Back to Journals » International Journal of Nanomedicine » Volume 20
Advancing Cancer Treatment: Innovative Materials in PDT and Diagnostic Integration
Authors Wang Y, Hu C, Li Y, Chen ZS, Zhang L
Received 31 December 2024
Accepted for publication 8 April 2025
Published 31 May 2025 Volume 2025:20 Pages 7037—7060
DOI https://doi.org/10.2147/IJN.S514937
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Yanan Wang,1– 3,* Chaohua Hu,4,* Yangjia Li,4 Zhe-Sheng Chen,5 Lei Zhang2,3,6
1College of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou, 350002, People’s Republic of China; 2State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, 350108, People’s Republic of China; 3Fujian College, University of Chinese Academy of Sciences, Fuzhou, 350108, People’s Republic of China; 4National Engineering Research Center for Sugarcane, Fujian Agriculture and Forestry University, Fuzhou, 350002, People’s Republic of China; 5Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences, St. John’s University, Queens, NY, 11439, USA; 6University of Chinese Academy of Sciences, Beijing, 100049, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Zhang, State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, 350108, People’s Republic of China, Email [email protected]
Abstract: The diagnosis and treatment of cancers have become a significant challenge in overcoming malignant diseases. Early detection of tumors and timely targeted therapy can greatly impede the rapid deterioration of cancers. In recent years, nano-systems based on photodynamic materials have shown great progress in tumor diagnosis and treatment applications. With the continuous exploration of tumor-specific targets and the development of photodynamic nanoparticles, the generation of new nanoparticles that are target-specific, highly sensitive, and biosafe for integrated diagnosis and therapy is realistic. This review introduces the rational basis for photosensitizer-based materials for integrating cancer diagnosis and anti-cancer therapy, types and characteristics of organic and inorganic photosensitizers currently used for PDT treatment, photosensitive nano-materials with dual detection and therapeutic properties the advancement in developing photo-dynamic nano-systems showing potential in integrated diagnosis and therapeutic applications. We also introduce current strategies for optimizing nano-systems with the properties for enhancing targeting ROS release and accurate imaging, combining therapeutic efficacy, as well as biosafety of the integrative materials for PDT application, providing references for the coordinated optimization of photosensitizer design and clinical translation.
Plain Language Summary: The integration of PDT with advanced diagnostic modalities represents a transformative approach in cancer theranostics, as evidenced by the comprehensive exploration of photosensitizer-based nanomaterials in this review. By leveraging the unique photophysical properties of organic and inorganic photosensitizers, researchers have developed innovative nano-systems capable of simultaneous tumor detection and targeted therapy. Key advancements include the rational design of aggregation-induced emission photosensitizers (AIE-PS) with redshifted absorption spectra, hypoxia-responsive nanomaterials for enhanced ROS generation, and multifunctional composites such as UCNP@MOF hybrids that address tumor microenvironment limitations. These systems demonstrate improved targeting precision, reduced off-target effects, and synergistic therapeutic outcomes when combined with chemotherapy or immunotherapy. Despite these strides, challenges persist in optimizing light penetration depth, mitigating photobleaching, and ensuring biosafety during clinical translation. The development of oxygen-independent Type III photosensitizers and stimuli-responsive delivery systems offers promising avenues to overcome hypoxia-related barriers. Furthermore, the integration of MOFs and UCNPs highlights the potential for real-time imaging-guided therapy. Future efforts should prioritize scalable synthesis, rigorous toxicological profiling, and combinatorial strategies to enhance therapeutic efficacy while minimizing systemic toxicity. By bridging nanotechnology, materials science, and clinical oncology, next-generation photodynamic platforms hold immense potential to redefine precision medicine in oncology and beyond. While PDT nanomaterials offer revolutionary potential in cancer theranostics, addressing toxicity, stability, and clinical scalability is critical. Integrating PDT with complementary modalities (chemotherapy, immunotherapy) and advancing TME-responsive designs will bridge the gap between preclinical innovation and clinical application.
Keywords: precision anti-cancer therapy, photodynamic therapy, integrated diagnosis and treatment, novel nano-systems, tumor targeting, advanced imaging technology
Introduction
The activity of tumor cells to evolve and spread rapidly frequently causes drug-resistance, recurrence, and metastasis of cancers, which are characterized by low survival rates. The integration of precise diagnosis, particularly for early-stage cancers, and treatment is essential for impeding cancer progression in patients.1,2 Accurate detection of cancer plays a crucial role not only in the early diagnosis and treatment of tumors but also in real-time imaging guidance during surgical resection and monitoring for metastasis and prognosis post-treatment.3,4 In addition to the side effects that can lead to wound infections and accelerate tumor metastasis, the residual or tumor lesions also frequently lead to cancer recurrence and metastasis.
Chemotherapy frequently encounters problems such as multidrug resistance (MDR).5 In recent years, various functional nanomaterials, such as photodynamic, photothermal, enzyme-catalyzed, and chemokinetic nanomaterials, have gained widespread attention in cancer treatment due to their controllable and high efficient tumor-eliminating capabilities, which also reduce the risk of inducing drug resistance. Meanwhile, the emergence of photo-sensitive and precise diagnostic technologies provided bases for the development of biocompatible systems with integrated cancer diagnosis and anti-cancer therapeutic application.
Photosensitive Molecules and Materials with Potential for Exploration of Integrated PDT and Cancer Diagnosis
Rational Basis for Photosensitizer-Based Materials for Integrated Cancer Diagnosis and Anti-Cancer Therapy
Compared to surgery, radiotherapy and chemotherapy, photodynamic therapy (PDT) exhibits excellent tumor cell elimination performance and is controllable during implementation; It also plays a role in enhancing the body’s immune response.6 PDT utilizes photosensitizers that, once introduced into the body and activated by specific light irradiation upon the tumor, generate reactive oxygen species (ROS) that can kill tumor cells. This treatment also minimizes damage to normal tissues and alleviates patient suffering, holding potential for achieving diagnosis and supportive treatment. To enhance the therapeutic efficacy of photosensitizers while integrating diagnostic functions for prompt patient treatment, an increasing number of nanosystems with favorable application effects have been developed and improved through modifications. To systematically evaluate the merits and challenges of PDT, we conducted a comparative analysis between PDT and conventional therapeutic modalities, with findings summarized in Table 1.
 |
Table 1 Benefits and Challenges of PDT, as Compared with Standard Treatments |
The range of light used in PDT generally spans from blue light to near-infrared (approximately 1350 nm), depending on the photo-characteristics of photo-sensitive reagents or materials (photosensitizers). This makes it possible for the selection and optimization of the nanosystems in different treatment applications. For example, for tumors located deeper, excitation light corresponds to the first near-infrared (NIR-I) biological window (750–1000 nm) has better penetration than visible light and is more ideal, because living tissues exhibit weak absorption of laser light, resulting in lower energy loss as the excitation light passes through normal tissues.7 To enhance the response of certain photosensitizers in vivo, red-shifting their absorption peaks through modifications are commonly applied. For example, our research group applied molecular engineering to obtain aggregation-induced emission photosensitizers (AIE-PS) with a red shift in their absorption spectrum, which largely improved the singlet oxygen quantum yield and anti-tumor efficiency.8 Combined use with upconversional materials can facilitate efficient use of energy through energy transfer, and generate a set of multiple phototherapy or imaging methods.9,10 During these modifications or combined application of two or more materials, reducing photon scattering and background fluorescence can enhance the signal-to-noise ratio, facilitating biomolecular imaging and accurate diagnosis of cancer cells.11
During photosensitive therapy, the incident photons exciting upon the chromophores undergo scattering, transmission, or absorption.12,13 The absorbed photons transfer part of their energy to the photosensitizers (PS) responsible for generating cytotoxic ROS. In the photosensitizer, a paired electron typically resides in a relatively stable and favorable molecular orbital known as the ground state (S0) (Figure 1). The excited-state electrons, however, are unstable and quickly, within approximately 10−6 seconds, undergo rapid radiative and non-radiative internal conversion processes returning to the lowest vibrational energy level.14–16 For photosensitizers, energy returns to the ground state through fluorescence, phosphorescence, or collision with the environment, generating ROS capable of damaging bio-molecules and realizing PDT against cancer cells. Modifications of photosensitizers can improve anti-cancer efficacy. For example, for materials whose properties change under aggregation, modifying the molecular structure to change intramolecular interactions holds potential for optimizing the photodynamic properties of the materials.17 These factors provide possibilities for enhancing the target performance of the materials.
 |
Figure 1 Schematic representation of the luminescence and photodynamic excitation mechanisms in photosensitizers. |
The designing of photosensitizers commonly follow the rational associated with conversion of light energy to fluorescence, phosphorescence or ROS. The electron in ground state (S0) of the material, upon absorbing light energy of a specific wavelength, causes the electron to undergo an electronic transition to the singlet (S1) excited state.15 The electron rapidly relaxes to the lowest vibrational energy level of the PS excited state. However, the S1 (Figure 1) excited-state electrons is short lived (~ 10−6 s) and tend to return to S0, which can occur through three primary pathways.15,18 The first pathway involves emission of photons in the form of fluorescence to return to S0. The second pathway involves the first transitioning of electron from S1 level 0 to the excited triplet state (T1) through the intersystem crossover (ISC) process during which the spin direction of an electron in the higher energy level of the electron pair changes,14 followed by the release of phosphorescence to return to S0. Fluorescence typically occurs instantaneously during the excited state, whereas phosphorescence is emitted over a period after the excited state ends. The third pathway leading to energy dissipation and a return to S0 involves external conversion through collisions with other molecules in the environment, which may lead to the quenching of fluorescence and phosphorescence. The external conversion processes are key to enabling photosensitizers to generate ROS for PDT of tumors. Some molecules in the T1 excited state can be represented as 3PS*. Through electron transfer, they can form the radical PS•− with substrates such as cell membranes, proteins, and liposomes. When PS•− reacts with O2, the cytotoxic superoxide anion radical can be generated, and when it reacts with hydrogen peroxide (H2O2), hydroxide ions (OH−) and the similarly cytotoxic hydroxyl radical (•OH) can be generated.19 Due to environmental variations, additional reactions such as SOD disproportionation and Fenton reactions may occur during the generation of reactive oxygen species, during which H2O2 can also be regenerated.20 The excited photosensitizer can transfer energy to O2 through energy transfer, converting ground-state O2 into the highly electrophilic singlet oxygen (1O2), which is a highly cytotoxic ROS.12,21 Excessive ROS can oxidize vital biomolecules like proteins and nucleic acids in cancer cells and in tumor microenvironment, inactivate biomolecules and lead to oxidative stress in the tumor cells and ultimately resulting in apoptosis and necrosis of tumor tissues. It can affect the membrane permeability of mitochondria, which are crucial energy-supplying organelles, leading to a decrease in mitochondrial membrane potential and inducing apoptosis. Furthermore, it damages cells and release tumor associated antigens which can enhance the body’s immune system.
Both fluorescence and phosphorescence can be used for cancer diagnosis, while ROS can be used for eliminating cancer cells. On the basis of the rational for exciting photosensitizers to generate fluorescence, phosphorescence or ROS, photosensitizers can be designed and optimized for integrated cancer diagnosis and anti-cancer therapy.
