Back to Journals » International Journal of Nanomedicine » Volume 20
Advances in Functionalized Nanoparticles for Osteoporosis Treatment
Authors Cai R , Jiang Y, Sun H, Du F , Zhu L, Tao J , Xiao L , Wang Z, Shi H
Received 29 January 2025
Accepted for publication 9 June 2025
Published 20 June 2025 Volume 2025:20 Pages 7869—7891
DOI https://doi.org/10.2147/IJN.S519945
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Krishna Nune
Rong Cai,1,* Yuanyuan Jiang,1,* Haiyan Sun,2,* Fengyi Du,3 Like Zhu,1 Jialing Tao,1 Long Xiao,1,* Zhirong Wang,1 Haiwei Shi1
1Translational Medical Innovation Center, Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Zhangjiagang, Jiangsu, 215600, People’s Republic of China; 2Department of Anesthesiology, Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Zhangjiagang, Jiangsu, 215600, People’s Republic of China; 3Department of Gastroenterology, Affiliated Hospital of Jiangsu University, Jiangsu University, Zhenjiang, Jiangsu, 212013, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhirong Wang, Translational Medical Innovation Center, Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Zhangjiagang, Jiangsu, 215600, People’s Republic of China, Email [email protected] Haiwei Shi, Translational Medical Innovation Center, Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine, Zhangjiagang, Jiangsu, 215600, People’s Republic of China, Email [email protected]
Abstract: Osteoporosis (OP) represents a significant global health burden, characterized by reduced bone density and an increased risk of fractures due to imbalances in bone remodeling processes. Traditional therapeutic strategies, while mitigating symptoms, often lack the precision to address the multifactorial nature of OP effectively. In recent years, functionalized nanoparticles have emerged as a versatile platform, offering enhanced drug delivery, targeted therapy, and the potential for theranostic applications in OP treatment. This review examines the various types and architectures of functionalized nanoparticles, emphasizing their unique capabilities in targeting bone tissue and modulating bone metabolism. By focusing on their roles in inflammation modulation, oxidative stress reduction, and promoting bone regeneration, we discuss the mechanisms by which these nanoparticles offer multifunctional, synergistic effects. Additionally, we address the challenges in achieving controlled drug release, biocompatibility, and effective bone tissue penetration, proposing future directions that integrate emerging nanotechnologies, biomechanics, and regenerative medicine approaches to optimize therapeutic outcomes. This comprehensive review provides a foundation for the future development of functionalized nanoparticle therapies, positioning them as promising tools for advanced, personalized OP treatment.
Keywords: functionalized nanoparticles, osteoporosis, bone tissue targeting, drug delivery
Introduction
Osteoporosis (OP) is a systemic metabolic bone disease characterized by decreased bone mineral density (BMD) and the deterioration of bone microarchitecture, resulting in increased bone fragility and a significantly elevated risk of fractures.1 The underlying pathophysiological mechanism involves a dynamic imbalance in bone remodeling, where bone resorption exceeds bone formation.2 Under normal physiological conditions, the continuous process of bone remodeling is regulated by the coordinated activity of osteoclasts, which resorb bone, and osteoblasts, which form new bone, thereby maintaining skeletal homeostasis.3 However, in patients with OP, heightened osteoclast activity leads to excessive bone resorption, while osteoblast function is diminished, resulting in inadequate bone formation. This imbalance is often exacerbated by various factors, including hormonal changes, genetic predispositions, nutritional deficiencies, and aging.4 As bone mass progressively decreases, the structural integrity of bone is compromised, leading to a marked reduction in bone strength and an increased susceptibility to fractures.5 Understanding the complex pathophysiological mechanisms of OP not only sheds light on the disease’s multifactorial nature but also provides a solid theoretical foundation for the development of targeted therapeutic strategies.
Despite the progress made in slowing bone loss and reducing fracture risk, current therapeutic approaches for OP still face numerous challenges and limitations.6 The most commonly used treatments include anti-resorptive agents (such as bisphosphonates), anabolic agents (such as parathyroid hormone analogs), and hormone replacement therapies.7 However, long-term use of these medications often comes with significant adverse effects, such as gastrointestinal discomfort, osteonecrosis of the jaw, and cardiovascular complications, all of which severely affect the quality of life for patients.8 Additionally, the efficacy of these therapies in promoting bone regeneration is limited, making it difficult to fully repair damaged bone tissue. Furthermore, the complex and multifactorial pathophysiology of OP renders single-treatment strategies inadequate in addressing the diverse factors influencing disease progression.9 As a result, some patients continue to face a heightened risk of fractures despite ongoing treatment. These limitations underscore the urgent need for innovative therapeutic approaches that combine efficacy, safety, and targeted delivery. Multifunctional treatment strategies must be developed to overcome the current bottlenecks in OP management and provide more comprehensive and personalized care.
Functionalized nanoparticles, as an emerging therapeutic modality, have demonstrated immense potential and distinct advantages in the treatment of OP.10 Owing to their unique physicochemical properties, nanoparticles not only enable efficient drug loading and targeted delivery but also allow for controlled release through intelligent design, concentrating therapeutic effects at the site of bone lesions.11 This significantly enhances drug efficacy while minimizing systemic side effects. Through surface modification or multi-functionalization, nanoparticles can be engineered to carry a variety of therapeutic agents,12 such as anti-inflammatory drugs and bone-promoting factors, and can integrate imaging capabilities to enable theranostics (Figure 1). This enables real-time monitoring and precise control throughout the treatment process, thereby improving therapeutic accuracy and outcomes.13 Compared to conventional treatment methods, functionalized nanoparticles can overcome the challenges associated with poor targeting of bone tissue.14,15 Surface modifications enhance the binding affinity of drugs to bone surfaces, increasing drug retention time and local concentration in bone tissue, which greatly improves bone regeneration capacity.16 Furthermore, functionalized nanoparticles can regulate the dynamic balance between osteoclasts and osteoblasts, promoting bone restoration and addressing the underlying pathophysiology of OP.17 The high adaptability and multifunctionality of functionalized nanoparticles make them a promising breakthrough in the future of OP therapy, offering patients safer, more effective, and personalized treatment options, ultimately improving their quality of life.
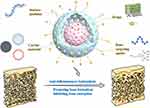 |
Figure 1 Schematic illustration of the composition and structural framework of functionalized nanoparticles for OP treatment. (Some parts of the figure by Figdraw, www.figdraw.com). |
Types and Architecture of Functionalized Nanoparticles
The diversity in the types and architecture of functionalized nanoparticles plays a pivotal role in determining their therapeutic efficacy, especially in the treatment of complex diseases like OP.18 The versatility of these nanoparticles allows for tailored modifications to enhance targeting precision, drug delivery, and controlled release mechanisms.19 From metal-based to polymer-based and even biomembrane-coated nanoparticles, each type brings unique advantages in terms of biocompatibility, mechanical properties, and drug-loading capabilities.20 By fine-tuning the surface characteristics and core materials, these nanoparticles can be optimized for specific biological environments, enabling a more effective and personalized therapeutic approach.
Surface Modification and Functionalization Techniques
Targeting
In the treatment of bone-related diseases such as OP, the targeted delivery of functionalized nanoparticles is of paramount importance. The structural characteristics of bone tissue, including the complex network of trabecular and cortical bone, as well as the dynamic balance of bone cells in bone metabolism, necessitate precise drug targeting to the affected areas to achieve optimal therapeutic outcomes.21 Achieving bone-targeting primarily relies on the affinity between specific targeting molecules, modified on the surface of functionalized nanoparticles, and bone tissue—particularly their interaction with hydroxyapatite.22 This ensures the effective accumulation of functionalized nanoparticles at the site of bone lesions, thereby enhancing therapeutic efficacy.
Currently, commonly used bone-targeting agents include bisphosphonates (such as alendronate sodium23), peptides (such as Asp24), and novel targeting molecules (such as cell membranes overexpressing CXCR425). These targeting agents promote the accumulation of functionalized nanoparticles in bone tissue through their chemical affinity to the bone matrix.26 However, there are several notable drawbacks associated with these existing bone-targeting agents. First, most designed bone-targeting functionalized nanoparticles only generally target bone tissue, lacking precise targeting for specific types of bone cells, such as osteoclasts or osteoblasts, leading to suboptimal therapeutic outcomes.27 In response to this challenge, Zhang and colleagues28 proposed a novel dual-targeting functionalized nanoparticle system capable of effectively targeting different stages of osteoclasts (OCs). The functionalized nanoparticles designed in this study utilize calcitonin gene-related peptide receptor (CGRPR) and anti-tartrate-resistant acid phosphatase (TRAP) as targeting ligands to specifically address the characteristics of OCs at different developmental stages, thereby achieving more precise drug delivery. This innovative design not only enhances targeting efficiency toward OCs but also ensures that the drug exerts its effects during various functional stages of OCs, significantly improving treatment precision. Additionally, the stability of some targeting agents is insufficient, as they may rapidly degrade in vivo, thus compromising their targeting efficiency.29 Moreover, in complex physiological environments, targeting molecules are prone to competitive binding or nonspecific interactions, which can further reduce their effectiveness.30
Therefore, to overcome these challenges, future research should focus on optimizing the chemical stability and biocompatibility of targeting agents, while also developing more effective targeting strategies to achieve precise targeting of specific bone cell types. By pursuing these advanced design approaches, bone-targeting functionalized nanoparticles hold great promise for enhanced clinical applications, providing patients with more effective therapeutic options.
Biocompatibility and Cytocompatibility
In the treatment of OP, surface modification of functionalized nanoparticles is critical for enhancing their biocompatibility and cytocompatibility. Good biocompatibility provides the foundation for the long-term presence of functionalized nanoparticles in vivo, while cytocompatibility ensures effective interaction with bone cells, promoting endocytosis and drug release.31 Therefore, improving the biocompatibility and cytocompatibility of functionalized nanoparticles is key to successful OP treatment. Surface modification can effectively enhance nanoparticle stability in vivo, reduce immune system rejection, and ultimately improve both therapeutic efficacy and safety.32 Functionalized nanoparticles with excellent biocompatibility and cytocompatibility can increase drug bioavailability, enhance the efficiency of targeted delivery, reduce side effects, and promote the accumulation of drugs within specific bone cells.33
Commonly used materials to enhance the biocompatibility and cytocompatibility of functionalized nanoparticles include polyethylene glycol (PEG),34 chitosan,35 and polyethylenimine (PEI).36 These materials exhibit excellent biocompatibility and biodegradability, forming protective layers or increasing surface hydrophilicity, which can significantly reduce the immunogenicity of functionalized nanoparticles while promoting their uptake by bone cells.37 For instance, the incorporation of PEG can effectively minimize protein adsorption and prolong the circulation time of functionalized nanoparticles,38 whereas chitosan facilitates endocytosis by interacting with the bone cell membrane.39
Real-Time Monitoring
In the rapid advancement of nanomedicine, real-time monitoring technologies have become a crucial tool for the early diagnosis of diseases and the assessment of therapeutic outcomes.40 Particularly in OP research, functionalized nanoparticles allow for the real-time observation of bone cell behavior and the efficacy of therapeutic interventions.41 The core of this technology lies in the functional surface modifications of nanoparticles, which bestow them with specific targeting and sensing capabilities, enabling highly sensitive and specific detection of biomarkers.42 These functional modifications not only empower nanoparticles to recognize and bind to target cells but also enable them to sense specific physiological changes within cells.43 Through this approach, nanoparticles can track the status and functional alterations of mature bone cells in real-time, providing critical insights into cellular activity, drug responses, and disease progression.
Matrix metalloproteinase-13 (MMP13), a critical matrix metalloproteinase, plays a key role in bone remodeling, particularly in regulating the activity of mature osteoclasts.44 Yan and colleagues45 utilized a functionalized nanoparticle surface modified with an MMP13-responsive probe (BHQ) to monitor MMP13 expression, enabling real-time assessment of bone cell functional status and the dynamic process of bone remodeling. As illustrated in Figure 2, the surface of the functionalized nanoparticles is capped with a “hat” (BHQ), which quenches the fluorescence of the nanoparticles. Upon activation by 980 nm near-infrared light, the drug encapsulated within the functionalized nanoparticles is released, inducing osteogenic differentiation of mesenchymal stem cells (MSCs), leading to the production of MMP13. MMP13, in turn, cleaves the MMP13-sensitive peptide, removing the BHQ and restoring the fluorescence of the functionalized nanoparticles. Moreover, this real-time monitoring technology allows for tracking the impact of therapeutic interventions on bone cell activity throughout the treatment process, providing valuable data to support the development of personalized treatment strategies.46
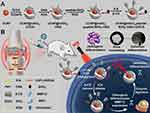 |
Figure 2 (A) Synthesis and structural design of the UCNP nanocomplex; (B) Near-infrared (NIR) light-triggered osteogenic differentiation of MSCs coupled with real-time monitoring of cellular differentiation, demonstrated both in vitro and in vivo. Reproduced from Yan R, Guo Y, Wang X, et al. Near-infrared light-controlled and real-time detection of osteogenic differentiation in mesenchymal stem cells by upconversion nanoparticles for osteoporosis therapy. ACS Nano. 2022;16(5):8399–8418.45 Abbreviations: TEOS, tetraethyl orthosilicate; TEDATS, N-(3-trimethoxysilyl)propyl ethylenediamine triacetic acid trisodium salt; ONA, oligonucleotide; PEG, polyethylene glycol; BHQ, black hole quencher; RGD, Arg-Gly-Asp; ICA, Icariin; β-CD, β-Cyclodextrin; UCNP, upconversion nanoparticles; MMP13, matrix metalloproteinase 13; MSC, mesenchymal stem cells. |
Real-time monitoring technology not only enhances our ability to gain deeper insights into the physiological state of bone cells but also opens new possibilities for the early diagnosis of OP and the assessment of therapeutic efficacy.47 Leveraging the advantages of functionalized nanoparticles, researchers can achieve highly sensitive and specific in vivo monitoring, enabling the timely detection of dynamic changes in bone cells under the influence of therapeutic agents.48 This innovative strategy marks a significant step forward in the evolution of nanomedicine toward more precise and personalized treatments.
