Back to Journals » International Journal of Nanomedicine » Volume 20
Advancements in Nano-Delivery Systems for Photodynamic and Photothermal Therapy to Induce Immunogenic Cell Death in Tumor Immunotherapy
Authors Zhao R , Li S , Zhao J, Yao C
Received 27 December 2024
Accepted for publication 4 June 2025
Published 26 June 2025 Volume 2025:20 Pages 8221—8248
DOI https://doi.org/10.2147/IJN.S514659
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Jie Huang
Rui Zhao,1,2,* Shuai Li,1,* Jingyuan Zhao,3 Chenhui Yao4
1Department of Pharmacy, The First Affiliated Hospital of Dalian Medical University, Dalian, People’s Republic of China; 2College of Pharmacy, Dalian Medical University, Dalian, People’s Republic of China; 3Clinical Laboratory Center, Central Hospital of Dalian University of Technology, Dalian, People’s Republic of China; 4Department of General Surgery, The First Affiliated Hospital of Dalian Medical University, Dalian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chenhui Yao, Department of general surgery, The First Affiliated Hospital of Dalian Medical University, Dalian, People’s Republic of China, Email [email protected] Jingyuan Zhao, Clinical Laboratory Center, Central Hospital of Dalian University of Technology, Dalian, People’s Republic of China, Email [email protected]
Abstract: Inducing immunogenic cell death (ICD) can tackle the issue of low immune response levels in tumor immunotherapy. Photodynamic therapy (PDT) uses photosensitizers to generate reactive oxygen species that kill tumor cells, while photothermal therapy (PTT) directly uses heat from photothermal converters to destroy tumor cells. However, these single treatments have their limitations. The development of optical therapy nanodelivery systems, along with the combination of PTT/PDT with chemotherapy and immunotherapy, offers new strategies for tumor immunotherapy. This article explores the application of PDT and PTT mediated by nanodelivery systems in promoting immunogenic cell death in tumor cells. This article explores nano-delivery systems that precisely release photosensitizers to boost efficacy and reduce toxicity. It also discusses their potential for combination therapy through co-delivery, utilizing multimodal strategies to enhance both phototherapy and chemotherapy, thus improving anticancer effectiveness.
Keywords: photodynamic therapy, photothermal therapy, immunogenic cell death, delivery system, nano
Introduction
The incidence and mortality rate of cancer continue to rise, and traditional treatments such as surgery, radiotherapy, and chemotherapy have certain limitations. Surgery may damage surrounding tissues and is difficult to treat metastatic tumors; radiotherapy can damage normal tissues, and some tumors are also insensitive to it; chemotherapy has severe side effects and can easily lead to drug resistance. Compared with traditional chemotherapy or radiotherapy, immunotherapy has fewer side effects and less impact on the quality of life of patients. Among them, checkpoint inhibitors, as a means of tumor immunotherapy, restore the activity of tumor-specific T cells by blocking immune checkpoint pathways such as PD-1/PD-L1, allowing the immune system to more effectively identify and attack tumor cells.1 Checkpoint inhibitors have made significant progress in the treatment of tumors, but the treatment of cold tumors remains a challenge. Cold tumors are characterized by a lack of T cell infiltration and a weak immune response, making them less responsive to treatments like checkpoint inhibitors. The relationship between cold tumors and ICD is pivotal because ICD has the potential to transform cold tumors into “hot” tumors.2 ICD triggers the release of tumor antigens and DAMPs, which attract and activate immune cells, particularly dendritic cells. These cells then present the antigens to T cells, initiating a robust immune response.3,4 This process is vital for overcoming the immunosuppressive microenvironment of cold tumors and enhancing the efficacy of immunotherapy.5 The immune microenvironment of cold tumors affects the efficacy of tumor immunotherapy through a variety of mechanisms.6 First, cold tumors have less T cell infiltration, especially effector T cells, resulting in a weak immune response. Second, tumor cells may express fewer tumor-associated antigens or have defective antigen presentation mechanisms, making it difficult for the immune system to recognize and attack tumor cells. In addition, high levels of immunosuppressive factors and immunosuppressive cells are present in the cold tumor microenvironment, and these factors work together to create an inhibitory microenvironment that reduces the function and activity of immune cells.7,8 These factors work together to result in a poor response to current immunotherapy strategies in cold tumors.9
Immunogenic cell death is a form of programmed cell death that usually involves the stress response within tumor cells, leading to the release of damage-associated molecular patterns (DAMPs) that can activate the immune system and promote the recognition of tumors by the immune system. Immunogenic cell death is different from traditional apoptosis or necrosis, can activate the body’s immune response, triggering a specific immune response and enhancing anti-tumor immunity. Immunogenic cell death (ICD) is a special form of programmed cell death characterized by the ability to activate the body’s immune system, thereby enhancing the anti-tumor immune response. The mechanism of ICD involves a series of molecular events, including endoplasmic reticulum stress (ER stress), the release of heat shock proteins (HSPs), the release of the high-mobility group protein B1 (HMGB1), and the release of adenosine triphosphate (ATP).10 Endoplasmic reticulum stress results in the transfer of calreticulin (CRT) from the lumen of the endoplasmic reticulum to the outer surface of the cell membrane, where it is recognized and engulfed by dendritic cells (DCs) as a “eat me” signal.11 HSPs, such as HSP70 and HSP90, bind to CD91 receptors on the surface of DCs, promoting their maturation and activation. HMGB1, as a nuclear protein, is released into the extracellular space after CRT exposure and binds to the TLR4 receptor on the surface of DCs to enhance antigen presentation ability. The release of ATP acts as a “find me” signal, attracting DCs and promoting their maturation and activation. Photodynamic therapy (PDT) induces endoplasmic reticulum stress through the production of reactive oxygen species (ROS), leading to CRT exposure and HMGB1 release, while promoting ATP release, activating DCs. Photothermal therapy (PTT) uses photothermal agents to convert light energy into heat energy, disrupt cell membranes and release DAMPs (such as CRT, HSPs, and HMGB1) to attract and activate immune cells.12 Although PDT and PTT have shown great potential in inducing ICD, their clinical translation still faces challenges such as light penetration, nanoparticle clearance, and biocompatibility. Future studies should optimize the biocompatibility of nanomaterials and verify their efficacy and safety through clinical trials, while exploring their combination with other therapies (such as immune checkpoint inhibitors) to further improve the therapeutic effect13 Conditions that can induce immunogenic cell death include viral infection, chemotherapy drugs such as anthracyclines and oxaliplatin, specific radiotherapy, and photodynamic therapy, which can lead to the release of danger signal molecules that are recognized by the immune system, thereby initiating the relevant immune response. Compared to drug-induced immunogenic cell death, photothermal therapy (PTT) and photodynamic therapy (PDT) have advantages. PTT can be repeatedly treated, has strong targeting, achieves high localization, can be applied to difficult-to-operate areas, and can improve efficacy when combined with chemotherapy. PDT has the advantages of high selectivity, low side effects, high spatiotemporal precision and resistance to multidrug resistance, and has been rapidly developed in clinical tumor treatment.
In recent years, significant progress has been made in the clinical application of PDT and PTT. FDA-approved photosensitizers like Photofrin® and Verteporfin are now used to treat lung cancer, esophageal cancer, and AMD by generating ROS or localized heat to induce tumor cell death. However, challenges such as tumor hypoxia and limited light penetration depth persist. Nanotechnology has emerged as a transformative solution to these limitations. Nano-delivery systems—including liposomes, polymeric nanoparticles, and MOFs—enhance the solubility, stability, and tumor-targeting precision of photosensitizers and photothermal agents. These systems can be engineered to respond to pH, temperature, or enzymes, further improving therapeutic specificity. Despite these advances, critical gaps remain: while PDT/PTT are known to induce ICD, the immunological role of nano-delivery systems in amplifying ICD and anti-tumor immunity is underexplored. Most research focuses on material properties rather than immune outcomes, and clinical translation requires rigorous validation of safety and efficacy.
This review uniquely bridges these gaps by systematically analyzing how nano-delivery systems optimize ICD induction. Unlike prior works that emphasized PDT/PTT mechanisms or immunotherapy combinations and focused more on specific aspects, such as the role of PDT in inducing immunogenic cell death and the application of ROS in cancer therapy, we highlight nano-carriers as immunological modulators. We detail strategies to overcome hypoxia, improve targeting, and a more comprehensive perspective, covering the combined application of PDT and PTT, the combination application with chemotherapy, offering a roadmap for clinical implementation. Our approach provides a holistic perspective, integrating nanomaterial design with immune activation, while addressing challenges in scalability and biocompatibility.
A critical evaluation of the potential risks, cost-effectiveness, and scalability of nano delivery-based phototherapies is essential to provide a balanced perspective. While nano delivery systems offer significant advantages, they also introduce potential risks such as biocompatibility concerns and long-term toxicity. The cost of developing and manufacturing these complex nanostructures can be prohibitive, and their scalability for widespread clinical use remains uncertain. In addition, conflicting results from existing studies highlight the variability of treatment outcomes, with some case reports of success, for example, Au@PDA@DOX nanoparticles achieved 80% tumor regression in prostate cancer models by DAMP release. Others fail due to tumor resistance or suboptimal nanoparticle delivery. For example, in hypoxic pancreatic tumors, graphene-based PTTs failed to elicit ICDs due to insufficient HSP70 release, whereas PDT resistance occurred in CSCs overexpressing the ABCG2 transporter; Only 0.7–5% of intravenously administered nanoparticles reach tumors, and the stromal barrier in fibroproliferative neoplasms further reduces accumulation by >50%. This underscores the need for more standardized and rigorous research methodologies to ensure consistent and reliable results.
In this review, we explain the mechanism by which phototherapy induces immunogenic cell death, as well as the advantages of using nanodelivery systems to deliver photosensitizers that trigger immunogenic cell death (Figure 1).
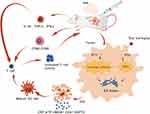 |
Figure 1 Immunogenic cell death induced by PDT/ PTT in vivo. |
Mechanisms of Immunogenic Cell Death Induced by Photodynamic Therapy
Photodynamic Therapy (PDT) is a therapeutic approach based on photosensitizers and light irradiation. When the photosensitizer is activated by light at a specific wavelength, it reacts with oxygen molecules, generating reactive oxygen species (ROS).14 The highly active ROS produced by these photosensitizers can cause irreversible damage to tumor cells, leading to cell death (Table 1). The first-generation photosensitizers are typically based on naturally occurring compounds, such as porphyrins and riboflavin. These photosensitizers can generate singlet oxygen (^1O2) upon irradiation with light at specific wavelengths, and induce target cell damage through oxidative reactions. The primary mechanism of action is through photoexcitation of the photosensitizer, generating ROS that directly leads to cell death. The drawbacks of the first-generation photosensitizers are their limited light absorption range and uneven distribution in the body, which can lead to non-specific damage. The second-generation photosensitizers have been chemically improved upon the first-generation, such as aluminum phthalocyanine. These photosensitizers usually have higher light absorption efficiency and stronger tumor selectivity, allowing them to accumulate more effectively in tumor tissues. The mechanism of action not only relies on ROS generation, but also involves interactions with cell membranes, mitochondria, and other cellular structures.15,16 The design of these photosensitizers aims to improve their accumulation in the tumor site, thereby reducing damage to normal tissues and enhancing the therapeutic effect. The third-generation photosensitizers are typically synthetic organic molecules or nanomaterials. These photosensitizers overcome the problems of hydrophobicity and targeting through active targeting (conjugation with tumor-targeting moieties) and passive targeting (utilizing the EPR effect, loading into nano-carriers), thereby improving the efficacy and safety of the treatment.
 |
Table 1 Representative PDT Photosensitizers and Their Mechanisms |
In photodynamic therapy, tumor cells die due to the generation of ROS, which in turn triggers an immune response. Photodynamic therapy causes tumor cell lysis, releasing tumor antigens that can activate dendritic cells (DCs). These DCs can recognize and engulf the tumor antigens, thereby activating T cells and promoting the development of a specific immune response. The generation of ROS can also promote the release of pro-inflammatory cytokines22 such as IL-1, IL-6, and TNF-α, which play an important role in the activation and proliferation of immune cells. After photodynamic therapy, the tumor-derived antigens are captured by DCs, which then present them to CD4+ and CD8+ T cells via MHC molecules, activating their proliferation and differentiation. T cell stimulation and proliferation are key to generating an effective immune response, the specific T cells can recognize and attack tumor cells. Photodynamic therapy can also promote the activation of natural killer (NK) cells, which can directly recognize and kill tumor cells, enhancing the anti-tumor immune response.
The photosensitizer plays a crucial role in the efficacy of PDT for tumor treatment. However, most photosensitizers have high hydrophobicity, low bioavailability, and poor tumor targeting, which may also damage normal tissues after administration, limiting their application in cancer treatment. Nano-delivery System can increase the solubility of photosensitizers, improve their stability, and enhance tumor targeting. Furthermore, Nano-delivery System can combine PDT with other cancer treatment modalities to improve the overall therapeutic effect.
