Back to Journals » International Journal of Nanomedicine » Volume 19
Advancing Ovarian Cancer Therapeutics: The Role of Targeted Drug Delivery Systems
Authors Lin Q, Li J, Abudousalamu Z, Sun Y, Xue M, Yao L, Chen M
Received 15 May 2024
Accepted for publication 6 August 2024
Published 10 September 2024 Volume 2024:19 Pages 9351—9370
DOI https://doi.org/10.2147/IJN.S478313
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Jie Huang
Qianhan Lin,1 Jiajia Li,1 Zulimire Abudousalamu,1 Yating Sun,1 Mengyang Xue,1 Liangqing Yao,2 Mo Chen1
1Department of Gynecologic Oncology, Obstetrics and Gynecology Hospital, Fudan University, Shanghai, People’s Republic of China; 2Department of Gynecologic Oncology, Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, People’s Republic of China
Correspondence: Liangqing Yao; Mo Chen, Email [email protected]; [email protected]
Abstract: Ovarian cancer (OC) is the most lethal reproductive system cancer and a leading cause of cancer-related death. The high mortality rate and poor prognosis of OC are primarily due to its tendency for extensive abdominal metastasis, late diagnosis in advanced stages, an immunosuppressive tumor microenvironment, significant adverse reactions to first-line chemotherapy, and the development of chemoresistance. Current adjuvant chemotherapies face challenges such as poor targeting, low efficacy, and significant side effects. Targeted drug delivery systems (TDDSs) are designed to deliver drugs precisely to the tumor site to enhance efficacy and minimize side effects. This review highlights recent advancements in the use of TDDSs for OC therapies, including drug conjugate delivery systems, nanoparticle drug delivery systems, and hydrogel drug delivery systems. The focus is on employing TDDS to conduct direct, effective, and safer interventions in OC through methods such as targeted tumor recognition and controlled drug release, either independently or in combination. This review also discusses the prospects and challenges for further development of TDDSs. Undoubtedly, the use of TDDSs shows promise in the battle against OCs.
Keywords: targeted drug delivery, ovarian cancer, drug conjugates, nanoparticle, hydrogel
Introduction
Among gynecological malignancies, ovarian cancer (OC) has the highest mortality rate and the poorest prognosis.1,2 The latest statistics indicate that in 2022, there were 324,398 new cases and 206,839 deaths worldwide.3 Of these, 61,100 new cases and 32,600 deaths occurred in China.4 It is estimated that there will be approximately 19,680 new cases and 12,740 cancer-related deaths in the United States in 2024.5 Invasive epithelial ovarian cancer (EOC) is a heterogeneous disease, with high-grade serous ovarian carcinoma (HGSOC) being the predominant cancer type.6 Debulking surgery and adjuvant chemotherapy remain the mainstays of treatment for OC.2 Clinically, OC is often characterized by a trio of concerning statistics, commonly referred to as the “three 70%”: Firstly, 70% of patients receive their diagnosis at an advanced stage. Secondly, after undergoing treatment, 70% of patients experience a disease recurrence. Lastly, 70% of patients ultimately succumb to the disease.
In recent years, researchers have continuously sought to innovate drugs, explore drug combinations, alter routes of administration, and improve pharmacokinetics. Although novel antitumor drugs have been developed, most are unable to accurately distinguish between normal and tumor cells, leading to significant side effects.7 Efficacy and efficiency can be significantly improved by applying appropriate carriers, dosage forms, and routes of administration. Currently, drug delivery technology is advancing rapidly due to developments in medicine, pharmacy, nanoscience, and biomaterials. The distribution of drugs within an organism is regulated in terms of space, time, and dose through this method. It has demonstrated outstanding outcomes in controlled drug release, drug stability, and biocompatibility. A targeted drug delivery system (TDDS) is a device that enables the effective delivery of active pharmaceutical ingredients to the targeted site to achieve therapeutic effects. In recent years, there have been tremendous advances in TDDSs applied to OC, which can be classified into drug conjugate delivery systems, nanoparticle (NP) drug delivery systems (NDDS), and hydrogel drug delivery systems according to their materials. Figure 1 depicts a schematic of the TDDSs targeting OCs and generating efficacy.
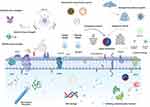 |
Figure 1 A schematic of the TDDSs targeting OCs and generating efficacy. Created by BioRender. com. |
Clinically, the high mortality and poor prognosis of OC can be attributed to a multitude of factors. TDDS emerges as a promising therapeutic strategy. Firstly, in contrast to cervical and uterine cancers, OC is particularly susceptible to extensive abdominal implantation metastasis due to the ovaries’ deep pelvic location and the lack of a substantial peritoneal covering. Moreover, due to the insidious onset and atypical symptoms, OC is typically diagnosed in the advanced or metastatic stage, which presents a challenge to debulking surgery. In this procedure, residual tumor cells that remain after surgery serve as the “seeds“ for the subsequent recurrence. However, TDDSs have the potential to enhance drug delivery directly to the tumor site, especially targeting these residual cells. By leveraging the unique characteristics of the tumor microenvironment (TME) and tumor cell targets, researchers can design drug carriers with precise physicochemical properties or configurations, ensuring more accurate and specific delivery of therapeutic agents to tumor tissues. Concurrently, OC exhibits an immunosuppressive TME, rendering it particularly susceptible to recurrence. In response, numerous TDDSs focus on simultaneously delivering immune adjuvants and cytotoxic drugs to not only eradicate tumor cells but also to reverse the immune-suppressive TME, thereby converting immunologically “cold” tumors into ”hot” ones. Additionally, the first-line chemotherapy regimens for OC, namely platinum and paclitaxel, are frequently associated with severe side effects like third-degree myelosuppression, leading to chemotherapy delay or non-completion. Such interruptions allow residual tumor cells to proliferate, triggering recurrence. TDDSs, by concentrating drugs at the tumor site while minimizing exposure to normal tissues, can mitigate these adverse effects. This strategy not only improves the quality of life but also enhances patient compliance and reduces the incidence of treatment delays and discontinuation. Furthermore, as the number of recurrence-related chemotherapy treatments increases, patients gradually develop acquired resistance to chemotherapeutic drugs,2,8 which further leads to a poorer prognosis and higher mortality rate. Nevertheless, several current TDDS targets are also novel therapeutic targets for tumors. The targeted attachment of specific antibodies and small molecule drugs to these targets facilitates precise drug delivery, while also offering tumor inhibitory functions. The innovative strategy of combining drugs may show great potential for acquired drug-resistant tumor cells. These underscore the potential of TDDSs to significantly impact the treatment landscape of OC, offering a beacon of hope for improving patient outcomes.
