Back to Journals » Infection and Drug Resistance » Volume 18
Advances in the Study of Acute Necrotizing Encephalopathy
Received 28 November 2024
Accepted for publication 20 March 2025
Published 2 April 2025 Volume 2025:18 Pages 1713—1720
DOI https://doi.org/10.2147/IDR.S508092
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Oliver Planz
Zi-Jie Cao,1,2 Yi Sun,1,2 Feng-Ling Li1,2
1Department of Neurology, Affiliated Hospital of Shandong Second Medical University, Weifang, 261000, People’s Republic of China; 2School of Clinical Medicine, Shandong Second Medical University, Weifang, 261000, People’s Republic of China
Correspondence: Feng-Ling Li, Department of Neurology,Affiliated Hospital of Shandong Second Medical University, No. 2428 of Yuhe Road, Dayu District, Weifang, 261000, People’s Republic of China, Tel +8615953647606, Fax +8605363081268, Email [email protected]
Abstract: Acute necrotizing encephalopathy (ANE) is a rare and rapidly progressing acute encephalopathy characterized by seizures and changes in consciousness. This condition typically affects the thalamus, brainstem, and cerebellum in a symmetrical manner, and if left untreated, is associated with significantly higher mortality rates. In recent years, with in-depth research on the etiology and pathogenesis of ANE, as well as continuous advances in clinical diagnosis and treatment techniques, the understanding of this disease has gradually increased. However, the complexity and diversity of ANE still pose significant challenges to clinical diagnosis and treatment. In addition, due to its rarity, many clinical doctors have insufficient understanding of it, resulting in a high rate of misdiagnosis and missed diagnosis. Therefore, this article reviews the etiology, pathogenesis, risk factors, pathology, clinical manifestations, auxiliary examinations, diagnostic methods, treatment strategies, and prognosis related to ANE. Its purpose is to enhance clinical doctors’ ability to identify and manage ANE, provide reference for accurate diagnosis and effective treatment, and support ongoing research aimed at gaining a deeper understanding of this serious disease.
Keywords: acute necrotizing encephalopathy, inflammation, MRI, RanBP2, viral infection
Background
Acute necrotizing encephalopathy (ANE) is an uncommon and severe form of acute encephalopathy characterized by seizures or altered levels of consciousness. It typically manifests as multiple symmetrical lesions in the thalamus, brainstem, and cerebellum following viral infection. First documented by Mizuguchi in 1995, ANE predominantly affects children under the age of five, with occurrences in adults being exceedingly rare.1 There are various types of acute necrotizing encephalopathy, including diffuse acute necrotizing encephalopathy (ANE1), SARS-CoV-2-related ANE, ANE caused by other viruses, and recurrent ANE, in addition to the most common sporadic ANE. Different types may be associated with viral infections, genetic susceptibility, metabolic disorders, or immune abnormalities. The primary trigger for ANE is associated with viral infections, though the exact etiology remains unclear and may involve genetic mutations, including RanBP2, CPTII, and SCN1A. ANE onset often involves a cytokine storm and excessive immune response, leading to an acute progression of the disease. The median interval between the onset of prodromal influenza symptoms and ANE is approximately three days. ANE presents with high fever, impaired consciousness, and seizures, with a poor prognosis and high mortality rate. Diagnosis relies on imaging, electrophysiological studies, and cerebrospinal fluid markers, with severity assessed using the ANE-SS rating scale. Treatment requires prompt interventions, including immunotherapy, anti-IL6 therapy, hypothermia, CRRT, and supportive care; however ANE has not been widely recognized among clinicians.
- Literature search: Relevant literature was searched through databases such as PubMed, Web of Science, Google Scholar, etc. The search keywords included “acute necrotizing encephalopathy”, “cytokine storm”, “RANBP2 gene”, “diagnosis and treatment”, etc.
- Inclusion and exclusion criteria: The included studies include peer-reviewed journal articles, review articles, and clinical guidelines. The exclusion criteria include peer-reviewed articles, conference abstracts, and duplicate studies.