Mechanisms of Immunogenic Cell Death Induced by Photothermal Therapy
Photothermal therapy (PTT) is a non-invasive cancer treatment that uses photothermal agents (PTAs) to convert light energy into heat energy23 and plays an important role in biomedicine (Table 2). When PTAs are exposed to near-infrared (NIR) light, they absorb photon energy, transitioning from the ground singlet state to the excited singlet state. The excited energy is then dissipated through non-radiative decay, such as vibrational relaxation, resulting in an increase in the kinetic energy of the surrounding molecules, thereby generating heat.24 When the tissue temperature reaches 41°C, the body initiates a heat shock response, producing heat shock proteins (HSPs) to resist the initial thermal damage.25 At 42°C, the tissue will undergo irreversible damage; maintaining the temperature at 42–46°C for 10 minutes can cause cell necrosis; further increasing the temperature to 46–52°C will lead to rapid cell death due to ischemia from microvascular thrombosis; and temperatures above 60°C can instantly cause cell death due to protein denaturation and membrane disruption.26 In the process of tumor photothermal therapy, the local tumor temperature is a critical factor determining the success of the treatment.27 On one hand, to achieve the desired photothermal therapeutic effect, the local tumor temperature typically needs to be raised above 50°C. On the other hand, such high temperatures may also damage the normal tissues surrounding the tumor and limit the depth of laser penetration. Therefore, mild photothermal therapy (MPTT) (<45°C) is gaining attention as it can minimize damage to normal tissues, although it may reduce the tumor-killing efficacy. Fortunately, combining MPTT with other drugs or therapies to create a multi-modal synergistic treatment system can compensate for this limitation. In this system, the performance of the PTA is crucial.
 |
Table 2 Representative PTT Photosensitizers and Their Mechanisms |
The ideal PTA should have high water solubility, good biocompatibility, high photothermal conversion efficiency, and good tumor targeting ability. These properties directly affect the efficacy of photothermal therapy. PTAs can be inorganic materials such as gold nanostructures33 and carbon-based materials, or organic materials such as cyanine dyes, all of which play important roles in tumor treatment. Photothermal conversion agents can be broadly classified into the following categories: 1) Metal nanoparticles,34–36 such as gold nanoparticles, which exhibit excellent photothermal conversion efficiency. Under near-infrared light irradiation, gold nanoparticles can effectively convert light energy into thermal energy, leading to a localized temperature increase that induces tumor cell death. 2) Carbon-based materials also possess good photothermal conversion properties. Their high thermal conductivity allows for rapid heat propagation under light illumination, enabling effective killing of tumor cells. Furthermore, their excellent biocompatibility and modifiability make them widely applicable in the biomedical field. 3) Polymer-based materials can have relatively high photothermal conversion efficiency. Their structures can be tuned through different synthesis methods to optimize their photothermal characteristics. Some of these materials are often derived from natural plants, exhibiting good biocompatibility and biodegradability, allowing for efficient thermal energy conversion under light exposure.
The mechanisms of immunogenic cell death induced by photothermal therapy primarily include the following aspects. Changes in cell membrane: Photothermal therapy increases the temperature of tumor cells, leading to changes in the structure of the cell membrane. The disruption of the cell membrane exposes tumor-associated antigens, which can be recognized by immune cells, thereby triggering a specific immune response. Studies have shown that after photothermal treatment, the morphological changes in the tumor cell membrane result in the formation of antigen particles that can be captured and processed by dendritic cells (DCs). Release of DAMPs:1 During the process of photothermal therapy, the death of tumor cells is accompanied by the release of a series of DAMPs, such as heat shock proteins (HSPs), high-mobility group box 1 (HMGB1), and adenosine triphosphate (ATP). These molecular signals can attract and activate immune cells. The release of DAMPs not only enhances the immune cells’ ability to recognize tumor antigens but also promotes the activation of immune cells, further strengthening the anti-tumor immune response. Antigen presentation: The released tumor antigens and DAMPs35 are engulfed and presented by dendritic cells to T cells. This process is a crucial step in activating the adaptive immune response. Dendritic cells present the antigens to cytotoxic CD8+ T cells and helper CD4+ T cells through their surface major histocompatibility complex (MHC)37 molecules, and the latter play an important role in enhancing the anti-tumor activity of T cells. Activation of immune cells: The immunogenic cell death induced by photothermal therapy not only causes the release of antigens and DAMPs from tumor cells but also directly activates immune cells. Through the released DAMPs, immune cells are activated and begin to attack the tumor cells. When T cells recognize infected or transformed cells, they release cytokines (such as interferon-γ), further enhancing the body’s immune response. Establishment of memory immune response: During the process of immunogenic cell death induced by photothermal therapy, the generated memory T cells can provide protection against future tumor recurrence.38 These memory cells can quickly recognize and attack the reappearing tumor cells, thereby reducing the risk of recurrence and improving the patient’s survival rate. Photothermal therapy not only can directly kill tumor cells but also can enhance the anti-tumor capacity of the immune system by the induction of immunogenic cell death. This comprehensive treatment strategy makes photothermal therapy a promising option in in improving treatment efficacy and reducing the risk of tumor recurrence.
Research on Nano Delivery for PDT-Induced ICD for Tumor Immunotherapy
Based on whether the endoplasmic reticulum (ER) is the primary target, ICD-inducing agents can be classified into type I and type II. Type I agents do not primarily target the ER, but induce mild ER stress and the release of immunogenic molecules in tumor cells. Radiotherapy and most chemotherapeutic drugs belong to this category. In contrast, type II agents selectively target the ER, triggering ER stress-dependent release of danger signals and apoptotic signals through reactive oxygen species (ROS)-dependent mechanisms,39 leading to more robust ER stress and higher ROS levels, and the release of more damage-associated molecular patterns, thereby generating a stronger anti-tumor immune response.40 In a study, Liu et al successfully constructed a Mn@CaCO3/ICG@siRNA nanometer platform that aims to enhance the effect of photodynamic therapy and inhibit the resistance/evasion of tumor cells by integrating PDT with immunotherapy (Figure 2A).41 The preparation of Mn@CaCO3/ICG nanoparticles involves the reduction of potassium permanganate by PAH with MnO2 nanoparticles as the core, followed by the modification of the pH-responsive CaCO3 layer on the surface of MnO2, and the simultaneous encapsulation of ICG molecules (Mn@CaCO3/ICG). The PD-L1-targeting siRNA is loaded onto a positively charged Mn@CaCO3/ICG through electrostatic interaction to form the final nanoplatform (Mn@CaCO3/ICG@siRNA). In the tumor microenvironment, the nanoplatform not only exhibits enhanced photodynamic therapy effects in vitro due to the protective effect of the CaCO3 layer on ICG and the oxygen produced by MnO2, but also effectively alleviates tumor hypoxia by decomposing endogenous hydrogen peroxide into oxygen.42 This dual action significantly boosts the production of reactive oxygen species (ROS) and promotes the release of damage-associated molecular patterns (DAMPs), thereby activating dendritic cells (DCs) and triggering a robust anti-tumor immune response. In vivo experiments further confirmed that the nanoplatform can effectively deliver drugs to tumor tissues and significantly improve tumor hypoxia, thereby enhancing the in vivo therapeutic effect of PDT. Additionally, the synergistic effect of siRNAs silences the checkpoint gene PD-L1, which mediates immune resistance/evasion, producing a therapeutic effect that awakens the immune system and leads to tumor cell apoptosis and necrosis, as illustrated in the cancer immunity cycle activation diagram.
 |
Figure 2 (A) A schematic illustration of the synthetic route of Mn@CaCO₃/ICG@siRNA and the mechanism of the nanoprobe-mediated photodynamic tumor immunotherapy in vivo. Reproduced from Liu Y, Pan Y, Cao W, Xia F, Liu B, Niu J, Alfranca G, Sun X, Ma L, de la Fuente JM, Song J, Ni J, Cui D. A tumor microenvironment responsive biodegradable CaCO₃/MnO₂-based nanoplatform for the enhanced photodynamic therapy and improved PD-L1 immunotherapy. Theranostics. 2019 Sep 21;9(23):6867-6884. © The author(s).Creative Commons Attribution License.41 (B) The cancer immunity cycle and roles of PDT and immune checkpoint inhibitors. Reproduced from Sasaki M, Tanaka M, Kojima Y, et al. Anti-tumor immunity enhancement by photodynamic therapy with talaporfin sodium and anti-programmed death 1 antibody. Molecular Therapy-Oncolytics. 2023;28:118–131. © 2023 The Authors. CC BY license.43 |
The cancer immunity cycle involves a series of steps that generate and amplify anti-cancer immune responses. Sasaki M et al elaborated that tumor cell killing by Tumor-Seeking Photodynamic Therapy (TS-PDT) initiates this cycle by promoting the release of neoantigens and the induction of Damage-Associated Molecular Patterns (Figure 2B).43 Specifically, TS-PDT employs light-activated photosensitizers to induce tumor cell death, which leads to the release of tumor-specific antigens and DAMPs.44 These molecules are then captured and processed by dendritic cells (DCs), which migrate to lymph nodes and present the antigens to T cells via major histocompatibility complex (MHC) molecules. This antigen presentation activates CD4+ helper T cells and CD8+ cytotoxic T cells, enhancing their proliferation and differentiation. The activated CD8+ T cells infiltrate the tumor microenvironment and recognize tumor cells expressing the presented antigens, leading to their destruction. Additionally, the release of pro-inflammatory cytokines such as IL-12 and IL-18 further amplifies the immune response, promoting the maturation and activation of DCs and the recruitment of more immune cells to the tumor site. The blockade of the anti-PD-1/PD-L1 pathway using immune checkpoint inhibitors further enhances the cytotoxic activity of T cells against cancer cells by preventing the suppression of T cell function mediated by these checkpoints.45 These steps collectively induce a synergistic effect, enhancing the overall antitumor immune response and leading to sustained tumor regression and improved patient outcomes. Lan et al46 constructed a novel nanophotosensitizer Fe-TBP, which consists of Fe3O clusters and 5,10,15,20-tetrakis (p-benzoate) porphyrin (TBP) ligands. The loading efficiency of Fe-TBP was reported to be 85%, and the release efficiency under hypoxic conditions was 90% after 2 hours of irradiation. This MOF can effectively stimulate the ICD effect of the tumor, thereby inducing cytotoxic T cells to infiltrate the tumor and generate an anti-tumor immune response.47,48 Fe-TBP-mediated PDT can also significantly enhance the efficacy of anti-programmed death ligand 1 (α-PD-L1) therapy, resulting in tumor regression of more than 90%. Other group designed a serum albumin (SA)-coated boehmite (“B”; aluminum hydroxide) organic-inorganic framework for loading the photosensitizers chlorin e6 (Ce6) and melittin (MLT) peptides, denoted as Ce6/MLT@SAB. The loading efficiency of Ce6/MLT@SAB was 92%, and the release efficiency under physiological conditions was 88% after 4 hours of irradiation. In vitro experiments demonstrated that compared with free MLT, Ce6/MLT@SAB could significantly reduce hemolysis, and MLT could enhance the inhibitory effect of PDT on tumor cells. Compared with Ce6@SAB, Ce6/MLT@SAB enhanced the penetration of Ce6 into cancer cells in vitro and in vivo, thereby enhancing the production of intracellular ROS. Ce6/MLT@SAB-treated cells showed significantly increased ICD levels and the ability to activate dendritic cells. Under laser irradiation, a single injection of Ce6/MLT@SAB eradicated one-third of subcutaneous tumors in treated mice. The combination of Ce6/MLT@SAB phototherapy with immune checkpoint blockade can further generate more CD4+ and CD8+ T cells in the tumor and reduce myeloid-derived suppressor cells, thereby enhancing the anti-tumor effect.49,50
The clinical efficacy of PDT is still limited by cancer stem cells (CSCs) within tumor tissues. CSC can not only regenerate itself or become ordinary cancer cells to resist the attack of drugs, but also repair itself after its own DNA damage, which makes the tumor difficult to treat and easily leads to tumor recurrence and metastasis. Recently, Zhao et al designed a nano-targeted micelle that combines olaparib (OLA), which hinders CSC DNA repair, dihydroporphyrin e6 (Ce6), which produces reactive oxygen species (ROS) under infrared light, and hyaluronic acid (HA), which can recognize CD44 receptors on the surface of CSC (Figure 3).51 HA can bind specifically to CD44 receptors overexpressed on the surface of CSCs, targeting the delivery of OLA-laden micelles (HCCOs) to CSCs within tumors. Ce6 enables PDT treatment of tumors by producing ROS, and induces tumor immunogenic cell death (ICD) and activates anti-tumor immunity, characterized by the release of calreticulin (CRT) and high-mobility group box 1 (HMGB1). These damage-associated molecular patterns (DAMPs) are recognized and processed by immature dendritic cells (DCs), promoting their maturation and activation. The mature DCs then present tumor antigens to CD4+ and CD8+ T cells, enhancing the anti-tumor immune response. The combination of Ce6-mediated PDT and OLA-mediated inhibition of DNA damage repair not only coordinates the enhancement of ICD action but also inhibits the infiltration of myeloid-derived suppressor cells (MDSCs), thereby effectively reversing the therapeutic resistance of CSC and improving the anti-tumor efficacy of PDT. This multifaceted approach leads to the death of CSCs, reduces tumor burden, and significantly inhibits the recurrence and metastasis of tumors, as demonstrated by the reduced incidence of metastatic lesions and tumor recurrence in animal models. The combination of Ce6-mediated PDT and OLA-mediated inhibition of DNA damage repair can coordinate the enhancement of ICD action and inhibit the infiltration of myeloid-derived suppressor cells (MDSCs),52 thereby effectively reversing the therapeutic resistance of CSC and improving the anti-tumor efficacy of PDT. It can effectively inhibit the recurrence and metastasis of tumors. PDT is crucial in treating various tumors. Let us examine some typical applications.53
 |
Figure 3 Schematic diagram of HCCO reversing drug resistance and enhancing PDT in cancer stem cells. (A) Preparation of HCCOs. (B) HCCO reverses the CSC immunosuppressive microenvironment and induces irreversible DNA damage to inhibit breast cancer recurrence and metastasis. Reproduced from Wang T, Tao J, Wang B, et al. Reversing resistance of cancer stem cells and enhancing photodynamic therapy based on hyaluronic acid nanomicelles for preventing cancer recurrence and metastasis. Adv Healthcare Mater. 2024;13(4):2302597. © 2023 Wiley-VCH GmbH.51 |
Melanoma
PDT, an immunotherapy approach, demonstrates effectiveness in tumor eradication with minimal surgical intervention, albeit its application in melanoma metastatic lesions is somewhat restricted. Marina M. Simões et al54 investigated the effect of photodynamic therapy (PDT) on mouse melanoma B16-F10 cells by preparing solid lipid nanoparticles and SLN containing photosensitizer aluminum phthalocyanine chloride (SLN-AlPc), The results showed that SLN-AlPc could significantly reduce the viability of B16-F10 cells, induce the production of ROS, promote ICD, activate DCs, and increase the production of pro-inflammatory cytokines under PDT treatment. Liao et al55 investigated a simple nanoparticle called HTCS, capable of co-delivering the STING agonist 2’3’-cyclic guanosine monophosphate-adenosine monophosphate (2’3’-cGAMP) and a mitochondria-targeting modified photosensitizer (TPP-PEI-Ce6). When intravenously administered to mice, HTCS was internalized by tumor cells through hyaluronic acid-mediated active targeting. Subsequently, TPP-PEI-Ce6 was delivered to the mitochondria, generating substantial ROS and efficiently eradicating tumor cells. The resultant tumor cell debris triggered immunogenic cell death, which played a pivotal role in modulating the immune response. Furthermore, the 2’3’-cGAMP present in the cell debris activated the STING pathway, enhancing the release of inflammatory cytokines and promoting the maturation of DCs.