Present Photosensitizers Used for PDT Treatment
Types of Photosensitizers
At present, major photosensitizers can be divided to two Types. Type I photosensitizers primarily react with biomolecules to generate ROS through processes such as electron transfer and hydrogen abstraction. Upon activation by light, these photosensitizers produce free radicals, including •OH and superoxide anions (O2•⁻).22 These highly reactive species can inflict oxidative damage on cellular components, such as lipids, proteins, and nucleic acids.23 As a result, Type I photosensitizers can induce cytotoxicity and cell death, even under hypoxic conditions, making them versatile agents in photodynamic therapy.19
In contrast, Type II photosensitizers function by transferring energy to molecular oxygen (O2) to generate ¹O2, a highly reactive form of oxygen.24 This mechanism requires the presence of molecular oxygen and occurs when the excited photosensitizer interacts with O2.25 Singlet oxygen can then react with various biomolecules, leading to oxidative damage and cellular apoptosis.22 Type II photosensitizers are particularly effective in photodynamic therapy for cancer treatment, as they specifically target tumor tissues while minimizing damage to surrounding healthy cells in the presence of adequate oxygen levels.
Organic Photosensitizers
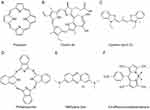
Photosensitizers can be developed based on both organic and inorganic nanomaterials. Common organic photodynamic sensitizers include porphyrins, chlorin e6 (Ce6), Cyanine dyes, and phthalocyanine.26 Porphyrin is a dye molecule characterized by a large conjugated π system and is composed of four pyrrole units linked by methylene bridges (Figure 2A). Due to their unique optoelectronic properties, porphyrins and their derivatives can perform well in applications such as photodynamic therapy agents, biological imaging probes, and near-infrared fluorescent dyes. The inherent affinity of porphyrins for tumor cells allows for selective accumulation in tumor cells.27 Porphyrin derivatives also exhibit good biocompatibility and ease of modification. Derivatives containing heavy atoms and cations demonstrate high yields of reactive oxygen species, making them suitable for PDT treatment on tumors. Zinc protoporphyrin IX (ZP) shows a competitive advantage in binding to Heme oxygenase-1 (HO-1), which is highly expressed in solid tumors and has antioxidant defense functions, thus enhancing the PDT effect.28,29 The first-generation photosensitizer, Hematoporphyrin derivative (HPD), with its main effective component being dihematoporphyrin ether or ester, was the first photodynamic drug approved by the US Food and Drug Administration (FDA) in 1993 for the treatment of bladder cancer, showcasing exciting photodynamic effects while also making researchers aware of its shortcomings including low targeting ability of mixed porphyrin agents, poor water solubility, and insufficient photosensitivity during light treatment. Additionally, porphyrins typically exhibit their maximum absorption peaks between 400 and 600 nm, with inadequate tissue penetration while causing photosensitivity when excited by sunlight.30 The modification with heavy metals can block some pathways of fluorescence dissipation in porphyrins, while the addition of lanthanide ions, Gadolinium (III) (Gd3+), and Lutetium (III) (Lu3+) can enhance the ISC of porphyrins while preventing fluorescence quenching.31 Through strategies for enhancing their performance include amphiphilic modification, physical encapsulation, the construction of supramolecular polymers, and the creation of porphyrin organic frameworks, the application of porphyrins as photosensitizers in tumor therapy could be optimized.32
Subsequent discoveries and in-depth studies have been conducted on dyes such as Ce6, cyanine dyes, and methylene blue, where researchers are increasingly inclined to choose photosensitizers with longer absorption wavelengths and materials capable of selective accumulation. On this basis, strategies such as modification can induce a red shift in the absorption peak of the materials without affecting or even enhancing the ROS generation ability of the photosensitizers.33–35 Ce6, derived from natural chlorophyll,36 is a well-defined chemical compound (Figure 2B) with a significant infrared absorption coefficient and photosensitivity, offering advantages for PDT of tumors due to its favorable singlet oxygen generation efficiency. In a clinical study with a sample size of 52 people, intravenous injections of the Ce6 photosensitizer demonstrated an 80.8% lesion regression rate in the treatment of human papillomavirus (HPV) and cervical intraepithelial neoplasia.37 Additionally, Ce6 exhibits certain photothermal properties, facilitating a combined PDT and PPT approach.38,39 Nevertheless, Ce6 still faces limitations such as low targeting capability, drug resistance, and hydrophobicity, which restrict its clinical efficacy in tumor treatment. Therefore, there is a need to combine it with other materials to synergistically enhance its performance.
Cyanine dyes are compounds consisting of two heterocyclic units connected by a polymethine chain core structure,40 exhibiting favorable biocompatibility and fluorescence properties. For instance, Cyanine dye (C11) may have the potential to cross the blood-brain barrier. (Figure 2C).41 Modified anthocyanin derivatives retain their fluorescent properties while exhibiting potential for cancer treatment, with modifications including halogenation, incorporation of metal atoms, and etc. Among them, indocyanine green (ICG) is the only cyanine dye approved by the FDA.40 ICG demonstrates high selectivity towards liver cancer cells, aiding in the identification of pathological sites during surgery. The Sulfo-Cyanine7 (Cy7) cyanine dye series from modified anthocyanins shows high selectivity for cancer cells and enhanced retention in tumor tissues than in normal tissues when forming covalent complex with albumin, accompanied with sustained fluorescence indicating tumors.42 Its downside includes poor hydrophobicity, necessitating collaborative actions with other materials. In addition to characteristic fluorescence, anthocyanins also possess potential for improved photodynamic performance through ROS generation. Amplifying the fluorescence and shifting the emission towards the red spectrum constitutes an effective strategy to bolster the fluorescence and phototherapeutic efficacy of anthocyanins. For instance, Jin et al introduced naphthalimides into the anthocyanin backbone, resulting in a novel near-infrared dye, ML880, which, when co-loaded with chemotherapeutic agents into mesoporous silica, exhibited significantly enhanced PDT and PPT effects in vivo.33 The introduction of heavy atoms in the fluorophore can improve the spin-orbit coupling (SOC) efficiency, thus enhancing ISC efficiency.43,44
The halogenation modifications of anthocyanin dyes can lead to heavy halogen effects that enhance the generation of free radicals,45,46 thus can be used to improve anthocyanin derivatives. For instance, Cao et al modified Cy7 with the heavy atom iodine to create CyI, which increased ROS production while also generating heat, maintaining its fluorescent properties, and enabling non-invasive imaging during treatment.47 Heavy metal like gold or silver was referenced to modify anthocyanin dyes to achieve both photodynamic and photothermal effects.48,49
Phthalocyanine (Pc) is a macrocyclic compound formed by the condensation of four isoindole molecules bridged by nitrogen atoms.50 Its structure is very similar to that of porphyrins widely existing in nature, possessing an 18-electron large conjugated system and a unique two-dimensional conjugated π-electron structure (Figure 2D). Their extensive conjugated system provides strong light absorption predominantly in the near-infrared Q band range from 670 to 850 nm, which corresponds to relatively decent tissue penetration.51 The diverse molecular structures and ease of modification, combined with strong coordination abilities, allow for the formation of distinct derivatives. The vacant centers of phthalocyanine can coordinate with most metals, and the physicochemical properties of the resulting complexes are significantly influenced by the nature of the coordinating metals. For example, complexes formed by substituting the central atom of phthalocyanine with zinc (II) (Zn2+), aluminum (III) (Al3+), gallium (III) (Ga3+), or silicon can increase the cytotoxicity of phthalocyanine.52 Clinically, phthalocyanine demonstrate superior light and thermal stability and therapeutic effects compared to ICG.53 However, their drawback lies in poor water solubility, which can lead to aggregation in aqueous media.54 The introduction of sulfonic acid or other substituents can improve this to some extent.55
Methylene blue (MB) is a compound used as a dye, showing favorable lipophilicity (Figure 2E).56,57 It exhibits photodynamic activity upon irradiation at wavelengths of 630–680 nm, generating reactive oxygen species and demonstrating in vitro anticancer efficacy.58,59 Taldaev et al revealed that MB exhibits photodynamic cytotoxicity against colorectal and breast cancer cells in mouse models.57 However, MB’s low affinity for cancer cells restricts its ability to exert anticancer effects as a photodynamic agent.60 From early local injections to later modifications involving targeted conjugation, delivery vehicles, and combination with other materials, all demonstrate the potential of MB as a photodynamic agent for cancer therapy.61,62
Another photosensitizer, boron-dipyrrin (BODIPY) derives from a parent structure consisting of two pyrrole rings connected by a methylene bridge with a boron-nitrogen six-membered ring in the middle, forming a rigid conjugated planar structure (Figure 2F).34 It has relatively low molecular weight and considerably lower biological toxicity in the form of monomers. Additionally, the absence of ionic charges in its molecular structure allows BODIPY to avoid electrostatic interactions with other ions in solution. This reduction of interference sources enhances the photo-sensitivity of BODIPY as a fluorescent dye. BODIPY emits fluorescence at around 500 nm, which might limit its application in the red to near-infrared light regions. The functional modifications of the BODIPY core can be conducted to balance its various properties during applications. The BODIPY derivatives may possess high fluorescence quantum yields, good photothermal stability, ease of structural modification, and minimal influence from environmental pH variations, ensuring stable spectral analysis signals. Therefore, they are widely used for labeling proteins and DNA, with applications in biological detection and photodynamic therapy. The high molar extinction coefficients also endow them with certain photosensitizing properties. BODIPY fluorophores can also aggregate due to strong intermolecular π-π interactions, leading to aggregation-caused quenching (ACQ) effects, which not only result in fluorescence quenching and reduced luminescence but also increase the cross-interaction efficiency between material systems.20,43 Kang et al designed materials for reversible activation and deactivation of ISC to allow more flexible applications of these fluorescent materials in integrated tumor diagnostics and therapy.43
Inorganic Materials for Photodynamic Therapy
Inorganic photodynamic photosensitizers such as noble metals, carbon-based nanomaterials, and upconversional nanomaterials, have shown great potential in PDT therapy. Metallic photosensitizers play a crucial role in PDT due to their excellent photodynamic properties, long-lived triplet state fluorescence, and good photostability.63 By rational design and ligand modification, novel metal complexes can be constructed as dual-photon photosensitizers for anticancer research.64 Additionally, metal-doped carbon-based photosensitizers have shown excellent performance in photocatalytic oxidation, with FeCu-doped carbon dots (FeCu-CDs) and Co-N-doped carbon materials exhibiting enhanced electron and energy transfer efficiency by introducing unsaturated metal states, thereby improving photocatalytic oxidation activity.65–67 These metallic photosensitizers have the potential to be used in various applications, including the development of novel metal complex photosensitizers based on Ru(II), Ir(III), and Pt(II) complexes, and the design of metal-doped carbon-based photosensitizers for enhanced photocatalytic oxidation activity.68