- Data extraction and analysis: Conduct a systematic analysis of the included studies to extract key information, including etiology, pathogenesis, diagnostic methods, treatment strategies, and prognosis evaluation. Pay special attention to recent research progress and knowledge gaps.
- Quality assessment: Conduct a quality assessment of the included studies, with a focus on study design, sample size, follow-up time, and statistical methods.
- This article aims to fill the knowledge gap in the etiology, pathogenesis, diagnosis, and treatment of ANE, and provide more accurate diagnosis and treatment guidance for clinical doctors. By identifying and addressing these gaps, we have the potential to improve the prognosis of ANE patients and drive future research directions.
Etiology
Viruses
Isolated sporadic ANE is predominantly associated with viral infections, notably those caused by influenza virus, HHV-6, SARS-CoV-2, enterovirus, and COVID-19. Current evidence indicates limited direct viral invasion of the central nervous system, as these viruses rarely cross the blood-brain barrier to reach brain tissue. ANE cases typically arise spontaneously, although familial occurrences have been documented.
Genetic Factors
Ran-binding protein 2 (RanBP2 or Nucleoporin358) constitutes a major component of the cytoplasmic filaments in the nuclear pore complex and plays a role in regulating viral infections through its interactions with various viruses.2 In 2009, Neilson et al identified an association between familial cases of recurrent ANE and missense mutations in the RANBP2 gene, with ANE linked to RanBP2 mutations classified as ANE1.3 Mutations in the CPTII gene have also been implicated in ANE;4 Shinohara et al reported CPTII mutations in pediatric cases, while Kobayashi et al documented these mutations in adult ANE patients, noting the absence of RANBP2 mutations in these cases.5,6 Additionally, Saitoh et al reported a variant in the SCN1A gene that may contribute to the etiology of ANE.7 The RANBP2 gene mutation has clear pathogenicity in familial ANE, but its role in sporadic ANE is not yet clear.
For the study of the relationship between genetic factors and ANE, although gene mutations such as RanBP2, CPTII, SCN1A have been found to be associated with ANE, current research is mostly case reports or small sample studies, with limited sample sizes, making it difficult to determine the prevalence and pathogenicity of these mutations in different populations. For the study of the relationship between cytokine storm and ANE, although the levels of cytokines such as IL-6 and TNF - α in patients’ serum and cerebrospinal fluid are elevated, there is still controversy over whether cytokine storm is the direct cause of ANE, and some studies have failed to clarify the causal relationship between cytokines and disease occurrence and development. In depth research on the interaction between genetic background and environmental factors may provide new ideas for prevention and treatment.
Pathogenesis
ANE pathogenesis is associated with cytokine storms and hyperactive immune responses. Cytokine storms are marked by an excessive release of cytokines following viral infection, and studies have demonstrated significantly elevated levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) in the serum and cerebrospinal fluid of patients with ANE.8 Additionally, RanBP2 is thought to play a role in modulating innate immune response pathways; excessive immune activation can lead to upregulated gene transcription of IL-6, IL-10, and TNF-α, thereby causing damage to specific regions of the central nervous system.9 RanBP2 is ubiquitously expressed and has diverse cellular functions that may contribute to the development of ANE1. These functions include roles in maintaining protein homeostasis, chemokine signaling, intracellular metabolism, mitochondrial distribution, nucleocytoplasmic transport, and cellular metabolic regulation, as well as in cytokine storm responses and abnormal mitochondrial localization.10 Although elevated cytokine levels are closely related to the clinical manifestations of ANE, there is still controversy over whether it is a direct cause.
