Back to Journals » International Journal of Nanomedicine » Volume 20
Advancements in Nanotherapeutics for the Treatment of Depression via Intranasal Pathway: A Review
Authors Patel M , Minglani VV , Vaddadi H, Jha LL, Patel LD, Huanbutta K, Sangnim T
Received 1 March 2025
Accepted for publication 22 May 2025
Published 9 June 2025 Volume 2025:20 Pages 7323—7342
DOI https://doi.org/10.2147/IJN.S525759
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Meenakshi Patel,1 Vahid Vikram Minglani,1 Hatasha Vaddadi,1 Lalit Lata Jha,1 LD Patel,2 Kampanart Huanbutta,3 Tanikan Sangnim4
1Department of Pharmaceutics, School of Pharmacy, Faculty of Pharmacy, Parul University, Vadodara, Gujarat, 391760, India; 2Faculty of Pharmacy, Parul University, Vadodara, Gujarat, India; 3Department of Manufacturing Pharmacy, College of Pharmacy, Rangsit University, Pathum Thani, 12000, Thailand; 4Faculty of Pharmaceutical Sciences, Burapha University, Muang, Chonburi, 20131, Thailand
Correspondence: Tanikan Sangnim, Faculty of Pharmaceutical Sciences, Burapha University, 169, Seansook, Muang, Chonburi, 20131, Thailand, Email [email protected]
Abstract: Depression is a complex psychiatric disorder marked by persistent emotional disturbances such as sadness, hopelessness, and fatigue, frequently accompanied by psychosocial impairments. Current treatment approaches are hindered by limited efficacy, poor patient adherence, and the inability of many therapeutic agents to effectively penetrate the blood-brain barrier (BBB). The BBB, a selective and protective interface between the bloodstream and brain tissue, restricts drug delivery to the central nervous system (CNS), resulting in suboptimal concentrations of antidepressants at the target site and delayed therapeutic responses. This review explores the limitations of conventional drug delivery systems for depression and highlights the intranasal route as a promising non-invasive alternative for direct brain targeting. Intranasal delivery bypasses hepatic first-pass metabolism and systemic degradation, offering rapid drug absorption and CNS access through olfactory and trigeminal neural pathways. Among emerging strategies, nanotherapeutics have gained increasing attention due to their capacity to improve solubility, protect labile compounds, and provide sustained drug release. Nanoparticles can encapsulate both hydrophilic and lipophilic drugs, enhancing their pharmacokinetics and stability. When administered intranasally, these nanocarriers can directly reach the brain, potentially reducing dosage frequency and enhancing therapeutic outcomes, while minimizing systemic side effects. This review focuses on the latest advancements in intranasal nanotherapeutic formulations for depression, such as polymeric nanoparticles, nanoemulsions, solid lipid nanoparticles, and nanostructured lipid carriers. The synergistic integration of nanotechnology and targeted CNS delivery offers a transformative approach to overcome the challenges posed by the BBB and improve depression management. While preclinical findings are promising, further clinical studies are necessary to confirm safety, efficacy, and long-term outcomes. Overall, intranasal nanotherapeutics represent a compelling direction for the development of next-generation antidepressant therapies, aiming to achieve faster onset, improved adherence, and enhanced quality of life for patients suffering from depression.
Keywords: nanotherapeutics, intranasal, nanocarriers, depression, blood brain barrier
Introduction
Depression is the most commonly occurring psychological disorder and psychosocial illness. It is distinguished from general feelings of sadness and a demoralizing state of mind.1 It is accompanied by serotonin depletion and dopamine depletion in the CNS, which generates difficulties processing emotions, pessimism, self-loathing, and lack of motivation.2 Alterations in serotonin levels further disrupt the circadian rhythm, leading to changes in the sleep cycles, sleep quality, and cognitive function in addition to diurnal mood variations.3 Depression treatment has a high recurrence rate and low success rate, often leading patients to self-harm or suicidal tendencies if effective intervention is not provided promptly.4
Depression as a disease is subdivided into various categories, each having its own unique aetiology and characteristics of onset and progression.5 The global epidemiology of depression has been extensively researched. A 2023 report found that since the onset of the COVID-19 pandemic, depression and anxiety rates went up by 25% worldwide. Globally, it has been observed that over 350 million people suffer from depression; by 2030, it is estimated to be the cause of the highest disease burden globally. Epidemiological findings reveal that depression is more prevalent in regions such as the Middle East, North Africa, South Asia, and the United States compared to other areas.6,7 Depression treatment aims to minimize and later eliminate avoidable suffering and loss of life by the use of psychotherapy and concurrent medication with antidepressants.8
Antidepressants are a broad class of psychoactive drugs used to treat depression. Selective Serotonin Reuptake Inhibitors (SSRIs), Tricyclic Antidepressants and Monoamine Oxidase Inhibitors (MAOIs) are the three most widely prescribed drugs in the United States. Upon assessing recent findings by Centres for Disease Control and Prevention, the use of antidepressants has risen by 65% over the last 15 years.9 It is usually found that the first-line therapy of medication often leads to therapeutic failures. The causes of these recurrent failures are attributed to the first-pass metabolism and the incomplete and limited drug permeation into the CNS.10,11 To overcome the issues in delivering therapeutic agents to the CNS, various treatment methods have been developed, ranging from invasive approaches to methods involving disruption of the BBB. Nonetheless, these methods are not as promising as the development of nanotechnology-oriented approaches which have numerous.12 A concise representation of the application of nanotherapeutic agents for the treatment of depression is given in Figure 1.
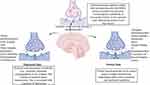 |
Figure 1 Restoration of neurotransmitter levels through nanotherapeutic delivery. |
Since the biggest challenge for CNS-targeting medications is crossing the BBB, developing a nanocarrier-based formulation that effectively crosses or bypasses the BBB is crucial for next-generation drug therapy. This requirement is fulfilled by delivering the drug via the intranasal route, which utilizes the olfactory pathway and provides drug localization at the site of absorption.13,14 However, this route has certain challenges, such as rapid mucociliary clearance. To avoid this, the addition of mucoadhesive substances and in-situ gelling systems has proved beneficial.15
This study aims to explore the latest advances in nanotherapeutic techniques designed for the intranasal delivery of antidepressants. It highlights the challenges of traditional treatments, especially the difficulties posed by the BBB, and how new nanotechnology offers solutions for more effective drug delivery to the brain. Nanocarriers like polymeric nanoparticles, solid lipid nanoparticles, nanostructured lipid carriers, nanoemulsions, and nanosuspensions have shown promise in improving drug solubility, stability, and bioavailability. The review also emphasizes the progress made in bypassing the BBB using the intranasal route, allowing for targeted delivery and reducing unwanted side effects. These innovative nanotherapies not only offer better treatment outcomes but could also help address issues like the delayed onset of action and patient noncompliance often seen with conventional antidepressants. Overall, the future looks promising for faster-acting, non-invasive, and highly effective treatments for depression by combining nanotechnology and neuroscience.
Depression
Aetiology and Pathophysiology of Depression
Over the centuries, public conceptions of what causes depression have changed. The initial justifications emphasized supernatural causes. But scientists put out the hypothesis that depression is caused by a chemical imbalance in the brain in the late 1950s and early 1960s.16 The neurotransmitter norepinephrine was initially considered to be the reason for this condition, but by the middle of the 1960s, attention had switched to serotonin, a different transmitter, which eventually resulted in the invention of selective serotonin reuptake inhibitors.17 The deficiency of serotonin was established as the root cause of depression after gaining clinical credibility due to the association of experimentally decreased central serotonin with mood-congruent memory bias, changed reward-related behaviors, and disturbance of inhibitory affective processing.18 Additionally, a decrease in serotonin synthesis as an uncommon risk factor for depression may be explained by mutations that cause the brain enzyme tryptophan hydroxylase-2 to work improperly. The monoamine system is the main focus of treatment for almost all recognized antidepressants.19
According to studies conducted by Baillie et al (2009), it was found that depression is a multifactorial condition arising from instances in a patient’s life. Important factors that influence the onset of depression were the effects of physical illness (including pregnancy), diet, alcohol, personality traits, unconscious conflicts, abnormal thinking, childhood factors, recent life events, relationship difficulties, parenting, social isolation, stress, and lifestyle. Therefore signifying, that psychological conditions and social factors are responsible for depression.20 Although various internal and external factors may contribute to cause depression, the most widely accepted theory is the chemical imbalance theory which lays emphasis on the brain’s neurotransmitter levels and their subsequent effect on mental ability and causes depression. According to this theory, the underlying cause of depression is the decline in the concentrations of the neurotransmitters serotonin, norepinephrine, or dopamine in the CNS.
Molecular and Genetic Mechanisms of Depression
Depression, or major depressive disorder (MDD), is a multifaceted mental illness involving the interplay of neurobiological, genetic, immune, endocrine, and environmental factors. One of the earliest and most widely accepted hypotheses is the monoamine neurotransmitter theory, which suggests that reduced levels of serotonin (5-HT), dopamine (DA), and norepinephrine (NE) contribute to mood dysregulation and emotional disturbances. These neurotransmitters are vital for regulating emotions, motivation, and cognitive functions. In MDD, disturbances in these levels disrupt neural communication and contribute to symptoms such as low mood, anhedonia, and fatigue. Astrocytes, supporting glial cells in the brain, also regulate the availability of these neurotransmitters. Dysfunction of astrocytes can impair neurotransmitter recycling and exacerbate depressive symptoms.21 Current antidepressants, such as SSRIs and SNRIs, work by increasing the levels of these monoamines in the brain, although they often require several weeks to show therapeutic effects and may not work for all patients.