Drug Loading and Controlled Release
In the treatment of OP, many drugs require modification through materials on the surface of functionalized nanoparticles due to poor water solubility, inadequate stability, or low bioavailability.49 This method of drug loading offers several advantages, including enhanced solubility and stability, prolonged circulation time in vivo, improved targeting, and controlled release at the diseased site.50 For instance, the use of polymers such as polylactic acid (PLA) or polyvinyl alcohol (PVA) as carriers helps form stable drug complexes, preventing rapid degradation in the body. However, this approach also presents certain limitations.51 The interactions between the drug and the carrier may reduce the drug’s activity, or result in uneven release rates in vivo, which can negatively impact therapeutic outcomes.
To overcome these challenges, controlled drug release technologies have emerged, playing a crucial role in the treatment of OP. These technologies ensure sustained drug release in the body, avoiding sharp fluctuations in plasma drug concentrations, thereby enhancing therapeutic outcomes.52 Functionalized nanoparticles exhibit unique advantages in this regard, as they can intelligently modulate drug release rates in response to varying physiological conditions such as pH, temperature, or light.53 For instance, Liu and colleagues54 demonstrated the application of carbon dots modified on the surface of nanoparticles, enabling phase transitions in hydrogels upon near-infrared light irradiation. This design mimics the natural pulsatile and sustained secretion pattern of PTHrP-2 (parathyroid hormone-related protein-2), achieving efficient drug release to bone cells. This light-responsive mechanism not only improves drug bioavailability but also allows for precise, time-controlled drug release, ensuring a stable therapeutic effect. Meanwhile, Cui and colleagues55 explored the use of polyethyleneimine-dimethyl methacrylamide (PEI-DMMA) modified on the surface of functionalized nanoparticles, conferring pH sensitivity. In neutral or alkaline environments, the PEI-DMMA layer remains hydrophobic, retaining the encapsulated drug. However, in acidic conditions, the PEI-DMMA layer undergoes protonation, transforming into a hydrophilic state. This protonation process leads to the expansion of the nanoparticle hydrogel core, disrupting the outer shell and triggering rapid drug release. This environment-responsive mechanism enables functionalized nanoparticles to release drugs precisely under specific pathological conditions, such as at sites of bone resorption, providing a more effective solution for OP treatment.56
Thus, functionalized nanoparticles not only effectively load OP therapeutic agents but also enable precise controlled drug release.57 These technological innovations have opened new possibilities for the application of nanomedicine in the treatment of diseases such as OP.58 Future research should continue to explore novel materials and modification strategies to further optimize drug loading and release mechanisms, providing stronger support for clinical applications.
Types of Material
To advance OP treatment, various multifunctional nanoparticles have been developed, each crafted from distinct materials that confer specific therapeutic properties.59 These materials—ranging from metals and polymers to biomembranes and carbon-based nanomaterials—exhibit unique characteristics that enhance their utility in drug delivery, bone regeneration, and targeted therapy.60,61 As summarized in Table 1, metal-based nanoparticles stand out for their exceptional physicochemical stability, tunable optical and magnetic properties, and diverse surface functionality, making them ideal for targeted delivery applications. In contrast, polymer-based nanoparticles offer superior biocompatibility and customizable release profiles,62 while membrane-based systems provide innate targeting abilities and intracellular delivery, effectively enhancing cellular uptake.63 Carbon-based nanoparticles, with their distinctive optical properties, not only facilitate targeted drug delivery but also enable simultaneous imaging, thereby supporting both diagnostic and therapeutic goals.64 Each material type brings particular advantages and challenges to OP treatment, necessitating careful selection based on the therapeutic requirements.
 |
Table 1 Overview of the Types of Materials Used in Functionalized Nanoparticles for OP Treatment |
Metal-Based Multifunctional Nanoparticles
Metal-based multifunctional nanoparticles have demonstrated significant advantages in the treatment of OP, primarily due to their excellent biocompatibility, superior mechanical strength, and tunable drug release properties.78 These multifunctional nanoparticles are typically synthesized through chemical reduction, sol-gel processes, or physical evaporation methods, with their formation relying on the reduction and aggregation of metal ions into nanoparticles.79 They can be categorized into several types, including iron-based nanoparticles,80 gold-based nanoparticles,69 copper-based nanoparticles,67 and other transition metal nanoparticles.81 Each type of metal nanoparticle has distinct characteristics in terms of functionalization and application, allowing for tailored designs to meet specific biological environments and therapeutic needs.
Metal-based multifunctional nanoparticles have shown unique advantages in the treatment of OP. For example, Fe3O4 nanoparticles synthesized by Lee and colleagues65 not only effectively promote the proliferation and differentiation of osteoblasts but also improve the bone microenvironment, enhancing the synthesis and mineralization of the bone matrix. These multifunctional nanoparticles interact with bone cells, stimulating osteoblast activity, thereby increasing bone density and strength. However, the potential risk of ferroptosis induced by Fe3O4 nanoparticles cannot be overlooked, as it may lead to apoptosis and further bone tissue damage.82 Despite their positive role in bone regeneration, caution is needed in their clinical application. In contrast, Fe2O3 nanoparticles offer advantages due to their lower iron load and better biocompatibility in vivo, effectively avoiding ferroptosis,83 which partially compensates for the drawbacks of Fe3O4 nanoparticles. Additionally, Fe2O3 enhances the activity of antioxidant enzymes, boosting bone cells’ resistance to oxidative stress, providing a new approach for OP treatment.84 However, the poor solubility of Fe2O3 nanoparticles in vivo may limit their therapeutic efficacy, suggesting future research should focus on improving their bioavailability to fully realize their potential in OP treatment.85 Optimizing a combination of Fe3O4 and Fe2O3 nanoparticles could lead to more effective outcomes for OP therapy. At the same time, in the field of optical therapy, upconversion nanoparticles (UCNPs) synthesized with different rare-earth metal ions demonstrate varying capabilities in spectral conversion.86 Ma and colleagues87 synthesized UCNPs-1, which can effectively convert near-infrared (NIR) light into visible light, significantly reducing potential harm to biological tissues. As shown in Figure 3A, the upconversion luminescence (UCL) spectrum of UCNPs-1 under 808 nm NIR excitation emits visible light peaks at around 540 nm (green) and 650 nm (red), both within the visible light range of 400 to 700 nm. Therefore, it is clear that UCNPs-1 emit visible light under 808 nm NIR excitation. This visible light exhibits lower phototoxicity and better biocompatibility, as its lower energy minimizes the risk of DNA damage or other photochemical reactions, thereby avoiding potential harm to cells and tissues.88 In contrast, the UCL spectrum of UCNPs-2, synthesized by Ye and colleagues,89 emits light peaks in the blue/ultraviolet region below 500 nm (Figure 3B). Although this higher-energy light can more effectively activate certain photosensitive molecules or processes, it also carries a higher risk, as blue or ultraviolet light has higher energy, potentially leading to DNA base dimerization or other photodamage reactions, increasing the risk of phototoxicity.90 Therefore, selecting appropriate rare-earth dopants and optimizing the shape and size of upconversion nanoparticles is crucial to achieving safer light conversion. In addition, cerium oxide nanoparticles (CeO2 NPs) are gaining attention in OP treatment due to their ability to modulate the intracellular redox state, striking a balance between antioxidation and oxidation.91 CeO2 nanoparticles possess dual oxidation states, allowing them to switch between oxidation and reduction, depending on the physiological conditions. In the acidic microenvironment of bone resorption, CeO2 promotes the expression of antioxidant enzymes (such as superoxide dismutase and glutathione peroxidase), effectively scavenging excess reactive oxygen species (ROS) and reducing oxidative stress-induced damage to bone cells.92 In contrast, in the neutral bone microenvironment, the oxidative properties of CeO2 stimulate osteoblast proliferation and differentiation, promoting bone matrix synthesis.93 This mechanism not only mitigates oxidative damage to bone cells but also promotes their self-renewal and repair, ultimately enhancing bone density. Therefore, combining and optimizing the design of CeO2 nanoparticles holds promise for providing more effective solutions for OP treatment, advancing clinical applications.
 |
Figure 3 Upconversion luminescence (UCL) spectra of (A) UCNPs-1 and (B) UCNPs-2. Figure 3A Reproduced from Ma X, Luan Z, Zhao Q, et al. NIR-triggered release of nitric oxide by upconversion-based nanoplatforms to enhance osteogenic differentiation of mesenchymal stem cells for osteoporosis therapy. Biomater Res. 2024;28:0058. Distributed under a Creative Commons Attribution License 4.0 (CC BY 4.0). https://creativecommons.org/licenses/by/4.0/.87 Figure 3B Reproduced from Ye J, Jiang J, Zhou Z, et al. Near-infrared light and upconversion nanoparticle defined nitric oxide-based osteoporosis targeting therapy. ACS Nano. 2021;15(8):13692–13702.89 Abbreviations: UCNP, upconversion nanoparticles; BHQ, black hole quencher; FL, fluorescence; UCPA, upconversion nanoparticle-based photosensitizing gas nanoplatform with alendronate modification; BNN, N,N’-Di-sec-butyl-N,N’-dinitroso-1,4-phenylenediamine. |
The advantages of metal-based multifunctional nanoparticles lie in their excellent biocompatibility, controlled release properties, and targeted drug delivery capabilities, which can significantly enhance the therapeutic outcomes for OP. However, current studies have also revealed certain limitations, such as the potential for metal accumulation in the body, the risk of toxicity, and inconsistent release profiles.94,95 Future research should focus on optimizing the design of metal-based multifunctional nanoparticles to improve their stability in biological environments, ensuring both their safety and efficacy. These advancements will better position them for effective application in the treatment of OP.
Polymer-Based Multifunctional Nanoparticles
Polymer-based multifunctional nanoparticles, due to their excellent biocompatibility and tunability, have broad applications in the treatment of OP.96 Common types of polymer-based multifunctional nanoparticles include liposomes and hydrogel microspheres. Liposomes are favored for their superior drug-carrying capacity and biocompatibility,97 while hydrogel microspheres are widely studied for their controlled release properties and exceptional drug encapsulation capabilities.98 Both types of polymer-based multifunctional nanoparticles offer distinct advantages in OP treatment.
The synthesis of liposomes typically employs the thin-film hydration method, which efficiently encapsulates drugs of varying properties.99,100 Specifically, hydrophobic drugs are usually embedded in the hydrophobic region of the lipid bilayer, while hydrophilic drugs are encapsulated within the aqueous core.101,102 This dual drug-loading capability allows for the simultaneous transport of both hydrophobic and hydrophilic drugs, enhancing therapeutic diversity and efficacy. However, the design of conventional liposomes focuses primarily on drug encapsulation, with limited control over drug release, which restricts their flexibility and effectiveness in clinical applications.103 To address this limitation, functionalized liposomes have emerged, enabling more precise controlled drug release. One innovative example in this field is the thermosensitive liposome synthesized by Che and colleagues,104 which incorporates polydopamine modifications to create a temperature-sensitive system. As the ambient temperature increases, the structure of polydopamine changes, causing a phase transition in the liposomal membrane and leading to the rapid release of encapsulated drugs. This thermosensitive controlled-release mechanism allows for the timed release of drugs in response to body temperature changes, significantly improving therapeutic outcomes. On the other hand, Yang and colleagues105 introduced a pH-responsive functionalized liposome, which is modified with hexachlorocyclotriphosphazene, enabling structural changes in acidic environments. When the environmental pH decreases, the polymer chains of the liposome undergo a structural shift, increasing hydrophilicity and triggering rapid drug release. This pH-responsive mechanism facilitates precise drug release under bone resorption conditions, offering a novel approach for OP treatment. Hydrogel microspheres, on the other hand, are typically synthesized through polymer cross-linking, forming a three-dimensional network structure via physical or chemical cross-linking.106 In this structure, drugs can be effectively encapsulated through soaking or copolymerization. In OP applications, hydrogel microspheres maintain stability in physiological environments and can control drug release rates by adjusting cross-linking density and environmental conditions.107 This highly tunable release characteristic allows hydrogel microspheres to offer personalized drug release profiles based on the specific needs of the patient, thereby enhancing the therapeutic efficacy in OP treatment.
