j.ejps.2010.06.016
20. Jeevanandam J, San Chan Y, Danquah MK. Nano-formulations of drugs: recent developments, impact and challenges. Biochimie. 2016;128-129:99–112. doi:10.1016/j.biochi.2016.07.008
21. Alotaibi HF, Khafagy ES, Abu Lila AS, et al. Anticancer potentials of metformin loaded coconut oil nanoemulsion on MCF-7, HepG2 and HCT-116 cell lines. Artif Cells Nanomed Biotech. 2023;51(1):419–427. doi:10.1080/21691401.2023.2246145
22. Hatem S, Elkheshen SA, Kamel AO, et al. Functionalized chitosan nanoparticles for cutaneous delivery of a skin whitening agent: an approach to clinically augment the therapeutic efficacy for melasma treatment. Drug Deliv. 2022;29(1):1212–1231. doi:10.1080/10717544.2022.2058652
23. Ding F, Liu S, Wu G, et al. A novel process for developing a quantitative chromatographic fingerprint analysis method based on analytical quality by design: a case study of Xiaochaihu capsules. Microchem J. 2023;194:109253. doi:10.1016/j.microc.2023.109253
24. Łapińska A, Grochowska N, Antonowicz J, et al. Influence of the filler distribution on PDMS-graphene based nanocomposites selected properties. Sci Rep. 2022;12(1):19038. doi:10.1038/s41598-022-23735-3
25. Xu X, Khan MA, Burgess DJ. A quality by design (QbD) case study on liposomes containing hydrophilic API: i. Formulation, processing design and risk assessment. Int J Pharm. 2011;419(1–2):52–59. doi:10.1016/j.ijpharm.2011.07.012
26. Sreeharsha N, Rajpoot K, Tekade M, et al. Development of metronidazole loaded chitosan nanoparticles using QbD approach—A novel and potential antibacterial formulation. Pharmaceutics. 2020;12(10):920. doi:10.3390/pharmaceutics12100920
27. Rathi R, Sanshita K, Vishvakarma A, et al. Advancements in Rectal Drug Delivery Systems: clinical Trials, and Patents Perspective. Pharmaceutics. 2022;14(10):2210. doi:10.3390/pharmaceutics14102210
28. Sharma A, Sharma US. Liposomes in drug delivery: progress and limitations. Int J Pharm. 1997;154(2):123–140. doi:10.1016/S0378-5173(97)00135-X
29. Kaur S, Goyal A, Rai A, Sharma A, Ugoeze KC, Singh I. Quercetin nanoformulations: recent advancements and therapeutic applications. Adv Nat Sci: Nanosci Nanotechnol. 2023;14(3):033002.
30. Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed. 2006;1(3):297.
31. Nikolova MP, Kumar EM, Chavali MS. Updates on responsive drug delivery based on liposome vehicles for cancer treatment. Pharmaceutics. 2022;14(10):2195. doi:10.3390/pharmaceutics14102195
32. Gulati M, Grover M, Singh S, Singh M. Lipophilic drug derivatives in liposomes. Int J Pharm. 1998;165(2):129–168. doi:10.1016/S0378-5173(98)00006-4
33. Liang W, Dong Y, Shao R, et al. Application of nanoparticles in drug delivery for the treatment of osteosarcoma: focussing on the liposomes. J Drug Target. 2022;30(5):463–475. doi:10.1080/1061186X.2021.2023160
34. Guimarães D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571. doi:10.1016/j.ijpharm.2021.120571
35. Alavi S, Mahjoob MA, Haeri A, et al. Multivesicular liposomal depot system for sustained delivery of risperidone: development, characterization, and toxicity assessment. Drug Dev Ind Pharm. 2021;47(8):1290–1301. doi:10.1080/03639045.2021.1989454
36. Das B, Sen SO, Maji R, Nayak AK, Sen KK. Transferosomal gel for transdermal delivery of risperidone: formulation optimization and ex vivo permeation. J Drug Deliv Sci Technol. 2017;38:59–71. doi:10.1016/j.jddst.2017.01.006
37. Biswas P, Wu CY. Nanoparticles and the environment. J Air Waste Manag Assoc. 2005;55(6):708–746. doi:10.1080/10473289.2005.10464656
