Back to Journals » International Journal of Nanomedicine » Volume 19
Advances in Nanotherapy for Targeting Senescent Cells
Authors Shi Y, Zhang Y, Zhang Y, Yao J, Guo J, Xu X , Wang L
Received 15 March 2024
Accepted for publication 17 August 2024
Published 27 August 2024 Volume 2024:19 Pages 8797—8813
DOI https://doi.org/10.2147/IJN.S469110
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kamakhya Misra
Yurou Shi,1,2,* Yingjie Zhang,1,* Yaxuan Zhang,2 Jiali Yao,2 Junping Guo,3 Xiaoling Xu,2 Lijun Wang1
1Geriatric Medicine Center, Department of Endocrinology, Zhejiang Provincial People’s Hospital (Affiliated People’s Hospital), Hangzhou Medical College, Hangzhou, Zhejiang, 310015, People’s Republic of China; 2Shulan International Medical College, Zhejiang Shuren University, Hangzhou, 310015, People’s Republic of China; 3Rainbowfish Rehabilitation and Nursing School, Hangzhou Vocational & Technical College, Hangzhou, 310018, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoling Xu, Shulan International Medical College, Zhejiang Shuren University, 8 Shuren Street, Hangzhou, 310015, People’s Republic of China, Email [email protected] Lijun Wang, Geriatric Medicine Center, Department of Endocrinology, Zhejiang Provincial People’s Hospital, No. 158 Shangtang Road, Hangzhou, Zhejiang, 310015, People’s Republic of China, Email [email protected]
Abstract: Aging is an inevitable process in the human body, and cellular senescence refers to irreversible cell cycle arrest caused by external aging-promoting mechanisms. Moreover, as age increases, the accumulation of senescent cells limits both the health of the body and lifespan and even accelerates the occurrence and progression of age-related diseases. Therefore, it is crucial to delay the periodic irreversible arrest and continuous accumulation of senescent cells to address the issue of aging. The fundamental solution is targeted therapy focused on eliminating senescent cells or reducing the senescence-associated secretory phenotype. Over the past few decades, the remarkable development of nanomaterials has revolutionized clinical drug delivery pathways. Their unique optical, magnetic, and electrical properties effectively compensate for the shortcomings of traditional drugs, such as low stability and short half-life, thereby maximizing the bioavailability and minimizing the toxicity of drug delivery. This article provides an overview of how nanomedicine systems control drug release and achieve effective diagnosis. By presenting and analyzing recent advances in nanotherapy for targeting senescent cells, the underlying mechanisms of nanomedicine for senolytic and senomorphic therapy are clarified, providing great potential for targeting senescent cells.
Keywords: senescent cells, calcium carbonate nanoparticles, liposomes, nanoemulsions, mesoporous silica nanoparticles
Graphical Abstract:

Introduction
With the trend of population aging becoming increasingly obvious, the burden on social, economic, and healthcare systems continues to increase, necessitating sustainable and long-lasting solutions. Cellular senescence is a biological process in which cells undergo a stable cell cycle arrest state,1,2 which has been implicated in the promotion of aging through a diverse array of mechanisms (Figure 1). The phenomenon of cellular senescence is multifaceted, with both beneficial and detrimental implications for various physiological processes.
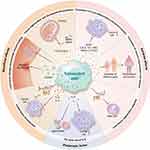 |
Figure 1 Biological consequences of cellular senescence. |
On the one hand, senescent cells play a crucial role in guiding tissue regeneration and embryonic development by secreting fibroblast growth factors (FGF4 and FGF8) and matrix metalloproteinases 2 and 9 (MMP2 and MMP9), which contribute to shaping placental structure and function. Additionally, senescent cells serve to limit tissue damage by suppressing excessive cell proliferation and facilitating wound healing through the secretion of platelet-derived growth factor-AA (PDGF-AA). Moreover, one of the key functions of senescence is its ability to suppress tumorigenesis. Senescent cells impede tumor development by inducing cell cycle arrest in a cell-autonomous manner via the upregulation of key regulatory proteins such as p53, p16, and p21, as well as by promoting senescence in neighboring cells through the secretion of interleukin-6 (IL-6) and interleukin-8 (IL-8).3
Conversely, senescent cells can contribute to the establishment of a proinflammatory microenvironment and facilitate tumor progression in adjacent tissues through the release of various components of the senescence-associated secretory phenotype (SASP).4 This proinflammatory state induced by senescent cells has been implicated in the pathogenesis of age-related diseases, with SASP factors such as IL-6, IL-1 receptor antagonist (IL-1RA), growth-related oncogene-α (GROα), and interferon-γ (IFNγ) playing key roles in promoting chronic inflammation.5 Furthermore, the dysregulation of tissue architecture and the promotion of inflammation and tumorigenesis can be exacerbated by the action of SASP factors such as MMPs. Moreover, the upregulation of cell cycle inhibitory proteins such as p16 and p21 in senescent cells can lead to functional impairment of stem or progenitor cells, thereby compromising the regenerative potential of tissues. This disruption in the supply of new cells from the tissue may further contribute to the limitations in tissue regeneration associated with cellular senescence.6
Notably, the immune response induced by the SASP has a protective effect and helps recruit immune cells capable of clearing senescent cells and promoting tissue repair.7 However, with increasing age, the clearance rate of senescent cells by immune cells decreases, leading to the continuous accumulation of senescent cells.8,9 The accumulation of senescent cells can easily lead to aging and age-related diseases such as neurodegenerative diseases,10,11 cardiovascular diseases,12,13 arthritis, and even tumor formation. The incidence of these pathological conditions increases exponentially with age. Some studies have shown that the selective elimination of senescent cells can alleviate aging and age-related diseases, which has greatly inspired the development of senolytic drugs.14–17 Therefore, senescent cells serve as potential therapeutic targets and are key to current research.
Studies have shown that blocking essential pathways involved in the survival of cancer cells may be a valuable research direction for senotherapy.18 For example, resistance to apoptosis is a common feature of both tumor cells and stem cells. By analyzing the proteomic and transcriptomic data of senescent cells and no senescent cells, senescence-associated antiapoptotic pathways (SAAPs) can be identified, and siRNAs targeting the corresponding antiapoptotic molecules can be utilized to block these pathways. The corresponding molecular targets include B-cell lymphoma-2 (Bcl-2), B-cell lymphoma-xL (Bcl-xL), phosphatidylinositol 3-kinase (PI3K), p53, p21, plasminogen activator inhibitor-1/2 (PAI-1/2), estrogen receptor (ER), tyrosine kinase (TK), hypoxia-inducible factor-1α (HIF-1α), and HSP90.19 Furthermore, studies have shown that the use of senolytics to target SAAPs can induce apoptosis in certain senescent cells,20 paving the way for the development of small molecule inhibitors targeting fundamental survival pathways in senescent cells.
Senotherapy includes both senolytic and senomorphic agents. Senolytic drugs can mainly be classified into tyrosine kinase inhibitors (such as dasatinib), natural compounds (such as quercetin), fisetin, Bcl-2 family inhibitors (such as navitoclax, ABT-737, A1331852, and A1155463), panobinostat, FOXO modulators, and HSP90 inhibitors (such as 17-DMAG). Senomorphic therapy interferes with the proinflammatory nature of senescent cells and normalizes SASP processes in the senescent microenvironment by modulating various biochemical pathways involving mTOR, p38MAPK, NF-κB, JAK/STAT, ROCK, glucocorticoid receptors, and neutralizing antibodies. The famous anti-aging drugs rapamycin and metformin belong to this class of drugs.