Another key component in the pathophysiology of depression is the dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, which controls the body’s response to stress. Chronic stress leads to the persistent activation of the HPA axis and excessive secretion of glucocorticoids such as cortisol. Prolonged exposure to high cortisol levels can damage brain structures such as the hippocampus, which is essential for learning, memory, and mood regulation. Normally, the HPA axis is regulated through a negative feedback loop, but in depression, this feedback becomes impaired, leading to a cycle of stress sensitivity and neurodegeneration. Additionally, hormonal imbalances involving thyroid hormones and sex hormones may further influence the risk and progression of depressive symptoms.22
Genetic and epigenetic factors also contribute significantly to MDD. Studies estimate that depression has a heritability of around 30–50%, and several genes associated with neurotransmission (eg, DRD2), neuroplasticity (eg, BDNF), and stress regulation (eg, FKBP5) have been identified. However, genes alone do not fully explain the disorder. Epigenetic mechanisms—such as DNA methylation and histone modification—regulate how genes are expressed in response to environmental factors. Early-life stress, trauma, and chronic adversity can lead to long-lasting epigenetic changes that increase vulnerability to depression.22
Emerging research supports the inflammatory hypothesis of depression, which suggests that chronic low-grade inflammation plays a role in its development. Elevated levels of pro-inflammatory cytokines, such as IL-1β, IL-6, TNF-α, and CRP, have been observed in patients with MDD. These cytokines can influence brain function by crossing the blood brain barrier (BBB) or activating neural pathways. Inflammation disrupts neurotransmitter metabolism, inhibits the growth of new brain cells, and contributes to neuroplastic deficits. One particular mechanism of interest is the NLRP3 inflammasome, a protein complex within immune cells that is activated during stress and inflammation. Once activated, it promotes the release of inflammatory mediators, leading to further neural damage and mood disturbances. Interestingly, some antidepressants have shown anti-inflammatory properties, suggesting dual mechanisms of action.23
The neuroplasticity hypothesis of depression posits that reduced synaptic plasticity and impaired neurogenesis are central to its pathophysiology. Brain-derived neurotrophic factor (BDNF), a protein that supports neuron growth and function, is often found at lower levels in people with depression. Chronic stress suppresses BDNF production, which may lead to neuronal atrophy and reduced connectivity in critical brain areas such as the hippocampus and prefrontal cortex. Antidepressant therapies have been shown to restore BDNF levels, contributing to functional recovery. Intracellular signalling pathways like ERK, PI3K/AKT, and CREB also play key roles in maintaining synaptic health and are often impaired in MDD.24
Glial cells, particularly astrocytes and microglia, are gaining attention in depression research. Astrocytes help regulate neurotransmitters, maintain ion balance, and support neurons metabolically. In MDD, astrocyte density and function are often reduced, contributing to disrupted brain signalling and energy imbalances. Microglia are the brain’s resident immune cells and become activated in response to stress or injury. Overactivated microglia release harmful cytokines and reactive oxygen species, further damaging neurons and promoting a pro-inflammatory brain environment. Altered communication between neurons and microglia, involving receptors like CX3CR1 and CD200R, has been linked to depressive symptoms. Neuroimaging studies have shown structural and functional changes in the brains of individuals with depression. Key areas affected include the prefrontal cortex, hippocampus, amygdala, and nucleus accumbens. These regions often exhibit reduced volume, synaptic density, and neural activity. Such changes are associated with symptoms like low motivation, memory problems, and emotional instability. Importantly, these alterations are not necessarily permanent—successful treatment can sometimes reverse or improve brain structure and function through neuroplasticity.25
Beyond the brain, systemic factors like the gut-brain axis and oxidative stress also contribute to depression. The gut microbiota plays a crucial role in regulating mood and brain function. Disruption in gut bacteria (dysbiosis) can increase gut permeability, allowing toxins like LPS to enter the bloodstream and trigger inflammation that affects the brain. Additionally, depression is linked to oxidative stress, a state in which the body’s ability to neutralize harmful free radicals is compromised. This results in damage to neurons and further promotes inflammation and energy deficits.26
In conclusion, depression is a disorder with a broad biological footprint, involving neurotransmitter imbalances, HPA axis dysfunction, inflammation, impaired neuroplasticity, glial cell dysfunction, genetic and epigenetic influences, structural brain changes, and systemic metabolic imbalances. These interconnected pathways offer valuable insights for developing more effective and personalized treatments.
Types of Depression
The need for appropriate therapy and medication is ascertained after the measurement of severity and categorization of the type of depression. Severity of depression can be graded into mild, moderate severe, and psychotic.19 These grades are assigned based on evaluation of instances of recurrence of symptoms along with the severity of functional impairment of the affected individual. The classification of depression is given as per the grading parameters of the Hamilton Depression Rating Scale and Montgomery–Asberg Depression Rating Scale.27,28 As presented in Table 1, the various types of depression are described as follows.29
 |
Table 1 Classification of Depression and Descriptions |
Major depressive disorder is the most prevalent form of depression, characterized by persistent feelings of sadness, exhaustion, and a lack of motivation lasting at least two weeks. Individuals may also experience sleep disturbances and appetite loss. The severity of the disorder varies, ranging from mild to severe, depending on the number and intensity of symptoms. While some people experience only a single episode of depression, others may suffer from recurrent episodes, in which case it is classified as recurrent depressive disorder.30 Similarly, persistent depressive disorder, also known as chronic depression, lasts for more than two years. Although the severity of symptoms fluctuates, individuals rarely experience symptom-free periods, making it particularly challenging. Some people may experience a milder form of persistent mood disorder, where symptoms are less severe but still distressing due to their long-lasting nature. If persistent depressive disorder occurs alongside major depressive disorder, it is termed “double depression”.31
Other specific types of depression include seasonal affective disorder (SAD), postnatal depression, and premenstrual dysphoric disorder (PMDD).32,33 SAD occurs during the darker months of autumn and winter due to reduced sunlight exposure and typically resolves in spring. Postnatal depression affects some mothers after childbirth, causing severe mood swings and sadness, sometimes making it difficult for them to care for their baby. This condition can be especially distressing as societal expectations often pressure new mothers to feel happiness, leading to feelings of guilt and isolation. PMDD, on the other hand, affects some women in the latter half of their menstrual cycle, causing extreme mood swings, irritability, fatigue, and difficulty concentrating, often accompanied by physical symptoms like abdominal cramps and breast tenderness.
Bipolar disorder, also known as manic depression, is another condition in which individuals experience alternating episodes of depression and mania. During depressive phases, individuals exhibit typical depression symptoms, while manic phases bring heightened energy, extreme self-confidence, and impulsive behavior. Manic episodes may cause individuals to lose touch with reality, leading to reckless decisions, financial troubles, or dangerous situations. The drastic mood swings of bipolar disorder make it a particularly complex condition, requiring careful management and treatment.34
Conventional Therapy for Depression
Measurement of severity and categorization of the type of depression are the usual steps followed to ascertain the need for appropriate therapy. Various approaches involved in the treatment of depression include various non-drug therapies like music therapy and family therapy. Medically, gene therapy and drug delivery therapy are used to treat depression. The psychiatrist often determines the treatment plan based on the patient’s comfort level, medical history, and overall health.35,36
Non-Drug Therapy
Music therapy draws its effectiveness from the therapeutic relationship between the therapist and the patient and relies on emotions and feelings to be expressed, discussed, and evaluated on the basis of playing an instrument, listening to a song, and talking about how it makes them feel.37–39 Another therapy is family therapy, where family members of the affected individual are trained to become a support system for the patient which speeds up the recovery process.35,40,41 Electroconvulsive therapy is another way of treating depression, which involves the implementation of electrical current on the scalp in order to initiate a generalized epileptic seizure.37,38 Electroconvulsive therapy is believed to cause rapid alterations in brain chemistry, leading to rapid reversal in the symptoms of depression. Furthermore, cognitive therapy is a direct active and systematically structured approach for the treatment of depression.42
Gene Therapy
This therapy is aimed at correcting the cause of depression by a protein-mediated process. In the nucleus accumbens area of the brain, a protein p11 is responsible for the regulation of serotonin receptors 5-HT1B and 5-HT4, in the absence of this protein or due to other conditions where faulty synthesis of this protein is taking place the patient is highly prone to depression. To overcome this, the insertion of p11 protein by gene therapy can be performed to avoid depression.43
Chemical Drug Therapy
Treatment involves drug substances designed to rectify the biochemical causes of depression in the brain is termed as drug delivery therapy. In this type of therapy, the key lies in restabilizing the imbalance of neurotransmitters in the brain. The drugs employed to treat depression are categorized by their mechanism of action, which they act on to inhibit the aforementioned reuptake process include SSRIs, Selective Noradrenaline Reuptake Inhibitors (SNRIs), MAOIs and atypical antidepressants.44 SNRIs drugs are also found to be useful for certain chronic pain conditions as well.45 Using MAOIs requires a strict diet because of supposedly fatal interactions with foods containing tyramine such as certain cheeses, pickles, grapes and wine.46 Atypical antidepressants are employed when no improvement is seen with SSRIs. They include bupropion, venlafaxine, mirtazapine, and nefazodone.47
Drawbacks of Conventional Therapy
Antidepressant medications have many challenges, due to which a novel drug delivery mechanism is the need of the hour for patients across the globe.48 Additionally, there is no standardized approach to treat depression and healthcare professionals usually must rely on a highly specific and customized treatment regime.49 Though in the last 10 years more than 500 trials have been conducted to examine the effects of antidepressant medication.50
Nanotechnology Based Brain Drug Delivery
Ideally, a targeted delivery must exclusively transport the administered dose of the medicament to the targeted site of action, and minimal drug should reach the nearby tissues or organs. Strategies that are employed for targeting the drug to the brain have been divided into invasive and non-invasive categories. By utilizing nanotechnology, promising results have been obtained for treating many neurological disorders.51–53 A number of nanoparticulate strategies for therapeutic agents have been used to deliver medications to the brain.54 Due to their small size and surface functionalization with target-specific ligands, nanoparticles with dimensions ranging from 1 to 100 nm make it much simpler for drugs to pass through the BBB.55 The various approaches for the treatment of depression by the utilization of nanocarriers include polymeric nanoparticles, solid lipid nanoparticles, nanostructured lipid carriers, co-polymeric micelles, nanoemulsions, nanosuspensions, polymeric core-shell nanoparticles, magnetic nanoparticles, smart nanovehicles, liposomal nanoconstructs, and recently developed nanocubic vesicles.56
Though the various novel nanotherapeutic ways for drug delivery mentioned above the route of administration for these nanocarriers is often met with limited and nonconventional choices due to various complexities arising due to conventional delivery routes. The novel approach of the intranasal route of drug delivery is of great importance. This method of drug delivery is a highly advantageous route to transport medicaments into the brain without the intervention of the BBB.57 The intranasal route, however advantageous as it may seem, is not void of drawbacks. First of all, the volume of the intranasal cavity is quite small, that limits the amount of drug that can be instilled into the mucosal linings. Secondly, due to the low drug retention time, the administered drug has limited absorption. Further reduction in absorption and subsequent reduction in bioavailability are caused by mucociliary clearance and enzymatic degradation.
