Back to Journals » International Journal of Nanomedicine » Volume 19
Advancements and Challenges of Nanostructured Lipid Carriers for Wound Healing Applications
Authors Wathoni N , Suhandi C , Elamin KM , Lesmana R, Hasan N, Mohammed AFA, El-Rayyes A, Wilar G
Received 19 May 2024
Accepted for publication 26 July 2024
Published 15 August 2024 Volume 2024:19 Pages 8091—8113
DOI https://doi.org/10.2147/IJN.S478964
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. RDK Misra
Nasrul Wathoni,1,* Cecep Suhandi,1,* Khaled M Elamin,2,* Ronny Lesmana,3,4,* Nurhasni Hasan,5,* Ahmed Fouad Abdelwahab Mohammed,6,* Ali El-Rayyes,7,* Gofarana Wilar8,*
1Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Jatinangor, 45363, Indonesia; 2Graduate School of Pharmaceutical Sciences, Kumamoto University, Kumamoto, 862-0973, Japan; 3Physiology Division, Department of Biomedical Science, Faculty of Medicine, Universitas Padjadjaran, Bandung, Indonesia; 4Biological Activity Division, Central Laboratory, Universitas Padjadjaran, Bandung, Indonesia; 5Department of Pharmacy Science and Technology, Faculty of Pharmacy, Universitas Hasanuddin, Makassar, 90245, Indonesia; 6Department of Pharmaceutics, Faculty of Pharmacy, Minia University, Minia, 61519, Egypt; 7Department of Chemistry, College of Science, Northern Border University, Arar, Saudi Arabia; 8Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Universitas Padjadjaran, Jatinangor, 45363, Indonesia
*These authors contributed equally to this work
Correspondence: Nasrul Wathoni, Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Jatinangor, 45363, Indonesia, Tel +62-22-842-888-888, Email [email protected]
Abstract: The current treatments for wound healing still exhibit drawbacks due to limited availability at the action sites, susceptibility to degradation, and immediate drug release, all of which are detrimental in chronic conditions. Nano-modification strategies, offering various advantages that can enhance the physicochemical properties of drugs, have been employed in efforts to maximize the efficacy of wound healing medications. Nowadays, nanostructured lipid carriers (NLCs) provide drug delivery capabilities that can safeguard active compounds from environmental influences and enable controlled release profiles. Consequently, NLCs are considered an alternative therapy to address the challenges encountered in wound treatment. This review delves into the application of NLCs in drug delivery for wound healing, encompassing discussions on their composition, preparation methods, and their impact on treatment effectiveness. The modification of drugs into the NLC model can be facilitated using relatively straightforward technologies such as pressure-based processes, emulsification techniques, solvent utilization methods, or phase inversion. Moreover, NLC production with minimal material compositions can accommodate both single and combination drug delivery. Through in vitro, in vivo, and clinical studies, it has been substantiated that NLCs can enhance the therapeutic potential of various drug types in wound healing treatments. NLCs enhance efficacy by reducing the active substance particle size, increasing solubility and bioavailability, and prolonging drug release, ensuring sustained dosage at the wound site for chronic wounds. In summary, NLCs represent an effective nanocarrier system for optimizing the bioavailability of active pharmacological ingredients in the context of wound healing.
Keywords: drug delivery, nanocarrier, nanostructured lipid carriers, wound healing
Graphical Abstract:

Introduction
Wound healing encompasses a myriad of intricate physiological processes aimed at regenerating cells and tissues to replace damaged components.1 Pharmacological agents play a vital role in supporting and expediting the wound healing process. Among these agents, anti-inflammatories are employed to mitigate complications stemming from an excessive immune response at the wound site.2 Antibacterial agents are equally indispensable to thwart the growth and infiltration of bacteria that may breach the injured skin’s surface.3,4 Furthermore, various biological preparations, including growth factors, have gained widespread application in accelerating the regeneration of damaged tissue.5,6 Additionally, the application of wound dressings proves beneficial in shielding the wound from environmental conditions that might impede or exacerbate the healing process.7
Nevertheless, the challenge remains to identify optimal drug delivery preparations for expediting wound healing.8 Currently, formulators encounter an array of issues concerning drug delivery to wounds. These issues encompass mechanical hurdles where maintaining local effects of preparations during patients’ daily activities proves arduous.8 In cases of infected tissues, wounds tend to be chronic, necessitating prolonged therapeutic interventions.9 Mechanical barriers within the wound necessitate the optimization of an ideal drug delivery system to wound sites, a task complicated by the challenge of maintaining the local effect of therapeutic agents. Additionally, the body’s defense system hosts a multitude of protease enzymes, further raising concerns about the effective administration of epidermal growth activator biological agents, typically protein components.8 Supporting this, Zhang et al’s study revealed that topical administration of epidermal growth factor (EGF) failed to significantly reduce the time to improvement in wound conditions compared to controls (6.8 ± 0.12 vs 7.5 ± 0.13 days, respectively).10 These myriad challenges underscore the importance of developing preparations with extended retention times in the wound area, ensuring long-term release, and providing protection during application at the wound site.
Recent advances in nanotechnology offer a promising avenue for overcoming barriers in drug formulation.11–15 In the realm of wound healing, nano-delivery systems (nanocarriers) present opportunities to enhance the physicochemical characteristics of preparations.16–18 Various nanomaterials suitable for drug delivery in wound care encompass inorganic options such as gold and silver nanoparticles, organic choices including polymeric and lipid nanoparticles, as well as nanofibers, hydrogels, nanospheres, and scaffolds.19–21 Notably, a recent clinical study employing a nano-silver modification technique failed to achieve an optimal timeframe for wound treatment using EGF compared to controls (6.2 ± 0.32 vs 7.5 ± 0.13 days, respectively). Encouragingly, promising results emerged from studies involving wound drug delivery via lipid nanoparticles modified with essential oils. This modification accelerated in vitro wound healing, achieving complete wound closure within 48 hours.10 Lipid nanoparticles, exemplified by α-Gal liposomes, accelerate wound healing by enhancing macrophage activation and promoting wound closure across various conditions, as evidenced by multiple studies.22–27 This superiority is attributed to the lipid nanocarriers’ composition, consisting of lipids compatible with mucosal and skin components.28,29
Lipid nanoparticles are categorized into two types: solid lipid nanoparticles (SLNs), the first generation of lipid nanoparticles, and nanostructured lipid carriers (NLCs), the second generation.30 The utilization of SLNs has largely been discontinued due to inherent limitations such as a high polymorphic tendency, low stability, and inefficient drug loading capacity.31,32 In contrast, NLCs, as the second generation, offer numerous advantages.33 Beyond overcoming the shortcomings of SLNs, NLCs exhibit controlled release capabilities,34 are ease of manufacture,35 and skin hydration properties,36 thereby enhancing their suitability for wound treatment. Furthermore, NLCs exhibit higher entrapment efficiency compared to SLNs, with NLCs achieving up to 88% drug entrapment, whereas SLNs can only entrap about 80%. Additionally, unlike the burst effect seen with SLNs, the regulated release of NLCs keeps the drug at an efficacious dose, maintaining the therapeutic window and preventing toxic levels.14 Its advantages are also highly beneficial for wound healing applications, as wounds often require a sustained-release drug mechanism for extended recovery periods.
This review aims to explore the utilization of NLCs as nanocarriers for therapeutic agents in wound therapy. This review was compiled based on a literature search conducted in electronic databases, including PubMed (MEDLINE), Scopus, ScienceDirect, and Google Scholar. The searches utilized the keywords “Nanostructured Lipid Carrier” AND Wound, “Nanostructured Lipid Carrier” AND Ulcer, NLC AND Wound, AND NLC AND Ulcer. Articles published from 2013 to 2023 were considered. The initial section provides an overview of wound pathologies to elucidate the role of therapeutic agents and the significance of NLCs in wound treatment. It subsequently offers a comprehensive examination of NLCs as nanocarriers. Finally, the review summarizes various research findings discussing the application of NLCs in drug delivery for wound treatment, encompassing in vitro, in vivo, and clinical studies. Figure 1 illustrates the strategy employed in structuring this review.