Recent advancements regarding the application of TDDSs for the treatment of OC, include drug conjugate delivery systems, NDDSs, and hydrogel drug delivery systems, are highlighted in this review, with an emphasis on how each system achieves efficient tumor-targeted drug delivery. Additionally, we discuss the clinical translation potential and development prospects of these systems. An overall schematic of this review is shown in Figure 2.
 |
Figure 2 An overall schematic of this review. Created by BioRender.com. |
Drug Conjugate Delivery Systems
Antibody‒drug Conjugates
Antibody drugs represent a rapidly expanding class of therapeutics, with numerous approved for therapeutic use and many more in development.9 Antibody‒drug conjugates (ADCs) represent a revolutionary class of targeted drugs that integrate highly specific monoclonal antibodies (mAbs) with the potent activity of small molecule cytotoxins. This combination aims to improve the on-target effects of antitumor agents while reducing their side effects.10–12 The antibody component provides precision targeting, while the small molecule cytotoxic drug provides most of the toxicity. The antibody component itself can also contribute to toxicity. mAbs and cytotoxic drugs are connected by a linker. The conjugate enters the cells via a receptor-mediated endocytosis pathway once the mAb binds to the target antigen receptor on the tumor cell surface. Then, the ADC is transported to the lysosome for degradation, releasing a cytotoxic toxin that damages DNA or prevents tumor cell division, ultimately resulting in cell death. As an emerging therapeutic candidate for OC, ADCs have been vigorously developed and have progressed into multi-stage clinical trials (Tables 1 and 2). The development of ADCs affords new opportunities for OC therapy.
 |
Table 1 ADCs in Ovarian Cancer |
 |
Table 2 Clinical Trials of ADCs in Ovarian Cancer |
FRα-ADC
There are three major subtypes of the folate receptor (FR): FRα, FRβ, and FRγ. FRα is a transmembrane cell surface protein that transfers folate unidirectionally into the cell.13,14 Compared to its limited distribution in normal cells, FRα is overexpressed specifically in many epithelial tumor cells, including OC cells.15 Clinically, FRα is overexpressed in 85–90% of OCs, and FRα expression is positively related to the histological grade.16 Therefore, FRα has become a widely researched therapeutic target for the treatment of OC.15 Many studies have been conducted to develop ADCs based on FRα targets.
Mirvetuximab soravtansine (MIRV) is the sole approved ADC by the Food and Drug Administration (FDA) for platinum-resistant epithelial OC (PROC) treatment and has demonstrated promising results.17 MIRV is formed by the combination of a mAb against FRα and the microtubule inhibitor maytansinoid DM4 (DM4). MIRV showed promising anticancer efficacy and satisfactory tolerability and security in PROC patients with high FRα expression in the single-arm Phase III SORAYA trial (NCT04296890).18 A recent global, validated, randomized, controlled phase III trial, the MIRASOL study (NCT04209855), showed that MIRV treatment was superior to chemotherapy for progression-free survival (PFS), overall survival (OS) and objective remission rate (ORR) in FRα-positive PROC patients.19 Compared with chemotherapy, MIRV significantly improved long-term survival in patients with FRα-overexpressing PROC; it also decreased the incidence of disease progression by 35% (median PFS: 5.62 vs 3.98 months, hazard ratio (HR) =0.65, P<0.0001), and the risk of all-cause mortality by 33% (median OS: 16.46 vs 12.75 months, HR=0.67, P=0.0046). Additionally, MIRV improved patient PFS2 and reduced the risk of secondary disease progression by 37% (PFS2: 11.04 vs 8.05 months, HR=0.63). MIRV improved the ORR significantly compared to chemotherapy. The MIRV group had an ORR of 42.3%, while the chemotherapy group had an ORR of 15.9%. The most common toxicities of MIRV are ocular toxicity and gastrointestinal toxicity. Compared with chemotherapy, MIRV had a superior safety profile, with a lower incidence of adverse events rated as grade 3 or higher, serious adverse events of any grade, and discontinuation rates (41.7% vs 54.1%, 23.9% vs 32.9%, and 9.2% vs 15.9%, respectively), as well as significantly reduced risk of hematological events. Currently, ongoing clinical trials are evaluating the effectiveness of MIRV in OC patients, including a phase III clinical trial in platinum-sensitive OC (PSOC) patients with high FRα expression (NCT05445778) and Phase II clinical trials in recurrent PSOC patients (NCT05887609) and newly diagnosed and advanced serous OC patients with high FRα expression (NCT04606914).
Additionally, several other ADCs that target FR α with promising clinical efficacy are currently under evaluation for OC treatment. Examples include farletuzumab ecteribulin (MORAb-202) and luveltamab tazevibulin (STRO-002), both of which are undergoing phase II clinical trials.
NaPi2b-ADC
Sodium-dependent phosphate transport protein 2b (NaPi2b), which is encoded by SLC34A2, is responsible for intestinal phosphate absorption.20,21 In OC and non-small cell lung cancer (NSCLC), NaPi2b expression is upregulated in tumors compared to normal tissues.22–24 Currently, various ADCs targeting NaPi2b have been studied in OC patients.
Monomethyl auristatin E (MMAE) is a potent antimitotic agent that blocks the polymerization of tubulin, often utilized as a therapy payload for ADCs. Lifastuzumab vedotin (LIFA) is a conjugate of an anti-NaPi2b mAb and MMAE, linked by a protease-labile linker.25,26 In a phase II clinical trial (NCT01991210), PROC patients were randomly assigned to intravenous LIFA (2.4 mg/kg, every 3 weeks) or pegylated liposomal doxorubicin (PLD) (40 mg/m2, every 4 weeks). LIFA was well tolerated and resulted in an improved ORR compared to PLD in intent-to-treat (ITT) patients (34% vs 15%, P=0.03) and NaPi2b-high patients (36% vs 14%, P=0.02). However, PFS showed a moderate but not significant improvement in the ITT patients (5.3 months vs 3.1 months, NaPi2b =0.78, P=0.343) and the NaPi2b-high patients (5.3 months vs 3.4 months, HR=0.71, P=0.243). It is important to note that there was strong (2+/3+) expression of NaPi2b in the majority of patients included. This suggests that the methodology used to assess the high-expression population of NaPi2b in this study may require improvement.25
Upifitamab rilsodotin (UpRi) is another ADC that consists of a unique humanized anti-NaPi2b mAb conjugated to auristatin F-hydroxypropylamide.27 The initial efficacy of UpRi was explored in a phase Ib study (NCT03319628) involving 97 HGSOC patients. The data on efficacy and tolerability supported the potential clinical benefit of UpRi, with an ORR of 34% and a median duration of remission of up to 5 months in NaPi2b-positive HGSOC patients.28 However, UpRi did not meet the primary endpoint in the next Phase II, the single-arm UPLIFT study (NCT03319628), which recruited PROC patients who had undergone one to four previous treatment therapies. Enrollment of new patients in two ongoing OC studies, UP-NEXT (NCT05329545) and UPGRADE (NCT04907968), has been temporarily suspended due to five patient deaths.
Msln-Adc
Mesothelin (MSLN) is a 40 kDa glycoprotein located on the cell membranes.29,30 It is rarely expressed in normal tissue but is highly expressed in a number of different epithelial tumor tissues.30 In preclinical studies, MSLN has been identified as a key protein in the multistep process by which OC spreads through the peritoneum.31 Multiple ADCs targeting MSLN are now being investigated in a growing number of tumors.
Anetumab ravtansine (BAY 94–9343) is an ADC that consists of the fully human immunoglobulin G1 anti-MSLN mAb and DM4 linked by a reducible disulfide bond. A multicenter, Phase I study recruited patients with advanced or metastatic solid tumors, including 64 patients with OC, to conduct a preliminary exploration of the safety and efficacy of BAY 94–9343 (NCT01439152). 94–9343 demonstrated a tolerable safety profile and promising initial antitumor efficacy, as well as a superior pharmacokinetics profile.32 The side effects of bevacizumab and BAY 94–9343 or paclitaxel (PTX) are being investigated in an ongoing phase II clinical trial involving PROC patients (NCT03587311).