Mechanism of Drug and Nanoparticles Absorption via Nasal Route
The nasal cavity is made up of three distinct regions, respiratory, olfactory, and nasal vestibule, based on their respective roles and anatomical structure. The nasal vestibular region occupies the anterior nasal cavity and has a relatively modest surface area of only 0.6 cm2. The competence of antidepressants to get absorbed in the nasal vestibule is limited by nasal hairs and mucus as they filter fine particles and debris. The middle turbinate, the corresponding part of the septum, and the medial surface of the superior turbinate are where the olfactory region is situated and approximately 10 cm2 of surface area is accessible.58 The somewhat floppy layer of connective tissue underneath the epithelial cells, known as the lamina propria (LP), includes lymphatic, blood, and nerve vessels. As the name indicates, ciliated cells have cilia that enhance their surface area. They aid in the mucociliary clearance process by assisting the mucus in moving toward the nasopharynx. As a result, the nasal respiratory region is a site for substantial drug transfer into the systemic circulation, as well as a high degree of vascularization.59
Another neurological pathway that extends from the nose to the brain is the trigeminal nerve. The nerve originates from the pons, from which the ocular and maxillary branches of the trigeminal nerve arise and supply the nasal cavity. The trigeminal nerve mostly comprises the myelin sheath, which is composed of Schwann cells.60 The olfactory mucosal vasculature receives its blood supply from vessels regulated by non-myelinated branches of the trigeminal nerve, which modulate blood flow. In a study involving intranasal administration, fluorescently labeled insulin, known to exhibit antidepressant properties, was delivered and subsequently observed to enter the extracellular matrix surrounding the trigeminal nerve, facilitating its transport to the brain.61
The nasal cavity, particularly the olfactory epithelium, facilitates drug and nanoparticle absorption through three primary pathways: intracellular, transcellular, and paracellular routes, as shown in Figure 2.62 The intracellular route allows direct penetration of drugs into epithelial cells, influenced heavily by molecular size and lipophilicity, whereas the transcellular pathway enables molecules, including nanoparticles, to traverse epithelial barriers via processes such as endocytosis and receptor-mediated transport. Nanoparticles particularly excel in this mechanism due to customizable attributes like size, surface chemistry, and targeting capabilities.63 The paracellular pathway, involving the diffusion of drugs through tight junctions between adjacent cells, is vital for hydrophilic substances and large biomolecules, such as peptides and proteins. Enhancing this route’s permeability often requires nanoparticle-based formulations or permeation enhancers to modulate tight junction openings effectively. Carefully designed nanoparticles with optimized size, surface charge, and mucoadhesion characteristics significantly improve mucosal residence time and absorption efficiency. Ultimately, harnessing these absorption mechanisms through strategic nanoparticle engineering presents substantial potential in improving nasal delivery systems, enabling rapid systemic drug uptake, and facilitating direct nose-to-brain transport, thus circumventing traditional barriers like the BBB and offering innovative therapeutic approaches for neurological and systemic diseases.64
The nasal route has certain limitations for the delivery of the drug. Only the small dose of the drug can be accommodated due to the small surface area. Also, the low molecular weight drugs can travel down the axons from the olfactory cortex to the cerebellum through the olfactory bulb, but larger molecular weight drugs face issues in the absorption. About 95% of nasal secretions are made up of water, 2% of which is mucin, 1% is salt, 1% is other proteins, which include albumin, immunoglobulins, lysozymes, and lactoferrin, and 1% is lipids. Particles are cleaned from the nose every 15 to 20 minutes because they pass through the nose at a pace of around 5 to 6 mm/min. Additionally, the nasal cavity contains enzymes such as glutathione S-transferases, carboxylesterases, and isoforms of the cytochrome P450 (CYP1A, CYP2A, and CYP2E).65 Biologically adhesive (mucoadhesive) excipients can be added to the formulation for enhancing the residence time of the drug in the nasal cavity.66
 |
Figure 2 Schematic representation of nasal regions and drug absorption pathways across the olfactory epithelium. |
Nanotechnology for Depression Treatment Through Nasal Route
Various nanotherapeutic agents have been developed for administration via the olfactory route to treat depression, as illustrated in Figure 3. The following section highlights several notable and promising approaches employing these nanocarriers for the intranasal delivery of antidepressant medications.
 |
Figure 3 Types of nanotherapeutic agents used to treat depression. |
Polymeric Nanoparticles (PNPs)
PNPs are small, colloidal systems that can be comprised of both organic and synthetic polymers and have a wide variety of sizes on the nanometric scale. They are made up of a hydrophilic crown that gives the nanoparticles stability and a dense polymeric matrix core that is ideal for encasing drugs with a lipophilic structure.67
Nanocapsules and nanospheres are the two primary varieties of polymeric PNPs.68 The medicament is either deposited on top of the nanosphere or preserved inside it, due to the continuous polymeric network that reinforces the nanospheres. Jani et al incorporated agomelatine into poly-lactic-coglycolic acid (PLGA) nanoparticles for the intranasal delivery of the drug to treat depression. It was discovered that when compared to a simple drug solution, the drug-loaded PLGA nanoparticles had a higher drug release and greater tissue permeability.69 To enhance the pharmacokinetic and pharmacodynamic characteristics of duloxetine a medication used to treat MDD, nasal chitosan-grafted polymeric nanoparticles were created by Salem et al. They found that in contrast to duloxetine solution, the chitosan-grafted polymeric nanoparticles showed a fourfold enhancement in drug permeation after 24 hours, according to an ex vivo permeation investigation.70
Further work should focus on in vivo biodistribution studies pharmacokinetic profiling of drug concentration in plasma and brain, and chronic toxicity studies on nasal mucosa. Mucoadhesion and nasal residence time should be assessed for formulation retention. Behavioral studies, including cognitive and anxiety models, can support antidepressant activity. Comparative studies with other delivery routes should also be assessed.
Solid Lipid Nanoparticles (SLNs)
SLNs are matrix nanoparticles comprised of solid lipids distributed in water or an aqueous surfactant solution. They have outstanding physical stability with no organic solvents used in their synthesis and have strong biocompatibility and tolerability. They also allow regulated drug release and lengthen the nasal retention period of the formulation due to their occlusive action and adherence to the mucosa.71 They also have the limitation of possible drug expulsion during storage due to the crystallization process and an undesirable increase in particle size due to agglomeration, which can result in an immediate and unwanted drug release.72 Polyethylene glycol (PEG) is a hydrophilic and biocompatible polymer that can be employed to improve the problem of poor stability. PEG also functions as a penetration enhancer and nanoparticle stabilizer.73
Zolmitriptan, a 5-HT-1B agonist, is clinically advised for the treatment of migraine, but studies have revealed its potential use for the treatment of depression and conjoined loss of memory.74 Intranasal administration of zolmitriptan solid lipid nanostructure via the olfactory route achieved a drug targeting potential of around 90% when assessed as compared to traditional zolmitriptan tablets.75 Further, the implementation of SLNs for depression treatment was done by Yasir et al by the formulation of buspirone-loaded SLNs. The results indicated that an intranasally administered formulation comprising SLNs had 2.18 times greater bioavailability in the brain than intranasal buspirone solution. Furthermore, the intranasal SLNs of the drug showed 2.66 times more bioavailability than intravenously administered SLNs of buspirone. The study also used confocal laser scanning microscopy (CLSM) imaging with rhodamine 123 (ROD-123) dye to assess brain targeting as shown in Figure 4. Results showed that intranasally administered SLNs delivered more ROD-123 to the brain compared to the ROD-123 solution. This improved delivery may be due to the mucoadhesive properties of SLNs, which reduce mucociliary clearance, and their ability to bypass P-glycoprotein (P-gp) efflux, which otherwise limits brain retention of the free dye. This indicated the better targeting efficiency of created SLNs through the nasal route.76
 |
Figure 4 CLSM images of brain at 30 min after intranasal administration of ROD-123 tagging buspirone -SLNs (A); ROD-123 Sol (B). Reproduced from Yasir M, Chauhan I, Zafar A, et al. Buspirone loaded solid lipid nanoparticles for amplification of nose to brain efficacy: formulation development, optimization by Box-Behnken design, in-vitro characterization and in-vivo biological evaluation. J Drug Delivery Sci Technol. 2021;61:102164.chi. © 2020 Elsevier B.V. All rights reserved.76 |
Advanced behavioral studies should be conducted using additional models to confirm antidepressant efficacy and assess improvements in cognitive function. Chronic toxicity and immunogenicity can be evaluated via animal toxicity studies, including blood biochemistry and organ histology. To enhance the formulation, mucoadhesive polymers can be added to improve nasal retention and absorption.
