Back to Journals » International Journal of Nanomedicine » Volume 20
Advancements, Challenges, and Future Prospects of Nanotechnology in Sepsis Therapy
Authors Liu Y, Liu J, Wang D, Xu L, Li Z, Bai X, Zhu H, Wang Y
Received 21 July 2024
Accepted for publication 16 May 2025
Published 16 June 2025 Volume 2025:20 Pages 7685—7714
DOI https://doi.org/10.2147/IJN.S488026
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Dongwoo Khang
Yukun Liu,1 Jiafeng Liu,1 Dongfang Wang,2 Ligang Xu,2 Zhanfei Li,2 Xiangjun Bai,2 Hao Zhu,3 Yuchang Wang2
1Department of Plastic and Cosmetic Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China; 2Trauma Center/Department of Emergency and Traumatic Surgery, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China; 3Department of Orthopedics Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430030, People’s Republic of China
Correspondence: Hao Zhu, Email [email protected] Yuchang Wang, Email [email protected]
Abstract: Sepsis is a life-threatening systemic inflammatory syndrome, typically triggered by infection, that can lead to multi-organ failure and high mortality rates. Traditional treatments for sepsis often have limited efficacy and significant side effects, necessitating the exploration of innovative therapeutic strategies. In recent years, the application of nanotechnology in sepsis therapy has garnered widespread attention due to its potential to modulate immune responses, reduce inflammation and oxidative stress, and eliminate bacterial toxins. This review aims to provide an overview of the latest advancements, challenges, and future prospects of nanotechnology in sepsis treatment. By analyzing recent developments in anti-inflammatory, immunomodulatory, antioxidant, and detoxification applications of nanotechnology, key findings and therapeutic potential are summarized, including the use of nanocarriers, biomimetic nanoparticles, and self-assembled nanomaterials. Furthermore, this review addresses the challenges in clinical translation, such as drug targeting, long-term safety, and biocompatibility. Future research will require large-scale clinical trials and interdisciplinary collaboration to validate the efficacy of nanotechnology in sepsis treatment and facilitate its integration into clinical practice. Overall, nanotechnology presents unprecedented opportunities for sepsis management, and this review seeks to offer insights into ongoing research while promoting further advancements in this field.
Keywords: sepsis, MODS, nanotechnology, nanomedicine delivery methods, nanoimmunotherapy
Introduction
Currently, sepsis is defined as a state in which the body’s reaction to infection becomes unregulated, leading to potentially fatal dysfunction of organs. ICU patients worldwide are progressively dying as a result of this.1,2 The World Health Organization (WHO) recognizes sepsis as a significant threat to both patient well-being and public health. Between 1990 and 2017, there were documented 49 million instances of sepsis worldwide, leading to 11 million fatalities. These fatalities caused by sepsis were 19.7% of the total number of deaths globally.3 Thus, sepsis remains a prominent global health issue with potentially lethal consequences, requiring focused endeavours, especially in the domains of timely identification and the adoption of inventive and effective treatment approaches.
Timely identification and prompt medical treatment are essential for enhancing clinical results and decreasing mortality in sepsis.4–6 The main emphasis of sepsis management guidelines is on three key areas: (1) maintaining stable blood flow, (2) controlling the infection, and (3) regulating the body’s response to sepsis.7–9 Additional procedures encompass non-specific techniques to support organ function, such as administering oxygen therapy, employing mechanical breathing, providing hemodynamic support, administering corticosteroids, and utilizing renal replacement therapy.6,10–12 The treatment of sepsis requires a combination of different therapeutic techniques depending on the severity of the condition. Mild cases with failure in a single organ may only require moderate supportive care, while cases with dysfunction in many organs may require more invasive treatments.13,14 Wide-ranging antibiotics are essential in treating sepsis, but the development of resistance in pathogens presents considerable difficulties, leading to a notable rise in fatality rates.15–17 Aside from antimicrobial drugs, other supplementary treatments such as synthetic antimicrobial peptides, anti-inflammatory medications, immunomodulators, blood purification, and antioxidants have demonstrated certain advantages in improving sepsis survival.9,18–21 However, these alternative medicines have not shown optimal efficacy in clinical practice. Possible causes of these differences may involve alterations in medication distribution caused by sepsis, which can negatively impact the effectiveness and safety of treatment and increase the likelihood of treatment failure or the development of resistance.22,23 Additional obstacles that impact the effectiveness of treatment include the limited duration of pharmacological activity, the absence of selectivity for certain tissues or cells, and the inadequate solubility in water and absorption of anti-inflammatory medicines and antioxidants. Moreover, several medications, specifically peptide-based drugs, demonstrate substantial anti-inflammatory properties when tested in laboratory conditions (in vitro). However, these compounds are unable to provide the same effects when tested in living organisms (in vivo) due to their breakdown by enzymes within cells.24,25
Current research is actively exploring nanotechnology-based ways to address the limits of existing treatment methods for sepsis. These approaches have shown significant potential in both the detection and treatment of sepsis.25,26 Nanoparticles (NPs) have distinct physicochemical properties, including size, shape, and a high surface area-to-volume ratio, which enable them to circulate in the body for longer periods and achieve more precise distribution. This enhances their antimicrobial, anti-inflammatory, and antioxidant effects.27–30 Furthermore, NP surfaces can be modified to accomplish desired effects on particular cell types.31–33 Several polymer and carrier components, both organic and inorganic, have shown antibacterial and anti-inflammatory characteristics when used in nano-systems. This offers extra advantages in addressing problems related to antibiotic resistance.29,34 The supplementary and cooperative antimicrobial effects assist in tackling difficulties in medication therapy and can also be utilized as a component of measures to decrease antibiotic consumption.35 Hence, nano-based technologies offer a framework for advancements in various domains, including precise medication administration, mitigating drug-induced side effects, and improving therapeutic effectiveness, as well as efficient diagnosis and treatment of sepsis.36,37 Several studies have shown encouraging outcomes of using nanoparticle-based and nanoformulation-based therapies for sepsis in both laboratory and animal models. Therefore, it is necessary to conduct a comprehensive evaluation of innovative sepsis therapy approaches utilizing nanotechnology.
This study aims to offer a thorough examination of nano-therapeutics and nano-technological preventive measures in relation to their potential for treating sepsis. Initially, we will revise the agreed-upon definition of sepsis and investigate its underlying physiological processes, along with the difficulties encountered in identifying and managing sepsis. In the following, we will provide a concise overview of the most recent advancements in nanoformulations that are applicable to the treatment of sepsis. Ultimately, we will highlight the possibilities and capacity of these goods in enhancing the results of sepsis treatment.
Definition of Sepsis
The understanding of sepsis has undergone substantial changes over time. In 1991, the American College of Chest Physicians and the Society of Critical Care Medicine established definitions for systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock.2 Early definitions of sepsis described it as systemic inflammatory response syndrome (SIRS) resulting from infection. Severe sepsis was identified by the presence of organ dysfunction, hypoperfusion, or hypotension even after receiving sufficient fluid resuscitation. Septic shock was characterized by arterial hypotension despite adequate fluid resuscitation.2 Since then, there have been multiple modifications to the definition of sepsis. In 2016, sepsis was redefined according to the “Sepsis-3” criteria.1,38 The new definition states that sepsis is a condition where the body’s response to infection becomes disordered, leading to life-threatening organ dysfunction. This definition highlights the fact that the body’s response to infection can be dangerous even beyond the infection itself and emphasizes the need for timely diagnosis.11,39,40 Septic shock is a condition characterized by vasodilation and distributive shock. Clinically, it is characterized as sepsis accompanied by ongoing low blood pressure that necessitates the use of vasopressors to maintain a mean arterial pressure (MAP) of at least 65 mmHg, while receiving sufficient fluid resuscitation. Additionally, it is identified by a serum lactate level over 2 mmol/L (or 18 mg/dL).19 The current definition emphasizes the seriousness associated with sepsis, stating that sepsis is caused by invasive infections and results in an uncontrolled reaction from the host.41
The Pathophysiology of Sepsis
The pathophysiology of sepsis is currently known to consist of an early phase characterized by excessive inflammation, which is then followed by a subsequent period of immune suppression.38,42 The mortality rate among septic patients is highest during two specific periods. The first phase, known as the early peak phase, usually lasts for a few days. The second phase, called the immune suppression phase, can last for weeks to months.43,44 The primary cause of early death in sepsis is typically related to excessive inflammation, sometimes referred to as the “cytokine storm”. This condition is marked by symptoms such as high temperature, unresponsive shock, insufficient resuscitation, and failure of the heart or lungs.12,42 Subsequent fatalities occur as a result of prolonged weakening of the immune system and subsequent infections, ultimately leading to damage or malfunction of organs12,43,44 (Figure 1). Due to improvements in sepsis treatment and critical care abilities, the risk of early death has been greatly reduced. However, patients may still die as a result of extended periods of weakened immune systems and dysfunction that can last for months or even years.41,45 Hence, it is imperative to possess a comprehensive comprehension of the pathophysiological processes associated with inflammatory imbalance and the pathological conditions of immune suppression that ultimately result in patient fatality, in order to effectively decrease mortality rates.
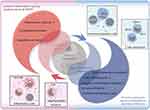 |
Figure 1 Pathophysiological changes caused by infection-induced sepsis. Reproduced from Liu D, Huang SY, Sun JH, et al. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res. 2022;9(1):56. Copyright 2022, Springer Nature. This article is licensed under a Creative Commons Attribution 4.0 International License.12 |
Based on the most recent consensus, it is widely recognized that gaining a thorough understanding of the mechanisms that cause an imbalance in the immune response is an essential and crucial stage.46 In the initial phases of sepsis, the inflammatory process consists of several sequential events that trigger the cytokine “cascade” effect.47 When pathogens invade, macrophages engulf them and release a sequence of pro-inflammatory cytokines, which activate the innate immune system and initiate a chain reaction of cytokines.47 The activation of the innate immune system is primarily carried out by pattern recognition receptors (PRRs). These receptors can specifically identify damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs). Once recognized, the immune cells are activated and the expression of genes related to pro-inflammatory cytokines is increased.48 PAMPs largely consist of external factors derived from pathogens, such as lipopolysaccharide (LPS). On the other hand, DAMPs predominantly encompass internal factors that are released by injured cells, such as high mobility group box 1 (HMGB-1) protein. Binding to specific pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), C-type lectin receptors (CLRs), RIG-I-like receptors (RLRs), and NOD-like receptors (NLRs), triggers the activation of downstream signaling pathways.48–50 One of these pathways involves myeloid differentiation factor 88 (MyD88), which in turn activates c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase 1/2 (ERK1/2), p38 mitogen-activated protein kinase (MAPK), and nuclear factor-kappa B (NF-κB) pathways. This activation leads to the release of pro-inflammatory mediators, including cytokines, chemokines, interferons, reactive oxygen species (ROS), and reactive nitrogen species (RNS). The purpose of this acute inflammation response is to control pathogens and facilitate tissue repair.51,52 Severe systemic inflammation, known as sepsis, can occur as a result of exaggerated responses caused by the genetic makeup of the host and/or the ability of the pathogen to evade the immune system.51,53 Soluble cytoplasmic pattern recognition receptors (PRRs), such as nucleotide-binding oligomerization domain-like receptors (NLRs), also play a role in the immunological imbalance caused by sepsis.54 NLRs, which consist of nucleotide-binding oligomerization domain (NOD) and leucine-rich repeat (LRR) domains similar to TLRs, are involved in the regulation of inflammation. They interact with the adaptor protein receptor-interacting protein kinase 2 (RIP2), also known as RICK, resulting in the activation of NF-κB and AP-1. On the other hand, other NLRs such as NLRP and NLRC4 are responsible for the formation of inflammasomes.55 As an illustration, the NLRP3 inflammasome breaks down pro-caspase-1 into active caspase-1, which then breaks down pro-IL-1β and pro-IL-18, leading to the production of pro-inflammatory cytokines.56 The CLR family, which consists of Dectin, DC-SIGN, and mannose-binding lectin, is classified as the third class of PRRs.57 Dectin triggers the production of reactive oxygen species (ROS) by activating Src and Syk kinases, leading to inflammatory reactions. On the other hand, DC-SIGN plays a role in identifying Leishmania, viruses, and fungi, and its effects are carried out through signaling pathways that are mediated by Raf-1.58 These events trigger the “inflammatory state”, which activates leukocytes, complement, and coagulation pathways. This leads to the disruption of endothelial, cellular, and cardiovascular functions, which are characteristic hallmarks of sepsis.45,59 Sepsis causes disturbances in the immunological and neuroendocrine systems, which disrupt the normal balance of cellular energy metabolism and affect the function of endothelium and epithelial cells. This ultimately leads to dysfunction at the organ level.46,60
The extended period of immune suppression is an intricate and multifaceted process triggered by unregulated cellular apoptosis, which is the main cause of immune suppression in sepsis.12 The immune system’s ability to fight off infections is compromised in sepsis due to various mechanisms. These mechanisms include alterations in HLA-DR, decreased replication of lymphocytes, activation of programmed cell death (apoptosis), heightened production of anti-inflammatory chemicals, and enhanced activity of inhibitory receptors and ligands on cells12,45 (Figure 1). The emergence of immunological imbalance in sepsis has been associated with excessive death of immune cells,61 pyroptosis62,63 and ferroptosis.64,65 Nevertheless, neutrophil death occurs at a relatively late stage, while Treg cells exhibit greater resistance to apoptosis produced by sepsis.66 Determining whether sepsis is in the first stage of excessive inflammation or the later stage of immune suppression is a difficult and unresolved task. Thus, considering our present comprehension of the pathophysiology of sepsis, biomarkers, such as expression patterns, can assist in categorizing patients into more uniform categories or devising specific treatment strategies.