BMS-986148 is another ADC directed toward the cell surface protein MSLN. It comprises a fully human IgG1 anti-MSLN mAb linked to tubulysin via a valine-citrulline linker.33 Tubulysin is a cytotoxic compound that inhibits microtubule assembly, thus resulting in defective proliferation and consequent apoptotic death.34 BMS-986148 was assessed in an international phase I/IIa trials (NCT02341625) involving patients suffering from MSLN-positive tumors, including mesothelioma, OC, NSCLC, pancreatic cancer, and gastric cancer. Twenty-seven patients with OC received BMS-986148 monotherapy, and two patients with OC received BMS-986148 in combination with navulizumab. The primary endpoints of the study were safety and tolerability. The prevalent therapy-related AEs (≥10%) were elevated levels of alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase. BMS-986148 ± nivolumab has shown preliminary clinical efficacy, which is safe and well tolerated.33
Her2-Adc
Human epidermal growth factor receptor 2 (HER2) is a member of the HER family. Among gynecological malignancies, HER2 is most commonly overexpressed in endometrial (17–30%) and ovarian (5–60%) cancers.35–38 Additionally, in OC patients, HER2 overexpression has been related to chemoresistance and a worse prognosis,35 making it an ideal target for ADC treatment. Trastuzumab-deruxtecan (T-DXd) is an ADC comprising the anti-HER2 mAb trastuzumab and the payload DXd, a topoisomerase I inhibitor. T-DXd received accelerated approval from the FDA as a therapy for HER2-mutant NSCLC and HER2-positive and HER2-low unresectable or metastatic breast cancer.39–42 A multicenter, open-label phase II clinical trial (NCT04482309) recently assessed T-DXd (5.4 mg/kg every 3 weeks) in solid tumors expressing HER2. A total of 267 patients suffering from solid tumors were recruited, including 40 patients with OC. The ORRs were 45.0% (95% CI, 29.3 to 61.5) in OC patients and 63.6% (95% CI, 30.8 to 89.1]) in HER2 immunohistochemistry 3+ OC patients. In the overall study population, the median OS was 13.2 months, and 20.0 months for the median PFS. The prevalent AEs reported were nausea (55.1%), anemia (27.7%), diarrhea (25.8%), fatigue (24.7%), and vomiting (24.7%).43
Peptide-Drug Conjugates
Peptide‒drug conjugates (PDCs) provide a promising avenue for targeted therapy following ADCs. Antibodies have certain limitations due to their large molecular weight, inability to effectively diffuse into cells, and high cost of synthesis. Using small molecule cancer cell targeting peptides (CTPs) instead of traditional mAbs appears to be a promising approach. The toxic reagent payload is coupled to CTP through a linker to produce a new targeted therapeutic drug. CTPs capable of binding with superior affinity to overexpressed receptors on the tumor cell surface offer precise targeting effects. PDCs have several core advantages over ADCs, including strong tissue permeability, relatively inexpensive synthesis, and low immunogenicity.44 Furthermore, it is worth noting that PDCs are quickly metabolized through the kidneys, in contrast to ADCs, which have more intricate pharmacokinetics.45
Cox et al reported a knottin peptide-drug conjugate and further validated it as a high-potency inhibitor against OC cell lines by selectively targeting OC cells expressing tumor-associated integrins.46 Sortilin (SORT1) is an intrinsic transmembrane protein that is overexpressed in OC.47,48 TH1902 is a PDC that contains two molecules of PTX connected to a peptide (TH19P01) that targets SORT1.49 In preclinical studies, TH1902 has demonstrated significant antitumor effects as a single agent or in combination with other agents, both in vitro and in xenograft mice models.49–51 Currently, a Phase 1 open-label clinical trial is underway to evaluate the efficacy of TH1902 (NCT04706962) in patients with advanced solid tumors, including OC. The study involves both dose escalation and expansion.
Aptamer-Drug Conjugates
An aptamer is a short, single-stranded oligonucleotide, composed of either RNA or DNA, that can bind exclusively and specifically to a target molecule with high affinity.52,53 The aptamer interacts with the target molecule through the folding of secondary and tertiary structures, forming a specific three-dimensional spatial conformation. Aptamer-drug conjugates (ApDCs) represent an efficient targeted delivery system. Compared to ADCs, ApDCs are smaller and more chemically stable, less immunogenic, penetrate rapidly into tissues, and have a simpler engineering design.52,54 Several aptamers have been validated for their potential to specifically bind to OC-related membrane proteins, such as CD44, nucleolin, and programmed cell death-ligand 1.55–57
Li et al constructed and characterized a highly hydrophilic nucleolin aptamer-PTX conjugate. Using the nucleolin-binding aptamer to specifically bind to nucleolin on the tumor cell membrane surface, this ApDC can specifically deliver PTX to tumor sites. Promising results were shown in vitro and in vivo using xenograft mice models. Even though multiple ApDCs have been reported by scientists, only a few ApDCs have progressed to the clinical trial stage. The application of ApDCs is restricted by their limited in vivo specificity, poor serum stability, and rapid renal elimination.56
ADCs are currently the fastest-developing drug conjugates. MIRV is the only approved ADC for OC therapy. Research on tumor-specific targets has expanded, leading to the rapid development of various drug conjugates targeting different targets. Exploration of the payload is underway. A wide variety of payloads, including proteins, liposomes, nucleic acids, bacteria, viruses and even cells, are now utilized beyond small molecule cytotoxic drugs.
Nanoparticle Drug Delivery Systems
NDDSs are sub-particulate DDSs composed of inorganic or polymeric materials in the nanoscale microcosmic range with superior biocompatibility, stability, safety, and modifiability. Nanoparticles with a diameter of <100 nm or materials with a diameter of 100–1000 nm that exhibit NP properties are used as materials for NDDSs. According to their properties, nanocarriers can be divided into two main categories: organic and inorganic. Organic nanocarriers mainly include liposomes, polymer micelles, dendritic macromolecules, polymers, and so on, while inorganic nanocarriers mainly include silica, metal-organic frameworks, carbon nanotubes, quantum dots, and so on.
Nanocarriers can encapsulate water-insoluble drugs by forming a lipophilic vesicle cavity. The external structure of nanocarriers is hydrophilic, which can improve the dissolution and absorption of these drugs, thereby increasing their bioavailability.58–60 Nanocarriers can also extend the half-life and duration of action of drugs and reduce their degradation in vitro and in vivo, thereby increasing their stability.61,62 In the case of poorly biocompatible drugs, the encapsulation of the drug in nanocarriers can enhance the bioavailability of the system and prevent enzymatic cleavage or hydrolysis of the drug.63 Several antitumor NDDSs, including doxorubicin hydrochloride liposomes and PTX liposomes, are currently being used to treat OC.
Targeting Mechanism
The targeting mechanism within NDDS is distinguished into passive and active targeting. Passive targeting utilizes the body’s inherent distribution mechanisms and the unique biological characteristics of diseased tissues. On the other hand, active targeting entails the precise attachment to specific molecules present on the surface of diseased cells. These two critical mechanisms, passive and active targeting, serve as the foundation of targeted drug delivery in NDDS, each presenting distinct advantages and limitations.