38. Mohanraj VJ, Chen YJ. Nanoparticles-a review. Trop J Pharm Res. 2006;5(1):561–573.
39. Salarvand M, Ramezani V, Salarvand F, Darabi ZA, Akrami M. Improvement of drug delivery properties of risperidone via preparation of fast dissolution tablet containing nanostructured microparticles. Iran J Pharm Res. 2021;20(2):183. doi:10.22037/ijpr.2020.112230.13621
40. Cekić ND, Savić SM, Ilić TM, Savić SD. The reverse dialysis bag method for the assessment of in vitro drug release from parenteral nanoemulsions: a case study of risperidone. Adv Technol. 2020;9(1):5–12. doi:10.5937/savteh2001005C
41. Walia N, Zhang S, Wismer W, Chen L. A low energy approach to develop nanoemulsion by combining pea protein and Tween 80 and its application for vitamin D delivery. Food Hydrocoll Healt. 2022;2:100078. doi:10.1016/j.fhfh.2022.100078
42. Smirnov A, Terekhina S, Tarasova T, Hattali L, Grigoriev S. From the development of low-cost filament to 3D printing ceramic parts obtained by fused filament fabrication. Int J Adv Manuf Technol. 2023;128(1–2):511–529. doi:10.1007/s00170-023-11849-5
43. Delmas T, Piraux H, Couffin AC, et al. How to prepare and stabilize very small nanoemulsions. Langmuir. 2011;27(5):1683–1692. doi:10.1021/la104221q
44. Ostertag F, Weiss J, McClements DJ. Low-energy formation of edible nanoemulsions: factors influencing droplet size produced by emulsion phase inversion. J Colloid Interface Sci. 2012;388(1):95–102. doi:10.1016/j.jcis.2012.07.089
45. Kumar P, Sharma V, Jaggi C, Malik P, Raina KK. Orientational control of liquid crystal molecules via carbon nanotubes and dichroic dye in polymer dispersed liquid crystal. Liq Cryst. 2017;44(5):843–853. doi:10.1080/02678292.2016.1247476
46. Đorđević SM, Cekić ND, Savić MM, et al. Parenteral nanoemulsions as promising carriers for brain delivery of risperidone: design, characterization and in vivo pharmacokinetic evaluation. Int J Pharm. 2015;493(1–2):40–54. doi:10.1016/j.ijpharm.2015.07.007
47. Karami Z, Zanjani MR, Hamidi M. Nanoemulsions in CNS drug delivery: recent developments, impacts and challenges. Drug Discov Today. 2019;24(5):1104–1115. doi:10.1016/j.drudis.2019.03.021
48. Đorđević SM, Santrač A, Cekić ND, et al. Parenteral nanoemulsions of risperidone for enhanced brain delivery in acute psychosis: physicochemical and in vivo performances. Int J Pharm. 2017;533(2):421–430. doi:10.1016/j.ijpharm.2017.05.051
49. Soni H, Sharma S. Current update on nanoemulsion: a review. Sch Int J Anat Physiol. 2021;4(1):6–13.
50. Fayez H, Daihom B, Abd El-Aleem Y, Ibrahim IT, Motaleb MA. Brain nanotargeted [131I] I-Rolapitant as a model for brain imaging: intranasal formulation, radiolabelling, biodistribution, and comparative study. J Drug Deliv Sci Technol. 2023;24:104705. doi:10.1016/j.jddst.2023.104705
51. Saha P, Kathuria H, Pandey MM. Nose-to-brain delivery of rotigotine redispersible nanosuspension: in vitro and in vivo characterization. J Drug Deliv Sci Technol. 2023;79:104049. doi:10.1016/j.jddst.2022.104049
52. Ellenbroek BA, JIN ZHANGXX, JIN G-Z. Effects of (‐) stepholidine in animal models for schizophrenia 1. Acta Pharmacol Sin. 2006;27(9):1111–1118. doi:10.1111/j.1745-7254.2006.00365.x
53. Pınar SG, Oktay AN, Karaküçük AE, Çelebi N. Formulation Strategies of Nanosuspensions for Various Administration Routes. Pharmaceutics. 2023;15(5):1520. doi:10.3390/pharmaceutics15051520
54. Wang Y, Zheng Y, Zhang L, Wang Q, Zhang D. Stability of nanosuspensions in drug delivery. J Control Release. 2013;172(3):1126–1141. doi:10.1016/j.jconrel.2013.08.006
55. Du J, Li X, Zhao H, et al. Nanosuspensions of poorly water-soluble drugs prepared by bottom-up technologies. Int J Pharm. 2015;495(2):738–749. doi:10.1016/j.ijpharm.2015.09.021
56. Cosco D, Paolino D, Muzzalupo R, et al. Novel PEG-coated niosomes based on bola-surfactant as drug carriers for 5-fluorouracil. Biomed Microdevices;2009. 1115–1125. doi:10.1007/s10544-009-9328-2
57. Paolino D, Muzzalupo R, Ricciardi A, et al. In vitro and in vivo evaluation of Bola-surfactant containing niosomes for transdermal delivery. Biomed Microde. 2007:421–433.