Membrane-Based Multifunctional Nanoparticles
Membrane-based multifunctional nanoparticles have shown unique advantages in the treatment of OP. These nanoparticles typically combine the properties of cell membranes and exosomes, enhancing their targeting ability, biocompatibility, and therapeutic efficacy.108,109 Tian and colleagues74 demonstrated a novel multifunctional nanoparticle system, where nanoparticles were coated with osteoblast membranes or exosome membranes, significantly improving their targeting capabilities toward specific bone cells. This design not only leverages the biocompatibility of cell membranes but also allows the nanoparticles to evade recognition by the immune system, thus extending their circulation time and enhancing their bioavailability. Furthermore, Hu and colleagues75 explored the critical role of exosomes in drug delivery. Their research demonstrated that exosomes can effectively encapsulate various types of therapeutic agents, including small-molecule drugs and nucleic acids such as siRNA and mRNA. Specifically, these exosomes deliver drugs to target cells through an endocytosis mechanism, facilitating efficient cellular uptake of siRNA, which subsequently exerts its function by significantly inhibiting the expression of specific genes within target cells. This exosome-mediated delivery of siRNA offers a novel strategy for OP treatment, as modulating the gene expression of osteoblasts can effectively improve bone metabolism.110
Carbon-Based Multifunctional Nanoparticles
Although research on carbon-based multifunctional nanoparticles for OP is still relatively limited, the application of carbon quantum dots is gradually becoming a focus in this field.111 As a novel nanomaterial, carbon quantum dots have garnered significant attention due to their excellent biocompatibility and tunable surface properties, which enable effective interactions with bone cells and promote the accumulation of drugs at the target site.112 Furthermore, the unique optical properties of carbon quantum dots allow them to function as imaging agents without the need for additional fluorescent markers, enabling real-time monitoring of bone cell status and drug release.113 This dual functionality, seamlessly integrating both therapeutic and diagnostic capabilities, offers new possibilities for the clinical management of OP.
Design Strategies for Multifunctional Nanoparticles
The design of functionalized nanoparticles plays a critical role in enabling precise and efficient treatment, especially for complex diseases like OP. These functionalized nanoparticles offer a flexible platform capable of addressing multiple therapeutic targets by integrating various functionalities such as drug encapsulation, controlled release, and targeted delivery.114,115 By incorporating a combination of therapeutic agents, functionalized nanoparticles can simultaneously modulate inflammation, promote bone regeneration, and inhibit bone resorption.116 Additionally, their structural adaptability allows for enhanced drug stability and targeting precision, ensuring that therapeutic agents are delivered directly to the site of action while minimizing side effects.117 Functionalized nanoparticles, through advanced design strategies, hold great potential for improving treatment outcomes and advancing the field of precision medicine.
Combined Drug Delivery
Functionalized nanoparticles offer vast potential in the field of drug delivery, with their core advantage being the ability to effectively encapsulate small-molecule drugs within their internal cavity or porous structure.118,119 Their nanoscale size and unique physicochemical properties enable them to protect drugs from degradation by external environmental factors, thus enhancing drug stability and bioavailability in vivo.120,121 Additionally, functionalized nanoparticles can be surface-modified with specific functional groups or ligands, allowing for targeted drug loading and controlled release. This process relies on multiple mechanisms, including chemical bonding, electrostatic adsorption, or hydrophobic interactions, ensuring precise drug targeting to specific pathological sites.122 However, the complexity of OP often requires more than single-drug delivery to address its multifaceted pathophysiology. A combined drug delivery strategy not only broadens the scope and depth of treatment but also reduces the required dosage of each individual drug, minimizing adverse reactions and enhancing overall therapeutic efficacy.
The combined drug delivery strategy enables the co-delivery of drugs with antioxidant, anti-inflammatory, bone formation-promoting, and bone resorption-inhibiting properties, providing a comprehensive therapeutic approach to OP.123 For instance, through the structural design of functionalized nanoparticles, antioxidant agents and bone-regenerating drugs can simultaneously target damaged sites, achieving synergistic effects.124 Beyond the delivery of single-function drugs, functionalized nanoparticles are also capable of delivering dual- or triple-function drugs, which possess both anti-inflammatory and bone regeneration-promoting properties.73 However, the therapeutic mechanisms and targeting specificity of such multifunctional drugs are often limited, making it challenging to address the full complexity of OP pathophysiology.125 Moreover, the advantage of combined drug delivery is not limited to the co-delivery of drugs with different therapeutic effects but also extends to the simultaneous delivery of drugs with different characteristics. Li and colleagues126 encapsulated both free vancomycin and vancomycin-loaded liposomes in a hydrogel, achieving a dual release profile of rapid and sustained drug release in vivo. This design significantly enhanced the effectiveness of anti-infective therapy, particularly in combating fracture-related infections associated with OP. Through this combined delivery strategy, nanoparticle carriers can flexibly meet various therapeutic needs, ensuring precise drug release at different time points, thereby providing more sustained and effective treatment.
Integrated Application of Imaging and Therapy
Functionalized nanoparticles not only efficiently deliver therapeutic agents but also offer the advantage of imaging, allowing real-time monitoring of drug distribution and release during treatment.127 In the context of OP therapy, the imaging capability of functionalized nanoparticles is particularly important, as it aids in precisely targeting bone tissue and tracking therapeutic outcomes.128 Different types of functionalized nanoparticles, with their unique optical or magnetic properties, enable the visualization of drug distribution within the bone. Cheng and colleagues129 developed a bone-targeting nanocarrier based on near-infrared (NIR) emission, utilizing the NIR-II region (>1000 nm) to achieve high-resolution whole-body skeletal imaging. This system leverages the luminescence properties of lanthanide elements, significantly enhancing imaging depth and spatial resolution, making it especially suitable for non-invasive bone imaging. As shown in Figure 4, a strong NIR-II signal was observed three hours after the in vivo injection of these multifunctional nanoparticles, demonstrating their successful accumulation in the bones and targeted delivery, which plays a crucial role in improving drug bioavailability and therapeutic efficacy. On the other hand, Chen and colleagues130 synthesized functionalized nanoparticles utilizing the principle of upconversion luminescence (UCL). These nanoparticles, doped with rare-earth ions, convert low-energy excitation into high-energy emission, enabling both in vivo and in vitro imaging. The key difference between these two methods lies in their luminescence mechanisms: the former provides deeper imaging through NIR emission, while the latter’s upconversion luminescence offers higher optical stability and lower background noise. Despite the differences in their light emission mechanisms, both techniques harness the unique optical properties of functionalized nanoparticles to integrate bone imaging and therapy, providing new possibilities for the diagnosis and treatment of OP.131,132 These methods not only enhance drug targeting but also enable real-time monitoring of therapeutic outcomes, advancing the development of precision medicine.
 |
Figure 4 Bone-targeting effect and biotoxicity evaluation of LMEK. (a) Whole-body NIR-II imaging of OVX mice; (b) NIR-II imaging of the forelimbs of mice; (c) NIR-II imaging of the hindlimbs of mice; (d) Ex vivo NIR-II imaging of bones dissected from the LMEK group. Reprinted from Acta Biomaterialia, Cheng C, Xing Z, Hu Q, et al. A bone-targeting near-infrared luminescence nanocarrier facilitates alpha-ketoglutarate efficacy enhancement for osteoporosis therapy. 2024;173:442–456. With permission from Elsevier.129 Abbreviations: OVX, ovariectomy; LnNP, lanthanide nanoparticles; LMEK, β-NaYF4:7%Yb,60%Nd@NaLuF4@mSiO2-EDTA-AKG; EDTA, ethylenediaminetetraacetic acid; AKG, Alpha-ketoglutarate. |
Multi-Layer Functional Modification
Multilayer functionalization of nanoparticles has shown immense potential in the treatment of OP. By applying multi-tiered modifications to the surface of functionalized nanoparticles, their biocompatibility, targeting specificity, and controlled release capabilities can be significantly enhanced.133 This multilayer structure enables nanoparticles to carry multiple drugs or bioactive factors simultaneously, achieving synergistic effects during therapy and improving therapeutic outcomes.134 Moreover, multilayer functionalization increases the stability of nanoparticles, prolonging their circulation time in vivo and reducing potential side effects.135
As a promising bone substitute material, calcium phosphate cement (CPC) is commonly used in the treatment of OP.136,137 However, its setting process can be disrupted by alendronate (ALN), making it difficult to use both simultaneously in OP therapy.138 To overcome this challenge, Huang and colleagues139 employed a multilayer functionalization strategy, incorporating a layer of poly(lactic-co-glycolic acid) (PLGA) between the CPC and ALN. This CPC-PLGA-ALN multilayer design not only enhanced the mechanical strength of the functionalized nanoparticles but also allowed CPC and ALN to act independently without interfering with each other. In contrast, Zhang and colleagues140 synthesized functionalized nanoparticles specifically designed for gene drug delivery. By constructing a polyelectrolyte core-shell structure, where the core is composed of cationic polymers for loading and releasing small interfering RNA (siRNA), and the outer shell is modified with polyethylene glycol (PEG) to improve biocompatibility and prolong circulation time in vivo, this design is particularly suited for precision gene therapy. It regulates bone resorption by inhibiting osteoclast maturation while promoting new bone formation, offering broad potential in OP treatment. Ma and colleagues141 developed a hyaluronic acid-gelatin composite hydrogel, forming microspheres with a hierarchical structure. These microspheres, featuring inverse-protein architecture with nano- and microscale pores, not only increased drug loading capacity but also promoted cell adhesion and osteogenesis. Xu and colleagues142 introduced a magnetic core-shell structured functionalized nanoparticle, where the core consists of magnetic nanoparticles, and the outer shell comprises extracellular vesicles loaded with miR-15b-5p. This core-shell design leverages the targeting ability of the magnetic core and the gene drug-loading capacity of the outer layer to achieve precise drug delivery. The system, enhanced by magnetic targeting, delivers miR-15b-5p directly to osteoclasts, downregulating GFAP expression, inhibiting osteoclast differentiation, and promoting bone formation.
Thus, it is evident that different multilayer functionalization strategies each offer unique advantages. The multilayer structure endows functionalized nanoparticles with remarkable flexibility and versatility, enabling them to meet various complex clinical need.143 On one hand, the enhanced mechanical strength provided by the multilayer design allows functionalized nanoparticles to play a crucial role in long-term support and stabilization in bone repair.144 On the other hand, multilayer modifications can load a variety of drugs, combining anti-inflammatory effects, promotion of osteogenesis, and inhibition of osteoclasts, providing multiple therapeutic effects.145 This characteristic demonstrates the strong synergistic potential of multilayer functionalization in the multidimensional treatment of OP. However, multilayer structures also present certain challenges. First, the increased complexity of the structure can complicate the synthesis process, raising both costs and technical barriers.146 Moreover, precisely controlling the release time and concentration of drugs from different layers, to avoid premature depletion or insufficient release during treatment, remains a key challenge in design.147 This is particularly true for gene therapy, where highly complex structures may introduce uncertainty, potentially affecting the final therapeutic outcome.148 Therefore, selecting the appropriate multilayer functionalization strategy must balance various functionalities based on therapeutic needs. If the goal is to enhance mechanical properties, prioritizing material strength is crucial. Conversely, if drug targeting and controlled release are paramount, the focus should be on layers that ensure precise release, ultimately providing the most personalized and effective treatment plan for the patient.
Application Mechanism of Functionalized Nanoparticles in the Treatment of OP
The treatment of OP presents significant challenges due to the complex interplay of inflammation, oxidative stress, and disrupted bone metabolism. Traditional therapeutic approaches often focus on isolated pathways, such as reducing inflammation or inhibiting bone resorption, but rarely address the multifactorial nature of the disease.149,150 The advent of functionalized nanoparticles offers a promising solution, providing a versatile platform that can simultaneously target multiple pathogenic mechanisms. By enhancing drug delivery precision, controlling release rates, and minimizing systemic side effects, these nanoparticles enable more effective management of osteoporosis.151 This chapter explores the mechanisms by which functionalized nanoparticles are employed to combat OP, focusing on their roles in modulating inflammation, combating oxidative stress, promoting bone formation, and inhibiting osteoclastogenesis, as detailed in Table 2. These strategies represent a significant advancement in osteoporosis treatment, opening doors to more comprehensive and personalized therapeutic approaches.
 |
Table 2 Overview of the Therapeutic Efficacy of Functionalized Nanoparticles in OP Treatment |
Anti-Inflammatory and Antioxidant
Inflammation and oxidative stress play critical roles in the onset and progression of OP, with their interplay exacerbating bone tissue damage and disrupting the balance between bone resorption and regeneration.168,169 Inflammation activates osteoclasts through the release of pro-inflammatory factors such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), thereby increasing bone resorption.170 Simultaneously, oxidative stress generates excessive reactive oxygen species (ROS), directly damaging bone cells and inducing apoptosis.171 The combined effects of these two pathological states not only result in decreased bone mineral density and increased bone fragility but also significantly elevate the risk of fractures. Specifically, inflammation accelerates the formation of osteoclasts and inhibits the function of osteoblasts, aggravating the progression of OP. Meanwhile, oxidative stress impairs both osteoblast and osteoclast functions, disrupting normal bone remodeling and reducing the synthesis of the bone matrix.