Risk Factors
Key factors in assessing mortality risk for ANE include sex, Glasgow Coma Scale (GCS) score, Degree of brainstem involvement, Multiple organ dysfunction, IL-6 concentration, and brainstem involvement.11 Although gender itself is not an independent risk factor, some studies have shown that the prognosis of male children may be worse.A GCS score below 5 upon admission is significantly associated with high mortality and severe neurological sequelae. Imaging shows that patients with brainstem involvement have a worse prognosis and higher mortality rate.Patients with combined shock, cerebral herniation, or multiple organ dysfunction syndrome (MODS) have a poor prognosis.Genetic studies indicate that polymorphisms in IL6 (rs1800796) and IL10 (rs1800781/rs1800782) may play a role in ANE pathogenesis. Functional analyses reveal that the IL-10 rs1800781/rs1800782 polymorphisms influence IL-10 expression, whereas the IL6 rs1800796 variant does not appear to impact IL-6 expression. Clinically, IL-6 levels exceeding 6,000 pg/mL are linked to brainstem dysfunction, and no survival has been reported at levels above 15,000 pg/mL.12 The IL10 rs1800781/rs1800782 polymorphisms are thus considered genetic risk factors for ANE, while the pathogenic relevance of IL6 rs1800796 polymorphisms requires further investigation.9 Additionally, ferritin levels may serve as a prognostic marker for ANE, as hyperferritinemia is associated with increased risk of adverse neurological outcomes, with an approximately eight-fold heightened risk of poor prognosis at ferritin concentrations exceeding 1,823 ng/mL.13 Although certain genetic polymorphisms are associated with the onset of ANE, their specific applications in clinical settings, such as risk prediction and therapeutic targets, still require further research.
Pathology
Pathological findings in patients with ANE typically include vasogenic and cytotoxic edema, petechial or macro-hemorrhage, cystic cavitation, and tissue necrosis.14 Vasogenic edema is caused by increased vascular permeability. The immune system’s overreaction triggers a cytokine storm, leading to the breakdown of the blood-brain barrier and the formation of brain tissue edema. Edema usually occurs in the peripheral area of the lesion.Cytotoxic edema is caused by an increase in intracellular water content. Cytokine storms directly damage neurons and glial cells. This type of damage leads to the accumulation of intracellular fluid, typically in the central area of the lesion.These hemorrhages usually occur at the center of the lesion and are related to inflammation and cell necrosis around the blood vessels. Stasis bleeding is one of the typical pathological manifestations of ANE.As the lesion progresses, damaged brain tissue may undergo liquefaction and cystic cavitation. This change usually occurs during the subacute or chronic phase of the disease, manifested as the formation of a cystic cavity in brain tissue.Neurons and glial cells may undergo necrosis.
Manifestations
The clinical presentation of ANE is nonspecific, with primary features including high fever, altered consciousness, and seizures. Other manifestations vary widely, encompassing symptoms such as headache, hypotonia, and brainstem dysfunction. The disease progression in ANE generally follows three stages: prodromal infection, acute encephalopathy, and recovery. The prodromal phase often includes fever and respiratory or gastrointestinal symptoms such as cough, vomiting, and diarrhea, which rapidly progress to the encephalopathy stage marked by neurological decline, typically presenting as altered consciousness, coma, seizures, and dystonia. Patients often experience rapid deterioration; however, some survivors demonstrate partial neurological recovery after the acute phase.
A retrospective cross-sectional study identified neurogenic shock as significantly associated with worse clinical outcomes.15 Children with ANE frequently have abnormalities in liver, kidney, and cardiac function, indicative of multiorgan involvement or failure.16 The short-term mortality rate for acute necrotizing encephalopathy of childhood (ANEC) is estimated at 30–50%, with survivors often experiencing severe neurological sequelae, such as cognitive impairment, motor disorders, and white matter atrophy, necessitating long-term rehabilitation.