58. Allam A, El-Mokhtar MA, Elsabahy M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation. J Pharm Pharmacol. 2019;71(8):1209–1221. doi:10.1111/jphp.13106
59. Mohamed HB, El-Shanawany SM, Hamad MA, Elsabahy M. Niosomes: a strategy toward prevention of clinically significant drug incompatibilities. Sci Rep. 2017;7(1):6340. doi:10.1038/s41598-017-06955-w
60. Chen S, Hanning S, Falconer J, Locke M, Wen J. Recent advances in non-ionic surfactant vesicles (niosomes): fabrication, characterization, pharmaceutical and cosmetic applications. Eur J Pharm Biopharm. 2019;144:18–39. doi:10.1016/j.ejpb.2019.08.015
61. Sadeghi-Ghadi Z, Ebrahimnejad P, Talebpour Amiri F, Nokhodchi A. Improved oral delivery of quercetin with hyaluronic acid containing niosomes as a promising formulation. J Drug Target. 2021;29(2):225–234. doi:10.1080/1061186X.2020.1830408
62. Ghafelehbashi R, Akbarzadeh I, Yaraki MT, et al. Preparation, physicochemical properties, in vitro evaluation and release behavior of cephalexin-loaded niosomes. Int J Pharm. 2019;569:118580. doi:10.1016/j.ijpharm.2019.118580
63. Chen F, Liu H, Wang B, et al. Physiologically based pharmacokinetic modeling to understand the absorption of risperidone orodispersible film. Front Pharmacol. 2020;10:1692. doi:10.3389/fphar.2019.01692
64. Shivaji Ashok Chakravarthy P, Grandhi S, Rajan S, Inderbir S. Optimization of quercetin loaded fast dissolving films by employing qbd as a designing tool for improved bioavailability. Eur Chem Bull. 2023;12(5):69–80.
65. Chue P. Risperidone long-acting injection. Expert Rev Neurother. 2003;3(4):435–446. doi:10.1586/14737175.3.4.435
66. Chawla A, Sharma P, Pawar P. Eudragit S-100 coated sodium alginate microspheres of naproxen sodium: formulation, optimization and in vitro evaluation. Acta Pharm Sin B. 2012;62(4):529–545. doi:10.2478/v10007-012-0034-x
67. D’Souza S, Faraj JA, Giovagnoli S, DeLuca PP. IVIVC from long acting olanzapine microspheres. Int J Biomater. 2014;1. doi:10.1155/2014/407065
68. Huanbutta K, Suwanpitak K, Weeranoppanant N, et al. Continuous flow synthesis: a promising platform for the future of nanoparticle-based drug delivery. J Drug Deliv Sci Technol. 2023;16:105265.
69. Shiny J, Ramchander T, Goverdhan P, Habibuddin M, Aukunuru JV. Development and evaluation of a novel biodegradable sustained release microsphere formulation of paclitaxel intended to treat breast cancer. Int J Pharm Investig. 2013;3(3):119. doi:10.4103/2230-973X.119212
70. Yerragunta B, Jogala S, Chinnala KM, Aukunuru J. Development of a novel 3-month drug releasing risperidone microspheres. J Pharm Bioallied Sci. 2015;7(1):37. doi:10.4103/0975-7406.148777
71. Castberg I, Spigset O. Serum concentrations of risperidone and 9-hydroxyrisperidone after administration of the long-acting injectable form of risperidone: evidence from a routine therapeutic drug monitoring service. Ther Drug Monit. 2005;27(1):103–106. doi:10.1097/00007691-200502000-00019
72. Schoretsanitis G, Spina E, Hiemke C, de Leon J. A systematic review and combined analysis of therapeutic drug monitoring studies for long-acting risperidone. Expert Rev Clin Pharmacol. 2017;10(9):965–981. doi:10.1080/17512433.2017.1345623
73. Rukmangathen R, Yallamalli IM, Yalavarthi PR. Formulation and biopharmaceutical evaluation of risperidone-loaded chitosan nanoparticles for intranasal delivery. Drug Dev Ind Pharm. 2019;45(8):1342–1350. doi:10.1080/03639045.2019.1619759
74. Lugasi L, Grinberg I, Rudnick-Glick S, Okun E, Einat H, Margel S. Designed proteinoid polymers and nanoparticles encapsulating risperidone for enhanced antipsychotic activity. J Nanobiotechnology. 2020;18:1–6. doi:10.1186/s12951-020-00709-z