Colorectal Adenocarcinoma and Lung Cancer
Xu et al56 studied the effect of PDT on HT29 human colorectal adenocarcinoma cells and A549 human lung cancer cells by designing zinc(II)phthalocyanine derivatives ZnPc-2NO and ZnPc-4NO. The experimental results showed that the two conjugates were able to inhibit mitochondrial respiration and reduce intracellular oxygen consumption by releasing nitric oxide (NO) under PDT treatment, thereby producing ROS and inducing cytotoxicity under hypoxic conditions. The photodynamic effect of ZnPc-2NO can trigger the release of damage-related molecular patterns, promote the maturation of dendritic cells, and trigger anti-tumor immune responses. This immunogenic cell death induced by oxygen-depleting PDT has been demonstrated by in vitro and in vivo experiments.
Colorectal Cancer
Currently, the mortality rate associated with advanced colorectal cancer remains high, and PDT holds promise as a treatment option for eradicating this disease. However, the efficacy of PDT is significantly hindered by tumor hypoxia. To address this challenge, He et al57 designed a core-shell gold nanocage coated with manganese dioxide and hyaluronic acid (AMH). This innovative nanostructure targets colorectal tumors for precise delivery and achieves in situ oxygenation, thereby enhancing the immunogenicity of phototherapy. Notably, AMH nanoparticles further boost the PDT efficacy of the system under near-infrared (NIR) irradiation by generating an abundant oxygen medium due to their weakly acidic properties. Additionally, AMH-based PDT induces immunogenic cell death (ICD) in tumor cells, accompanied by the release of damage-associated molecular patterns (DAMPs), which promotes the maturation of dendritic cells (DCs) and enhances systemic anti-tumor immunity against advanced tumors. In vivo experiments demonstrate that AMH nanoparticles not only possess tumor-targeting capabilities but also generate sufficient oxygen in situ to alleviate tumor hypoxia. Wang et al58 have successfully induced ICD in human colorectal cancer cell lines DLD-1 and HCT116 using sodium Chinese porphyrin (DVDMS) as a photosensitizer, in combination with oxaliplatin treatment and NIR laser irradiation. The combined therapy of DVDMS-derived PDT and oxaliplatin induced the release of ICD markers, such as increased ATP and HMGB1 levels in the supernatant, as detected by ELISA. The release of these DAMPs is indicative of immunogenic cell death, which activates DCs, promotes their maturation, and attracts cytotoxic T lymphocytes, ultimately enhancing the systemic anti-tumor immune response against advanced tumors. In vivo experiments further revealed that pretreatment of CT26 cells with DVDMS and subsequent injection into mice significantly inhibited primary tumor growth, suggesting that the combination of DVDMS-derived PDT and oxaliplatin also triggers immunogenic cell death in vivo. This finding offers a promising therapeutic strategy for the treatment of colorectal cancer.
Head and Neck Tumors
It is understood that the photobiological effects of PDT can be used to modulate tumor vasculature and stromal. PDT can also sensitize tumors to immunotherapy by inducing a cascade of molecular events, such as ICDs. Bhandari Chanda et al59 utilized liposomal benzoporphyrin derivatives in conjunction with 690 nm light to achieve dual objectives in mouse models with AT-84 head and neck tumors: enhancing the delivery of α-PD-L1 antibody by twofold and inducing ICDs in vivo. Notably, a significant increase, ranging from 3 to 11 times, in the exposure of tumor cells to ICDs displaying damage-associated molecular patterns (such as calreticulin, HMGB1, and HSP70) was observed. These findings collectively indicate that PDT holds promise for improving immunotherapy outcomes in head and neck cancer. This is achieved by addressing both the physical hurdles that impede the delivery of immune checkpoint inhibitors and the biochemical mechanisms that underpin immunosuppression.
Lung Cancer
Suppression of the immune microenvironment stands as a pivotal endogenous contributor to the inefficacy of lung cancer treatment. Yu et al60 conducted an in-depth exploration of the immunogenic impact of Photodynamic Therapy (PDT) on lung cancer, uncovering its underlying mechanisms. Their findings revealed that Chlorine e6 (Ce6)-based PDT not only augments the immunogenicity of lung cancer cells but also triggers apoptosis. Furthermore, additional experimental data demonstrated that Ce6-PDT generates Reactive Oxygen Species (ROS), which subsequently induce Endoplasmic Reticulum (ER) stress, facilitating the translocation of Calreticulin (CRT) to the cell membrane. This, in turn, triggers a DNA Damage Response (DDR) that leads to the expression and secretion of nuclear HMGB1 and HSP90, further potentiating the immunogenicity of lung cancer cells.
PDT can effectively activate the body’s anti-tumor immune response and enhance therapeutic efficacy by inducing ICD in various tumor cells.61 This not only provides a new approach for the application of PDT monotherapy, but also lays a theoretical foundation for the combined use of PDT and immunotherapy in tumor treatment. Compared to traditional cancer therapies, the advantages of photodynamic therapy (PDT) lie in its ability to provide precise and effective treatment, with relatively minor side effects.62,63 However, there are still some challenges and limitations in its practical application. Selectivity of photosensitizers: The selective accumulation of effective photosensitizers in tumor tissues remains a key issue. Improving the targeting of photosensitizers and reducing damage to normal tissues is one of the current research priorities. Limitations in treatment depth: The efficacy of PDT is limited by the penetration depth of light, especially when treating deep-seated tumors, where the depth of light penetration may be insufficient, affecting the treatment outcome. This problem needs to be overcome by improving light sources and techniques. Further exploration of the molecular mechanisms of PDT-induced ICD and optimization of the combination regimen of PDT-ICD and immunotherapy will undoubtedly bring new hope for improving the prognosis of cancer patients.
Research on Nano Delivery for PTT-Induced ICD for Tumor Immunotherapy
PTT has the ability of photothermal agents to absorb near-infrared light energy and convert it to thermal energy, causing thermal damage and ablation to tumor tissues or cells.64 Photothermal therapy can inhibit tumor growth and metastasis by activating the body’s immune effector cells and promoting the secretion of cytokines.65,66 Additionally, mild hyperthermia can also modulate the tumor microenvironment (TME), which is beneficial for the initiation of an immune response.37,67,68 Deng et al69 loaded the HSP90 inhibitor (SNX2112) onto an oxidized graphene carrier and performed NIR irradiation, which was found to downregulate the expression of HSP90 and PD-L1 in tumor cells, while upregulating the expression of the T cell activation marker CD69. This indicates that photothermal therapy can reactivate T cells and relieve the suppression of the immune system by tumor cells. The efficacy of tumor immunotherapy largely depends on the expression of PD-L1 in the tumor tissue and the recruitment of tumor-infiltrating lymphocytes (TILs).70 Li et al71 loaded the NIR photothermal agent IR820 and the programmed death ligand 1 (aPD-L1) antibody into a thermoreversible lipid hydrogel, and controlled the release of aPD-L1 by regulating the NIR irradiation. This strategy effectively increased the recruitment of TILs in the tumor microenvironment, enhanced T cell activity, and converted the “cold” tumor lacking lymphocytes into a “hot” tumor, thereby synergistically enhancing the efficacy of immunotherapy.35,72
In the field of cancer treatment, the combination of phototherapy and immune checkpoint blockade (ICB) has become an innovative treatment strategy. Yu et al described the relevant mechanism of phototherapy combined with ICB in the treatment of cancer in the study (Figure 4).73 First of all, phototherapy, combined with a nanoparticle-loaded photosensitizer or photothermal agent, can effectively enrich tumor tissues under laser irradiation. These nanoparticles produce ROS and thermal energy during phototherapy, which play a role in PDT and PTT, respectively, to induce ICD in tumor cells. The death of tumor cells leads to the release of tumor neoantigens and damage-associated molecular patterns (DAMPs) that further stimulate dendritic cell (DC) maturation and activate T cells. This results in the recruitment of CD4+ and CD8+ T cells into the tumor microenvironment. The combination with immune checkpoint inhibitors (ICIs), such as anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies,74,75 further enhances the cytotoxic activity of T cells against cancer cells. This synergistic approach effectively transforms immunologically “cold” tumors, which have minimal T cell infiltration and are less responsive to immunotherapy, into “hot” tumors characterized by increased T cell presence and activity.76 The enhanced immune response not only targets the primary tumor but also has the potential to combat metastatic lesions, offering a promising therapeutic strategy for a wide range of cancer types.77
 |
Figure 4 Schematic of phototherapy combined with ICB in cancer treatment. Reproduced from Yujie Z, Xu L, Xinyu L, et al. Combination of phototherapy with immune checkpoint blockade: theory and practice in cancer. Front Immunol. 2022;13:955920. Copyright © 2022 Zhao, Liu, Liu, Yu, Bai, Wu, Guo, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY).72 |
In a recent study, researchers discovered that a dual-targeted nano delivery system known as GOx@FeNPs, in conjunction with an αPD-L1 immune checkpoint blocker, exhibits potent inhibitory effects on colorectal cancer (CRC) progression. This inhibitory action is mediated through PTT, the induction of ferroptosis, and the stimulation of an anti-tumor immune response. Enhancing the efficacy of PD-1/PD-L1 therapy for CRC hinges on the effective induction of immunogenicity and the disruption of the immunosuppressive tumor microenvironment. Jin et al78 developed a corn-like Au/Ag nanorod (NR) that can induce ICD in tumor cells under 1064 nm light irradiation. The loading efficiency of Au/Ag NRs was 78%, and the release efficiency under NIR-II light irradiation was 85% after 3 hours. Au/Ag NRs under NIR-II light irradiation triggers immunogenic cancer cell death. The in vivo results showed that this treatment strategy significantly increased the tumor infiltration of T cells and triggered a systemic immune response to reverse the tumor immune microenvironment, transforming cold tumors into hot tumors; synergistic treatment with ICB antibodies, especially aCTLA4, can effectively inhibit distant tumors growth. This therapeutic strategy elicited a powerful immune memory effect that prevented tumor recurrence. Researchers used gold nanorods to design a combination therapeutic drug-gold nanorod (AuNR)/doxorubicin (DOX) gel, in which the gel can control the persistence of DOX freed. The loading efficiency of AuNR/DOX gel was 82%, and the release efficiency under 880 nm laser irradiation was 80% after 2 hours. DOX acts as an inducer of immunogenic tumor cell death (ICD), triggering the production of damage-associated molecular patterns (DAMPs). Mild photothermal therapy (Mild PTT) generated by 880 nm laser-irradiated AuNRs also produced tumor-associated antigens. To further promote antigen presentation, the researchers designed maleimide-modified liposomes (L-Mals) as antigen capture agents to promote the uptake of tumor antigens by DCs. The results of the study showed that CD8+ T cells in tumor tissues were significantly increased and regulatory T cells (Tregs) were significantly decreased under the effect of combination therapy, and this immune response enhanced the anti-tumor response of PD-L1 antibody. The findings demonstrate that this combination strategy based on gold nanoparticles promotes the efficiency of cancer immunotherapy. However, it is noteworthy that the immunogenicity associated with cell death induced solely by PTT is generally mild.79,80 To address this challenge, the study employed a synergistic strategy by incorporating ferroptosis, a newly identified and distinct form of regulated cell death that offers unique advantages in antitumor therapy. Li et al (Figure 5A)81 found in the study that the nanotherapy platform Fe3O4 obtained @PDA-PEG-cRGD-AA@Gox (abbreviated as GOx@FeNPs), possesses dual-targeting capabilities, photothermal conversion properties, the ability to induce ferroptosis, and magnetic resonance imaging (MRI) functionality. Once GOx@FeNPs actively bind to the tumor site, the individual components are thermally released under the influence of a near-infrared (NIR) laser. The Fe3O4@PDA component of the nanotherapy platform demonstrates robust photothermal conversion properties, which are capable of inducing apoptosis and immunogenic cell death (ICD) in tumor cells. This process involves the generation of reactive oxygen species (ROS) and the induction of ferroptosis, a form of regulated cell death characterized by lipid peroxidation and iron accumulation. The extensive release of tumor-specific antigens and damage-associated molecular patterns (DAMPs), such as calreticulin (CRT), ATP, and high-mobility group box 1 (HMGB1),82 resulting from ICD and ferroptosis, promotes the maturation of dendritic cells (DCs) and their migration to lymph nodes. Here, mature DCs present tumor antigens to T cells, activating their proliferation and differentiation into cytotoxic T lymphocytes (CTLs).83 These activated CTLs infiltrate the tumor microenvironment, leading to the recognition and killing of cancer cells. Additionally, the combination of photothermal therapy with immune checkpoint blockade further enhances T cell activity and tumor cell killing. This multifaceted approach not only directly destroys tumor cells but also stimulates a systemic immune response, effectively inhibiting tumor growth and reducing the risk of metastasis.84
Immunotherapy has become one of the important means of cancer treatment,85 the photothermal therapy inducing immunogenic death of tumor cells, releasing a large number of tumor antigens, and activating the body’s immune system, thereby inhibiting further tumor growth. Photothermal immunotherapy can directly kill local tumor cells, also stimulate systemic anti-tumor immune responses, inhibiting the growth and metastasis of the primary tumor. Compared with traditional surgery, chemotherapy, and radiotherapy, photothermal immunotherapy has the advantages of small trauma, low side effects, and long-lasting treatment effects. It is especially advantageous for some refractory tumors, playing an important role in the treatment of various immune-related diseases. Let us discuss several typical application cases in detail.
Breast Cancer
Zhu et al86 introduced a multifaceted nanoplatform, termed IDNP, that integrates chemophotothermal therapy by seamlessly blending therapeutic drugs with photothermal converting agents. This innovative approach is designed to eradicate primary tumors and simultaneously induce ICD. The cell death triggered by IDNP is accompanied by the liberation of damage-associated molecular patterns (DAMPs), which includes the translocation of calreticulin (CRT) from the endoplasmic reticulum (ER) to the cell membrane, the release of HMGB1 protein from the nucleus, and the secretion of adenosine triphosphate (ATP). These DAMPs significantly amplify the immunogenicity of cancer cells, thereby initiating a series of cellular responses that convert breast cancer from a non-immunogenic “cold” tumor into an immunogenic “hot” tumor. Consequently, this transformation activates a robust anti-tumor immune response. Zheng et al87 developed a PTT-induced feed-back carbon nanosystem, termed LCTi, for enhanced breast cancer therapy by extracellular matrix remodeling. This innovative approach aims to address the issue that the dense extracellular matrix (ECM) in breast cancer severely impedes drug delivery and immune cell infiltration, resulting in poor therapeutic effects.After intravenous injection, LCTi accumulates in the tumor through iRGD-mediated active targeting and subsequently destroys tumor cells and induces immunogenic cell death (ICD) under 808 nm laser irradiation. Simultaneously, losartan is photothermal-responsively released from LCTi to remodel the ECM. This process alleviates hypoxia and improves the tumor immune microenvironment, thereby enhancing the efficacy of photothermal therapy (PTT). Focusing on ECM remodeling, this study provides an attractive “PTT-reinforced PTT” feed-back strategy for future breast cancer therapy.