Traditional Senotherapy
Senolytic
Recently, various anti-aging strategies have been developed using a combination of in vitro aging models and in vivo animal models. In senescent cells, negative regulators of apoptosis, including BCL-2 family members (including BCL-2, BCL-W, and BCL-X), which confer resistance to apoptosis-inducing signals, are frequently upregulated.21–23 The anti-senescent agents ABT-737 and ABT-263 (also known as navitoclax) inhibit the activity of members of the BCL-2 family, thereby allowing senescent cells to initiate apoptosis. Recently, the cardiac glycoside ouabain has been shown, at least in part, to exhibit senescence-resolving activity by inducing the proapoptotic BCL-2 family protein NOXA. Various natural flavonoids, including quercetin and fisetin, which are used alone or in combination with the pantyrosine kinase inhibitor dasatinib, can stimulate senescence under a variety of conditions in vitro and in vivo,24 and fisetin selectively induces apoptosis in senescent human umbilical vein endothelial cells (HUVECs).25 Importantly, the administration of dasatinib and quercetin (D + Q) has been shown to be effective in reducing p16 and SA-β-gal expression in Phase 1 trials in patients with diabetic nephropathy and idiopathic lung disease.26–28 Other aging-resolving drugs, including HSP90 inhibitors and piperamine, have also been shown to be selective for senescent cells.29–32 Recently, clinically approved antibiotics have been reported to have anti-aging effects on DNA damage-induced senescent cells through metabolic changes.33–35 Collectively, these strategies target a wide range of cellular pathways, suggesting that senescent cells can be removed by multiple pathways.
Senomorphic
The principle of senomorphics is to inhibit SASP production and secretion while keeping cells alive. Reducing the SASP is essential for preventing the spread of senescence to proximal cells or tissues. Senomorphic therapy interferes with the proinflammatory nature of senescent cells and normalizes SASP processes in the senescent microenvironment by modulating various biochemical pathways involving mTOR, p38MAPK, NF-κB, JAK/STAT, ROCK, glucocorticoid receptors, and neutralizing antibodies.36 Such therapies include metformin and rapamycin. Metformin mainly prevents the nuclear translocation of NF-κB pathway components and their subsequent transactivation at the promoters of target genes. Rapamycin is an mTOR inhibitor. Interference with the mTOR pathway can reduce NF-κB and inhibit proinflammatory SASP factors at the translational level. Roxastat also interferes with the expression of various SASP factors by attenuating mTOR activation and has been shown to reduce aging-mediated adverse events in a mouse model of doxorubicin-induced aging.37
However, the clinical utility of these drugs is hindered by numerous challenges, including systemic administration-related toxic side effects and low specificity.38–40 Clinical trials have revealed that administering the senolytic drug ABT263 systemically to patients can result in adverse effects such as thrombocytopenia, characterized by a rapid decrease in platelet count.41–43 D+Q drug therapy for aging has been associated with the development of pulmonary edema. Although navitoclax currently stands out as the most promising anti-aging drug in clinical cancer models, its efficacy in combating aging may vary. For instance, senescent LNCaP aging prostate cancer cells treated with anti-androgen therapy do not respond to ABT-263 but exhibit sensitivity to anti-aging effects when exposed to PARP inhibitors or radiotherapy.44 However, certain limitations are associated with the use of senolytic drugs. For example, piperlongumine, while demonstrating moderate anti-aging activity in ovarian cancer patients treated with olaparib, was ineffective at eliminating senescent prostate cells. Similarly, fisetin did not induce cell death in senescent ovarian cancer cells.45 Moreover, senolytics lack specificity and are unable to differentiate between pathological and physiological senescent cells, posing an additional limitation to their potential use as anti-aging therapeutic agents.
Therefore, there is an urgent need to develop precise senotherapies to eliminate senescent cells or reduce SASP-related cytokine production.
Nanotherapies Targeting Senescent Cells
Nanotechnology applications in the biomedical field are revolutionizing the fight against aging by enabling scientists to manipulate materials at the molecular and atomic levels. This precision allows for the creation of miniature tools capable of penetrating human cells and directly addressing the root causes of aging.46
One significant application of nanotechnology is targeted drug delivery using nanoparticles. These nanoparticles can be engineered to transport drugs, genes, or other therapeutic molecules directly on senescent cells or damaged tissues, thereby enhancing treatment efficacy and minimizing side effects.47
Nanomethods
Exosomes
As natural nanoparticles of signaling molecules, exosomes have potential applications in drug delivery and disease therapy. Exosomes have a vesicular structure that can carry various molecules both on the membrane and inside and deliver payloads to target cells by fusion, phagocytosis, or recognition of binding receptors. Studies have shown that natural exosomes can load nucleic acids (such as mRNAs, miRNAs, siRNAs, long noncoding RNAs (lncRNAs) and DNA), proteins, and metabolites.48 Therefore, efforts have been made to explore the potential of exosomes in the treatment of aging-related diseases. Aging impairs the function of tendon stem/progenitor cells (AT-SCs) and tendon homeostasis, and there is no effective treatment for tendon diseases caused by aging. However, as naturally derived nanoparticles containing bioactive molecules, exosomes have attracted much attention in the fields of tissue engineering and regenerative medicine. Liu et al reported that young exosomes (SHED-Exos) secreted by human deciduous tooth stem cells have abundant anti-aging effects. These young bionanoparticles alleviate the aging phenotype of aged AT-SCs and maintain their tenogenic capacity. Mechanistically, SHED-Exos reversed AT-SC aging by regulating histone methylation and repressing nuclear factor (NF)-κB. Systemic administration of SHED-Exos bionanoparticles delayed tendon degeneration, and locally delivered SHED-Exos reduced ectopic bone formation by senescent cells in a naturally aging mouse model, thereby functionally and structurally restoring endogenous tendon regeneration and repair capacity in aging rats. Taken together, these findings indicate that SHED-Exos have great potential as natural bioactive nanoparticles for the treatment of aging-related diseases.
Microneedle
Microneedle-mediated transdermal administration of nanoparticles further increases the efficiency of drug delivery and prevents loss after oral or intravenous administration because this method of administration can directly pierce the stratum corneum of the skin and inject therapeutic molecules into the viable epidermis and dermis in a painless, efficient and minimally invasive manner, thus significantly improving the bioavailability of drugs while avoiding the pain associated with injection. The development of microfabrication technology and nanotechnology will improve drug stability during the preparation of nanoparticles and microneedles, increase the amount of drug per nanoparticle patch, and promote the sustained release of drugs under the skin. This will effectively reduce the frequency of drug administration and improve patient compliance, breaking through the bottleneck of the treatment of aging diseases. There is a risk of various health complications in wound healing in the elderly population, which results in substantial economic and psychological burdens on patients. The low activity of aged dermal fibroblasts (A-FBs) and a disturbed local immune response in the deep dermis result in delayed wound healing. Therefore, the complex deep local microenvironment requires further treatment. Jiang et al presented a novel bilayer hyaluronic acid methacrylate (HAMA)/polyvinyl alcohol (PVA) MN patch (MNP) encapsulated with young fibroblast-derived exosomes (Y-EXOs) (Y-EXOs @ HAMA/PVA MNPs) for deep drug delivery, aging, wound healing, and immunomodulation. In this study, we aimed to design portable MNs with anti-aging and immunomodulatory properties for aged unhealed wounds.49 Fibroblast-derived young exosomes (YEXOs) (Y-EXOs @ Zn-HAMA/PVA MNPs) were coated with the addition of zinc to the MN tips of hyaluronic acid methacrylate (HAMA)/polyvinyl alcohol (PVA) MNPs. HAMA MNPs were initially frozen in liquid nitrogen to create ultralow temperature conditions. When the exosomal solution was sprayed onto the MN tip surface, the water was turned into ice to protect the HAMA from swelling. The MNPs were then placed in a freeze dryer to remove excess water, and the above steps were repeated. The mechanical strength after freeze-drying increased, and the characteristics of the MN tips before and after cycling also differed. When the coated MN tip penetrates the wound area, the MN tip is separated from the substrate, and the MN tip remains in the wound. The outer layer of exosomes is rapidly released into actively aging dermal fibroblasts (A-FBs) and regulates T-cell IL-17A production. In vitro, Y-EXOs promoted the proliferation and migration of A-FBs and affected the differentiation of Th17 cells and the production of IL-17A. In vivo, exosomes promote wound healing in aging skin by regulating collagen deposition and IL-17A production. In addition, this study sequenced the microRNA content of Y-EXOs and A-EXOs. This study initially demonstrated that Y-EXOs have effective antiaging and anti-inflammatory effects, and Y-EXOs@HAMA/PVA MNPs are expected to constitute a new therapeutic strategy.