Carbon-based nanomaterials (CBNs), such as graphene (GE), graphene oxide (GO), carbon dots (C-Dot, CDs), and carbon nanotubes (CNT), consist of a core made from graphitized carbon and polymer surface groups, with no distinct boundary between the carbon core and the polymer.69 The advantages of CBNs include good water solubility, biocompatibility, tunable photoluminescence, high photothermal/photostability, high photoluminescence quantum yields, and a rich variety of functional groups. These characteristics bestow surface-functionalized CBNs with photodynamic and photothermal properties. CBNs are small in size, easily functionalized, and capable of penetrating natural biological barriers, exhibiting significant distribution advantages in vivo.70 They can also serve as carriers for drug delivery. Apart from being components of phototherapy composite materials, graphene quantum dot (GQDs) are also frequently utilized in cellular imaging, sensing, and drug delivery due to their low biological toxicity and excellent biocompatibility.71,72 GO is commonly an important component in nanocomposites; for instance, prepared nanographene oxides (NGO) and coated AIE nanoparticles with them to improve their ability to produce ROS upon light illumination.73 Additionally, reduced graphene oxide (r-GO), obtained by removing surface functional groups from GO through various reduction methods, is gradually being applied in photosensitive therapeutic materials for tumors, assisting composites in achieving synergistic PDT and PTT or significantly enhancing the effectiveness of one component.74 For example, Campbell et al combined nitrogen-doped GQD, hyaluronic acid (HA) and ferrocene (Fc) composite materials to increase the ROS production to 3 times that of Fc.75 Due to the structural advantages of GO, some synthesized composite materials can also trace live cells while exhibiting phototherapeutic effects.76,77 Fluorescent carbon particles with sizes less than 10 nanometers, including graphene quantum dots, carbon nanodots, and carbonized polymer dots, can be collectively referred to as CDs, which exhibit certain structural transformation relationships and relatively simple synthesis.78 During the preparation of CDs, various functional groups such as -OH, -COOH, -CHO, -NH2, and -SH can be introduced on the surface of CDs depending on the precursors used, and the number of functional groups will also influence their properties.69 In applications of tumor photodynamic therapy, the amount of active oxygen produced by CDs under light as photosensitizers is relatively limited, so they are often used in conjunction with traditional photosensitizers. Furthermore, the water solubility provided by CDs allows for better application of composites in biological contexts.69
Another nanomaterial with unique optical properties, upconversion nanoparticles (UCNPs), can convert two or more low-energy photons into a single high-energy photon, transforming long-wavelength light into short-wavelength light, with broad emission spectral bands that can be adjusted based on material structure or modifications. The short-wavelength light can be used to excite the photosensitizers located in deep tumors. The optical properties of UCNPs are not only unique but also exhibit high fluorescence quantum yields, stability, and dispersion characteristics. Their excellent water dispersibility, biocompatibility, and low toxicity make them promising candidates for in vivo therapeutic applications. Furthermore, their surfaces are rich in common functional groups such as amino, carboxyl, and hydroxyl groups, which provide greater potential for surface modifications. Nanomaterials capable of achieving integrated tumor diagnosis and treatment are highly anticipated, and this design necessitates a high degree of compatibility among material components. Liu et al have designed a nanomotor, UCNPs@mSiO2-Au-Cys, based on the optical properties of UCNPs and nanomechanical technology, which can be used for multimodal imaging of tumors and combined PTT and PDT.79 The ability of UCNPs in this material to emit at 659 nm and 800 nm under 808 nm laser irradiation holds promise for real-time online diagnosis and treatment. Additionally, the self-propulsion generated by UCNPs under this laser irradiation can enhance the permeability of the tumor tissue, while the gold nanoparticles (Au NPs) act as catalysts for oxygen generation alongside facilitating photothermal, photodynamic therapy, and photothermal imaging.79 Following this, Hu et al designed down-/up-conversational nanoparticles (D/UCNPs) (wavelengths at 660, 1060, and 1550 nm) by conjugating dual-ligands (endogenous glutathione-responsive and doxorubicin-loaded)-stabilized gold nanoclusters (cgAuNCs), effectively achieving ratiometric NIR-II fluorescence imaging combined with chemo-photodynamic therapy toward tumors upon exposure to an 808 nm laser (Figure 3).80
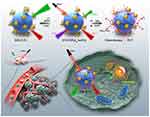 |
Figure 3 Single-excitation three-emission D/UCNPs for tumor microenvironment-responsive fluorescence imaging and chemo/photo-dynamic combination therapy. The NIR-II fluorescence imaging of cgAuNCs was enhanced by endogenous glutathione response, while doxorubicin loaded on the nanoparticles was utilized for chemical therapy, and the photosensitizer methylene blue exerted its photodynamic therapy effects. Source: Reprinted from Hu S, Huang L, Zhou L et al. Single-excitation triple-emission down-/up-conversion nanoassemblies for tumor microenvironment-enhanced ratiometric NIR-II fluorescence imaging and chemo-/photodynamic combination therapy. Anal Chem. 2023;95(7):3830–3839. Copyright 2023, with permission from American Chemical Society.80 |
Despite having a high fluorescence quantum yield, the luminescence intensity and ROS generation capability of UCNPs are deemed insufficient to meet the demands of photodynamic therapy for tumor cells directly, particularly in deeper tissue regions. Thus, one direction to overcome this limitation is to incorporate targeted, environment-responsive switches to enhance their therapeutic efficacy, such as pH-responsive assembled nanoparticles. Another approach involves increasing oxygen supply within the tumor microenvironment, utilizing carriers like metal-organic frameworks (MOFs) and cyanobacteria to assist in oxygen delivery.10,81 This strategy is also an effective approach employed to improve the photodynamic performance of other photosensitizers.
Photosensitive Nano-Materials with Dual Detection and Therapeutic Properties
With the continuous improvement in the targeting precision of nanoparticles, many drug-delivery nanomaterials have also shown promising therapeutic and prognostic effects. By combining nanomaterials with different properties and functions to leverage their complementary advantages, significant improvements can be achieved; for instance, the addition of nanoparticles often enhances the PDT efficacy of poorly soluble photosensitizers. Furthermore, the strong specificity of targeted detection materials previously demonstrated can be coupled with therapeutic agents to optimize their effectiveness in controlling tumor progression early and even achieving tumor elimination while facilitating timely monitoring of treatment outcomes.82,83
Some new nanomaterials have been developed with dual functionalities for detection and therapy, aligning with the principles of integrated diagnosis and treatment. The phthalocyanine compounds, which possess photosensitive properties, can effectively balance fluorescence emission with the generation of singlet oxygen.84 Chow et al developed biotin-coupled trimer analogs of phthalocyanine compounds, which were directed towards HeLa tumor cells. These compounds were cleaved and activated by glutathione (GSH), leading to the formation of a self-quenching complex through disulfide-linked monomers that enter into an activated fluorescent state upon cleavage, generating fluorescence signals while simultaneously producing singlet oxygen to achieve PDT (Figure 4).85 Similarly, the IR-822 dye, used as a fluorophore, exhibits a good molar extinction coefficient and a tendency to preferentially accumulate in tumors.86 When conjugated with the pH-responsive proton acceptor ethane-1,2-diamine, significant fluorescence within the acidic tumor microenvironment can enable both fluorescence and photoacoustic dual imaging.86 Karaca et al synthesized DNA/Au/PEDOT/Pt (poly(3,4-ethylenedioxythiophene)/platinum) micromotor probes labeled with6-FAM (6-carboxyfluorescein) to enhance the targeting of tumor marker miRNA-21, in which, Pt nanoparticles are often designed as photosensitizers. This material effectively recognizes MCF-7 and SJSA-1 cells, revealing changes in fluorescence intensity, while simultaneously upregulating endogenous pro-apoptotic proteins such as Bax, Bim, and Bad, downregulating anti-apoptotic factors Bcl-2 and Bcl-w, and activating transmembrane receptors of the TNF family, which regulate the extrinsic apoptotic pathway, thereby inhibiting breast cancer growth to some extent).87
 |
Figure 4 Cell-selective biotin conjugated glutathione-responsive tris (phthalocyanine) for smart targeted photodynamic therapy, which achieves fluorescence activation and photodynamic treatment through the cleavage by glutathione near tumor cells and generates phthalocyanine monomers. Source: Reprinted from Chow SYS, Zhao S, Lo PC et al. A cell-selective glutathione-responsive tris(phthalocyanine) as a smart photosensitiser for targeted photodynamic therapy. Dalton Trans. 2017;46(34):11,223–11229. Copyright 2017, with permission conveyed through Copyright Clearance Center, Inc.85 |
MOFs are composite materials consisting of metal nodes or clusters linked by coordinate bonds to organic components. They serve as an effective design strategy or model, allowing for the selection of components with specific characteristics based on the requirements of the composite material. The resulting structures can vary widely, ranging from one-dimensional to three-dimensional forms, showcasing remarkable plasticity. The highly flexible structure of MOFs includes their high porosity, which provides sufficient space for the coupling of other component materials as well as for drug loading and delivery. For the three main novel tumor treatment strategies mentioned above, MOFs can enhance tumor targeting capabilities, such as by enabling the attachment of targeting molecules and alleviating hypoxic constraints in PDT. The adaptable structure and ease of modification make MOFs capable of modulating their light absorption characteristics to enhance PTA performance. Similarly, they can alter electron transfer pathways in PDT to increase the yield of ROS. In recent years, MOFs often employed in the synthesis of nanomaterials, have emerged as promising components to help traditional photosensitizers overcome their limitations, for instance, in addressing the oxygen-deprivation challenges that hinder the photodynamic performance of UCNPs in the tumor microenvironment. Zhang et al integrated MOF and UCNPs into an innovative hybrid nanomaterial using a dual-ligand-assisted assembly approach. This heterostructure can exploit the excess H2O2 present in the tumor microenvironment to continuously generate oxygen, thereby facilitating Type II photodynamic action of the UCNPs while promoting effective energy transfer from UCNPs to the MOF, resulting in enhanced near-infrared activated PDT.10 In addition, the UCNP@MnOx-Hyp designed by Zhao et al disintegrates upon stimulation with glutathione, releasing Mn2+ as a coordination center. The photosensitizer Hyp coordinates with Mn2+ and encapsulates the lanthanide UCNPs as energy donors, forming a cross-linked aggregate UCNP@Mn2+-Hyp. During this process, the absorbance of Hyp experiences a redshift, increasing the spectral overlap with the light emitted by the UCNPs, thereby enhancing the energy transfer efficiency from UCNPs to Hyp by 5.6 times. Simultaneously, the cross-linking enhances the luminescent intensity of UCNPs by resisting aqueous quenching, and the increase in material size due to cross-linking also aids in the accumulation of UCNPs and Hyp within cells (Figure 5).88 Chen et al constructed a near-infrared activated material, TPP-UCNPs@MOF-Pt, consisting of UCNP cores and a porphyrin-based MOF shell containing platinum nanoparticles, which effectively alleviates the limitations imposed by tumor hypoxia in photodynamic therapy.89 MOFs can alter electron transfer pathways through structural design and corresponding modifications, thereby increasing the yield of ROS.90 The ligands used for MOFs in photodynamic therapy are often well-established photosensitizers, including derivatives of porphyrin, chlorin, and bacteriochlorin with carboxylic acid functional groups.