Neuroblastoma
Neuroblastoma is a malignant tumor that originates in the nervous system, usually in infants or young children, and is one of the most common solid tumors in children. Juliana Cano-Mejia et al88 utilized a nanoparticle called CpG-PBNP, which induces ICD by binding PTT and the immunoadjuvant CpG. CpG-PBNP nanoparticles release CpG, a DNA sequence capable of activating the immune system, under specific conditions, such as pH changes or photothermal effects. The liberation of CpG plays a pivotal role in activating dendritic cells (DCs), which subsequently fosters the proliferation and activation of tumor-specific T cells. In our current study, tumor cells treated with CpG-PBNP-PTT released damage-associated molecular patterns (DAMPs), including ATP, HMGB1, and calreticulin. These DAMPs are capable of stimulating the immune system and eliciting a potent immune response against tumors.This immune response helps to remove residual tumor cells and may generate immune memories that prevent tumor recurrence. In summary, CpG-PBNP nanoparticles effectively induce ICD by binding PTT and the release of the immunoadjuvant CpG, and have shown significant anti-tumor effects in animal models.
Prostate Cancer
Prostate cancer is a common male malignancy that complicates treatment when it progresses to metastasis, especially when bone metastases occur, as tumors often exhibit multidrug resistance. Ran et al89 used a dopamine coating to enhance the photothermal conversion rate of Au and grafted the chemotherapy drug doxorubicin (DOX) onto the dopamine coating. Au@PDA@DOX nanoparticles generate heat when irradiated with near-infrared light, which directly kills tumor cells through thermal ablation. At the same time, thermal ablation also promotes immunogenic cell death, causing tumor cells to release damage-related molecular patterns (DAMPs), such as ATP, HMGB1, and calreticulin, thereby activating the immune system and triggering an immune response against tumors. By activating dendritic cells (DCs) and promoting the proliferation and activation of tumor-specific T cells, Au@PDA@DOX nanoparticles effectively activate anti-tumor immune responses in vivo, inhibit tumor growth, and alleviate tumor damage to bone. Au@PDA@DOX nanoparticles effectively induce ICD by binding PTT and drug release, and have shown significant anti-tumor effects in animal models, providing a new therapeutic strategy for bone metastasis of prostate tumors.
Melanoma
Sun et al80 developed a novel NIR-dependent IDO-inhibiting ethosomes (INES) for treating melanoma through the synergy of PTT/PDT/immunotherapy. This innovative approach leverages the combination of phototherapy and immunotherapy to significantly enhance the treatment of melanoma. INES is composed of the photosensitizer IR251 and the Indoleamine-2,3-dioxygenase (IDO) inhibitor NLG919. Under 808 nm laser irradiation, INES demonstrates excellent phototherapeutic properties, strong phototoxicity, and a notable ability to inhibit IDO. It effectively induces immunogenic cell death (ICD) in melanoma cells, releasing damage-associated molecular patterns (DAMPs) such as calreticulin (CRT) and high mobility group box 1 protein (HMGB1), which promote the maturation of dendritic cells (DCs) and activate naive T cells to produce effector T cells (specifically CD4⁺ and CD8⁺ T cells) that target and kill tumor cells. In vivo experiments show that INES injection into primary tumors not only treats the primary tumor but also exerts a strong inhibitory effect on distant tumors, with favorable biosafety. In conclusion, INES represents a promising strategy for melanoma treatment through the combination of phototherapy and immunotherapy, providing new insights and a theoretical basis for the clinical treatment of melanoma.
Colorectal Cancer
Li et al90 have engineered a sophisticated photothermal Fe₃O₄ nanoparticle-based nanoplatform, designated as GOx@FeNPs, to orchestrate a synergistic therapeutic strategy for colorectal cancer (CRC) by inducing immunogenic ferroptosis and leveraging photothermal therapy (PTT). This advanced system integrates PTT with ferroptosis to surmount the immunosuppressive tumor microenvironment (TIME) and amplify the efficacy of cancer immunotherapy. The GOx@FeNPs system employs dual-targeting ligands—cyclic arginine-glycyl-aspartate (cRGD) peptide and anisamide (AA)—to facilitate precise delivery to tumor sites. Upon irradiation with near-infrared light, these nanoparticles exhibit robust photothermal conversion efficiency, enabling effective PTT and the induction of immunogenic cell death (ICD). Simultaneously, the depletion of intracellular glutathione (GSH) and catalysis of reactive oxygen species (ROS) generation from endogenous H₂O₂ further potentiate the Fenton reaction, thereby enhancing the ferroptotic process. This dual mechanism results in the release of tumor-specific antigens, which not only stimulate dendritic cell (DC) maturation in lymph nodes but also promote the infiltration of CD8⁺ cytotoxic T lymphocytes into the tumor microenvironment, thereby orchestrating a robust anti-tumor immune response. In conjunction with αPD-L1 immune checkpoint blockade, the GOx@FeNPs system achieves a tumor inhibition rate exceeding 90%, underscoring its potent synergistic efficacy against CRC. Moreover, the presence of Fe³⁺ within the nanoparticles endows GOx@FeNPs with superior T2-weighted magnetic resonance imaging (MRI) capabilities, facilitating real-time monitoring and integrated diagnostic-therapeutic applications for CRC management. By constructing this dual-targeted GOx@FeNPs nanoplatform, the study demonstrates a paradigm-shifting approach where PTT is synergistically combined with ferroptosis to enhance immunotherapeutic outcomes. This innovation not only addresses the challenges posed by the immunosuppressive tumor microenvironment but also provides a novel and highly effective strategy for the immunotherapy of colorectal cancer, paving the way for future translational applications in clinical oncology.
PTT has shown great promise, yet it also faces several limitations.91,92 Light source selection and control: PTT relies on specific wavelengths of light to activate photothermal agents, generating heat to kill cancer cells.92 Selecting the appropriate light source and ensuring effective irradiation within the tumor tissue is a challenge. Different light sources vary in terms of penetration depth, tissue selectivity, and energy output, and how to choose the optimal light source to maximize the therapeutic effect is an ongoing research topic.93,94 Selection and optimization of photothermal agents: The type and characteristics of photothermal agents directly impact the treatment efficacy.95,96 Currently, nanoparticles are widely used in PTT, but the biocompatibility, stability, and in vivo distribution and clearance of different materials still require further research and optimization. Additionally, the performance of photothermal agents in the tumor microenvironment and their interactions with the host immune response need to be thoroughly investigated. Combination of multiple treatment modalities: Combining PTT with other treatment modalities (such as immune checkpoint blockade) may produce synergistic effects and improve anti-tumor efficacy.97,98 However, how to effectively integrate multiple treatment modalities to optimize the treatment regimen is still an urgent problem to be solved.
Combination of Phototherapy and Chemotherapy to Induce ICD for Tumor Treatment
Combination therapy is gaining more attention in tumor treatment, especially the combined use of photodynamic therapy, photothermal therapy, and chemotherapy. Using PDT and PTT together with chemotherapy not only boosts the anti-tumor effects but also alters the way tumor cells die, particularly in inducing immunogenic cell death. The diagram below illustrates the process of how these three therapies work together to induce immunogenic cell death (Figure 5B). The combination therapy begins with the application of PDT and PTT, which generate reactive oxygen species (ROS) and thermal energy, respectively. These agents directly kill tumor cells and induce ICD, leading to the release of damage-associated molecular patterns (DAMPs) such as calreticulin (CRT), ATP, and HMGB1. Simultaneously, chemotherapy drugs enhance the cytotoxicity and further disrupt tumor cells, releasing additional tumor-specific antigens. The released antigens and DAMPs are captured by immature dendritic cells (DCs), prompting their maturation. Mature DCs then migrate to lymph nodes, where they present the antigens to T cells, stimulating their activation and proliferation. This results in the recruitment of CD4+ and CD8+ T cells into the tumor microenvironment, leading to the recognition and killing of cancer cells. The synergy between PDT, PTT, and chemotherapy not only increases the efficiency of tumor cell death but also amplifies the immune response. The release of tumor antigens and DAMPs from dying cells activates the innate and adaptive immune systems, creating a sustained anti-tumor effect. Additionally, the combination therapy can reduce the dose of individual treatments, minimizing side effects while maintaining or even enhancing therapeutic efficacy.42 This multi-modal approach offers a promising strategy for overcoming treatment resistance and improving outcomes in various cancer types.
 |
Figure 5 (A) Schematic representation of GOx@FeNPs-facilitated PTT synergizing with ferroptosis through the induction of ICD to enhance colorectal cancer immunotherapy. Reproduced from Li Y, Chen J, Xia Q, et al. Photothermal Fe3O4 nanoparticles induced immunogenic ferroptosis for synergistic colorectal cancer therapy. J Nanobiotechnol. 2024;22(1):630. Copyright © 2024, The Author(s). Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.80 (B) The process of inducing cell immunogenicity death by PDT/PTT/chemotherapy. |
Studies have shown that when photodynamic therapy is combined with chemotherapy, tumor cell mortality is significantly increased. This combination not only improves the efficiency of cell death, but also shortens the time to treatment.99 For example, the action of chemotherapy drugs can make tumor cells more susceptible to attack by photodynamic therapy, allowing for synergies. He and colleagues100 successfully synthesized core-shell nanoparticles called NCP, which co-delivered PS pyrolipid and OXA for combination therapy of PDT and chemotherapy. The loading efficiency of NCP was 88%, and the release efficiency under light irradiation was 85% after 3 hours. Pyrolipid-triggered PDT with OXA releasing induced immune responses, resulting in the inhibition of distant tumors in bilateral syngeneic mouse tumor models of CT26 and MC38. The results of in vitro experiments demonstrated NCP@pyrolipid with light irradiation could induce ICD with secretion levels of CRT, IFN-γ, IL-6, TNF-α significantly increased. Liu and colleagues101 have discovered a series of novel small-molecule inhibitors targeting programmed death-ligand 1 (PD-L1), which exhibit potent in vivo anti-tumor immune activity. These inhibitors hold significant promise for cancer immunotherapy due to their remarkable inhibitory activity against the PD-1/PD-L1 interaction and their ability to effectively counteract the immunosuppressive effects of PD-L1 on T cells. Crystallographic studies have elucidated the binding mode of the lead compound X18 with PD-L1, providing critical insights into the molecular mechanisms underlying its high affinity and specificity. Through rational prodrug design, the team has successfully optimized the oral pharmacokinetic properties of X22, addressing the common issue of poor oral bioavailability associated with small-molecule PD-L1 inhibitors. This optimization is crucial for enhancing the therapeutic potential of these inhibitors in clinical settings. Notably, X22 demonstrated robust antitumor efficacy in murine models of MC38 and CT26 colon cancer. The mechanism of action involves the upregulation of tumor infiltration and cytotoxicity of CD8⁺ T cells, partially contributing to the observed therapeutic effects. These findings underscore the potential of these novel PD-L1 inhibitors to significantly improve the outcomes of cancer immunotherapy by enhancing the body’s immune response against tumors. Overall, this study offers a significant advancement in the development of small-molecule PD-L1 inhibitors, providing a strong foundation for their further exploration as innovative agents in the field of cancer immunotherapy. Combination therapy can promote the occurrence of immunogenic death. Photodynamic therapy and photothermal therapy can increase the release of these immune signals while inducing cell death, thereby improving the immune system’s ability to recognize and eliminate tumor cells. Combination therapies can affect the death pathways of tumor cells. Different treatments may act on tumor cells through different mechanisms.102,103 For example, chemotherapy typically induces apoptosis, while photodynamic therapy may cause cell death through mechanisms such as necrosis or autophagy. Therefore, the implementation of combination therapies may cause tumor cells to experience multiple pathways of death, thereby increasing the effectiveness of treatment.104 Tumor cells often become resistant to a single therapy, and combination therapies can reduce this risk through multi-targeted action.105 Studies have shown that the combination of treatments with different mechanisms can effectively overcome drug resistance in tumor cells and improve the success rate of treatment.106 Combination therapy can reduce the dose of chemotherapy drugs while still maintaining a good therapeutic outcome, and improves the overall tolerability and quality of life of patients.107 Combination therapies can activate both tumor-specific and non-specific immune responses. This dual immune activation allows patients to obtain stronger anti-tumor immunity after treatment, reducing the risk of tumor recurrence.108
Combination of Photodynamic Therapy and Photothermal Therapy
The combination of photodynamic therapy and photothermal therapy can produce a variety of synergistic effects. For example, photothermal therapy can improve the accumulation and penetration of photosensitizers at tumor sites. This combination not only improves the mortality of tumor cells, but also reduces the side effects. Combined therapy can also trigger immunogenic death, activating the body’s immune response, further enhancing the effect of treatment. Monotherapy with either PTT or PDT often provides suboptimal results, whereas the combined treatment of the two modalities has clear advantages.
PDT utilizes photosensitizers that react with oxygen to generate reactive oxygen species, thereby inducing apoptosis of tumor cells. However, the strong reducing glutathione (GSH) in the tumor microenvironment can neutralize ROS, and the hypoxic nature of tumors can also compromise the efficacy of PDT. The localized hyperthermia generated by PTT can increase oxygen penetration in the tumor site, alleviating tissue hypoxia and enhancing the effects of PDT. Some photothermal agents have dual functions of both PTT and PDT,109 such as the Pd@Au-Ce6/MnO2 nanoplatform, where MnO2 generates oxygen to reverse tumor hypoxia and amplify PDT, while the thermal effects of Pd@Au and Ce6 mediate PTT. Additionally, other strategies have been explored to overcome tumor hypoxia, such as the PDA-Pt-CD@RuFc nanoplatform where platinum catalyzes oxygen generation, and photothermal-induced vasodilation.110 Similarly, encapsulating Ce6 within NIR-absorbing Au/Ag-modified hollow mesoporous MnO2 allows MnO2 to generate ROS for PDT while using acid-sensitive control of Ce6 release. Furthermore, incorporating porphyrin IV within polymeric materials can trigger the degradation of the polymer under PTT to synergistically activate PDT.