Management and Challenge in Treating Sepsis
Managing sepsis is a multifaceted and demanding undertaking. Although there are guidelines based on evidence for treating sepsis, there is still a shortage of specific therapeutic drugs that have been shown effective.42,67 The management of sepsis encompasses various components, such as prompt resuscitation, utilization of vasoactive medications, provision of ventilatory assistance, evaluation of steroid therapy, maintenance of glycemic control, administration of anticoagulants and anti-inflammatory agents, among other interventions.5,68,69 Nevertheless, despite these therapeutic standards, there are still numerous obstacles that continue to exist in clinical practice.
Effective fluid management is essential in the treatment of sepsis, however, it remains difficult to determine the precise type and volume of fluids that should be administered. Overly generous administration of fluids can result in reduced flexibility of the arteries, leading to the widening of blood vessels and an overly active condition, which can have a negative impact on the functioning of organs.70,71 In order to ensure stable blood flow, it is often advised to administer vasopressors promptly, with norepinephrine being the preferred choice for individuals experiencing septic shock. Nevertheless, the safety and optimal dosage of the majority of vasopressors are still uncertain, despite the fact that using a combination of many additional drugs with norepinephrine treatment has proven to be beneficial in managing severe septic shock.72 Antibiotic treatment is essential in managing sepsis, and a delay in administering the right antibiotics is linked to higher fatality rates in sepsis. Nevertheless, additional investigation is needed to determine the correlation between the timing of antibiotic administration and negative consequences.73–75 Furthermore, the efficiency of antibiotic therapy is influenced by various aspects, including microbiological analytical sampling, the immune and organ functioning status of the patient, the pharmacokinetics of different antibiotics, and the potential creation of biofilm.76 These considerations require the creation of customized anti-infection tactics. Sepsis patients frequently experience immunological failure, rendering them vulnerable to secondary infections, such as those caused by multidrug-resistant bacteria.66 Hence, it is necessary to employ targeted approaches to reinstate the functionality of the immune response. These approaches may involve the utilization of several supplementary treatments, such as the suppression of pro-inflammatory cytokines, immunological modulation, and antioxidant medicines.67,77,78 To summarize, managing sepsis is a multifaceted undertaking that necessitates comprehensive treatment options and vigilant monitoring. Subsequent investigations have the potential to offer novel insights and approaches to enhance the likelihood of survival and recovery for those suffering from sepsis.
The Potential Role and Mechanisms of Nanomedicine in Sepsis Treatment
The rise of multidrug-resistant germs exacerbates the challenge and reduces the success rate of sepsis treatment. The growing emphasis towards the possible application value of nanotechnology in the field of antimicrobial resistance is due to the sluggish development of new effective antibiotics, along with the associated dangers and uncertainties.79 Nanotechnology enables the creation and production of drug carriers at the nanoscale, which enhances the effectiveness of delivering antibiotics.80,81 In comparison to conventional antibiotics, nanotechnology has several advantages. It enables the targeted delivery of medications to infection sites within living organisms, resulting in reduced drug wastage and lower biological toxicity. Additionally, nanotechnology enhances the effectiveness of antibiotics and allows for lower dosages to be administered.82,83 In addition, nanoparticle formulations have the ability to extend the amount of time that antibiotic carriers remain in the body, serving as controlled release systems that decrease the frequency of dosing and enhance the effectiveness of treatment.84 Metal and metal oxide nanoparticles can have antimicrobial effects through multiple mechanisms, such as disrupting protein function, physically destroying cell structures, generating reactive oxygen species, depleting antioxidants, damaging cell membranes, interfering with nutrient absorption, and dephosphorylating tyrosine residues. These actions alter signal transduction and inhibit bacterial growth.60,85–87(Figure 2)
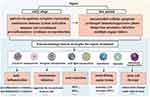 |
Figure 2 Overview of the pathophysiological process of sepsis and nanotechnology-based sepsis treatment strategies. Reproduced from Zhao Y, Pu M, Zhang J, et al. Recent advancements of nanomaterial-based therapeutic strategies toward sepsis: bacterial eradication, anti-inflammation, and immunomodulation. Nanoscale. 2021;13(24):10726–10747. © Royal Society of Chemistry 2021.79 |
Utilizing Nanoparticles to Formulate Antibiotics
The 2016 International Guidelines for Management of Sepsis and Septic Shock highlight the importance of promptly administering potent antibiotics with a wide range of effectiveness, such as carbapenems or broad-spectrum β-lactamase inhibitors, as part of combination therapy.88 Nevertheless, these medications exhibit certain drawbacks in their actual implementation, including resistance and a brief duration of action. Several studies have discovered that chitosan nanoparticles containing meropenem have advantages in terms of preservation and also demonstrate greater antibacterial effectiveness compared to unbound drugs in laboratory experiments. In a sepsis rat model of Klebsiella pneumoniae pneumonia, notable enhancements were observed in terms of survival and elimination of bacteria.89 Gold nanoparticles enhance the effectiveness of carbapenem antibiotics (imipenem and meropenem) by increasing their specificity and safety, while also preventing the development of bacterial resistance to carbapenems.84 Vancomycin-loaded nanoparticles made of poly(lactic-co-glycolic acid) were created using an oil-in-water double emulsion technique. These nanoparticles were designed to be taken up by endothelial cells and effectively inhibit the growth of Staphylococcus aureus bacteria within the cells. This approach resulted in a considerable reduction in intracellular bacterial growth.90 Ascorbic acid stearate (AS) was used as a powerful inhibitor of hyaluronidase to create vancomycin-loaded solid lipid nanoparticles (VCM-AS-SLNs). These nanoparticles were developed to have biomimetic and enzyme-responsive properties, allowing them to effectively bind to vancomycin and prevent its harmful effects even at lower drug concentrations. In addition, the use of silicon dioxide nanoparticles (SNPs) greatly increases the effectiveness of vancomycin in killing bacteria91 (Figure 3A). Conversely, many research have endeavoured to enhance the control of the immune response elicited by sepsis through the targeting of infection sites and the simultaneous administration of antibiotics and anti-inflammatory medications in specific contexts. An experiment was conducted to test the effectiveness of nanoparticles built of copolymers that are sensitive to pH and bacterial enzymes. These nanoparticles were loaded with antibiotics (ciprofloxacin) and anti-inflammatory drugs ((2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide) to treat sepsis. The results of the experiment showed that these nanoparticles efficiently eased sepsis, as shown in (Figure 3B).92 Other methods that are similar involve using nanoparticles (NPs) that contain antibiotics (sparfloxacin, SFX) and immunosuppressants (tacrolimus, TAC) with a broad range of effectiveness. These nanoparticles are designed to attach specifically to intercellular adhesion molecule-1 (ICAM-1), which is found in high levels on the surface of inflamed endothelial cells. By doing so, they effectively reduce inflammation and immune responses in mice with acute pulmonary infections93 (Figure 3C). This strategy also showcases the ability to specifically target sepsis-related objectives. Prior research has utilized porous poly(ε-caprolactone) nanoparticles for the purpose of administering a combination of antibiotics and antioxidant-based anti-inflammatory medications.94 Using nanoparticles to provide antibiotics can have several advantages compared to regular antibiotics. These include targeted distribution, increased effectiveness against microorganisms, regulated release, reduction of inflammation, and reduced development of resistance. Nevertheless, further research and clinical trials are necessary to ascertain the efficacy and safety of its implementation. (Table 1)
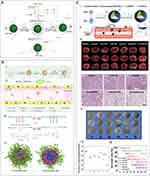 |
Figure 3 (A) I. Schematic illustration of the synthesis of SNP. II. Functionalization with aminopropyl groups. III. Functionalization with propyl methyl phosphonate groups. IV. Functionalization with octadecyl methyl groups. Reproduced from Ndayishimiye J, Cao Y, Kumeria T, Blaskovich MAT, Falconer JR, Popat A. Engineering mesoporous silica nanoparticles towards oral delivery of vancomycin. J Mater Chem B. 2021;9(35):7145–7166. © The Royal Society of Chemistry 2021.91 (B) I. Design and bio-functionalization of IME-responsive nanoparticles (NPs) for targeted drug delivery. II. Schematic of multifunctional NP design. III. Drug-loaded NPs-anti-ICAM-1 specifically target activated endothelial cells at infection sites after intravenous injection, binding to them, crossing blood vessels, and releasing drugs in response to local infection signals. IV. Structure and pH/enzyme-sensitive amphiphilic block copolymers. V. Coarse-grained simulation of CIP-PMs self-assembly using DPD methods. Reproduced from Zhang CY, Gao J, Wang Z. Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv Mater. 2018;30(43):e1803618. © 2018 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.92 (C) γ3 peptide grafted onto nanoparticle (NP) surfaces enhances uptake by activated endothelial cells (ECs) and specifically targets the lungs to alleviate inflammation. I. Schematic of the preparation of γ3 peptide-modified PLGA NPs loaded with SFX and TAC. II. Schematic of targeted therapy in mice with lung infection via intravenous injection of NPs. III. Digital images of lungs from mice with acute lung injury (ALI) infection treated with different formulations. IV. H&E sections of lungs from ALI mice receiving different treatments. V. Digital images of plated lungs from mice with acute lung infection caused by Pseudomonas aeruginosa receiving different treatments. VI. Colony-forming unit (CFU) counts. VII. Survival rate of different groups. I: PBS, II: free SFX, III: free TAC, IV: free S + T, V: PLGA/S + T, VI: γ3-PLGA/S + T. Data are shown as mean ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001. Reproduced from Yang Y, Ding Y, Fan B, et al. Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J Control Release. 2020;321:463–474. Copyright © 2020 Elsevier B.V. All rights reserved.93 |
 |
Table 1 A Summary of Utilizing Nanoparticles to Formulate Antibiotics |
Nanoformulations of Antimicrobial Peptides
Antibacterial peptides (AMPs) are viewed as effective remedies for infections caused by multidrug-resistant (MDR) bacteria and biofilm-related diseases. This is because they possess strong antibacterial properties without promoting bacterial resistance.95,96 Antimicrobial peptides (AMPs) differ from conventional antibiotics in that they employ various ways to eliminate germs, hence minimizing the likelihood of bacterial resistance. The initial interaction involves electrostatic interactions between the negatively charged bacterium membranes. Subsequently, the bacterial membrane is disrupted through hydrophobic interactions with membrane phospholipids, finally resulting in bacterial death. This versatile mechanism mitigates the likelihood of bacterial resistance to antimicrobial peptides (AMPs) and is seen as a promising alternative for addressing illnesses caused by bacteria that are resistant to multiple drugs95–97 (Figure 4A). Nevertheless, antimicrobial peptides (AMPs) encounter obstacles such as breakdown by bacterial proteases and inconsistent pharmacokinetic characteristics. Nanotechnology can address challenges such as cytotoxicity, proteolytic degradation, and target site efficiency.94,96 (Figure 4B)
 |
Figure 4 (A) Schematic presentation of the antibacterial mechanism of AMPs. Reproduced from Zhang R, Xu L, Dong C. Antimicrobial peptides: an overview of their structure, function and mechanism of action. Protein Pept Lett. 2022;29(8):641–650. Copyright© Bentham Science Publishers.97 (B) I. Illustration of a passive nanosystem, demonstrating antimicrobial peptide delivery into an infected cell. II. Illustration of an active nano system demonstrating antimicrobial peptide delivery into an infected cell. Reproduced from Biswaro LS, da Costa Sousa MG, Rezende TMB, Dias SC, Franco OL. Antimicrobial peptides and nanotechnology, recent advances and challenges. Front Microbiol. 2018;9:855. Copyright © 2018 Biswaro, da Costa Sousa, Rezende, Dias and Franco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY).94 (C) I. Synthesis of SNAPPs by ring-opening polymerization of lysine and valine N-carboxyanhydrides (NCAs), with poly(amidoamine) (PAMAM) dendrimer terminal amines as initiators. II. Comparison of the antibacterial mechanisms of typical membrane-disrupting cationic antimicrobial peptides (AMPs) and SNAPPs against Gram-negative bacteria. a. Cationic AMPs bind to the outer membrane (OM) of Gram-negative bacteria via electrostatic interactions, penetrate the OM through membrane destabilization, and disrupt the cytoplasmic membrane (CM) physical integrity via “barrel-stave”, “toroidal pore”, or “carpet” mechanisms (not shown). b. SNAPPs, whether aggregated or not, interact with the OM, peptidoglycan (PG), and CM layers of Gram-negative bacteria via electrostatic attraction, destabilizing/disrupting the OM and potentially the CM, leading to uncontrolled ion movement and cell death through apoptosis-like pathways (not shown). Reproduced from Lam SJ, O’Brien-Simpson NM, Pantarat N, et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat Microbiol. 2016;1(11):16162. Copyright © 2016, Macmillan Publishers Limited.98 (D) I. Schematic of Ts-LPs-LEV preparation. II. Schematic of Ts-LPs-LEV bactericidal process. III. Kaplan–Meier survival curve representing mortality data of levofloxacin formulations in a multidrug-resistant (MDR) clinical isolate-induced septic shock model. Reproduced from Fan X, Fan J, Wang X, Wu P, Wu G. S-thanatin functionalized liposome potentially targeting on Klebsiella pneumoniae and its application in sepsis mouse model. Front Pharmacol. 2015;6:249. Copyright © 2015 Fan, Fan, Wang, Wu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY).99 |
Recently developed structured nanoengineered antimicrobial peptide polymers (SNAPPs) have been found to effectively kill Gram-negative bacteria. This is because they contain arginine and valine residues, which destabilize the outer membrane of the bacteria. As a result, ions are able to penetrate through the cytoplasmic membrane without regulation, leading to the activation of apoptotic-like death pathways and ultimately causing the death of the bacteria98 (Figure 4C). Hence, SNAPPs could offer a viable remedy for combating infections caused by antibiotic-resistant Gram-negative bacteria. Fan et al (2015) created liposomes that included a combination of the short antimicrobial peptide S-Thanatin (Ts) and the antibiotic levofloxacin. These liposomes were referred to as Ts-LEV-LPs. The study discovered that the combination of levofloxacin and Ts had a synergistic impact at certain concentrations. This resulted in a significant decrease in mortality and rapid elimination of bacteria in a mouse model that was infected with clinically multidrug-resistant (MDR) isolates99 (Figure 4D). Several processes have been suggested, such as the specific delivery of antimicrobial peptides, increased permeability through liposomes, alteration of the structure of bacterial cytoplasmic membranes, and regulated release of drugs. These mechanisms work together to produce a synergistic effect.99 Nevertheless, in vitro experiments are unable to completely replicate in vivo settings. Although AMPs show great promise, they nevertheless encounter obstacles in their use in therapeutic settings, including non-specific effects and the possibility of damage to host cells.98 (Table 2)
 |
Table 2 Nanoformulations of Antimicrobial Peptides |
Other Antimicrobial Nanoformulations
Due to the growing resistance of bacteria to typical antibiotic formulations, it is necessary to search for alternative antimicrobial agents derived from natural sources.111 In addition to the previously stated nanoantibiotic formulations and antimicrobial peptides, there has been an increased interest in antimicrobial chemicals derived from natural sources, as well as metal and metal oxide nanoparticles107 (Figure 5A) chemicals found in medicinal and aromatic plants (MAPs), specifically terpenes and aromatic chemicals such as carvacrol, eugenol, cinnamaldehyde, and β-caryophyllene, have been acknowledged for their capacity to fight against microorganisms.107–109 Aromatic compounds display potent antibacterial properties as a result of their phenolic, aldehydic, and olefinic constituents. Furthermore, the combination of these molecules produces synergistic effects.110 Nevertheless, their tendency to rapidly change and their limited capacity to dissolve in water result in reduced availability for biological processes. In order to address these problems, Montagu et al (2014) documented the creation of lipid nanocapsules (LNCs) for the purpose of enclosing lipophilic active substances like cinnamaldehyde and eugenol.105 Despite the need for surface modification and functionalization to specifically target bacteria, these nanoformulations have not yet shown enhanced antibacterial activity in living organisms.