Nanobased Drug Delivery Systems
Nanosystems, also known as nanomedicines, have provided many benefits for disease prevention, diagnosis, and treatment. One important aspect of nanomedicine development is the delivery and controlled release of drugs to disease sites. By combining nanobased drug delivery systems, the effectiveness of treatment can be improved. Traditional drug delivery systems commonly have deficiencies such as low stability, poor solubility, low bioavailability, short half-life, nontargeted delivery, and challenges posed by their large size materials. Therefore, the use of new drug delivery systems that can target specific body sites may be a viable option to address these key issues. Compared to traditional systems, nanosystems have been found to be more suitable for therapeutic purposes, as nanodrugs can improve drug delivery routes by altering drug solubility, release curves, diffusion rates, bioavailability, and immunogenicity, thereby reducing toxicity, minimizing side effects, improving biodistribution, and extending drug lifespan. It has been reported that nanostructures help prevent drugs from being damaged in the gastrointestinal tract and deliver small amounts of water-soluble drugs to their target locations. Nanodrugs exhibit typical absorption and endocytosis mechanisms in the body, indicating increased oral bioavailability. Nanostructures remain in the circulatory system for longer periods and can release drugs at specified doses. Therefore, they cause minimal plasma fluctuations and have fewer adverse reactions.50 Additionally, nanoformulations used for cancer treatment and diagnostics have been used to deliver drugs to solid tumors at relatively high concentrations, with minimal toxicity to surrounding normal cells or tissues.51 Different nanomaterials with varying structures can improve the bioavailability of insoluble or unstable drugs. The development of nanotechnology has led to the exploration of different nanomaterials with structural sizes ranging from 1 to 100 nm, which are lighter, stronger, faster, and more durable and can move more freely in the human body, exhibiting their unique structural, chemical, mechanical, magnetic, and biological properties. Common nanocarrier materials include polymer nanoparticles (PNPs), liposomes (LNPs), micelles, dendrimers, quantum dots (QDs), fullerenes, carbon nanotubes, etc. Given the aforementioned circumstances, the high drug-loading capacity and preferential delivery of drugs to targets provided by nanomaterials greatly modulate the characteristics of candidate drugs, offering new hope for targeting, detecting, and eliminating senescent cells.
Senolytics are a new class of drugs with significant potential for improving aging-related diseases.52 However, some of the discovered senolytic drugs lack specificity and may even have strong side effects. According to the mid-term results of a clinical trial on knee osteoarthritis patients, the first clinical trial using candidate drugs failed to surpass the placebo in relieving joint pain and stiffness. Therefore, developing precise senotherapies based on targeted drug delivery strategies to eliminate senescent cells and improve safety is crucial. Multifunctional nanosystem treatment approaches can be considered second-generation senolytic nanotherapies, which not only have high drug-loading capacity but also preferentially deliver drugs to targets without adverse effects, providing new hope for targeting, detecting, and eliminating senescent cells.53,54 One strategy for second-generation senolytics is to differentiate senescent cells from normal cells. In this regard, senescence-associated β-galactosidase activity (SA-β-gal) is one of the characteristics of senescent cells. Senescent cells display elevated levels of lysosomal enzymes, such as β-galactosidase or α-fucosidase.55 Low oligosaccharide-encapsulated nanoparticles (GalNPs) preferentially release their cargo on senescent cells.56 Senolytics are encapsulated in nanoparticles with a galactose coating, and the high level of SA-β-gal in senescent cells enhances drug release, making drug release more pronounced. In theory, encapsulated senolytics reduce the systemic toxicity of drugs.57 Additionally, the expression of senescent cell markers such as CD9, B2M, CD36, and CD47 can be used to enhance delivery accuracy.58–61 Therefore, nanoparticles can also be used in combination with different cargoes for imaging or killing senescent cells.62
Currently, progress has been made in the development of nanoparticles for diagnosis and treatment, including mesoporous calcium carbonate nanoparticles, mesoporous silica nanoparticles, carbon quantum dots, iron oxide, and molecularly imprinted polymer nanoparticles, which can encapsulate senolytics/senomorphic/fluorescent agents in nanomaterials to achieve targeted detection or elimination of senescent cells. All the nanosystems targeting senescent cells are summarized in Table 1.