Although the nanomaterials used for combined PTT/PDT therapy still have some limitations, such as complex fabrication and poor stability,111 the advantages of single-wavelength-activated PTT/PDT are evident.112 Recently, some new conjugated small molecules like IID-Th TPA and DPP-BDT NPs have been successfully applied in single-wavelength-activated synergistic PTT/PDT, demonstrating promising photothermal and photodynamic therapeutic effects, warranting further exploration.113 In summary, rationally designed PTT and PDT combination therapies can fully leverage the advantages of both modalities, effectively overcoming the defects of the tumor microenvironment, and significantly enhancing anti-cancer treatment outcomes. This combination therapy strategy provides a new approach for precise tumor treatment.
Combination of Photodynamic Therapy and Chemotherapy
The combined use of photodynamic therapy and chemotherapy aims to enhance the therapeutic effect through the synergistic action of the two treatment modalities, while reducing the side effects of chemotherapy. PDT can increase the concentration of drugs in the tumor tissue by utilizing the accumulation of photosensitizers in the tumor tissue and cells. This can significantly reduce the damage to normal cells caused by chemotherapeutic drugs. During chemotherapy, tumor cells may develop resistance to the chemotherapeutic drugs. PDT can induce cell death and suppress the proliferation of resistant cells, thereby enhancing the effect of chemotherapy. This combination strategy helps to reduce the occurrence of drug resistance and improve the overall therapeutic efficacy. PDT can induce immunogenic cell death, leading to better presentation of tumor antigens and triggering the body’s immune response. By combining with chemotherapy, the cross-presentation of tumor antigens can be further enhanced, improving the immune surveillance capability of the host. PDT has a strong targeting capability, as it can activate the photosensitizers under specific wavelengths of light. This targeting feature allows PDT to be combined with chemotherapeutic drugs to achieve a more precise treatment regimen, reducing the damage to normal tissues. The integration of photodynamic therapy with chemotherapy has demonstrated promising application prospects.
Triple Negative Breast Cancer Cells
Beyzanur Erk114 conducted an investigation into the effectiveness of cisplatin-based chemotherapy in combination with 5-aminolevulinic acid (5-ALA)/ PDT in vitro, focusing on TNBC (triple-negative breast cancer) cells and healthy breast cells. The results revealed a significant reduction in the viability of MDA-MB-231 TNBC cells when treated with the combined therapy, compared to when either cisplatin or 5-ALA/PDT was used as a monotherapy. These findings hint at the potential of cisplatin and 5-ALA/PDT simultaneous combination therapy as a novel and promising strategy for TNBC treatment. However, additional studies are needed to delve deeper into the molecular mechanisms underlying the efficacy of this combined treatment approach.
Prostate Cancer
A novel therapeutic-diagnostic agent, PSMA-1-MMAE-Pc413, has been developed by Aditi A. Shirke,115 integrating a PSMA-targeting ligand, the photosensitizer Pc413, and the microtubule inhibitor Monomethyl auristatin E (MMAE) to achieve synergistic therapeutic effects in the treatment of prostate cancer. Studies on in vitro uptake have revealed that PSMA-1-MMAE-Pc413 exhibits selective and specific uptake exclusively in PSMA-positive PC3pip cells, as opposed to PSMA-negative PC3flu cells. Cytotoxicity assays conducted in vitro have demonstrated that PSMA-1-MMAE-Pc413 exhibits a synergistic effect upon exposure to light. Furthermore, in vivo imaging studies have shown the selective uptake of PSMA-1-MMAE-Pc413 in tumors. An in vivo study has also indicated that PSMA-1-MMAE-Pc413 significantly enhances treatment outcomes for prostate cancer by offering a synergistic effect that surpasses the efficacy of each individual treatment modality within tumors. These findings hint at the strong potential of combining photodynamic therapy with chemotherapy for clinical application in improving the treatment of prostate cancer.
Hepatocyte (HCC) Orthotopic Xenograft Model
Recently, the combination of PDT with concurrent chemotherapy has exhibited synergistic effects, resulting in superior cancer treatment outcomes. In a study conducted by Park Jae Sun et al,116 a patient-derived orthotopic xenograft (PDoX) model was employed to assess the efficacy of combining chemotherapeutic agents with PDT using indocyanine green (ICG) both in vitro and in vivo. The researchers independently administered sorafenib or doxorubicin for PDT and chemotherapy in Huh-7 and Hep3B cell lines, systematically increasing the concentrations of these drugs and the total energy of 808 nm light. Their findings revealed a significant and rapid decrease in the viability of Huh-7 and Hep3B cells as the concentrations of sorafenib or doxorubicin and the total energy of 808 nm light were incremented. Notably, the cell viability of Huh-7 and Hep3B cell lines when treated with the combined therapy of PDT and chemotherapy was notably lower compared to those treated with either PDT or chemotherapy alone.
Cholangiocarcinoma
Chen et al117 conducted an umbrella review on adjuvant photodynamic therapy (PDT) for the palliative treatment of cholangiocarcinoma, systematically evaluating existing meta-analyses and clinical studies to refine survival outcomes. Cholangiocarcinoma is a rare and often fatal malignancy, with limited treatment options, especially for unresectable cases. Numerous studies have shown promising outcomes and survival rates associated with PDT as an adjuvant therapy. The findings indicated that combining PDT with stenting or chemotherapy significantly improves overall survival and reduces mortality rates in cholangiocarcinoma patients, without increasing the risk of adverse events such as cholangitis or abscess formation. For extrahepatic cholangiocarcinoma, adding PDT to stenting notably improves the 2-year survival rate, while for hilar cholangiocarcinoma, the addition of chemotherapy to PDT enhances the 1-year survival rate. The current evidence suggests that PDT combined with stenting or chemotherapy decreases overall mortality and enhances overall survival without increasing the incidence of adverse events, highlighting its potential as a valuable component in the palliative management of cholangiocarcinoma.
Breast Cancer
Gao et al118 have developed a sophisticated multifunctional nanoplatform, termed CuS-EPI-AIPH@SF-PDA-FA, for the synergistic treatment of breast cancer through the integration of photothermal therapy (PTT), photodynamic therapy (PDT), and chemotherapy. This innovative approach leverages the unique properties of copper sulphide (CuS) nanoparticles and polydopamine (PDA) to address the limitations of conventional breast cancer therapies, such as drug resistance and systemic toxicity. The CuS-EPI-AIPH@SF-PDA-FA nanoparticles were synthesized using a microfluidic-assisted method, encapsulating the chemotherapeutic drug Epirubicin (EPI), the PTT/PDT agent CuS, and the heat-activated, oxygen-independent alkyl radical generator AIPH. The nanoparticles were coated with polydopamine (PDA) to enhance photothermal effects and functionalized with surface-bound folic acid (FA) for targeted delivery to breast cancer cells. The synthesized nanoparticles achieved a controlled size of 378 nm, exhibiting strong near-infrared (NIR) absorption and high photothermal conversion efficiency. Under 808 nm NIR irradiation, these nanoparticles selectively triggered the release of alkyl radicals and Epirubicin, significantly improving intracellular drug levels and effectively killing various breast cancer cell lines while demonstrating low toxicity to non-cancerous cells.
This study demonstrates that the novel core–shell CuS-EPI-AIPH@SF-PDA-FA nanoparticles represent a multifunctional nanoplatform that integrates PTT, PDT, and chemotherapy for targeted, synergistic breast cancer treatment. This approach not only overcomes the limitations of single-modality treatments but also enhances therapeutic efficacy by leveraging the combined strengths of multiple therapeutic modalities.
In conclusion, the combination of photodynamic therapy and chemotherapy is a promising cancer treatment strategy. By combining the advantages of both, it is possible to not only improve the effectiveness of treatment, but also reduce side effects and enhance the patient’s immune response. With the deepening of research and the promotion of clinical application, this combination therapy is expected to become a more effective cancer treatment option, bringing better prognosis and quality of life to patients.
Combination of Photothermal Therapy and Chemotherapy
The combination of photothermal therapy and chemotherapy is mainly through synergistic effects to enhance the anti-tumor effect. Chemotherapy drugs usually work by inhibiting the proliferation of tumor cells and inducing cell death, but their clinical application is often limited by drug resistance and toxic side effects. The introduction of photothermal therapy has opened up a new possibility for chemotherapy.107 Synergistic enhancement effect: Photothermal therapy can improve the sensitivity of tumor cells to chemotherapy drugs. For example, after photothermal therapy, the growth rate of tumor cells may be slowed, reducing drug resistance, making subsequent chemotherapy more effective. Immunogenic death: Photothermal therapy can not only directly kill tumor cells, but also induce tumor cells to release tumor-related antigens and stimulate the body to produce specific anti-tumor immune responses. This ICD can be combined with chemotherapy to further enhance the immune system’s recognition and attack on the tumor.99 Reduce toxic side effects: The local therapeutic properties of photothermal therapy enable it to reduce the systemic effects of chemotherapy drugs to a large extent, thereby reducing toxic side effects. The combination of photothermal therapy and chemotherapy has shown promising prospects in multiple tumor types. Studies have shown significant improvements in tumor shrinkage and survival in combination therapy strategy.
Breast cancer: Sun et al119 developed a drug delivery system that combines the local administration of Lycium barbarum polysaccharides (LBP) and photothermal material polypyrrole nanoparticles (PPY NPs). In vitro cytotoxicity experiments showed that under NIR (near-infrared) laser irradiation, NPs inhibited 4T1 cells more effectively than the same concentration of DOX alone (64% vs 8%). The in vivo anti-tumor experiment demonstrated that the tumor inhibition rate reached 87.86%. H&E staining and biochemical assays revealed that the reduced systemic toxicity, with significantly decreased liver damage. In summary, the combination of NPs and photothermal therapy can enhance the therapeutic effect of DOX on breast cancer while reducing its side effects.
Liver cancer: Gong et al120 grafted thyrocalcitonin (TCA) and folic acid (FA) on Fe3O4-modified graphene oxide (MGO), and successfully loaded DOX to form MGO-TCA-FA@DOX composite nanomaterials. In vitro and in vivo experiments have proved that the nanomedicine carrier can specifically target hepatocellular carcinoma cells with significant killing effect, and the tumor inhibition rate of photothermal therapy/chemotherapy synergistic therapy is about 85%, which is significantly higher than that of monotherapy. MGO-TCA-FA@DOX nanomaterials have a variety of HCC targeting, multifactor-triggered drug release, high drug loading rate, excellent photothermal conversion performance and good biocompatibility, providing a good platform for the synergistic treatment of HCC with photothermal therapy/chemotherapy.
Lung cancer: Li et al121 rationally designed a targeted nanomedicine that incorporates intelligent reactive polydopamine (PDA) for the delivery of cinobufagin, aiming to enhance its anti-cancer efficacy in lung cancer treatment. This nanomedicine is modified with folic acid, which mediates tumor targeting by interacting with the folic acid receptor present on tumor cells. Once inside the lysosomal compartment, the PDA nanodrug responds to the low pH environment, triggering the release of its payload into the tumor microenvironment. When used in conjunction with photothermal therapy, this nanomedicine demonstrates an even more pronounced therapeutic effect on lung cancer. Maryam Deinavizadeh et al122 have proposed an innovative synergistic approach for lung cancer treatment by utilizing gold nanorods (AuNRs) loaded onto sulfhydryl-functionalized mesoporous silica, combined with chemophotothermal therapy. Notably, these nanocomposites exhibit dual-responsive drug release behavior to both pH and near-infrared (NIR) light, enabling precise and targeted drug delivery. In addition, they exhibit exceptional biocompatibility and efficient internalization by A549 lung cancer cells. The combination of AuNR@S-MCM-41-DOX with photothermal chemotherapy has shown superior cancer cell killing efficacy compared to either chemotherapy or photothermal therapy alone.
Based on the above discussion, we have summarized the following table for classification according to tumors (Table 3).
 |
Table 3 Various Tumors and Their Corresponding Phototherapy Treatments |
In summary, the combination of photothermal therapy and chemotherapy provides a new idea and direction for cancer treatment. Although the combination of photothermal therapy and chemotherapy has achieved some success in clinical application, there are still some challenges. Technology optimization: Improve the selectivity and safety of photothermal agents, and develop more efficient near-infrared light sources to improve the precision and effectiveness of treatment. Mechanistic studies: in-depth study of the molecular mechanism of the combination of photothermal therapy and chemotherapy in order to better understand its mechanism of action and provide a theoretical basis for future clinical applications.
The technologies discussed in this review have shown significant promise in preclinical studies and hold potential for real-time practical use. However, several challenges must be addressed for successful clinical translation.123,124 Industrial implications include the development of nano-delivery systems for PDT and PTT, which offer improved targeting and efficiency, reducing damage to healthy tissues and enhancing ICD induction. This makes them valuable for cancer immunotherapy and could lead to the development of targeted therapies with reduced side effects and improved patient outcomes.125,126 Additionally, the synergistic effects of combining phototherapy with chemotherapy or immunotherapy can enhance treatment efficacy, particularly for patients with advanced or resistant tumors, offering a more comprehensive treatment strategy.127 Despite these potential benefits, there are drawbacks for real-time use. The development and manufacturing of nano-delivery systems and combination therapies are complex, requiring specialized expertise and infrastructure. This complexity can increase costs and limit accessibility. Safety and toxicity concern also arise, as some nano-materials may have long-term toxicity or biocompatibility issues that need thorough evaluation before clinical use. Regulatory hurdles pose another challenge, with the approval process for new combination therapies and nano-delivery systems being lengthy and demanding extensive preclinical and clinical data to demonstrate safety and efficacy.93,128 Lastly, the high development and production costs of these advanced therapies may make them expensive, potentially limiting their availability to patients, especially in low-resource settings. Despite these challenges, ongoing research and technological advancements are working towards overcoming these barriers, bringing these innovative therapies closer to real-time clinical application.129,130
Recent advancements in nanotechnology have introduced several innovative approaches that hold promise for enhancing cancer treatment and drug delivery. Biodegradable nanocarriers, designed to degrade safely in the body after delivering their payload, reduce long-term toxicity concerns and are suitable for sustained drug release or targeting specific tissues. CRISPR-based approaches offer precise gene editing capabilities, which can be used to enhance the efficacy of cancer treatments by targeting specific genetic mutations, though challenges such as efficient delivery and minimizing off-target effects need to be addressed.131,132 AI-driven drug delivery uses machine learning algorithms to predict optimal drug formulations, enhance targeting, and personalize treatment plans based on patient-specific data, revolutionizing how drugs are developed and administered.133,134 These emerging nanotechnologies are expected to play a significant role in shaping the future of cancer treatment, offering more personalized, effective, and safer therapeutic options.