Nanostructured Lipid Carriers (NLCs)
NLCs are made up of a combination of solid and liquid lipids that combine to produce an imperfect crystalline matrix into which drugs can be introduced. NLCs are biodegradable and typically consist of physiological lipids, resulting in minimal toxicity and tolerance in the body. Because hydrophobic molecules are more soluble in liquid lipids than in solid lipids, this nanosystem can achieve a better drug encapsulation efficiency.77,78
Alam et al performed the pharmacodynamic studies on rats to investigate the efficiency of duloxetine-enriched NLCs and the simple solution form of the drug following intranasal administration. The results indicated the presence of a high concentration of duloxetine-loaded NLCs in the brain of the study animal as compared to simple duloxetine solution.79 In another study, the efficacy of intranasal venlafaxine NLCs was checked by Shah et al and the results showed significantly higher drug penetration across the goat nasal mucosa.80
The studies should also have focused on investigating the mucoadhesive properties and nasal residence time to ensure optimal formulation performance and sustained therapeutic efficacy. Furthermore, exploring combination therapies by co-encapsulating other neuroprotective agents or synergistic antidepressants could provide additional benefits.
Nanoemulsions (NEs)
A liquid nano-dispersion system, created by two types of insoluble liquid, stabilized by surfactant and co-surfactant, is known as a nanoemulsion. NEs are colloidal particulate systems with submicron particle sizes that serve as drug carrier molecules. Their sizes range between 10 and 1000 nm. These carriers are solid spheres with an amorphous, lipophilic, negatively charged surface. They improve the therapeutic efficiency of the drug while minimizing side effects and toxic responses.81 Ghazwani et al developed an intranasal thermoreversible NE containing mirtazapine for the treatment of depression. Based on the results, it was concluded that the developed formulation has great potential to be used as an intranasal gel for treating depression in patients.82 In another study, Ahmad et al developed an NE containing melatonin with mucoadhesive properties for increased retention time in the nasal mucosa. The developed intranasal NEs of the drug were subjected to pharmacokinetic and pharmacodynamic studies. The results showed the increased drug concentration in the brain leading to improvement in brain bioavailability and treatment of depression.83
To fully assess the safety during administration, human nasal cell line testing is essential, and the study should also incorporate in vitro BBB models to simulate in vivo conditions more accurately so as to provide a better understanding of the efficacy of the formulation. Long-term safety on nasal mucosa after repeated administration should also be addressed.
Nanosuspensions
Nanosuspensions are colloidal dispersions of nanosized drug particles stabilized by surfactants that are submicron in size. Nanosuspensions are made up of a weakly water-soluble medication suspended in a dispersion of matrix material.84 These can be used to improve the solubility of medications that are insoluble in water and lipid environments. Because of the smaller particle size, it is possible to administer poorly soluble medications intravenously without clogging the blood capillaries.85
Khalil et al formulated a nanosuspension consisting of Hypericum perforatum, which showed better antidepressant activity in rat brain.86 In another study, the nanosuspension of nortriptyline hydrochloride, an antidepressant drug, was prepared for the brain targeting through nasal route. The optimized formulation had an average particle size of 10–100 nm with an increase in solubility, and the desired viscosity to adhere to the nasal mucosa. It was concluded that the nanosuspension is one of the best possible approaches for targeting drugs to the brain through the nasal route.87
The potential for irritation due to the small particle size and surface properties of the nanoparticles could lead to mucosal toxicity, especially with repeated administration. Challenges of maintaining optimal osmolarity to prevent irritation or damage to the nasal mucosa need to be addressed. The formulation must be designed to balance effective absorption while minimizing potential adverse effects on the nasal epithelium, ensuring both safety and efficacy in long-term use.
Nanogels
By possessing a small volume, nanogels are potential drug nanocarriers that can reach the tiniest capillary veins and permeate tissues through transcellular routes. By means of their interpenetrating network structure, nanogels encapsulate medications well and can be beneficial in delivering the drugs through the nasal route.88 Nanogel-based nanocarriers have overcome the constraint of regulating medication distribution throughout the body, boosting the efficacy of targeted medicines. These nanocarriers have improved permeability and retention effects, enabling stable and responsive treatments.89 The recent implementation of nanogels for the treatment of depression was done by Xu et al by the application of albiflorin into a nanogel for the treatment of depression. The results indicated the quick entry of the drug into the brain after intranasal delivery.90 Abdelnabi et al prepared an in situ nanovesicular gel of buspirone for the treatment of anxiety associated with depression, wherein the in vivo study data showed 3.26 times increase in bioavailability when compared to a drug solution applied via the nasal route.91
Neurobehavioral evaluations are necessary to strengthen the claims of antidepressant efficacy such as the Open Field Test and Tail Suspension Test, which should be performed to successfully gauge antidepressant activity. The biocompatibility and potential toxicity of the gel matrix and the incorporated materials need thorough evaluation to ensure they do not cause adverse effects especially with repeated dosing. The stability of nanogels in biological fluids and their potential for degradation or aggregation over time are also concerns that need to be addressed for long-term therapeutic efficacy.
Magnetic Nanoparticles (MNPs)
MNPs are a type of nanoparticle that may be tweaked using magnetic fields. These magnetic fields can be used for the magnetoporation of drugs, which refers to the capacity of cells to create temporary gaps in their membranes, such as the BBB endothelium, in order to increase targeting and delivery.92 MNPs with a diameter of 50–200 nanometers are magnetic nanoparticle clusters formed of a number of individual magnetic nanoparticles. These clusters serve as the foundation for their subsequent magnetic assembly into magnetic nanochains. Researchers are growing increasingly interested in the use of magnetic nanomaterials in neuroscience, with some spectacular discoveries on brain control based on targeted binding of magnetic nanoparticles to neurons. The magneto-thermal effect can be used to regulate brain function by activating ion channels. The effects of magneto-electric nanoparticles on intrinsic neuro-electric signals have been predicted and researched.93 The application of MNPs has been explored for the treatment of depression. Lu et al demonstrated that by the use of gold MNPs, the depression-like symptoms of mice can be treated by the utilization of superparamagnetic nanodrugs.94
One major issue is the potential toxicity of MNPs as the accumulation of magnetic nanoparticles may lead to adverse effects over time. The surface modification of MNPs is crucial for ensuring biocompatibility as well as for controlling the drug release and preventing aggregation. Additionally, there is a need for benchmarking against other nanoparticle systems and conventional delivery options to evaluate the relative safety and efficacy. This comparative analysis will provide insights into the advantages and limitations of the prepared formulation in relation to other established drug delivery methods.
Smart Nanovesicles (SNVs)
SNVs are a class of nanotherapeutic agents that are utilized for specific targeting of DNA, proteins, and cellular metabolites to provide the necessary therapeutic action. The SNVs are made up of a diagnostic agent coated on the surface and/or a drug encapsulated in a biocompatible polymeric core with suitable surface properties and size.95,96 Agyare et al developed smart nanovehicles that targeted the amyloid deposits and plaques in the parenchyma of the animal brain.97 Taymouri et al developed an in-situ gel containing nanotranserosomes which were loaded with aripiprazole. The in-vivo data revealed that, when compared to the other treatment groups, the group of rats receiving aripiprazole-loaded nanotransferosomes had a significant increase in swimming and climbing time and a decrease in locomotor activity and immobility time. As a result, aripiprazole-loaded nanotransferosomes were discovered to offer favorable properties for therapeutic improvement.98
The incurred costs for preparing the formulation and chances of scale-up for production also need to be considered as the formulation should be feasible for mass production and economically viable for the patients. Further research needs to be done to address the pharmacodynamic and pharmacokinetic aspects of the prepared formulations. The summary of the key outcomes of each nanotherapeutic agent developed for the treatment of depression via the intranasal route is mentioned in Table 2.
 |
Table 2 Key Outcomes from Each Developed Intranasal Nanoformulations for Depression |
Comparative Analysis of Intranasal Nanotherapeutic Approaches for Brain-Targeted Drug Delivery in Depression Treatment
As described in the previous section, the advancement of nanotechnology has provided a promising avenue for the treatment of depression by improving intranasal drug delivery to the brain. This delivery route bypasses the BBB, one of the most formidable challenges in neuropharmacology, which blocks approximately 98% of small-molecule drugs and nearly all large-molecule therapeutics. Nanotechnology plays a central role in enhancing the efficacy of intranasal drug delivery. Nanoparticles, due to their small size and modifiable surface properties, can encapsulate a wide range of antidepressant agents and protect them from degradation, enhance their solubility, and improve their pharmacokinetic profiles. Each of these nanocarriers possesses distinct advantages and limitations (Table 3), making their selection crucial for optimizing brain-targeted drug delivery.12
 |
Table 3 Comparative Analysis of Intranasal Nanotherapeutic Approaches for Brain-Targeted Drug Delivery in Depression Treatment |
Lipid-based nanocarriers, such as liposomes, SLNs, and NLCs, are particularly well-suited for this purpose. These systems are typically composed of biocompatible and biodegradable lipids that can encapsulate both hydrophilic and hydrophobic molecules. Liposomes, for instance, have been shown to significantly increase the brain bioavailability of drugs like donepezil when administered intranasally, with minimal toxicity to peripheral organs. Similarly, SLNs and NLCs have demonstrated improved brain targeting, controlled drug release, and enhanced therapeutic effects in animal models of neurodegenerative diseases and depression.99,100
Polymer-based nanoparticles represent another important class of carriers for intranasal delivery. Natural polymers such as chitosan and alginate are widely used due to their mucoadhesive properties and ability to open tight junctions in the nasal epithelium, thereby facilitating paracellular drug transport. Chitosan, for instance, becomes positively charged in the slightly acidic environment of nasal mucus, which helps it adhere to the negatively charged mucosal surfaces, increasing the residence time of the drug.101,102 Modified derivatives like thiolated-chitosan (TC) have been shown to further enhance mucoadhesion and drug permeation. In one study, selegiline-loaded TC nanoparticles administered intranasally significantly improved behavioral markers of depression and restored mitochondrial function in rodent models, outperforming unmodified chitosan formulations.103
Comparing lipid-based and polymer-based systems reveals distinct strengths and limitations for brain-targeted delivery in depression therapy. Lipid-based carriers generally excel in transporting lipophilic drugs and facilitating transcellular absorption through the nasal epithelium due to their membrane-mimetic properties. However, they may suffer from stability issues or limited drug loading in the case of hydrophilic compounds. In contrast, polymer-based systems offer greater flexibility in surface modification and drug release kinetics, often leading to better bioadhesion and sustained drug exposure in the brain.104 For example, venlafaxine-loaded alginate nanoparticles showed superior targeting efficiency and behavioral outcomes in depression models compared to the same drug administered in solution form.105 Thus, the choice of carrier should be tailored to the physicochemical properties of the therapeutic agent and the desired pharmacological outcome.