 |
Figure 1 Reviewing flow chart. |
Wound Pathology
Understanding wound pathology is of paramount importance in the development of drugs and drug delivery strategies for wound treatment. A wound refers to a condition in which the skin becomes damaged or torn due to mechanical stress, violence, trauma, injury, or other factors stemming from physiological processes.37 Wounds can be categorized based on both the depth of the affected area and the duration required for the healing process. In terms of depth, wounds fall into three distinct categories,:38 superficial wounds, where damage is limited to the epidermal layer; partial-thickness wounds, where both the epidermal and deeper dermal layers are affected; and full-thickness wounds, where damage extends to deeper layers, including subcutaneous fat.39 Furthermore, wounds are classified based on their healing timeframes as acute or chronic wounds.40 Acute wounds are characterized by a relatively short healing process, while chronic wounds, often associated with pathological conditions such as infection and diabetic ulcers, require a longer healing duration.38,40,41
Given the intricacy of wound healing as a physiological process, it comprises four principal stages, as illustrated in Figure 2. These stages encompass the hemostasis phase, inflammation phase, proliferation phase, and remodeling phase.42,43
 |
Figure 2 Phases of wound healing processes. |
Haemostasis Phase
This phase initiates immediately following tissue damage, and it holds significant importance in preventing exsanguination by regulating vascularity.37 Platelets play a pivotal role in stemming exsanguination through the formation of aggregates, a process known as clotting, which effectively obstructs the flow of blood.44,45 Clot formation involves intricate molecular mechanisms, commencing with the activation of platelet receptors triggered by type I collagen, serving as indicators of the extravascular or extracellular matrix (ECM).46 Activated platelets subsequently release various substances that collaborate to form a clot. These include adhesive glycoproteins like fibronectin, fibrinogen, fibrin, thrombospondin, and vitronectin, which function akin to glue, binding all components together to form aggregates that impede blood flow.47 Moreover, growth factors released as a result of platelet activation play crucial roles in various immune recruitment mechanisms.48,49 These growth factors encompass tumor growth factor-β (TGF-β) and platelet-derived growth factor (PDGF), which participate in recruiting monocytes and neutrophils, as well as tumor growth factor-α (TGF-α), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF), contributing to the activation of endothelial cells, inducing angiogenesis.49 Additionally, PDGF is known for its role in activating fibroblasts, ultimately initiating collagen and extracellular matrix formation.50
Remarkably, platelets also serve to prevent excessive aggregation or clotting,47 achieved through various molecular mechanisms. This includes the role of prostacyclin, which directly inhibits platelet aggregation, bolstered by antithrombin III’s function in inhibiting thrombin activity.51 Additionally, the complement protein C5a plays a role in curbing excessive aggregation by facilitating the degradation of coagulation factors V and VII.52
Inflammation Phase
The inflammation phase, which is the second phase, typically occurs within a timeframe ranging from 24 hours to 2 weeks following the injury.37 Innate immunity takes precedence in addressing potential pathogenic agents that may infiltrate exposed skin or mucosal areas due to the wounds.53 This response is mediated by various immune cells, including macrophages, Langerhans cells, and mast cells.53 Additionally, T cell activation plays a critical role by releasing pro-inflammatory molecules, contributing to vasodilation, which, in turn, facilitates the migration of neutrophils to the wounded area.54 Once neutrophils reach the wound site, they embark on eliminating damaged cells and shielding the wound from invading pathogens, achieved through the release of reactive oxygen species (ROS) or phagocytosis.55
Furthermore, the role of cytokines and chemokines in adaptive immunity holds significant importance, particularly in cases of chronic infective wounds.56 Inflammatory cytokines such as IL-1, IL-6, and IL-8 are instrumental in cell signaling processes that facilitate fibroblast infiltration and proliferation, keratinocyte chemotaxis, and collagen synthesis.57 These processes are vital preparations for the subsequent phases of wound healing. Chemokines, small regulatory proteins, also play a crucial role in recruiting various immune cells.56 Among the chemokines involved in the inflammatory phase of wound healing are α-chemokines (CXC), β-chemokines (CC), and γ-chemokines (C and CXXXC).58 Under injury conditions, these chemokines participate in recruiting neutrophils, activated T lymphocytes, eosinophils, basophils, monocytes, and natural killer cells.58
Proliferation Phase
During the proliferation phase, fibroblasts take center stage in the process of wound closure.37,59 This phase typically commences approximately 12 hours after the initial injury.37 Migrating fibroblasts transport soluble mediators, forming granulation tissue to replace the fibrin-rich matrix.59 The migration of these fibroblasts is initiated through interactions with integrin receptors present in the fibrin-rich matrix.60 The direction of fibroblast movement is contingent upon the composition and concentration of matrix constituents, which include various cytokines, chemokines, and growth factors.60 Additionally, keratinocytes play a pivotal role in epithelialization.61,62 They release matrix metalloproteinases (MMPs) to establish a basal membrane that serves as a framework for the extracellular matrix in the formation of new epithelial tissue.63
In addition to extracellular matrix production, the proliferation phase also witnesses the formation of new collagen.37 Primarily, migrating fibroblasts contribute to the production of this new collagen.59 The collagen generated is utilized by fibroblasts as a component in the formation of granulation tissue, alongside elastin and proteoglycans.64 The predominant type of collagen synthesized during the proliferation phase is type III collagen.65 Furthermore, damaged blood vessels undergo repair through angiogenesis during this phase.66 Vascular endothelial cells are produced by macrophages and various growth factors known as angiogenic factors. This angiogenesis process is specifically regulated by vascular endothelial growth factor (VEGF) as an endothelial cell growth factor and counteracted by endostatin and angiostatin, which function as anti-angiogenic factors.66
Remodeling Phase
The remodelling phase constitutes the final stage of the wound healing process, commencing from the onset of fibrin clot formation and extending until the complete maturation of type I collagen, a process that can span several years.37 Throughout this phase, fibroblasts continue to play a pivotal role.67 However, in contrast to their role in the proliferation phase, fibroblasts’ involvement in the formation of granulation tissue diminishes during the remodelling phase.67 Instead, fibroblasts initiate the production and transport of hyaluronan and proteoglycans, thereby replacing the fibrin clot.68 Additionally, extracellular matrix proteins such as matrix metalloproteinases (MMPs), which also contribute to the proliferation phase, participate in the remodelling process by degrading fibrillar collagen molecules and proteoglycans.63 The degradation of protein components within the extracellular matrix is mediated by serine proteases, particularly α1-protease inhibitor.63
The completion of the remodelling phase serves as an indicator for the type of collagen predominating in the healed tissue. In contrast to the proliferative phase, the predominant collagen type in this phase is typically type I collagen.69 Type I collagen undergoes maturation to replace type III collagen, continuing until the ideal composition of healthy tissue is attained. In healthy adult tissues, a typical ratio of type I collagen to type III collagen is 80:10. Notably, type I collagen boasts superior tensile strength compared to type III collagen.37,70 Consequently, this process reinforces the tender scar until it attains a protective function similar to that of natural skin.70
Nanostructured Lipid Carriers
Subsequent to acquiring an understanding of wound pathology, delving into the nanostructured lipid carriers (NLCs) system becomes imperative for their application in our wound drug candidates. NLCs represent nanocarriers characterized by their formulation as oil-in-water emulsions within the nanoscale range, typically falling within the 10–1000 nm spectrum.71 NLCs essentially serve as an alternative to solid lipid nanoparticles (SLNs), a concept first introduced by Professor R.H. Müller and Professor M. Gasco in 1990.30 NLCs offer several notable advantages, including the safeguarding of drugs against unfavorable environmental conditions, facile large-scale synthesis facilitated by the high-pressure homogenization technique, biocompatibility, and biodegradability.72 Moreover, NLCs stand out due to their ability to encapsulate both solid lipid components and liquid lipid constituents, thereby mitigating the rigidity often associated with nanocarriers, a primary limitation of SLNs.73 A comprehensive depiction of the general structure of NLCs is provided in Figure 3. Subsequently, a detailed exploration of NLC composition, types, and preparation methods is described below.
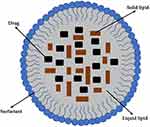 |
Figure 3 General structure of nanostructured lipid carrier. |
Composition of Nanostructured Lipid Carriers
As previously mentioned, NLCs represent carrier systems comprising both solid and liquid lipids.73 The distinctions between solid lipids and liquid lipids within NLCs are discernible through their physical properties, melting characteristics, and crystalline attributes. Solid lipids, exemplified by glyceryl behenate, glyceryl palmitostearate, and stearic acid, maintain solidity at both ambient and physiological temperatures, with a melting point typically exceeding 37°C, thus ensuring structural integrity in NLC formulations. In contrast, liquid lipids such as oleic acid, medium-chain triglycerides, and squalene remain fluid at these temperatures, possessing a lower melting point below 37°C, which enhances the lipid matrix’s fluidity. Solid lipids exhibit higher crystallinity levels, impacting drug encapsulation efficiency and release kinetics, whereas liquid lipids contribute to a less ordered lipid matrix, promoting enhanced drug loading capacity and modulation of drug release profiles within NLCs.74,75
NLCs achieve stability by mitigating the risk of aggregation, a function primarily attributed to surfactants.76 The drugs are housed within the lipid phase, serving as a reservoir, and are subsequently released as the surfactant layer disintegrates.77 A compilation of commonly utilized solid lipids, liquid lipids, and surfactants in the development of NLCs for drug delivery in wound healing is presented in Table 1.
 |
Table 1 Common Ingredients in Developing NLC Loaded with Wound Healing’s Active Pharmacological Agent |
Types of Nanostructured Lipid Carriers
NLCs are categorized into three distinct types: imperfect types, amorphous types, and multiple types. These varying classifications of NLCs exert an influence on the spatial arrangement of the loaded drugs.79 Each type of NLC possesses a distinctive structure, as depicted in Figure 4.