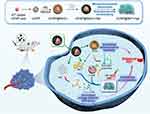 |
Figure 5 An in situ convertible nanoplatform with supramolecular cross-linking-triggered complementary functions—UCNP@MnOx-Hyp to enhance cancer photodynamic therapy. Source: Reprinted from Zhao M, Zhuang H, Li B et al. In situ transformable nanoplatforms with supramolecular cross-linking triggered complementary function for enhanced cancer photodynamic therapy. Adv Mater. 2023;35(20):e2209944. Copyright 2023, with permission from Wiley-Blackwell. © 2023 Wiley-VCH GmbH.88 |
The π-π interactions among rigid monomers within covalent organic frameworks can frequently lead to aggregation and quenching of the photosensitizers, negatively impacting the rate of ROS generation. In contrast, flexible organic frameworks can effectively suppress the aggregation and quenching of porphyrins. For example, the cationic flexible organic framework nanoparticles (PEI-Por NPs) designed by the researchers address this issue well.91 Zeng et al developed a metal-organic framework (TBP-MOF) based on benzoporphyrin, featuring 10 connected Zr6 clusters. The π-extended benzoporphyrin linkers promote the production of 1O2 while exhibiting a red-shifted absorption band, strong near-infrared luminescence for bioimaging, and the ability to stimulate an anti-tumor immune response.92
Currently, there are many composite materials ingeniously combined to leverage the advantages of each component based on the needs of integrated tumor diagnosis and therapy, achieving synergistic effects. Compared to the previously mentioned trimeric phthalocyanine analogs that are activated by the tumor microenvironment for therapeutic applications, our research group developed a universally applicable and efficient strategy for intracellular activation therapy by designing a GSH-activatable multifunctional prodrug. The prodrug B-BDP-CL-CPT consists of a GSH-responsive boron-dipyrromethene (BDP) photosensitizer, the chemotherapeutic agent camptothecin (CPT), and a ROS sensitive linker. After the biotin moiety connecting BDP to CPT is effectively internalized by GSH-positive cancer cells, the overexpressed GSH by the cells cleaves the terminator DNBS (2,4-dinitrobenzenesulfonate), thereby activating BDP to generate ROS. This process not only kills tumor cells but also breaks the thioether bond to release CPT, resulting in a combined photodynamic therapy (PDT) and chemotherapy effect. Additionally, the prodrug emits fluorescence upon GSH activation (Figure 6).93
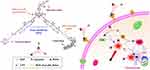 |
Figure 6 B-BDP-CL-CPT used for integrated cancer diagnosis and anti-cancer treatment. B-BDP-CL-CPT is activated in GSH-positive cancer cells, then emits fluorescence, realizing the generation of ROS and release of CPT, a chemotherapeutic drug. Source: Reprinted from Hu P, Xu G, Yang D-C et al. An advanced multifunctional prodrug combining photodynamic therapy with chemotherapy for highly efficient and precise tumor ablation. Dyes Pigm. 2022;205:110500. Copyright 2022, with permission from Elsevier.93 |
Approaches for Optimizing Photosensitizers
Conventional photosensitizers exhibit intrinsic limitations including light exposure constraints, limited diffusion penetration and suboptimal targeting, as well as issues related to ROS generation efficiency and oxygen dependence. These drawbacks necessitate continuous refinement through iterative material design processes. Current nanomaterial development efforts demonstrate multifaceted optimization strategies addressing these limitations. An optimized taxonomical framework for solution strategies was established through systematic classification refinement, representative design examples are categorized and described in the Table 2 below.
 |
Table 2 Challenges Faced by Photosensitizers in Realizing Photodynamic Functions and Effective Solutions |
Improving ROS Yields
The conventional photosensitizers have limitations such as restrictions related to light exposure, poor diffusion penetration and targeting, or issues regarding the efficiency of ROS generation and oxygen dependence. These factors require continual optimization during material design. Even though different materials have preferred ranges of light sources or relatively effective light source parameters, many materials face the issue of photobleaching, where the dye’s polymethine chains are oxidized by singlet oxygen species, leading to decomposition and loss of fluorescence.102 At this stage, photosensitizers exposed to a single or singular light treatment may struggle to achieve ideal therapeutic effects.103 Better light stability enables photosensitizers to continuously generate ROS, addressing the limitations of the limited tissue penetration of NIR light. For instance, Juengpanich et al combined the porphyrin derivative Pu18 with UCNPs doped with lanthanide elements that possess excellent biocompatibility and high light stability, forming a nanomaterial called stimuli-sensitive tumor-targeted photodynamic nanoparticles (STPN). Prior to intravenous injection, STPN was subjected to NIR radiation stimulation, allowing it to accumulate at tumor sites and subsequently enter cells via mediation by HER2 receptors. The continuously luminescent STPN not only generates ROS but can also be used for diagnostics via magnetic resonance imaging (MRI) and intraoperative NIR navigation.9 However, light exposure may lead to reactions such as skin allergies and edema in affected areas.104 Inappropriate dosing and timing of light exposure may also provoke immune.22,105 Hydrophobic photosensitizers may accumulate in blood vessels, potentially inducing thrombosis. Meanwhile, the ROS generated during light exposure could damage the vasculature. Therefore, selecting optimal light exposure conditions for achieving the best therapeutic performance must remain within specific biocompatibility limits, alongside precise control over drug activation timing and tracking to mitigate side effects.21
Many photosensitizers, particularly organic dye-based ones, exhibit inefficient intersystem crossing from S1 to T1, making it challenging to achieve ideal high singlet oxygen generation efficiency independently.94 Thus, enhancing the ROS generation efficiency is a direct method to improve PDT performance of the photosensitizers. Jiang et al designed dual-emissive semiconducting polymer nanoparticles (SPNs) with near-infrared phosphorescent iridium (III) as the donor of BODIPY fluorescent derivatives, achieving an excellent singlet oxygen yield of 0.97. The phosphorescence quenching phenomenon of iridium(III) complexes can be used to assist in the early diagnosis of cancer (Figure 7).94 Additionally, Wan et al optimized the intramolecular charge transfer strategy of electron-rich anion-π+ AIE-active luminogens (AIEgens) to enhance the generation efficiency of ROS by partially suppressing non-radiative internal conversion while promoting radiative transitions and intersystem crossings.106
 |
Figure 7 Dual-emissive semiconducting polymer nanoparticles (SPNs) generate phosphorescent quenching signals and ROS through FRET effect after exposure to light in vivo. Source: Reprinted from Jiang J, Qian Y, Xu Z et al. Enhancing singlet oxygen generation in semiconducting polymer nanoparticles through fluorescence resonance energy transfer for tumor treatment. Chem Sci. 2019;10(19):5085–5094. Copyright 2019, with permission from Royal Society of Chemistry.94 |
In addition to the above factors, the size of the materials can significantly impact ROS yield and localization, making size an important consideration in the entire process of nanoparticle design and application the body.
Activating PDT Materials Using Hypoxic Nature of the Tumor Environment
Alternatively, the hypoxic nature of the tumor environment can be exploited as a signal to activate the photodynamic effects of materials. For instance, Yang et al designed a hypoxia-responsive human serum albumin (HSA) nanosystem (HCHOA) that cross-links hypoxia-sensitive azo-benzene between the photosensitizer Ce6 conjugated HSA (HC) and oxaliplatin to create a prodrug HSA (HO).107 Upon exposure to the hypoxic tumor microenvironment, it rapidly decomposes into ultra-small HC and HO therapeutic nanoparticles with diameter less than 10 nm, which significantly enhanced their infiltration ability within tumors. Furthermore, the quenching fluorescence of Ce6 in the resultant HC nanoparticles can provide bioimaging signals.107
Developing Photosensitizers Independent of Oxygen Molecules
In 2010, Allison et al proposed a Type III mechanism based on the aforementioned Type I and II mechanisms, which occurs independently of oxygen concentration.96 In addition to being unaffected by this critical factor, the photosensitizers exhibiting this mechanism also possess highly desirable targeting capabilities, specifically toward biomolecules such as proteins within tumor cells.96 Following this line of thought, Yao et al developed a family of novel photosensitizers NBEX (X = S, Se, Te), which employs the Type III mechanism with specific targeting functionalities. Upon exposure to light energy, these sensitizers can directly target RNA within tumor cells, effectively killing cancer cells and inhibiting their metastasis while also inducing immune responses.108
Some therapeutic agents based on BODIPY have the advantage of generating photodynamic and photothermal effects without oxygen, enhancing their tumor treatment efficacy.109 The excellent therapeutic outcomes of these photosensitizers also drive the continuous optimization of materials, with accumulated experience in material modification and synthesis providing greater potential and controllability for the performance of nanomaterials, including their integration into diagnosis and therapy.
Targeted Delivery Systems for Enhancing PDT
Targeted delivery systems demonstrate significant potential in improving PDT by precisely delivering photosensitizers to diseased areas, thereby greatly enhancing therapeutic efficacy and reducing side effects. Traditional PDT often suffers from the non-specific distribution of photosensitizers throughout the body, leading to phototoxicity in normal tissues and insufficient light penetration depth. Targeted delivery systems, such as antibody conjugates, ligand-modified nanoparticles, or exosomes, achieve selective accumulation of photosensitizers at the lesion sites by recognizing tumor-specific biomarkers (such as EGFR and folate receptors) or responding to tumor microenvironments (such as low pH and high enzymatic activity).9,110,111 Furthermore, nanoparticle carriers can encapsulate photosensitizers and integrate imaging probes, enabling a therapeutic-diagnostic synergy. Some systems can also co-deliver therapeutic agents (such as anti-angiogenic drugs or immune modulators) to overcome tumor resistance through multi-modal treatment approaches.112 This strategy not only enhances the spatiotemporal control of PDT but also provides new avenues for treating deep-seated tumors and metastatic lesions.