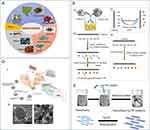 |
Figure 5 (A) Summary of natural extracts from various natural sources, including plants, animals, or microbial species, for antibacterial applications. Reproduced from Chen C, Chen L, Mao C, et al. Natural extracts for antibacterial applications. Small. 2024;20(9):e2306553. © 2023 Wiley‐VCH GmbH.107 (B) Schematic of the synthesis of metal nanoparticles/nanocellulose composite materials. I. Simple mixing of cellulose source with prepared inorganic nanoparticles. II. Synthesis of nanoparticles using an external reducing agent. III. Synthesis of nanoparticles through surface modification of nanocellulose. IV. Synthesis of nanoparticles using nanocellulose itself without an external reducing agent. (C) Illustration of the synthesis of CNC/Ag composite nanoparticles. Reproduced from Oun AA, Shankar S, Rhim JW. Multifunctional nanocellulose/metal and metal oxide nanoparticle hybrid nanomaterials. Crit Rev Food Sci Nutr. 2020;60(3):435–460. Rights managed by Taylor & Francis.100 (D) I. Schematic of surface modification of Curcumin and SNP-immobilized Cu-MSN and their possible antibacterial mechanism. II. TEM images of Cur-Cu-MSN-SNP samples, scale bars: (a) 50 nm and (b) 200 nm. Reproduced from Kuthati Y, Kankala RK, Busa P, et al. Phototherapeutic spectrum expansion through synergistic effect of mesoporous silica trio-nanohybrids against antibiotic-resistant gram-negative bacterium. J Photochem Photobiol B. 2017;169:124–133. opyright © 2017 Elsevier B.V. All rights reserved.102 |
Conversely, metal and metal oxide nanoparticles, often known as “nanoantibiotics”, have a wide range of antibacterial effects and bacteria have a relatively hard time developing resistance to them100,101 (Figure 5B and C). Nanoparticles can eliminate germs by multiple processes, such as membrane breakage, protein denaturation, and DNA damage. Silver nanoparticles are a prime illustration,103 and they demonstrate significant antibacterial effectiveness against clinically resistant pathogens in both planktonic and biofilm phases.104,105 Nevertheless, the use of these applications in living organisms is still difficult due to concerns regarding safety. The researchers have altered the silver nanoparticles on the surface of amine-functionalized Cu-MSN by coordinating them with silver ions and then further reducing them to silver nanoparticles using formaldehyde. The affinity interactions between copper metal and curcumin strengthen the mesoporous structure of Cu-MSN, leading to an increase in the release dynamics of silver ions when exposed to light. This, in turn, results in the generation of significant quantities of reactive oxygen species (ROS). This increases the effectiveness of killing antibiotic-resistant Escherichia coli102 (Figure 5D). These ternary nanohybrids showcase a novel photodynamic deactivation technique that can be used as an alternative to antibiotics for combating resistant Escherichia coli. Moreover, diamond nanoparticles (NDs) have demonstrated significant promise in several biomedical settings as a valuable material. The glucose-modified nanodiamonds (NDs) were discovered to be highly efficient in disrupting the survival of Staphylococcus aureus, but they did not have any impact on the growth of Escherichia coli. In contrast, particles that include lactose units have a contrary effect on the proliferation of Staphylococcus aureus.106 This unique and infrequent technique still requires additional investigation in the future.
To summarize, antimicrobial chemicals derived from natural sources and metal nanoparticles have the potential to serve as effective antimicrobial agents, offering a possible solution to the problem of bacterial resistance. Nevertheless, additional investigation is necessary to tackle concerns like as bioavailability, targeting, and safety in order to establish these methods as feasible alternatives for clinical therapy. Nanoformulations offer significant benefits in addressing microbial resistance, particularly in terms of their ability to effectively distribute throughout the body and administer targeted treatments. (Table 3)
 |
Table 3 Summary of Antimicrobial Nanoformulations |
Nanotechnology and Anti-Inflammatory, Immunomodulatory
The dysregulated pro-inflammatory and anti-inflammatory responses, impaired inflammation resolution, and sustained inflammation are important factors in both the acute and chronic stages of sepsis. It is believed that suppressing cytokine storms after immune cell activation could be an effective treatment for sepsis.112 The inflammatory response in sepsis is triggered by the activation of pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) such as peptidoglycan, lipopolysaccharides, and bacterial DNA, which in turn activate inflammatory pathways.10,21 Throughout this procedure, pro-inflammatory cytokines such as IL-6, IL-8, and TNF-α are discharged into the extracellular space, resulting in persistent inflammation. Higher levels of these pro-inflammatory substances are strongly linked to a negative patient outlook, and suppressing the excessive release of cytokines can enhance the prognosis of individuals with sepsis.62,113,114 Nanotechnology is essential in understanding the underlying mechanisms of sepsis, namely in regulating the inflammatory and anti-inflammatory responses mediated by cytokines. This knowledge opens up several possibilities for future therapeutic interventions79,115,116 (Figure 6A).
 |
Figure 6 (A) I. Approaches for the design and fabrication of diverse stimuli-responsive biomaterials and their utilization in the formulation of stimuli-responsive nano-delivery systems. R, S, and COO- are ROS-responsive moieties. II. Strategies for designing and fabricating biomimetic nanoparticles targeting sepsis microenvironments. Ismail EA, Devnarain N, Govender T, Omolo CA. Stimuli-responsive and biomimetic delivery systems for sepsis and related complications. J Control Release. 2022;352:1048–1070. Copyright © 2022 Elsevier B.V. All rights reserved.115 (B) I. Nano-antibody production process using phage display technology. II. Effect of administration times on survival rate of rats. Reproduced from Liao S, Liu S, Zhang Y. Preparation of anti toll-like receptor-4 nano-antibody and its effect on gram negative sepsis. J Nanosci Nanotechnol. 2021;21(2):1048–1053. Copyright 2021, American Scientific Publishers.117 |
TLR4 functions as a receptor that facilitates increased cellular activation and the release of inflammatory mediators.118 Experimental studies conducted both in vitro and in vivo have shown that anti-TLR4 nanobodies have a significant impact on reducing the production of inflammatory cytokines and improving animal survival rates. These nanobodies are particularly effective when the C-terminal and middle domains are in a closed state117 (Figure 6B). Particles derived from poly(lactic-co-glycolic acid) (PLGA) and poly(lactic acid) (PLA) exhibit strong immunomodulatory capabilities that are influenced by their molecular weight, polymer composition, and charge. These properties include the reduction of co-stimulatory molecule production and cytokine release caused by TLR. Particles synthesized utilizing the anionic surfactant poly(ethylene glycol)-maleic acid (PEMA) effectively reduce the responses of antigen-presenting cells to TLR4 (lipopolysaccharide) and TLR9 (CpG-ODN) agonists. These particles demonstrate a wide-ranging inhibitory effect on both external and intracellular TLR ligands. Nanoparticles made from poly(lactic acid) (PLA) and PEMA (PLA-PEMA nanoparticles) have a strong negative charge and show improved anti-inflammatory effects. They are able to reduce the expression of co-stimulatory molecules and pro-inflammatory cytokines (such as IL-6, MCP-1, and TNF-α) that are induced by TLR. These effects are more pronounced compared to nanoparticles made with polyvinyl alcohol (PVA)119 (Figure 7A–C). Moreover, macrophages have a vital function in protecting the host against infections and in the development of sepsis-related immune system dysfunction. Several studies utilize different design tactics, such as surface functionalization, modulation of monocyte/macrophage recognition of nanoparticles, and their phagocytosis, to control their activity in sepsis, addressing shortcomings in conventional methods120 (Figure 8A–C).