 |
Figure 4 Types of nanostructured lipid carriers, including (a) imperfect type, (b) amorphous type, and (c) multiple type. |
Imperfect Type
The imperfect type, commonly referred to as the imperfect crystal type, is a classification of NLCs characterized by a lower ratio of liquid lipids to solid lipids in its composition.72,80 Moreover, variations in the composition of the solid lipid constituents within the container have been observed, including differences in the structure of hydrocarbon chains (whether long or branched) and polymorphic forms. These variations result in irregularities in the spatial arrangement, creating more opportunities for drug molecules to occupy the gaps within the lipid matrix.80 However, this imperfect type has a vulnerability in terms of stability, as the system tends to undergo structural breakdown due to the tendency of solid lipid crystallization.79
Amorphous Type
In the amorphous type, the solid lipid constituents loaded into the system exist in an amorphous state, thus averting the risk of leakage caused by crystallization.81 Amorphous lipids differ significantly from crystalline lipids as they lack a well-defined, orderly structure. Instead of possessing a regular, repeating molecular arrangement, amorphous lipids exhibit a random, disordered configuration. Amorphous clusters are predominantly generated through the utilization of the cold homogenization approach, particularly when incorporating highly lipophilic drugs, as opposed to the hot homogenization method.80 Examples of lipids suitable for creating the amorphous type encompass isopropyl myristate, hydroxy octacosanol hydroxy stearate, dibutyl adipate, and certain triglycerides.81,82 As this type boasts a more orderly spatial arrangement, the drug loading capacity is generally lower than that of the imperfect type.81
Multiple Type
In this type, liquid oils serve as prominent high-dissolved drug reservoirs, effectively substituting the role of liquid lipids.76 The utilization of this oil phase has implications for the occurrence of phase separation between solid lipids and oils during the cooling process. Given that drugs are housed within the oil phase, their release proceeds at a slower rate. Consequently, this type is exceptionally well-suited for the administration of controlled-release drugs.76,79
Preparation Method of Nanostructured Lipid Carriers
The preparation methods of NLCs vary and are contingent upon the stability of the active substance and other technical factors. Additional considerations encompass process simplicity, process viability, and the desired particle attributes.81 NLCs can be prepared using the methods described below:
High-Pressure Homogenization Method
The high-pressure approach employed in NLCs manufacturing is highly environmentally friendly, particularly as it does not necessitate the use of organic solvents. Furthermore, this method is highly versatile, suitable for both small-scale production and scaling up to higher capacities.81 This method has been successfully employed to produce NLCs containing the active ingredients thymoquinone, zerumbone, and cinnamon oil. Suitable solid lipids for use in this method include shea butter, while appropriate liquid lipids include argan oil. The process of NLCs production commences with the melting of the lipid component until it reaches a temperature 5–10°C above its melting point.71 The active substance is prepared as a mixture with the molten lipid. Typically, the hot process is preferred over the cold process. In the hot process, elevated temperatures induce melting, resulting in material dispersion at extremely small particle sizes, often approaching molecular dispersion conditions. In contrast, the cold process involves lowering the temperature to trigger the recrystallization of the mixed ingredients, yielding a stable crystalline state that is still easily broken down into smaller particles. In the next step, the mixture is directly incorporated into the surfactant solution when using the hot temperature method. The surfactant is preheated to the same temperature as in the melting stage.83 Conversely, in the cold temperature process, the homogeneous melt obtained is first cooled to induce crystallization and form a solid phase. The resultant solid is subsequently pulverized before being encapsulated into a water solution containing surfactants at lower temperatures.84 Regardless of whether the process involves hot or cold temperatures, the mixture is further homogenized using high pressure through a narrow gap.81 The flow chart depicting the development of NLCs using high-pressure homogenization in both hot and cold processes is illustrated in Figure 5. Homogenizing the cold-processed mixture generally requires higher pressure and more process cycles. Compared to the cold process, the equipment required for hot-high-pressure homogenization is relatively compact. However, the hot process is not suitable for processing thermolabile active substances.84
 |
Figure 5 High pressure homogenization flow chart. |
High Shear Homogenization Method
In this method, the drug is initially prepared as a mixture within a molten lipid phase. Subsequently, the mixture is homogenized using a high-speed stirrer to create a nano-dispersion.81 Before pouring, the surfactant solution, which may consist of poloxamers, lecithins, polysorbates, and polyethoxylated monoglycerides, is heated to the melting temperature of the lipid phase. Achieving a homogeneous mixture with smaller particle sizes is contingent upon the gradual integration of the lipid mixture. A higher stirring speed can yield smaller particles. Additionally, this method is frequently combined with ultrasonication techniques, employing probe-type ultrasonication to disintegrate agglomerates or large-sized globules.85 This method has been effectively utilized to create NLCs with the active compounds Eucalyptus essential oil (EEO) and Rosemary essential oil (REO). In this approach, soya lecithin can serve as both a solid lipid and a liquid lipid.
Melt Emulsification Method
The melt emulsification technique is almost identical to high shear homogenization.86 The distinction lies in the point at which the nano-dispersion of drugs is formed. This method is suitable for loading peptide-based drugs, such as the LL37 peptide. Initially, the drug is blended with the molten solid and liquid lipids. The mixture of surfactants and co-surfactants, such as lecithin as a surfactant and sodium taurodeoxycholate as a co-surfactant, is then heated to the melting temperature of the lipid phase. These two mixtures are homogenized to produce an oil-in-water (o/w) emulsion phase. Following homogenization, the mixture is promptly immersed in cold water and agitated. Dilution and rapid temperature changes induce emulsions that initially have larger sizes to become smaller particles.87 This technique is considered a straightforward method for NLC formation. However, it has a drawback in that the particle sizes obtained are often on a micro scale.88
Double Emulsion Technique
This technique is essentially a variation of the melt emulsification method used specifically for incorporating hydrophilic active substances.30 This method has proven effective in producing NLCs incorporating epidermal growth factor (EGF) and curcumin as active ingredients. Solid lipids such as glyceryl palmitostearate (Precirol®) are suitable for this process, along with liquid lipids like eicosapentaenoic acid (EPA) or omega-3. Initially, the active substances are dissolved in water, which is then dispersed in the melted lipid phase to create a water-in-oil (w/o) emulsion system. Subsequently, this mixture is introduced to a surfactant solution as a stabilizer to produce a nano-water-in-oil-in-water (w/o/w) emulsion phase. The speed of stirring and process temperature are the primary factors influencing the size of the resulting particles.89
Phase Inversion Method
This method can be regarded as a novel approach in the preparation of NLCs. This technique leverages the surfactants’ ability to exhibit different hydrophilic-lipophilic balance (HLB) values at varying temperatures.90 At high temperatures, surfactants will have a relatively low HLB. At a specific temperature threshold, the surfactant undergoes a change in HLB value, which can alter the emulsion phase formed. The formation of the NLCs system with this method begins by mixing the active substances, lipid phase, and surfactant, followed by stirring. The mixture is later subjected to drastic temperature changes for up to three cycles (85°C – 60°C – 85°C – 60°C – 85°C) to produce an oil-in-water (o/w) emulsion phase. Subsequently, the mixture is vigorously introduced to cold water (0°C) to invert the phase into an oil-in-water (o/w) emulsion phase. This technique is highly suitable for thermolabile active substances, especially when addressing limitations associated with high-temperature organic solvent evaporation.91 This method has been successfully applied to create NLCs containing the active ingredients ferulic acid (FA) and Lavandula essential oil (LEO). Solid lipids suitable for this method include glyceryl palmitostearate (Precirol®), while appropriate liquid lipids include propylene glycol monocaprylate (Capryol® 90).
Solvent Evaporation Method
In the solvent evaporation technique, both the active substance and the lipid phase are dissolved in an organic solvent.92 The organic solvent used must be immiscible with water. The organic phase is subsequently mixed into the surfactant solution to create an oil-in-water (o/w) emulsion system. The resulting emulsion is then heated to facilitate the evaporation of the organic solvent.93 The remaining lipid phase precipitates into nanoscale particles. This technique is seldom employed due to concerns regarding the potential for organic solvents to leave behind toxic residues.71 This method has been effectively employed for encapsulating drug such as pioglitazone.
Solvent Diffusion Method
This technique closely resembles the solvent evaporation method, with the distinction lying in the type of organic solvent and solvent removal approach employed.84 To clarify, the differing processes involved in developing NLCs using these two methods can be observed in Figure 6. In this method, the organic solvent employed must be water miscible. Initially, both the active substance and the lipid phase are dissolved in the organic solvent. The organic phase is then mixed with a surfactant solution to create an oil-in-water (o/w) emulsion. Subsequently, the organic solvent is removed through dilution in water (at a 1:10 ratio).71 The organic solvent undergoes diffusion into the water phase, leaving behind nano-precipitates. Following this, the solid phase can be separated, either through ultrafiltration or lyophilization.30 This method has been successfully employed to encapsulate drug such as simvastatin.
 |
Figure 6 Solvent evaporation and solvent diffusion method comparation. |
In NLC research, the incorporation efficiency of active substances (drugs) into the carriers and the handling of non-incorporated substances can vary significantly. Encapsulation efficiency refers to the percentage of drug successfully embedded within the lipid matrix relative to the total amount utilized during formulation, influenced by lipid composition, preparation techniques, and drug-specific properties.
Non-incorporated substances, remaining as free molecules within the suspension post-formulation, can impact the overall stability and therapeutic efficacy of NLCs. Strategies often involve optimizing formulation parameters to enhance drug-lipid interactions and increase encapsulation efficiency. Alternatively, purification methods may be employed to selectively remove non-incorporated substances from the NLC suspension, aiming to concentrate carriers with higher drug content and purity. Approaches to managing non-incorporated substances are tailored to meet specific objectives, such as maximizing drug loading capacity, improving stability profiles, or refining targeted drug delivery strategies within the context of NLC development.