Auxiliary Examinations
Magnetic Resonance
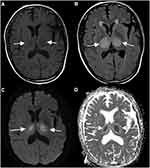
Radiographic findings characteristic of ANE are key to its diagnosis, with hallmark features including bilateral symmetrical thalamic lesions observed in all cases, along with lesions in periventricular white matter, internal capsule, putamen, brainstem, and cerebellum.17 In some cases, additional involvement of the hippocampus, amygdala, medial temporal lobe, and spinal cord may be seen.18 Early in the disease, brain MRI often reveals long T1 (Figure 1A) and T2 (Figure 1B) signals, hyperintensity on diffusion-weighted imaging (DWI) (Figure 1C), and hypointensity on apparent diffusion coefficient (ADC) (Figure 1D) imaging, indicative of cytotoxic edema. As ANE progresses, lesions may develop a “concentric” or “lamellar” pattern due to punctate hemorrhages and nerve cell necrosis. This pattern appears as a central hypointense core on DWI surrounded by hyperintensity, which represents cytotoxic edema, while the outermost layer presents hypointensity due to vasogenic edema, forming a “trichromatic” or “target” pattern on ADC imaging.19 In milder cases without central hemorrhage or necrosis, the ADC may display a “bichromatic” pattern, with central hypointensity (cytotoxic edema) and peripheral hyperintensity (vasogenic edema). Although the three-layer appearance on ADC imaging is often described, it does not manifest in all patients.The use of advanced imaging techniques, such as functional magnetic resonance imaging, to study the dynamic changes of brain injury may provide more accurate basis for prognosis evaluation.
Diagnostic tools to assess ANE severity on imaging have been proposed. Wong et al developed an MRI scoring system based on the presence of hemorrhage, cyst formation, brainstem injury, and damage to cerebral or cerebellar white matter, assigning scores from 0 to 4. Higher scores were correlated with worse prognoses, particularly when brainstem and white matter were affected.20 Additionally, a lower Glasgow Coma Scale (GCS) score has been associated with poorer outcomes, potentially linked to the extent of brain involvement.21 The scoring system established by Khandwala et al is a more comprehensive method, and awards points based on the severity and location of imaging markers, including hemorrhage, cavitation, enhancement, and diffusion restriction, with scores for specific brain regions such as basal ganglia, brainstem, white matter, and cerebellum.15 Total scores range from 0 to 10 and are categorized as mild (0–3), moderate (4–7), and severe (8–10).Existing prognostic scoring systems (e g, ANE-SS and MRI scores) have yielded inconsistent results across studies and lack prospective validation.Developing more accurate prognostic assessment tools may help clinicians to better develop individualized treatment options.
In a retrospective study of 13 adults with ANE, Lin et al observed that the clinical presentation, imaging characteristics, and laboratory findings in adults largely parallel those seen in pediatric cases, underscoring the consistency of ANE features across age groups.22
Electroencephalography
Continuous electroencephalography monitoring, including evaluation of sleep spindles, may help in the intensive care management of ANE The presence of normal sleep spindles has been linked to improved functional outcomes in children with ANE.23
Cerebrospinal Fluid
In most cases of ANEC, cerebrospinal fluid (CSF) analysis reveals elevated protein levels, a positive Pandy test, normal white blood cell counts, normal chloride levels, and no reduction in glucose levels.16 Finding specific biomarkers, such as cytokines or gene expression profiles in cerebrospinal fluid, may help with early diagnosis and personalized treatment.
Differentiation
Due to the absence of distinct symptoms specific to ANE, it is essential to differentiate it from conditions such as Reye syndrome, Wernicke encephalopathy, and Leigh syndrome. Other acute encephalopathy syndromes and ADEM, which also involve thalamic damage, should not be completely ruled out. Key differential diagnoses to consider include ADEM, Reye syndrome, and infectious encephalitis.24
Diagnosis
The ANE-SS score developed by Yamamoto et al evaluates prognosis in ANE patients and ranges from 0 to 9 points. In the first patient group, brainstem lesions visible on MRI and shock at disease onset revealed a significant correlation with patient outcomes, while factors such as age > 48 months, elevated cerebrospinal fluid protein levels, and reduced platelet counts were also associated with prognosis. However, no specific treatment type was found to correlate with outcomes. The scoring system allocates 3 points for shock, 2 points for brainstem lesions, 2 points for age > 48 months, 1 point for a platelet count below 100,000/μL, and 1 point for cerebrospinal fluid protein levels exceeding 60 mg/dL. Patients are categorized into low-risk (ANE-SS 0–1), intermediate-risk (ANE-SS 2–4), and high-risk (ANE-SS 5–9) groups.25 This scoring system enhances the diagnostic criteria initially proposed by Mizuguchi in 1997.26 Although the ANE-SS scoring system has shown some effectiveness in prognostic assessment, its application still has some limitations: the ANE-SS scoring system is mainly based on retrospective analysis of early cases and may not be fully applicable to all patient populations. For example, some studies have found that the incidence of shock and DIC is lower in patients who receive early immunotherapy, which may affect the accuracy of the ANE-SS score. The imaging findings may vary depending on the speed of lesion progression and the location of involvement. For example, the imaging manifestations of SARS-CoV-2-related ANE may be more complex and involve a wider range.