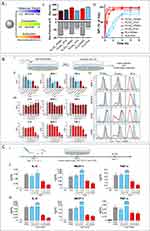 |
Figure 7 (A) Synthesis and characterization of immunomodulatory particles. I. The particles are formulated from polymers with different molecular weights (low or high), compositions (glycolic acid (GA) and lactic acid (LA)), or surfactants (PVA or PEMA). II. Measurements of particle size and zeta potential. III. The interaction kinetics between particles and bone marrow-derived macrophages (BMMØs) depend on the emulsifying surfactant. (B) Particles regulate innate inflammatory responses. I. Schematic of cytokine inhibition assay. II. Inhibition of inflammatory cytokine production in BMMØs treated with particles (300 μg/mL) and stimulated with 100 ng/mL LPS. III. Particle-induced inhibition of BMMØ cytokine production is dependent on particle and LPS concentration. IV. Evaluation of live cell surface marker expression in BMMØs by flow cytometry. (C) Intravenous injection of PLA particles alters cytokine responses of splenocytes to LPS and CpG-ODN stimulation in mice. I. Evaluation of inflammatory cytokine secretion by splenocytes. II. Cytokine secretion after in vitro stimulation with LPS (100 ng/mL). III. Cytokine secretion after in vitro stimulation with CpG-ODN (100 ng/mL). Cytokines were measured by ELISA after 2 days of culture. Reproduced from Casey LM, Kakade S, Decker JT, et al. Cargo-less nanoparticles program innate immune cell responses to toll-like receptor activation. Biomaterials. 2019;218:119333. © 2019 Elsevier Ltd. All rights reserved.119 |
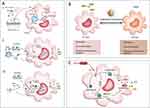 |
Figure 8 (A) Interaction of nanoparticles (NPs) with macrophages and the regulation of macrophage pro/anti-inflammatory functions by NPs. (I) Upon entering the body, NPs bind to plasma proteins and are internalized by macrophages. NPs are endocytosed into endosomes, degraded, and then released extracellularly to exert their active effects; other endosomes fuse with lysosomes to form endolysosomes, exerting intracellular effects. (II) First, NPs can eliminate macrophage activation by phagocytosis and restriction of pathogen-associated molecular patterns (PAMPs); second, they inhibit the interaction between PAMPs and pattern recognition receptors (PRRs); third, NPs that enter the cytoplasm inhibit the transmission of inflammatory signaling pathways; finally, NPs inhibit the release of active products from inflammatory pathways, controlling cell and tissue damage caused by overactivated macrophages. (III) NPs modulate the pro-inflammatory activity of macrophages. NPs can enhance PRR activation to initiate macrophage inflammatory responses. Once in the cytoplasm, NPs activate downstream pathways and inflammasomes to induce the production of pro-inflammatory factors. NPs, nanoparticles; PAMPs, pathogen-associated molecular patterns; ROS, reactive oxygen species; ILs, interleukins; TNFs, tumor necrosis factors; MAPK, mitogen-activated protein kinase; NF-kB, nuclear factor-κB. (B) Regulation of macrophage polarization by nanoparticles (NPs). M1-type macrophages typically function during the cytokine storm phase of sepsis, releasing large amounts of pro-inflammatory mediators, including reactive oxygen species (ROS), interferons (IFNs), interleukins (ILs), and tumor necrosis factors (TNFs). In contrast, M2-type macrophages usually appear during the immune paralysis phase, secreting anti-inflammatory mediators, most notably IL-10. During excessive inflammatory stimulation, the continuous release of pro-inflammatory mediators by M1-type macrophages causes damage to the body, whereas, during immune paralysis, excessive activation of M2-type macrophages increases the risk of secondary infections. NPs can modulate macrophage polarization at different stages of sepsis to improve prognosis. NPs, nanoparticles; ROS, reactive oxygen species; ILs, interleukins; IFNs, interferons; TNFs, tumor necrosis factors. (C) Interference of macrophage pyroptosis by nanoparticles (NPs). NPs can block the activation of caspase-1 and caspase-4/5/11, reducing the release of DAMPs (damage-associated molecular patterns) and preventing unnecessary tissue and cell damage. NPs, nanoparticles; LPS, lipopolysaccharide; IL-1b, interleukin-1b; DAMPs, damage-associated molecular patterns; GSDMD, gasdermin D. Reproduced from Song C, Xu J, Gao C, Zhang W, Fang X, Shang Y. Nanomaterials targeting macrophages in sepsis: a promising approach for sepsis management. Front Immunol. 2022;13:1026173. Copyright © 2022 Song, Xu, Gao, Zhang, Fang and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY).120 |
Cao et al developed a technique using a membrane-cloaked metal-organic framework (MOF) to deliver plasmid DNA (pDNA) as a treatment for sepsis.121 The antimicrobial gene LL37 was successfully enclosed within pH-responsive Metal-Organic Frameworks (MOFs) and adapted to be compatible with the membranes of macrophages. The process of macrophage membrane coating enables the specific delivery of LL37 to macrophages, resulting in the creation of macrophage factories that continuously produce antimicrobial peptides. Nanoparticles that have been transformed with primary bone marrow mesenchymal stem cell-derived macrophage membranes have been found to greatly improve the survival of immunodeficient septic mice. This is achieved through a combination of synergistic gene therapy and the extraction of inflammatory cytokines. The effectiveness of this approach is demonstrated in Figure 5B and has been shown to be much better than using bare nanoparticles alone121 (Figure 9A–D). The combination of curcumin (Cur) and cerium oxide (CeO2) incorporated into octenyl succinic anhydride (OSA) functionalized chitosan nanoparticles (Cur@Ce/OCS) creates a medication delivery method for sepsis that is responsive to changes in pH. Experimental results conducted in living organisms show that OCS NPs containing CeO2 lower the amount of germs in sepsis models and effectively decrease inflammation. This enhances their potential as a treatment for pulmonary infections, as demonstrated in the study122 (Figure 9E). Nano-parthenolide (PTL) has gained significant attention as a therapy approach for sepsis in recent years. Both ordinary PTL and nano-PTL improve the survival and survival time of septic rats by increasing the levels of 5-HTR2A, reducing mean arterial pressure and serum inflammatory cytokine levels, protecting liver and kidney function, increasing transmembrane resistance (TEER) values, and decreasing extracellular oxidative stress (ROS) and apoptosis. Nano-PTL demonstrates superior performance compared to ordinary PTL123 (Figure 10A–D). Ultimately, nanoparticles have the capacity to cure sepsis by regulating immune responses and inhibiting excessive inflammatory reactions, hence potentially enhancing patient survival rates.
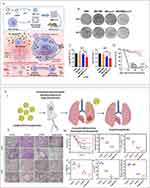 |
Figure 9 (A) Design, synthesis, and hypothesized mechanism of macrophage membrane-coated MOFs (MMD-LL37) for delivering pLL37 in the treatment of sepsis. (I) MMD-LL37 inhibits potential inflammatory responses in sepsis by adsorbing pro-inflammatory cytokines. (II) Homologous targeting and intracellular delivery of antibacterial plasmids. (III) Transfection with the LL37 plasmid induces macrophages to act as “factories” for continuous LL37 production. (IV) Systematic elimination of bacteria hidden within macrophages and circulating in the bloodstream. (B) Representative images of blood plate colonies at 24 hours and 48 hours post-treatment. (C) Bacterial load in the blood at 24 hours and 48 hours post-administration. (D) Survival rate of septic mice under different treatment regimens. Reproduced from Cao H, Gao Y, Jia H, et al. Macrophage-membrane-camouflaged nonviral gene vectors for the treatment of multidrug-resistant bacterial sepsis. Nano Lett. 2022;22(19):7882–7891. Copyright © 2022 American Chemical Society.121 (E) (I) Design and schematic illustration elaborating the formulation of curcumin and CeO2-loaded nanoformulations for the treatment of lung infectious sepsis. (II) Histopathological observations of lung tissue with H&E and MTS staining 20 hours post-treatment following Pseudomonas aeruginosa infection. (III) Observations of the therapeutic potential of curcumin-loaded Ce/OCS nanoparticles in lung sections of induced bacterial sepsis; (a) Survival rate of mice in the peritonitis-induced sepsis model after treatment with different nanoformulations, (b) white blood cell count, (c) TNF-α, (d) IL-1β, (e) IL-6, and (f) protein content in peritoneal fluid after treatment with different formulations (including control (PBS), free curcumin, Ce/OCS, and Cur@Ce/OCS nanoparticles). Reproduced from Teng L, Zhang Y, Chen L, Shi G. Fabrication of a curcumin encapsulated bioengineered nano-cocktail formulation for stimuli-responsive targeted therapeutic delivery to enhance anti-inflammatory, anti-oxidant, and anti-bacterial properties in sepsis management. J Biomater Sci Polym Ed. 2023;34(12):1716–1740. Rights managed by Taylor & Francis.122 |
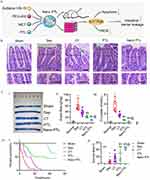 |
Figure 10 (A) The schematic diagram illustrating the mechanism of the therapeutic effect of Nano PTL on intestinal barrier function after sepsis. (B) Representative microscopic images of H&E-stained intestinal sections (scale bar, 100 µm, n=8 per group). (C) (I and II) Intestinal Evans Blue (EB) leakage after treatment with Nano PTL (n=8 per group). (III) D-lactate levels in septic rats. (D) Protective effect of Nano PTL on septic rats. (I) Survival rate. (II) Survival time of each group. Reproduced from Guo NK, She H, Tan L, et al. Nano parthenolide improves intestinal barrier function of sepsis by inhibiting apoptosis and ROS via 5-HTR2A. Int J Nanomed. 2023;18:693–709. © 2023 Guo et al. Dove Medical press, Creative Commons Attribution – Non Commercial.123 |
Tumor necrosis factor (TNF)-related apoptosis-inducing ligands (TRAIL) is a commonly employed therapeutic strategy that induces programmed cell death in inflammatory cells by activating the extrinsic apoptosis pathway.124 The use of antimicrobial peptide-crosslinked nano-gels containing TRAIL can effectively suppress Klebsiella pneumoniae infection and regulate the activity of overactivated macrophages. Administering TRAIL-nano-gels to mice with sepsis substantially increases their lifespan and decreases the number of germs in their bloodstream.125 Neutrophils have a crucial role in the immunological response of the host to sepsis.126 Sepsis is believed to be caused by either overstimulation or malfunction of neutrophil function.127,128 Exerting precise control over the process of neutrophil death can effectively resolve inflammation and reinstate a state of immunological homeostasis. Zhang et al developed protein nanoparticles (NPs) that are coupled with doxorubicin (DOX). These NPs have the ability to specifically target inflammatory neutrophils in their natural location, deliver DOX into the cells, and trigger apoptosis (cell death) in the neutrophils. The acidic milieu within neutrophils induces the release of DOX, thereby impeding neutrophil migration and inflammatory reactions. DOX-conjugated nanoparticles (NPs) have a notable impact on the survival of mice with sepsis and protect the brain from injury during cerebral ischemia/reperfusion. Importantly, this effect is achieved without compromising the overall immunological function of the body.129 (Table 4)
 |
Table 4 Nanotechnology in Anti-Inflammatory and Immunomodulatory Applications |
Despite the potential benefits of anti-inflammatory medication in enhancing the treatment of sepsis, its practical implementation remains difficult. Nanotechnology presents a novel method that has the potential to overcome resistance and address other challenges in the treatment of sepsis. Nevertheless, the clinical implementation of this treatment still requires the resolution of concerns regarding toxicity, efficacy of drug administration, and long-term safety. Hence, forthcoming investigations must tackle these concerns in order to further the pragmatic implementation of nanotechnology in the treatment of sepsis.
Nanotechnology and Antioxidants in Sepsis
Multiple studies have demonstrated the potential advantages of different antioxidants, including vitamins C and E, polyphenols, melatonin, β-glucans, N-acetylcysteine, mitochondria-targeted antioxidants (MitoQ, MitoE, and dimethylthiourea-related peptides), selenium salts, and organic selenium compounds, in enhancing oxidative stress and sepsis outcomes.130,131 The protective effects of melatonin are of particular interest since it inhibits NF-κB and NLRP3 inflammasome activation.132,133 Nevertheless, melatonin’s therapeutic potential is hindered by its brief half-life (t1/2) and limited bioavailability.134 In 2012, Volti et al discovered that Poly-D, l-lactic-co-glycolic acid (PLGA [NP-A]) and polyethylene glycol-co-(poly-D, l-lactic-co-glycolic acid) (PLGA-peg [NP-B]) were utilized to create nanocarriers containing melatonin at a dosage of 10 mg/kg. During sepsis, melatonin demonstrates notable antioxidant properties and can be enhanced through targeted drug delivery systems.135 Nano-curcumin (NC) has been found to decrease inflammation indicators and safeguard endothelial function in septic patients during clinical study. Additionally, it has been observed to drastically diminish oxidative stress markers such as malondialdehyde (MDA), nuclear factor-2 (Nrf-2), catalase, and superoxide dismutase (SOD).136,137
Stimuli-responsive drug delivery systems are now being used as additional treatments for sepsis.138 Cerium oxide nanoparticles exhibit remarkable efficacy in the removal of reactive oxygen species (ROS) and have been utilized in the management of illnesses associated with ROS139–141 (Figure 11A and B). Nevertheless, cerium oxide nanoparticles cannot specifically target mitochondria, and ultra-small cerium oxide nanoparticles tend to form aggregates. To address these limitations and enhance the effectiveness of therapy, Yu et al made alterations to cerium oxide nanoparticles by including triphenylphosphine (TCeria NPs). Additionally, they applied a ROS-responsive organic polymer (mPEG-TK-PLGA) to coat the cerium oxide nanoparticles and loaded them with atorvastatin (Atv/PTP-TCeria NPs). Atv/PTP-TCeria nanoparticles aggregate specifically in the kidneys and possess potent drug-release capabilities that are responsive to reactive oxygen species (ROS). On the other hand, TCeria nanoparticles target mitochondria to effectively reduce excessive ROS. Laboratory experiments have demonstrated that Atv/PTP-TCeria nanoparticles exhibit favourable antioxidant and anti-apoptotic properties. Atv/PTP-TCeria NPs have been shown in in-vivo experiments to significantly decrease oxidative stress and inflammatory reactions caused by sepsis in AKI mice. They also safeguard the structure of cellular mitochondria, reduce the death of renal tubular cells, and prevent renal tubular necrosis142 (Figure 11C). In a study conducted in 2017, Chen et al discovered that self-assembled NP created from polyethylene glycol (PEG) and polypropylene sulfide (PPS) diblock copolymers have the potential to be highly efficient drug delivery systems due to their ability to respond to active oxygen. mPEG-b-PPS-NPs demonstrate biocompatibility and are superior in mitigating oxidative stress, inflammatory response, and eventual liver injury compared to equal amounts of unbound medications143 (Figure 11D). The research findings suggest that the use of antioxidants and nanotechnology could offer potential advancements in the treatment of oxidative stress and sepsis. (Table 5) However, additional research is required to confirm their clinical applicability.