Application of Nanostructured Lipid Carrier on Wound Healing
Numerous studies have explored the incorporation of active ingredients into NLCs, highlighting the significant role these carriers play in wound drug delivery. The mechanism behind the enhanced wound healing facilitated by NLCs can be attributed to their superior bioadhesive properties. Supporting evidence comes from a study conducted by Saporito et al, which demonstrated that NLCs exhibited the highest level of bioadhesion compared to both control and unloaded drugs. Furthermore, this study revealed that NLCs demonstrated excellent biocompatibility, maintaining fibroblast viability within the range of 90–100%. This can be attributed to the sustained release mechanism of NLCs, ensuring that the drug content does not induce excessive toxicity in fibroblasts.94 The impact of employing NLCs as nanocarriers in wound treatment has been assessed in vitro, in vivo, and in clinical studies, as summarized in Figure 7. Generally, NLCs have a significant influence on enhancing the effectiveness of active pharmacological agents. The application of NLCs for drug delivery in wound healing treatments is summarized in Table 2.
 |
Table 2 NLC Development and Application on Delivering Active Agents for Wound Healing |
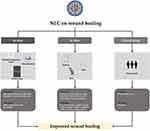 |
Figure 7 Benefits of NLC on wound healing based on in vitro, in vivo, and clinical study. |
In vitro Study
In vitro studies have been conducted using various pharmacologically active substances loaded into NLCs. NLCs have proven effective for delivering biologically active agents such as growth factors (eg, epidermal growth factor (EGF) and recombinant human epidermal growth factor (rhEGF)),95,96 and nucleic acids (small interfering ribonucleic acid (Si-RNA)).97 The methods employed for constructing biomolecule-loaded NLCs include double emulsion (w/o/w), ultrasonication, and emulsification followed by ultrasonication.95–97 These methods are particularly suitable for biomolecule agents due to their thermosensitivity, as hot homogenization can degrade bioactive substances.124 Conversely, thermal techniques are incompatible with the preparation of volatile agents such as essential oil-loaded NLCs, as they can vaporize during the process. An exception is found in the studies by Carbone et al and Costa-Fernandez et al, where a high-temperature method was employed. For instance, in the manufacturing of tea tree oil-loaded NLCs, tea tree oil was added at 34°C after the melting process at 40°C, preventing the essential oil from evaporating during mixing.99,100 Similarly, Lavandula essential oil was used in the phase inversion temperature (PIT) method, which involved low energy levels at temperatures of 65–73°C, preventing essential oil degradation during the inversion process.99 Other agent-loaded NLCs can be developed using high-pressure homogenization, high-shear homogenization, hot emulsification, and solvent diffusion, either followed by or without ultrasonication.93,94,98,102,104
Generally, in vitro assays for evaluating the wound-healing effectiveness of NLC preparations have involved cell migration assays, scratch assays, and biomarker expression analyses. The migration cell assay, or scratch assay, assesses wound healing performance based on the ability to induce cell migration on a two-dimensional surface.125 Some cell lines used in performing these assays include normal human dermal fibroblasts (NHDF), mouse embryonic fibroblasts (NIH-3T3), murine fibroblasts (CCL-3T3), human umbilical vein endothelial cells (HUVEC), and human epidermal keratinocytes (HaCaT).93,94,96,98,99,104 Effectiveness is measured by the degree of gap closure over a certain time (% wound closure) from the initial scratch area. These assays are performed using fibroblasts, keratinocytes, and endothelial cells. For instance, Tezgel et al evaluated wound healing effectiveness based on its impact on the expression of ERK1 (extracellular signal-regulated kinase 1), a biomarker of the healing process.97 Inhibition of ERK1 can suppress the inflammatory process, thereby enhancing wound recovery.126 Gainza et al assessed effectiveness by examining the drug’s residence time over approximately 48 hours, given that the preparation is intended as a wound dressing. Residence time was determined through cell diffusion tests using Franz cells, with pharmacological agents evaluated after the diffusion process at specified time points.95 Additionally, the inhibition of nitric oxide (NO) production, which suppresses inflammatory activity, can serve as a valuable predictor of wound healing efficacy.127
Based on in vitro evaluations, NLCs as nanocarriers have demonstrated remarkable efficacy in enhancing wound treatment. Results from cell migration or scratch assays consistently showed that NLCs loaded with various active pharmacological agents exhibited superior wound closure rates compared to control groups and even unloaded active agents or blank NLCs. Notably, Si-RNA-loaded NLCs demonstrated exceptional effectiveness in wound closure, surpassing unloaded Si-RNA and control groups, as evidenced by ERK1 expression levels.97 NLCs also exhibited prolonged effects due to their controlled release properties, making them suitable for use as wound dressings.95 Recent studies have shown that NLC systems can effectively encapsulate propolis in combination with α-mangostin, a xanthone isolate compound from mangosteen pericarp.14,15,128 These findings underscore the NLC system’s significant loading capacity for various types of drugs, even though they were not directly confirmed for wound healing treatment. In vitro test results indicated that NLC preparations containing the combination of propolis and α-mangostin produced a significant antioxidant effect compared to unloaded drugs and controls (p < 0.01).14,129,130
In vivo Study
Preclinical studies evaluating pharmacologic agent-loaded NLCs for wound treatment were conducted using various animal models, including mice, rats, and rabbits.108,109,115 These NLCs were applied to both acute and chronic wounds, as indicated by the use of a diabetic animal model. The effectiveness of wound healing was assessed based on parameters such as the percentage of wound closure or reduction in wound area after administering the preparation for a specified period. In cases of ulcer conditions, including diabetic ulcers or gastric ulcers, effectiveness was determined by the preparation’s ability to reduce the ulcer index value.131 The ulcer index is a standardized method for evaluating the presence of lesions, bleeding, and erosions, typically scored on a scale of 0–5 points.117,118,132 Additionally, the reduction in the ulcer area served as an indicator of ulcer improvement. Preclinical studies also assessed the wound healing effect on animals with corneal injuries.113 The clarity of the cornea, correlated with lower or absent neovascularization activity, was indicative of the healing ability of the samples in corneal repair.113 In light of the correlation between wounds and inflammatory conditions, which play a pivotal role in wound healing acceleration, the analysis of inflammatory markers emerged as a valuable indicator of wound healing. The regulation of cytokines and chemokines, in particular, was closely monitored to assess inflammatory activity, which indirectly influences the rate of wound healing.133 Moreover, antioxidant activity was examined as an indicator of the body’s ability to counteract reactive oxygen species (ROS), which can exacerbate wounds and hinder the healing process.134 Increased expression of growth factors was also a significant focus, as these factors promote the growth and repair of damaged cells and tissues.49
Furthermore, in vivo studies demonstrated that the application of NLCs as nanocarriers for wound treatment showed promising potential. Generally, the application of NLCs led to a reduction in inflammatory factors, as indicated by increased levels of TGF-β, IL-10, IL-3, and SDF-1α, along with decreased levels of IL-6, IL-1β, TNF-α, TGFβ1, NF-κB, CXCL-5, MMP-1, MMP-3, MMP-9, COX-2, Col Іα1, Col Іα2, and Fn.98,106,107,109,111–114,122 Additionally, antioxidant activity increased, as evidenced by elevated levels of SOD, CAT, GPx, and reduced LPO.96 Furthermore, the growth factors, including VEGF, FGF-2, b-FGF, EGF, and PDGF, were upregulated due to the modulatory role of NLCs.98,106,107,109,111–114,122 Notably, histopathological examinations of wounds revealed significant repair, as demonstrated by increased levels of hydroxyproline (collagen).106,122 Furthermore, the delivery of biologic agents such as the LL37 peptide, which has an immunomodulatory effect by inactivating macrophages, resulted in a dose-dependent and superior wound closure rate compared to controls.135 NLCs were found to be highly compatible with the delivery of various active compounds in the form of essential oils, whether prepared using non-thermal or low thermal energy methods. The nanomodification strategy employed was also effective in optimizing the wound healing ability of anti-inflammatory agents such as thymoquinone and zerumbone,107,120–122 as well as antibacterial agents like rapamycin.113 Additionally, NLCs were found to be suitable for enhancing the wound healing properties of extract-based agents like propolis, which contains various antioxidant and anti-inflammatory compounds such as flavonoids and lipid glue-like components.108 An intriguing discovery was made in a study by Örgul et al, which demonstrated an enhanced wound healing effect of simvastatin, an agent unfamiliar for topical application. NLCs were believed to play a significant role in enhancing the angiogenic properties of simvastatin, resulting in increased vascular endothelial growth factor (VEGF) activity.119,136
Clinical Trial
Clinical studies evaluating the effectiveness of drug delivery using NLCs are still quite limited, with only two study conducted by Samadi et al and Motawea et al utilizing tretinoin (TRE) and phenytoin (PHT), respectively, as the active compound loaded into NLCs.123 These two studies similarly employed a prospective double-blinded randomized controlled design. Tretinoin, as an anti-acne agent, has been proven to be more effective in acne treatment when modified into an NLC system. The use of tretinoin significantly decreased skin lesions caused by acne vulgaris, including blackheads, whiteheads, and papules. After 8 weeks of treatment, the differences were significant compared to unmodified tretinoin (p value < 0.001). Besides its efficacy, tretinoin-loaded NLCs were proven safe in a Phase 1 clinical study, demonstrating that this system is typically safe for short-term application.78
In Motawea et al’s study, phenytoin, the chosen active compound, was selected due to its established wound healing properties. The mechanism of action of phenytoin in wound treatment is believed to involve nerve restoration,137 although other potential mechanisms include the stimulation of collagen deposition, enhancement of fibroblast proliferation, glucocorticoid inhibition, and antibacterial action.123 In this study, the loaded drugs were prepared in a hydrogel formulation (0.5% w/v) and subsequently evaluated in patients with diabetic foot ulcers (DFUs). Following twice-daily application for 8 weeks, patients using PHT-loaded NLCs exhibited the highest percentage of wound healing (95.82 ± 2.22) compared to those using unloaded PHT (47.10 ± 4.23) and blank (−34.91 ± 28.33) formulations (p < 0.001).123 Several factors likely contributed to this advantage, including the small particle size, which enhances solubility and increases surface contact area, as well as the lipid components that facilitate improved drug penetration into the subcutaneous layer of the skin. However, it’s important to acknowledge that this study had certain limitations, primarily its relatively small sample size. Therefore, conducting larger-scale clinical studies is highly recommended to further validate the efficacy of NLCs as nanocarriers in wound treatment.123
Challenge and Limitation
The utilization of nanostructured lipid carriers (NLCs) technology for topical wound healing is fraught with various challenges and obstacles. One significant issue is the regulatory framework governing the development of nano-scale pharmaceuticals. Guidelines for testing and quality assurance processes are typically designed for simple, unmodified drugs. The inclusion of modifications within the NLCs system necessitates the standardization of methods, instrumentation, and specifications in the quality assurance process.138 Furthermore, different formulations and manufacturing methods can introduce variability in the physicochemical properties of the preparations, thereby requiring specific testing methods for each. Additionally, the incorporation of extra materials, processes, and energy to develop preparations within the NLC system inevitably increases production costs, leading to higher product prices. This presents a challenge for developers to carefully balance efficacy and cost, ensuring that the final product remains attractive and affordable to the target users.139
The application of NLCs also faces limitations based on the materials used in their formulation. For instance, the use of polysorbate 80 (Tween 80) as a stabilizer is restricted to preparations intended for adult patients, as it poses a risk of skin irritation in pediatric patients.140 Consequently, formulators must conduct preformulation studies to ensure the compatibility of the materials used in each development. Additionally, given that NLC systems are typically oil-in-water emulsions,14 it is challenging to formulate drug-loaded NLCs into solid dosage forms. Techniques such as freeze-drying can be employed to obtain powder forms from NLC systems; however, these methods only yield a small amount of powdered product.141 Therefore, further development of techniques to convert liquid NLCs into solid forms is essential to maximize the potential of NLCs as a drug delivery modification system.