To overcome the limitations of existing diagnostic tools, future research should seek specific biomarkers that can identify ANE early, such as cytokines, genetic markers, etc., to improve the accuracy and timeliness of diagnosis. Combining functional imaging techniques such as apparent diffusion coefficient maps and magnetic resonance spectroscopy imaging to further optimize imaging evaluation and improve predictive ability for lesion progression and prognosis. Validate and optimize the ANE-SS scoring system through multicenter, prospective studies to ensure its universality and accuracy across different populations.
Treatment
Currently, no evidence-based guidelines, approved medications, or randomized trials exist for ANE treatment. The primary therapeutic approaches include immunotherapy, anti-IL6 therapy, hypothermia therapy, CRRT, and supportive care.27 Immunotherapy primarily consists of glucocorticoids, gamma globulin, and plasma exchange. In severe ANE cases, combining tocilizumab with methylprednisolone (MTP) and intravenous immunoglobulin (IVIG) has revealed a potential trend toward improved survival rates.28 This effect may stem from the ability of the immunoglobulins to modulate cytokine production and cellular immune function, thus stabilizing immune responses, reducing cytokine-induced neuronal damage, and providing neuroprotection.
High-dose steroids and IVIG are recommended initially, with plasmapheresis as a consideration if there is no clinical improvement. Influenza vaccination and vigilant monitoring during flu season are advised to lower the risk of recurrence. Although oseltamivir, IVIG, and plasma exchange appear ineffective in patients with ANE /type 1, favorable outcomes have been observed when pulsed steroids, IVIG, and early plasmapheresis are administered. Given that genetic mutations may contribute to immune dysregulation, plasmapheresis could be beneficial.10 Additional treatments under consideration include hypothermia and anti-cytokine therapies, with cytokine receptor monoclonal antibodies, such as anti-IL6, revealing promise as potential therapeutic targets.IL-6 inhibitors have shown potential in some studies, but still require large-scale clinical trials for validation. Although high-dose methylprednisolone and immunoglobulin are considered protective factors, their optimal dosage and course of treatment still need to be validated through large-scale randomized controlled trials.
Most of the current studies were single-center or retrospective studies, with a small sample size and a short follow-up time. Moreover, the management strategies for recurrent ANE and familial ANE remain unclear.
Conclusion
Rapid neuroimaging is essential in previously healthy individuals who present with seizures, encephalopathy, or sudden loss of consciousness following fever and upper respiratory tract infection. ANE should be considered when brain MRI reveals bilateral thalamic concentric lesions or diffuse lesions in specific areas, provided other conditions are ruled out. Early diagnosis and treatment can significantly improve prognosis. High fever or ultrahyperpyrexia following infection, combined with acute neurological symptoms, are early indicators of ANE, while the presence of neurogenic shock, coagulopathy, and significantly elevated cytokines (IL-6, IL-8, IL-10) signals a high mortality risk. Close monitoring of brain, circulatory, and coagulation functions, as well as inflammatory cytokine levels, alongside early intervention, may contribute to improved outcomes.