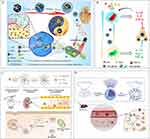 |
Figure 11 (A) Schematic illustration of the synthesis, antibacterial mechanism, and regulation of macrophage polarization by CeO2@Ce6 nanocomposites for the treatment of periodontal disease. The enhanced antibacterial efficiency of CeO2@Ce6 may rely on ROS produced by aPDT and the inherent antibacterial activity of CeO2. Nanocomposites containing CeO2 exhibit SOD- and CAT-mimetic activities, capable of scavenging excess ROS by switching between Ce(III) and Ce(IV) states. CeO2@Ce6 nanocomposites can actively modulate macrophage polarization from the pro-inflammatory M1 phenotype to the anti-inflammatory (regenerative) M2 phenotype. Ce6: chlorin e6; aPDT: antimicrobial photodynamic therapy; ROS: reactive oxygen species; SOD: superoxide dismutase; CAT: catalase. (B) Schematic images illustrating the shift of macrophage polarization between the M1 phenotype and the M2 phenotype with the regulation of regenerative functions. Zhou X, Zhou Q, He Z, et al. ROS balance autoregulating core-shell CeO(2)@ZIF-8/Au nanoplatform for wound repair. Nanomicro Lett. 2024;16(1):156. Copyright © 2024, The Author(s). Creative Commons CC BY license.141 (C) Schematic diagram of Atv/PTP-TCeria NPs used for acute kidney injury (AKI). Atv/PTP-TCeria NPs can be passively targeted to the kidney and release the drug in response to high levels of ROS, while TCeria NPs target mitochondria to scavenge excess ROS, thereby improving AKI. Reproduced from Yu H, Jin F, Liu D, et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics. 2020;10(5):2342–2357. Creative Commons Attribution License.142 (D) Schematic illustration depicting the on-demand delivery of melatonin to the liver during sepsis and its mechanism for reducing excess ROS and inflammation. Reproduced from Chen G, Deng H, Song X, et al. Reactive oxygen species-responsive polymeric nanoparticles for alleviating sepsis-induced acute liver injury in mice. Biomaterials. 2017;144:30–41. Copyright © 2017 Elsevier Ltd. All rights reserved.143 |
 |
Table 5 Nanotechnology and Antioxidants in Sepsis |
The Application of Nanotechnology in the Removal of Toxins
Bacteria can secrete several toxins that can bind to specific receptors on host cells, resulting in injury to the host.144,145 The mechanisms include membrane contact, which results in structural disruption, interference with membrane-related activities and signal transduction, as well as interference with intracellular processes such as protein synthesis.146 Bacterial infections often release cholesterol-dependent cytolysins (CDCs), α-hemolysins, or bacterial phospholipase C toxins. These toxins function as pore-forming toxins, causing harm to the membranes of host cells. They play a crucial role in the development of infectious disorders.147 At present, different techniques such distillation, ethylene oxide treatment, filtering, and irradiation are employed to eliminate endotoxins from contaminated materials. However, these treatments have a low reduction efficiency and pose obstacles.148 Current detoxification methods, such as antiserum, monoclonal antibodies, small molecule inhibitors, and molecularly imprinted polymers, work by specifically targeting the molecular structure of toxins. However, these methods have limitations in terms of their effectiveness in neutralizing toxins and require customized treatments for different diseases148,149 (Figure 12A and B). Nevertheless, nanoparticles possess inherent attributes such as extended circulation durations and the capacity for engaging with numerous poisons, instilling optimism for addressing this issue150 (Figure 12C).
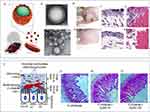 |
Figure 12 (A) (I) Schematic and the actual structure of the system. Schematic representation of the structure of toxin nanosponges and their mechanism for neutralizing pore-forming toxins (PFTs). (II) TEM (transmission electron microscopy) images of nanosponges mixed with α-toxin (scale bar, 80 nm) and an enlarged view of a single toxin-adsorbed nanosponge (scale bar, 20 nm). Samples were negatively stained with uranyl acetate before TEM imaging. (B) In vivo toxin neutralization. (I–III) Skin lesions in mice three days after injection with α-toxin. (IV–VI) No skin lesions in mice injected with α-toxin/nanosponge mixture. Each group had n = 6 mice. Reproduced from Hu CM, Fang RH, Copp J, Luk BT, Zhang L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat Nanotechnol. 2013;8(5):336–340. Copyright © 2013, Springer Nature Limited.149 (C) Antibacterial effect of AuNS100 on biofilms of V. cholerae adhering to epithelial surfaces. (I) Schematic illustration of V. cholerae interacting with villi in the inner mucus layer to form an intestinal biofilm. (II–IV) Microscopic images of small intestine sections from mice infected with V. cholerae before and after treatment. Reproduced from Chatterjee T, Saha T, Sarkar P, Hoque KM, Chatterjee BK, Chakrabarti P. The gold nanoparticle reduces Vibrio cholerae pathogenesis by inhibition of biofilm formation and disruption of the production and structure of cholera toxin. Colloids Surf B Biointerfaces. 2021;204:111811. Copyright © 2021. Published by Elsevier B.V.150 |
Another method entails the use of biomimetic toxin nanosponges, which enhance the stability of nano-sponges by creating a polymer core and then enveloping them with natural red blood cell membranes. This allows them to effectively absorb different types of pore-forming toxins. Hu et al developed a biomimetic poison nanosponge that acts as a decoy for toxins in living organisms. The nanosponges are composed of a central polymeric nanoparticle enclosed by red blood cell membranes. These membranes have the ability to absorb toxins that interfere with cell membranes and redirect them away from their intended cellular targets. In a murine model, nanosponges effectively mitigated the toxicity of Staphylococcus aureus alpha-toxin (α-toxin), leading to improved survival rates in mice that were intoxicated.149 Koo et al developed biomimetic “nanosponges” by encapsulating donor red blood cells (RBCs) around a polymer core. These nanoparticles have a receptor library similar to host cells, allowing them to effectively trap and neutralize a wide range of bacterial toxins, host pro-inflammatory chemokines, and cytokines. This strategy demonstrates significant potential in mitigating infections151 (Figure 13A). Studies have demonstrated that this approach effectively safeguards mice from deadly methicillin-resistant Staphylococcus aureus (MRSA) infection caused by full-secreted protein (wSP). It achieves this by minimizing lung damage and blocking the activation of splenic nuclear factor kappa B152 (Figure 13B). Researchers have recently created nanoparticles by surrounding polymer cores with macrophage cell membranes. These nanoparticles have antigenic surfaces that are identical to the cells they came from. These nanoparticles mimic macrophages and effectively bind and destroy endotoxins, therefore limiting immunological activation. In addition, these nanoparticles with macrophage-like properties effectively separate pro-inflammatory cytokines and prevent them from promoting the cascade responses of septicemia. In a mouse model of Escherichia coli bacteremia, the administration of simulated macrophage nanoparticles (MΦ-NPs) resulted in a decrease in levels of pro-inflammatory cytokines, inhibited the spread of germs, and eventually improved the survival rate of infected mice153 (Figure 13C). Shen et al used a hydrothermal technique to create Fe3O4 nanoparticles that were modified with polyethyleneimine (PEI). They then generated Fe3O4@MMs nanoparticles by pressing Fe3O4 nanoparticles and macrophage cell membranes together through a 100-nm porous polycarbonate membrane. Nanoparticles, which were created to target and reduce LPS, were found to have a substantial impact on the immune response in mice treated with endotoxin. These nanoparticles reduced inflammation reactions, improved survival rates154 (Figure 13D). Disulfiram (DSF) has the potential to be used in the treatment of inflammatory illnesses. Lactoferrin (LF) is a glycoprotein with multiple functions, including strong antibacterial and anti-inflammatory properties. It can neutralize toxins in the bloodstream and stimulate cellular responses. Ou et al created a nano-system called DSF-LF NP, which combines the immune-suppressive actions of DSF and LF. DSF-LF nanoparticles efficiently inhibit macrophage apoptosis and the secretion of inflammatory cytokines. DSF-LF nanoparticles exhibit a substantial therapeutic impact on sepsis produced by lipopolysaccharide (LPS). DSF-LF-NPs have the ability to efficiently inhibit macrophage-mediated inflammatory responses and enhance the treatment of sepsis and UC-induced sequelae.155 In addition, chitosan (CH) is enclosed within nanoemulsions loaded with ciprofloxacin-sodium deoxycholate surfplex (CFn- sdc) to improve the penetration of CFn into tissues and eliminate endotoxins (lipopolysaccharide or LPS) that are generated during antibiotic therapy.156 Overall, nanoparticle-based detoxification approaches provide a promising solution for treating sepsis by utilizing nanotechnology to efficiently neutralize toxins created by bacteria and safeguard individuals from the risks of infection. (Table 6) Nevertheless, additional investigation and rigorous clinical trials are required to substantiate the safety and effectiveness of these approaches.
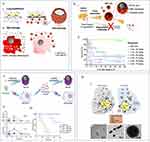 |
Figure 13 (A) (I) Schematic illustration demonstrating the ability of human red blood cell-derived nanosponges (hRBC-NS) to capture β-H/C, thereby reducing cytotoxic damage to lung epithelial cells and mitigating macrophage inflammasome activation, leading to inhibited IL-1β production (II). Reproduced from Koo J, Escajadillo T, Zhang L, Nizet V, Lawrence SM. Erythrocyte-coated nanoparticles block cytotoxic effects of group B streptococcus beta-hemolysin/cytolysin. Front Pediatr. 2019;7:410. Copyright © 2019 Koo, Escajadillo, Zhang, Nizet and Lawrence. Creative Commons Attribution License (CC BY).151 (B) (I) Schematic diagram showing the preparation and characterization of red blood cell nanosponges (RBC-NS) and their use in treating mice subjected to MRSA toxin-induced toxic shock. (II) The schematic includes the survival rate of mice within 24 hours of treatment (n = 6 per cohort). Reproduced from Chen Y, Zhang Y, Chen M, et al. Biomimetic nanosponges suppress in vivo lethality induced by the whole secreted proteins of pathogenic bacteria. Small. 2019;15(6):e1804994. © 2019 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.152 (C) (I) Schematic illustration of using macrophage-derived nanoparticles (MΦ-NPs) to neutralize endotoxins and pro-inflammatory cytokines as a two-step process for sepsis management. (II) Levels of pro-inflammatory cytokines, including TNF-α and IL-6, in plasma (n = 6). (III) Survival rate (n = 10). Reproduced from Thamphiwatana S, Angsantikul P, Escajadillo T, et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc Natl Acad Sci U S A. 2017;114(43):11488–11493.153 (D) (I) Schematic representation and detoxification process of Fe3O4@MMs. (II) Schematic structure of Fe3O4@MMs and the mechanism by which they neutralize LPS. (III) Transmission electron microscopy (TEM) image of PEI-modified Fe3O4 nanoparticles. (IV) TEM image of Fe3O4@MMs nanoparticles. (V) Enlarged view of a single Fe3O4@MMs nanoparticle. Reproduced from Shen S, Han F, Yuan A, et al. Engineered nanoparticles disguised as macrophages for trapping lipopolysaccharide and preventing endotoxemia. Biomaterials. 2019;189:60–68. Copyright © 2018 Elsevier Ltd. All rights reserved.154 |
 |
Table 6 Application of Nanotechnology in Toxin Removal |
Challenges and Prospects for the Future
Complexity
Nanoparticles exhibit considerable potential for the treatment of sepsis; yet, they encounter certain limitations and obstacles. Sepsis, an acute and life-threatening infection usually caused by bacteria, can result in widespread inflammation and malfunction of multiple organs.9,157 The cause and development of sepsis are extremely intricate, encompassing many physiological systems at different points in the progression of the disease.158,159 The intricate nature of this situation requires specialized nanoparticle treatments that may require customisation for various types of infections and disease processes, hence intensifying the challenge of treatment.
Drug Delivery Systems
Nanoparticles have the potential to be used as carriers for delivering drugs. However, effectively targeting and releasing drugs at specific infection sites still presents difficulties.25,160 It is essential to ensure efficient transportation of drugs to infected areas while minimizing any negative effects on healthy tissues. Multiple nanoparticle formulations have been documented,26,95,161 showing that surface functionalization with antimicrobial peptides can achieve targeted distribution and strong antimicrobial effects. The inclusion of antimicrobial peptides in nanoparticle formulations enables targeted delivery of antibiotics to specific areas within bacteria, such as the cell walls/membranes, cytoplasm, or nucleus. This approach enhances the effectiveness of bacterial eradication. However, it is important to validate these findings through well-designed clinical studies.
Immunoreactivity
Nanoparticles can be employed to transport anti-inflammatory medications for the purpose of controlling systemic inflammatory reactions caused by sepsis.162–164 This could potentially enhance patient outcomes and mitigate the likelihood of multi-organ dysfunction. Nevertheless, nanoparticles have the potential to trigger immunological reactions, which could lead to allergy responses or the activation of the immune system, hence heightening the likelihood of systemic inflammatory reactions. Therefore, a thorough examination of the immunocompatibility and toxicity of nanoparticles is essential. Additionally, nanoparticles show potential as vehicles for delivering vaccines to combat infections caused by sepsis and boosting the immunological responses of patients.165,166
Drug Resistance
The susceptibility of antibiotics is a crucial factor in determining which antibiotics to use, as certain infections can become resistant, making treatment more difficult.167,168 Sepsis causes alterations in the circulation of patients, making it difficult to reach the optimal levels of antibiotic distribution in the body. In sepsis patients, it is common to encounter infections caused by multidrug-resistant (MDR) pathogens or polymicrobial infections. Therefore, combination therapy based on sensitivity is necessary to treat these infections.169 Nevertheless, there is a scarcity of evidence regarding the use of nanoparticle antibiotic combination medicines, which calls for additional research to investigate their potential in nanoparticle formulations. Nanoparticles have the capability to transport different kinds of antibiotics or other pharmaceuticals that fight against infections. This has the potential to address drug resistance by increasing the amount of drugs at the site of infection and decreasing the likelihood of antibiotic resistance.