Future Perspective
NLCs represent highly valuable nanocarrier systems in the realm of drug delivery for wound treatment. The composition of solid lipids and liquid lipids within these systems enables the delivery of hydrophobic compounds and enhances the permeability of the formulation through mucosal and dermal surface membranes.142 The utility of NLCs extends beyond chemical compounds to encompass the delivery of biologically active substances, such as EGF, rhEGF, rhTM, and siRNA.95–97,105 Moreover, NLCs for wound treatment can be formulated using various suitable methods, each offering distinct advantages. Nevertheless, despite the demonstrated effectiveness of NLCs in enhancing the wound healing potential of delivered compounds, there is still a dearth of discussion regarding aspects such as biodistribution, toxicity, and the underlying molecular mechanisms of NLCs systems. Furthermore, clinical studies investigating the application of NLCs in wound treatment remain scarce and typically involve small study populations. Consequently, there is an imperative need for additional clinical studies, especially those involving larger and more diverse populations, to substantiate the efficacy of NLCs as nanocarriers for drug delivery in the context of wound healing.
Conclusion
Nanostructured lipid carriers (NLCs) present substantial advantages in enhancing the therapeutic efficacy of pharmacologically active substances for wound treatment. Numerous studies have validated the effectiveness of NLCs in delivering hydrophobic chemical compounds, essential oils, crude extracts, and biologically active substances, such as proteins and nucleic acids, for wound healing purposes. NLCs improve the stability, bioavailability, and controlled release of these substances, thereby promoting wound repair and regeneration more effectively. The adaptability of NLC formulations is reflected in the variety of solid and liquid lipids, as well as surfactants used, and the diverse preparation methods available, including high-pressure homogenization, solvent evaporation, and microemulsion techniques. These methods enable the customization of NLCs to address specific therapeutic requirements and optimize the delivery of various pharmacologically active compounds. Since NLCs provide sustained drug release, they are also particularly beneficial for chronic wounds that require long-term drug availability. Nevertheless, further research is necessary to explore the use of NLCs in wound treatment with a broader array of pharmacologically active substances, including newer synthetic and natural agents. Additionally, comprehensive safety evaluations are essential to ensure the secure application of NLCs, especially for chronic wounds, burns, and diabetic ulcers. This involves assessing the long-term effects, potential toxicity, and biocompatibility of NLCs to confirm their safe and effective use in clinical settings.
Acknowledgments
The authors express their gratitude to the rector of Universitas Padjadjaran for the APC. The authors also would like to extend their appreciation to the Deanship of Scientific Research at Northern Border University, Arar, KSA, for funding this research work with the project number “NBU-FPEJ-2024-2985-03”.
Funding
This research was funded by the National Research and Innovation Agency (BRIN) for the Riset Inovasi dan Indonesia Maju (RIIM).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Negut I, Grumezescu V, Grumezescu AM. Treatment strategies for infected wounds. Molecules. 2018;23(9):2392. doi:10.3390/molecules23092392
2. Zhu J, Zhong K, Zong Y, et al. A mussel-inspired wet-adhesion hydrogel with hemostasis and local anti-inflammation for managing the development of acute wounds. Mater Des. 2022;213. doi:10.1016/j.matdes.2021.110347
3. Sacco P, Travan A, Borgogna M, Paoletti S, Marsich E. Silver-containing antimicrobial membrane based on chitosan-TPP hydrogel for the treatment of wounds. J Mater Sci Mater Med. 2015;26(3). doi:10.1007/s10856-015-5474-7
4. Wathoni N, Suhandi C, Purnama MFG, et al. Alginate and chitosan-based hydrogel enhance antibacterial agent activity on topical application. Infect Drug Resist. 2024;17. doi:10.2147/IDR.S456403
5. Yamakawa S, Hayashida K. Advances in surgical applications of growth factors for wound healing. Burns Trauma. 2019;7. doi:10.1186/s41038-019-0148-1
6. Suhandi C, Mohammed AFA, Wilar G, El-Rayyes A, Wathoni N. Effectiveness of mesenchymal stem cell secretome on wound healing: a systematic review and meta-analysis. Tissue Eng Regen Med. 2023. doi:10.1007/s13770-023-00570-9
7. Shi C, Wang C, Liu H, et al. Selection of appropriate wound dressing for various wounds. Front Bioeng Biotechnol. 2020;8. doi:10.3389/fbioe.2020.00182
8. Whittam AJ, Maan ZN, Duscher D, et al. Challenges and opportunities in drug delivery for wound healing. Adv Wound Care. 2016;5(2). doi:10.1089/wound.2014.0600
9. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4(9). doi:10.1089/wound.2015.0635
10. Zhang K, Li Y, He J, et al. Therapeutic effect of epidermal growth factor combined with nano silver dressing on diabetic foot patients. Front Pharmacol. 2021;12. doi:10.3389/fphar.2021.627098
11. Saeedi M, Eslamifar M, Khezri K, Dizaj SM. Applications of nanotechnology in drug delivery to the central nervous system. Biome Pharmacother. 2019;111. doi:10.1016/j.biopha.2018.12.133
12. Saadh MJ, Jadullah RK. Nanotechnology in drug delivery. Pharmacologyonline. 2021;3. doi:10.17148/ijarcce.2016.55255
13. Aldeeb MME, Wilar G, Suhandi C, Elamin KM, Wathoni N. Nanosuspension-based drug delivery systems for topical applications. Int J Nanomed. 2024;19:825–844. doi:10.2147/IJN.S447429
14. Suhandi C, Wilar G, Lesmana R, et al. Propolis-based nanostructured lipid carriers for α-mangostin delivery: formulation, characterization, and in vitro antioxidant activity evaluation. Molecules. 2023;28(16). doi:10.3390/molecules28166057
15. Megantara S, Wathoni N, Mohammed AFA, Suhandi C, Ishmatullah MH, Mffd P. In silico study: combination of α-mangostin and chitosan conjugated with trastuzumab against human epidermal growth factor receptor 2. Polymers. 2022;14(13). doi:10.3390/polym14132747
16. Kushwaha A, Goswami L, Kim BS. Nanomaterial-based therapy for wound healing. Nanomaterials. 2022;12(4). doi:10.3390/nano12040618
17. Wang H, Ding L, Xu F, et al. Construction of novel amphiphilic chitosan-polylactide graft copolymer nanodroplets for contrast enhanced ultrasound tumor imaging. J Biomater Appl. 2021;36(4). doi:10.1177/08853282211011766
18. Chen J, Zhao Q, Peng J, Yang X, Yu D, Zhao W. Antibacterial and mechanical properties of reduced graphene-silver nanoparticle nanocomposite modified glass ionomer cements. J Dent. 2020;96. doi:10.1016/j.jdent.2020.103332
19. Fu L. Delivery systems in wound healing and nanomedicine. In: Wound Healing - New Insights into Ancient Challenges. IntechOpen; 2016. doi10.5772/63763
20. Hua S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front Pharmacol. 2015;6. doi:10.3389/fphar.2015.00219
21. Mihai MM, Dima MB, Dima B, Holban AM. Nanomaterials for wound healing and infection control. Materials. 2019;12(13). doi:10.3390/ma12132176
22. Samadi A, Buro J, Dong X, et al. Topical α-gal nanoparticles enhance wound healing in radiated skin. Skin Pharmacol Physiol. 2022;35(1). doi:10.1159/000518015
23. Kaymakcalan OE, Abadeer A, Goldufsky JW, et al. Topical α-gal nanoparticles accelerate diabetic wound healing. Exp Dermatol. 2020;29(4). doi:10.1111/exd.14084
24. Galili U. α-gal nanoparticles in wound and burn healing acceleration. Adv Wound Care. 2017;6(3). doi:10.1089/wound.2016.0703
25. Hurwitz ZM, Ignotz R, Lalikos JF, Galili U. Accelerated porcine wound healing after treatment with α-gal nanoparticles. Plast Reconstr Surg. 2012;129(2). doi:10.1097/PRS.0b013e31823aebb1
26. Wigglesworth KM, Racki WJ, Mishra R, Szomolanyi-Tsuda E, Greiner DL, Galili U. Rapid recruitment and activation of macrophages by anti-Gal/α-Gal liposome interaction accelerates wound healing. J Immunol. 2011;186(7). doi:10.4049/jimmunol.1002324
27. Galili U, Wigglesworth K, Abdel-Motal UM. Accelerated healing of skin burns by anti-Gal/α-gal liposomes interaction. Burns. 2010;36(2). doi:10.1016/j.burns.2009.04.002
28. Sandhu SK, Kumar S, Raut J, et al. Systematic development and characterization of novel, high drug-loaded, photostable, curcumin solid lipid nanoparticle hydrogel for wound healing. Antioxidants. 2021;10(5). doi:10.3390/antiox10050725
29. Hosny KM, Naveen NR, Kurakula M, et al. Design and development of neomycin sulfate gel loaded with solid lipid nanoparticles for buccal mucosal wound healing. Gels. 2022;8(6). doi:10.3390/gels8060385
30. Chauhan I, Yasir M, Verma M, Singh AP. Nanostructured lipid carriers: a groundbreaking approach for transdermal drug delivery. Adv Pharm Bull. 2020;10(2). doi:10.34172/apb.2020.021
31. López-García R, Ganem-Rondero A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC): occlusive effect and penetration enhancement ability. J Cosmet Dermatological Sci Appl. 2015;05(02). doi:10.4236/jcdsa.2015.52008
32. Doktorovova S, Souto EB, Silva AM. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers - A systematic review of in vitro data. Eur J Pharm Biopharm. 2014;87(1). doi:10.1016/j.ejpb.2014.02.005
33. Poonia N, Kharb R, Lather V, Pandita D. Nanostructured lipid carriers: versatile oral delivery vehicle. Future Sci OA. 2016;2(3). doi:10.4155/fsoa-2016-0030
34. Nagaich U, Gulati N. Nanostructured lipid carriers (NLC) based controlled release topical gel of clobetasol propionate: design and in vivo characterization. Drug Deliv Transl Res. 2016;6(3). doi:10.1007/s13346-016-0291-1
35. Chandana KV, Gupta NV, Kanna SA. Nanostructured Lipid Carriers: the Frontiers In Drug Delivery. Asian J Pharm Clin Res. 2019. doi:10.22159/ajpcr.2019.v12i7.33595
36. Loo CH, Basri M, Ismail R, et al. Effect of compositions in nanostructured lipid carriers (NLC) on skin hydration and occlusion. Int J Nanomed. 2013;8. doi:10.2147/IJN.S35648