Although some studies have revealed the partial pathogenesis and treatment strategies of ANE, current research still has the following limitations: the current understanding of ANE mostly focuses on virus correlation, cytokine storm, and genetic susceptibility, but these studies lack original insights or novel perspectives. The existing diagnostic criteria are mainly based on clinical features and imaging findings, but lack specific biomarkers. The treatment strategies are mostly empirical treatments, lacking precise treatments targeting the pathogenesis. The existing prognostic scoring systems have inconsistent results in different studies and lack prospective validation. Given the limitations mentioned above, the purpose of writing this article is to fill the knowledge gap in the etiology, pathogenesis, diagnosis, and treatment of ANE, and to provide more accurate diagnosis and treatment guidance for clinical doctors.
Abbreviations
Acute necrotizing encephalopathy (ANE);interleukin-6 (IL-6);continuous renal replacement therapy (CRRT);intravenous immunoglobulins (IVIG);tumor necrosis factor-α,(TNF-α).
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to acknowledge the hard and dedicated work of all the staff who implemented the intervention and evaluation components of the study.
Disclosure
The authors declare that they have no competing interests.
References
1. Mizuguchi M, Abe J, Mikkaichi K, et al. Acute necrotising encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry. 1995;58(5):555–561. doi:10.1136/jnnp.58.5.555
2. Jiang J, Wang YE, Palazzo AF, Shen Q. Roles of nucleoporin RanBP2/Nup358 in acute necrotizing encephalopathy type 1 (ANE1) and viral infection. Int J mol Sci. 2022;23(7):3548. doi:10.3390/ijms23073548
3. Neilson DE, Adams MD, Orr CM, et al. Infection-triggered familial or recurrent cases of acute necrotizing encephalopathy caused by mutations in a component of the nuclear pore, RANBP2. Am J Hum Genet. 2009;84(1):44–51. doi:10.1016/j.ajhg.2008.12.009
4. Li KC, Hao CJ, Qian SY, Wang TY. Progress in the genetics of acute necrotizing encephalopathy in children. Zhonghua Er Ke Za Zhi. 2020;58(4):336–338. doi:10.3760/cma.j.cn112140-20190812-00507
5. Shinohara M, Saitoh M, Takanashi J, et al. Carnitine palmitoyl transferase II polymorphism is associated with multiple syndromes of acute encephalopathy with various infectious diseases. Brain Dev. 2011;33(6):512–517. doi:10.1016/j.braindev.2010.09.002
6. Kobayashi Y, Kanazawa H, Hoshino A, et al. Acute necrotizing encephalopathy and a carnitine palmitoyltransferase 2 variant in an adult. J Clin Neurosci. 2019;61:264–266. doi:10.1016/j.jocn.2018.11.045
7. Saitoh M, Shinohara M, Hoshino H, et al. Mutations of the SCN1A gene in acute encephalopathy. Epilepsia. 2012;53(3):558–564. doi:10.1111/j.1528-1167.2011.03402.x
8. Diagnosis and treatment of acute necrotic encephalopathy in children (2023 version). Quanke Yixue Yu Jiaoyu. 2023;21(10):868–871.