The physicochemical characteristics of nanoparticles including size, surface charge, and lipophilicity profoundly influence their transport through the nasal cavity and subsequent delivery to the brain. Smaller particles, generally under 100 nm, penetrate nasal mucus more efficiently and exhibit prolonged residence times, allowing for better absorption.106,107 Positively charged nanoparticles tend to interact more robustly with the negatively charged mucosal surfaces, enhancing adhesion and paracellular transport.108 Moreover, the hydrophilic or hydrophobic nature of a carrier can determine the pathway of brain delivery, with hydrophilic carriers often showing wider CNS distribution via the cerebrospinal fluid,109 while hydrophobic carriers concentrate around the olfactory bulb.110 These variables must be carefully balanced during formulation to optimize drug delivery and therapeutic efficacy.
In summary, intranasal nanotherapeutic strategies offer a highly promising approach for delivering antidepressants directly to the brain, bypassing traditional barriers and improving treatment outcomes. The comparative analysis of lipid-based and polymer-based nanocarriers underscores the importance of aligning formulation characteristics with therapeutic goals. Both systems have demonstrated significant potential in preclinical models of depression, offering enhanced brain bioavailability, sustained drug release, and behavioral improvements. Future research should continue to refine these technologies by integrating advanced pharmacokinetic modeling, evaluating long-term safety, and exploring combination therapies that leverage the unique benefits of each nanocarrier system. With further clinical development, intranasal nanotherapeutics could become a cornerstone in the management of depression and other CNS disorders.
Clinical Trials and Real-World Applications
Despite their considerable potential for delivering antidepressant and anxiolytic drugs, nanoparticles face significant challenges, including scalability issues, high production costs, and a lack of extensive clinical trials. These barriers have impeded their widespread adoption in the pharmaceutical market. Consequently, only a limited number of commercial products have been launched to date.111 However, numerous drug candidates and products are currently in various stages of clinical trials, highlighting ongoing efforts to overcome these challenges and advance nanoparticle-based therapeutics.
The only FDA-approved nasal spray currently available on the market for the treatment of treatment-resistant depression (TRD) is Esketamine (SPRAVATO™). SPRAVATO™ is developed and manufactured by Janssen Pharmaceuticals and has been approved by the FDA as a novel antidepressant that operates through a unique mechanism in the brain. Unlike traditional antidepressants, which typically take weeks to show effects, esketamine acts within hours, providing a rapid treatment option for approximately 5 million individuals in the US who do not respond to conventional therapies. Although IV ketamine infusion has a higher success rate (83%) compared to esketamine nasal spray (40%), patient compliance with the nasal spray is generally better due to its ease of administration. Esketamine requires only twice-weekly sessions for four weeks, followed by weekly or biweekly maintenance, whereas IV ketamine involves 5–6 infusions over 2–3 weeks, followed by periodic booster sessions.112,113 From an economic perspective, IV ketamine is generally more cost-effective for healthcare systems than esketamine nasal spray in the treatment of treatment-resistant depression (TRD). Although both therapies require clinical administration and incur significant medication and facility-related costs, IV ketamine is often considered the more favorable option in base-case health economic models. Barbara et al (2024) report that the incremental cost-effectiveness ratio (ICER) for IV ketamine ranges from $867,606 to over $7 million per quality-adjusted life year (QALY), depending on whether clinical trial data or real-world evidence is used. Despite this, esketamine offers substantial financial advantages for patients. As an FDA-approved treatment, esketamine is eligible for commercial insurance coverage and manufacturer assistance programs, which significantly reduce out-of-pocket expenses—benefits typically not available for off-label IV ketamine. While esketamine is associated with higher overall healthcare costs, it remains more accessible to insured individuals. However, the limited follow-up duration in current studies (up to 90 days) restricts the understanding of its long-term economic implications.114,115
Challenges and Future Prospects in Nanocarrier Based Intranasal Treatment of Depression
Issues related to mental health, particularly depression, are expected to be the leading cause of mortality and morbidity worldwide by 2030. According to the World Health Organization, by 2030, depression will be the biggest single healthcare burden, costing $6 trillion globally. It is further estimated that by 2050, over 46 million individuals aged 18 and above will have been diagnosed with a depressive condition, only in the USA. The rise will be most dramatic, by 125%, among males aged 65 and over.116
Treatment of depression has various challenges, including the unacceptability of the patient about their condition, the expectation of immediate treatment benefits and the requirement of long-term therapy.117 Antidepressant intranasal delivery opens up new options for the treatment of the individuals suffering from depression. In comparison to traditional delivery systems, the creation of various nasal administration devices, such as Vianase™, has considerably increased the deposition of antidepressant active components in the olfactory area of the upper nasal cavity.118 It is generally recognized that the progress of intranasal drug transportation benefits from nanocarrier-based formulations. More studies are needed to increase drug delivery performance as well as selectivity of the nanoformulation for brain tissue with reduced neurotoxicity. While there are presently no nanosystems-based pharmaceuticals in the market for the treatment of depression, several studies have demonstrated their effectiveness.119 The majority of the formulations based on nanosystems, as well as those employing neuropeptides and cellular treatments, are in the preclinical stage of the pharmaceutical product development pipeline.120 No nanomedicines have yet been authorized for the treatment of depression. Hence, more research is needed to assess the full potential of intranasal nanosystems for antidepressants, particularly in terms of clinical trial efficacy and safety. Furthermore, the scale-up issues of these formulations should be studied and addressed properly. This indicates the requirement to conduct extensive research to obtain nanosystems with less complicated manufacturing techniques and lower-cost components so that intranasal nanoparticles containing antidepressant drugs are able to enter the market and improve the therapeutic outcomes of these diseases someday.
Published Patents Focusing on the Intranasal Delivery of Nanotherapeutics for the Treatment of Depression
In response to the limitations associated with conventional antidepressant therapies, recent advancements have focused on innovative drug delivery systems. Among these, intranasal nanotherapeutics have gained increasing attention, and several patents have been published in this area. A recently published patent WO2020193838 consists of a pharmaceutical composition for intranasal treatment of depression using nanoparticles of a serotonin-norepinephrine reuptake inhibitor drug encapsulated in PLGA polymer and stabilized with cryoprotectants like trehalose. The nanoparticles are spherical, negatively charged, and sized between 100–500 nm, ensuring effective delivery. Administered intranasally, it bypasses the BBB, enhancing bioavailability and rapid onset. The formulation is stable, efficient, and reduces systemic side effects, offering a promising and innovative approach to managing depression effectively.121
Another notable approach is presented in patent US20180177744 highlighting the use of an advanced method for administering a combination esketamine-arketamine formulation for treating depression. The nanoparticle composition utilizes chitosan or pectin excipients, enabling effective intranasal delivery past the nasal valve to bypass the BBB. With a particle size of 200–300 nm, it offers enhanced bioavailability, rapid therapeutic effects, and reduced systemic side effects. The formulation is versatile for liquid and dry powder forms, offering innovative solutions for treating depression.122
Further advancement is demonstrated in WO2023105415 the patent discusses compositions for the intranasal administration of drugs with high plasma protein binding to treat central nervous system disorders. The compositions are specifically designed for drugs that bind at a level of at least 90% to plasma proteins, such as SSRIs including sertraline and piroxicam. The intranasal administration can be single or multiple and is suitable for diseases like depression, PTSD, social anxiety, Parkinson’s, Alzheimer’s, autoimmune encephalitis, and brain tumors. The formulations for intranasal administration include options like nanoemulsion, a nanoparticle, a nanostructured lipid carrier, a chitosan nanoparticle and may include excipients or additives. Devices for intranasal delivery are also highlighted, offering targeted and sustained brain delivery to enhance therapeutic outcomes.123
Conclusion
Since depression is still the biggest cause of morbidity worldwide, an antidepressant with a swift onset of action might alter how this chronic condition is dealt with. The intranasal antidepressant delivery is an appealing prospect in this sector since it is non-invasive and provides quick action, increased bioavailability, and medication dosage reduction, as well as the capacity to circumvent the BBB and lessen some systemic adverse effects. Furthermore, incorporating pharmaceuticals into nanosystems has also shown the improvement in treatment effectiveness, with intranasal administration being particularly important. Currently, only one intranasal antidepressant, esketamine (in conjunction with an oral antidepressant), is authorized for the treatments. It is important to conduct more research in the future. In the future years, the pharmaceutical industry must evolve and improve the novel techniques for producing accurate, consistent, and precise intranasal dosage forms.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Evans-Lacko S, Aguilar-Gaxiola S, Al-Hamzawi A. et al. Socio-economic variations in the mental health treatment gap for people with anxiety, mood, and substance use disorders: results from the WHO World Mental Health (WMH) surveys. Psychological Medicine. 2018;48(9):1560–1571. doi:10.1017/S0033291717003336
2. Yadav K, Rani A, Tyagi P, Babu MR. The Application of Nanotechnology and Nanomaterials in Depression: an Updated Review. Precision Nanomed. 2023;6(2):1023–1047. doi:10.33218/001c.82215
3. Peeters F, Berkhof J, Delespaul P, Rottenberg J, Nicolson NA. Diurnal mood variation in major depressive disorder. Emotion. 2006;6(3):383. doi:10.1037/1528-3542.6.3.383
4. Jin Z, Han Y, Zhang D, et al. Application of intranasal administration in the delivery of antidepressant active ingredients. Pharmaceutics. 2022;14(10):2070. doi:10.3390/pharmaceutics14102070
5. Nemeroff CB. The state of our understanding of the pathophysiology and optimal treatment of depression: glass half full or half empty? Am J Psychiatry. 2020;177(8):671–685. doi:10.1176/appi.ajp.2020.20060845