37. Wilkinson H; Biology MHO, 2020 U. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223.
38. Wang W, Lu KJ, Yu CH, Huang QL, Du YZ. Nano-drug delivery systems in wound treatment and skin regeneration. J Nanobiotechnol. 2019;17(1). doi:10.1186/s12951-019-0514-y
39. Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: a review of patents and commercial products. Eur Polym J. 2015;65. doi:10.1016/j.eurpolymj.2014.11.024
40. Saghazadeh S, Rinoldi C, Schot M, et al. Drug delivery systems and materials for wound healing applications. Adv Drug Deliv Rev. 2018;127. doi:10.1016/j.addr.2018.04.008
41. Lei M, Guo X, Yao Y, et al. Trelagliptin relieved cognitive impairment of diabetes mellitus rats: involvement of PI3K/Akt/GSK-3β and inflammation pathway. Exp Gerontol. 2023;182. doi:10.1016/j.exger.2023.112307
42. Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173(2). doi:10.1111/bjd.13954
43. Li J, Amaya E. Investigating the cellular and molecular mechanisms of wound healing in xenopus oocytes and embryos. Cold Spring Harb Protoc. 2019;2019(4). doi:10.1101/pdb.prot100982
44. Golebiewska EM, Poole AW. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29(3). doi:10.1016/j.blre.2014.10.003
45. Long T, Li C, Xu F, Xiao J. Therapeutic efficacy of platelet-rich fibrin on surgical site wound healing in patients undergoing oral carcinoma resection: a meta-analysis. Int Wound J. 2024;21(1). doi:10.1111/iwj.14386
46. Bai Y, Niu Y, Qin S, Ma G. A new biomaterial derived from aloe vera—acemannan from basic studies to clinical application. Pharmaceutics. 2023;15(7). doi:10.3390/pharmaceutics15071913
47. Locatelli L, Colciago A, Castiglioni S, Maier JA. Platelets in Wound Healing: what Happens in Space? Front Bioeng Biotechnol. 2021;9. doi:10.3389/fbioe.2021.716184
48. Ashika Riswana N, Don KR. Role of growth factors in wound healing. Drug Invention Today. 2019;11(2):458.
49. Vaidyanathan L. Growth factors in wound healing ⇓ a review. Biomed Pharmacol J. 2021;14(3). doi:10.13005/bpj/2249
50. Demaria M, Ohtani N, Youssef SA, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31(6). doi:10.1016/j.devcel.2014.11.012
51. Luengo-Gil G, Calvo MI, Martín-Villar E, et al. Antithrombin controls tumor migration, invasion and angiogenesis by inhibition of enteropeptidase. Sci Rep. 2016;6. doi:10.1038/srep27544
52. Denzinger M, Held M, Daigeler A, Krajewski S, Link A. Complement activation at the interface of wound dressings and blood does not influence keratinocyte migration/proliferation in vitro. Wound Repair Regener. 2020;28(4). doi:10.1111/wrr.12817
53. Versey Z, da Cruz Nizer WS, Russell E, et al. Biofilm-innate immune interface: contribution to chronic wound formation. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.648554
54. Li X, Xiang Y, Li F, Yin C, Li B, Ke X. WNT/β-catenin signaling pathway regulating T cell-inflammation in the tumor microenvironment. Front Immunol. 2019;10. doi:10.3389/fimmu.2019.02293
55. Mortaz E, Alipoor SD, Adcock IM, Mumby S, Koenderman L. Update on neutrophil function in severe inflammation. Front Immunol. 2018;9. doi:10.3389/fimmu.2018.02171
56. Hahn J, Schauer C, Czegley C, et al. Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. FASEB J. 2019;33(1). doi:10.1096/fj.201800752R
57. Zhou Z, Xi R, Liu J, et al. TAS2R16 activation suppresses lps-induced cytokine expression in human gingival fibroblasts. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.726546
58. Bakogiannis C, Sachse M, Stamatelopoulos K, Stellos K. Platelet-derived chemokines in inflammation and atherosclerosis. Cytokine. 2019;122. doi:10.1016/j.cyto.2017.09.013
59. Cheng F, Shen Y, Mohanasundaram P, et al. Vimentin coordinates fibroblast proliferation and keratinocyte differentiation in wound healing via TGF-β-Slug signaling. Proc Natl Acad Sci U S A. 2016;113(30). doi:10.1073/pnas.1519197113
60. Katayama Y, Naitoh M, Kubota H, et al. Chondroitin sulfate promotes the proliferation of keloid fibroblasts through activation of the integrin and protein kinase B pathways. Int J Mol Sci. 2020;21(6). doi:10.3390/ijms21061955
61. Pastar I, Stojadinovic O, Yin NC, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care. 2014;3(7). doi:10.1089/wound.2013.0473
62. Holt JR, Zeng WZ, Evans EL, et al. Spatiotemporal dynamics of piezo1 localization controls keratinocyte migration during wound healing. Elife. 2021;10. doi:10.7554/eLife.65415
63. Zhang C, Lim J, Jeon HH, et al. FOXO1 deletion in keratinocytes improves diabetic wound healing through MMP9 regulation. Sci Rep. 2017;7(1). doi:10.1038/s41598-017-10999-3
64. Slade EA, Thorn RMS, Young A, Reynolds DM. An in vitro collagen perfusion wound biofilm model; with applications for antimicrobial studies and microbial metabolomics. BMC Microbiol. 2019;19(1). doi:10.1186/s12866-019-1682-5
65. Tahir T, Febrianti N, Wahyuni S, Rabia, Syam Y. Evaluation of acute wound healing potential of red dragon fruit (Hylocereus Polyrhizus) extract cream on type III collagen and Epidermal Growth Factor (EGF) levels: an animal study. Medicina Clinica Practica. 2020;3. doi:10.1016/j.mcpsp.2020.100091
66. Lu Y, Yang Y, Xiao L, Li S, Liao X, Liu H. Autocrine and paracrine effects of vascular endothelial cells promote cutaneous wound healing. Biomed Res Int. 2021;2021. doi:10.1155/2021/6695663
67. Kim HS, Hwang HJ, Kim HJ, et al. Effect of decellularized extracellular matrix bioscaffolds derived from fibroblasts on skin wound healing and remodeling. Front Bioeng Biotechnol. 2022;10. doi:10.3389/fbioe.2022.865545
68. Mara JN, Zhou LT, Larmore M, et al. Ovulation and ovarian wound healing are impaired with advanced reproductive age. Aging. 2020;12(10). doi:10.18632/aging.103237
69. Monaghan M, Browne S, Schenke-Layland K, Pandit A. A collagen-based scaffold delivering exogenous MicroRNA-29B to modulate extracellular matrix remodeling. Mol Ther. 2014;22(4). doi:10.1038/mt.2013.288
70. Mori HM, Kawanami H, Kawahata H, Aoki M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-β in a rat model. BMC Complement Altern Med. 2016;16(1). doi:10.1186/s12906-016-1128-7
71. Garg J, Pathania K, Sah SP, Pawar SV. Nanostructured lipid carriers: a promising drug carrier for targeting brain tumours. Futur J Pharm Sci. 2022;8(1). doi:10.1186/s43094-022-00414-8
72. Sharma A, Baldi A. Nanostructured lipid carriers: a review journal. J Dev Drugs. 2018;7(2). doi:10.4172/2329-6631.1000187
73. Sarhadi S, Gholizadeh M, Moghadasian T, Golmohammadzadeh S. Moisturizing effects of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) using deionized and magnetized water by in vivo and in vitro methods. Iran J Basic Med Sci. 2020;23(3). doi:10.22038/IJBMS.2020.39587.9397
74. Apostolou M, Assi S, Fatokun AA, Khan I. The effects of solid and liquid lipids on the physicochemical properties of nanostructured lipid carriers. J Pharm Sci. 2021;110(8). doi:10.1016/j.xphs.2021.04.012
75. Rehman M, Madni A, Ihsan A, et al. Solid and liquid lipid-based binary solid lipid nanoparticles of diacerein: in vitro evaluation of sustained release, simultaneous loading of gold nanoparticles, and potential thermoresponsive behavior. Int J Nanomed. 2015;10. doi:10.2147/IJN.S67147
76. Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biome Pharmacother. 2018;103. doi:10.1016/j.biopha.2018.04.055
77. Miranda M, Cruz MT, Vitorino C, Cabral C. Nanostructuring lipid carriers using Ridolfia segetum (L.) Moris essential oil. Mater Sci Eng C. 2019;103. doi:10.1016/j.msec.2019.109804