Summary and Outlook
Photodynamic therapy (PDT) and photothermal therapy (PTT) have emerged as promising strategies in cancer treatment, offering non-invasive, repeatable, and minimally invasive approaches that can induce immunogenic cell death (ICD) and trigger the body’s immune response against tumor cells. The integration of nanodelivery systems with these phototherapies has further enhanced their efficacy and precision. PDT utilizes photosensitizers activated by light to generate reactive oxygen species (ROS), leading to tumor cell death and the release of damage-associated molecular patterns (DAMPs) that activate immune responses. At the same time the design of photosensitizers from first-generation natural compounds to third-generation synthetic molecules and nanomaterials, have improved treatment efficacy and reduced off-target effects. PDT has demonstrated significant potential in various cancers, including melanoma, colorectal adenocarcinoma, lung cancer, and head and neck tumors, often through the induction of ICD and enhancement of tumor antigen presentation. PTT employs photothermal agents (PTAs) to convert light energy into heat, causing thermal damage to tumor cells and releasing tumor antigens and DAMPs. The ideal PTA should possess high water solubility, good biocompatibility, efficient photothermal conversion, and effective tumor targeting. PTT has shown promise in treating breast cancer, neuroblastoma, prostate cancer, and other malignancies, with the ability to modulate the tumor microenvironment and enhance immune cell infiltration and activation. Nano delivery systems have revolutionized phototherapy by improving the solubility, stability, and tumor targeting of photosensitizers and PTAs. The integration of PDT/PTT with chemotherapy and immunotherapy, creating synergistic effects that enhance overall therapeutic outcomes. Examples include the ICG@siRNA nanoplatform for PDT and the GOx@FeNPs system for PTT, which combine precise drug delivery with immune modulation to overcome treatment resistance and improve antitumor efficacy.
Despite the progress made in PDT and PTT, there are still some challenges, and the effectiveness of both PDT and PTT is limited by the depth of light penetration, especially for deep tumors, and therefore, improved light sources and techniques are needed to address this limitation. At the same time, the biocompatibility, stability, and clearance of nanoparticles from the body need to be further optimized to ensure long-term safety. While phototherapy is usually minimally invasive, localized overheating during PTT can damage surrounding normal tissue, so strategies to improve temperature control and aiming accuracy are essential. Future research should focus on the development of nanomaterials with enhanced biocompatibility, targeting capabilities, and PDT/PTT efficiency. This includes exploring new materials and surface modifications to improve performance in the tumor microenvironment. Translating novel nanodelivery systems and light therapy into clinical practice requires rigorous safety and efficacy studies to meet regulatory standards. On top of this, further exploration of combination regimens, such as PDT/PTT versus immunotherapy or chemotherapy, is needed to optimize treatment options and overcome resistance mechanisms in various cancer types. Ongoing clinical trials are investigating the safety and efficacy of PDT and PTT in various cancer types, including trials that combine phototherapy with immune checkpoint inhibitors. The implementation of these therapies in the real world will require standardized light transmission, dosimetry, and more to ensure consistent results across different healthcare settings. In conclusion, PDT and PTT, combined with advanced nanodelivery systems, offer transformative potential for cancer treatment. Addressing the remaining challenges and pursuing strategic research directions will pave the way for these therapies to become a mainstream choice in precision oncology, ultimately improving patient outcomes and quality of life.
Photodynamic therapy and photothermal therapy have become new strategies for cancer treatment. They are non-invasive, repeatable, and minimally invasive approaches that can induce immunogenic cell death, triggering the body’s immune response to attack tumor cells, showing great clinical potential. To overcome the limitations of single therapies, the combined use of PDT, PTT, and chemotherapy has shown significant advantages, providing new treatment options for cancer patients and offering fresh perspectives for future cancer treatment strategies. With the advent of nano-delivery technologies and optimized strategies for photosensitizers, challenges that PDT and PTT still face, such as low ROS production, local overheating leading to damage to normal tissues, and limited impact on in situ cancers, will be addressed. Despite promising preclinical results, clinical translation of nano delivery-based phototherapies faces multifaceted barriers. First, their size, composition, and surface properties critically influence in vivo behavior, yet these characteristics dynamically change post-administration through biological interactions, complicating performance evaluation. Second, rapid clearance by the reticuloendothelial system (RES) reduces tumor bioavailability while potentially causing liver/spleen toxicity, with current solutions like PEGylation only partially mitigating this issue. Third, tumor targeting remains problematic as both active (ligand-mediated) and passive (EPR effect) delivery mechanisms show inconsistent accumulation due to tumor heterogeneity and microenvironmental barriers. Fourth, safety concerns persist regarding nanotoxicity, including poor biodegradation, DNA damage, metal-induced neurotoxicity, and chronic immune effects that may outweigh therapeutic benefits. While promising in preclinical studies, these combined challenges - encompassing manufacturing reproducibility (~30% batch consistency), biological barriers (5–40x patient variability in ICD markers), and clinical practicality ($8,500/dose cost) - currently limit widespread adoption. Overcoming these hurdles will require innovations in nanoparticle design (simplified formulations), targeting strategies (TME-responsive carriers), and toxicity management (immune-compatible materials) to bridge the gap between laboratory promise and clinical reality. We believe that new treatment options based on PDT and PTT will gradually mature. Light therapies will play an increasingly important role in cancer diagnosis and treatment, providing new choices for precision medicine and benefiting more patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. Rui Zhao and Shuai Li are co-first authors.
Funding
This research received no external funding.
Disclosure
The authors declare no potential conflicts of interest.
References
1. Li B, Ashrafizadeh M, Jiao T. Biomedical application of metal-organic frameworks (MOFs) in cancer therapy: stimuli-responsive and biomimetic nanocomposites in targeted delivery, phototherapy and diagnosis. Int J Biol Macromol. 2024;260(P2):129391. doi:10.1016/j.ijbiomac.2024.129391
2. Abreu MM, Chocron AF, Smadja DM. From cold to hot: mechanisms of hyperthermia in modulating tumor immunology for enhanced immunotherapy. Front Immunol. 2025;16:1487296. doi:10.3389/fimmu.2025.1487296
3. Wang K, Li L, Liang G, et al. Sonodynamic activated nanoparticles with Glut1 inhibitor and cystine-containing polymer stimulate disulfidptosis for improved immunotherapy in bladder cancer. Biomaterials. 2025;319:123178. doi:10.1016/j.biomaterials.2025.123178
4. Wu Q, Liu M, Zhang H, et al. WO 3-x @ferrocene-folic acid composites induce cancer cell death and activate immunity via PTT/CDT. Small. 2025;21(16):e2500104. doi:10.1002/smll.202500104
5. Kennedy BE, Noftall EB, Dean C, et al. Targeted intra-tumoral hyperthermia using uniquely biocompatible gold nanorods induces strong immunogenic cell death in two immunogenically ‘cold’ tumor models. Front Immunol. 2025;15:1512543. doi:10.3389/fimmu.2024.1512543
6. Zhang F. Immune checkpoint inhibitors: the mechanisms, limitations, and improvements. 2023;9.
7. Xu T, Liu K, Mi S, et al. Cyclooxygenase-2/prostaglandin E2 inhibition remodulated photodynamic therapy-associated immunosuppression for enhanced cancer immunotherapy. Mater Today Bio. 2025;31:101530. doi:10.1016/j.mtbio.2025.101530
8. Zhang T, Tang D, Wu P, et al. NIR-II photo-accelerated polymer nanoparticles boost tumor immunotherapy via PD-L1 silencing and immunogenic cell death. Bioact Mater. 2025;46:285–300. doi:10.1016/j.bioactmat.2024.12.018
9. Li J, Lv W, Han Z, et al. Mitoxantrone-encapsulated ZIF-8 enhances chemo-immunotherapy via amplified immunogenic cell death. Adv Sci. 2025;12(14):e2501542. doi:10.1002/advs.202501542
10. Zhang Y, Li H, Zhao Y, et al. Macrocarpal I induces immunogenic cell death and synergizes with immune checkpoint inhibition by targeting tubulin and PARP1 in colorectal cancer. Cell Death Discovery. 2025;11(1):73. doi:10.1038/s41420-025-02360-9
11. Shen T, Li M, Bai Q, et al. A powerful organic photosensitizer for effective treatment of malignant tumor via activating antitumor immune response. Chembiochem Europ J Chem Biol. 2025;26(5):e202400975. doi:10.1002/cbic.202400975
12. Wang B, Tang X, Xiao C, et al. Nucleus-targeted ruthenium(II) complex triggers immunogenic cell death and sensitizes melanoma to anti-PD-1 therapy by activating cGAS–STING pathway. J Inorganic Biochemi. 2025;267:112871. doi:10.1016/j.jinorgbio.2025.112871
13. Biagioni A, Petroni G, Corbet C, Andreucci E. Editorial: turning cold tumors hot: insights into molecular mechanisms and clinical applications of immunogenic cell death. Front Cell Develop Biol. 2024;12:1496001. doi:10.3389/fcell.2024.1496001
14. Shi B, Huang Y, Zhao J, Xiong Y, Liao X, Wang J. Photodynamic antimicrobial activity against S. aureus of metallic Iridium(III) complexes with Helicin pendant. J Organomet Chem. 2024;1008:123044. doi:10.1016/j.jorganchem.2024.123044
15. Wang Q, Tao H, Wang H, et al. Albiflorin inhibits osteoclastogenesis and titanium particles-induced osteolysis via inhibition of ROS accumulation and the PI3K/AKT signaling pathway. Int Immunopharmacol. 2024;142(PB):113245. doi:10.1016/j.intimp.2024.113245
16. Xu Y, Zhang J, Wang Z, et al. Water-soluble AIE photosensitizer in short-wave infrared region for albumin-enhanced and self-reporting phototheranostics. Biomaterials. 2025;314:122847. doi:10.1016/j.biomaterials.2024.122847
17. Dhaini B, Arnoux P, Daouk J, et al. Energy transfer between AGuIX nanoparticles and photofrin under light or X-ray excitation for PDT applications. Pharmaceuticals. 2024;17(8):1033. doi:10.3390/ph17081033
18. Daniela M, BS M, Carmen C, et al. Foscan and foslip based photodynamic therapy in osteosarcoma in vitro and in intratibial mouse models. Inter J Cancer. 2017;140(7):1680–1692. doi:10.1002/ijc.30572
19. G-EA M, AA A, Afaf A, BH J, RB M, HN M. Perftoran improves Visudyne-photodynamic therapy via suppressing hypoxia pathway in murine lung cancer. J Radiat Res Appl Sci. 2022;15(1):238–244. doi:10.1016/j.jrras.2022.03.011
20. Fievet C, Vicentini C, Maire C, Staumont D, Mordon S, Mortier L. Traitement par PDT avec textile lumineux chez 2 patientes atteintes de maladie de Paget vulvaire. Annales de dermatologie et de venereologie. 2017;144(12S):S339–S339. doi:10.1016/j.annder.2017.09.578
21. Yasuyuki T, Satoko K, Naoki Y, Koji A, Hitoshi T, Shunichi S. Transvascular delivery of talaporfin sodium to subcutaneous tumors in mice by nanosecond pulsed laser-induced photomechanical waves. Photodiagn Photodyn Ther. 2023;44:103861. doi:10.1016/j.pdpdt.2023.103861
22. ChiaYu L, WeiLin C, YuChun H, Li LC, ChaoHsun Y. Gum Arabic in combination with IFN-γ promotes the M1 polarization in macrophage. Int J Biol Macromol. 2022;209(PA):506–512. doi:10.1016/j.ijbiomac.2022.04.024
23. Houjuan Z, Penghui C, Peng C, Kanyi P. Recent progress in the development of near-infrared organic photothermal and photodynamic nanotherapeutics. Biomater Sci. 2018;6(4):746–765. doi:10.1039/C7BM01210A
24. Dongwon Y, Heeyeong J, Seung-Hyun N, Jae-Hyun L, Jinwoo C. Magnetically triggered dual functional nanoparticles for resistance-free apoptotic hyperthermia. Angew Chem. 2013;52(49):13047–13051. doi:10.1002/anie.201306557
25. Seiko T-B, Steven F. Local tumour hyperthermia as immunotherapy for metastatic cancer. Inter J Hyperthermia. 2014;30(8):531–539. doi:10.3109/02656736.2014.968640
26. Science CoM, Optoelectronic Technology UoCAoS, Beijing, 100049, China. Nanomaterials KLfBEo, Song L, Dong X, Zhu S, et al. Bi 2 S 3 –tween 20 nanodots loading PI3K inhibitor, LY294002, for mild photothermal therapy of LoVo cells in vitro and in vivo. Adv Healthcare Mater. 2018;7(22):e1800830. doi:10.1002/adhm.201800830.
27. Chuanhui S, Xinyu Z, Zichen C, et al. Regulating tumor cholesterol microenvironment to enhance photoimmunotherapy in oral squamous cell carcinoma. Chem Eng J. 2023;462:142160.
28. Gao Y, Huo S, Chen C, et al. Gold nanorods as biocompatible nano-agents for the enhanced photothermal therapy in skin disorders. J Biomed Res. 2024;39(1)11–17.
29. Farbod M, Sharif L, Rezatofighi SE. Photothermal performance of carbon nanotubes in the visible and near-infrared regions: applications in water evaporation and destroying cancer cells. Colloid Polym Sci. 2024;303(1);1–7.