6. Datta S, Suryadevara U, Cheong J. Mood disorders. Continuum. 2021;27(6):1712–1737.
7. Institute of Health Metrics and Evaluation. Global Health Data Exchange (Ghdx). Seattle, WA, USA: Institute of Health Metrics and Evaluation; 2021.
8. Ramnath S, Suri G. Managing depression in India: opportunities for a targeted smartphone app. Int J Social Psychiatry. 2021;67(8):1035–1045. doi:10.1177/00207640211032253
9. Panek M, Kawalec P, Pilc A, Lasoń W. Developments in the discovery and design of intranasal antidepressants. Expert Opin Drug Discov. 2020;15(10):1145–1164. doi:10.1080/17460441.2020.1776697
10. Xu J, Tao J, Wang J. Design and application in delivery system of intranasal antidepressants. Front Bioeng Biotechnol. 2020;8:626882. doi:10.3389/fbioe.2020.626882
11. Fong CW. Permeability of the blood–brain barrier: molecular mechanism of transport of drugs and physiologically important compounds. J Memb Biol. 2015;248(4):651–669. doi:10.1007/s00232-015-9778-9
12. Antunes JL, Amado J, Veiga F, Paiva-Santos AC, Pires PC. Nanosystems, drug molecule functionalization and intranasal delivery: an update on the most promising strategies for increasing the therapeutic efficacy of antidepressant and anxiolytic drugs. Pharmaceutics. 2023;15(3):998. doi:10.3390/pharmaceutics15030998
13. Zorkina Y, Abramova O, Ushakova V, et al. Nano carrier drug delivery systems for the treatment of neuropsychiatric disorders: advantages and limitations. Molecules. 2020;25(22):5294. doi:10.3390/molecules25225294
14. Alberto M, Paiva-Santos AC, Veiga F, Pires PC. Lipid and polymeric nanoparticles: successful strategies for nose-to-brain drug delivery in the treatment of depression and anxiety disorders. Pharmaceutics. 2022;14(12):2742. doi:10.3390/pharmaceutics14122742
15. Trivedi R, Minglani VV, El-Gazzar AM, et al. Optimization of pramipexole-loaded in situ thermosensitive intranasal gel for Parkinson’s disease. Pharmaceuticals. 2024;17(2):172. doi:10.3390/ph17020172
16. Mutingwende FP, Kondiah PP, Ubanako P, Marimuthu T, Choonara YE. Advances in nano-enabled platforms for the treatment of depression. Polymers. 2021;13(9):1431. doi:10.3390/polym13091431
17. Schroder HS, Duda JM, Christensen K, Beard C, Björgvinsson T. Stressors and chemical imbalances: beliefs about the causes of depression in an acute psychiatric treatment sample. J Affective Disorders. 2020;276:537–545. doi:10.1016/j.jad.2020.07.061
18. Hasler G. Pathophysiology of depression: do we have any solid evidence of interest to clinicians? World Psychiatry. 2010;9(3):155. doi:10.1002/j.2051-5545.2010.tb00298.x
19. Baxter LR, Schwartz JM, Phelps ME, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiatry. 1989;46(3):243–250. doi:10.1001/archpsyc.1989.01810030049007
20. Baillie D, McCABE R, Priebe S. Aetiology of depression and schizophrenia: current views of British psychiatrists. Psychiatric Bulletin. 2009;33(10):374–377. doi:10.1192/pb.bp.108.021899
21. Sălcudean A, Popovici R-A, Pitic DE, et al. Unraveling the Complex Interplay Between Neuroinflammation and Depression: a Comprehensive Review. Int J Mol Sci. 2025;26(4):1645. doi:10.3390/ijms26041645
22. Benatti BM, Adiletta A, Sgadò P, Malgaroli A, Ferro M, Lamanna J. Epigenetic Modifications and Neuroplasticity in the Pathogenesis of Depression: a Focus on Early Life Stress. Behav Sci. 2024;14(10):882. doi:10.3390/bs14100882
23. Correia AS, Cardoso A, Vale N. Oxidative stress in depression: the link with the stress response, neuroinflammation, serotonin, neurogenesis and synaptic plasticity. Antioxidants. 2023;12(2):470. doi:10.3390/antiox12020470
24. Humo M, Lu H, Yalcin I. The molecular neurobiology of chronic pain–induced depression. Cell Tissue Res. 2019;377(1):21–43. doi:10.1007/s00441-019-03003-z
25. Cui L, Li S, Wang S, et al. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduc Target Ther. 2024;9(1):30. doi:10.1038/s41392-024-01738-y
26. Edition F. Diagnostic and statistical manual of mental disorders. Am Psychiatr Assoc. 2013;21(21):591–643.
27. Edition F. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American psychiatric association; 1980:205–224.
28. Van Loo HM, De Jonge P, Romeijn J-W, Kessler RC, Schoevers RA. Data-driven subtypes of major depressive disorder: a systematic review. BMC Med. 2012;10(1):1–12. doi:10.1186/1741-7015-10-156
29. InformedHealth.org. Depression: Learn More – Types of Depression. 2024.
30. Marx W, Penninx BW, Solmi M, et al. Major depressive disorder. Nature Reviews Disease Primers. 2023;9(1):44. doi:10.1038/s41572-023-00454-1
31. Walter HJ, Abright AR, Bukstein OG, et al. Clinical practice guideline for the assessment and treatment of children and adolescents with major and persistent depressive disorders. J Am Acad Child Adolesc Psychiatry. 2023;62(5):479–502. doi:10.1016/j.jaac.2022.10.001
32. Slyepchenko A, Minuzzi L, Frey BN. Comorbid premenstrual dysphoric disorder and bipolar disorder: a review. Frontiers in Psychiatry. 2021;12:719241. doi:10.3389/fpsyt.2021.719241
33. Chen Z-W, Zhang X-F, Tu Z-M. Treatment measures for seasonal affective disorder: a network meta-analysis. J Affective Disorders. 2024;350:531–536. doi:10.1016/j.jad.2024.01.028
34. Nierenberg AA, Agustini B, Köhler-Forsberg O, et al. Diagnosis and treatment of bipolar disorder: a review. JAMA. 2023;330(14):1370–1380. doi:10.1001/jama.2023.18588
35. Ciraulo DA, Shader RI, Greenblatt DJ. Clinical pharmacology and therapeutics of antidepressants. Pharmacother Depression. 2011;2011;33–124.
36. Lenox RH, Frazer A. Mechanism of action of antidepressants and mood stabilizers. Neuropsychopharmacology: the fifth generation of progress. 2002;2022:1139–1163.
37. De Witte M, AdS P, Stams G-J, Moonen X, Bos AE, Van Hooren S. Music therapy for stress reduction: a systematic review and meta-analysis. Health Psychol Rev. 2022;16(1):134–159. doi:10.1080/17437199.2020.1846580
38. Aalbers S, Fusar‐Poli L, Freeman RE, et al. Music therapy for depression. Cochrane Database Syst Rev. 2017;2017(11). doi:10.1002/14651858.CD004517.pub3.
39. Erkkilä J, Punkanen M, Fachner J, et al. Individual music therapy for depression: randomised controlled trial. Br J Psychiatry. 2011;199(2):132–139. doi:10.1192/bjp.bp.110.085431
40. Henken T, Huibers MJ, Churchill R, Restifo KK, Roelofs JJ. Family therapy for depression. Cochrane Database Syst Rev. 1996;2010(1):1.