Passive Targeting
Nanocarriers can be passively enriched in tumor tissue that is rich in blood vessels and poor in lymphatic drainage via the enhanced permeability and retention (EPR) effect, thereby achieving passive targeting.64–67 This targeting is termed “passive” because it does not depend on any specific molecular recognition mechanisms on the surface of the nanoparticles. Instead, it leverages the physical and biochemical disparities between diseased and healthy tissues. The EPR effect has been recognized as a major contributor to nanocarrier enrichment in tumor tissue since its first report in the late 1980s.68 The principal advantage of this method is its simplicity and wide applicability. However, it is not effective for tumors or diseased tissues that do not exhibit a significant EPR effect. Additionally, the impact of the EPR effect on nanomedicine has been the subject of controversy.69,70 Sindhwani et al investigated the mechanism of NP penetration into solid tumors.71 There is sufficient evidence to suggest that transcytosis may be the primary mechanism for NP accumulation at the tumor site. These findings have inspired researchers to develop NDDSs that utilize active transcytosis, thereby improving drug delivery efficiency. Focal adhesion kinase (FAK) is a major driver of desmoplasia in pancreatic ductal adenocarcinoma (PDAC), affecting drug and immune cell infiltration into deep tumors. Dual-responsive NPs were designed, prepared, and assembled to codeliver the chemotherapeutic drug gemcitabine and the FAK selective inhibitor defactinib, which are capable of inducing transcytosis. The stromal cells of PDAC, which are initially responsible for drug entry, actively transport NPs to the deeper parts of the lesion, resulting in chemotherapy sensitization.72
Active Targeting
Targeted therapy can be achieved through surface functionalization of nanocarriers to deliver loaded drugs to specific sites and tissues, reducing nonspecific distribution in other organs and tissues. This can be achieved by targeting receptors (eg, FRα, NaPi2b, MSLN, HER2), specific enzymes, receptors, and antigens overexpressed on the surface of OC cells. The bioavailability of drugs can be enhanced and systemic toxicity can be decreased by active targeting. Satpathy et al reported that cisplatin (CDDP)-encapsulated magnetic iron oxide NP conjugated with a HER2 affibody. In an OC xenograft model in nude mice, the growth of primary tumors and metastatic peritoneal and lung tumors was significantly inhibited.73 Similarly, in 2021, Wang et al designed a CDDP-loaded NP modified by Her2 specific affibody allowing specific targeting, which can be used in tumor-enhanced chemo-radiotherapy.74 All of these NPs were designed to target the tumor cell membrane based on the specific binding of the HER2 affibody conjugated with the extracellular HER2. In addition, TMTP1 is a peptide that targets tumor lesions and metastases by specifically binding to aminopeptidase P, which is highly expressed on OC cell membrane.75,76 Wang et al proposed the use of adavosertib-olaparib (Ola)-loaded NPs decorated with TMTP1. In both OVCAR8 xenograft mice models and patient-derived OC xenograft models, NPs demonstrated excellent selectivity in targeting OC cells and exhibited stronger tumor suppression and less toxicity than the free drug combination.77 In another study, by using another tumor-targeting peptide, cyclic-Arg-Gly-Asp (cRGD), which specifically binds to integrin αvβ3, heparin NPs loaded with Ola and CDDP were able to effectively inhibit the proliferation and metastatic spread of CDDP-resistant OC.78 (Figure 3).
 |
Figure 3 (A) Schematic illustration of integrin αvβ3 target DDP-Ola co-delivered nanoparticle for dual drug therapy for CDDP-resistant OC. Excised ovarian primary tumors (B), primary tumor weights (C), primary tumor volumes (D), metastatic tumor weights (E) and number of metastatic nodules (F) of each treatment group. *P<0.05, **P<0.01, ***P<0.001. Reprinted with permission from Liang X, Yang Y, Huang Cet al cRGD-targeted heparin nanoparticles for effective dual drug treatment of cisplatin-resistant ovarian cancer. J Control Release. 2023;356:691–701. Copyright (2023) with permission from Elsevier.78 |
Biomimetic Nano Delivery Systems
In recent years, there has been a proliferation of novel types of NDDS, which leverage the distinctive properties of their delivery materials to facilitate more efficient transportation of drugs to the site of disease action, including target tissues and cells. It is crucial to recognize that the incorporation of these specialized NDDS with a targeting strategy (passive or active) enhances the specificity and efficacy of drug delivery without altering the original targeting strategy.
Biomimetic nano delivery systems (BNDSs), such as extracellular vesicles (EVs), natural cell membranes (CMs), and platelets, have recently shown unprecedented potential in the treatment of cancer.79–82 BNDSs have received considerable attention due to their low immunogenicity, long cycle time, superior cellular uptake ability, and intrinsic transcytosis. Owing to their advantageous characteristics such as nanosize, biocompatibility and excellent stability, EVs have greater potential for use as drug carriers than synthetic nanocarriers.83–86 Specifically, EVs have outstanding cellular internalization and intrinsic capacity for transcytosis, allowing them to rapidly traverse physiological barriers and penetrate the dense intratumoral tissues.87,88 In addition, EVs exhibit a degree of intrinsic active targeting capability. While not always precise or efficient, EVs can be modified through biotechnology techniques to enhance their active targeting capability.
Smart-Responsive NDDS
Moreover, in recent years, smart-responsive NDDSs, also referred to as “stimulus sensitive” NDDSs, which primarily function by influencing the payload release mechanism rather than the initial targeting/accumulation mechanism, have emerged as a prominent area of research. They can exhibit different responsive release characteristics that can result in immediate and dramatic physical and chemical alterations when stimulated by both external and internal stimulus. Two different approaches can be employed to construct stimuli-responsive NDDSs. The first approach involves using stimulus-responsive natural and synthetic polymeric materials directly, rather than through additional modification, by utilizing sensitive moieties in structural framework. The other is to attach sensitive functional groups or moieties to the backbone of the polymer by modification of side chains, end groups, or block junctions of the polymer framework.89–91 The design of an NDDS that responds to the TME is based on the unique variances of cancer and normal tissues. These variances include a slightly acidic environment, redox potential, activated oxygen-enriched environment, hypoxic environment, and enzyme expression in tumor tissue. Smart-responsive NDDSs normally remain in a “closed” station when in normal tissue, but convert to an “open” station when penetrating TME, thus enabling the controlled, selective drug release. In addition to responding to specific endogenous stimuli, smart-responsive NDDSs can also respond to exogenous stimuli (light, magnetism, and ultrasound) and release payloads in the desired region. In response to pH changes, studies on the redox potential and the effects of exogenous stimuli such as ultrasound are currently popular in stimulus-responsive nanocarrier research.