Compounding this complexity is the fact that the presence of OP further intensifies inflammation and oxidative stress, creating a vicious cycle.172 Studies have shown that levels of inflammatory cytokines and oxidative stress markers are significantly elevated in patients with OP, leading to ongoing bone tissue damage.173 To address this issue, the delivery of antibiotics and/or antioxidants via multifunctional nanoparticles has emerged as a promising therapeutic approach.152,153 This strategy not only targets the underlying causes of the disease but also minimizes systemic drug exposure, reducing potential side effects. By functionalizing nanoparticles, drug concentrations can be increased at the site of damage, enhancing anti-inflammatory effects and ultimately promoting bone regeneration and repair.174 Chen and colleagues encapsulated quercetin into ROS-responsive nanoparticles to leverage its antioxidant, anti-inflammatory, and glucose metabolism-regulating properties, thereby significantly enhancing the osteogenic differentiation potential of mesenchymal stem cells (MSCs) for osteoporotic bone repair.124 Gao and colleagues ingeniously utilized disulfide bonds to attach the antioxidant α-lipoic acid (LA) to the surface of nanoparticles, thereby achieving efficient drug delivery.154 Yu and colleagues skillfully encapsulated Oroxylin A, which holds anti-inflammatory potential, within the core of pH-responsive nanoparticles to achieve precise treatment for OP.155 This precise drug delivery method offers a new perspective for the treatment of OP and holds potential to improve bone health and the overall quality of life for patients.
Promote Bone Formation and Inhibit Bone Resorption
Anti-inflammatory and antioxidant strategies play a crucial role in the treatment of OP, effectively promoting bone formation and inhibiting bone resorption. By alleviating the inflammatory response and reducing oxidative stress levels, these approaches create a favorable environment for the normal function of bone cells, thereby enhancing the synthesis and mineralization of the bone matrix.175 However, the use of anti-inflammatory drugs and antioxidants alone often falls short of fully achieving bone formation and/or resorption inhibition.176 This limitation arises from the fact that these agents typically lack targeted delivery and precise mechanistic control, which may prevent them from sufficiently activating osteoblasts or effectively suppressing osteoclast activity.
Thus, utilizing drugs specifically designed to promote bone formation and/or inhibit bone resorption can significantly enhance the efficacy of OP treatment. Hydroxyapatite, a widely used biomaterial, plays a crucial role in bone regeneration due to its unique chemical and physical properties.177 Studies have shown that hydroxyapatite promotes the adhesion and proliferation of osteoblasts, providing excellent biocompatibility and structural support, thereby further enhancing the synthesis and mineralization of the bone matrix.178 Its porous structure not only facilitates the infiltration of bone cells and angiogenesis but also provides ample space for mineral deposition, accelerating the bone healing process.157 Parathyroid hormone (PTH) promotes bone formation by activating osteoblastic signaling pathways, enhancing calcium absorption and redistribution, thereby improving bone density.159 Moreover, simvastatin, a statin commonly used for cholesterol reduction, has been found to regulate bone metabolism and promote bone formation.160 This effect may be related to its ability to improve osteoblast function and upregulate osteogenesis-related gene expression, offering a novel therapeutic option for OP. In terms of inhibiting bone resorption, alendronate sodium is a commonly used drug that binds to the surface of osteoclasts, preventing their activation and function, thereby reducing bone matrix degradation and maintaining bone density.179 Additionally, bicarbonate has been suggested to mitigate the negative effects of bone resorption by regulating the body’s acid-base balance, helping to preserve bone health.180
While anti-inflammatory and antioxidant strategies positively impact the treatment of OP, adopting therapeutic approaches that directly promote bone formation and inhibit bone resorption can provide a more effective means of addressing the disease and offering patients a comprehensive treatment plan.181 Future research should continue to explore the optimal combinations and applications of these therapies to achieve improved clinical outcomes.
Other
In the context of OP, inflammatory states and immune dysregulation can lead to the abnormal activation of T cells.182 These over-activated T cells not only secrete pro-inflammatory cytokines but also promote osteoclastogenesis, accelerating bone resorption and resulting in decreased bone density and increased bone fragility.183 To address this issue, Yang and colleagues184 developed multifunctional nanoparticles loaded with monocyte chemoattractant protein-1 (MCP-1) and Fas ligand (FasL) to enhance their targeting specificity and therapeutic efficacy. MCP-1 is a key chemokine that directs the migration of over-activated T cells to inflamed sites, while FasL regulates immune responses by inducing apoptosis in T cells.185,186 This design allows the nanoparticles to effectively target activated T cells and suppress their function, thereby reducing their contribution to bone resorption. The accumulation of senescent cells is also considered a major contributor to OP, as these cells release pro-inflammatory factors that induce local inflammation and inhibit normal bone remodeling.187 As shown in Figure 5, Xing and colleagues188 used multifunctional nanoparticles loaded with senolytic agents to selectively target and eliminate senescent cells, significantly improving bone density and strength. Once the underlying “crises” inducing OP have been mitigated, a healthy bone-vascular coupling is essential to ensure adequate nutrient and oxygen supply to bone tissue, which is necessary for maintaining its normal structure and function. Research has shown that the synergy between angiogenesis and bone remodeling is fundamental to bone health, and damage to either process can lead to decreased bone density and an increased risk of fractures.189 Therefore, strategies to promote bone-vascular coupling are critical for alleviating the symptoms of OP. Zheng and colleagues190 functionalized nanoparticles by modifying their surface with specific bioactive molecules such as vascular endothelial growth factor (VEGF) and bone-related factors to enhance their affinity for both bone tissue and vascular endothelial cells. These nanoparticles are capable of selectively delivering bioactive molecules, promoting both angiogenesis and the proliferation of osteoblasts. Through this strategy, functionalized nanoparticles not only improve the nutritional supply to bone tissue but also enhance the repair of the bone matrix, thereby alleviating the symptoms of OP.191 This innovative therapeutic approach provides a new perspective for managing OP, highlighting the importance of bone-vascular coupling in bone health, and pointing to new directions for future research.
 |
Figure 5 Preparation and Characterization of Bone-Targeted Liposomes. (A) Schematic illustration of the binding of the bone-targeting peptide (DSS)6 to bone tissue; (B) Synthetic route of DSPE-PEG2000-(DSS)6; (C) Structural diagram of quercetin-loaded bone-targeted liposomes; (D) Schematic illustration of the preparation process of bone-targeted liposomes loaded with quercetin; (E) Digital photograph of the uniform suspension of quercetin-loaded bone-targeted liposomes in saline. Reprinted from Acta Biomaterialia, Xing X, Tang Q, Zou J, et al. Bone-targeted delivery of senolytics to eliminate senescent cells increases bone formation in senile osteoporosis. 2023;157:352–366. With permission from Elsevier.188 Abbreviations: DSS, Asp-Ser-Ser; HA, hydroxyapatite; DSPE, 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine; PEG, polyethylene glycol; MAL, maleimide. |
The pathogenesis of OP is complex, involving the interplay of multiple factors, including inflammation, oxidative stress, imbalances in bone metabolism, and intercellular signaling.192 Relying solely on traditional approaches such as anti-inflammatory, antioxidant, osteogenic promotion, and osteoclast inhibition strategies is often insufficient to fully address these issues, making it difficult to achieve comprehensive disease improvement. As our understanding of the mechanisms underlying OP deepens, researchers are exploring more innovative therapeutic approaches to tackle this multifaceted pathology, such as targeting over-activated immune cells, clearing senescent cells, and enhancing bone-vascular coupling.193,194 By integrating these novel strategies, future treatments are expected to be more precise and personalized, offering significant improvements in bone health and patient quality of life. This comprehensive treatment philosophy not only emphasizes the restoration of bone integrity but also takes into account the body’s overall physiological state, opening new avenues for the effective management of OP.
Future Development Direction and Challenges
With the rapid advancement of personalized and precision medicine, functionalized nanoparticles offer unprecedented possibilities for individualized treatments. Leveraging nanotechnology, therapeutic interventions can be precisely tailored to each patient’s unique pathological characteristics, enabling highly targeted, personalized therapies.195 By modifying their surface with specific targeting ligands or biomolecules, functionalized nanoparticles can accurately identify and target diseased sites, minimizing drug accumulation in healthy tissues and significantly reducing side effects.196 Additionally, the multifunctional design of nanoparticles allows for the co-delivery of multiple therapeutic agents or biological factors, creating bespoke treatment regimens that maximize therapeutic efficacy.197 Moreover, the design of personalized nanoparticles can integrate real-time imaging and diagnostic technologies, enabling dynamic monitoring of the treatment process and timely adjustment of dosing strategies, ensuring both the safety and effectiveness of therapy.198 However, despite their immense potential in personalized treatment, several challenges remain before functionalized nanoparticles can be widely applied, including long-term stability in the human body, immune response management, and technical barriers in large-scale production.199 Nonetheless, with the continued advancement of nanotechnology, bioengineering, and precision medicine, the application of functionalized nanoparticles in individualized therapies holds a promising future. These innovations are set to provide more flexible and efficient solutions for precision medicine, ushering in a new era of highly personalized healthcare.
To further enhance the efficacy of personalized therapies, the combined use of functionalized nanoparticles with other treatment modalities is emerging as a pivotal approach for addressing complex diseases. The synergistic effects of functionalized nanoparticles with physical therapies and gene therapies, in particular, are opening new avenues for the treatment of conditions like osteoporosis. As precise drug carriers, functionalized nanoparticles can efficiently deliver small-molecule drugs, gene fragments, or bioactive factors, while combining with external physical stimuli—such as ultrasound or magnetic fields—to promote targeted drug release and precision treatment.200 For instance, magnetic nanoparticles can achieve targeted drug delivery under the guidance of an external magnetic field, while ultrasound can enhance the permeability of cell membranes, facilitating the penetration and absorption of nanoparticle-carried genes or drugs.201 This combination of physical therapy and nanoparticle-based treatments not only increases the concentration of therapeutic agents at specific pathological sites but also allows for precise control of drug release timing through external stimuli. Similarly, the synergy between gene therapy and nanoparticles shows great promise. Functionalized nanoparticles can carry specific gene fragments and deliver them to targeted cells, addressing genetic defects or regulating pathological processes at their root.202 Furthermore, the multilayer functionalization of nanoparticles protects gene vectors from enzymatic degradation in vivo, prolonging their activity and providing a safer and more effective pathway for gene therapy. The integration of functionalized nanoparticles with other treatment strategies not only enhances therapeutic efficacy but also reduces the side effects associated with single therapies, creating a synergistic effect that exceeds the sum of individual components.203 This combined therapeutic strategy is poised to become a cornerstone in the treatment of complex diseases, offering more diverse and precise solutions for clinical applications.
In the study of functionalized nanoparticles for the treatment of osteoporosis, despite their significant potential for precision delivery and multifunctionality, several critical bottlenecks remain. First, the mechanisms governing the in vivo distribution and clearance of functionalized nanoparticles are not yet fully understood. Inter-individual metabolic differences may lead to uneven drug distribution, inconsistent therapeutic outcomes, and even unforeseen toxicity in some patients.204 Additionally, overcoming challenges related to poor blood supply or the unfavorable vascular microenvironment in bone tissue, which hinder the effective penetration of drugs into deep pathological sites, remains a key issue for clinical translation.205 To address these challenges, future solutions should focus on two main areas. First, nanotechnology can be combined with biomechanics and microfluidics to develop “mechanosensitive” or “dynamically responsive” functionalized nanoparticles. These nanoparticles could detect mechanical changes within bone tissue, such as microdamage or pressure fluctuations, and release drugs precisely at targeted locations, potentially even inducing bone regeneration. Furthermore, research could explore combining functionalized nanoparticles with cell or immunotherapy, using nanoparticles as carriers for cellular or immune modulators to regulate the immune microenvironment within bone tissue, thus enhancing the bone regeneration process. Additionally, with advances in synthetic biology, it may become possible to design nanoparticles with “self-healing” capabilities. These materials could work synergistically with bone cells, promoting new bone formation as the nanoparticles degrade, while simultaneously activating local repair signaling pathways to help restore the physiological function of bone tissue. By integrating interdisciplinary innovations, the application of functionalized nanoparticles in osteoporosis treatment will become more targeted and long-lasting, overcoming current bottlenecks and providing a more comprehensive therapeutic approach.
Conclusion
The research on functionalized nanoparticles for osteoporosis treatment has made remarkable progress, showcasing significant advantages. Through the precise delivery and controlled release of drugs via functionalized nanoparticles, therapeutic agents can efficiently target bone tissue, reducing systemic side effects while increasing the local drug concentration. Additionally, multilayer functionalized nanoparticles not only carry multiple drugs but also integrate imaging, diagnostic, and therapeutic functionalities, advancing the concept of theranostics. These technological advancements provide powerful tools for personalized osteoporosis treatment, enhancing the targeting and efficacy of therapies. Future research can further optimize the design of functionalized nanoparticles, making them more adaptable to changes in the bone microenvironment, such as mechanical stress or physiological signals. Moreover, with the continuous development of synthetic biology, cell therapy, and immunotherapy, functionalized nanoparticles could be integrated into multimodal treatment strategies, overcoming the limitations of single therapies. The combination of personalized medicine and big data technology will further enhance the application of functionalized nanoparticles in precision medicine, allowing them to better address physiological variations among patients and offer customized therapeutic solutions. In conclusion, functionalized nanoparticles bring new opportunities to the treatment of osteoporosis. Despite certain technical challenges, their multifunctionality and efficiency make them a promising tool for future medical applications. As interdisciplinary technologies converge and clinical research deepens, functionalized nanoparticles are poised to become a cornerstone in osteoporosis treatment, providing patients with safer, more effective, and personalized therapies.