Optimizing Targeting Activity of ROS
Target strategies highlight an promising pathway for developing photosensitizers (PSs) with selectivity and deep penetration. Many organic small molecule dyes, which have been studied more recently, also exhibit certain levels of tumor targeting; however, they still require continuous improvement to meet the increasingly precise targeting demands for nano-oncological diagnostic and therapeutic materials. This challenge is a key issue throughout various materials and therapeutic modalities. Our research group employed non-pathogenic Escherichia coli to target tumor delivery of photosensitizers, enhancing photodynamic therapy and tumor imaging.113
The water solubility of many photosensitizers used in photodynamic therapy does not meet expectations during tumor target applications. Besides modifying the hydrophilicity of the photosensitizers, researchers have attempted to enhance the assembly of porphyrin nanofibers through apoptosis, which allows the material to be cleaved by apoptosis-related enzyme caspase-3 (Casp3) and self-assemble into nanofibers upon laser irradiation, increasing the yield of ROS while inducing cellular apoptosis.114 The short lifespan and action radius of ROS necessitate their generation close to tumor cells to achieve optimal tumor-killing effects while preventing damage to surrounding healthy cells. Thus, in addition to red-shifting the absorption peaks of materials to increase treatment depth, optimizing the targeting of photosensitizer-generated ROS towards tumor cells is crucial.13 Besides introducing tumor-targeting ligands such as the well-known antibodies, RGD peptide, folate acids and et al,3,115 enhancing the rate of photodynamic generation, increasing the sensitivity of photosensitizer excitation, to magnify the own property of photosensitizers are promising approaches to improving tumor-specific efficacy.83 In another case, attaching charge-modified moieties with environment-responsive cell membrane anchoring functionality to porphyrin IX (PpIX) results in a self-transformable pH-driven membrane anchoring photosensitizer (pHMAPS) that exhibit an α-helical structure and integrate into cell membranes under mildly acidic tumor environments. Under 630 nm light exposure, they can generate ROS to disrupt cell membranes and induce cell death.116 Some natural plant active compounds, such as curcumin and its derivative β-diketone ligands, exhibit good antitumor and antibacterial activities. However, they suffer from poor water solubility, necessitating the use of carriers to improve their dispersion. In our research group, the nanoparticles (C086@HSA) formed from human serum albumin (HSA) loaded with curcumin derivative C086 showed not only increased tumor cell uptake of C086 but also enhanced in vivo stability and tumor targeted location through enhanced permeability and retention effect (EPR) effects, resulting in improved photodynamic therapy efficacy against tumors.100
One of the conditions for the photodynamic action, whether through Type I or Type II mechanisms, is a sufficient concentration of oxygen; however, within solid tumors, the interior often exists in a hypoxic state. This limitation significantly hinders the therapeutic effectiveness of photodynamic therapy. One approach is to increase the oxygen content in the surrounding environment. For instance, Tao et al cross-linked 5,10,15,20-tetra (4-hydroxyphenyl) porphyrin (THPP) with perfluorohexanoic acid (PFSEA) and polyethylene glycol (PEG) through a one-pot esterification reaction, preparing a fluorinated covalent organic polymer (COP) to achieve simultaneous tumor oxygenation and photodynamic therapy.117 Lv et al addressed the dependency on oxygen during the generation of singlet oxygen by photosensitizers, modifying Pt(II) porphyrin with cationic oligo-fluorene. These materials can monitor tumor hypoxia and maintain good reactive oxygen generation performance.118 Similarly, Liang et al dispersed perfluorooctyl bromide (PFOB) stably in porphyrin-grafted lipid (PGL) nanoparticles. The ordered arrangement of porphyrin and alkyl chains within the PGL NPs allows for high fluorescence without affecting the singlet oxygen yield of porphyrin itself while effectively enhancing the oxygen content in tumors.119 To address the weak tumor-targeting ability and oxygen dependence of Ce6, Zhao et al modified it with adamantane and used hydrogen peroxide enzyme-encapsulated β-cyclodextrin-hyaluronic acid nanoparticles as carriers, increasing the oxygen concentration and CD44 receptor targeting in the low-oxygen environment for the composite material HA-CAT@aCe6, consequently achieving a better anti-tumor effect.120
The tumor microenvironment and the abnormal expression of growth factor receptors on the surface of malignant tumors represent exploitable recognition and therapeutic targets.121 As summarized by Sharma et al (Figure 8).122 The specific characteristics in the tumor microenvironment that can be utilized to target materials or initiate drug release include pH,86 hypoxia, and elevated glutathione levels.123 These related biomarkers can be harnessed to design “responsive switches”, which not only facilitate tumor detection but also trigger the release of drugs loaded in nanoparticles.124,125 For instance, Zhao et al designed a convertible UCNP@MnOx-Hyp (Hyp, photosensitizer hypericin) system that, in tumor cells, reacts to glutathione and H2O2 and trigger the release Mn2+ and Hyp, PDT of the materials was improved by in-situ crosslinking.88
 |
Figure 8 Major targets for anti-cancer therapy using nanoparticles. Some representative targets associated with solid cancerous tumors are depicted. CAFs, cancer-associated fibroblasts; ECs, endothelial cells; TAMs, tumor asso_x0002_ciated macrophages. Source: Reprinted from Sharma N, Bietar K, Stochaj U et al. Targeting nanoparticles to malignant tumors. BBA-REV CANCER. 2022;1877(3):188703. Copyright 2022, with permission from Elsevier.122 |
Enhancing Stability
Serum components often lead to the disintegration of composite nanomaterials, resulting in off-target effects, necessitating the addition of protective components. For example, our research group designed a nanostructured self-assembling peptide-photodynamic agent carrier (NSPC) made from conjugated phthalocyanine derivatives (MCPZnPc) and ε-poly-L-lysine (EPL) for the delivery of chemotherapeutic drugs (CT). By the interactions between the core and outer layer, we engineered a highly stable CT@ME NSPC in serum that does not disintegrate, effectively protecting the chemotherapeutic agent while enabling a combined photodynamic and chemotherapeutic treatment.126
Discussion
Nanomaterials are effectively utilized in the diagnosis and treatment of tumors due to their excellent biological penetration properties. However, these same characteristics may also result in certain biological toxicity.127 For instance, metallic nanoparticles can induce the production of cytotoxic factors, trigger inflammatory responses, and generate ROS, which can be harmful to the organism.128,129 As more nanomaterials are incorporated into disease diagnosis and treatment research, it is imperative for researchers to not only focus on the significant specificity and functionality of new materials but also pay particular attention to the underexplored toxicological characteristics of different materials, as well as considerations regarding the residuals and metabolism of nanomaterials once they enter the body. The toxicity of nanoparticles is often related to their size, shape, surface charge, and other characteristics. Therefore, when modifying and engineering these materials, a cautious and thorough experimental approach is essential; haste should be avoided. Additionally, biomarkers and related expressions vary at different stages and degrees of tumor progression. Consequently, precise identification and comprehensive treatment under such circumstances may prove challenging.122
In recent years, nanomaterials are gaining increasing attention for their potential to be widely applied in tumor therapy due to their favorable properties compared to traditional therapeutic agents, such as good water solubility,130 small size that allows for better penetration and circulation,131 and their capability as drug delivery carriers. However, the integration of diagnosis and treatment still faces numerous challenges, particularly the multiple biological barriers that nanomaterials encounter during delivery of the photosensitizers to the tumor site. Both traditional chemotherapy drugs and nanoparticles first encounter the body’s natural physical barriers upon entering the system, with the primary barriers being the blood-brain barrier (BBB) and the peritoneal membrane.122 Once introduced into the body, photosensitive nanomaterials can more readily penetrate tumor tissues but not normal tissues due to the phenomenon of enhanced EPR.132 However, a portion of these materials may also accumulate in non-target tissues.122 The interstitial fluid and tumor microenvironment within this hydrogel system represent significant barriers that nanoparticles must overcome.133 The interstitial fluid pressure restricts the entry of nanoparticles to the vicinity of tumor cells.134 Convective interstitial flow can aid in the clearance of nanoparticles from the tumor cell surface. The interstitial fluid also contains the mononuclear phagocytic system (MPS), which includes phagocytic cells that clear the nanoparticles from the cell surface, with accumulation occurring in the spleen and liver. MPS recognition presents another critical barrier preventing nanomaterials from reaching tumors.122 Moreover, phagocytic cells exhibit a shape preference for nanoparticles. Macrophages preferentially uptake spherical nanoparticles, while neutrophils tend to ingest rod-shaped nanoparticles.135 More specifically, mouse leukemia monocytic macrophages more efficiently uptake triangular gold nanoparticles with larger adhesion areas.136,137 Additionally, the cellular membrane can restrict the access of nanoparticles into intracellular compartments.138 Presence of all these barriers calls for development of better designed photosensitive nanomaterials with enhanced penetration, prolonged half-life, low toxicity and the activity to evade non-target cell internalization.
Several agents have been FDA-approved, including aminolevulinic acid (ALA) and Photofrin, primarily for the treatment of skin cancers and precancerous lesions.103,139 Additionally, various novel photosensitizers are currently being investigated in clinical trials, including red-light-sensitive agents and nanoparticle-enhanced formulations.140 Preliminary results from these trials indicate that PDT has shown considerable success in treating specific malignancies, like squamous cell carcinoma and actinic keratosis, while offering advantages such as reduced side effects and faster recovery times compared to traditional therapies. These advancements provide new hope for future cancer treatment options.
Photodynamically generated ROS demonstrate multidisciplinary utility: clinically validated in dermatology (acne, psoriasis, vitiligo) and ophthalmology, combating antimicrobial resistance (MRSA) and biofilm-related infections. Emerging roles span atherosclerotic plaque stabilization, immunomodulation, and aesthetic rejuvenation via collagen remodeling. PDT’s dual action—direct cytotoxicity and immunostimulation—supports efficacy in dermatological/ocular therapies and expands into cardiovascular, immunological, and cosmetic domains. Based on this performance, our research group also designed a series of photosensitizer material with good antibacterial performance.141
In recent years, we have focused on pH and temperature-sensitive materials, such as phase-changeable hydrogels and degradable polymeric biomaterials, which are responsive to environmental conditions. The nano-to-micro drug delivery and release systems incorporating these materials show significant potential for applications in integrated tumor-targeting treatment and diagnosis. Meanwhile, in our present under-going studied, we are exploring novel biochar systems which are highly-applicable because they are prepared from plant and other organic materials at high temperature, showing decent biocompatibility and stability, which are promising for facilitation of the efficacy of the PDT materials in combination with diagnosis functions in vivo.
Photodynamic materials exhibit great potentials across diverse applications; however, their inherent toxicological risks, instability issues, and translational bottlenecks remain significant challenges, requiring systematic investigation. Future research should prioritize the development of photodynamic materials with highly-secured safety and stability, while providing refined and standardized clinical treatment protocols. Concerted interdisciplinary innovation integrating nanotechnology, materials science, and clinical oncology will be instrumental in overcoming existing limitations. Such collaborative advancements are poised to expand PDT’s applicability in oncological interventions, anti-infection treatments, and regenerative medicine, ultimately realizing their full translational potential through iterative technological refinement.