Due to accelerated proliferation and metabolism in tumors, the extracellular pH is approximately 6.5–7.2, compared to approximately 7.4 in normal tissues. In addition, it is further reduced in endosomes and lysosomes.92–95 Accordingly, multiple kinds of pH-responsive nanomaterials are being engineered to control drug release at specific tumor sites. Wlodarczyk et al synthesized and characterized a novel pH-responsive NP system providing active targeting and substantial delivery of platinum (II) to tumors. By combining stimulus-responsive release with an active targeting mechanism, the NPs showed increased uptake via receptor-mediated endocytosis at pH 6.8 in comparison with pH 7.4, with approximately three- and two-times greater uptake in the A2780 and CP70 cell lines.96
Another feature that can be used to regulate drug release from smart NDDSs is the redox potential. Glutathione (GSH) is the principal factor affecting the reducing environment and is highly abundant in the TME. Therefore, GSH is considered an ideal intracellular stimulator that triggers NDDS dissociation and rapid drug release.91 Reduction-sensitive disulfide linkers and diselenide linkers are commonly employed in redox-responsive DDSs and are reduced by GSH, leading to conformational changes and subsequent drug release.97,98 Zou et al constructed a redox-sensitive NP self-assembled from disulfide-linked PTX and tetramethylpyrazine (TMP). Amphiphilic PTX-ss-TMP spontaneously self-assembles in aqueous solution to form stable NPs. When tumor cells were exposed to high levels of GSH, disulfide bonds were broken, and PTX/TMP was released. Compared to free drugs, PTX-ss-TMP NPs in A2780 xenograft mice demonstrated superior tumor-specific enrichment and therapeutic efficacy.99 Qu et al designed a programmable disulfide cross-linked micelles loaded with betulinic acid (BA) and paclitaxel (PDCM@PTX), whose programmable switches comprise ester bonds and disulfide cross-links. In TME, the disulfide linkers were reduced by GSH and subsequently the ester bonds were cleaved by intracellular esterase. The in-situ release of PTX and BA leads to an enhanced anti-tumor effect in multi-drug resistant OC.100 (Figure 4)
 |
Figure 4 (A) Schematic illustration of glutathione and esterase response cross-linked micelle. (B–C) Advanced T1-weight MR images-measured tumor volume and quantitative analysis for the PTX-resistant mouse model with different treatments. (D) Tumor weight for the PTX-resistant mouse model with different treatments. *P<0.05, ***P<0.001. Reprinted with permission from Qu H, Yang J, Li S, et al. Programmed-response cross-linked nanocarrier for multidrug-resistant ovarian cancer treatment. J Control Release. 2023;357:274–286. Copyright (2023) wiht permission from Elsevier.100 |
Functional nanomaterials can also be exploited as nanocarriers in response to exogenous stimuli such as ultrasound, light, temperature, magnetic, microwave, or electric fields to release encapsulated drugs. For instance, ultrasound can be employed to trigger spatial and temporal drug release. Its biocompatibility and low attenuation in tissues have great potential for remote activation, steadily advancing the field of intelligent drug delivery systems. Baghbani et al developed alginate nanodroplets that co-deliver doxorubicin and curcumin, which are responsive to ultrasound. Low-frequency ultrasound irradiation significantly increased the cytotoxicity of nanodroplets to A2780 human OC cells and resulted in efficient tumor regression in OC tumor-bearing mice models.101
Drug carriers that trigger drug release in responding to dual and multiple stimuli, such as pH-redox and pH-temperature, have been rapidly advanced to further enhance drug release and therapeutic potency.102–104 In this regard, Fathi et al constructed pH/thermo-responsive magnetic NPs loaded with the tyrosine kinase inhibitor erlotinib for targeted OC therapy.105
The advancement of nanomedicine has resulted in the development of sophisticated NDDSs that target tumors and respond to the TME and external energy. These systems have improved drug efficacy and decreased toxic effects in the adjunctive management of OC. Drug resistance and metastasis are the primary causes of high morbidity and mortality in OC patients. Nanodrugs have unique advantages in delaying drug resistance and inhibiting metastasis due to their structural features and multitargeted mode of action. Notably, nanomedicine studies have focused mainly on animal models, with most experimental data coming from rats and mice. It should be noted, however, that there is a certain species heterogeneity between humans and mice.
Hydrogel Drug Delivery System
Hydrogels are hydrophilic polymer networks formed by physically or chemically crosslinked macromolecules that retain relatively large amounts of fluid and are compatible with the water-rich biological environment of the organism.106–109 Hydrogels mimic living tissues for their high-water component, soft structure, and high porosity, with outstanding biocompatibility, biodegradability, and sustained encapsulation.110–112 Hydrogels can be classified as macrogels, microgels (0.5–10 μm), or nanogels (NGs) (<200 nm) based on their size. In contrast to macrogels, microgels, and NGs are characterized by smaller particles, which is beneficial for systemic administration. Microgels smaller than 5 μm can be administered orally or inhaled, and NGs can be given by intravenous injection.113 They are available for various routes of administration, including intravenous, in situ injection or implantation, transdermal delivery, oral delivery, pulmonary delivery, and transarterial chemoembolization.114 Hydrogels are promising new options for DDSs due to multiple scales and various administration methods, allowing them to be used to target a wide variety of cancers Hydrogel materials are frequently employed as a precisely controlled DDS for a variety of substances, including chemo drugs, radionuclides, immune suppressants, thermotherapeutic agents, phototherapeutic agents, and others. They are widely used in cancer treatment through various therapies, such as radiotherapy, chemotherapy, immunotherapy, thermotherapy, photodynamic therapy, and photothermal therapy.115–117
Similarly, the targeting mechanism of a hydrogel delivery system can be classified into two categories: passive targeting and active targeting, which are analogous to those observed in NDDSs. Hydrogel DDSs can be given active targeting ability by modifying functional groups that specifically bind to tumor targets. Another member of the HER family is epidermal growth factor receptor (EGFR, HER1), which is also expressed in up to 60% of malignant OCs.118 EGFR not only is a biological target but also actively participates in maintaining the proliferation and survivability of cancer cells.119 Coencapsulated polymeric NGs loaded with CDDP and neratinib (NRT) were developed by Xi et al. The drug-loaded NGs was modified with two target ligands binding with EGFR to provide tumor-specific targeting capability. These NGs enable the delivery of combinations of CDDP and NRT to tumor cells that overexpress EGFR. The combination of CDDP and NRT demonstrated synergistic toxicity to cancer cells. Codelivery of drugs with NGs, compared to mixtures of free drugs and single-agent NGs administered at the same dosages, significantly enhanced their inhibition of tumor growth in EGFR-positive intraperitoneal OC xenografts. Significant tumor growth inhibition and improved animal survival were achieved by the targeted fusion approach with coadministered drugs.120
Smart-responsive hydrogel DDS
The elaborate design of hydrogels allows for the construction of “stimulus-responsive” hydrogel DDSs using different approaches, which are promising strategies for controlled drug release therapies. Hydrogels can be categorized as normal or responsive hydrogels based on their responsiveness.114 Hydrogel materials that are responsive to changes in their environment through both internal and external stimuli, such as pH, light, temperature, enzymes, redox potential, and magnetism.114,121–130 Responsive hydrogel DDSs, which enable remote control and on-demand release of agents, are critical for effectively delivering antitumor drugs to the intended target, minimizing systemic toxicity, and enhancing their potency. Salt-induced kinase 2 (SIK2) is overexpressed in both primary and metastatic lesions of OC, promoting tumor occurrence and metastasis.131,132 Hua et al constructed an SIK2-reactive supramolecular hydrogel loaded with an SIK2 inhibitor for intraperitoneal injection, which exhibited gel-to-sol transitions upon activation by SIK2. Hydrogelators are efficiently converted to hydrophilic phosphates, which are activated by SIK2 overexpression in tumor lesions, resulting in the degradation of the nanofibers and the sustained release of SIK2 inhibitors. Consequently, SIK2 inhibitors downregulate SIK2 activity and promote OC cell apoptosis.130 (Figure 5)
 |
Figure 5 (A) Schematic illustration of the formation and drug release of Gel Nap-S+HG and its mechanism of action. (B) The gel-sol transitions before and after incubation with PBS or cell lysates. (C and D) Omental metastasis tumors after different treatments. (E) Tumor weights with different treatments of each treatment group. *P<0.05, **P<0.01. Reprinted with permission from Hua Y, Yin H, Liu X, et al. Salt-Inducible Kinase 2-Triggered Release of Its Inhibitor from Hydrogel to Suppress Ovarian Cancer Metastasis. Adv Sci (Weinh). 2022;9(22):e2202260.130 |
Topical Administration Hydrogel DDS
Additionally, optimizing the administration route can improve biodistribution, thereby facilitating controlled release of drugs at the target site in another efficient way. For instance, topical administration hydrogel DDSs, the direct delivery of a drug to the target site, can be used as an alternative to systemic administration. The properties of biodegradable and injectable hydrogels make them suitable for topical administration.
The injectability of hydrogels makes them excellent carriers for topical drug application. The sol-gel state transformability of hydrogels enables researchers to design morphological transformations in specific environments, thereby controlling the localisation of drugs and the rate of drug release. The application of in situ triggered gel technology after injection provides a minimally invasive approach to in situ injection of hydrogel DDSs for almost any organ or tissue.132,133 Drugs within the hydrogel are injected directly into the tumor sites and are localized within a cross-linked network of the hydrogel, increasing local retention of the therapeutic agents within the tumor tissue and limiting drug toxicity to a localized area where tumor cells are located. This approach differs from systemic administration because it can bypass the hepatic first-pass effect and enhance the drug concentration in tumor lesions, resulting in effective tumor suppression. Therefore, it can load drugs or cooperate with other DDSs to deliver medication more accurately, increase concentration at the tumor lesion, and decrease toxicity.