30. Mahnaz A, Hosein GS, Leila M, Mohammad A. Graphene-based hybrid composites for cancer diagnostic and therapy. J Transl Med. 2024;22(1):611. doi:10.1186/s12967-024-05438-7
31. Nandi S, Karati D, Mukherjee S. Cancer therapy by MXene-based nanosystems: an explicative review. Inorg Chem Commun. 2024;170(P1):113214. doi:10.1016/j.inoche.2024.113214
32. Guo N, Wu Q, Gan H, et al. MnO2 nanozyme boosts synergistic photodynamic/photothermal therapy of bacterial biofilm infections. Chem Eng J. 2024;499:156172. doi:10.1016/j.cej.2024.156172
33. Catherine M. Mini gold nanorods and their plasmonic properties. Abstracts Papers Ame Chem Soc. 2018;256.
34. Fan J, Qin Y, Qiu W, et al. Gamabufotalin loaded micro-nanocomposites for multimodal therapy of metastatic TNBC by efficiently inducing ICD. Biomaterials. 2025;314:122851. doi:10.1016/j.biomaterials.2024.122851
35. Xiaoli F, Tian L, Dong C, et al. Mitochondria-associated ER stress evokes immunogenic cell death through the ROS-PERK-eIF2α pathway under PTT/CDT combined therapy. Acta Biomater. 2023;160:211–224. doi:10.1016/j.actbio.2023.02.011
36. Yang S, Pan H, George BP, et al. Bridging the gaps in cancer photothermal therapy through the intersection of nanotechnology and cell membrane coating. Chem Eng J. 2024;484(149641):149641. doi:10.1016/j.cej.2024.149641
37. Shankar S, Partha SN, Laxmanan K, Krishnamurthy S, Raju V. Ultra-small NIR-responsive nanotheranostic agent for targeted photothermal ablation induced damage-associated molecular patterns (DAMPs) from post-PTT of tumor cells activate immunogenic cell death. Nanotheranostics. 2023;7(1):41–60. doi:10.7150/ntno.76720
38. Mian Y, Weiwei Z, Yaqi O, et al. ATP-exhausted nanocomplexes for intratumoral metabolic intervention and photoimmunotherapy. Biomaterials. 2022;284:121503. doi:10.1016/j.biomaterials.2022.121503
39. Aebisher D, Woźnicki P, Aebisher DB. Photodynamic therapy and adaptive immunity induced by reactive oxygen species: recent reports. Cancers. 2024;16(5):967. doi:10.3390/cancers16050967
40. He M, Zhang M, Xu T, et al. Enhancing photodynamic immunotherapy by reprograming the immunosuppressive tumor microenvironment with hypoxia relief. J Controlled Release. 2024;368:233–250. doi:10.1016/j.jconrel.2024.02.030
41. Liu, Y, Pan, Y, Cao, W et al. A tumor microenvironment responsive biodegradable Caco3/mno2- based nanoplatform for the enhanced photodynamic therapy and improved Pd-l1 immunotherapy. Theranostics. 2019 9 23; 6867–6884. doi:10.7150/thno.37586
42. Zhu L. The applications of nanomaterials in phototherapy against cancer. HSET. 2023;45:122–128. doi:10.54097/hset.v45i.7332
43. Sasaki M, Tanaka M, Kojima Y, et al. Anti-tumor immunity enhancement by photodynamic therapy with talaporfin sodium and anti-programmed death 1 antibody. Molecular Therapy-Oncolytics. 2023;28:118–131. doi:10.1016/j.omto.2022.12.009
44. Li, B, Hao, G, Sun, B, Gu, Z, Xu, ZP, et al. Engineering a therapy‐induced “immunogenic cancer cell death” amplifier to boost systemic tumor elimination. Adv Funct Mater. 2020;30(22):1909745 doi:10.1002/adfm.201909745.
45. Zeng R, Zhou R, Zhen L, et al. Tumor-targeted nanosystem with hypoxia inducible factor 1α inhibition for synergistic chemo-photodynamic therapy against hypoxic tumor. Colloids Surf B. 2025;248:114456. doi:10.1016/j.colsurfb.2024.114456
46. Guangxu L, Kaiyuan N, Ziwan X, VS S, Yang S, Wenbin L. Nanoscale metal-organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J Am Chem Soc. 2018;140(17):5670–5673. doi:10.1021/jacs.8b01072
47. Hu X, Zhang M, Quan C, Ren S, Chen W, Wang J. ROS-responsive and triple-synergistic mitochondria-targeted polymer micelles for efficient induction of ICD in tumor therapeutics. Bioact Mater. 2024;36:490–507. doi:10.1016/j.bioactmat.2024.06.038
48. Kim M, Lee J, Choi J, Kim SY. Reactive oxygen species-responsive nanocomposite hydrogels for accurate drug delivery and localized PDT/PTT/chemo synergistic cancer therapy. Eur Polym J. 2025;224:113683. doi:10.1016/j.eurpolymj.2024.113683
49. Xu Z, Fan R, Zhang X, et al. Regulation of ROS balance in the tumor microenvironment achieves reversal of immune suppression and deep penetration of nanomedicines. Chem Eng J. 2025;505:159716. doi:10.1016/j.cej.2025.159716
50. Yunlong L, Xinyu S, Chao L, et al. SERD-NHC-Au(I) complexes for dual targeting ER and TrxR to induce ICD in breast cancer. Pharmacol Res. 2023;190:106731. doi:10.1016/j.phrs.2023.106731
51. Wang T, Tao J, Wang B, et al. Reversing resistance of cancer stem cells and enhancing photodynamic therapy based on hyaluronic acid nanomicelles for preventing cancer recurrence and metastasis. Adv Healthcare Mater. 2024;13(4):2302597. doi:10.1002/adhm.202302597
52. Chen Q, He Y, Wang Y, et al. Penetrable nanoplatform for “cold” tumor immune microenvironment reeducation. Adv Sci. 2020;7(17). doi:10.1002/advs.202000411.
53. Mai X, Zhang Y, Fan H, et al. Integration of immunogenic activation and immunosuppressive reversion using mitochondrial-respiration-inhibited platelet-mimicking nanoparticles. Biomaterials. 2020;232:119699. doi:10.1016/j.biomaterials.2019.119699
54. Simões MM, Paiva KLR, Souza I, et al. The Potential of photodynamic therapy using solid lipid nanoparticles with aluminum phthalocyanine chloride as a nanocarrier for modulating immunogenic cell death in murine melanoma in vitro. Pharmaceutics. 2024;16(7):941. doi:10.3390/pharmaceutics16070941
55. Liao X, Cao Y, Zhong W, et al. A multifunctional nanoparticle dual loading with chlorin e6 and STING agonist for combinatorial therapy of melanoma. ACS Appl Bio Mater. 2024;7(10):6768–6779. doi:10.1021/acsabm.4c00896
56. Xu F, Wang M, Dotse E, Chow KT, Lo PC. Inducing immunogenic cancer cell death through oxygen-economized photodynamic therapy with nitric oxide-releasing photosensitizers. Angewandte Chemie. 2024;63(37):e202404561–e202404561. doi:10.1002/anie.202404561
57. He H, Liu L, Liang R, Guangdong Key Laboratory of Nanomedicine C-HJLfB, Shenzhen Engineering Laboratory of Nanomedicine, Nanoformulations IoB, Biotechnology SIoAT, Chinese Academy of Sciences, Shenzhen, 518055, PR China, et al. Tumor-targeted nanoplatform for in situ oxygenation-boosted immunogenic phototherapy of colorectal cancer. Acta Biomater. 104;2020:188–197. doi:10.1016/j.actbio.2020.01.012
58. Xiaobo W, Lei R, Linhan Y, Jing C. Photodynamic therapy augments oxaliplatin-induced immunogenic cell death in colorectal cancer. Central-Europ J Immunol. 2023;48(3):189–202. doi:10.5114/ceji.2023.132053
59. Chanda B, Azophi M, John F, et al. A single photodynamic priming protocol augments delivery of ⍺-PD-L1 mAbs and induces immunogenic cell death in head and neck tumors. Photochem Photobiol. 2023;100(6):1647–58.
60. Ting-Ting Y, Jun H, Qi-Rui L, et al. Chlorin e6-induced photodynamic effect facilitates immunogenic cell death of lung cancer as a result of oxidative endoplasmic reticulum stress and DNA damage. Int Immunopharmacol. 2023;115:109661.
61. Zheng Z, Lu S, Wu Y, et al. Modulating glucose metabolism and relieving hypoxia for enhanced chemo/photoimmunotherapy with photosensitizing enzymatic nanodrugs. Chem Eng J. 2024;498:155024. doi:10.1016/j.cej.2024.155024
62. Alexander D, Maxim A, Tatyana G, et al. Exploring the potential of photodynamic therapy in the treatment of non-melanoma skin cancer: analysis of two clinical cases. Photodiagn Photodyn Ther. 2023;44:103748. doi:10.1016/j.pdpdt.2023.103748
63. Ravera S, Pasquale C, Panfoli I, et al. Assessing the effects of curcumin and 450 nm photodynamic therapy on oxidative metabolism and cell cycle in head and neck squamous cell carcinoma: an in vitro study. Cancers. 2024;16(9):1642. doi:10.3390/cancers16091642
64. Yue D, Han Y, Junxiao G, et al. Immune modulator and low-temperature PTT-induced synergistic immunotherapy for cancer treatment. ACS Appl Bio Mater. 2021;4(2):1524–1535. doi:10.1021/acsabm.0c01397
65. Zhaowei L, Long R. A homotypic membrane-camouflaged biomimetic nanoplatform with gold nanocrystals for synergistic photothermal/starvation/immunotherapy. ACS Appl Mater Interfaces. 2021;13(20):23469–80.
66. ZhenHan F, ZhanTao L, Shuang Z, et al. A combination strategy based on an Au nanorod/doxorubicin gel via mild photothermal therapy combined with antigen-capturing liposomes and anti-PD-L1 agent promote a positive shift in the cancer-immunity cycle. Acta Biomater. 2021;136:495–507. doi:10.1016/j.actbio.2021.09.052
67. Guiquan Z, Xuemei C, Xu C, et al. Click-reaction-mediated chemotherapy and photothermal therapy synergistically inhibit breast cancer in mice. ACS nano. 2023;17(15):14800–13.
68. Qilin L, Huiling F, Yunruo X, et al. NIR-responsive hollow germanium nanospheres mediate photothermal/photodynamic therapy and restrain immunosuppression to cooperatively eradicate primary and metastatic tumors. Chem Eng J. 2023;458:141314.
69. Department of Orthopaedic Surgery UH, Tongji Medical College, Huazhong University of Science, Technology W, China. Chemistry Do, Deng X, Guan W, Qing X, et al. Ultrafast low-temperature photothermal therapy activates autophagy and recovers immunity for efficient antitumor treatment. ACS Appl Mater Interfaces. 2020;12(4):4265–4275. doi:10.1021/acsami.9b19148.
70. Suwan L, Miaomiao Z, Huijun Y, et al. Immunoinducible carbon dot-incorporated hydrogels as a photothermal-derived antigen depot to trigger a robust antitumor immune response. ACS Appl Mater Interfaces. 2023;15(6):7700–12.
71. National Engineering Research Center for Nanomedicine CoLS, Technology HUoS, Technology W, 430074, China, et al. Mild photothermal therapy potentiates anti-PD-L1 treatment for immunologically cold tumors via an all-in-one and all-in-control strategy. Nat Commun. 2019;10(1):4871.
72. Kim M, Hwang JE, Lee JS, et al. Development of indocyanine green/methyl-β-cyclodextrin complex-loaded liposomes for enhanced photothermal cancer therapy. ACS Appl Mater Interfaces. 2024;16(26):32945–56.
73. Yujie Z, Xu L, Xinyu L, et al. Combination of phototherapy with immune checkpoint blockade: theory and practice in cancer. Front Immunol. 2022;13:955920. doi:10.3389/fimmu.2022.955920
74. Ezzaldeen EM, Yaguchi T, Imagawa R, et al. Beneficial effects on T cells by photodynamic therapy with talaporfin enhance cancer immunotherapy. Inter immunol. 2025:dxaf003.
75. Galsky MD, Kockx M, Roels J, et al. Different PD-L1 assays reveal distinct immunobiology and clinical outcomes in urothelial cancer. Cancer Immunol Res. 2025;13(4):476–486. doi:10.1158/2326-6066.CIR-24-0649
76. Liu T, Sun T, Chen X, et al. Targeting ARPC1B overcomes immune checkpoint inhibitor resistance in glioblastoma by reversing pro-tumorigenic macrophage polarization. Cancer Res. 2025.
77. Mayadev J, Zamarin D, Deng W, et al. Neoadjuvant or concurrent atezolizumab with chemoradiation for locally advanced cervical cancer: a randomized Phase I trial. Nat Commun. 2025;16(1):553. doi:10.1038/s41467-024-55200-2
78. Liangjie J, Song S, Youju H, Dongdong L, Xianzhu Y. Corn-like Au/Ag nanorod-mediated NIR-II photothermal/photodynamic therapy potentiates immune checkpoint antibody efficacy by reprogramming the cold tumor microenvironment. Biomaterials. 2021;268(120582).
79. Chen M, Shen Y, Pu Y, et al. Biomimetic inducer enabled dual ferroptosis of tumor and M2-type macrophages for enhanced tumor immunotherapy. Biomaterials. 2023;303:122386. doi:10.1016/j.biomaterials.2023.122386
80. Zeng L, Ding S, Cao Y, et al. A MOF-based potent ferroptosis inducer for enhanced radiotherapy of triple negative breast cancer. ACS nano. 2023;17(14):13195–13210. doi:10.1021/acsnano.3c00048
81. Li Y, Chen J, Xia Q, et al. Photothermal Fe3O4 nanoparticles induced immunogenic ferroptosis for synergistic colorectal cancer therapy. J Nanobiotechnol. 2024;22(1):630. doi:10.1186/s12951-024-02909-3
82. Tariq HK, Liang Z, Rabiu L, et al. Blockade of TIPE2-mediated ferroptosis of myeloid-derived suppressor cells achieves the full potential of combinatory ferroptosis and anti-PD-L1 cancer immunotherapy. Cells. 2025;14(2):108. doi:10.3390/cells14020108
83. Palomero J, Galvao V, Creus I, et al. Preclinical data and design of a phase I clinical trial of neoantigen-reactive TILs for advanced epithelial or ICB-resistant solid cancers. Immuno-Oncol Technol. 2025;25:101030. doi:10.1016/j.iotech.2024.101030
84. Zhang L, Jiang H, Ma H. Progress in immune microenvironment, immunotherapy and prognostic biomarkers in pediatric osteosarcoma. Front Immunol. 2025;16:1548527. doi:10.3389/fimmu.2025.1548527
85. Bei L, Yi F, Maodi X, et al. Gold-based nanoparticles realize photothermal and photodynamic synergistic treatment of liver cancer and improve the anaerobic tumor microenvironment under near-infrared light. Front Bioeng Biotechnol. 2022;10:957349. doi:10.3389/fbioe.2022.957349
86. Hui Z, Ke Y, Huan Y, et al. Multifunctional nanoplatform-mediated chemo-photothermal therapy combines immunogenic cell death with checkpoint blockade to combat triple-negative breast cancer and distant metastasis. Int J Nanomed. 2023;18:3109–3124. doi:10.2147/IJN.S408855
87. Zheng M, Zhang H, Dai M, et al. A PTT-induced feed-back carbon nanosystem for enhanced breast cancer therapy by extracellular matrix remodeling. Nano Lett. 2025;25(8):3180–3190. doi:10.1021/acs.nanolett.4c05625
88. Juliana C-M, BM L, SE E, JC M, Rohan F. Prussian blue nanoparticle-based antigenicity and adjuvanticity trigger robust antitumor immune responses against neuroblastoma. Biomater Sci. 2019;7(5):1875–1887. doi:10.1039/C8BM01553H
89. Bin R, Zhu C, Yunjia R, et al. Tumor growth inhibition and induced the immunogenic death of tumor cells in prostate tumor bone metastases by photothermal therapy mediated with au nanoparticles modified with PDA. Adv Ther. 2024;7(8):2300433. doi:10.1002/adtp.202300433
90. Sun C, He Q, Yang X, et al. A novel NIR-dependent IDO-inhibiting ethosomes treatment melanoma through PTT/PDT/immunotherapy synergy. Colloids Surf B. 2025;251:114565. doi:10.1016/j.colsurfb.2025.114565
91. Huining D, Qing X, Jiaqi S, Chunyun Z, Yongtai Z, Nianping F. Advances and prospects of tumor immunotherapy mediated by immune cell-derived biomimetic metal-organic frameworks. Colloids Surf B. 2023;232:113607.