Within the current therapeutic paradigm of oncology, a dynamic interplay exists among diverse therapeutic modalities, each exhibiting distinct mechanistic profiles and clinically validated efficacy advantages. PDT and PTT therapies utilize light-activated ROS generation or hyperthermia for localized tumor destruction, yet struggle with deep/metastatic tumors. Immunotherapies (eg, ICIs) enable immune-driven tumor clearance with durable efficacy but face variable responses and toxicity. Chemotherapy, a systemic DNA/RNA disruptor, remains widely used despite off-target effects and drug resistance. Emerging synergy arises in combo strategies—PDT/PTT-enhanced immunogenic cell death amplifies immunotherapy, while nanoplatforms integrate chemo-photothermal action to overcome resistance. Therapeutic dominance hinges on tumor context, with precision approaches combining microenvironment modulation, immune activation, and targeted delivery gaining traction.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by National Natural Science Foundation of China (21877113), the Natural Science Foundation of Fujian Province (2024Y0051, 2020I0036), and Innovation Foundation of Fujian Agriculture and Forestry University (KFB23187A). We would also like to sincerely thank the anonymous reviewers for their valuable comments, which have greatly improved this paper.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. doi:10.3322/caac.21820
2. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
3. Song J, Zhang N, Zhang L, et al. IR780-loaded folate-targeted nanoparticles for near-infrared fluorescence image-guided surgery and photothermal therapy in ovarian cancer. Int J Nanomed. 2019;14:2757–2772. doi:10.2147/IJN.S203108
4. Ali ES, Sharker SM, Islam MT, et al. Targeting cancer cells with nanotherapeutics and nanodiagnostics: current status and future perspectives. Semin Cancer Biol. 2021;69:52–68. doi:10.1016/j.semcancer.2020.01.011
5. Marin JJG, Macias RIR, Monte MJ, et al. Molecular bases of drug resistance in hepatocellular carcinoma. Cancers. 2020;12(6):1663. doi:10.3390/cancers12061663
6. Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193. doi:10.1038/ncomms13193
7. Weissleder R. A clearer vision for in vivo imaging. Nat Biotechnol. 2001;19(19):316–317. doi:10.1038/86684
8. Zhang Y, Pan X, Shi H, et al. Molecular engineering to red-shift the absorption band of AIE photosensitizers and improve their ROS generation ability. J Mater Chem B. 2023;11(14):3252–3261. doi:10.1039/d2tb02829h
9. Juengpanich S, Li S, Yang T, et al. Pre-activated nanoparticles with persistent luminescence for deep tumor photodynamic therapy in gallbladder cancer. Nat Commun. 2023;14(1):5699. doi:10.1038/s41467-023-41389-1
10. Zhang X, Cui J, Liu J, Chen X, Chen M, Wang J. Dual ligand-assisted assembly of metal-organic frameworks on upconversion nanoparticles for NIR photodynamic therapy against hypoxic tumors. J Mater Chem B. 2023;11:9516–9524. doi:10.1039/d3tb01398g
11. Meng X, Zhang J, Sun Z, et al. Hypoxia-triggered single molecule probe for high-contrast NIR II/PA tumor imaging and robust photothermal therapy. Theranostics. 2018;8(21):6025–6034. doi:10.7150/thno.26607
12. Ng KK, Zheng G. Molecular interactions in organic nanoparticles for phototheranostic applications. Chem Rev. 2015;115(19):11012–11042. doi:10.1021/acs.chemrev.5b00140
13. Zheng Q, Liu X, Zheng Y, et al. The recent progress on metal–organic frameworks for phototherapy. Chem Soc Rev. 2021;50(8):5086–5125. doi:10.1039/d1cs00056j
14. Ethirajan M, Chen Y, Joshi P, Pandey RK. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem Soc Rev. 2011;40(1):340–362. doi:10.1039/b915149b
15. Lismont M, Dreesen L, Wuttke S. Metal-organic framework nanoparticles in photodynamic therapy: current status and perspectives. Adv Funct Mater. 2017;27(14):1606314. doi:10.1002/adfm.201606314
16. Marian CM. Spin-orbit coupling and intersystem crossing in molecules. Wiley Interdiscip Rev Comput Mol Sci. 2012;2(2):187–203. doi:10.1002/wcms.83
17. Liu S, Li Y, Kwok RTK, Lam JWY, Tang BZ. Structural and process controls of AIEgens for NIR-II theranostics. Chem Sci. 2021;12(10):3427–3436. doi:10.1039/d0sc02911d
18. Lan G, Ni K, Lin W. Nanoscale metal-organic frameworks for phototherapy of cancer. Coord Chem Rev. 2019;379:65–81. doi:10.1016/j.ccr.2017.09.007
19. Sharman WM, Allen CM, Lier J. Photodynamic therapeutics: basic principles and clinical applications. J Am Chem Soc. 1999;10(3):148–154. doi:10.1016/s1359-6446(99)01412-9
20. Benson CR, Kacenauskaite L, VanDenburgh KL, et al. Plug-and-play optical materials from fluorescent dyes and macrocycles. Chem. 2020;6(8):1978–1997. doi:10.1016/j.chempr.2020.06.029
21. Felsher DW. Cancer revoked: oncogenes as therapeutic targets. Nat Rev Cancer. 2003;3(5):375–380. doi:10.1038/nrc1070
22. Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250–281. doi:10.3322/caac.20114
23. Bacellar I, Tsubone T, Pavani C, Baptista M. Photodynamic efficiency: from molecular photochemistry to cell death. Int J Mol Sci. 2015;16(9):20523–20559. doi:10.3390/ijms160920523
24. Foote CS. Mechanisms of photosensitized oxidation. There are several different types of photosensitized oxidation which may be important in biological systems. Science. 1968;162(29):963–970. doi:10.1126/science.162.3857.963
25. Van Straten D, Mashayekhi V, de Bruijn HS, Oliveira S, Robinson DJ. Oncologic photodynamic therapy: basic principles, current clinical status and future directions. Cancers. 2017;9(2):19. doi:10.3390/cancers9020019
26. Pham TC, Nguyen V-N, Choi Y, Lee S, Yoon J. Recent strategies to develop innovative photosensitizers for enhanced photodynamic therapy. Chem Rev. 2021;121(21):13454–13619. doi:10.1021/acs.chemrev.1c00381
27. Zhang Q, Yu W, Liu Z, et al. Design, synthesis, antitumor activity and ct-DNA binding study of photosensitive drugs based on porphyrin framework. Int J Biol Macromol. 2023;230:123147. doi:10.1016/j.ijbiomac.2023.123147
28. Cheng Y, Chang Y, Feng Y, et al. Bismuth sulfide nanorods with retractable zinc protoporphyrin molecules for suppressing innate antioxidant defense system and strengthening phototherapeutic effects. Adv Mater. 2019;31(10):e1806808. doi:10.1002/adma.201806808
29. Ryter SW, Choi AM. Therapeutic applications of carbon monoxide in lung disease. Curr Opin Pharm. 2006;6(3):257–262. doi:10.1016/j.coph.2006.03.002
30. Zheng B, He Q, Li X, Yoon J, Huang J. Phthalocyanines as contrast agents for photothermal therapy. Coord Chem Rev. 2021;426:213548. doi:10.1016/j.ccr.2020.213548
31. Yang ZS, Yao Y, Sedgwick AC, et al. Rational design of an “all-in-one” phototheranostic. Chem Sci. 2020;11(31):8204–8213. doi:10.1039/d0sc03368e
32. Tian J, Huang B, Nawaz MH, Zhang W. Recent advances of multi-dimensional porphyrin-based functional materials in photodynamic therapy. Coord Chem Rev. 2020;420:213410. doi:10.1016/j.ccr.2020.213410
33. Jin T, Cheng D, Jiang G, et al. Engineering naphthalimide-cyanine integrated near-infrared dye into ROS-responsive nanohybrids for tumor PDT/PTT/chemotherapy. Bioact Mater. 2022;14:42–51. doi:10.1016/j.bioactmat.2021.12.009
34. Li Y, Jiang M, Yan M, et al. Near-infrared boron–dipyrrin (BODIPY) nanomaterials: molecular design and anti-tumor, therapeutics. Coord Chem Rev. 2024;506:215718. doi:10.1016/j.ccr.2024.215718
35. Zhao D, Zhou A, Dong X, et al. Dual-purposing disulfiram for enhanced chemotherapy and afterglow imaging using chlorin e6 and semiconducting polymer combined strategy. Theranostics. 2024;14(13):5141–5151. doi:10.7150/thno.96136
36. Nasr S, Rady M, Sebak A, et al. A naturally derived carrier for photodynamic treatment of squamous cell carcinoma: in vitro and in vivo models. Pharmaceutics. 2020;12(6):494. doi:10.3390/pharmaceutics12060494
37. Gilyadova A, Ishchenko A, Shiryaev A, et al. Phototheranostics of cervical neoplasms with chlorin e6 photosensitizer. Cancers. 2022;14(1):211. doi:10.3390/cancers14010211
38. Chen M, Liao H, Bu Z, et al. Pyroptosis activation by photodynamic-boosted nanocatalytic medicine favors malignancy recession. Chem Eng J. 2022;441:136030. doi:10.1016/j.cej.2022.136030
39. Xu Z, Chen J, Li Y, et al. Yolk-shell Fe3O4@Carbon@Platinum-Chlorin e6 nanozyme for MRI-assisted synergistic catalytic-photodynamic-photothermal tumor therapy. J Colloid Interface Sci. 2022;628(Pt A):1033–1043. doi:10.1016/j.jcis.2022.08.006
40. Lange N, Szlasa W, Saczko J, Chwilkowska A. Potential of cyanine derived dyes in photodynamic therapy. Pharmaceutics. 2021;13(6):818. doi:10.3390/pharmaceutics13060818
41. Congdon EE, Figueroa YH, Wang L, et al. Inhibition of tau polymerization with a cyanine dye in two distinct model systems. J Biol Chem. 2009;284(31):20830–20839. doi:10.1074/jbc.M109.016089
42. Usama SM, Park GK, Nomura S, Baek Y, Choi HS, Burgess K. Role of albumin in accumulation and persistence of tumor-seeking cyanine dyes. Bioconj Chem. 2020;31(2):248–259. doi:10.1021/acs.bioconjchem.9b00771