9. Hoshino A, Takahashi N, Oka A, Mizuguchi M. Association of IL6 and IL10 gene promotor polymorphisms with susceptibility to acute necrotizing encephalopathy. Front Neurosci. 2023;17:1231957. doi:10.3389/fnins.2023.1231957
10. Levine JM, Ahsan N, Ho E, Santoro JD. Genetic acute necrotizing encephalopathy associated with RANBP2: clinical and therapeutic implications in pediatrics. Mult Scler Relat Disord. 2020;43:102194. doi:10.1016/j.msard.2020.102194
11. Fang Y, Gao Q, Jin W, et al. Clinical characteristics and prognostic analysis of acute necrotizing encephalopathy of childhood: a retrospective study at a single center in China over 3 years. Front Neurol. 2023;14:1308044. doi:10.3389/fneur.2023.1308044
12. Shukla P, Mandalla A, Elrick MJ, Venkatesan A. Clinical manifestations and pathogenesis of acute necrotizing encephalopathy: the interface between systemic infection and neurologic injury. Front Neurol. 2021;12:628811. doi:10.3389/fneur.2021.628811
13. Lee EP, Lin JJ, Chang HP, et al. Ferritin as an effective predictor of neurological outcomes in children with acute necrotizing encephalopathy. Pediatr Neurol. 2024;152:162–168. doi:10.1016/j.pediatrneurol.2023.12.029
14. Biswas A, Varman M, Gunturi A, Yoganathan S, Gibikote S. Teaching neuroimages: acute necrotizing encephalopathy of childhood: neuroimaging findings. Neurology. 2018;90(2):e177–e178. doi:10.1212/WNL.0000000000004800
15. Khandwala K, Hilal K, Jafri SK, et al. Clinical prognostication in acute necrotizing encephalopathy of childhood: the role of magnetic resonance imaging severity assessment. Pediatr Radiol. 2024;54:2026–2035. doi:10.1007/s00247-024-06058-5
16. Lin X, Wang Y, Li X, et al. Acute necrotizing encephalopathy in children with COVID-19: a retrospective study of 12 cases. Front Neurol. 2023;14:1184864. doi:10.3389/fneur.2023.1184864
17. Marco EJ, Anderson JE, Neilson DE, Strober JB. Acute necrotizing encephalopathy in 3 brothers. Pediatrics. 2010;125(3):e693–8. doi:10.1542/peds.2009-1984
18. Neilson DE. The interplay of infection and genetics in acute necrotizing encephalopathy. Curr Opin Pediatr. 2010;22(6):751–757. doi:10.1097/MOP.0b013e3283402bfe
19. Mizuguchi M, Ichiyama T, Imataka G, et al. Guidelines for the diagnosis and treatment of acute encephalopathy in childhood. Brain Dev. 2021;43(1):2–31. doi:10.1016/j.braindev.2020.08.001
20. Wong AM, Simon EM, Zimmerman RA, Wang HS, Toh CH, Ng SH. Acute necrotizing encephalopathy of childhood: correlation of MR findings and clinical outcome. AJNR Am J Neuroradiol. 2006;27(9):1919–1923. PMID: 17032866; PMCID: PMC7977901.
21. Chow CK, Ma C. Presentation and outcome of acute necrotizing encephalopathy of childhood: a 10-year single-center retrospective study from Hong Kong. J Child Neurol. 2020;35(10):674–680. doi:10.1177/0883073820927915
22. Lin YY, Lee KY, Ro LS, Lo YS, Huang CC, Chang KH. Clinical and cytokine profile of adult acute necrotizing encephalopathy. Biomed J. 2019;42(3):178–186. doi:10.1016/j.bj.2019.01.008
23. Appavu B, Foldes S, Fox J, et al. Treatment timing, EEG, neuroimaging, and outcomes after acute necrotizing encephalopathy in children. J Child Neurol. 2021;36(7):517–524. doi:10.1177/0883073820984063
24. Chen H, Lan SC, Tseng YL, Chang YY, Lu YT, Lan MY. Acute necrotizing encephalopathy in adult patients with influenza: a case report and review of the literature. BMC Infect Dis. 2024;24(1):931. doi:10.1186/s12879-024-09844-6
25. Yamamoto H, Okumura A, Natsume J, Kojima S, Mizuguchi M. A severity score for acute necrotizing encephalopathy. Brain Dev. 2015;37(3):322–327. doi:10.1016/j.braindev.2014.05.007
26. Mizuguchi M. Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997;19(2):81–92. doi:10.1016/s0387-7604(96)00063-0
27. Qin N, Wang J, Peng X, Wang L. Pathogenesis and management of acute necrotizing encephalopathy. Expert Rev Neurother. 2023;23(7):641–650. doi:10.1080/14737175.2023.2224503
28. Lee V, Khoo TB, Teh CM, et al. Factors associated with outcomes of severe acute necrotizing encephalopathy: a multicentre experience in Malaysia. Dev Med Child Neurol. 2023;65(9):1256–1263. doi:10.1111/dmcn.15536
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.