92. Ke Y, Shaojing Z, Baoling L, Benhua W, Minhuan L, Xiangzhi S. Low temperature photothermal therapy: advances and perspectives. Coord Chem Rev. 2021;454:214330.
93. Qi G, Chen K, Guan W, et al. One-pot synthesis of a pH-sensitive MOF Integrated with glucose oxidase for amplified tumor photodynamic/photothermal therapy. ACS Appl Mater Interfaces. 2024;16(44):61395. doi:10.1021/acsami.4c17184
94. Zhang Y, Jiang ZT, Wang Y, et al. A supramolecular nanoformulation with adaptive photothermal/photodynamic transformation for preventing dental caries. ACS nano. 2024:18(40):27340–57.
95. Zhang X, Shen Y, Xu S, et al. Intracellular pH-propelled assembly of smart carbon nanodots and selective photothermal therapy for cancer cells. Colloids Surf B. 2020;188:110724. doi:10.1016/j.colsurfb.2019.110724
96. Peipei Y, Minglong C, Wanbing Q, et al. Effective photothermal therapy mediated by indocyanine green nanoparticle tip-loaded microneedles to enhance checkpoint inhibitor immunotherapy for melanoma treatment. ACS Appl Nano Mater. 2021;4(6):5921–5931. doi:10.1021/acsanm.1c00832
97. Ma R, Liu S, Liu G, Liu P, Cai K. A triple-mode strategy combining low-temperature photothermal, photodynamic, and chemodynamic therapies for treating infectious skin wounds. Biomater Sci. 2024;12(21):5521–5533. doi:10.1039/D4BM00859F
98. Qi C, Li A, Wu B, Wang P. Multi-sensitive Au NCs/5-FU@Carr-LA composite hydrogels for targeted multimodal anti-tumor therapy. Molecules. 2024;29(17):4051. doi:10.3390/molecules29174051
99. Jiaqi X, Mengdi S, Zhou F, Lanxi Z, Jun W, Kehai L. A chemoimmunotherapy-based strategy to enhance tumor therapy by cross-linking a novel size-variable nanocluster via a bifunctional peptide. Mater Des. 2023;233:112232.
100. Chunbai H, Demin L, Wenbin L. Self-assembled core-shell nanoparticles for combined chemotherapy and photodynamic therapy of resistant head and neck cancers. ACS nano. 2015;9(1):991–1003. doi:10.1021/nn506963h
101. Liu L, Zhang H, Hou J, et al. Discovery of novel PD-L1 small-molecular inhibitors with potent in vivo anti-tumor immune activity. J Med Chem. 2024;67(6):4977–9.
102. Sandbhor P, Palkar P, Bhat S, John G, Goda JS. Nanomedicine as a multimodal therapeutic paradigm against cancer: on the way forward in advancing precision therapy. Nanoscale. 2024;16(13):6330–6364. doi:10.1039/D3NR06131K
103. Shanmugavadivu A, Lekhavadhani S, Miranda PJ, Selvamurugan N. Current approaches in tissue engineering-based nanotherapeutics for osteosarcoma treatment. Biomed Mater. 2024;19(2):022003. doi:10.1088/1748-605X/ad270b
104. Bhattacharya S, Beninger P. The emerging role of cell membrane-coated nanomaterials in cancer therapy. Curr Pharm Des. 2024;30(10):727–741. doi:10.2174/0113816128295414240221063434
105. Alavijeh RK, Akhbari K. Cancer therapy by nano MIL-n series of metal-organic frameworks. Coord Chem Rev. 2024;503:215643.
106. Zhang H, Yuan W. Self-healable oxide sodium alginate/carboxymethyl chitosan nanocomposite hydrogel loading Cu2+-doped MOF for enhanced synergistic and precise cancer therapy. Int J Biol Macromol. 2024;262(P2):129996. doi:10.1016/j.ijbiomac.2024.129996
107. Liu F, Li Y, Wei Q, Liu J. Degradable bifunctional phototherapy composites based on upconversion nanoparticle-metal phenolic network for multimodal tumor therapy in the near-infrared biowindow. J Colloid Interface Sci. 2024;663:436–448. doi:10.1016/j.jcis.2024.02.173
108. Li Y, Qi H, Geng Y, Li L, Cai X. Research progress of organic photothermal agents delivery and synergistic therapy systems. Colloids Surf B. 2024;234(113743):113743. doi:10.1016/j.colsurfb.2024.113743
109. Su M, Zhang Y, Yang L, et al. Camptothecin-loaded and IR780-doped polydopamine nanomedicine used for multifunctional chemo-photothermal-photodynamic therapy for lung cancer. J Drug Delivery Sci Technol. 2024;97(105657):105657. doi:10.1016/j.jddst.2024.105657
110. Zheng X, et al. A light‐triggered j‐aggregation‐regulated therapy conversion: from photodynamic/photothermal therapy to long‐lasting chemodynamic therapy for effective tumor ablation. Angew Chem. 2024;136(23):e202404395.
111. Yi J, Liu L, Gao W, et al. Advances and perspectives in phototherapy-based combination therapy for cancer treatment. J Mat Chem B. 2024;12(26):6285–6304. doi:10.1039/D4TB00483C
112. Wang Y, Chang L, Gao H, Yu C, Gao Y, Peng Q. Nanomaterials-based advanced systems for photothermal / photodynamic therapy of oral cancer. Eur J Med Chem. 2024;272:116508. doi:10.1016/j.ejmech.2024.116508
113. Yang ZC, Gu QS, Chao JJ, et al. Glutathione-activated biotin-targeted dual-modal imaging probe with improved PDT/PTT synergistic therapy. Anal Chim Acta. 2024;1316(342860):342860. doi:10.1016/j.aca.2024.342860
114. Erk B, Kamanli AF, Eskiler GG. The therapeutic efficacy of 5-ALA based photodynamic therapy and chemotherapy combination in triple negative breast cancer cells. Lasers Med Sci. 2024;39(1):191. doi:10.1007/s10103-024-04141-9
115. Shirke AA, Walker E, Chavali S, et al. A synergistic strategy combining chemotherapy and photodynamic therapy to eradicate prostate cancer. Int J Mol Sci. 2024;25(13):7086. doi:10.3390/ijms25137086
116. Sun PJ, Sohyun P, SangJae P, SeokKi K. Synergistic effects of concurrent photodynamic therapy with indocyanine green and chemotherapy in hepatocellular carcinoma cell lines and mouse models. J Photochem Photobiol B Biol. 2022;239:112642. doi:10.1016/j.jphotobiol.2022.112642
117. Chen H, Li H, Li H, Zhang Z. Umbrella review of adjuvant photodynamic therapy for cholangiocarcinoma palliative treatment. Photodiagn Photodyn Ther. 2025;51:104472. doi:10.1016/j.pdpdt.2025.104472
118. Gao Z, Mansor MH, Howard F, MacInnes J, Zhao X, Muthana M. Microfluidic-assisted silk nanoparticles co-loaded with epirubicin and copper sulphide: a synergistic photothermal–photodynamic chemotherapy against breast cancer. Nanomaterials. 2025;15(3):221. doi:10.3390/nano15030221
119. Lina S, Cuiling Z, Xinxin L, et al. Combined photothermal therapy and Lycium barbarum polysaccharide for topical administration to improve the efficacy of doxorubicin in the treatment of breast cancer. Pharmaceutics. 2022;14(12):2677. doi:10.3390/pharmaceutics14122677
120. Tao G, Xiaoyu W, Qing M, et al. Triformyl cholic acid and folic acid functionalized magnetic graphene oxide nanocomposites: multiple-targeted dual-modal synergistic chemotherapy/photothermal therapy for liver cancer. J Inorganic Biochemi. 2021;223(prepublish):111558. doi:10.1016/j.jinorgbio.2021.111558
121. Jianwen L, Zhanxia Z, Haibin D, Zhan Z. Cinobufagin-loaded and folic acid-modified polydopamine nanomedicine combined with photothermal therapy for the treatment of lung cancer. Front Chem. 2021;9:637754. doi:10.3389/fchem.2021.637754
122. Deinavizadeh M, Kiasat AR, Shafiei M, et al. Synergistic chemo-photothermal therapy using gold nanorods supported on thiol-functionalized mesoporous silica for lung cancer treatment. Sci Rep. 2024;14(1):4373. doi:10.1038/s41598-024-54778-3
123. Chandra J, Nasir N, Wahab S, Sahebkar A, Kesharwani P. Harnessing the power of targeted metal nanocarriers mediated photodynamic and photothermal therapy. Nanomedicine. 2024;11–19.
124. Hedaoo A, Khairnar P, Vambhurkar G, et al. Unveiling the potential of inorganic nanoarchitecture-mediated photothermal therapy: an illustration on melanoma. Eur Polym J. 2024;216:113282. doi:10.1016/j.eurpolymj.2024.113282
125. Huang Z, Tian K, Xue Y, Luo F. A promising role of noble metal NPs@MOFs in chondrosarcoma management. Nanoscale. 2024;17(6):2961–84.
126. Liu Y, Yang K, Wang J, Tian Y, Song B, Zhang R. Hypoxia-triggered degradable porphyrinic covalent organic framework for synergetic photodynamic and photothermal therapy of cancer. Mater Today Bio. 2024;25:100981. doi:10.1016/j.mtbio.2024.100981
127. Negin A, Parvaneh M, Fani PA, Majid R, Amir A. Antibody-modified gold nanobiostructures: advancing targeted photodynamic therapy for improved cancer treatment. Curr Pharm Des. 2023;29(39):3103–22.
128. Song Y, Tan KB, Zhou SF, Zhan G. Biocompatible copper-based nanocomposites for combined cancer therapy. ACS Biomater Sci Eng. 2024;10(6):3673–3692. doi:10.1021/acsbiomaterials.4c00586
129. Zhang W, Huo F, Yin C, Yin C. Advances on fluorescence bioimaging and photo-mediated therapy of NIR-II small molecule dyes. Coord Chem Rev. 2025;534:216587. doi:10.1016/j.ccr.2025.216587
130. Zhao L, Sun Y, Fu Q, Xiao W. Emerging nanoradiosensitizers and nanoradioprotectants for enhanced cancer theranostics. Chem Eng J. 2024;501:157554.
131. Low SJ, O’Neill M, Kerry WJ, et al. PathoGD: an integrative genomics approach to primer and guide RNA design for CRISPR-based diagnostics. Commun Biol. 2025;8(1):147. doi:10.1038/s42003-025-07591-1
132. Mukhtiar A, Mahmood A, Khan MA, Ameen M, Khayri JMA, Qari SH. Transforming field crops with CRISPR/Cas: a new era in genome editing. Rendiconti Lincei Scienze Fisiche e Naturali. 2025;1–14.
133. Pandey H, Misra V, Mall AK, Sharma A, Hillary VE, Ceasar SA. Enhancing sugar crop resilience to abiotic stress using CRISPR/Cas tools. Sugar Tech. 2025;1–18.
134. Rachappanavar V. Utilizing CRISPR-based genetic modification for precise control of seed dormancy: progress, obstacles, and potential directions. Molecular Biol Rep. 2025;52(1):204. doi:10.1007/s11033-025-10285-w
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Near-Infrared Light-Activated Oxygen Generator a Multidynamic Photo-Nanoplatform for Effective Anti-Cutaneous Squamous Cell Carcinoma Treatment
Zhang X, Bu X, Jia W, Ying Y, Lv S, Jiang G
International Journal of Nanomedicine 2022, 17:5761-5777
Published Date: 28 November 2022
Combined Photodynamic and Photothermal Therapy and Immunotherapy for Cancer Treatment: A Review
Kong C, Chen X
International Journal of Nanomedicine 2022, 17:6427-6446
Published Date: 16 December 2022
Smart Nanosystem-Mediated Inhibition of Mitochondrial Respiration for Enhanced Phototherapy-Induced Antitumor Immunity
Zhao Y, Lv B, Xue G, Sun Y, Cao J
International Journal of Nanomedicine 2023, 18:3443-3457
Published Date: 26 June 2023
A Multifunctional MIL-101-NH2(Fe) Nanoplatform for Synergistic Melanoma Therapy

Shang J, Chen Y, Wang F, Yang J, Li Y, Yang L, Liu X, Zhong Z, Yue C, Zhou M
International Journal of Nanomedicine 2025, 20:969-988
Published Date: 22 January 2025
Antibacterial and Antitumor Application of Carbon Dots Based on Natural Products for Photodynamic/Photothermal Effects
Romero MP, Lagos KJ, Cuadrado CF, Garzón-Romero CC, Salazar MA, Solorzano G, Gardener JA, González MA, Rivera M
International Journal of Nanomedicine 2025, 20:7893-7914
Published Date: 19 June 2025