 |
Table 1 Nanodrug Delivery System Targeting Senescent Cells |
Mesoporous Silica Nanoparticles
Mesoporous silica nanoparticles (MSNs), as novel inorganic nanomaterials, are excellent candidates for various biomedical applications due to their good biocompatibility, large pore size, and small size. They can interact with drugs through ionic bonds, hydrogen bonds, and electrostatic interactions, making it easy to regulate pore volume and pore size.73 Functional polymers or targeting coatings can be used to further protect and encapsulate cargoes until they reach the target area for therapeutic effects.74 In particular, they can minimize the systemic side effects of anticancer drugs.66,75 The first reported chemical therapeutic nanoparticles designed by Agostini et al achieved selective and controlled release of molecules within senescent cells by using mesoporous silica nanoparticles (MSNs) coated with low oligosaccharides (GOS) and containing the cargo molecule rhodamine B. GOS, as a substrate for senescence-associated β-galactosidase (SA-β-gal), degrades only the low oligosaccharide shell and releases the drug in senescent cells while protecting the cargo in other cells. Experiments have demonstrated that the nanocarrier preferentially releases the drug molecule in patients with congenital keratinization disorder and human senescent fibroblasts, indicating the effectiveness of this drug delivery system in vivo.74,76 Muñoz-Espín et al further demonstrated the efficacy of this drug carrier system in vitro cell and mouse models of pulmonary fibrosis and cancer. These models showed that the carrier was more effectively absorbed by senescent cells in diseased tissues, preventing drug exposure to nonlesioned tissues and minimizing associated adverse reactions. Galiana et al encapsulated navitoclax in MSNs terminated with low oligosaccharides, selectively releasing it in senescent cells54 (Figure 2). This approach inhibited tumor development and reduced metastasis while also reducing the systemic toxicity of navitoclax.77 Experimental evidence suggests that CD9 antibody-modified mesoporous silica nanoparticles exhibit high cellular uptake in senescent foam macrophages and senescent endothelial cells in vitro models, reducing reactive oxygen species levels,78 high-density lipoprotein oxidation, and the production of TNF-α and IL-6 while attenuating the aging process.63
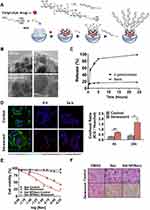 |
Figure 2 β-Galactosidase-dependent Capped Nanoparticles Selectively Release Their Payload. (A) A schematic illustration of the process used to create capped, mesoporous silica nanoparticles loaded with either navitoclax or ICG. After the nanoparticles are loaded, 3-aminopropyl triethoxysilane is used to functionalize their exterior surface. The galactooligosaccharide is then covalently grafted onto the outside surface of the nanoparticles by creating a hemiaminal link. (B) Exemplary high-resolution TEM images showing the release of Gal·NPs. Black and white longitudinal stripes are observed on spherical particles, approximately 100 nm in size, with a honeycomb structure of the porous scaffold or channels. The lower values indicate 50 nm, whereas the upper figures indicate 100 nm. (C) Absorption emission spectrometry measurements of Gal·NP(ICG) nanoparticle cargo release experiments. The graph shows the cargo release patterns at the specified time points when Aspergillus oryzae β-galactosidase is present in water at pH 4.5 at room temperature and when it is absent (blank). (D) Senescent 4 T1 cells preferentially take up Gal·NPs (ICG). Confocal imaging of senescent and control cells after they were exposed to fluorophore-loaded nanoparticles for six or twenty-four hours. Blue: WGA; Green: Hoechst Red: ICG; Alexa Fluor 488 Conjugate. The graph on the left displays the mean ± standard error of the mean values obtained from confocal imaging. The statistical significance was determined using a two-tailed Student’s t test: *p <0.05, **p <0.01. E–F) The application of Gal·NPs (Nav) to senescent 4 T1 cells induced by palbociclib causes cell death. (E) Luminous ATP detection in response to increasing doses or (F) crystal violet assay at the free navitoclax IC50 concentration (0.3 μM) for cell viability investigations of free or encapsulated navitoclax. Reprinted from J Controlled Release, volume 323, Galiana I, Lozano-Torres B, Sancho M et al. Preclinical antitumor efficacy of senescence-inducing chemotherapy combined with a nanoSenolytic. 624–634, copyright 2020, with permission from Elsevier.55 |
Calcium Carbonate Nanoparticles
Calcium carbonate (CaCO3) nanoparticles have been widely used in biomedical applications, particularly in drug delivery, due to their availability, low cost, safety, biocompatibility, pH sensitivity, and slow biodegradability.79 CaCO3 nanoparticles (CaCO3NPs) have shown great potential as drug carriers for targeting tumor tissues and cells. Their pH-dependent properties and potential for functionalization with targeted drugs make them unique in targeted delivery systems for anticancer drugs. Moreover, the slow degradation of the CaCO3 matrix can be utilized for sustained release systems, allowing drugs to remain in cancer tissues for a longer period of time. Previous studies have demonstrated that functionalized porous calcium carbonate nanoparticles loaded with the mTOR inhibitor rapamycin (CD9-Lac/CaCO3/Rapa) can selectively target senescent cells and exhibit anti-aging effects by blocking the SASP (IL-6 and IL-1β), reducing β-galactosidase and p53/p21/CD9/cyclin D1 expression, decreasing population doubling time, enhancing the proliferation and migration of aged dermal fibroblasts, and preventing cell cycle arrest.80 These results support the efficacy of this novel nanoparticle system for targeted therapy of senescent cells.
Liposomes
Liposomes are self-assembled hollow spheres composed of phospholipid molecules. The concept of using liposomes as drug delivery systems was initially established by Gregory Gregoriadis.81–83 Liposomes have similar compositions and structures to cell membranes, making them biocompatible and effective at protecting and releasing loaded drugs. With particle sizes ranging from 20 nm to 200 nm, liposomes can be easily metabolized by enzymes in the human body, allowing efficient drug absorption. They have been proven to be the optimal drug delivery system for hydrophobic drugs such as rapamycin. The unique structure of liposomes reduces the side effects of encapsulated drugs compared with free drugs, making them promising for the development of anticancer nanomedicines.67,84 Nguyen et al conducted a study on the association of a CD9 monoclonal antibody (CD9 mAb) with liposomal nanoparticles.70 CD9 monoclonal antibodies can specifically recognize the CD9 protein on cell membranes. The therapeutic effect of liposomes can be enhanced by PEGylation to reduce the uptake of liposomes by the reticuloendothelial system (RES) and prolong blood circulation time. They conjugated CD9 mAb to the surface of PEGylated liposomes for targeted delivery of rapamycin to overcome cellular senescence in cells overexpressing CD9 receptors. The results showed that senescent human dermal fibroblasts (HDFs) exhibited greater uptake of CD9-targeted liposomes than young HDFs. Furthermore, the LR-CD9 mAb significantly enhanced the antiproliferative, cell cycle-regulating, and wound healing effects of rapamycin on migration. These results suggest that CD9-targeted PEGylated liposomes may serve as a promising drug delivery platform for targeting senescent cells. Ashiba et al developed lipid nanoparticle (LNP) formulations with dual drug encapsulation to inhibit cellular senescence in human adipose tissue-derived mesenchymal stem cells (hAT-MSenescent cells) and natural killer (NK) cells by removing dysfunctional mitochondria from senescent cells.69 After LNP treatment, SA-β-Gal activity in hAT-MSenescent cells decreased by 20%, and the upregulation of p21 was inhibited. LNP treatment also significantly downregulated the expression of senescence markers in expanded NK cells. These results suggest the potential of LNP therapy for inhibiting or reversing cell senescence.
Nanoemulsions
In addition to liposomes, lipid-based organic nanoparticles include nanoemulsions. Belcastro et al developed a maleimide-modified Labrafac nanoemulsion for the transplantation of anti-VCAM-1 antibodies, aiming to target senescent endothelial cells.85 In addition to being developed as therapeutic approaches, these nanosystems can also be considered for diagnostic purposes when loaded with lipophilic fluorescent dyes. The 4N1K peptide derived from the TSP10 protein exhibits senolytic activity by targeting the CD47 receptor present on the surface of senescent cells. Jatal et al chemically conjugated 4N1K peptides to PEGylated hydrophobic chains, allowing them to bind to sphingomyelin nanoemulsion systems (SNs) and expose them on their surface.86 The nanoemulsion serves as a delivery system for the peptide to effectively target senescent cells. The results showed enhanced senolytic activity of SNs-4N1Ks (SNs-Ks) in a chemotherapy-induced breast cancer cell senescence model, demonstrating for the first time the potential of combining SNs with the 4N1K peptide in the development of innovative senolytic therapies for cancer (Figure 3).