75. Gohil D, Shah N, Maheshwari RA. Effect of Formulation Variables on Fabrication of Risperidone Loaded Nanoparticles for Sustained Drug Delivery. Indian J Pharm Educ Res. 2022;56:58–64. doi:10.5530/ijper.56.1.8
76. Dilawar N, Ur-Rehman T, Shah KU, Fatima H, Alhodaib A. Development and evaluation of PLGA nanoparticle-loaded organogel for the transdermal delivery of risperidone. Gels. 2022;8(11):709. doi:10.3390/gels8110709
77. Muthu MS, Singh SP. nanosuspensions of risperidone for parenteral delivery: formulation and in-vitro evaluation. Curr Drug Deliv. 2009;6(1):62–68. doi:10.2174/156720109787048302
78. Nair A, Khunt D, Misra M. Application of quality by design for optimization of spray drying process used in drying of Risperidone nanosuspension. Powder Technol. 2019;342:156–165. doi:10.1016/j.powtec.2018.09.096
79. Sambhakar S, Paliwal SK, Sharma S, Sati B, Singh B. Formulation and development of risperidone loaded niosomes for improved bioavailability: in vitro and in vivo study. Acta Pol Pharm. 2017;74(6):1859–1873.
80. Imam SS, Aqil M, Akhtar M, Sultana Y, Ali A. Formulation by design-based proniosome for accentuated transdermal delivery of risperidone: in vitro characterization and in vivo pharmacokinetic study. Drug Deliv. 2015;22(8):1059–1070. doi:10.3109/10717544.2013.870260
81. Kumar M, Pathak K, Misra A. Formulation and characterization of nanoemulsion-based drug delivery system of risperidone. Drug Dev Ind Pharm. 2009;35(4):387–395. doi:10.1080/03639040802363704
82. Kumar M, Misra A, Babbar AK, et al. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int J Pharm. 2008;358(1–2):285–291. doi:10.1016/j.ijpharm.2008.03.029
83. Vatankhah M, Dadashzadeh S, Mahboubi A, et al. Preparation of multivesicular liposomes for the loco-regional delivery of Vancomycin hydrochloride using active loading method: drug release and antimicrobial properties. J Liposome Res. 2023;7:1.
84. Narayan R, Singh M, Ranjan O, et al. Development of risperidone liposomes for brain targeting through intranasal route. Life Sci. 2016;163:38–45. doi:10.1016/j.lfs.2016.08.033
85. Mudhakir D, Wibisono C, Rachmawati H. Encapsulation of risperidone into chitosan-based nanocarrier via ionic binding interaction. Procedia Chem. 2014;13:92–100. doi:10.1016/j.proche.2014.12.011
86. Singh S, Soni R, Rawat MK, et al. In vitro and in vivo evaluation of buccal bioadhesive films containing salbutamol sulphate. Chem Pharm Bull. 2010;58(3):307–311. doi:10.1248/cpb.58.307
87. Muthu MS, Rawat MK, Mishra A, Singh S. PLGA nanoparticle formulations of risperidone: preparation and neuropharmacological evaluation. Nanomedicine. 2009;5(3):323–333. doi:10.1016/j.nano.2008.12.003
88. Shahrokhian S, Hafezi Kahnamoui M, Salimian R. Surface modification of glassy carbon electrode with the functionalized carbon nanotube for ultrasensitive electrochemical detection of risperidone. J Iran Chem Soc. 2018;1485–1494. doi:10.1007/s13738-018-1346-7
89. Prieto MJ, Temprana CF, Del Río Zabala NE, et al. Optimization and in vitro toxicity evaluation of G4 PAMAM dendrimer–risperidone complexes. Eur J Med Chem. 2011;46(3):845–850. doi:10.1016/j.ejmech.2010.12.021
90. Gol D, Thakkar S, Misra M. Nanocrystal-based drug delivery system of risperidone: lyophilization and characterization. Drug Dev Ind Pharm. 2018;44(9):1458–1466. doi:10.1080/03639045.2018.1460377
91. D’Souza S, Faraj J, DeLuca P. Microsphere delivery of Risperidone as an alternative to combination therapy. Eur J Med Chem. 2013;85(3):631–639.