78. Samadi A, Sartipi Z, Ahmad Nasrollahi S, et al. Efficacy assessments of tretinoin-loaded nano lipid carriers in acne vulgaris: a double blind, split-face randomized clinical study. Arch Dermatol Res. 2022;314(6). doi:10.1007/s00403-021-02256-5
79. Subramaniam B, Siddik ZH, Nagoor NH. Optimization of nanostructured lipid carriers: understanding the types, designs, and parameters in the process of formulations. J Nanopart Res. 2020;22(6). doi:10.1007/s11051-020-04848-0
80. Müller RH, Radtke M, Wissing SA. Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharm. 2002;242(1–2). doi:10.1016/S0378-5173(02)00180-1
81. Elmowafy M, Al-Sanea MM. Nanostructured lipid carriers (NLCs) as drug delivery platform: advances in formulation and delivery strategies. Saudi Pharm J. 2021;29(9). doi:10.1016/j.jsps.2021.07.015
82. Faheim SH, Gardouh AR, Nouh AT, Ghorab MM. Review article on nanoemulsions and nanostructured lipid carriers. Records Pharm Biomed Sci. 2018;2(2). doi:10.21608/rpbs.2018.5223.1011
83. Salvi VR, Pawar P. Nanostructured lipid carriers (NLC) system: a novel drug targeting carrier. J Drug Deliv Sci Technol. 2019;51. doi:10.1016/j.jddst.2019.02.017
84. Ganesan P, Narayanasamy D. Lipid nanoparticles: different preparation techniques, characterization, hurdles, and strategies for the production of solid lipid nanoparticles and nanostructured lipid carriers for oral drug delivery. Sustain Chem Pharm. 2017;6. doi:10.1016/j.scp.2017.07.002
85. Fang CL, A Al-Suwayeh S, Fang JY. Nanostructured Lipid Carriers (NLCs) for drug delivery and targeting. Recent Pat Nanotechnol. 2012;7(1). doi:10.2174/18722105130105
86. Mendes IT, Ruela ALM, Carvalho FC, Freitas JTJ, Bonfilio R, Pereira GR. Development and characterization of nanostructured lipid carrier-based gels for the transdermal delivery of donepezil. Colloids Surf B Biointerfaces. 2019;177. doi:10.1016/j.colsurfb.2019.02.007
87. Qidwai A, Khan S, Md S, et al. Nanostructured lipid carrier in photodynamic therapy for the treatment of basal-cell carcinoma. Drug Deliv. 2016;23(4). doi:10.3109/10717544.2016.1165310
88. Joshi MD, Prabhu RH, Patravale VB. Fabrication of nanostructured lipid carriers (NLC)-based gels from microemulsion template for delivery through skin. In: Methods in Molecular Biology. Vol. 2000. Springer; 2019. doi:10.1007/978-1-4939-9516-5_19
89. Iqbal M, Zafar N, Fessi H, Elaissari A. Double emulsion solvent evaporation techniques used for drug encapsulation. Int J Pharm. 2015;496(2). doi:10.1016/j.ijpharm.2015.10.057
90. Ren G, Sun Z, Wang Z, Zheng X, Xu Z, Sun D. Nanoemulsion formation by the phase inversion temperature method using polyoxypropylene surfactants. J Colloid Interface Sci. 2019;540. doi:10.1016/j.jcis.2019.01.018
91. Jintapattanakit A. Preparation of nanoemulsions by phase inversion temperature (PIT) method. Pharm Sci Asia. 2018;45(1). doi:10.29090/psa.2018.01.001
92. Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure preparation and application. Adv Pharm Bull. 2015;5(3). doi:10.15171/apb.2015.043
93. Zheng X, Zhang W, Wang Z. Simvastatin preparations promote PDGF-BB secretion to repair LPS-induced endothelial injury through the PDGFRβ/PI3K/Akt/IQGAP1 signalling pathway. J Cell Mol Med. 2019;23(12):8314–8327. doi:10.1111/jcmm.14709
94. Saporito F, Sandri G, Bonferoni MC, et al. Essential oil-loaded lipid nanoparticles for wound healing. Int J Nanomed. 2018;13:175–186. doi:10.2147/IJN.S152529
95. Gainza G, Pastor M, Aguirre JJ, et al. A novel strategy for the treatment of chronic wounds based on the topical administration of rhEGF-loaded lipid nanoparticles: in vitro bioactivity and in vivo effectiveness in healing-impaired db/db mice. J Control Release. 2014;185(1):51–61. doi:10.1016/j.jconrel.2014.04.032
96. Lee HJ, Jeong M, Na YG, Kim SJ, Lee HK, Cho CW. An EGF- And curcumin-Co-encapsulated nanostructured lipid carrier accelerates chronic-wound healing in diabetic rats. Molecules. 2020;25(20). doi:10.3390/molecules25204610
97. Tezgel Ö, DiStasio N, Laghezza-Masci V, et al. Collagen scaffold-mediated delivery of NLC/siRNA as wound healing materials. J Drug Deliv Sci Technol. 2020;55. doi:10.1016/j.jddst.2019.101421
98. Sun D, Guo SY, Yang L, et al. Silicone elastomer gel impregnated with 20(S)-protopanaxadiol-loaded nanostructured lipid carriers for ordered diabetic ulcer recovery. Acta Pharmacol Sin. 2020;41(1):119–128. doi:10.1038/s41401-019-0288-7
99. Carbone C, Caddeo C, Grimaudo MA, Manno DE, Serra A, Musumeci T. Ferulic acid-nlc with lavandula essential oil: a possible strategy for wound-healing? Nanomaterials. 2020;10(5). doi:10.3390/nano10050898
100. Costa-Fernandez S, Matos JKR, Scheunemann GS, et al. Nanostructured lipid carriers containing chitosan or sodium alginate for co-encapsulation of antioxidants and an antimicrobial agent for potential application in wound healing. Int J Biol Macromol. 2021;183:668–680. doi:10.1016/j.ijbiomac.2021.04.168
101. Silva J, Mesquita R, Pinho E, et al. Incorporation of lipid nanosystems containing omega-3 fatty acids and resveratrol in textile substrates for wound healing and anti-inflammatory applications. SN Appl Sci. 2019;1(9). doi:10.1007/s42452-019-1049-4
102. Liakopoulou A, Mourelatou E, Hatziantoniou S. Exploitation of traditional healing properties, using the nanotechnology’s advantages: the case of curcumin. Toxicol Rep. 2021;8:1143–1155. doi:10.1016/j.toxrep.2021.05.012
103. Romić MD, Sušac A, Lovrić J, Cetina-čižmek B, Filipović-Grčić J, Hafner A. Evaluation of stability and in vitro wound healing potential of melatonin loaded (lipid enriched) chitosan based microspheres. Acta Pharm. 2019;69(4):635–648. doi:10.2478/acph-2019-0049
104. Alexander HR, Syed Alwi SS, Yazan LS, Zakarial Ansar FH, Ong YS. Migration and proliferation effects of Thymoquinone-Loaded Nanostructured Lipid Carrier (TQ-NLC) and Thymoquinone (TQ) on in vitro wound healing models. Evid Based Complement Alternat Med. 2019;2019:1–14. doi:10.1155/2019/9725738
105. Hsueh YS, Shyong YJ, Yu HC, et al. Nanostructured lipid carrier gel formulation of recombinant human thrombomodulin improve diabetic wound healing by topical administration. Pharmaceutics. 2021;13(9). doi:10.3390/pharmaceutics13091386
106. Natarajan J, Sanapalli BKR, Bano M, Singh SK, Gulati M, Karri VVSR. Nanostructured lipid carriers of pioglitazone loaded collagen/chitosan composite scaffold for diabetic wound healing. Adv Wound Care. 2019;8(10):499–513. doi:10.1089/wound.2018.0831
107. Albaayit SFA, Abdullah R, Noor MHM. Zerumbone-loaded nanostructured lipid carrier gel enhances wound healing in diabetic rats. Biomed Res Int. 2022;2022. doi:10.1155/2022/1129297
108. Elkhateeb OM, Badawy MEI, Noreldin AE, Abou-Ahmed HM, El-Kammar MH, Elkhenany HA. Comparative evaluation of propolis nanostructured lipid carriers and its crude extract for antioxidants, antimicrobial activity, and skin regeneration potential. BMC Complement Med Ther. 2022;22(1). doi:10.1186/s12906-022-03737-4
109. Tazehjani DAJ, Farahpour MR, Hamishehkar H. Effectiveness of topical caraway essential oil loaded into nanostructured lipid carrier as a promising platform for the treatment of infected wounds. Colloids Surf a Physicochem Eng Asp. 2021;610. doi:10.1016/j.colsurfa.2020.125748
110. Garcia-Orue I, Gainza G, Garcia-Garcia P, et al. Composite nanofibrous membranes of PLGA/Aloe vera containing lipid nanoparticles for wound dressing applications. Int J Pharm. 2019;556:320–329. doi:10.1016/j.ijpharm.2018.12.010