Future Perspectives and Challenges
In the near future, a promising avenue of research may be the integration of multiple therapeutic methods in a complex multi-stimulus response system. Once accumulated at the tumor site, capsule drugs can be released through distinct mechanisms, targeting different cells and releasing in sequence. With the advent of immunotherapy in the field of tumor treatment, ovarian cancer, which has been designated an “immune cold tumor”, has garnered attention in the context of reversing the immunosuppressive microenvironment of tumors through combined immunotherapy. Enhancing the infiltration of CD8+ T cells and inducing the differentiation of macrophages from M2 to M1 represent crucial steps in improving the immunosuppressive microenvironment. In the future, through meticulous design, immune adjuvants can be delivered to immune cells in the tumor microenvironment, and cell cytotoxic drugs can be specifically delivered to tumor cells, achieving efficient and multifunctional combination therapy while also circumventing the inflammatory storm caused by immune adjuvants throughout the body. In recent years, there has been a narrowing of the targeted targets from organs/tissues to cells. This is occurring concurrently with a deepening of research on the role of subcellular structures, especially organelles, in tumor progression. As a result, there is a growing interest in TDDS targeting organelles (such as the nucleus, mitochondria, lysosomes, endoplasmic reticulum, Golgi apparatus, etc). The targeting of more specific sites may result in enhanced therapeutic efficacy and a reduction in adverse effects, given that the treatment mode and target molecules of numerous drugs are present or active in these locations. For example, mitochondria, which function as the cell’s primary energy source, are responsible for producing the majority of ATP (through the oxidative phosphorylation process) and also participate in numerous other essential cellular processes, including apoptosis, the generation of ROS, and the regulation of signal transduction pathways. At present, a considerable number of studies are focused on the regulation of pivotal mitochondrial targets with the objective of alleviating tumor burden. The potential of TDDS to deliver drugs specifically to the interior of mitochondria represents a promising avenue for the development of intelligent and efficient treatment modalities, thereby facilitating the translation of theoretical research into tangible clinical benefits.
Despite these many benefits, clinical translation is hampered by several key problems and barriers that need to be addressed. First, with the exception of ADCs, which have been the subject of clinical trials in a variety of populations, most studies of TDDSs have been conducted primarily in animal models. A large number of human clinical trials are needed to ensure short- as well as long-term effects and to explore the potential of these systems. Furthermore, incorporating comprehensive genetic testing into the initial assessment of ovarian cancer represents a significant advancement in the era of personalized medicine, effectively matching patients with efficacious treatments. The application of uniform drug therapy has been demonstrated to have no impact on the survival rate of some patients, while frequently resulting in the onset of severe toxic side effects. Given that TDDS targets receptors overexpressed on tumors, it is essential to consider individual heterogeneity based on comprehensive genetic testing to benefit a greater number of patients. Ovarian cancer tumors may exhibit high heterogeneity, and target expression may change over time. Therefore, patient screening and target monitoring throughout the entire treatment process are of paramount importance. Moreover, it is essential to acknowledge that for multi-drug delivery strategies, if the target cells are situated in disparate locations, the delivery and release processes must be meticulously precise and accurate. To illustrate, the number of immune cells in the tumor microenvironment is restricted, with dendritic cells (DC) and CD8 T cells predominantly situated in lymph nodes. In the event that TDDS is unable to simultaneously deliver drugs to the tumor microenvironment and lymph nodes, it is essential to determine how to distinguish between different target cells and release the appropriate drugs in the appropriate locations. Moreover, the drugs must be released promptly upon reaching the target location to prevent transport or efflux to alternative sites. Consequently, the sensitivity and specificity of TDDS represent significant challenges. Finally, research on TDDS targeting specific organelles is still very limited, particularly in light of the considerable vesicular transport observed. Finally, it is noteworthy that research on TDDS targeting specific organelles remains scarce, particularly in light of the substantial vesicular transport observed between some organelles for the exchange of substances. This may potentially result in an early accumulation of drugs in the targeted organelles, which could subsequently be transferred to other organelles. Therefore, it is essential to gain a comprehensive understanding of the targeted section of the target organelle and the complex interconnectivity between organelles in order to achieve optimal drug accumulation in the intended organelles.
Conclusions
In conclusion, the battle against OC necessitates innovative approaches beyond the traditional paradigms of chemotherapy. The advent and evolution of TDDSs offer a novel pathway to significantly enhance therapeutic outcomes, with the potential to transcend the limitations of current treatments, addressing the critical challenges of poor targeting, low efficacy, and substantial side effects. The exploration of drug conjugate delivery systems, NDDSs, and hydrogel DDSs underscores a pivotal shift towards leveraging advanced technologies for direct, effective, and safer OC interventions. Additionally, this review highlights future directions and challenges.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (82002750 to Mo Chen) and the Shanghai Pujiang Programme (23PJD009 to Mo Chen). The funding source provided financial support for the study and did not have any other involvement in this study.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Zhang Y, Luo G, Li M, et al. Global patterns and trends in ovarian cancer incidence: age, period and birth cohort analysis. BMC Cancer. 2019;19(1):984. doi:10.1186/s12885-019-6139-6
2. Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384(9951):1376–1388. doi:10.1016/S0140-6736(13)62146-7
3. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
4. Zheng RS, Chen R, Han BF, et al. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi. 2024;46(3):221–231. doi:10.3760/cma.j.cn112152-20240119-00035
5. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. doi:10.3322/caac.21820
6. Azzalini E, Stanta G, Canzonieri V, Bonin S. Overview of tumor heterogeneity in high-grade serous ovarian cancers. Int J Mol Sci. 2023;24(20):15077. doi:10.3390/ijms242015077
7. He J, Li C, Ding L, et al. Tumor targeting strategies of smart fluorescent nanoparticles and their applications in cancer diagnosis and treatment. Adv Mater. 2019;31(40):e1902409. doi:10.1002/adma.201902409
8. Tavares V, Marques IS, Melo IG, Assis J, Pereira D, Medeiros R. Paradigm shift: a comprehensive review of ovarian cancer management in an era of advancements. Int J Mol Sci. 2024;25(3):1845. doi:10.3390/ijms25031845