Safety
Thorough analysis is necessary to ensure the safety of nanoparticles in drug delivery and treatment, despite their potential. There may be concerns regarding the potential toxicity and compatibility of nanoparticles. It is worth mentioning that certain anti-inflammatory medications have been tested in many preclinical and clinical investigations using nanoparticle formulations.170 A clinical experiment examined the efficacy of nano curcumin (NC) in reducing inflammation biomarkers, improving endothelial function, reducing oxidative stress indicators, optimizing biochemical variables, enhancing nutritional status, and improving clinical outcomes in patients with sepsis.136 The research was a clinical experiment conducted in an intensive care unit (ICU) with 40 patients. The patients were randomly assigned to either the NC or placebo group. They were given NC (160 mg) or a placebo through a tube in their nose, twice a day for 10 days. The trial was double-blind, meaning neither the patients nor the researchers knew which group they were in, and it was also placebo-controlled, meaning some patients received a fake treatment. Administering NC as a supplement resulted in a considerable decrease in both SOFA scores and the length of time mechanical ventilation was required. Additional research is needed to investigate the prolonged impact and safety of nanoparticles in the treatment of sepsis. Subsequent clinical trials are necessary to evaluate the safety and effectiveness of nanoparticles in the treatment of sepsis.
Conclusion
The field of sepsis treatment holds significant potential for utilizing nanotechnology to enhance therapeutic outcomes and alleviate patient suffering. Further advancements in this area will improve our understanding of the role of nanotechnology in treating sepsis and offer superior therapeutic options for patients. Ensuring the biocompatibility of nanoparticles and minimizing potential toxicity risks are critical in clinical applications. More in-depth research is needed to verify their long-term safety and efficacy. Additionally, ethical issues in clinical trials, including informed consent from patients and long-term monitoring of impacts, must receive adequate attention. Future research should not only focus on improving treatment outcomes but also prioritize ensuring safety and feasibility in real-world applications. Only when these issues are fully addressed can nanotechnology provide reliable and effective treatment options for sepsis patients, thereby advancing the development of this field.
Data Sharing Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Consent Statement
Not applicable. No individual personal data are included in the study.
Acknowledgments
Yukun Liu and Jiafeng Liu contributed equally to this study.
Author Contributions
All authors contributed to the design of the study and writing of the manuscript. Yukun Liu, Dongfang Wang undertook the research, Yukun Liu and Yuchang Wang wrote the main manuscript text and prepared figures. Zhanfei Li, Xiangjun Bai and Yuchang Wang revised the article critically for important intellectual content and final approval of the version to be submitted. All authors reviewed the manuscript.
Funding
This study was supported by grants from Hubei Provincial Natural Science Foundation of China (No. 2023AFB825) and Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. 2023A15).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
2. Sartelli M, Kluger Y, Ansaloni L, et al. Raising concerns about the Sepsis-3 definitions. World J Emerg Surg. 2018;13(1):6. doi:10.1186/s13017-018-0165-6
3. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–211. doi:10.1016/S0140-6736(19)32989-7
4. Kumar S, Tripathy S, Jyoti A, Singh SG. Recent advances in biosensors for diagnosis and detection of sepsis: a comprehensive review. Biosens Bioelectron. 2019;124-125:205–215. doi:10.1016/j.bios.2018.10.034
5. Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. doi:10.1136/bmj.i1585
6. Gavelli F, Castello LM, Avanzi GC. Management of sepsis and septic shock in the emergency department. Intern Emerg Med. 2021;16(6):1649–1661. doi:10.1007/s11739-021-02735-7
7. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi:10.1016/S0140-6736(18)30696-2
8. Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2(1):16045. doi:10.1038/nrdp.2016.45
9. Vincent JL. Current sepsis therapeutics. EBioMedicine. 2022;86:104318. doi:10.1016/j.ebiom.2022.104318
10. Zhang YY, Ning BT. Signaling pathways and intervention therapies in sepsis. Signal Transduct Target Ther. 2021;6(1):407. doi:10.1038/s41392-021-00816-9
11. Srzic I, Nesek Adam V, Tunjic Pejak D. Sepsis definition: what’s new in the treatment guidelines. Acta Clin Croat. 2022;61(Suppl 1):67–72. doi:10.20471/acc.2022.61.s1.11
12. Liu D, Huang SY, Sun JH, et al. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res. 2022;9(1):56. doi:10.1186/s40779-022-00422-y
13. Molnar Z, Giamarellos-Bourboulis EJ, Kumar A, Nierhaus A. Sepsis: diagnostic and Therapeutic Challenges. Biomed Res Int. 2016;2016:5786182. doi:10.1155/2016/5786182
14. Alhazzani W, Moller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically III adults with coronavirus disease 2019 (COVID-19). Crit Care Med. 2020;48(6):e440–e469. doi:10.1097/CCM.0000000000004363
15. Capsoni N, Bellone P, Aliberti S, et al. Prevalence, risk factors and outcomes of patients coming from the community with sepsis due to multidrug resistant bacteria. Multidiscip Respir Med. 2019;14(1):23. doi:10.1186/s40248-019-0185-4
16. Campion M, Scully G. Antibiotic Use in the intensive care unit: optimization and de-escalation. J Intensive Care Med. 2018;33(12):647–655. doi:10.1177/0885066618762747
17. Laxminarayan R, Matsoso P, Pant S, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387(10014):168–175. doi:10.1016/S0140-6736(15)00474-2
18. Monard C, Rimmele T, Ronco C. Extracorporeal blood purification therapies for sepsis. Blood Purif. 2019;47(Suppl 3):1–14. doi:10.1159/000499520
19. Esposito S, De Simone G, Boccia G, De Caro F, Pagliano P. Sepsis and septic shock: new definitions, new diagnostic and therapeutic approaches. J Glob Antimicrob Resist. 2017;10:204–212. doi:10.1016/j.jgar.2017.06.013
20. Rello J, Valenzuela-Sanchez F, Ruiz-Rodriguez M, Moyano S. Sepsis: a review of advances in management. Adv Ther. 2017;34(11):2393–2411. doi:10.1007/s12325-017-0622-8
21. Tosi M, Coloretti I, Meschiari M, De Biasi S, Girardis M, Busani S. The interplay between antibiotics and the host immune response in sepsis: from basic mechanisms to clinical considerations: a comprehensive narrative review. Antibiotics. 2024;13(5). doi:10.3390/antibiotics13050406
22. Charlton M, Thompson JP. Pharmacokinetics in sepsis. BJA Educ. 2019;19(1):7–13.
23. Selvaraj V, Manne ND, Arvapalli R, et al. Effect of cerium oxide nanoparticles on sepsis induced mortality and NF-kappaB signaling in cultured macrophages. Nanomedicine. 2015;10(8):1275–1288. doi:10.2217/nnm.14.205
24. Huang M, Cai S, Su J. The pathogenesis of sepsis and potential therapeutic targets. Int J Mol Sci. 2019;20(21):5376. doi:10.3390/ijms20215376
25. Papafilippou L, Claxton A, Dark P, Kostarelos K, Hadjidemetriou M. Nanotools for sepsis diagnosis and treatment. Adv Healthc Mater. 2021;10(1):e2001378. doi:10.1002/adhm.202001378
26. Pant A, Mackraj I, Govender T. Advances in sepsis diagnosis and management: a paradigm shift towards nanotechnology. J Biomed Sci. 2021;28(1):6. doi:10.1186/s12929-020-00702-6
27. Amreddy N, Babu A, Muralidharan R, et al. Recent advances in nanoparticle-based cancer drug and gene delivery. Adv Cancer Res. 2018;137:115–170.
28. Suk JS, Xu Q, Kim N, Hanes J, Ensign LM. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99(Pt A):28–51. doi:10.1016/j.addr.2015.09.012
29. Bag N, Bardhan S, Roy S, et al. Nanoparticle-mediated stimulus-responsive antibacterial therapy. Biomater Sci. 2023;11(6):1994–2019. doi:10.1039/D2BM01941H
30. Joudeh N, Linke D. Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J Nanobiotechnology. 2022;20(1):262. doi:10.1186/s12951-022-01477-8
31. Jelinkova P, Mazumdar A, Sur VP, et al. Nanoparticle-drug conjugates treating bacterial infections. J Control Release. 2019;307:166–185. doi:10.1016/j.jconrel.2019.06.013
32. Hu Y, Zhang W, Chu X, et al. Dendritic cell-targeting polymer nanoparticle-based immunotherapy for cancer: a review. Int J Pharm. 2023;635:122703. doi:10.1016/j.ijpharm.2023.122703
33. Cevaal PM, Ali A, Czuba-Wojnilowicz E, et al. In vivo T cell-targeting nanoparticle drug delivery systems: considerations for rational design. ACS Nano. 2021;15(3):3736–3753. doi:10.1021/acsnano.0c09514
34. Donahue ND, Acar H, Wilhelm S. Concepts of nanoparticle cellular uptake, intracellular trafficking, and kinetics in nanomedicine. Adv Drug Deliv Rev. 2019;143:68–96. doi:10.1016/j.addr.2019.04.008
35. Katterman C, Pierce C, Larsen J. Combining nanoparticle shape modulation and polymersome technology in drug delivery. ACS Appl Bio Mater. 2021;4(4):2853–2862. doi:10.1021/acsabm.1c00203
36. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi:10.1038/s41573-020-0090-8
37. Park K. Controlled drug delivery systems: past forward and future back. J Control Release. 2014;190:3–8. doi:10.1016/j.jconrel.2014.03.054
38. Shankar-Hari M, Phillips GS, Levy ML, et al; Sepsis Definitions Task, F. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock Sepsis-3. JAMA. 2016;315(8):775–787. doi:10.1001/jama.2016.0289
39. Chiu C, Legrand M. Epidemiology of sepsis and septic shock. Curr Opin Anaesthesiol. 2021;34(2):71–76. doi:10.1097/ACO.0000000000000958
40. Bracht H, Hafner S, Weiss M. [Sepsis update: definition and epidemiology]. Anasthesiol Intensivmed Notfallmed Schmerzther. 2019;54(1):10–20. doi:10.1055/a-0625-5492 Danish
41. Rodriguez A, Martin-Loeches I, Yebenes JC. New definition of sepsis and septic shock: what does it give us? Med Intensiva. 2017;41(1):38–40. doi:10.1016/j.medin.2016.03.008
42. Salomao R, Ferreira BL, Salomao MC, Santos SS, Azevedo LCP, Brunialti MKC. Sepsis: evolving concepts and challenges. Braz J Med Biol Res. 2019;52(4):e8595. doi:10.1590/1414-431x20198595
43. Zheng X, Chen W, Gong F, Chen Y, Chen E. The role and mechanism of pyroptosis and potential therapeutic targets in sepsis: a review. Front Immunol. 2021;12:711939. doi:10.3389/fimmu.2021.711939
44. Mira JC, Gentile LF, Mathias BJ, et al. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med. 2017;45(2):253–262. doi:10.1097/CCM.0000000000002074
45. van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17(7):407–420. doi:10.1038/nri.2017.36
46. Jacobi J. The pathophysiology of sepsis - 2021 update: part 2, organ dysfunction and assessment. Am J Health Syst Pharm. 2022;79(6):424–436. doi:10.1093/ajhp/zxab393
47. Reddy H, Javvaji CK, Malali S, Kumar S, Acharya S, Toshniwal S. Navigating the cytokine storm: a comprehensive review of chemokines and cytokines in sepsis. Cureus. 2024;16(2):e54275. doi:10.7759/cureus.54275
48. Raymond SL, Holden DC, Mira JC, et al. Microbial recognition and danger signals in sepsis and trauma. Biochim Biophys Acta Mol Basis Dis. 2017;1863(10 Pt B):2564–2573. doi:10.1016/j.bbadis.2017.01.013
49. Lamkanfi M. Emerging inflammasome effector mechanisms. Nat Rev Immunol. 2011;11(3):213–220. doi:10.1038/nri2936
50. McKernan DP. Pattern recognition receptors as potential drug targets in inflammatory disorders. Adv Protein Chem Struct Biol. 2020;119:65–109.