Acknowledgments
This work was supported by the Natural Science Foundation of Jiangsu Province (BK20241832); the Key Disciplines in Suzhou (LCZX202221, LCZX202331); the Suzhou Science and Technology Development Plan Project (SKY2023012, SKYD2023056, SYWD2024173); the Natural Science Foundation project of Nanjing University of Traditional Chinese Medicine (XZR2023029); and the Key project of University-land collaborative Innovation Research Project of Jiangsu Vocational College of Medicine (20239608, 20249061).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Reid IR, Billington EO. Drug therapy for osteoporosis in older adults. Lancet. 2022;399(10329):1080–1092. doi:10.1016/S0140-6736(21)02646-5
2. Brent MB. Pharmaceutical treatment of bone loss: from animal models and drug development to future treatment strategies. Pharmacol Ther. 2023;244:108383.
3. Black DM, Geiger EJ, Eastell R, et al. Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med. 2020;383(8):743–753. doi:10.1056/NEJMoa1916525
4. Wu J, Hu M, Jiang H, et al. Endothelial cell-derived lactate triggers bone mesenchymal stem cell histone lactylation to attenuate osteoporosis. Adv Sci. 2023;10(31):e2301300. doi:10.1002/advs.202301300
5. Liu H, Song P, Zhang H, et al. Synthetic biology-based bacterial extracellular vesicles displaying BMP-2 and CXCR4 to ameliorate osteoporosis. J Extracell Vesicles. 2024;13(4):e12429. doi:10.1002/jev2.12429
6. Wei X, Zheng Z, Feng Z, et al. Sigma-1 receptor attenuates osteoclastogenesis by promoting ER-associated degradation of SERCA2. EMBO Mol Med. 2022;14(7):e15373. doi:10.15252/emmm.202115373
7. Foessl I, Dimai HP, Obermayer-Pietsch B. Long-term and sequential treatment for osteoporosis. Nat Rev Endocrinol. 2023;19(9):520–533.
8. Reid IR, Horne AM, Mihov B, et al. Duration of fracture prevention after zoledronate treatment in women with osteopenia: observational follow-up of a 6-year randomised controlled trial to 10 years. Lancet Diabetes Endocrinol. 2024;12(4):247–256. doi:10.1016/S2213-8587(24)00003-2
9. Yu B, Wang CY. Osteoporosis and periodontal diseases - An update on their association and mechanistic links. Periodontol 2000. 2022;89(1):99–113. doi:10.1111/prd.12422
10. Li Q, Shi R, Xu H, et al. Thin-film freeze-drying of an influenza virus hemagglutinin mRNA vaccine in unilamellar lipid nanoparticles with blebs. J Control Release. 2024;375:829–838. doi:10.1016/j.jconrel.2024.09.030
11. Liu X, Li F, Dong Z, et al. Metal-polyDNA nanoparticles reconstruct osteoporotic microenvironment for enhanced osteoporosis treatment. Sci Adv. 2023;9(31):eadf3329. doi:10.1126/sciadv.adf3329
12. Hong L, Xu K, Yang M, et al. Vista antibody-loaded Fe3O4@TiO2 nanoparticles for sonodynamic therapy-synergistic immune checkpoint therapy of pancreatic cancer. Mater Today Bio. 2024;26:101106. doi:10.1016/j.mtbio.2024.101106
13. Guo Y, Liu Y, Shi C, et al. Remote-controllable bone-targeted delivery of estradiol for the treatment of ovariectomy-induced osteoporosis in rats. J Nanobiotechnology. 2021;19(1):248. doi:10.1186/s12951-021-00976-4
14. Liu Y, Zhu Z, Pei X, et al. ZIF-8-modified multifunctional bone-adhesive hydrogels promoting angiogenesis and osteogenesis for bone regeneration. ACS Appl Mater Interfaces. 2020;12(33):36978–36995. doi:10.1021/acsami.0c12090
15. Deng C, Zhang Q, He P, et al. Targeted apoptosis of macrophages and osteoclasts in arthritic joints is effective against advanced inflammatory arthritis. Nat Commun. 2021;12(1):2174. doi:10.1038/s41467-021-22454-z
16. Fu H, Wang L, Bao Q, et al. Acid neutralization and immune regulation by calcium-aluminum-layered double hydroxide for osteoporosis reversion. J Am Chem Soc. 2022;144(20):8987–8999. doi:10.1021/jacs.2c00749
17. Wang M, Wang C, Zhang Y, et al. Controlled release of dopamine coatings on titanium bidirectionally regulate osteoclastic and osteogenic response behaviors. Mater Sci Eng C Mater Biol Appl. 2021;129:112376. doi:10.1016/j.msec.2021.112376
18. Zha Y, Li Y, Lin T, et al. Progenitor cell-derived exosomes endowed with VEGF plasmids enhance osteogenic induction and vascular remodeling in large segmental bone defects. Theranostics. 2021;11(1):397–409. doi:10.7150/thno.50741
19. Joshi AS, Bapat MV, Singh P, et al. Viridibacillus culture derived silver nanoparticles exert potent anticancer action in 2D and 3D models of lung cancer via mitochondrial depolarization-mediated apoptosis. Mater Today Bio. 2024;25:100997. doi:10.1016/j.mtbio.2024.100997
20. Yu X, Zhu L. Nanoparticles for the treatment of bone metastasis in breast cancer: recent advances and challenges. Int J Nanomed. 2024;19:1867–1886. doi:10.2147/IJN.S442768
21. Clézardin P, Coleman R, Puppo M, et al. Bone metastasis: mechanisms, therapies, and biomarkers. Physiol Rev. 2021;101(3):797–855. doi:10.1152/physrev.00012.2019
22. Kim S, Lee H, Hong J, et al. Bone-targeted delivery of cell-penetrating-RUNX2 fusion protein in osteoporosis model. Adv Sci. 2023;10(28):e2301570. doi:10.1002/advs.202301570
23. Gilarska A, Hinz A, Bzowska M, et al. Addressing the osteoporosis problem-multifunctional injectable hybrid materials for controlling local bone tissue remodeling. ACS Appl Mater Interfaces. 2021;13(42):49762–49779. doi:10.1021/acsami.1c17472
24. Cai M, Yang L, Zhang S, et al. A bone-resorption surface-targeting nanoparticle to deliver anti-miR214 for osteoporosis therapy. Int J Nanomed. 2017;12:7469–7482. doi:10.2147/IJN.S139775
25. Zhang C, Zhang W, Zhu D, et al. Nanoparticles functionalized with stem cell secretome and CXCR4-overexpressing endothelial membrane for targeted osteoporosis therapy. J Nanobiotechnol. 2022;20(1):35. doi:10.1186/s12951-021-01231-6
26. Jiang Z, Qi G, He X, et al. Ferroptosis in osteocytes as a target for protection against postmenopausal osteoporosis. Adv Sci. 2024;11(12):e2307388. doi:10.1002/advs.202307388
27. Xi Y, Wang W, Ma L, et al. Alendronate modified mPEG-PLGA nano-micelle drug delivery system loaded with astragaloside has anti-osteoporotic effect in rats. Drug Deliv. 2022;29(1):2386–2402. doi:10.1080/10717544.2022.2086942
28. Zhang B, Zhao J, Yan H, et al. A novel nano delivery system targeting different stages of osteoclasts. Biomater Sci. 2022;10(7):1821–1830. doi:10.1039/D2BM00076H
29. Zheng J, Li X, Zhang F, et al. Targeting osteoblast-osteoclast cross-talk bone homeostasis repair microcarriers promotes intervertebral fusion in osteoporotic rats. Adv Healthc Mater. 2024;13(31):e2402117. doi:10.1002/adhm.202402117
30. Liu R, Chen Y, Liu L, et al. Long-term delivery of rhIGF-1 from biodegradable poly(lactic acid)/hydroxyapatite@Eudragit double-layer microspheres for prevention of bone loss and articular degeneration in C57BL/6 mice. J Mater Chem B. 2018;6(19):3085–3095. doi:10.1039/C8TB00324F
31. Chou W-C, Chen Q, Yuan L, et al. An artificial intelligence-assisted physiologically-based pharmacokinetic model to predict nanoparticle delivery to tumors in mice. J Control Release. 2023;361:53–63. doi:10.1016/j.jconrel.2023.07.040
32. Xu Y, Fourniols T, Labrak Y, et al. Surface modification of lipid-based nanoparticles. ACS Nano. 2022;16(5):7168–7196. doi:10.1021/acsnano.2c02347
33. Panáček D, Hochvaldová L, Bakandritsos A, et al. Silver covalently bound to cyanographene overcomes bacterial resistance to silver nanoparticles and antibiotics. Adv Sci. 2021;8(12):2003090. doi:10.1002/advs.202003090
34. Zhou H, He Z, Cao Y, et al. An injectable magnesium-loaded hydrogel releases hydrogen to promote osteoporotic bone repair via ROS scavenging and immunomodulation. Theranostics. 2024;14(9):3739–3759. doi:10.7150/thno.97412
35. Ma S, Xu S, Li M, et al. A bone targeting nanoparticle loaded OGP to restore bone homeostasis for osteoporosis therapy. Adv Healthc Mater. 2023;12(25):e2300560. doi:10.1002/adhm.202300560
36. Lozano D, Leiva B, Gómez-Escalonilla IS, et al. Pleiotrophin-loaded mesoporous silica nanoparticles as a possible treatment for osteoporosis. Pharmaceutics. 2023;15(2):658. doi:10.3390/pharmaceutics15020658
37. Guan Y, Zhang W, Mao Y, et al. Nanoparticles and bone microenvironment: a comprehensive review for malignant bone tumor diagnosis and treatment. Mol Cancer. 2024;23(1):246. doi:10.1186/s12943-024-02161-1
38. Dane EL, Belessiotis-Richards A, Backlund C, et al. STING agonist delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nat Mater. 2022;21(6):710–720. doi:10.1038/s41563-022-01251-z
39. He H, Qin Q, Xu F, et al. Oral polyphenol-armored nanomedicine for targeted modulation of gut microbiota-brain interactions in colitis. Sci Adv. 2023;9(21):eadf3887. doi:10.1126/sciadv.adf3887
40. Meghani N, Kim KH, Kim SH, et al. Evaluation and live monitoring of pH-responsive HSA-ZnO nanoparticles using a lung-on-a-chip model. Arch Pharm Res. 2020;43(5):503–513. doi:10.1007/s12272-020-01236-z
41. Xu H, Qiu Y, Xiong Z, et al. Tracking mesenchymal stem cells with Ir(III) complex-encapsulated nanospheres in cranium defect with postmenopausal osteoporosis. Mater Sci Eng C Mater Biol Appl. 2021;122:111842. doi:10.1016/j.msec.2020.111842
42. Pavlovic M, Szerlauth A, Muráth S, et al. Surface modification of two-dimensional layered double hydroxide nanoparticles with biopolymers for biomedical applications. Adv Drug Deliv Rev. 2022;191:114590. doi:10.1016/j.addr.2022.114590
43. Oberländer J, Champanhac C, da Costa Marques R, et al. Temperature, concentration, and surface modification influence the cellular uptake and the protein Corona of polystyrene nanoparticles. Acta Biomater. 2022;148:271–278. doi:10.1016/j.actbio.2022.06.028
44. Ponte F, Kim HN, Warren A, et al. Mmp13 deletion in mesenchymal cells increases bone mass and may attenuate the cortical bone loss caused by estrogen deficiency. Sci Rep. 2022;12(1):10257. doi:10.1038/s41598-022-14470-w
45. Yan R, Guo Y, Wang X, et al. Near-infrared light-controlled and real-time detection of osteogenic differentiation in mesenchymal stem cells by upconversion nanoparticles for osteoporosis therapy. ACS Nano. 2022;16(5):8399–8418. doi:10.1021/acsnano.2c02900
46. Park J, Lee S, Choi J, et al. Extra- and intracellular monitoring of TGF-β using single immunoplasmonic nanoprobes. ACS Sens. 2021;6(5):1823–1830. doi:10.1021/acssensors.0c02723
47. Feng Q, Fatima K, Yang A, et al. Multi-modal imaging for dynamic visualization of osteogenesis and implant degradation in 3D bioprinted scaffolds. Bioact Mater. 2024;37:119–131. doi:10.1016/j.bioactmat.2024.03.022
48. Zhang C, Ren J, He J, et al. Long-term monitoring of tumor-related autophagy in vivo by Fe3O4NO· nanoparticles. Biomaterials. 2018;179:186–198. doi:10.1016/j.biomaterials.2018.07.004
49. Liu Z, Yamada S, Otsuka Y, et al. Surface modification of hydroxyapatite nanoparticles for bone regeneration by controlling their surface hydration and protein adsorption states. Dalton Trans. 2022;51(25):9572–9583. doi:10.1039/D2DT00969B
50. Li J, Wei G, Liu G, et al. Regulating type H vessel formation and bone metabolism via bone-targeting oral micro/nano-hydrogel microspheres to prevent bone loss. Adv Sci. 2023;10(15):e2207381. doi:10.1002/advs.202207381
51. Ye W, Zhu F, Cai Y, et al. Improved paclitaxel delivery with PEG-b-PLA/zein nanoparticles prepared via flash nanoprecipitation. Int J Biol Macromol. 2022;221:486–495. doi:10.1016/j.ijbiomac.2022.09.021