9. Silver AB, Leonard EK, Gould JR, Spangler JB. Engineered antibody fusion proteins for targeted disease therapy. Trends Pharmacol Sci. 2021;42(12):1064–1081. doi:10.1016/j.tips.2021.09.009
10. Ruan DY, Wu HX, Meng Q, Xu RH. Development of antibody-drug conjugates in cancer: overview and prospects. Cancer Commun. 2024;44(1):3–22. doi:10.1002/cac2.12517
11. Wei Q, Li P, Yang T, et al. The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors. J Hematol Oncol. 2024;17(1):1. doi:10.1186/s13045-023-01509-2
12. Lopez de Sa A, Diaz-Tejeiro C, Poyatos-Racionero E, et al. Considerations for the design of antibody drug conjugates (ADCs) for clinical development: lessons learned. J Hematol Oncol. 2023;16(1):118. doi:10.1186/s13045-023-01519-0
13. Mai J, Wu L, Yang L, et al. Therapeutic strategies targeting folate receptor alpha for ovarian cancer. Front Immunol. 2023;14:1254532. doi:10.3389/fimmu.2023.1254532
14. Zhao R, Diop-Bove N, Visentin M, Goldman ID. Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr. 2011;31(1):177–201. doi:10.1146/annurev-nutr-072610-145133
15. Scaranti M, Cojocaru E, Banerjee S, Banerji U. Exploiting the folate receptor alpha in oncology. Nat Rev Clin Oncol. 2020;17(6):349–359. doi:10.1038/s41571-020-0339-5
16. Martin LP, Konner JA, Moore KN, et al. Characterization of folate receptor alpha (FRalpha) expression in archival tumor and biopsy samples from relapsed epithelial ovarian cancer patients: a phase I expansion study of the FRalpha-targeting antibody-drug conjugate mirvetuximab soravtansine. Gynecol Oncol. 2017;147(2):402–407. doi:10.1016/j.ygyno.2017.08.015
17. Dilawari A, Shah M, Ison G, et al. FDA approval summary: mirvetuximab soravtansine-Gynx for FRalpha-positive, platinum-resistant ovarian cancer. Clin Cancer Res. 2023;29(19):3835–3840. doi:10.1158/1078-0432.CCR-23-0991
18. Matulonis UA, Lorusso D, Oaknin A, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study. J Clin Oncol. 2023;41(13):2436–2445. doi:10.1200/JCO.22.01900
19. Moore KN, Angelergues A, Konecny GE, et al. Mirvetuximab soravtansine in fralpha-positive, platinum-resistant ovarian cancer. N Engl J Med. 2023;389(23):2162–2174. doi:10.1056/NEJMoa2309169
20. Forster IC, Hernando N, Biber J, Murer H. Phosphate transporters of the SLC20 and SLC34 families. Mol Aspects Med. 2013;34(2–3):386–395. doi:10.1016/j.mam.2012.07.007
21. Marks J. The role of SLC34A2 in intestinal phosphate absorption and phosphate homeostasis. Pflugers Arch. 2019;471(1):165–173. doi:10.1007/s00424-018-2221-1
22. Banerjee S, Drapkin R, Richardson DL, Birrer M. Targeting NaPi2b in ovarian cancer. Cancer Treat Rev. 2023;112:102489. doi:10.1016/j.ctrv.2022.102489
23. Lin K, Rubinfeld B, Zhang C, et al. Preclinical development of an anti-NaPi2b (SLC34A2) antibody-drug conjugate as a therapeutic for non-small cell lung and ovarian cancers. Clin Cancer Res. 2015;21(22):5139–5150. doi:10.1158/1078-0432.CCR-14-3383
24. Rangel LB, Sherman-Baust CA, Wernyj RP, Schwartz DR, Cho KR, Morin PJ. Characterization of novel human ovarian cancer-specific transcripts (HOSTs) identified by serial analysis of gene expression. Oncogene. 2003;22(46):7225–7232. doi:10.1038/sj.onc.1207008
25. Banerjee S, Oza AM, Birrer MJ, et al. Anti-NaPi2b antibody-drug conjugate lifastuzumab vedotin (DNIB0600A) compared with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer in a randomized, open-label, phase II study. Ann Oncol. 2018;29(4):917–923. doi:10.1093/annonc/mdy023
26. Gerber DE, Infante JR, Gordon MS, et al. Phase Ia Study of Anti-NaPi2b antibody-drug conjugate lifastuzumab vedotin DNIB0600A in patients with non-small cell lung cancer and platinum-resistant ovarian cancer. Clin Cancer Res. 2020;26(2):364–372. doi:10.1158/1078-0432.CCR-18-3965
27. Bodyak ND, Mosher R, Yurkovetskiy AV, et al. The dolaflexin-based antibody-drug conjugate XMT-1536 targets the solid tumor lineage antigen SLC34A2/NaPi2b. Mol Cancer Ther. 2021;20(5):896–905. doi:10.1158/1535-7163.MCT-20-0183
28. Richardson D, Hamilton E, Barve M, et al. Updated Results from the phase 1 expansion study of upifitamab rilsodotin (UpRi; XMT-1536), a NaPi2b-directed Dolaflexin Antibody Drug Conjugate (ADC) in Ovarian Cancer (076). Gynecologic Oncol. 2022; 166:S48.
29. Chang K, Pai LH, Batra JK, Pastan I, Willingham MC. Characterization of the antigen (CAK1) recognized by monoclonal antibody K1 present on ovarian cancers and normal mesothelium. Cancer Res. 1992;52(1):181–186.
30. Weidemann S, Gagelmann P, Gorbokon N, et al. Mesothelin expression in human tumors: a tissue microarray study on 12,679 tumors. Biomedicines. 2021;9(4):397. doi:10.3390/biomedicines9040397
31. Coelho R, Ricardo S, Amaral AL, et al. Regulation of invasion and peritoneal dissemination of ovarian cancer by mesothelin manipulation. Oncogenesis. 2020;9(6):61. doi:10.1038/s41389-020-00246-2
32. Hassan R, Blumenschein GR Jr, Moore KN, et al. First-in-human, multicenter, phase i dose-escalation and expansion study of anti-mesothelin antibody-drug conjugate anetumab ravtansine in advanced or metastatic solid tumors. J Clin Oncol. 2020;38(16):1824–1835. doi:10.1200/JCO.19.02085
33. Rottey S, Clarke J, Aung K, et al. Phase I/IIa Trial of BMS-986148, an anti-mesothelin antibody-drug conjugate, alone or in combination with nivolumab in patients with advanced solid tumors. Clin Cancer Res. 2022;28(1):95–105. doi:10.1158/1078-0432.CCR-21-1181
34. Khalil MW, Sasse F, Lunsdorf H, Elnakady YA, Reichenbach H. Mechanism of action of tubulysin, an antimitotic peptide from myxobacteria. Chembiochem. 2006;7(4):678–683. doi:10.1002/cbic.200500421
35. Editors PO. Expression of Concern: the prognostic value of HER2 in ovarian cancer: a meta-analysis of observational studies. PLoS One. 2022;17(12):e0279960. doi:10.1371/journal.pone.0279960
36. Luo H, Xu X, Ye M, Sheng B, Zhu X. The prognostic value of HER2 in ovarian cancer: a meta-analysis of observational studies. PLoS One. 2018;13(1):e0191972. doi:10.1371/journal.pone.0191972
37. Diver EJ, Foster R, Rueda BR, Growdon WB. The therapeutic challenge of targeting HER2 in endometrial cancer. Oncologist. 2015;20(9):1058–1068. doi:10.1634/theoncologist.2015-0149
38. Tuefferd M, Couturier J, Penault-Llorca F, et al. HER2 status in ovarian carcinomas: a multicenter GINECO study of 320 patients. PLoS One. 2007;2(11):e1138. doi:10.1371/journal.pone.0001138
39. Osterweil N. FDA Gives Nod to T-DXd for HER2-Mutant NSCLC. Cancer Discov. 2022;12(10):2224. doi:10.1158/2159-8290.CD-NB2022-0053
40. Andrikopoulou A, Zografos E, Liontos M, Koutsoukos K, Dimopoulos MA, Zagouri F. Trastuzumab Deruxtecan (DS-8201a): the latest research and advances in breast cancer. Clin Breast Cancer. 2021;21(3):e212–e219. doi:10.1016/j.clbc.2020.08.006
41. Narayan P, Osgood CL, Singh H, et al. FDA approval summary: fam-trastuzumab deruxtecan-Nxki for the treatment of unresectable or metastatic HER2-positive breast cancer. Clin Cancer Res. 2021;27(16):4478–4485. doi:10.1158/1078-0432.CCR-20-4557
42. Narayan P, Dilawari A, Osgood C, et al. US food and drug administration approval summary: fam-trastuzumab deruxtecan-nxki for human epidermal growth factor receptor 2-low unresectable or metastatic breast cancer. J Clin Oncol. 2023;41(11):2108–2116. doi:10.1200/JCO.22.02447
43. Meric-Bernstam F, Makker V, Oaknin A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 Phase II Trial. J Clin Oncol. 2024;42(1):47–58. doi:10.1200/JCO.23.02005
44. Wang M, Liu J, Xia M, et al. Peptide-drug conjugates: a new paradigm for targeted cancer therapy. Eur J Med Chem. 2024;265:116119. doi:10.1016/j.ejmech.2023.116119
45. Vhora I, Patil S, Bhatt P, Misra A. Protein- and Peptide-drug conjugates: an emerging drug delivery technology. Adv Protein Chem Struct Biol. 2015;98:1–55.