41. Waraan L, Rognli EW, Czajkowski NO, Aalberg M, Mehlum L. Effectiveness of attachment-based family therapy compared to treatment as usual for depressed adolescents in community mental health clinics. Child Adolescent Psychiatry Mental Health. 2021;15(1):1–14. doi:10.1186/s13034-021-00361-x
42. Beck A, Rush A, Shaw B, Emery G. Cognitive Therapy of Depression Guilford Press. New York; 1979.
43. Alexander B, Warner-Schmidt J, Eriksson TM, et al. Reversal of depressed behaviors in mice by p11 gene therapy in the nucleus accumbens. Sci, trans med. 2010;2(54):54ra76–54ra76. doi:10.1126/scitranslmed.3001079
44. Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs). Int Clinical Psychopharmacol. 1994;9:19–26. doi:10.1097/00004850-199403001-00004
45. Stahl SM, Grady MM, Moret C, Briley M. SNRIs: the pharmacology, clinical efficacy, and tolerability in comparison with other classes of antidepressants. CNS Spectrums. 2005;10(9):732–747. doi:10.1017/S1092852900019726
46. Thase ME, Trivedi MH, Rush AJ. MAOIs in the contemporary treatment of depression. Neuropsychopharmacology. 1995;12(3):185–219. doi:10.1016/0893-133X(94)00058-8
47. Horst WD, Preskorn SH. Mechanisms of action and clinical characteristics of three atypical antidepressants: venlafaxine, nefazodone, bupropion. J Affective Disorders. 1998;51(3):237–254. doi:10.1016/S0165-0327(98)00222-5
48. Cuijpers P, Stringaris A, Wolpert M. Treatment outcomes for depression: challenges and opportunities. Lancet Psychiatry. 2020;7(11):925–927. doi:10.1016/S2215-0366(20)30036-5
49. Blackburn TP. Depressive disorders: treatment failures and poor prognosis over the last 50 years. Pharmacol Res Perspect. 2019;7(3):e00472. doi:10.1002/prp2.472
50. Cuijpers P, Miguel C, Harrer M, et al. Psychological treatment of depression: a systematic overview of a ‘Meta-Analytic Research Domain’. J Affective Disorders. 2023;335:141–151. doi:10.1016/j.jad.2023.05.011
51. Nguyen TT, Nguyen TTD, Vo TK, Nguyen MK, Van Vo T, Van Vo G. Nanotechnology-based drug delivery for central nervous system disorders. Biomed. Pharmacother. 2021;143:112117. doi:10.1016/j.biopha.2021.112117
52. Naqvi S, Panghal A, Flora S. Nanotechnology: a promising approach for delivery of neuroprotective drugs. Front Neurosci. 2020;14:494. doi:10.3389/fnins.2020.00494
53. Rathi R, Mehetre NM, Goyal S, Singh I, Huanbutta K, Sangnim T. Advanced drug delivery technologies for enhancing bioavailability and efficacy of risperidone. Int J Nanomed. 2024;Volume 19:12871–12887. doi:10.2147/IJN.S492684
54. Nagpal K, Singh SK, Mishra DN. Drug targeting to brain: a systematic approach to study the factors, parameters and approaches for prediction of permeability of drugs across BBB. Expert Opin Drug Delivery. 2013;10(7):927–955. doi:10.1517/17425247.2013.762354
55. Silva GA. Nanotechnology approaches to crossing the blood-brain barrier and drug delivery to the CNS. BMC Neuro. 2008;9(Suppl 3):S4. doi:10.1186/1471-2202-9-S3-S4
56. Duong V-A, Nguyen -T-T-L, Maeng H-J. Recent advances in intranasal liposomes for drug, gene, and vaccine delivery. Pharmaceutics. 2023;15(1):207. doi:10.3390/pharmaceutics15010207
57. Saeedi M, Eslamifar M, Khezri K, Dizaj SM. Applications of nanotechnology in drug delivery to the central nervous system. Biomed. Pharmacother. 2019;111:666–675. doi:10.1016/j.biopha.2018.12.133
58. Mathison S, Nagilla R, Kompella UB. Nasal route for direct delivery of solutes to the central nervous system: fact or fiction? J Drug Targeting. 1998;5(6):415–441. doi:10.3109/10611869808997870
59. Wang Z, Xiong G, Tsang WC, Schätzlein AG, Uchegbu IF. Nose-to-brain delivery. J Pharmacol Exp Ther. 2019;370(3):593–601. doi:10.1124/jpet.119.258152
60. Crowe TP, Greenlee MHW, Kanthasamy AG, Hsu WH. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018;195:44–52. doi:10.1016/j.lfs.2017.12.025
61. Erdő F, Bors LA, Farkas D, Bajza Á, Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res. Bull. 2018;143:155–170. doi:10.1016/j.brainresbull.2018.10.009
62. Marcello E, Chiono V. Biomaterials-enhanced intranasal delivery of drugs as a direct route for brain targeting. Int J Mol Sci. 2023;24(4):3390. doi:10.3390/ijms24043390
63. Huanbutta K, Sriamornsak P, Suwanpitak K, et al. Key fabrications of chitosan nanoparticles for effective drug delivery using flow chemistry reactors. Int J Nanomed. 2023;Volume 18:7889–7900. doi:10.2147/IJN.S433756
64. Gandhi S, Shastri DH, Shah J, Nair AB, Jacob S. Nasal delivery to the brain: harnessing nanoparticles for effective drug transport. Pharmaceutics. 2024;16(4):481. doi:10.3390/pharmaceutics16040481
65. Minn A, Leclerc S, Heydel J-M, et al. Drug transport into the mammalian brain: the nasal pathway and its specific metabolic barrier. J Drug Targeting. 2002;10(4):285–296. doi:10.1080/713714452
66. Wu H, Hu K, Jiang X. From nose to brain: understanding transport capacity and transport rate of drugs. Expert Opin Drug Delivery. 2008;5(10):1159–1168. doi:10.1517/17425247.5.10.1159
67. Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B. 2010;75(1):1–18. doi:10.1016/j.colsurfb.2009.09.001
68. Rabiee N, Ahmadi S, Afshari R, et al. Polymeric nanoparticles for nasal drug delivery to the brain: relevance to Alzheimer’s disease. Adv Ther. 2021;4(3):2000076. doi:10.1002/adtp.202000076
69. Jani P, Vanza J, Pandya N, Tandel H. Formulation of polymeric nanoparticles of antidepressant drug for intranasal delivery. Therapeutic Delivery. 2019;10(11):683–696. doi:10.4155/tde-2019-0060
70. Salem HF, Ali AA, Rabea YK, El-Ela FIA, Khallaf RA. Optimization and appraisal of chitosan-grafted PLGA nanoparticles for boosting pharmacokinetic and pharmacodynamic effect of duloxetine HCl using box-benkhen design. J Pharmaceut Sci. 2023;112(2):544–561. doi:10.1016/j.xphs.2022.08.034
71. Prajapati JB, Patel GC. Nose to brain delivery of Rotigotine loaded solid lipid nanoparticles: quality by design based optimization and characterization. J Drug Delivery Sci Technol. 2021;63:102377. doi:10.1016/j.jddst.2021.102377
72. Satapathy MK, Yen T-L, Jan J-S, et al. Solid lipid nanoparticles (SLNs): an advanced drug delivery system targeting brain through BBB. Pharmaceutics. 2021;13(8):1183. doi:10.3390/pharmaceutics13081183
73. Naseri N, Valizadeh H, Zakeri-Milani P. Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application. Adv Pharm Bull. 2015;5(3):305. doi:10.15171/apb.2015.043
74. Afzal A, Ahmad S, Agha F, et al. Administration of 5-HT-1B agonist ameliorates pseudodementia induced by depression in rats. Pak J Pharm Sci. 2018;31(5):2179.
75. Mostafa DAE, Khalifa MK, Gad SS. Zolmitriptan brain targeting via intranasal route using solid lipid nanoparticles for migraine therapy: formulation, characterization, in-vitro and in-vivo assessment. Int J Appl Pharm. 2020;12(2):86–93. doi:10.22159/ijap.2020v12i2.36812
76. Yasir M, Chauhan I, Zafar A, et al. Buspirone loaded solid lipid nanoparticles for amplification of nose to brain efficacy: formulation development, optimization by Box-Behnken design, in-vitro characterization and in-vivo biological evaluation. J Drug Delivery Sci Technol. 2021;61:
77. Jiang Y, Pan X, Yu T, Wang H. Intranasal administration nanosystems for brain-targeted drug delivery. Nano Res. 2023;16(12):13077–13099. doi:10.1007/s12274-023-6026-y
78. Huanbutta K, Suwanpitak K, Ponlakorn P, et al. Development and evaluation of drug-loaded niosomes fabricated by flow chemistry: a novel vortex tube reactor approach. OpenNano. 2025;23:100243. doi:10.1016/j.onano.2025.100243
79. Alam MI, Baboota S, Ahuja A, Ali M, Ali J, Sahni JK. Intranasal administration of nanostructured lipid carriers containing CNS acting drug: pharmacodynamic studies and estimation in blood and brain. J Psychiatr Res. 2012;46(9):1133–1138. doi:10.1016/j.jpsychires.2012.05.014
80. Shah B, Khunt D, Bhatt H, Misra M, Padh H. Intranasal delivery of venlafaxine loaded nanostructured lipid carrier: risk assessment and QbD based optimization. J Drug Delivery Sci Technol. 2016;33:37–50. doi:10.1016/j.jddst.2016.03.008
81. Bahadur S, Pardhi DM, Rautio J, Rosenholm JM, Pathak K. Intranasal nanoemulsions for direct nose-to-brain delivery of actives for CNS disorders. Pharmaceutics. 2020;12(12):1230. doi:10.3390/pharmaceutics12121230
82. Ghazwani M, Vasudevan R, Kandasamy G, et al. Formulation of intranasal mucoadhesive thermotriggered in situ gel containing mirtazapine as an antidepressant drug. Gels. 2023;9(6):457. doi:10.3390/gels9060457
83. Ahmad N, Khalid MS, Al Ramadhan AM, et al. Preparation of melatonin novel-mucoadhesive nanoemulsion used in the treatment of depression. Polym Bull. 2023;80(7):8093–8132. doi:10.1007/s00289-022-04436-3
84. Chen Y, Liu Y, Xie J, et al. Nose-to-brain delivery by nanosuspensions-based in situ gel for breviscapine. Int J Nanomed. 2020;Volume 15:10435–10451. doi:10.2147/IJN.S265659
85. Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3(9):785–796. doi:10.1038/nrd1494
86. Khalil HM, Mahmoud DB, El-Shiekh RA, et al. Antidepressant and cardioprotective effects of self-nanoemulsifying self-nanosuspension loaded with Hypericum perforatum on post-myocardial infarction depression in rats. AAPS Pharm Sci Tech. 2022;23(7):243. doi:10.1208/s12249-022-02387-6
87. Amkar AJ, Rane BR, Jain AS. Development and Evaluation of Nanosuspension Loaded Nanogel of Nortriptyline HCl for Brain Delivery. Eng Proceed. 2023;56(1):58.