111. Khezri K, Farahpour MR, Mounesi Rad S. Efficacy of Mentha pulegium essential oil encapsulated into nanostructured lipid carriers as an in vitro antibacterial and infected wound healing agent. Colloids Surf A Physicochem Eng Asp. 2020;589. doi:10.1016/j.colsurfa.2020.124414
112. Ghodrati M, Farahpour MR, Hamishehkar H. Encapsulation of Peppermint essential oil in nanostructured lipid carriers: in-vitro antibacterial activity and accelerative effect on infected wound healing. Colloids Surf A Physicochem Eng Asp. 2019;564:161–169. doi:10.1016/j.colsurfa.2018.12.043
113. Zahir-Jouzdani F, Khonsari F, Soleimani M, et al. Nanostructured lipid carriers containing rapamycin for prevention of corneal fibroblasts proliferation and haze propagation after burn injuries: in vitro and in vivo. J Cell Physiol. 2019;234(4):4702–4712. doi:10.1002/jcp.27243
114. Khezri K, Farahpour MR, Mounesi Rad S. Accelerated infected wound healing by topical application of encapsulated Rosemary essential oil into nanostructured lipid carriers. Artif Cells Nanomed Biotechnol. 2019;47(1):980–988. doi:10.1080/21691401.2019.1582539
115. Varrica C, Carvalheiro M, Faria-Silva C, Eleutério C, Sandri G, Simões S. Topical allopurinol-loaded nanostructured lipid carriers: a novel approach for wound healing management. Bioengineering. 2021;8(12). doi:10.3390/bioengineering8120192
116. Wen MM, Abdelwahab IA, Aly RG, El-Zahaby SA. Nanophyto-gel against multi-drug resistant Pseudomonas aeruginosa burn wound infection. Drug Deliv. 2021;28(1):463–477. doi:10.1080/10717544.2021.1889720
117. Ahmed OAA, Fahmy UA, Bakhaidar R, et al. Pumpkin oil-based nanostructured lipid carrier system for antiulcer effect in NSAID-induced gastric ulcer model in rats. Int J Nanomed. 2020;15:2529–2539. doi:10.2147/IJN.S247252
118. Hosny KM, Sindi AM, Ali S, et al. Development, optimization, and evaluation of a nanostructured lipid carrier of sesame oil loaded with miconazole for the treatment of oral candidiasis. Drug Deliv. 2022;29(1):254–262. doi:10.1080/10717544.2021.2023703
119. Örgül D, Eroğlu H, Tiryaki M, Pınarlı FA, Hekimoglu S. In-vivo evaluation of tissue scaffolds containing simvastatin loaded nanostructured lipid carriers and mesenchymal stem cells in diabetic wound healing. J Drug Deliv Sci Technol. 2021;61. doi:10.1016/j.jddst.2020.102140
120. Abdelwahab SI, Sheikh BY, Taha MME, et al. Thymoquinone-loaded nanostructured lipid carriers: preparation, gastroprotection, in vitro toxicity, and pharmacokinetic properties after extravascular administration. Int J Nanomed. 2013;8:2163–2172. doi:10.2147/IJN.S44108
121. Fahmy UA, L. Alaofi A, Awan ZA, Alqarni HM, Alhakamy NA. Optimization of thymoquinone-loaded coconut oil nanostructured lipid carriers for the management of ethanol-induced ulcer. AAPS Pharm Sci Tech. 2020;21(5). doi:10.1208/s12249-020-01693-1
122. Albaayit SFA, Rasedee A, Abdullah N. Zerumbone-loaded nanostructured lipid carrier gel facilitates wound healing in rats. Rev Bras Farmacogn. 2020;30(2):272–278. doi:10.1007/s43450-020-00023-7
123. Motawea A, Abd El-Gawad AEGH, Borg T, Motawea M, Tarshoby M. The impact of topical phenytoin loaded nanostructured lipid carriers in diabetic foot ulceration. Foot. 2019;40:14–21. doi:10.1016/j.foot.2019.03.007
124. Ashwini M, Sudheer P, Sogali BS. Custom design perspective in the process parameter optimization of nano lipid carriers. Int J Appl Pharm. 2020;12(6). doi:10.22159/ijap.2020v12i6.39565
125. Qiang L, Sample A, Liu H, Wu X, He YY. Epidermal SIRT1 regulates inflammation, cell migration, and wound healing. Sci Rep. 2017;7(1). doi:10.1038/s41598-017-14371-3
126. Lee BC, Song J, Lee A, Cho D, Kim TS. Visfatin promotes wound healing through the activation of ERK1/2 and JNK1/2 pathway. Int J Mol Sci. 2018;19(11):3642. doi:10.3390/ijms19113642
127. Abd El-Aleem SA, Abdelwahab S, AM-Sherief H, Sayed A. Cellular and physiological upregulation of inducible nitric oxide synthase, arginase, and inducible cyclooxygenase in wound healing. J Cell Physiol. 2019;234(12):23618–23632. doi:10.1002/jcp.28930
128. Pomalingo DR, Suhandi C, Megantara S, Muchtaridi M. The optimization of α-mangostin as a new drug candidate through molecular docking and dynamic simulations. Rasayan J Chem. 2021;14(2):698–704. doi:10.31788/RJC.2021.1425770
129. Suhandi C, Alfathonah SS, Hasanah AN. Potency of xanthone derivatives from Garcinia mangostana L. for COVID-19 treatment through angiotensin-converting enzyme 2 and main protease blockade: a computational study. Molecules. 2023;28(13):5187. doi:10.3390/molecules28135187
130. Suharyani I, Suhandi C, Rizkiyan Y, et al. Molecular docking in prediction of α-mangostin/cyclodextrin inclusion complex formation. AIP Conf Proc. 2023;2706(1). doi:10.1063/5.0120782
131. Qin W, Liu K, Su H, et al. Tibial cortex transverse transport promotes ischemic diabetic foot ulcer healing via enhanced angiogenesis and inflammation modulation in a novel rat model. Eur J Med Res. 2024;29(1). doi:10.1186/s40001-024-01752-4
132. Fife CE, Horn SD, Smout RJ, Barrett RS, Thomson B. A predictive model for diabetic foot ulcer outcome: the wound healing index. Adv Wound Care. 2016;5(7). doi:10.1089/wound.2015.0668
133. Parikh U, Masri L, Saravanan S, Li H, Miao Q, Balaji S. Role of cytokines and chemokines in wound healing. In: Wound Healing, Tissue Repair, and Regeneration in Diabetes. Academic Press; 2020. doi10.1016/B978-0-12-816413-6.00011-3
134. Zhang J, Wang Y, Bao C, et al. Curcumin-loaded PEG-PDLLA nanoparticles for attenuating palmitate-induced oxidative stress and cardiomyocyte apoptosis through AMPK pathway. Int J Mol Med. 2019;44(2). doi:10.3892/ijmm.2019.4228
135. Garcia-Orue I, Gainza G, Girbau C, et al. LL37 loaded nanostructured lipid carriers (NLC): a new strategy for the topical treatment of chronic wounds. Eur J Pharm Biopharm. 2016;108:310–316. doi:10.1016/j.ejpb.2016.04.006
136. Orgul D, Eroglu H, Hekimoglu S. Formulation and characterization of tissue scaffolds containing simvastatin loaded nanostructured lipid carriers for treatment of diabetic wounds. J Drug Deliv Sci Technol. 2017;41:280–292. doi:10.1016/j.jddst.2017.08.001
137. Kumar CS, Vasudeva N, Rao DV, Naidu CRSA. Outcomes of topical phenytoin in the management of traumatic wounds. J Clin Orthop Trauma. 2021;13. doi:10.1016/j.jcot.2020.11.019
138. Allan J, Belz S, Hoeveler A, et al. Regulatory landscape of nanotechnology and nanoplastics from a global perspective. Regul Toxicol Pharmacol. 2021;122. doi:10.1016/j.yrtph.2021.104885
139. Taheri RA, Goodarzi V, Allahverdi A. Mixing performance of a cost-effective split-and-recombine 3D micromixer fabricated by xurographic method. Micromachine. 2019;10(11). doi:10.3390/mi10110786
140. Kriegel C, Festag M, Kishore RSK, Roethlisberger D, Schmitt G. Pediatric safety of polysorbates in drug formulations. Children. 2020;7(1). doi:10.3390/children7010001
141. Emanet M, Şen Ö, Pignatelli F, Lavarello C, Petretto A, Ciofani G. Hazelnut extract-loaded nanostructured lipid carriers and evaluation of their antioxidant properties. Front Bioeng Biotechnol. 2022;10. doi:10.3389/fbioe.2022.953867
142. Sun D, Xue A, Zhang B, Lou H, Shi H, Zhang X. Polysorbate 80-coated PLGA nanoparticles improve the permeability of acetylpuerarin and enhance its brain-protective effects in rats. J Pharm Pharmacol. 2015;67(12). doi:10.1111/jphp.12481
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