43. Kang Y-F, Chen W-K, Teng K-X, et al. Aggregation turns BODIPY fluorophores into photosensitizers: reversibly switching intersystem crossing on and off for smart photodynamic therapy. CCS Chem. 2022;4(11):3516–3528. doi:10.31635/ccschem.021.202101600
44. Kamkaew A, Lim SH, Lee HB, Kiew LV, Chung LY, Burgess K. BODIPY dyes in photodynamic therapy. Chem Soc Rev. 2013;42(1):77–88. doi:10.1039/c2cs35216h
45. Gorman A, Killoran J, O’Shea C, Kenna O, Gallagher WM, O’Shea DF. In vitro demonstration of the heavy-atom effect for photodynamic therapy. J Am Chem Soc. 2004;126(34):10619–10631. doi:10.1021/ja047649e
46. Atchison J, Kamila S, Nesbitt H, et al. Iodinated cyanine dyes: a new class of sensitisers for use in NIR activated photodynamic therapy (PDT). ChemComm. 2017;53(12):2009–2012. doi:10.1039/c6cc09624g
47. Cao J, Chi J, Xia J, Zhang Y, Han S, Sun Y. Iodinated cyanine dyes for fast near-infrared-guided deep tissue synergistic phototherapy. ACS Appl Mater Interfaces. 2019;11(29):25720–25729. doi:10.1021/acsami.9b07694
48. Kuo WS, Chang YT, Cho KC, et al. Gold nanomaterials conjugated with indocyanine green for dual-modality photodynamic and photothermal therapy. Biomaterials. 2012;33(11):3270–3278. doi:10.1016/j.biomaterials.2012.01.035
49. Ghorbani F, Attaran-Kakhki N, Sazgarnia A. The synergistic effect of photodynamic therapy and photothermal therapy in the presence of gold-gold sulfide nanoshells conjugated indocyanine green on HeLa cells. Photodiagn Photodyn Ther. 2017;17:48–55. doi:10.1016/j.pdpdt.2016.10.002
50. Li X, Zheng B-D, Peng X-H, et al. Phthalocyanines as medicinal photosensitizers: developments in the last five years. Coord Chem Rev. 2019;379:147–160. doi:10.1016/j.ccr.2017.08.003
51. Wong RCH, Lo P, Ng DKP. Stimuli responsive phthalocyanine-based fluorescent probes and photosensitizers. Coord Chem Rev. 2019;379:30–46. doi:10.1016/j.ccr.2017.10.006
52. Roguin LP, Chiarante N, Garcia Vior MC, Marino J. Zinc(II) phthalocyanines as photosensitizers for antitumor photodynamic therapy. Int J Biochem Cell Biol. 2019;114:105575. doi:10.1016/j.biocel.2019.105575
53. Liu J, Liang H, Li M, et al. Tumor acidity activating multifunctional nanoplatform for NIR-mediated multiple enhanced photodynamic and photothermal tumor therapy. Biomaterials. 2018;157:107–124. doi:10.1016/j.biomaterials.2017.12.003
54. Jiang Z, Shao J, Yang T, Wang J, Jia L. Pharmaceutical development, composition and quantitative analysis of phthalocyanine as the photosensitizer for cancer photodynamic therapy. J Pharm Biomed Anal. 2014;87:98–104. doi:10.1016/j.jpba.2013.05.014
55. Günsel A, Bilgiçli AT, Barut B, et al. Synthesis of water soluble tetra-substituted phthalocyanines: investigation of DNA cleavage, cytotoxic effects and metabolic enzymes inhibition. J Mol Struct. 2020;1214:128210. doi:10.1016/j.molstruc.2020.128210
56. Rojas JC, Bruchey AK, Gonzalez-Lima F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Prog Neurobiol. 2012;96(1):32–45. doi:10.1016/j.pneurobio.2011.10.007
57. Taldaev A, Terekhov R, Nikitin I, et al. Methylene blue in anticancer photodynamic therapy: systematic review of preclinical studies. Front Pharmacol. 2023;14:1264961. doi:10.3389/fphar.2023.1264961
58. Lim D-J. Methylene blue-based nano and microparticles: fabrication and applications in photodynamic therapy. POLYMERS-BASEL. 2021;13(22):3955. doi:10.3390/polym13223955
59. Boltes Cecatto R, Siqueira de Magalhães L, Fernanda Setúbal Destro Rodrigues M, et al. Methylene blue mediated antimicrobial photodynamic therapy in clinical human studies: the state of the art. Photodiagnosis Photodyn Ther. 2020;31:101828. doi:10.1016/j.pdpdt.2020.101828
60. Kofler B, Romani A, Pritz C, et al. Photodynamic effect of methylene blue and low level laser radiation in head and neck squamous cell carcinoma cell lines. Int J Mol Sci. 2018;19(4):1107. doi:10.3390/ijms19041107
61. Jia X, Bai J, Ma Z, Jiang X. BSA-exfoliated WSe2 nanosheets as a photoregulated carrier for synergistic photodynamic/photothermal therapy. J Mater Chem B. 2017;5(2):269–278. doi:10.1039/c6tb02525k
62. Xu X, Mao H, Wu Y, et al. Fabrication of methylene blue-loaded ovalbumin/polypyrrole nanoparticles for enhanced phototherapy-triggered antitumour immune activation. J Nanobiotechnol. 2022;20(1):297. doi:10.1186/s12951-022-01507-5
63. Karges J. Clinical development of metal complexes as photosensitizers for photodynamic therapy of cancer. Angew Chem Int Ed. 2021;61(5):e202112236. doi:10.1002/anie.202112236
64. Wei X, Guo X-H, Guo J-F, et al. Photophysical exploration of Zn(II) polypyridine photosensitizers in two-photon photodynamic therapy: insights from theory. Inorg Chem. 2022;61(46):18729–18742. doi:10.1021/acs.inorgchem.2c03232
65. Li X, Fu Y, Zhao S, et al. Metal ions-doped carbon dots: synthesis, properties, and applications. Chem Eng J. 2022;430:133101. doi:10.1016/j.cej.2021.133101
66. Jia Q, Ge J, Liu W, et al. A magnetofluorescent carbon dot assembly as an acidic H2O2‐driven oxygenerator to regulate tumor hypoxia for simultaneous bimodal imaging and enhanced photodynamic therapy. Adv Mater. 2018;30(13):e1706090. doi:10.1002/adma.201706090
67. Zhang Q, Xu W, Han C, et al. Graphene structure boosts electron transfer of dual-metal doped carbon dots in photooxidation. Carbon. 2018;126:128–134. doi:10.1016/j.carbon.2017.10.006
68. McKenzie LK, Bryant HE, Weinstein JA. Transition metal complexes as photosensitisers in one- and two-photon photodynamic therapy. Coord Chem Rev. 2019;379:2–29. doi:10.1016/j.ccr.2018.03.020
69. Wang B, Cai H, Waterhouse GIN, Qu X, Yang B, Lu S. Carbon dots in bioimaging, biosensing and therapeutics: a comprehensive review. Small Sci. 2022;2(6):2200012. doi:10.1002/smsc.202200012
70. Simon J, Flahaut E, Golzio M. Overview of carbon nanotubes for biomedical applications. Materials. 2019;12(4):624. doi:10.3390/ma12040624
71. Lagos KJ, Buzza HH, Bagnato VS, Romero MP. Carbon-based materials in photodynamic and photothermal therapies applied to tumor destruction. Int J Mol Sci. 2021;23(1):22. doi:10.3390/ijms23010022
72. Yao X, Tian Z, Liu J, Zhu Y, Hanagata N. Mesoporous silica nanoparticles capped with graphene quantum dots for potential chemo-photothermal synergistic cancer therapy. Langmuir. 2017;33(2):591–599. doi:10.1021/acs.langmuir.6b04189
73. Sun X, Zebibula A, Dong X, et al. Aggregation-induced emission nanoparticles encapsulated with PEGylated nano graphene oxide and their applications in two-photon fluorescence bioimaging and photodynamic therapy in vitro and in vivo. ACS Appl Mater Interfaces. 2018;10(30):25037–25046. doi:10.1021/acsami.8b05546
74. Zhang DY, Zheng Y, Tan CP, et al. Graphene oxide decorated with Ru(II)-polyethylene glycol complex for lysosome-targeted imaging and photodynamic/photothermal therapy. ACS Appl Mater Interfaces. 2017;9(8):6761–6771. doi:10.1021/acsami.6b13808
75. Campbell E, Hasan MT, Gonzalez-Rodriguez R, et al. Graphene quantum dot formulation for cancer imaging and redox-based drug delivery. Nanomedicine. 2021;37:102408. doi:10.1016/j.nano.2021.102408
76. Zaharie-Butucel D, Potara M, Suarasan S, Licarete E, Astilean S. Efficient combined near-infrared-triggered therapy: phototherapy over chemotherapy in chitosan-reduced graphene oxide-IR820 dye-doxorubicin nanoplatforms. J Colloid Interface Sci. 2019;552:218–229. doi:10.1016/j.jcis.2019.05.050
77. Lu T, Wei L, Huang X, et al. A potentially valuable nano graphene oxide/USPIO tumor diagnosis and treatment system. Mat Sci Eng C-Mater. 2021;128:112293. doi:10.1016/j.msec.2021.112293
78. Zhu S, Song Y, Zhao X, Shao J, Zhang J, Yang B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res. 2015;8:355–381. doi:10.1007/s12274-014-0644-3
79. Liu H, Zhang J, Jia Y, et al. Theranostic nanomotors for tumor multimode imaging and photothermal/photodynamic synergistic therapy. Chem Eng J. 2022;442:135994. doi:10.1016/j.cej.2022.135994
80. Hu S, Huang L, Zhou L, Wu T, Zhao S, Zhang L. Single-excitation triple-emission down-/up-conversion nanoassemblies for tumor microenvironment-enhanced ratiometric NIR-II fluorescence imaging and chemo-/photodynamic combination therapy. Anal Chem. 2023;95(7):3830–3839. doi:10.1021/acs.analchem.2c05333
81. Zhang Y, Liu H, Dai X, et al. Cyanobacteria-based near-infrared light-excited self-supplying oxygen system for enhanced photodynamic therapy of hypoxic tumors. Nano Res. 2020;14(3):667–673. doi:10.1007/s12274-020-3094-0
82. Luby BM, Walsh CD, Zheng G. Advanced photosensitizer activation strategies for smarter photodynamic therapy beacons. Angew Chem Int Ed Engl. 2019;58(9):2558–2569. doi:10.1002/anie.201805246
83. Yan K, Zhang Y, Mu C, et al. Versatile nanoplatforms with enhanced photodynamic therapy: designs and applications. Theranostics. 2020;10(16):7287–7318. doi:10.7150/thno.46288
84. Nyokong T, Antunes E. Photochemical and photophysical properties of metallophthalocyanines. Handbook of Porphyrin Science. 2010:247–357.