88. Vashist A, Kaushik A, Vashist A, et al. Nanogels as potential drug nanocarriers for CNS drug delivery. Drug Discovery Today. 2018;23(7):1436–1443. doi:10.1016/j.drudis.2018.05.018
89. Hu Y, Zhao M, Wang H, et al. Exosome-sheathed ROS-responsive nanogel to improve targeted therapy in perimenopausal depression. J Nanobiotechnol. 2023;21(1):261. doi:10.1186/s12951-023-02005-y
90. Xu D, Qiao T, Wang Y, Wang Q-S, Cui Y-L. Alginate nanogels-based thermosensitive hydrogel to improve antidepressant-like effects of albiflorin via intranasal delivery. Drug Delivery. 2021;28(1):2137–2149. doi:10.1080/10717544.2021.1986604
91. Abdelnabi DM, Abdallah MH, Elghamry HA. Buspirone hydrochloride loaded in situ nanovesicular gel as an anxiolytic nasal drug delivery system: in vitro and animal studies. AAPS Pharm Sci Tech. 2019;20(3):1–14. doi:10.1208/s12249-018-1211-0
92. Leary JF. Magnetic Nanoparticles for Advanced Drug Delivery. Advanced Drug Delivery: Methods and Applications. Springer; 2023:179–199.
93. D’Agata F, Ruffinatti FA, Boschi S, et al. Magnetic nanoparticles in the central nervous system: targeting principles, applications and safety issues. Molecules. 2018;23(1):9. doi:10.3390/molecules23010009
94. Lu Q-B, Sun J-F, Yang Q-Y, et al. Magnetic brain stimulation using iron oxide nanoparticle-mediated selective treatment of the left prelimbic cortex as a novel strategy to rapidly improve depressive-like symptoms in mice. Zoological Res. 2020;41(4):381. doi:10.24272/j.issn.2095-8137.2020.076
95. Yu -K-K, Li K, Lu C-Y, et al. Multifunctional gold nanoparticles as smart nanovehicles with enhanced tumour-targeting abilities for intracellular pH mapping and in vivo MR/fluorescence imaging. Nanoscale. 2020;12(3):2002–2010. doi:10.1039/C9NR06347A
96. Ansari R, Sadati SM, Mozafari N, Ashrafi H, Azadi A. Carbohydrate polymer-based nanoparticle application in drug delivery for CNS-related disorders. Eur Polym J. 2020;128:109607. doi:10.1016/j.eurpolymj.2020.109607
97. Agyare EK, Curran GL, Ramakrishnan M, Yu CC, Poduslo JF, Kandimalla KK. Development of a smart nano-vehicle to target cerebrovascular amyloid deposits and brain parenchymal plaques observed in Alzheimer’s disease and cerebral amyloid angiopathy. Pharm Res. 2008;25(11):2674–2684. doi:10.1007/s11095-008-9688-y
98. Taymouri S, Shahnamnia S, Mesripour A, Varshosaz J. In vitro and in vivo evaluation of an ionic sensitive in situ gel containing nanotransfersomes for aripiprazole nasal delivery. Pharm Developm Technol. 2021;26(8):867–879. doi:10.1080/10837450.2021.1948571
99. Lee D, Minko T. Nanotherapeutics for nose-to-brain drug delivery: an approach to bypass the blood brain barrier. Pharmaceutics. 2021;13(12):2049. doi:10.3390/pharmaceutics13122049
100. Qizilbash FF, Ashhar MU, Zafar A, et al. Thymoquinone-enriched naringenin-loaded nanostructured lipid carrier for brain delivery via nasal route: in vitro prospect and in vivo therapeutic efficacy for the treatment of depression. Pharmaceutics. 2022;14(3):656. doi:10.3390/pharmaceutics14030656
101. Sangnim T, Dheer D, Jangra N, Huanbutta K, Puri V, Sharma A. Chitosan in oral drug delivery formulations: a review. Pharmaceutics. 2023;15(9):2361. doi:10.3390/pharmaceutics15092361
102. Kaur M, Sharma A, Puri V, et al. Chitosan-based polymer blends for drug delivery systems. Polymers. 2023;15(9):2028. doi:10.3390/polym15092028
103. Singh D, Rashid M, Hallan SS, Mehra NK, Prakash A, Mishra N. Pharmacological evaluation of nasal delivery of selegiline hydrochloride-loaded thiolated chitosan nanoparticles for the treatment of depression. Artif. Cells Nanomed. Biotechnol. 2016;44(3):865–877. doi:10.3109/21691401.2014.998824
104. Suwanpitak K, Huanbutta K, Weeranoppanant N, Sriamornsak P, Panpipat C, Sangnim T. Optimization of lipid-based nanoparticles formulation loaded with biological product using a novel design vortex tube reactor via flow chemistry. Int J Nanomed. 2024;Volume 19:8729–8750. doi:10.2147/IJN.S474775
105. Haque S, Md S, Sahni JK, Ali J, Baboota S. Development and evaluation of brain targeted intranasal alginate nanoparticles for treatment of depression. J Psychiatr Res. 2014;48(1):1–12. doi:10.1016/j.jpsychires.2013.10.011
106. Prasher P, Sharma M, Singh SK, et al. Targeting mucus barrier in respiratory diseases by chemically modified advanced delivery systems. Chem Biol Interact. 2022;365:110048. doi:10.1016/j.cbi.2022.110048
107. Alp G, Aydogan N. Lipid-based mucus penetrating nanoparticles and their biophysical interactions with pulmonary mucus layer. Eur J Pharm Biopharm. 2020;149:45–57. doi:10.1016/j.ejpb.2020.01.017
108. Huanbutta K, Sriamornsak P, Luangtana-Anan M, et al. Application of multiple stepwise spinning disk processing for the synthesis of poly (methyl acrylates) coated chitosan–diclofenac sodium nanoparticles for colonic drug delivery. Eur J Pharm Sci. 2013;50(3–4):303–311. doi:10.1016/j.ejps.2013.07.010
109. Householder K, Dharmaraj S, Sandberg D, Wechsler-Reya R, Sirianni R. Fate of nanoparticles in the central nervous system after intrathecal injection in healthy mice. Sci Rep. 2019;9(1):12587. doi:10.1038/s41598-019-49028-w
110. Sudheer P, Shetty KBC. Current advances in nose to brain drug delivery. Pharm Sci Asia. 2023;50(3):2.
111. Nirosha V, Gayathri V. Spravato (Esketamine). Asian J Nurs Educ Res. 2023;13(3):226–227.
112. Ketamineclinics.com. SPRAVATO™ (Esketamine) - Nasal Spray, Now FDA Approved. 2019.
113. Bahr R, Lopez A, Rey JA. Intranasal esketamine (SpravatoTM) for use in treatment-resistant depression in conjunction with an oral antidepressant. Pharm Ther. 2019;44(6):340. doi:10.1017/S0033291709006011
114. Brendle M, Robison R, Malone DC. Cost-effectiveness of esketamine nasal spray compared to intravenous ketamine for patients with treatment-resistant depression in the US utilizing clinical trial efficacy and real-world effectiveness estimates. J Affective Disorders. 2022;319:388–396. doi:10.1016/j.jad.2022.09.083
115. Barbara AM, Xie W, Mahood Q, Hamson A. Ketamine for Adults With Treatment-Resistant Depression or Posttraumatic Stress Disorder: a 2023 Update. Canad J Health Technol. 2024;4(1). doi:10.51731/cjht.2024.817
116. Pandey M, Jain N, Kanoujia J, Hussain Z, Gorain B. Advances and challenges in intranasal delivery of antipsychotic agents targeting the central nervous system. Front Pharmacol. 2022;13:865590. doi:10.3389/fphar.2022.865590
117. Alexander A, Agrawal M, Uddin A, et al. Recent expansions of novel strategies towards the drug targeting into the brain. Int J Nanomed. 2019;Volume 14:5895–5909. doi:10.2147/IJN.S210876
118. Vyas TK, Shahiwala A, Marathe S, Misra A. Intranasal drug delivery for brain targeting. Current Drug Delivery. 2005;2(2):165–175. doi:10.2174/1567201053586047
119. Dong X. Current strategies for brain drug delivery. Theranostics. 2018;8(6):1481. doi:10.7150/thno.21254
120. Keller L-A, Merkel O, Popp A. Intranasal drug delivery: opportunities and toxicologic challenges during drug development. Drug Delivery Transl Res. 2022;2022:1–23.
121. Martín Banderas L, Cayero Otero MD, Fernández Arévalo M, Berrocoso Domínguez E, Pérez Caballero L, Micó Segura JA. Pharmaceutical composition for use in the intranasal treatment of depression. Spain patent application WO/2020/193838. 2020.
122. Jay G. Method of treating pain and depression using a hybrid mixture of s-ketamine and r-ketamine. Google Patents. 2018.
123. Bairrada Fortuna AC, Celta Falcão Ramos Ferreira A, Pinheiro Vitorino CS, Bicker De Melo Alves Aparício J, Vieira Da Silva TS, Almeida Fonseca CA. Intranasal administration for a sustained brain delivery of highly protein-bound. Portugal patent application WO/2023/105415. 2023.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