51. Kumar V. Toll-like receptors in sepsis-associated cytokine storm and their endogenous negative regulators as future immunomodulatory targets. Int Immunopharmacol. 2020;89(Pt B):107087. doi:10.1016/j.intimp.2020.107087
52. Chen F, Zou L, Williams B, Chao W. Targeting toll-like receptors in sepsis: from bench to clinical trials. Antioxid Redox Signal. 2021;35(15):1324–1339. doi:10.1089/ars.2021.0005
53. Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi:10.1038/ni.1863
54. Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. doi:10.1016/j.cell.2010.01.040
55. Kumar V. Inflammasomes: pandora’s box for sepsis. J Inflamm Res. 2018;11:477–502. doi:10.2147/JIR.S178084
56. Danielski LG, Giustina AD, Bonfante S, Barichello T, Petronilho F. The NLRP3 inflammasome and its role in sepsis development. Inflammation. 2020;43(1):24–31. doi:10.1007/s10753-019-01124-9
57. Li K, Underhill DM. C-type lectin receptors in phagocytosis. Curr Top Microbiol Immunol. 2020;429:1–18. doi:10.1007/82_2020_198
58. Xing K, Murthy S, Liles WC, Singh JM. Clinical utility of biomarkers of endothelial activation in sepsis--a systematic review. Crit Care. 2012;16(1):R7. doi:10.1186/cc11145
59. Pool R, Gomez H, Kellum JA. Mechanisms of organ dysfunction in sepsis. Crit Care Clin. 2018;34(1):63–80. doi:10.1016/j.ccc.2017.08.003
60. Sygitowicz G, Sitkiewicz D. Molecular mechanisms of organ damage in sepsis: an overview. Braz J Infect Dis. 2020;24(6):552–560. doi:10.1016/j.bjid.2020.09.004
61. Cao C, Yu M, Chai Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis. 2019;10(10):782. doi:10.1038/s41419-019-2015-1
62. Wang YC, Liu QX, Liu T, et al. Caspase-1-dependent pyroptosis of peripheral blood mononuclear cells predicts the development of sepsis in severe trauma patients: a prospective observational study. Medicine. 2018;97(8):e9859. doi:10.1097/MD.0000000000009859
63. Wang Y, Liu Y, Liu Q, et al. Caspase-1-dependent pyroptosis of peripheral blood mononuclear cells is associated with the severity and mortality of septic patients. Biomed Res Int. 2020;2020(1):9152140. doi:10.1155/2020/9152140
64. Xl L, Gy Z, R G, N C. Ferroptosis in sepsis: the mechanism, the role and the therapeutic potential. Front Immunol. 2022;13:956361. doi:10.3389/fimmu.2022.956361
65. Guo R, Wang H, Cui N. Autophagy regulation on pyroptosis: mechanism and medical implication in sepsis. Mediators Inflamm. 2021;2021:9925059. doi:10.1155/2021/9925059
66. Venet F, Monneret G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat Rev Nephrol. 2018;14(2):121–137. doi:10.1038/nrneph.2017.165
67. Girardis M, Coloretti I, Antonelli M, et al. Adjunctive immunotherapeutic agents in patients with sepsis and septic shock: a multidisciplinary consensus of 23. J Anesth Analg Crit Care. 2024;4(1):28. doi:10.1186/s44158-024-00165-3
68. Guarino M, Perna B, Cesaro AE, et al. 2023 update on sepsis and septic shock in adult patients: management in the emergency department. J Clin Med. 2023;12(9):3188. doi:10.3390/jcm12093188
69. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181–1247. doi:10.1007/s00134-021-06506-y
70. Marik PE, Farkas JD. The changing paradigm of sepsis: early diagnosis, early antibiotics, early pressors, and early adjuvant treatment. Crit Care Med. 2018;46(10):1690–1692. doi:10.1097/CCM.0000000000003310
71. De Backer D, Cecconi M, Lipman J, et al. Challenges in the management of septic shock: a narrative review. Intensive Care Med. 2019;45(4):420–433. doi:10.1007/s00134-019-05544-x
72. Meresse Z, Medam S, Mathieu C, Duclos G, Vincent JL, Leone M. Vasopressors to treat refractory septic shock. Minerva Anestesiol. 2020;86(5):537–545. doi:10.23736/S0375-9393.20.13826-4
73. Liu VX, Fielding-Singh V, Greene JD, et al. The timing of early antibiotics and hospital mortality in sepsis. Am J Respir Crit Care Med. 2017;196(7):856–863. doi:10.1164/rccm.201609-1848OC
74. Asner SA, Desgranges F, Schrijver IT, Calandra T. Impact of the timeliness of antibiotic therapy on the outcome of patients with sepsis and septic shock. J Infect. 2021;82(5):125–134. doi:10.1016/j.jinf.2021.03.003
75. Kim RY, Ng AM, Persaud AK, et al. Antibiotic timing and outcomes in sepsis. Am J Med Sci. 2018;355(6):524–529. doi:10.1016/j.amjms.2018.02.007
76. Moser C, Lerche CJ, Thomsen K, et al. Antibiotic therapy as personalized medicine - general considerations and complicating factors. APMIS. 2019;127(5):361–371. doi:10.1111/apm.12951
77. Coopersmith CM, De Backer D, Deutschman CS, et al. Surviving sepsis campaign: research priorities for sepsis and septic shock. Intensive Care Med. 2018;44(9):1400–1426. doi:10.1007/s00134-018-5175-z
78. Badeaux JE, Martin JB. Emerging adjunctive approach for the treatment of sepsis: vitamin C and thiamine. Crit Care Nurs Clin North Am. 2018;30(3):343–351. doi:10.1016/j.cnc.2018.05.002
79. Zhao Y, Pu M, Zhang J, et al. Recent advancements of nanomaterial-based therapeutic strategies toward sepsis: bacterial eradication, anti-inflammation, and immunomodulation. Nanoscale. 2021;13(24):10726–10747. doi:10.1039/D1NR02706A
80. Al-Awsi GRL, Alameri AA, Al-Dhalimy AMB, Gabr GA, Kianfar E. Application of nano-antibiotics in the diagnosis and treatment of infectious diseases. Braz J Biol. 2023;84:e264946. doi:10.1590/1519-6984.264946
81. Van Giau V, An SSA, Hulme J. Recent advances in the treatment of pathogenic infections using antibiotics and nano-drug delivery vehicles. Drug Des Devel Ther. 2019;13:327–343. doi:10.2147/DDDT.S190577
82. Hussain S, Joo J, Kang J, et al. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nat Biomed Eng. 2018;2(2):95–103. doi:10.1038/s41551-017-0187-5
83. Birk SE, Boisen A, Nielsen LH. Polymeric nano- and microparticulate drug delivery systems for treatment of biofilms. Adv Drug Deliv Rev. 2021;174:30–52. doi:10.1016/j.addr.2021.04.005
84. Shaker MA, Shaaban MI. Formulation of carbapenems loaded gold nanoparticles to combat multi-antibiotic bacterial resistance: in vitro antibacterial study. Int J Pharm. 2017;525(1):71–84. doi:10.1016/j.ijpharm.2017.04.019
85. Zhong Y, Zheng XT, Zhao S, Su X, Loh XJ. Stimuli-activable metal-bearing nanomaterials and precise on-demand antibacterial strategies. ACS Nano. 2022;16(12):19840–19872. doi:10.1021/acsnano.2c08262
86. Alavi M, Kamarasu P, McClements DJ, Moore MD. Metal and metal oxide-based antiviral nanoparticles: properties, mechanisms of action, and applications. Adv Colloid Interface Sci. 2022;306:102726. doi:10.1016/j.cis.2022.102726
87. Stankic S, Suman S, Haque F, Vidic J. Pure and multi metal oxide nanoparticles: synthesis, antibacterial and cytotoxic properties. J Nanobiotechnology. 2016;14(1):73. doi:10.1186/s12951-016-0225-6
88. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377. doi:10.1007/s00134-017-4683-6
89. Abdelkader A, El-Mokhtar MA, Abdelkader O, Hamad MA, Elsabahy M, El-Gazayerly ON. Ultrahigh antibacterial efficacy of meropenem-loaded chitosan nanoparticles in a septic animal model. Carbohydr Polym. 2017;174:1041–1050. doi:10.1016/j.carbpol.2017.07.030
90. Nader D, Yousef F, Kavanagh N, Ryan BK, Kerrigan SW. Targeting internalized staphylococcus aureus using vancomycin-loaded nanoparticles to treat recurrent bloodstream infections. Antibiotics. 2021;10(5). doi:10.3390/antibiotics10050581
91. Ndayishimiye J, Cao Y, Kumeria T, Blaskovich MAT, Falconer JR, Popat A. Engineering mesoporous silica nanoparticles towards oral delivery of vancomycin. J Mater Chem B. 2021;9(35):7145–7166. doi:10.1039/D1TB01430G
92. Zhang CY, Gao J, Wang Z. Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv Mater. 2018;30(43):e1803618. doi:10.1002/adma.201803618
93. Yang Y, Ding Y, Fan B, et al. Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J Control Release. 2020;321:463–474. doi:10.1016/j.jconrel.2020.02.030
94. Biswaro LS, da Costa Sousa MG, Rezende TMB, Dias SC, Franco OL. Antimicrobial peptides and nanotechnology, recent advances and challenges. Front Microbiol. 2018;9:855. doi:10.3389/fmicb.2018.00855
95. Song X, Liu P, Liu X, et al. Dealing with MDR bacteria and biofilm in the post-antibiotic era: application of antimicrobial peptides-based nano-formulation. Mater Sci Eng C Mater Biol Appl. 2021;128:112318. doi:10.1016/j.msec.2021.112318
96. Li X, Zuo S, Wang B, Zhang K, Wang Y. Antimicrobial mechanisms and clinical application prospects of antimicrobial peptides. Molecules. 2022;27(9).
97. Zhang R, Xu L, Dong C. Antimicrobial peptides: an overview of their structure, function and mechanism of action. Protein Pept Lett. 2022;29(8):641–650. doi:10.2174/0929866529666220613102145
98. Lam SJ, O’Brien-Simpson NM, Pantarat N, et al. Combating multidrug-resistant Gram-negative bacteria with structurally nanoengineered antimicrobial peptide polymers. Nat Microbiol. 2016;1(11):16162. doi:10.1038/nmicrobiol.2016.162
99. Fan X, Fan J, Wang X, Wu P, Wu G. S-thanatin functionalized liposome potentially targeting on Klebsiella pneumoniae and its application in sepsis mouse model. Front Pharmacol. 2015;6:249. doi:10.3389/fphar.2015.00249
100. Oun AA, Shankar S, Rhim JW. Multifunctional nanocellulose/metal and metal oxide nanoparticle hybrid nanomaterials. Crit Rev Food Sci Nutr. 2020;60(3):435–460. doi:10.1080/10408398.2018.1536966
101. Alavi M, Li L, Nokhodchi A. Metal, metal oxide and polymeric nanoformulations for the inhibition of bacterial quorum sensing. Drug Discov Today. 2023;28(1):103392. doi:10.1016/j.drudis.2022.103392
102. Kuthati Y, Kankala RK, Busa P, et al. Phototherapeutic spectrum expansion through synergistic effect of mesoporous silica trio-nanohybrids against antibiotic-resistant gram-negative bacterium. J Photochem Photobiol B. 2017;169:124–133. doi:10.1016/j.jphotobiol.2017.03.003
103. Tang S, Zheng J. Antibacterial activity of silver nanoparticles: structural effects. Adv Healthc Mater. 2018;7(13):e1701503. doi:10.1002/adhm.201701503
104. Bi X, Bai Q, Liang M, et al. Silver peroxide nanoparticles for combined antibacterial sonodynamic and photothermal therapy. Small. 2022;18(2):e2104160. doi:10.1002/smll.202104160
105. Saulnier P, A M. Aromatic and terpenic compounds loaded in lipidic nanocapsules: activity against multi-drug resistant Acinetobacter baumannii assessed in vitro and in a murine model of sepsis. J Nanomed Nanotechnol. 2014;05(03). doi:10.4172/2157-7439.1000206
106. Khanal M, Raks V, Issa R, et al. Selective antimicrobial and antibiofilm disrupting properties of functionalized diamond nanoparticles against Escherichia coli and staphylococcus aureus. Part Part Syst Charact. 2015;32.