92. Shen J, Choi S, Qu W, Wang Y, Burgess DJ. In vitro-in vivo correlation of parenteral risperidone polymeric microspheres. J Control Release. 2015;218:2–12. doi:10.1016/j.jconrel.2015.09.051
93. de Souza LE, Eckenstaler R, Syrowatka F, Beck-Broichsitter M, Benndorf RA, Mäder K. Has PEG-PLGA advantages for the delivery of hydrophobic drugs? Risperidone as an example. J Drug Deliv Sci Technol. 2021;61:102239. doi:10.1016/j.jddst.2020.102239
94. Janich C, Friedmann A, de Souza M, et al. Risperidone-Loaded PLGA–Lipid Particles with Improved Release Kinetics: manufacturing and Detailed Characterization by Electron Microscopy and Nano-CT. Pharmaceutics. 2019;11(12):665. doi:10.3390/pharmaceutics11120665
95. Burns MJ. The pharmacology and toxicology of atypical antipsychotic agents. J Toxicol Clin Toxicol. 2001;39(1):1–4. doi:10.1081/CLT-100102873
96. Aldaz A, Bellés MD, Del Río R, Milara J, Rojo A. Using pharmacokinetics and pharmacogenetics to optimize psychiatric treatments: a systematic review. Farm Hosp. 2021;45:84–93.
97. May M, Beauchemin M, Vary C, Barlow D, Houseknecht KL, De Luca V. The antipsychotic medication, risperidone, causes global immunosuppression in healthy mice. PLoS One. 2019;14(6):e0218937. doi:10.1371/journal.pone.0218937
98. Alvarez-Herrera S, Escamilla R, Medina-Contreras O, et al. Immunoendocrine peripheral effects induced by atypical antipsychotics. Front Endocrinol. 2020;11:195.
99. Simpson DS, Oliver PL. ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants. 2020;9(8):743. doi:10.3390/antiox9080743
100. Cikánková T, Fišar Z, Bakhouche Y, Ľupták M, Hroudová J. In vitro effects of antipsychotics on mitochondrial respiration. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1209–1223. doi:10.1007/s00210-019-01665-8
101. Milani GP, Bianchetti MG, Mazzoni MB, et al. Arterial hypertension and posterior reversible cerebral edema syndrome induced by risperidone. Pediatrics. 2014;133(3):e771–774. doi:10.1542/peds.2013-1301
102. Cabaleiro T, Ochoa D, López‐Rodríguez R, et al. Effect of polymorphisms on the pharmacokinetics, pharmacodynamics, and safety of risperidone in healthy volunteers. Hum Psychopharmacol Clin Exp. 2014;29(5):459–469. doi:10.1002/hup.2420
103. Oshikoya KA, Carroll R, Aka I, Roden DM, Van Driest SL. Adverse events associated with risperidone use in pediatric patients: a retrospective biobank study. Drugs-Real World Outcomes. 2019;6:59–71. doi:10.1007/s40801-019-0151-7
104. Longo G, Cicolini A, Orsolini L, Volpe U. The Novel Antipsychotic Lumateperone (Iti-007) in the Treatment of Schizophrenia: a Systematic Review. Brain Sci. 2023;13(12):1641. doi:10.3390/brainsci13121641
105. Orzelska-Górka J, Mikulska J, Wiszniewska A, Biała G. New atypical antipsychotics in the treatment of schizophrenia and depression. Int J Mol Sci. 2022;23(18):10624. doi:10.3390/ijms231810624
106. Clinical trials.gov. National Library of Medicine 8600 Rockville Pike, Bethesda, MD Accessed from https://clinicaltrials.gov.
107. Xiaojie C, Menghua G, Xinshi W, Siqi Z Preparation method of paliperidone palmitate suspension. China patent, CN112451483B, 2020.
108. Eric D. Slow-broadcast formulation of risperidone compound. Japan Pat J. JP5795606;2008.
109. Mainde C, Nandlal Nagori R, Hanuman Sagar Boddu S. Aqueous oral formulations of risperidone. Switzerland patent, WO2007138462A2;2007.
110. Dadey E, Li Q, Lindemann M. Sustained delivery formulations of risperidone compound. United States patent, US11712475B2; 2008.
111. Souna A, Hui Y, Ruifang L, Jingxia D, Lengxin D. Risperidone nano-suspension temperature sensitive gel and its preparation method. Chinese patent; 2014.
112. Misra A. A pharmaceutical oil-in-water nano-emulsion European patent, EP3160444B1; 2015.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.