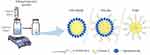 |
Figure 3 Diagrammatic representation of the production of the nanosystems (SNs-blank, SNs-Ks, and V-Ks) by the ethanol injection method. Reprinted from Int J Pharm, volume 617, Jatal R, Mendes Saraiva S, Vázquez-Vázquez C et al. Sphingomyelin nanosystems decorated with TSP-1 derived peptide targeting senescent cells. 121618, copyright 2022, with permission from Elsevier.69 |
Dendritic Polymers
Dendritic polymers are the smallest nanocarriers, with a tree-like structure, and are a class of highly branched and radially symmetric polymers that are easily functionalized. Due to their amphiphilic copolymers containing hydrophilic and hydrophobic monomer units, they can be used to carry drugs with poor solubility. Drugs can be covalently bound, electrostatically interact, or encapsulated into dendritic polymers. The branching structure gives dendritic polymers a very high surface area-to-volume ratio, allowing them to improve drug delivery efficiency.87,88 Dendritic polymers can be classified based on their functional groups, such as peptides (PPIs), polylysine (l-lysine), polyamidoamine (PAMAM), and PAMAM-organic silicon (PAMAMOS). PAMAM is the most commonly used dendritic polymer in nanomedicine and has been applied in many drug or gene delivery systems. Modification of dendritic polymers is usually carried out to reduce the toxicity caused by amino groups or eliminate them.72,89 Lewińska et al designed a nanodelivery system based on PAMAM dendritic polymers for lapatinib (L) and fulvestrant (F) and evaluated their effectiveness in improving the anticancer effects of L and F on three different receptor states of breast cancer cell models (ie, ER-positive, triple-negative, and HER2-positive cells).90 The experiments showed that the use of L-PAMAM and F-PAMAM nanodelivery systems not only enhanced the anticancer effects of free drugs mediated by apoptotic cell death and cytotoxic autophagy but also eliminated senescent breast cancer cells induced by doxorubicin, ie, senolytic therapy. Therefore, novel nanosenolytics may become part of a sequential treatment for breast cancer, promoting senescence and subsequently combating aging, but this hypothesis still needs to be confirmed through in vivo systems in further research.
Polymer Nanoparticles
Polymer nanoparticles have shown promise in drug delivery systems due to their controlled release properties, subcellular size, and biocompatibility with tissues and cells. They have been widely used for nanoencapsulation of various biologically active molecules and drugs.71 Common polymer nanoparticles include poly-d, l-lactic-co-glycolic acid (PLGA), polylactic acid (PLA), poly-ε-caprolactone (PCL), chitosan, and gelatin-polyacryloyl chloride (PAC).91 Among them, PLGA is one of the most successful biodegradable nanosystems for developing nanomedicines because it hydrolyzes in the body to produce the biodegradable monomers lactic acid and glycolic acid. Since the human body effectively processes these two monomers, the systemic toxicity of PLGA used for drug delivery or biomaterial applications is relatively low. Lim et al loaded the senolytic drug ABT263 into poly(lactic-co-glycolic acid) nanoparticles (PLGA-ABTs) and administered them to a rat model of needle puncture-induced intervertebral disc degeneration (IVDD) through disc delivery.64 This strategy selectively cleared senescent cells from degenerated IVDs, reduced the expression of proinflammatory cytokines and matrix metalloproteinases in IVDs, inhibited the progression of IVDD, and even restored IVD structure. This study demonstrated for the first time that local injection of a senolytic drug can effectively treat age-related IVDD. Similarly, Lee et al successfully eliminated stress-induced senescent cells in rat hearts using PLGA nanoparticles to deliver ABT263 locally, demonstrating for the first time that local delivery of senolytic drugs can effectively treat myocardial ischemia‒reperfusion (IR) injury and prevent systemic toxicity associated with the removal of senescent cells.92 Senescent cells are key driving factors in the formation and maturation of atherosclerotic plaques, and resveratrol (RSV) is an effective anti-aging drug that helps attenuate senescent cells and reduce aortic plaques. Pham et al loaded RSV into CD9-modified mesoporous silica nanoparticles coated with hyaluronic acid (HA), poly(l-lysine hydrochloride) (PLL), and methoxy poly(ethylene glycol)-block-poly(l-glutamic acid sodium salt) (PGA) (CD9-HMSN@RSV) and released them through the HAase reaction, which could precisely target aging plaques and senescent cells associated with atherosclerosis in ApoE-/- mice.
Fe3O4 Nanoparticles
Senolytic drugs specifically mediate the elimination of senescent cells. Lewińska et al used quercetin-functionalized Fe3O4 nanoparticles (MNPQs) and evaluated their senolytic and senostatic activities during hydrogen peroxide-induced human fibroblast aging in vitro.65 This study demonstrated that MNPQ promoted AMP-activated protein kinase (AMPK) activity, reduced senescence-related proinflammatory responses (decreased IL-8 and IFN-β levels), and effectively inhibited human fibroblast senescence, indicating the potential of MNPQ as a promising candidate for senolytic and senostatic-based antiaging therapy.
Molecularly Imprinted Nanoparticles
Molecularly imprinted polymers (MIPs), also known as plastic antibodies or artificial antibodies, are chemically synthesized affinity materials with custom-designed binding cavities that are complementary in shape, size, and functionality to template molecules. Due to the presence of imprinting cavities, MIPs can selectively recognize and bind template molecules. Compared to traditional biological ligands such as antibodies, aptamers, and lectins, MIPs have advantages such as ease of preparation, good stability, and low cost.68 In recent years, MIPs have gained prominence in mediating cell recognition, prompting researchers to expand their traditional application areas to more challenging biomedical applications.93 Regarding senolytic drugs, a new technique involving the loading of dasatinib into molecularly imprinted nanoparticles (nanoMIPs) and the validation of a set of novel senescence membrane markers, B2Ms, for detecting senescent cells in vitro and in vivo has been reported.94 The final results showed that nanoMIPs selectively killed senescent cells by delivering cytotoxic drugs to them. Furthermore, fluorescently labeled nanoMIPs could be used to detect senescent cells in vivo and were nontoxic when administered as a single dose.
Zinc Oxide Nanoparticles
Zinc oxide nanoparticles (ZnO NPs) are attractive due to their good biocompatibility, as zinc is an essential trace metal in the human body and is easily excreted. Wiesmann et al demonstrated that ZnO NPs can eliminate residual senescent tumor cells.95 This reveals the potential of ZnO NPs as an innovative adjunctive tumor therapy that can overcome radiation-induced senescence-associated radioresistance.
Summary
During the process of aging, the accumulation of senescent cells continuously drives the occurrence of chronic diseases. While senotherapy shows promise in addressing age-related illnesses, recent studies have highlighted its lack of specificity and potential side effects. In contrast, nanomedicine, as a drug delivery system, overcomes these limitations and has been validated experimentally. By optimizing the delivery route and overcoming physical and biological barriers, nanomedicine enables targeted drug delivery with specific characteristics such as altered drug solubility, release profiles, diffusion rates, bioavailability and immunogenicity, thereby improving drug distribution and extending drug lifespan.93 Comparisons between traditional anti-aging therapy and nanotherapy are listed below (Table 2).
 |
Table 2 Comparison Between Traditional Anti-Aging Therapy and Nanotherapy |
However, the application of nanosystems for targeting cellular senescence is limited by certain limitations. 1) There is significant heterogeneity in organs and aging states within patient populations.94 Current nanosystems for senotherapy have been developed using cellular senescence biomarkers as a basis. However, the markers of aging can differ depending on the specific condition and organ. While numerous inflammatory factors have been identified as components of the SASP, their expression levels lack specificity in pinpointing cellular senescence. Thus, there is a pressing need to identify more accurate biomarkers with heightened sensitivity and specificity to establish more precise nanosystems for senotherapy. 2) The different therapeutic responses of the developed nanosystems in animal models and humans. The immune system and skin exhibit varying responses across different animal species and human individuals. Therefore, while numerous anti-aging treatments have shown efficacy in animal models and in vitro studies, clinical trials evaluating targeted therapeutic approaches are essential to determine their safety and effectiveness in human patients.
Consequently, the guiding principles for the development of nanodrug delivery systems tailored for senotherapy in the future can be summarized as follows.