52. Catarata R, Azim N, Bhattacharya S, et al. Controlled drug release from polyelectrolyte-drug conjugate nanoparticles. J Mater Chem B. 2020;8(14):2887–2894. doi:10.1039/D0TB00012D
53. Cheng Z, Chen X, Zhai D, et al. Development of keratin nanoparticles for controlled gastric mucoadhesion and drug release. J Nanobiotechnol. 2018;16(1):24. doi:10.1186/s12951-018-0353-2
54. Liu S, Han Z, Hao JN, et al. Engineering of a NIR-activable hydrogel-coated mesoporous bioactive glass scaffold with dual-mode parathyroid hormone derivative release property for angiogenesis and bone regeneration. Bioact Mater. 2023;26:1–13. doi:10.1016/j.bioactmat.2023.02.008
55. Cui Y, Lv B, Li Z, et al. Bone-targeted biomimetic nanogels re-establish osteoblast/osteoclast balance to treat postmenopausal osteoporosis. Small. 2024;20(6):e2303494. doi:10.1002/smll.202303494
56. Du P, Wei Y, Liang Y, et al. Near-infrared-responsive rare earth nanoparticles for optical imaging and wireless phototherapy. Adv Sci. 2024;11(8):e2305308. doi:10.1002/advs.202305308
57. Zhang S, Zhao G, Mahotra M, et al. Chitosan nanofibrous scaffold with graded and controlled release of ciprofloxacin and BMP-2 nanoparticles for the conception of bone regeneration. Int J Biol Macromol. 2024;254(Pt 2):127912. doi:10.1016/j.ijbiomac.2023.127912
58. Xiao B, Liu Y, Chandrasiri I, et al. Bone-targeted nanoparticle drug delivery system-mediated macrophage modulation for enhanced fracture healing. Small. 2024;20(7):e2305336. doi:10.1002/smll.202305336
59. Zhang L, Haddouti EM, Welle K, et al. Local cellular responses to metallic and ceramic nanoparticles from orthopedic joint arthroplasty implants. Int J Nanomed. 2020;15:6705–6720. doi:10.2147/IJN.S248848
60. Wu Z, Yuan K, Zhang Q, et al. Antioxidant PDA-PEG nanoparticles alleviate early osteoarthritis by inhibiting osteoclastogenesis and angiogenesis in subchondral bone. J Nanobiotechnol. 2022;20(1):479. doi:10.1186/s12951-022-01697-y
61. Zhao W, Yan Y, Chen X, et al. Combining printing and nanoparticle assembly: methodology and application of nanoparticle patterning. Innovation. 2022;3(4):100253. doi:10.1016/j.xinn.2022.100253
62. Dosta P, Cryer AM, Dion MZ, et al. Investigation of the enhanced antitumour potency of STING agonist after conjugation to polymer nanoparticles. Nat Nanotechnol. 2023;18(11):1351–1363. doi:10.1038/s41565-023-01447-7
63. Cao S, Li Y, Shen L, et al. Functionalized virus nanoparticles alleviates osteoporosis via targeting the function of RANK-specific motifs. ACS Appl Mater Interfaces. 2023;15(27):32272–32280. doi:10.1021/acsami.3c06798
64. Liu G, Li B, Li J, et al. EGTA-derived carbon dots with bone-targeting ability: target-oriented synthesis and calcium affinity. ACS Appl Mater Interfaces. 2023;15(34):40163–40177. doi:10.1021/acsami.3c05184
65. Lee MS, Su CM, Yeh JC, et al. Synthesis of composite magnetic nanoparticles Fe3O4 with alendronate for osteoporosis treatment. Int J Nanomed. 2016;11:4583–4594. doi:10.2147/IJN.S112415
66. Yang L, Chen S, Shang T, et al. Complexation of injectable biphasic calcium phosphate with phosphoserine-presenting dendrons with enhanced osteoregenerative properties. ACS Appl Mater Interfaces. 2020;12(34):37873–37884. doi:10.1021/acsami.0c09004
67. Wang J, Ye J, Yang G, et al. Fenton-like reaction inspired “·OH catalyzed” osteogenic process for the treatment of osteoporosis. Adv Healthc Mater. 2024;13(15):e2304091. doi:10.1002/adhm.202304091
68. Zhao Y, Kang H, Wu X, et al. Multifunctional scaffold for osteoporotic pathophysiological microenvironment improvement and vascularized bone defect regeneration. Adv Healthc Mater. 2023;12(15):e2203099. doi:10.1002/adhm.202203099
69. Niveria K, ZafarYab M, Biswas L, et al. Leveraging selective knockdown of sost gene by Polyethyleneimine–siRNA–Chitosan reduced gold nanoparticles to promote osteogenesis in MC3T3-E1 & MEF cells. Nanomedicine. 2024;19(10):895–914. doi:10.2217/nnm-2023-0325
70. Li J, Wu J, Liu F, et al. Magnesium-organic framework-loaded bisphosphonate-functionalized gel scaffolds for enhanced bone regeneration. ACS Biomater Sci Eng. 2023;9(12):6849–6859. doi:10.1021/acsbiomaterials.3c01080
71. Jing C, Li B, Tan H, et al. Alendronate-decorated nanoparticles as bone-targeted alendronate carriers for potential osteoporosis treatment. ACS Appl Bio Mater. 2021;4(6):4907–4916. doi:10.1021/acsabm.1c00199
72. Chen L, Tang Y, Zhao K, et al. Sequential release of double drug (graded distribution) loaded gelatin microspheres/PMMA bone cement. J Mater Chem B. 2021;9(2):508–522. doi:10.1039/D0TB01452D
73. Li Y, Cai Z, Ma W, et al. A DNA tetrahedron-based ferroptosis-suppressing nanoparticle: superior delivery of curcumin and alleviation of diabetic osteoporosis. Bone Res. 2024;12(1):14. doi:10.1038/s41413-024-00319-7
74. Tian H, Gu C, Li W, et al. Neutralization of intracellular pH homeostasis to inhibit osteoclasts based on a spatiotemporally selective delivery system. Nano Lett. 2023;23(10):4101–4110. doi:10.1021/acs.nanolett.2c04295
75. Hu Y, Li X, Zhang Q, et al. Exosome-guided bone targeted delivery of antagomir-188 as an anabolic therapy for bone loss. Bioact Mater. 2021;6(9):2905–2913. doi:10.1016/j.bioactmat.2021.02.014
76. Li J, Zhang R, Du Y, et al. Osteophilic and dual-regulated alendronate-gene lipoplexes for reversing bone loss. Small. 2023;19(45):e2303456. doi:10.1002/smll.202303456
77. Zeng Y, Zhou M, Chen L, et al. Alendronate loaded graphene oxide functionalized collagen sponge for the dual effects of osteogenesis and anti-osteoclastogenesis in osteoporotic rats. Bioact Mater. 2020;5(4):859–870.
78. Wen J, Li H, Dai H, et al. Intra-articular nanoparticles based therapies for osteoarthritis and rheumatoid arthritis management. Mater Today Bio. 2023;19:100597. doi:10.1016/j.mtbio.2023.100597
79. Chen J, Pan S, Zhou J, et al. Assembly of bioactive nanoparticles via metal-phenolic complexation. Adv Mater. 2022;34(10):e2108624. doi:10.1002/adma.202108624
80. Saha R, Mondal B, Mukherjee PS. Molecular cavity for catalysis and formation of metal nanoparticles for use in catalysis. Chem Rev. 2022;122(14):12244–12307. doi:10.1021/acs.chemrev.1c00811
81. Wang D, Steffi C, Wang Z, et al. Beta-cyclodextrin modified mesoporous bioactive glass nanoparticles/silk fibroin hybrid nanofibers as an implantable estradiol delivery system for the potential treatment of osteoporosis. Nanoscale. 2018;10(38):18341–18353. doi:10.1039/C8NR05268A
82. Zhang J, Zhou K, Lin J, et al. Ferroptosis-enhanced chemotherapy for triple-negative breast cancer with magnetic composite nanoparticles. Biomaterials. 2023;303:122395. doi:10.1016/j.biomaterials.2023.122395
83. Yu P, Zheng L, Wang P, et al. Development of a novel polysaccharide-based iron oxide nanoparticle to prevent iron accumulation-related osteoporosis by scavenging reactive oxygen species. Int J Biol Macromol. 2020;165(Pt B):1634–1645. doi:10.1016/j.ijbiomac.2020.10.016
84. Mohamed HEA, Afridi S, Khalil AT, et al. Bio-redox potential of Hyphaene thebaica in bio-fabrication of ultrafine maghemite phase iron oxide nanoparticles (Fe2O3 NPs) for therapeutic applications. Mater Sci Eng C Mater Biol Appl. 2020;112:110890. doi:10.1016/j.msec.2020.110890
85. Xie Y, Jiang J, Tang Q, et al. Iron oxide nanoparticles as autophagy intervention agents suppress hepatoma growth by enhancing tumoricidal autophagy. Adv Sci. 2020;7(16):1903323. doi:10.1002/advs.201903323
86. Weng Z, Ye J, Cai C, et al. Inflammatory microenvironment regulation and osteogenesis promotion by bone-targeting calcium and magnesium repletion nanoplatform for osteoporosis therapy. J Nanobiotechnol. 2024;22(1):314. doi:10.1186/s12951-024-02581-7
87. Ma X, Luan Z, Zhao Q, et al. NIR-triggered release of nitric oxide by upconversion-based nanoplatforms to enhance osteogenic differentiation of mesenchymal stem cells for osteoporosis therapy. Biomater Res. 2024;28:0058. doi:10.34133/bmr.0058
88. Yu C, Yu L, Mohamed A, et al. Size-dependent visible-light-enhanced Cr(VI) bioreduction by hematite nanoparticles. Chemosphere. 2022;295:133633. doi:10.1016/j.chemosphere.2022.133633
89. Ye J, Jiang J, Zhou Z, et al. Near-infrared light and upconversion nanoparticle defined nitric oxide-based osteoporosis targeting therapy. ACS Nano. 2021;15(8):13692–13702. doi:10.1021/acsnano.1c04974
90. Bernard JJ, Gallo RL, Krutmann J. Photoimmunology: how ultraviolet radiation affects the immune system. Nat Rev Immunol. 2019;19(11):688–701. doi:10.1038/s41577-019-0185-9
91. Dou C, Li J, He J, et al. Bone-targeted pH-responsive cerium nanoparticles for anabolic therapy in osteoporosis. Bioact Mater. 2021;6(12):4697–4706.
92. Wang H, Zhang Y, Zhang Y, et al. Activating macrophage continual efferocytosis via microenvironment biomimetic short fibers for reversing inflammation in bone repair. Adv Mater. 2024;36(30):e2402968. doi:10.1002/adma.202402968
93. Ren S, Zhou Y, Zheng K, et al. Cerium oxide nanoparticles loaded nanofibrous membranes promote bone regeneration for periodontal tissue engineering. Bioact Mater. 2021;7:242–253. doi:10.1016/j.bioactmat.2021.05.037
94. Yu C, Yang W, Yang L, et al. Synergistic effect of magneto-mechanical bioengineered stem cells and magnetic field to alleviate osteoporosis. ACS Appl Mater Interfaces. 2023;15(16):19976–19988. doi:10.1021/acsami.3c01139
95. García-García P, Reyes R, García-Sánchez D, et al. Nanoparticle-mediated selective Sfrp-1 silencing enhances bone density in osteoporotic mice. J Nanobiotechnology. 2022;20(1):462. doi:10.1186/s12951-022-01674-5
96. Kim MK, Lee HN, Jenjob R, et al. Calcium-triggered pulsatile delivery of parathyroid hormone from microbeads for osteoporosis treatment. Biomacromolecules. 2017;18(10):3099–3105. doi:10.1021/acs.biomac.7b00750
97. Salave S, Shinde SD, Rana D, et al. Peptide engraftment on pegylated nanoliposomes for bone specific delivery of PTH (1-34) in osteoporosis. Pharmaceutics. 2023;15(2):608. doi:10.3390/pharmaceutics15020608
98. Xia X, Liu Y, Lu Y, et al. Retuning mitochondrial apoptosis/mitophagy balance via SIRT3-energized and microenvironment-modulated hydrogel microspheres to impede osteoarthritis. Adv Healthc Mater. 2023;12(32):e2302475. doi:10.1002/adhm.202302475
99. Lokugamage MP, Vanover D, Beyersdorf J, et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat Biomed Eng. 2021;5(9):1059–1068. doi:10.1038/s41551-021-00786-x
100. Large DE, Abdelmessih RG, Fink EA, et al. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176:113851. doi:10.1016/j.addr.2021.113851
101. Lian X, Chatterjee S, Sun Y, et al. Bone-marrow-homing lipid nanoparticles for genome editing in diseased and malignant haematopoietic stem cells. Nat Nanotechnol. 2024;19(9):1409–1417.
102. Grabowska J, Léopold V, Olesek K, et al. Platelets interact with CD169+ macrophages and cDC1 and enhance liposome-induced CD8+ T cell responses. Front Immunol. 2023;14:1290272. doi:10.3389/fimmu.2023.1290272
103. Zhu Y, Ma J, Shen R, et al. Screening for lipid nanoparticles that modulate the immune activity of helper T cells towards enhanced antitumour activity. Nat Biomed Eng. 2024;8(5):544–560.