107. Chen C, Chen L, Mao C, et al. Natural extracts for antibacterial applications. Small. 2024;20(9):e2306553. doi:10.1002/smll.202306553
108. Dai J, Han R, Xu Y, Li N, Wang J, Dan W. Recent progress of antibacterial natural products: future antibiotics candidates. Bioorg Chem. 2020;101:103922. doi:10.1016/j.bioorg.2020.103922
109. Mohan S, Ajay Krishna MS, Chandramouli M, et al. Antibacterial natural products from microbial and fungal sources: a decade of advances. Mol Divers. 2023;27(1):517–541. doi:10.1007/s11030-022-10417-5
110. Swamy MK, Akhtar MS, Sinniah UR. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid Based Complement Alternat Med. 2016;2016(1):3012462. doi:10.1155/2016/3012462
111. Tobal IE, Roncero AM, Moro RF, Diez D, Marcos IS. Antibacterial natural halimanes: potential source of novel antibiofilm agents. Molecules. 2020;25(7):1707. doi:10.3390/molecules25071707
112. Li Y, Zhang H, Chen C, et al. Biomimetic immunosuppressive exosomes that inhibit cytokine storms contribute to the alleviation of sepsis. Adv Mater. 2022;34(19):e2108476. doi:10.1002/adma.202108476
113. Wang Y, Liu Q, Liu T, et al. Early plasma monocyte chemoattractant protein 1 predicts the development of sepsis in trauma patients: a prospective observational study. Medicine. 2018;97(14):e0356. doi:10.1097/MD.0000000000010356
114. Leal YA, Alvarez-Nemegyei J, Lavadores-May AI, Giron-Carrillo JL, Cedillo-Rivera R, Velazquez JR. Cytokine profile as diagnostic and prognostic factor in neonatal sepsis. J Matern Fetal Neonatal Med. 2019;32(17):2830–2836. doi:10.1080/14767058.2018.1449828
115. Ismail EA, Devnarain N, Govender T, Omolo CA. Stimuli-responsive and biomimetic delivery systems for sepsis and related complications. J Control Release. 2022;352:1048–1070. doi:10.1016/j.jconrel.2022.11.013
116. Lasola JJM, Kamdem H, McDaniel MW, Pearson RM. Biomaterial-driven immunomodulation: cell biology-based strategies to mitigate severe inflammation and sepsis. Front Immunol. 2020;11:1726. doi:10.3389/fimmu.2020.01726
117. Liao S, Liu S, Zhang Y. Preparation of anti toll-like receptor-4 nano-antibody and its effect on gram negative sepsis. J Nanosci Nanotechnol. 2021;21(2):1048–1053. doi:10.1166/jnn.2021.18664
118. Coutinho-Wolino KS, Almeida PP, Mafra D, Stockler-Pinto MB. Bioactive compounds modulating Toll-like 4 receptor (TLR4)-mediated inflammation: pathways involved and future perspectives. Nutr Res. 2022;107:96–116. doi:10.1016/j.nutres.2022.09.001
119. Casey LM, Kakade S, Decker JT, et al. Cargo-less nanoparticles program innate immune cell responses to toll-like receptor activation. Biomaterials. 2019;218:119333. doi:10.1016/j.biomaterials.2019.119333
120. Song C, Xu J, Gao C, Zhang W, Fang X, Shang Y. Nanomaterials targeting macrophages in sepsis: a promising approach for sepsis management. Front Immunol. 2022;13:1026173. doi:10.3389/fimmu.2022.1026173
121. Cao H, Gao Y, Jia H, et al. Macrophage-membrane-camouflaged nonviral gene vectors for the treatment of multidrug-resistant bacterial sepsis. Nano Lett. 2022;22(19):7882–7891. doi:10.1021/acs.nanolett.2c02560
122. Teng L, Zhang Y, Chen L, Shi G. Fabrication of a curcumin encapsulated bioengineered nano-cocktail formulation for stimuli-responsive targeted therapeutic delivery to enhance anti-inflammatory, anti-oxidant, and anti-bacterial properties in sepsis management. J Biomater Sci Polym Ed. 2023;34(12):1716–1740. doi:10.1080/09205063.2023.2181554
123. Guo NK, She H, Tan L, et al. Nano parthenolide improves intestinal barrier function of sepsis by inhibiting apoptosis and ROS via 5-HTR2A. Int J Nanomed. 2023;18:693–709. doi:10.2147/IJN.S394544
124. Wilson TR, McLaughlin KM, McEwan M, et al. c-FLIP: a key regulator of colorectal cancer cell death. Cancer Res. 2007;67(12):5754–5762. doi:10.1158/0008-5472.CAN-06-3585
125. Chen YF, Chen GY, Chang CH, et al. TRAIL encapsulated to polypeptide-crosslinked nanogel exhibits increased anti-inflammatory activities in Klebsiella pneumoniae-induced sepsis treatment. Mater Sci Eng C Mater Biol Appl. 2019;102:85–95. doi:10.1016/j.msec.2019.04.023
126. Zhang H, Wang Y, Qu M, et al. Neutrophil, neutrophil extracellular traps and endothelial cell dysfunction in sepsis. Clin Transl Med. 2023;13(1):e1170. doi:10.1002/ctm2.1170
127. Park SY, Shrestha S, Youn YJ, et al. Autophagy primes neutrophils for neutrophil extracellular trap formation during sepsis. Am J Respir Crit Care Med. 2017;196(5):577–589. doi:10.1164/rccm.201603-0596OC
128. Qi X, Yu Y, Sun R, et al. Identification and characterization of neutrophil heterogeneity in sepsis. Crit Care. 2021;25(1):50. doi:10.1186/s13054-021-03481-0
129. Zhang CY, Dong X, Gao J, Lin W, Liu Z, Wang Z. Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Sci Adv. 2019;5(11):eaax7964. doi:10.1126/sciadv.aax7964
130. Prauchner CA. Oxidative stress in sepsis: pathophysiological implications justifying antioxidant co-therapy. Burns. 2017;43(3):471–485. doi:10.1016/j.burns.2016.09.023
131. Nagar H, Piao S, Kim CS. Role of mitochondrial oxidative stress in sepsis. Acute Crit Care. 2018;33(2):65–72. doi:10.4266/acc.2018.00157
132. Liu R, Luo X, Li J, et al. Melatonin: a window into the organ-protective effects of sepsis. Biomed Pharmacother. 2022;154:113556. doi:10.1016/j.biopha.2022.113556
133. Liu Y, Wang D, Li T, et al. Melatonin: a potential adjuvant therapy for septic myopathy. Biomed. Pharmacother. 2023;158:114209. doi:10.1016/j.biopha.2022.114209
134. Di S, Wang Z, Hu W, et al. The protective effects of melatonin against LPS-induced septic myocardial injury: a potential role of AMPK-mediated autophagy. Front Endocrinol. 2020;11:162. doi:10.3389/fendo.2020.00162
135. Li Volti G, Musumeci T, Pignatello R, et al. Antioxidant potential of different melatonin-loaded nanomedicines in an experimental model of sepsis. Exp Biol Med. 2012;237(6):670–677. doi:10.1258/ebm.2012.011425
136. Karimi A, Naeini F, Niazkar HR, et al. Nano-curcumin supplementation in critically ill patients with sepsis: a randomized clinical trial investigating the inflammatory biomarkers, oxidative stress indices, endothelial function, clinical outcomes and nutritional status. Food Funct. 2022;13(12):6596–6612. doi:10.1039/D1FO03746C
137. Naeini F, Tutunchi H, Razmi H, et al. Does nano-curcumin supplementation improve hematological indices in critically ill patients with sepsis? A randomized controlled clinical trial. J Food Biochem. 2022;46(5):e14093. doi:10.1111/jfbc.14093
138. Yu H, Gao R, Liu Y, Fu L, Zhou J, Li L. Stimulus-responsive hydrogels as drug delivery systems for inflammation targeted therapy. Adv Sci. 2024;11(1):e2306152. doi:10.1002/advs.202306152
139. Li X, Han Z, Wang T, et al. Cerium oxide nanoparticles with antioxidative neurorestoration for ischemic stroke. Biomaterials. 2022;291:121904. doi:10.1016/j.biomaterials.2022.121904
140. Sun Y, Sun X, Li X, et al. A versatile nanocomposite based on nanoceria for antibacterial enhancement and protection from aPDT-aggravated inflammation via modulation of macrophage polarization. Biomaterials. 2021;268:120614. doi:10.1016/j.biomaterials.2020.120614
141. Zhou X, Zhou Q, He Z, et al. ROS balance autoregulating core-shell CeO(2)@ZIF-8/Au nanoplatform for wound repair. Nanomicro Lett. 2024;16(1):156. doi:10.1007/s40820-024-01353-0
142. Yu H, Jin F, Liu D, et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics. 2020;10(5):2342–2357. doi:10.7150/thno.40395
143. Chen G, Deng H, Song X, et al. Reactive oxygen species-responsive polymeric nanoparticles for alleviating sepsis-induced acute liver injury in mice. Biomaterials. 2017;144:30–41. doi:10.1016/j.biomaterials.2017.08.008
144. Boss L, Kedzierska B. Bacterial toxin-antitoxin systems’ cross-interactions-implications for practical use in medicine and biotechnology. Toxins. 2023;15(6):380. doi:10.3390/toxins15060380
145. Ricci D, Demangel C. From bacterial toxin to therapeutic agent: the unexpected fate of mycolactone. Toxins. 2023;15(6):369. doi:10.3390/toxins15060369
146. Minasyan H. Sepsis: mechanisms of bacterial injury to the patient. Scand J Trauma Resusc Emerg Med. 2019;27(1):19. doi:10.1186/s13049-019-0596-4
147. Ramarao N, Sanchis V. The pore-forming haemolysins of bacillus cereus: a review. Toxins. 2013;5(6):1119–1139. doi:10.3390/toxins5061119
148. Donnell ML, Lyon AJ, Mormile MR, Barua S. Endotoxin hitchhiking on polymer nanoparticles. Nanotechnology. 2016;27(28):285601. doi:10.1088/0957-4484/27/28/285601
149. Hu CM, Fang RH, Copp J, Luk BT, Zhang L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat Nanotechnol. 2013;8(5):336–340. doi:10.1038/nnano.2013.54
150. Chatterjee T, Saha T, Sarkar P, Hoque KM, Chatterjee BK, Chakrabarti P. The gold nanoparticle reduces Vibrio cholerae pathogenesis by inhibition of biofilm formation and disruption of the production and structure of cholera toxin. Colloids Surf B Biointerfaces. 2021;204:111811. doi:10.1016/j.colsurfb.2021.111811
151. Koo J, Escajadillo T, Zhang L, Nizet V, Lawrence SM. Erythrocyte-coated nanoparticles block cytotoxic effects of group B streptococcus beta-hemolysin/cytolysin. Front Pediatr. 2019;7:410. doi:10.3389/fped.2019.00410
152. Chen Y, Zhang Y, Chen M, et al. Biomimetic nanosponges suppress in vivo lethality induced by the whole secreted proteins of pathogenic bacteria. Small. 2019;15(6):e1804994. doi:10.1002/smll.201804994
153. Thamphiwatana S, Angsantikul P, Escajadillo T, et al. Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc Natl Acad Sci U S A. 2017;114(43):11488–11493. doi:10.1073/pnas.1714267114
154. Shen S, Han F, Yuan A, et al. Engineered nanoparticles disguised as macrophages for trapping lipopolysaccharide and preventing endotoxemia. Biomaterials. 2019;189:60–68. doi:10.1016/j.biomaterials.2018.10.029
155. Ou AT, Zhang JX, Fang YF, et al. Disulfiram-loaded lactoferrin nanoparticles for treating inflammatory diseases. Acta Pharmacol Sin. 2021;42(11):1913–1920. doi:10.1038/s41401-021-00770-w
156. Jain V, Shukla P, Pal R, Mishra PR. Cationic nanoemulsions bearing ciprofloxacin surf-plexes enhances its therapeutic efficacy in conditions of E. coli induced peritonitis and sepsis. Pharm Res. 2014;31(10):2630–2642. doi:10.1007/s11095-014-1360-0
157. Ackerman MH, Ahrens T, Kelly J, Pontillo A. Sepsis. Crit Care Nurs Clin North Am. 2021;33(4):407–418. doi:10.1016/j.cnc.2021.08.003
158. Kellum JA, Formeck CL, Kernan KF, Gomez H, Carcillo JA. Subtypes and mimics of sepsis. Crit Care Clin. 2022;38(2):195–211. doi:10.1016/j.ccc.2021.11.013
159. Hunt A. Sepsis: an overview of the signs, symptoms, diagnosis, treatment and pathophysiology. Emerg Nurse. 2019;27(5):32–41. doi:10.7748/en.2019.e1926
160. Zhou C, Liu Y, Li Y, Shi L. Recent advances and prospects in nanomaterials for bacterial sepsis management. J Mater Chem B. 2023;11(45):10778–10792. doi:10.1039/D3TB02220J
161. Gafar MA, Omolo CA, Elhassan E, Ibrahim UH, Govender T. Applications of peptides in nanosystems for diagnosing and managing bacterial sepsis. J Biomed Sci. 2024;31(1):40.
162. Li Z, Feng Y, Zhang S, et al. A multifunctional nanoparticle mitigating cytokine storm by scavenging multiple inflammatory mediators of sepsis. ACS Nano. 2023;17(9):8551–8563. doi:10.1021/acsnano.3c00906
163. Lu L, Quan L, Li J, et al. Bioengineered stem cell membrane functionalized nanoparticles combine anti-inflammatory and antimicrobial properties for sepsis treatment. J Nanobiotechnology. 2023;21(1):170. doi:10.1186/s12951-023-01913-3
164. Li H, Yang T, Zhou H, Du J, Zhu B, Sun Z. Emodin combined with nanosilver inhibited sepsis by anti-inflammatory protection. Front Pharmacol. 2016;7:536. doi:10.3389/fphar.2016.00536
165. Bjanes E, Zhou J, Qayum T, et al. Outer membrane vesicle-coated nanoparticle vaccine protects against Acinetobacter baumannii pneumonia and sepsis. Adv Nanobiomed Res. 2023;3(2). doi:10.1002/anbr.202200130
166. Barry MA, Wang Q, Jones KM, et al. A therapeutic nanoparticle vaccine against Trypanosoma cruzi in a BALB/c mouse model of Chagas disease. Hum Vaccin Immunother. 2016;12(4):976–987. doi:10.1080/21645515.2015.1119346
167. Rhee C, Kadri SS, Dekker JP, et al. Program, C. D. C. P. E. prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Network Open. 2020;3(4):e202899. doi:10.1001/jamanetworkopen.2020.2899
168. Kalra SJS, Shankar H, Mansoori N, Gupta DL. Antibiotic-resistant bacteria originating from the gut may modulate the mucosal immune response during sepsis and septic shock. Drug Target Insights. 2022;16(1):81–87. doi:10.33393/dti.2022.2520
169. Karamouzos V, Giamarellos-Bourboulis EJ, Velissaris D, Gkavogianni T, Gogos C. Cytokine production and outcome in MDR versus non-MDR gram-negative bacteraemia and sepsis. Infect Dis. 2021;53(10):764–771. doi:10.1080/23744235.2021.1925738
170. Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res. 2016;33(10):2373–2387. doi:10.1007/s11095-016-1958-5
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