- Ultrasensitive biomarker-switchable nanosystems. Studies have shown that metabolic abnormalities in organelles such as lysosomes and mitochondria can be used as clear markers of cell senescence. β-galactosidase (SA-β-Gal) can catalyze the hydrolysis of β-galactoside in cells, but its activity is abnormally increased in senescent cells. Lipofuscin is a metabolite of lysosomal output that gradually deposits with cell senescence and eventually forms senile plaques on the body surface at advanced ages. Energy metabolism in senescent cells, including carbohydrates and lipids, is disordered, and the oxygen consumption rate of mitochondria, as the “energy factory” of cells, increases, which produces more free radicals while producing a large amount of ATP for the operation of protein machinery, resulting in further damage to various organelles.95
- Targeting upstream regulators in aging disease. Recent studies have shown that immunosenescence mediates changes in the inflammatory state of the body by releasing cytokines and changing immune capacity and subsequently participates in organ aging and disease. As the most powerful type of phagocytic cell, macrophages can phagocytose and eliminate senescent or dead cells, thus alleviating the aging phenomenon. At the same time, macrophages also undergo polarization, cytokine release and antigen presentation, which are closely related to inflammatory activation. Therefore, the aging of macrophages themselves may regulate their own inflammatory activation by inhibiting the cell cycle and may also reduce their phagocytic function and aggravate the accumulation of aging signals, which play a multifaceted role in organ aging and aging-related diseases. Yang et al discussed the specific molecular markers, cell function changes and regulatory network of macrophage senescence and described the critical role of macrophage senescence in organ aging and aging-related diseases. On this basis, nanosystems hijacking senescent macrophages show promise in reversing cellular senescence at an earlier stage.100
Overall, nanoscale drug delivery systems have great potential as nanotherapies for targeting senescent cells.101,102
Funding
This study was supported in part by the Zhejiang Provincial Natural Science Foundation of China (Grant Nos. LTGD23H150001 and LTGD24H070006), the Medical and Health Research Project of Zhejiang Province (Grant Nos. 2023KY212 and 2024KY631), and the Project of Zhejiang Province Administration of Traditional Chinese Medicine (Grant No. 2024ZL249).
Disclosure
The authors declare no competing interests in this work.
References
1. Aging CJ. Cellular Senescence, and Cancer. Annu Rev Physiol. 2013;75:685–705. doi:10.1146/annurev-physiol-030212-183653
2. Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest. 2018;128:1238–1246. doi:10.1172/JCI95148
3. Di Micco R, Krizhanovsky V, Baker D, d’Adda Di Fagagna F. Cellular senescence in aging: from mechanisms to therapeutic. Opport Nat Rev Mol Cell Biol. 2021;22:75–95.
4. McHugh D, Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol. 2018;217:65–77. doi:10.1083/jcb.201708092
5. J.-p. C, Patil CK, Rodier F, et al. Senescence-Associated Secretory Phenotypes Reveal Cell-Nonautonomous Functions of Oncogenic RAS and the p53 Tumor Suppressor. PLoS Biol. 2008;6:e301. doi:10.1371/journal.pbio.0060301
6. Khosla S, Farr JN, Tchkonia T, Kirkland JL. The role of cellular senescence in aging and endocrine disease. Nat Rev Endocrinol. 2020;16:263–275. doi:10.1038/s41574-020-0335-y
7. Lecot P, Alimirah F, Desprez P-Y, Campisi J, Wiley C. Context-dependent effects of cellular senescence in cancer development. Br J Can. 2016;114:1180–1184. doi:10.1038/bjc.2016.115
8. D HA, Tazearslan C, Tare A, et al. Age- and Tissue-Specific Expression of Senescence Biomarkers in Mice. Front Genet. 2018;9:59. doi:10.3389/fgene.2018.00059
9. Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech Aging Dev. 2007;128:36–44.
10. Kritsilis M, V Rizou S, Koutsoudaki PN, et al. Aging, Cellular Senescence and Neurodegenerative Disease. Int J Mol Sci. 2018;19:2937. doi:10.3390/ijms19102937
11. Baker DJ, Petersen RC. Cellular senescence in brain aging and neurodegenerative diseases: evidence and perspectives. J Clin Invest. 2018;128:1208–1216. doi:10.1172/JCI95145
12. Shakeri H, Lemmens K, Gevaert AB, De Meyer GRY, Segers VFM. Cellular senescence links aging and diabetes in cardiovascular disease. Am J Physiol Heart Circ Physiol. 2018;315:H448–H462. doi:10.1152/ajpheart.00287.2018
13. North BJ, Sinclair DA. The Intersection Between Aging and Cardiovascular Disease. Circ Res. 2012;110:1097–1108. doi:10.1161/CIRCRESAHA.111.246876
14. J BD, Wijshake T, Tchkonia T, et al. Clearance of p16Ink4a-positive senescent cells delays aging-associated disorders. Nature. 2011;479:232–236. doi:10.1038/nature10600
15. K PA, Tchkonia T, LeBrasseur NK, et al. Cellular Senescence in Type 2 Diabetes: a Therapeutic Opportunity. Diabetes. 2015;64:2289–2298. doi:10.2337/db14-1820
16. Tchkonia T, Zhu Y, Van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. doi:10.1172/JCI64098
17. Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases: curr. Opin Clin Nutr Metab Care. 2014;17:324–328.
18. Baker DJ. Naturally occurring p16Ink4a-positive cells shorten healthy lifespan. Nature. 2016;530:184–189. doi:10.1038/nature16932
19. Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi:10.1111/acel.12344
20. Wissler Gerdes EO, Zhu Y, Tchkonia T, Kirkland JL. Discovery, development, and future application of senolytics: theories and predictions. FEBS J. 2020;287:2418–2427. doi:10.1111/febs.15264
21. Montero JC, Seoane S, Ocaña A, Pandiella A. Inhibition of Src Family Kinases and Receptor Tyrosine Kinases by Dasatinib: possible Combinations in Solid Tumors. Clin Cancer Res. 2011;17:5546–5552. doi:10.1158/1078-0432.CCR-10-2616
22. Bruning A. Inhibition of mTOR Signaling by Quercetin in Cancer Treatment and Prevention. Anticancer Agents Med Chem. 2013;13:1025–1031. doi:10.2174/18715206113139990114
23. Childs BG, Baker DJ, Wijshake T, et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354:472–477. doi:10.1126/science.aaf6659
24. Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi:10.1016/j.ebiom.2018.12.052
25. Mahoney SA, Venkatasubramanian R, Darrah MA, et al. Intermittent supplementation with fisetin improves arterial function in old mice by decreasing cellular senescence. Aging Cell. 2024;23:e14060. doi:10.1111/acel.14060
26. Roos CM, Zhang B, Palmer AK, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15:973–977. doi:10.1111/acel.12458
27. Ogrodnik M, Miwa S, Tchkonia T, et al. Cellular senescence drives age-dependent hepatic steatosis. Nat Commun. 2017;8:15691. doi:10.1038/ncomms15691
28. Musi N, Valentine JM, Sickora KR, et al. Tau protein aggregation is associated with cellular senescence in the brain. Aging Cell. 2018;17:e12840. doi:10.1111/acel.12840
29. Dai H, Chen R, Gui C, et al. Eliminating senescent chondrogenic progenitor cells enhances chondrogenesis under intermittent hydrostatic pressure for the treatment of OA. Stem Cell Res Ther. 2020;11:199. doi:10.1186/s13287-020-01708-5
30. Zhu Y, Doornebal EJ, Pirtskhalava T, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging. 2017;9:955–963. doi:10.18632/aging.101202
31. Yousefzadeh MJ, Zhu Y, McGowan SJ, et al. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine. 2018;36:18–28. doi:10.1016/j.ebiom.2018.09.015
32. Chang J, Wang Y, Shao L, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi:10.1038/nm.4010
33. Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl‐2 family of anti‐apoptotic factors. Aging Cell. 2016;15:428–435. doi:10.1111/acel.12445
34. Tse C, Shoemaker AR, Adickes J, et al. ABT-263: a Potent and Orally Bioavailable Bcl-2 Family Inhibitor. Cancer Res. 2008;68:3421–3428. doi:10.1158/0008-5472.CAN-07-5836
35. Yoon S, Eom GH. HDAC and HDAC Inhibitor: from Cancer to Cardiovascular Diseases. Chonnam Med J. 2016;52:1. doi:10.4068/cmj.2016.52.1.1
36. Samaraweera L, Adomako A, Rodriguez-Gabin A, McDaid HM. A Novel Indication for Panobinostat as a Senolytic Drug in NSCLC and HNSCC. Sci Rep. 2017;7:1900. doi:10.1038/s41598-017-01964-1
37. Baar MP, Brandt RMC, Putavet DA, et al. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. 2017;169:132–147.e16. doi:10.1016/j.cell.2017.02.031
38. Fuhrmann-Stroissnigg H, Ling YY, Zhao J, et al. Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun. 2017;8:422. doi:10.1038/s41467-017-00314-z
39. Jeon OH, Kim C, Laberge R-M, et al. Local clearance of senescent cells attenuates the development of posttraumatic osteoarthritis and creates a pro-regenerative environment. Nat Med. 2017;23:775–781. doi:10.1038/nm.4324
40. Aguayo-Mazzucato C. Acceleration of β Cell Aging Determines Diabetes and Senolysis Improves Disease Outcomes. Cell Metab. 2019;30:129–142.e4.