104. Che L, Wang Y, Sha D, et al. A biomimetic and bioactive scaffold with intelligently pulsatile teriparatide delivery for local and systemic osteoporosis regeneration. Bioact Mater. 2022;19:75–87. doi:10.1016/j.bioactmat.2022.03.023
105. Yang X, Kuang Z, Yang X, et al. Facile synthesis of curcumin-containing poly(amidoamine) dendrimers as pH-responsive delivery system for osteoporosis treatment. Colloids Surf B Biointerfaces. 2023;222:113029.
106. Ouyang J, Deng B, Zou B, et al. Oral hydrogel microbeads-mediated in situ synthesis of selenoproteins for regulating intestinal immunity and microbiota. J Am Chem Soc. 2023;145(22):12193–12205.
107. Kim J, Choi YJ, Park H, et al. Fabrication of multifunctional alginate microspheres containing hydroxyapatite powder for simultaneous cell and drug delivery. Front Bioeng Biotechnol. 2022;10:827626. doi:10.3389/fbioe.2022.827626
108. Wu Z, Zhang H, Yan J, et al. Engineered biomembrane-derived nanoparticles for nanoscale theranostics. Theranostics. 2023;13(1):20–39. doi:10.7150/thno.76894
109. Kim J, Zhu Y, Chen S, et al. Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood-brain-barrier penetration and tumor microenvironment modulation. J Nanobiotechnology. 2023;21(1):253. doi:10.1186/s12951-023-02006-x
110. Zhao L, Gu C, Gan Y, et al. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Control Release. 2020;318:1–15. doi:10.1016/j.jconrel.2019.12.005
111. DuMez R, Miyanji EH, Corado-Santiago L, et al. In vivo characterization of carbon dots-bone interactions: toward the development of bone-specific nanocarriers for drug delivery. Drug Deliv. 2021;28(1):1281–1289. doi:10.1080/10717544.2021.1938753
112. Yu L, Li X, He M, et al. Antioxidant carboxymethyl chitosan carbon dots with calcium doping achieve ultra-low calcium concentration for iron-induced osteoporosis treatment by effectively enhancing calcium bioavailability in zebrafish. Antioxidants. 2023;12(3):583. doi:10.3390/antiox12030583
113. Zhou Y, Mintz KJ, Cheng L, et al. Direct conjugation of distinct carbon dots as Lego-like building blocks for the assembly of versatile drug nanocarriers. J Colloid Interface Sci. 2020;576:412–425. doi:10.1016/j.jcis.2020.05.005
114. Zhang Q, Jeppesen DK, Higginbotham JN, et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat Cell Biol. 2021;23(12):1240–1254. doi:10.1038/s41556-021-00805-8
115. Peruzzi JA, Vu TQ, Gunnels TF, et al. Rapid generation of therapeutic nanoparticles using cell-free expression systems. Small Methods. 2023;7(12):e2201718. doi:10.1002/smtd.202201718
116. Han X, Alameh MG, Gong N, et al. Fast and facile synthesis of amidine-incorporated degradable lipids for versatile mRNA delivery in vivo. Nat Chem. 2024;16(10):1687–1697. doi:10.1038/s41557-024-01557-2
117. Liang J, Wang H, Ding W, et al. Nanoparticle-enhanced chemo-immunotherapy to trigger robust antitumor immunity. Sci Adv. 2020;6(35):eabc3646. doi:10.1126/sciadv.abc3646
118. Nakamura T, Sato Y, Yamada Y, et al. Extrahepatic targeting of lipid nanoparticles in vivo with intracellular targeting for future nanomedicines. Adv Drug Deliv Rev. 2022;188:114417. doi:10.1016/j.addr.2022.114417
119. Pan P, Yue Q, Li J, et al. Smart cargo delivery system based on mesoporous nanoparticles for bone disease diagnosis and treatment. Adv Sci. 2021;8(12):e2004586. doi:10.1002/advs.202004586
120. Sheth V, Wang L, Bhattacharya R, et al. Strategies for delivering nanoparticles across tumor blood vessels. Adv Funct Mater. 2021;31(8):2007363. doi:10.1002/adfm.202007363
121. Fabozzi A, Della Sala F, Di Gennaro M, et al. Design of functional nanoparticles by microfluidic platforms as advanced drug delivery systems for cancer therapy. Lab Chip. 2023;23(5):1389–1409. doi:10.1039/D2LC00933A
122. Wang N, Li J, Wang J, et al. Shape-directed drug release and transport of erythrocyte-like nanodisks augment chemotherapy. J Control Release. 2022;350:886–897. doi:10.1016/j.jconrel.2022.09.005
123. Gong Y, Bu Y, Li Y, et al. Hydrogel-based delivery system applied in the local anti-osteoporotic bone defects. Front Bioeng Biotechnol. 2022;10:1058300. doi:10.3389/fbioe.2022.1058300
124. Chen M, Li M, Wei Y, et al. ROS-activatable biomimetic interface mediates in-situ bioenergetic remodeling of osteogenic cells for osteoporotic bone repair. Biomaterials. 2022;291:121878. doi:10.1016/j.biomaterials.2022.121878
125. Yao Z, Ayoub A, Srinivasan V, et al. Hydroxychloroquine and a low antiresorptive activity bisphosphonate conjugate prevent and reverse ovariectomy-induced bone loss in mice through dual antiresorptive and anabolic effects. Bone Res. 2024;12(1):52. doi:10.1038/s41413-024-00352-6
126. Li J, Leung SSY, Chung YL, et al. Hydrogel delivery of DNase I and liposomal vancomycin to eradicate fracture-related methicillin-resistant staphylococcus aureus infection and support osteoporotic fracture healing. Acta Biomater. 2023;164:223–239. doi:10.1016/j.actbio.2023.03.044
127. Berry ME, McCabe SM, Sloan-Dennison S, et al. Tomographic imaging and localization of nanoparticles in tissue using surface-enhanced spatially offset raman spectroscopy. ACS Appl Mater Interfaces. 2022;14(28):31613–31624. doi:10.1021/acsami.2c05611
128. Ostadhossein F, Benig L, Tripathi I, et al. Fluorescence detection of bone microcracks using monophosphonated carbon dots. ACS Appl Mater Interfaces. 2018;10(23):19408–19415. doi:10.1021/acsami.8b03727
129. Cheng C, Xing Z, Hu Q, et al. A bone-targeting near-infrared luminescence nanocarrier facilitates alpha-ketoglutarate efficacy enhancement for osteoporosis therapy. Acta Biomater. 2024;173:442–456. doi:10.1016/j.actbio.2023.11.022
130. Chen X, Zhu X, Hu Y, et al. EDTA-modified 17β-estradiol-laden upconversion nanocomposite for bone-targeted hormone replacement therapy for osteoporosis. Theranostics. 2020;10(7):3281–3292. doi:10.7150/thno.37599
131. Gan S, Wu W, Feng G, et al. Size optimization of organic nanoparticles with aggregation-induced emission characteristics for improved ros generation and photodynamic cancer cell ablation. Small. 2022;18(26):e2202242. doi:10.1002/smll.202202242
132. Liu C, Zheng X, Dai T, et al. Reversibly photoswitching upconversion nanoparticles for super-sensitive photoacoustic molecular imaging. Angew Chem Int Ed Engl. 2022;61(19):e202116802. doi:10.1002/anie.202116802
133. Finn JD, Smith AR, Patel MC, et al. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 2018;22(9):2227–2235. doi:10.1016/j.celrep.2018.02.014
134. Braz Gomes K, Zhang YN, Lee YZ, et al. Single-component multilayered self-assembling protein nanoparticles displaying extracellular domains of matrix protein 2 as a pan-influenza a vaccine. ACS Nano. 2023;17(23):23545–23567. doi:10.1021/acsnano.3c06526
135. Zhou Q, Hong P, Shi X, et al. Efficient degradation of tetracycline by a novel nanoconfinement structure Cu2O/Cu@MXene composite. J Hazard Mater. 2023;448:130995. doi:10.1016/j.jhazmat.2023.130995
136. Wu X, Tang Z, Wu K, et al. Strontium-calcium phosphate hybrid cement with enhanced osteogenic and angiogenic properties for vascularised bone regeneration. J Mater Chem B. 2021;9(30):5982–5997. doi:10.1039/D1TB00439E
137. Spicer CD, Pujari-Palmer M, Autefage H, et al. Synthesis of phospho-amino acid analogues as tissue adhesive cement additives. ACS Cent Sci. 2020;6(2):226–231. doi:10.1021/acscentsci.9b01149
138. Liu SM, Chen JC, Huang SM, et al. Enhanced cell osteogenic differentiation in alendronate acid and flufenamic acid drug-impregnated nanoparticles of mesoporous bioactive glass composite calcium phosphate bone cement in vitro. Pharmaceuticals. 2023;16(5):680. doi:10.3390/ph16050680
139. Huang L, Cai P, Bian M, et al. Injectable and high-strength PLGA/CPC loaded ALN/MgO bone cement for bone regeneration by facilitating osteogenesis and inhibiting osteoclastogenesis in osteoporotic bone defects. Mater Today Bio. 2024;26:101092. doi:10.1016/j.mtbio.2024.101092
140. Zhang Z, Ding P, Meng Y, et al. Rational polyelectrolyte nanoparticles endow preosteoclast-targeted siRNA transfection for anabolic therapy of osteoporosis. Sci Adv. 2023;9(10):eade7379. doi:10.1126/sciadv.ade7379
141. Ma Y, Su H, Li W, et al. The hyaluronic acid-gelatin hierarchical hydrogel for osteoporotic bone defect repairment. Int J Biol Macromol. 2024;276(Pt 1):133821. doi:10.1016/j.ijbiomac.2024.133821
142. Xu C, Wang Z, Liu Y, et al. Delivery of miR-15b-5p via magnetic nanoparticle-enhanced bone marrow mesenchymal stem cell-derived extracellular vesicles mitigates diabetic osteoporosis by targeting GFAP. Cell Biol Toxicol. 2024;40(1):52. doi:10.1007/s10565-024-09877-2
143. Panferov VG, Ivanov NA, Mazzulli T, et al. Electrophoresis-assisted multilayer assembly of nanoparticles for sensitive lateral flow immunoassay. Angew Chem Int Ed Engl. 2023;62(2):e202215548. doi:10.1002/anie.202215548
144. Hinz A, Szczęch M, Szczepanowicz K, et al. Fluorophore localization determines the results of biodistribution of core-shell nanocarriers. Int J Nanomed. 2022;17:577–588. doi:10.2147/IJN.S343266
145. Khan R, Haider S, Khan MUA, et al. Fabrication of amine-functionalized and multi-layered PAN-(TiO2)-gelatin nanofibrous wound dressing: in-vitro evaluation. Int J Biol Macromol. 2023;253(Pt 5):127169. doi:10.1016/j.ijbiomac.2023.127169
146. Sun Q, Gao T, Li X, et al. Layer-by-layer printing strategy for high-performance flexible electronic devices with low-temperature catalyzed solution-processed SiO 2. Small Methods. 2021;5(8):e2100263. doi:10.1002/smtd.202100263
147. Bai X, Dao X, Wang Q, et al. In-situ synthesized 2D MXene/TiO2/Fe hybrid with (001)-(101) surface heterojunction for degradation of tetracycline under visible light. Chemosphere. 2023;338:139546. doi:10.1016/j.chemosphere.2023.139546
148. He L, Chaudhary A, Lin X, et al. Single-component multilayered self-assembling nanoparticles presenting rationally designed glycoprotein trimers as Ebola virus vaccines. Nat Commun. 2021;12(1):2633. doi:10.1038/s41467-021-22867-w
149. Li B, Wang Y, Gong S, et al. Puerarin improves OVX-induced osteoporosis by regulating phospholipid metabolism and biosynthesis of unsaturated fatty acids based on serum metabolomics. Phytomedicine. 2022;102:154198. doi:10.1016/j.phymed.2022.154198
150. Abe Y, Kofman ER, Almeida M, et al. RANK ligand converts the NCoR/HDAC3 co-repressor to a PGC1β- and RNA-dependent co-activator of osteoclast gene expression. Mol Cell. 2023;83(19):3421–3437.e11. doi:10.1016/j.molcel.2023.08.029
151. Svarca A, Grava A, Dubnika A, et al. Calcium phosphate/hyaluronic acid composite hydrogels for local antiosteoporotic drug delivery. Front Bioeng Biotechnol. 2022;10:917765. doi:10.3389/fbioe.2022.917765
152. Wang J, Tao S, Jin X, et al. Calcium supplement by tetracycline guided amorphous calcium carbonate potentiates osteoblast promotion for synergetic osteoporosis therapy. Theranostics. 2020;10(19):8591–8605. doi:10.7150/thno.45142
153. Rasool N, Negi D, Singh Y. Thiol-functionalized antioxidant, and osteogenic mesoporous silica nanoparticles for osteoporosis. ACS Biomater Sci Eng. 2023;9(6):3535–3545. doi:10.1021/acsbiomaterials.3c00479
154. Gao W, Li JJ, Shi J, et al. Ångstrom-scale gold particles loaded with alendronate via alpha-lipoic acid alleviate bone loss in osteoporotic mice. J Nanobiotechnology. 2024;22(1):212.