85. Chow SYS, Zhao S, Lo PC, Dkp N. A cell-selective glutathione-responsive tris(phthalocyanine) as a smart photosensitiser for targeted photodynamic therapy. Dalton Trans. 2017;46(34):11223–11229. doi:10.1039/c7dt02086d
86. Meng X, Li W, Sun Z, et al. Tumor-targeted small molecule for dual-modal imaging-guided phototherapy upon near-infrared excitation. J Mater Chem B. 2017;5(47):9405–9411. doi:10.1039/c7tb02496g
87. Yurdabak Karaca G, Kuralay F, Bingol Ozakpinar O, et al. Catalytic Au/PEDOT/Pt micromotors for cancer biomarker detection and potential breast cancer treatment. Appl Nanosci. 2021;13(1):367. doi:10.1007/s13204-021-01735-5
88. Zhao M, Zhuang H, Li B, Chen M, Chen X. In situ transformable nanoplatforms with supramolecular cross-linking triggered complementary function for enhanced cancer photodynamic therapy. Adv Mater. 2023;35(20):e2209944. doi:10.1002/adma.202209944
89. Chen Y, Yang Y, Du S, et al. Mitochondria-targeting upconversion nanoparticles@MOF for multiple-enhanced photodynamic therapy in hypoxic tumor. ACS Appl Mater Interfaces. 2023;15(30):35884–35894. doi:10.1021/acsami.3c05447
90. Yu Z, Hu W, Zhao H, et al. Generating new cross-relaxation pathways by coating Prussian Blue on NaNdF 4 to fabricate enhanced photothermal agents. Angew Chem Int Ed Engl. 2019;58(25):8536–8540. doi:10.1002/anie.201904534
91. Hao K, Lin L, Sun P, et al. Cationic flexible organic framework for combination of photodynamic therapy and genetic immunotherapy against tumors. Small. 2021;17(19):e2008125. doi:10.1002/smll.202008125
92. Zeng JY, Zou MZ, Zhang M, et al. Π-extended benzoporphyrin-based metal-organic framework for inhibition of tumor metastasis. ACS Nano. 2018;12(5):4630–4640. doi:10.1021/acsnano.8b01186
93. Hu P, Xu G, Yang D-C, Liu J-Y, Chen Z, Huang M. An advanced multifunctional prodrug combining photodynamic therapy with chemotherapy for highly efficient and precise tumor ablation. Dyes Pigm. 2022;205:110500. doi:10.1016/j.dyepig.2022.110500
94. Jiang J, Qian Y, Xu Z, et al. Enhancing singlet oxygen generation in semiconducting polymer nanoparticles through fluorescence resonance energy transfer for tumor treatment. Chem Sci. 2019;10(19):5085–5094. doi:10.1039/c8sc05501g
95. Tian J, Wan S, Yang Z, et al. PDL1/HER2‐targeted lipid‐encapsulated oxygen nanobubbles combined with photodynamic therapy for HER2+ breast cancer immunotherapy. Adv Healthc Mater. 2024;13(31):e2400030. doi:10.1002/adhm.202400030
96. Allison RR, Sibata CH. Oncologic photodynamic therapy photosensitizers: a clinical review. Photodiagn Photodyn Ther. 2010;7(2):61–75. doi:10.1016/j.pdpdt.2010.02.001
97. Bevernaegie R, Doix B, Bastien E, et al. Exploring the phototoxicity of hypoxic active iridium(III)-based sensitizers in 3d tumor spheroids. JAmChemSoc. 2019;141(46):18486–18491. doi:10.1021/jacs.9b07723
98. Yang Z-C, Gu Q-S, Chao -J-J, et al. Glutathione-activated biotin-targeted dual-modal imaging probe with improved PDT/PTT synergistic therapy. Anal Chim Acta. 2024;131. doi:10.1016/j.aca.2024.342860
99. Wang R, Qiu M, Zhang L, et al. Augmenting immunotherapy via bioinspired MOF‐based ROS homeostasis disruptor with nanozyme‐cascade reaction. Adv Mater. 2023;35(49):e2306748. doi:10.1002/adma.202306748
100. He C, Zhang L, Liu W, et al. Albumin-based nanoparticles combined with photodynamic therapy enhance the antitumor activity of curcumin derivative C086. Dyes Pigm. 2021;189:109258. doi:10.1016/j.dyepig.2021.109258
101. Zhang Y, Jia R, Wang X, et al. Targeted delivery of catalase and photosensitizer ce6 by a tumor-specific aptamer is effective against bladder cancer in vivo. Mol Pharm. 2024;21(4):1705–1718. doi:10.1021/acs.molpharmaceut.3c01047
102. Zhao X, Yang Y, Yu Y, Guo S, Wang W, Zhu S. A cyanine-derivative photosensitizer with enhanced photostability for mitochondria-targeted photodynamic therapy. ChemComm. 2019;55(90):13542–13545. doi:10.1039/c9cc06157f
103. Baskaran R, Lee J, Yang SG. Clinical development of photodynamic agents and therapeutic applications. Biomater Res. 2018;22:25. doi:10.1186/s40824-018-0140-z
104. Song Y, Wang L, Xie Z. Metal-organic frameworks for photodynamic therapy: emerging synergistic cancer therapy. Biotechnol J. 2021;16(2):e1900382. doi:10.1002/biot.201900382
105. Celli JP, Spring BQ, Rizvi I, et al. Imaging and photodynamic therapy: mechanisms, monitoring, and optimization. Chem Rev. 2010;110(5):2795–2838. doi:10.1021/cr900300p
106. Hu JJ, Jiang W, Yuan L, et al. Recent advances in stimuli‐responsive theranostic systems with aggregation‐induced emission characteristics. Aggreg. 2021;2(1):48–65. doi:10.1002/agt2.10
107. Yang G, Phua SZF, Lim WQ, et al. A hypoxia-responsive albumin-based nanosystem for deep tumor penetration and excellent therapeutic efficacy. Adv Mater. 2019;31(25):e1901513. doi:10.1002/adma.201901513
108. Yao Q, Fan J, Long S, et al. The concept and examples of type-III photosensitizers for cancer photodynamic therapy. Chem. 2022;8(1):197–209. doi:10.1016/j.chempr.2021.10.006
109. Schneider L, Kalt M, Koch S, et al. BODIPY-based photothermal agents with excellent phototoxic indices for cancer treatment. J Am Chem Soc. 2023;145(8):4534–4544. doi:10.1021/jacs.2c11650
110. de Bruijn HS, Mashayekhi V, Schreurs TJL, et al. Acute cellular and vascular responses to photodynamic therapy using EGFR-targeted nanobody-photosensitizer conjugates studied with intravital optical imaging and magnetic resonance imaging. Theranostics. 2020;10(5):2436–2452. doi:10.7150/thno.37949
111. Fernandez M, Javaid F, Chudasama V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem Sci. 2018;9(4):790–810. doi:10.1039/c7sc04004k
112. Lin R, Zhang L, Ye B, et al. A multi-functional nano-system combining PI3K-110α/β inhibitor overcomes p-glycoprotein mediated mdr and improves anti-cancer efficiency. Cancer Lett. 2023;563:216181. doi:10.1016/j.canlet.2023.216181
113. Dai T, Ye F, Hu P, et al. A strategy for enhanced tumor targeting of photodynamic therapy based on Escherichia Coli-driven drug delivery system. Sci China Mater. 2020;64(1):232–240. doi:10.1007/s40843-020-1363-2
114. Liu X, Zhan W, Gao G, et al. Apoptosis-amplified assembly of porphyrin nanofiber enhances photodynamic therapy of oral tumor. J Am Chem Soc. 2023;145(14):7918–7930. doi:10.1021/jacs.2c13189
115. Song L, Chen B, Qin Z, et al. Temperature dependent cat like rgd bpns@smfn nanoplatform for PTT PDT self synergetic tumor phototherapy. Adv Healthc Mater. 2021;11(8):e2102298. doi:10.1002/adhm.202102298
116. Luo GF, Chen WH, Hong S, Cheng Q, Qiu WX, Zhang XZ. A self‐transformable Ph driven membrane anchoring photosensitizer for effective photodynamic therapy to inhibit tumor growth and metastasis. Adv Funct Mater. 2017;27(36):1702122. doi:10.1002/adfm.201702122
117. Tao D, Feng L, Chao Y, et al. Covalent organic polymers based on fluorinated porphyrin as oxygen nanoshuttles for tumor hypoxia relief and enhanced photodynamic therapy. Adv Funct Mater. 2018;28(43):1804901. doi:10.1002/adfm.201804901
118. Lv Z, Zou L, Wei H, Liu S, Huang W, Zhao Q. Phosphorescent starburst Pt(II) porphyrins as bifunctional therapeutic agents for tumor hypoxia imaging and photodynamic therapy. ACS Appl Mater Interfaces. 2018;10(23):19523–19533. doi:10.1021/acsami.8b05944
119. Liang X, Chen M, Bhattarai P, Hameed S, Dai Z. Perfluorocarbon@porphyrin nanoparticles for tumor hypoxia relief to enhance photodynamic therapy against liver metastasis of colon cancer. ACS Nano. 2020;14(10):13569–13583. doi:10.1021/acsnano.0c05617
120. Phua SZF, Yang G, Lim WQ, et al. Catalase-integrated hyaluronic acid as nanocarriers for enhanced photodynamic therapy in solid tumor. ACS Nano. 2019;13(4):4742–4751. doi:10.1021/acsnano.9b01087
121. Foster C, Watson A, Kaplinsky J, Kamaly N. Improved targeting of cancers with nanotherapeutics. Methods Mol Biol. 2017;1530:13–37. doi:10.1007/978-1-4939-6646-2_2
122. Sharma N, Bietar K, Stochaj U. Targeting nanoparticles to malignant tumors. BBA-Rev Cancer. 2022;1877(3):188703. doi:10.1016/j.bbcan.2022.188703
123. Chu S, Stochaj U. Exploring near-infrared absorbing nanocarriers to overcome cancer drug resistance. Cancer Drug Resist. 2020;3(3):302–333. doi:10.20517/cdr.2020.20
124. Ang MJY, Chan SY, Goh YY, Luo Z, Lau JW, Liu X. Emerging strategies in developing multifunctional nanomaterials for cancer nanotheranostics. Adv Drug Deliv Rev. 2021;178:113907. doi:10.1016/j.addr.2021.113907
125. Gao Q, Zhang J, Gao J, Zhang Z, Zhu H, Wang D. Gold nanoparticles in cancer theranostics. Front Bioeng Biotechnol. 2021;9:647905. doi:10.3389/fbioe.2021.647905
126. Chen J, Zhou Y, Song M, et al. A serum-stable supramolecular drug carrier for chemotherapeutics fabricated by a peptide-photosensitizer conjugate. J Colloid Interface Sci. 2023;646:959–969. doi:10.1016/j.jcis.2023.05.131
127. Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Penetration of intact skin by quantum dots with diverse physicochemical properties. Toxicol Sci. 2006;91(1):159–165. doi:10.1093/toxsci/kfj122
128. Valdiglesias V, Fernandez-Bertolez N, Kilic G, et al. Are iron oxide nanoparticles safe? Current knowledge and future perspectives. J Trace Elem Med Biol. 2016;38:53–63. doi:10.1016/j.jtemb.2016.03.017
129. Teleanu DM, Chircov C, Grumezescu AM, Volceanov A, Teleanu RI. Impact of nanoparticles on brain health: an up to date overview. J Clin Med. 2018;7(12):490. doi:10.3390/jcm7120490
130. Montane X, Bajek A, Roszkowski K, et al. Encapsulation for cancer therapy. Molecules. 2020;25(7):1605. doi:10.3390/molecules25071605
131. Uskokovic D, Stevanovic M. Poly(lactide-co-glycolide)-based micro and nanoparticles for the controlled drug delivery of vitamins. Curr Nanosci. 2009;5(1):1–14. doi:10.2174/157341309787314566
132. Bjornmalm M, Thurecht KJ, Michael M, Scott AM, Caruso F. Bridging bio-nano science and cancer nanomedicine. ACS Nano. 2017;11(10):9594–9613. doi:10.1021/acsnano.7b04855
133. Daly AC, Riley L, Segura T, Burdick JA. Hydrogel microparticles for biomedical applications. Nat Rev Mater. 2020;5(1):20–43. doi:10.1038/s41578-019-0148-6
134. Abyaneh HS, Regenold M, McKee TD, Allen C, Gauthier MA. Towards extracellular matrix normalization for improved treatment of solid tumors. Theranostics. 2020;10(4):1960–1980. doi:10.7150/thno.39995
135. Safari H, Kelley WJ, Saito E, et al. Neutrophils preferentially phagocytose elongated particles—an opportunity for selective targeting in acute inflammatory diseases. Sci Adv. 2020;6(24):1–10. doi:10.1126/sciadv.aba1474
136. Nambara K, Niikura K, Mitomo H, et al. Reverse size dependences of the cellular uptake of triangular and spherical gold nanoparticles. Langmuir. 2016;32(47):12559–12567. doi:10.1021/acs.langmuir.6b02064
137. Millstone JE, Wei W, Jones MR, Yoo H, Mirkin CA. Iodide ions control seed-mediated growth of anisotropic gold nanoparticles. Nano Lett. 2008;8(8):2526–2529. doi:10.1021/nl8016253
138. Kodiha M, Wang YM, Hutter E, Maysinger D, Stochaj U. Off to the organelles - killing cancer cells with targeted gold nanoparticles. Theranostics. 2015;5(4):357–370. doi:10.7150/thno.10657
139. Nordmann NJ, Michael AP. 5-aminolevulinic acid radiodynamic therapy for treatment of high-grade gliomas: a systematic review. Clin Neurol Neurosurg. 2021;201:106430. doi:10.1016/j.clineuro.2020.106430
140. Cardoso M, Marto CM, Paula A, et al. Effectiveness of photodynamic therapy on treatment response and survival in patients with recurrent oral squamous cell carcinoma: a systematic review. Photodiagnosis Photodyn Ther. 2024;48:104242. doi:10.1016/j.pdpdt.2024.104242
141. Zhang Y, Liu W, Huang Y, Wang Y, Chen X, Chen Z. Bacterial biofilm microenvironment responsive copper-doped zinc peroxide nanocomposites for enhancing chemodynamic therapy. Chem Eng J. 2022;446:137214. doi:10.1016/j.cej.2022.137214
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.