41. Rudin CM, Hann CL, Garon EB, et al. Phase II Study of Single-Agent Navitoclax (ABT-263) and Biomarker Correlates in Patients with Relapsed Small Cell Lung Cancer. Clin Cancer Res. 2012;18:3163–3169. doi:10.1158/1078-0432.CCR-11-3090
42. Gandhi L, Camidge DR, Ribeiro de Oliveira M, et al. Phase I Study of Navitoclax (ABT-263), a Novel Bcl-2 Family Inhibitor, in Patients With Small-Cell Lung Cancer and Other Solid Tumors. J Clin Oncol. 2011;29:909–916. doi:10.1200/JCO.2010.31.6208
43. Wilson WH, O’Connor OA, Czuczman MS, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–1159. doi:10.1016/S1470-2045(10)70261-8
44. Malaquin N, Vancayseele A, Gilbert S, et al. DNA Damage- However, Not Enzalutamide-Induced Senescence in Prostate Cancer Promotes Senolytic Bcl-xL Inhibitor Sensitivity. Cells. 2020;9:1593. doi:10.3390/cells9071593
45. Fleury H, Malaquin N, Tu V, et al. Exploiting interconnected synthetic lethal interactions between PARP inhibition and cancer cell reversible senescence. Nat Commun. 2019;10:2556. doi:10.1038/s41467-019-10460-1
46. Van Baarle D, Tsegaye A, Miedema F, Akbar A. Significance of senescence for virus-specific memory T-cell responses: rapid aging during chronic stimulation of the immune system. Immunol Lett. 2005;97:19–29. doi:10.1016/j.imlet.2004.10.003
47. Demaria M, Ohtani N, Youssef SA, et al. An Essential Role for Senescent Cells in Optimal Wound Healing through Secretion of PDGF-AA. Dev. Cell. 2014;31:722–733. doi:10.1016/j.devcel.2014.11.012
48. Jin S, Wang Y, Wu X, et al. Young Exosome Bio-Nanoparticles Restore Aging-Impaired Tendon Stem/Progenitor Cell Function and Reparative Capacity. Adv Mater. 2023;35(2211602).
49. Xu J, Lin S, Chen H, et al. Highly Active Frozen Nanovesicles Microneedles for Senile Wound Healing via Antibacteria. Immunother Skin Regen Adv Healthc Mater. 2024;13(2304315).
50. Patra JK. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(71).
51. Shi J, Votruba AR, Farokhzad OC, Langer R. Nanotechnology in Drug Delivery and Tissue Engineering: from Discovery to Applications. Nano Lett. 2010;10:3223–3230. doi:10.1021/nl102184c
52. Sun T. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew Chem Int Ed. 2014;53:12320–12364.
53. Dolgin E. Send in the senolytics. Nat Biotechnol. 2020;38:1371–1377. doi:10.1038/s41587-020-00750-1
54. Poblocka M, Bassey AL, Smith VM, et al. Targeted clearance of senescent cells using an antibody‒drug conjugate against a specific membrane marker. Sci Rep. 2021;11:20358. doi:10.1038/s41598-021-99852-2
55. Galiana I. Preclinical antitumor efficacy of senescence-inducing chemotherapy combined with a nanoSenolytic. J Control Release. 2020;323:624–634.
56. Hildebrand D, Lehle S, Borst A, et al. α-Fucosidase as a novel convenient biomarker for cellular senescence. Cell Cycle. 2013;12:1922–1927. doi:10.4161/cc.24944
57. Agostini A. Targeted Cargo Delivery in Senescent Cells Using Capped Mesoporous Silica Nanoparticles. Angew Chem Int Ed. 2012;51:10556–10560.
58. Hernández-Mercado E, Prieto-Chávez JL, Arriaga-Pizano LA, et al. Increased CD47 and MHC Class I Inhibitory Signals Expression in Senescent CD1 Primary Mouse Lung Fibroblasts. Int J Mol Sci. 2021;22:10215. doi:10.3390/ijms221910215
59. Cho JH, Kim E-C, Son Y, et al. CD9 induces cellular senescence and aggravates atherosclerotic plaque formation. Cell Death Differ. 2020;27:2681–2696. doi:10.1038/s41418-020-0537-9
60. Chong M, Yin T, Chen R, et al. CD36 initiates the secretory phenotype during the establishment of cellular senescence. EMBO Rep. 2018;19:e45274. doi:10.15252/embr.201745274
61. Wang H, Liu B, Wei J. Beta2-microglobulin(B2M) in cancer immunotherapies: biological function, resistance and remedy. Cancer Lett. 2021;517:96–104. doi:10.1016/j.canlet.2021.06.008
62. Muñoz‐Espín D, Rovira M, Galiana I, et al. A versatile drug delivery system targeting senescent cells. EMBO Mol Med. 2018;10:e9355. doi:10.15252/emmm.201809355
63. Thapa RK, Nguyen HT, Jeong J-H, et al. Progressive slowdown/prevention of cellular senescence by CD9-targeted delivery of rapamycin using lactose-wrapped calcium carbonate nanoparticles. Sci Rep. 2017;7:43299. doi:10.1038/srep43299
64. Lewinska A, Adamczyk-Grochala J, Bloniarz D, et al. AMPK-mediated senolytic and senostatic activity of quercetin surface functionalized Fe3O4 nanoparticles during oxidant-induced senescence in human fibroblasts. Redox Biol. 2020;28:101337. doi:10.1016/j.redox.2019.101337
65. E E-A-A. Detecting and targeting senescent cells using molecularly imprinted nanoparticles. Nanoscale Horiz. 2019;4:757–768.