155. Yu B, Gao Q, Sheng S, et al. Smart osteoclasts targeted nanomedicine based on amorphous CaCO3 for effective osteoporosis reversal. J Nanobiotechnology. 2024;22(1):153. doi:10.1186/s12951-024-02412-9
156. Lu S, Zhu Y, Lin J, et al. Controlled delivery of procyanidin through magnesium oxide nanoparticles (MgO NPs) to improve the activity and mineralization of osteoblasts under oxidative stressin vitro. Biomed Mater. 2024;19(4):10. doi:10.1088/1748-605X/ad5260
157. Li L, Yu M, Li Y, et al. Synergistic anti-inflammatory and osteogenic n-HA/resveratrol/chitosan composite microspheres for osteoporotic bone regeneration. Bioact Mater. 2020;6(5):1255–1266. doi:10.1016/j.bioactmat.2020.10.018
158. Huang L, Zhang S, Bian M, et al. Injectable, anti-collapse, adhesive, plastic and bioactive bone graft substitute promotes bone regeneration by moderating oxidative stress in osteoporotic bone defect. Acta Biomater. 2024;180:82–103.
159. Li Y, Zha Y, Hu W, et al. Monoporous microsphere as a dynamically movable drug carrier for osteoporotic bone remodeling. Adv Healthc Mater. 2023;12(16):e2201242. doi:10.1002/adhm.202201242
160. Lin CW, Lee CY, Lin SY, et al. Bone-targeting nanoparticles of a dendritic (aspartic acid)3-Functionalized PEG-PLGA biopolymer encapsulating simvastatin for the treatment of osteoporosis in rat models. Int J Mol Sci. 2022;23(18):10530. doi:10.3390/ijms231810530
161. Mora-Raimundo P, Lozano D, Manzano M, et al. Nanoparticles to knockdown osteoporosis-related gene and promote osteogenic marker expression for osteoporosis treatment. ACS Nano. 2019;13(5):5451–5464. doi:10.1021/acsnano.9b00241
162. Xiao L, Lin J, Chen R, et al. Sustained release of melatonin from gelma liposomes reduced osteoblast apoptosis and improved implant osseointegration in osteoporosis. Oxid Med Cell Longev. 2020;2020:6797154.
163. Chiang CW, Chen CH, Manga YB, et al. Facilitated and controlled strontium ranelate delivery using gcs-ha nanocarriers embedded into PEGDA coupled with decortication driven spinal regeneration. Int J Nanomed. 2021;16:4209–4224. doi:10.2147/IJN.S274461
164. Hedvičáková V, Žižková R, Buzgo M, et al. The effect of alendronate on osteoclastogenesis in different combinations of M-CSF and RANKL growth factors. Biomolecules. 2021;11(3):438. doi:10.3390/biom11030438
165. Lin W, Hu S, Li K, et al. Breaking osteoclast-acid vicious cycle to rescue osteoporosis via an acid responsive organic framework-based neutralizing and gene editing platform. Small. 2024;20(22):e2307595. doi:10.1002/smll.202307595
166. Kotak DJ, Devarajan PV. Bone targeted delivery of salmon calcitonin hydroxyapatite nanoparticles for sublingual osteoporosis therapy (SLOT). Nanomedicine. 2020;24:102153. doi:10.1016/j.nano.2020.102153
167. Liang H, Chen K, Xie J, et al. A bone-penetrating precise controllable drug release system enables localized treatment of osteoporotic fracture prevention via modulating osteoblast-osteoclast communication. Small. 2023;19(26):e2207195. doi:10.1002/smll.202207195
168. Liu Q, Yao Q, Li C, et al. Bone protective effects of the polysaccharides from Grifola frondosa on ovariectomy-induced osteoporosis in mice via inhibiting PINK1/Parkin signaling, oxidative stress and inflammation. Int J Biol Macromol. 2024;270(Pt 2):132370. doi:10.1016/j.ijbiomac.2024.132370
169. Iantomasi T, Romagnoli C, Palmini G, et al. Oxidative stress and inflammation in osteoporosis: molecular mechanisms involved and the relationship with microRNAs. Int J Mol Sci. 2023;24(4):3772. doi:10.3390/ijms24043772
170. Tao H, Li W, Zhang W, et al. Urolithin A suppresses RANKL-induced osteoclastogenesis and postmenopausal osteoporosis by, suppresses inflammation and downstream NF-κB activated pyroptosis pathways. Pharmacol Res. 2021;174:105967. doi:10.1016/j.phrs.2021.105967
171. Li M, Yu Y, Xue K, et al. Genistein mitigates senescence of bone marrow mesenchymal stem cells via ERRα-mediated mitochondrial biogenesis and mitophagy in ovariectomized rats. Redox Biol. 2023;61:102649. doi:10.1016/j.redox.2023.102649
172. Tao ZS, Hu XF, Wu XJ, et al. Protocatechualdehyde inhibits iron overload-induced bone loss by inhibiting inflammation and oxidative stress in senile rats. Int Immunopharmacol. 2024;141:113016. doi:10.1016/j.intimp.2024.113016
173. Stojanovic A, Veselinovic M, Draginic N, et al. The influence of menopause and inflammation on redox status and bone mineral density in patients with rheumatoid arthritis. Oxid Med Cell Longev. 2021;2021(1):9458587. doi:10.1155/2021/9458587
174. Wu Y, Zhang Y, Tang X, et al. Synergistic anti-oxidant and anti-inflammatory effects of ceria/resatorvid co-decorated nanoparticles for acute lung injury therapy. J Nanobiotechnology. 2023;21(1):502. doi:10.1186/s12951-023-02237-y
175. Li J, Li L, Wu T, et al. An injectable thermosensitive hydrogel containing resveratrol and dexamethasone-loaded carbonated hydroxyapatite microspheres for the regeneration of osteoporotic bone defects. Small Methods. 2024;8(1):e2300843. doi:10.1002/smtd.202300843
176. Yu Y, Li X, Li J, et al. Dopamine-assisted co-deposition of hydroxyapatite-functionalised nanoparticles of polydopamine on implant surfaces to promote osteogenesis in environments with high ROS levels. Mater Sci Eng C Mater Biol Appl. 2021;131:112473. doi:10.1016/j.msec.2021.112473
177. Arcos D, Gómez-Cerezo N, Saiz-Pardo M, et al. Injectable mesoporous bioactive nanoparticles regenerate bone tissue under osteoporosis conditions. Acta Biomater. 2022;151:501–511. doi:10.1016/j.actbio.2022.07.067
178. Kim H-Y, Cooley V, Kim E-J, et al. Adult dental epithelial stem cell-derived organoids deposit hydroxylapatite biomineral. Int J Oral Sci. 2023;15(1):55. doi:10.1038/s41368-023-00257-w
179. Zhao Z, Li G, Ruan H, et al. Capturing magnesium ions via microfluidic hydrogel microspheres for promoting cancellous bone regeneration. ACS Nano. 2021;15(8):13041–13054. doi:10.1021/acsnano.1c02147
180. Bai X, Gao Y, Zhang M, et al. Carboxylated gold nanoparticles inhibit bone erosion by disturbing the acidification of an osteoclast absorption microenvironment. Nanoscale. 2020;12(6):3871–3878. doi:10.1039/C9NR09698A
181. Nishiguchi A, Taguchi T. Osteoclast-responsive injectable bone of bisphosphonated-nanocellulose that regulates osteoclast/osteoblast activity for bone regeneration. Biomacromolecules. 2019;20(3):1385–1393. doi:10.1021/acs.biomac.8b01767
182. Li Q, Yue T, Du X, et al. HSC70 mediated autophagic degradation of oxidized PRL2 is responsible for osteoclastogenesis and inflammatory bone destruction. Cell Death Differ. 2023;30(3):647–659. doi:10.1038/s41418-022-01068-y
183. Huang L, Wang X, Cao H, et al. A bone-targeting delivery system carrying osteogenic phytomolecule icaritin prevents osteoporosis in mice. Biomaterials. 2018;182:58–71. doi:10.1016/j.biomaterials.2018.07.046
184. Yang X, Zhou F, Yuan P, et al. T cell-depleting nanoparticles ameliorate bone loss by reducing activated T cells and regulating the Treg/Th17 balance. Bioact Mater. 2021;6(10):3150–3163. doi:10.1016/j.bioactmat.2021.02.034
185. He Y, Qu Y, Meng B, et al. Mesenchymal stem cells empower T cells in the lymph nodes via MCP-1/PD-L1 axis. Cell Death Dis. 2022;13(4):365. doi:10.1038/s41419-022-04822-9
186. Kang J, Postigo-Fernandez J, Kim K, et al. Notch-mediated hepatocyte MCP-1 secretion causes liver fibrosis. JCI Insight. 2023;8(3):e165369. doi:10.1172/jci.insight.165369
187. Wang Y, Che L, Chen X, et al. Repurpose dasatinib and quercetin: targeting senescent cells ameliorates postmenopausal osteoporosis and rejuvenates bone regeneration. Bioact Mater. 2023;25:13–28. doi:10.1016/j.bioactmat.2023.01.009
188. Xing X, Tang Q, Zou J, et al. Bone-targeted delivery of senolytics to eliminate senescent cells increases bone formation in senile osteoporosis. Acta Biomater. 2023;157:352–366. doi:10.1016/j.actbio.2022.11.056
189. Rao VV, Wechsler ME, Cravens E, et al. Granular PEG hydrogels mediate osteoporotic MSC clustering via N-cadherin influencing the pro-resorptive bias of their secretory profile. Acta Biomater. 2022;145:77–87. doi:10.1016/j.actbio.2022.04.023
190. Zheng G, Ma HW, Xiang GH, et al. Bone-targeting delivery of platelet lysate exosomes ameliorates glucocorticoid-induced osteoporosis by enhancing bone-vessel coupling. J Nanobiotechnology. 2022;20(1):220. doi:10.1186/s12951-022-01400-1
191. Lin H, Weng E, Rong X, et al. ECM-mimicking strontium-doped nanofibrous microspheres for periodontal tissue regeneration in osteoporosis. ACS Appl Mater Interfaces. 2024;16(31):40555–40569. doi:10.1021/acsami.4c06286
192. Sang Z, Jiang Z, Liu S, et al. A green, efficient and stable platform based on hyperbranched quaternized hydrothermal magnetic chitosan nanospheres integrated cytomembranes for screening drug candidates from natural products. Int J Biol Macromol. 2024;258(Pt 2):129039. doi:10.1016/j.ijbiomac.2023.129039
193. Sun X, Wei J, Lyu J, et al. Bone-targeting drug delivery system of biomineral-binding liposomes loaded with icariin enhances the treatment for osteoporosis. J Nanobiotechnology. 2019;17(1):10. doi:10.1186/s12951-019-0447-5
194. Cotts KG, Cifu AS. Treatment of Osteoporosis. JAMA. 2018;319(10):1040–1041. doi:10.1001/jama.2017.21995
195. Tavares MT, Santos SC, Custódio CA, et al. Platelet lysates-based hydrogels incorporating bioactive mesoporous silica nanoparticles for stem cell osteogenic differentiation. Mater Today Bio. 2021;9:100096. doi:10.1016/j.mtbio.2021.100096
196. Jiang W, Hou F, Gu Y, et al. Local bone metabolism balance regulation via double-adhesive hydrogel for fixing orthopedic implants. Bioact Mater. 2021;12:169–184. doi:10.1016/j.bioactmat.2021.10.017
197. Wang X, Liu S, Sun Y, et al. Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat Protoc. 2023;18(1):265–291. doi:10.1038/s41596-022-00755-x
198. Kumaravel V, Nair KM, Mathew S, et al. Antimicrobial TiO2 nanocomposite coatings for surfaces, dental and orthopaedic implants. Chem Eng J. 2021;416:129071. doi:10.1016/j.cej.2021.129071
199. Stiepel RT, Pena ES, Ehrenzeller SA, et al. A predictive mechanistic model of drug release from surface eroding polymeric nanoparticles. J Control Release. 2022;351:883–895. doi:10.1016/j.jconrel.2022.09.067
200. Xu C, Wang Z, Liu Y, et al. Extracellular vesicles derived from bone marrow mesenchymal stem cells loaded on magnetic nanoparticles delay the progression of diabetic osteoporosis via delivery of miR-150-5p. Cell Biol Toxicol. 2023;39(4):1257–1274. doi:10.1007/s10565-022-09744-y
201. Van de Walle A, Figuerola A, Espinosa A, et al. Emergence of magnetic nanoparticles in photothermal and ferroptotic therapies. Mater Horiz. 2023;10(11):4757–4775. doi:10.1039/D3MH00831B
202. Whitley JA, Cai H. Engineering extracellular vesicles to deliver CRISPR ribonucleoprotein for gene editing. J Extracell Vesicles. 2023;12(9):e12343. doi:10.1002/jev2.12343
203. Yu S, Luk KH, Cheung ST, et al. Polysaccharide-protein complex-decorated selenium nanosystem as an efficient bone-formation therapeutic. J Mater Chem B. 2018;6(32):5215–5219. doi:10.1039/C8TB01084F
204. Ye Y, Zhong H, Huang S, et al. Reactive oxygen species scavenging hydrogel regulates stem cell behavior and promotes bone healing in osteoporosis. Tissue Eng Regen Med. 2023;20(6):981–992. doi:10.1007/s13770-023-00561-w
205. Liu X, Gu Y, Kumar S, et al. Oxylipin-PPARγ-initiated adipocyte senescence propagates secondary senescence in the bone marrow. Cell Metab. 2023;35(4):667–684.e6. doi:10.1016/j.cmet.2023.03.005
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