66. Pham LM, Kim E-C, Ou W, et al. Targeting and clearance of senescent foamy macrophages and senescent endothelial cells by antibody-functionalized mesoporous silica nanoparticles for alleviating aorta atherosclerosis. Biomaterials. 2021;269:120677. doi:10.1016/j.biomaterials.2021.120677
67. Nguyen HT, Thapa RK, Shin BS, et al. CD9 monoclonal antibody-conjugated PEGylated liposomes for targeted delivery of rapamycin in the treatment of cellular senescence. Nanotechnology. 2017;28:095101. doi:10.1088/1361-6528/aa57b3
68. Wiesmann N, Gieringer R, Viel M, et al. Zinc Oxide Nanoparticles Can Intervene in Radiation-Induced Senescence and Eradicate Residual Tumor Cells. Cancers. 2021;13:2989. doi:10.3390/cancers13122989
69. Jatal R. Sphingomyelin nanosystems decorated with TSP-1 derived peptide targeting senescent cells. Int J Pharm. 2022;617:121618. doi:10.1016/j.ijpharm.2022.121618
70. Belcastro E. Fluorescent nanocarriers targeting VCAM-1 for early detection of senescent endothelial cells. Nanomedicine. 2021;34:102379.
71. Lim S. Local Delivery of Senolytic Drug Inhibits Intervertebral Disc Degeneration and Restores Intervertebral Disc Structure. Adv Healthc Mater. 2022;11:2101483.
72. Lewińska A, Wróbel K, Błoniarz D, et al. Lapatinib- and fulvestrant-PAMAM dendrimer conjugates promote apoptosis in chemotherapy-induced senescent breast cancer cells with different receptor status. Biomater Adv. 2022;140:213047. doi:10.1016/j.bioadv.2022.213047
73. Lozano-Torres B, Blandez JF, Galiana I, et al. Real-Time In Vivo Detection of Cellular Senescence through the Controlled Release of the NIR Fluorescent Dye Nile Blue. Angew. Chem Int Ed Engl. 2020;59:15152–15156.
74. Jafari S, Derakhshankhah H, Alaei L, et al. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed Pharmacother. 2019;109:1100–1111. doi:10.1016/j.biopha.2018.10.167
75. Baeza A, Vallet-Regi M. Targeted Mesoporous Silica Nanocarriers in Oncology. Curr Drug Targets. 2018;19:213–224.
76. Pourpirali R, Mahmoudnezhad A, Oroojalian F, Zarghami N, Pilehvar Y. Prolonged proliferation and delayed senescence of the adipose-derived stem cells grown on the electrospun composite nanofiber coencapsulated with TiO2 nanoparticles and metformin-loaded mesoporous silica nanoparticles. Int J Pharm. 2021;604(120733). doi:10.1016/j.ijpharm.2021.120733
77. Dos Reis SRR, Pinto SR, de Menezes FD, et al. Senescence and the Impact on Biodistribution of Different Nanosystems: the Discrepancy on Tissue Deposition of Graphene Quantum Dots. Polycaprolactone Nanoparticle and Magnetic Mesoporous Silica Nanoparticles in Young and Elder Animals Pharm Res. 2020;37(40).
78. Maleki Dizaj S, Barzegar-Jalali M, Zarrintan MH, Adibkia K, Lotfipour F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin Drug Deliv. 2015;12:1649–1660. doi:10.1517/17425247.2015.1049530
79. Gregoriadis G. Liposomes as Immunoadjuvants and Vaccine Carriers: antigen Entrapment. ImmunoMethods. 1994;4:210–216. doi:10.1006/immu.1994.1022
80. Gregoriadis G, Ryman BE. Liposomes as carriers of enzymes or drugs: a new approach to the treatment of storage diseases. Biochem. J. 1971;124:58P–58P. doi:10.1042/bj1240058p
81. Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev. 2005;4:145–160. doi:10.1038/nrd1632
82. Tardi P, Choice E, Masin D, et al. Liposomal encapsulation of topotecan enhances anticancer efficacy in murine and human xenograft models. Cancer Res. 2000;60:3389–3393.
83. Guimarães D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571. doi:10.1016/j.ijpharm.2021.120571
84. Ashiba K, Mino K, Okido Y, Sato K, Kawakami H. Senescence recovering by dual drug-encapsulated liposomal nanoparticles for large-scale human cell expansion. J Artif Organs. 2023;26:246–250. doi:10.1007/s10047-022-01356-x
85. Santos A, Veiga F, Figueiras A. Dendrimers as Pharmaceutical Excipients: synthesis, Properties, Toxicity and Biomedical Applications. Materials. 2019;13:65. doi:10.3390/ma13010065
86. Duncan R, Izzo L. Dendrimer biocompatibility and toxicity. Adv Drug Deliv Rev. 2005;57:2215–2237. doi:10.1016/j.addr.2005.09.019
87. Abedi-Gaballu F, Dehghan G, Ghaffari M, et al. PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy. Appl. Mater. Today. 2018;12:177–190. doi:10.1016/j.apmt.2018.05.002
88. Wolinsky J, Grinstaff M. Therapeutic and diagnostic applications of dendrimers for cancer treatment☆. Adv Drug Deliv Rev. 2008;60:1037–1055. doi:10.1016/j.addr.2008.02.012
89. Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–347. doi:10.1016/s0169-409x(02)00228-4
90. Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75:1–18. doi:10.1016/j.colsurfb.2009.09.001
91. J.-r L, Park B-W, Park J-H, et al. Local delivery of a senolytic drug in ischemia and reperfusion-injured heart attenuates cardiac remodeling and restores impaired cardiac function. Acta Biomater. 2021;135:520–533. doi:10.1016/j.actbio.2021.08.028
92. BelBruno JJ. Molecularly Imprinted Polymers. Chem Rev. 2019;119:94–119. doi:10.1021/acs.chemrev.8b00171
93. Ostrovsky S, Kazimirsky G, Gedanken A, Brodie C. Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res. 2009;2:882–890.
94. Rad ME, Soylukan C, Kulabhusan PK, et al. Material and Design Toolkit for Drug Delivery: state of the Art. Trends, and Challenges ACS Appl Mater Interfaces. 2023;15:55201–55231.
95. Sun Y, Li Q, Kirkland JL. Targeting senescent cells for a healthier longevity: the roadmap for an era of global aging. Life Med. 2022;1:103–119.
96. Chen S, Han Y, Huang J, et al. Fabrication and Characterization of Layer-by-Layer Composite Nanoparticles Based on Zein and Hyaluronic Acid for Codelivery of Curcumin and Quercetagetin. ACS Appl Mater Interfaces. 2019;11:16922–16933. doi:10.1021/acsami.9b02529
97. Zhang H, Xu X, Shou X, et al. Senolytic Therapy Enabled by Senescent Cell-Sensitive Biomimetic Melanin Nano-Senolytics. Adv Healthc Mater. 2024.
98. Park W, Seong KY, Han HH, et al. Dissolving microneedles delivering cancer cell membrane coated nanoparticles for cancer immunotherapy. RSC Adv. 2021;11:10393–10399.
99. Park GH, Kwon HH, Seok J, et al. Efficacy of combined treatment with human adipose tissue stem cell-derived exosome-containing solution and microneedling for facial skin aging: a 12-week prospective, randomized, split-face study. J Cosmet Dermatol. 2023;22:3418–3426.
100. Wang L, Hong W, Zhu H, et al. Macrophage senescence in health and diseases. Acta Pharm Sin B. 2024;14:1508–1524. doi:10.1016/j.apsb.2024.01.008
101. Calcinotto A, Kohli J, Zagato E, et al. Cellular Senescence: aging. Canc Injury Physiol Rev. 2019;99:1047–1078.
102. Pan G, Shinde S, Yeung SY, et al. An Epitope-Imprinted Biointerface with Dynamic Bioactivity for Modulating Cell-Biomaterial Interactions. Angew Chem Int Ed Engl. 2017;56:15959–15963. doi:10.1002/anie.201708635
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