46. Cox N, Kintzing JR, Smith M, Grant GA, Cochran JR. Integrin-targeting knottin peptide-drug conjugates are potent inhibitors of tumor cell proliferation. Angew Chem Int Ed Engl. 2016;55(34):9894–9897. doi:10.1002/anie.201603488
47. Ghaemimanesh F, Ahmadian G, Talebi S, et al. The effect of sortilin silencing on ovarian carcinoma cells. Avicenna J Med Biotechnol. 2014;6(3):169–177.
48. Hemmati S, Zarnani AH, Mahmoudi AR, et al. Ectopic expression of sortilin 1 (NTR-3) in patients with ovarian carcinoma. Avicenna J Med Biotechnol. 2009;1(2):125–131.
49. Currie JC, Demeule M, Charfi C, et al. The peptide-drug conjugate TH1902: a new sortilin receptor-mediated cancer therapeutic against ovarian and endometrial cancers. Cancers (Basel). 2022;14(8):1877. doi:10.3390/cancers14081877
50. Demeule M, Charfi C, Currie JC, et al. The TH1902 docetaxel peptide-drug conjugate inhibits xenografts growth of human SORT1-positive ovarian and triple-negative breast cancer stem-like cells. Pharmaceutics. 2022;14(9):1910. doi:10.3390/pharmaceutics14091910
51. Charfi C, Demeule M, Currie JC, et al. New peptide-drug conjugates for precise targeting of SORT1-Mediated vasculogenic mimicry in the tumor microenvironment of TNBC-Derived MDA-MB-231 breast and ovarian ES-2 clear cell carcinoma cells. Front Oncol. 2021;11:760787. doi:10.3389/fonc.2021.760787
52. He S, Du Y, Tao H, Duan H. Advances in aptamer-mediated targeted delivery system for cancer treatment. Int J Biol Macromol. 2023;238:124173. doi:10.1016/j.ijbiomac.2023.124173
53. Liang C, Guo B, Wu H, et al. Aptamer-functionalized lipid nanoparticles targeting osteoblasts as a novel RNA interference-based bone anabolic strategy. Nat Med. 2015;21(3):288–294. doi:10.1038/nm.3791
54. He J, Duan Q, Ran C, Fu T, Liu Y, Tan W. Recent progress of aptamer‒drug conjugates in cancer therapy. Acta Pharm Sin B. 2023;13(4):1358–1370. doi:10.1016/j.apsb.2023.01.017
55. Eaton RM, Shallcross JA, Mael LE, et al. Selection of DNA aptamers for ovarian cancer biomarker HE4 using CE-SELEX and high-throughput sequencing. Anal Bioanal Chem. 2015;407(23):6965–6973. doi:10.1007/s00216-015-8665-7
56. Li F, Lu J, Liu J, et al. A water-soluble nucleolin aptamer-paclitaxel conjugate for tumor-specific targeting in ovarian cancer. Nat Commun. 2017;8(1):1390. doi:10.1038/s41467-017-01565-6
57. Yazdian-Robati R, Ramezani M, Khedri M, Ansari N, Abnous K, Taghdisi SM. An aptamer for recognizing the transmembrane protein PDL-1 (programmed death-ligand 1), and its application to fluorometric single cell detection of human ovarian carcinoma cells. Mikrochim Acta. 2017;184(10):4029–4035. doi:10.1007/s00604-017-2436-4
58. Liu Y, Fens M, Lou B, et al. pi-pi-Stacked Poly(epsilon-caprolactone)-b-poly(ethylene glycol) Micelles Loaded with a Photosensitizer for Photodynamic Therapy. Pharmaceutics. 2020;12(4):338. doi:10.3390/pharmaceutics12040338
59. Bozzer S, Dal Bo M, Grimaldi MC, Toffoli G, Macor P. Nanocarriers as a delivery platform for anticancer treatment: biological limits and perspectives in B-cell malignancies. Pharmaceutics. 2022;14(9):1965. doi:10.3390/pharmaceutics14091965
60. Tsouris V, Joo MK, Kim SH, Kwon IC, Won YY. Nano carriers that enable co-delivery of chemotherapy and RNAi agents for treatment of drug-resistant cancers. Biotechnol Adv. 2014;32(5):1037–1050. doi:10.1016/j.biotechadv.2014.05.006
61. Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi:10.1016/j.addr.2012.09.037
62. Guo D, Shi C, Wang X, Wang L, Zhang S, Luo J. Riboflavin-containing telodendrimer nanocarriers for efficient doxorubicin delivery: high loading capacity, increased stability, and improved anticancer efficacy. Biomaterials. 2017;141:161–175. doi:10.1016/j.biomaterials.2017.06.041
63. Wang C, Zhang Y. Current application of nanoparticle drug delivery systems to the treatment of anaplastic thyroid carcinomas. Int J Nanomed. 2023;18:6037–6058. doi:10.2147/IJN.S429629
64. Shi Y, van der Meel R, Chen X, Lammers T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics. 2020;10(17):7921–7924. doi:10.7150/thno.49577
65. Maeda H. Macromolecular therapeutics in cancer treatment: the EPR effect and beyond. J Control Release. 2012;164(2):138–144. doi:10.1016/j.jconrel.2012.04.038
66. Maeda H. Polymer therapeutics and the EPR effect. J Drug Target. 2017;25(9–10):781–785. doi:10.1080/1061186X.2017.1365878
67. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1–2):271–284. doi:10.1016/S0168-3659(99)00248-5
68. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392.
69. Nichols JW, Bae YH. EPR: evidence and fallacy. J Control Release. 2014;190:451–464. doi:10.1016/j.jconrel.2014.03.057
70. Danhier F. To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release. 2016;244(Pt A):108–121. doi:10.1016/j.jconrel.2016.11.015
71. Sindhwani S, Syed AM, Ngai J, et al. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19(5):566–575. doi:10.1038/s41563-019-0566-2
72. Chen H, Song H, Luo Y, et al. Transcytosis mediated deep tumor penetration for enhanced chemotherapy and immune activation of pancreatic cancer. Adv. Funct. Mater. 2023;33(28):2214937. doi:10.1002/adfm.202214937
73. Satpathy M, Wang L, Zielinski RJ, et al. Targeted drug delivery and image-guided therapy of heterogeneous ovarian cancer using HER2-targeted theranostic nanoparticles. Theranostics. 2019;9(3):778–795. doi:10.7150/thno.29964
74. Wang H, Jia D, Yuan D, et al. Dimeric Her2-specific affibody mediated cisplatin-loaded nanoparticles for tumor enhanced chemo-radiotherapy. J Nanobiotechnology. 2021;19(1):138. doi:10.1186/s12951-021-00885-6
75. Yang W, Luo D, Wang S, et al. TMTP1, a novel tumor-homing peptide specifically targeting metastasis. Clin Cancer Res. 2008;14(17):5494–5502. doi:10.1158/1078-0432.CCR-08-0233
76. Li F, Yuan Y, Dai Y, et al. M11: a tropism-modified oncolytic adenovirus arming with a tumor-homing peptide for advanced ovarian cancer therapies. Hum Gene Ther. 2022;33(5–6):262–274. doi:10.1089/hum.2021.247
77. Wang W, Xiong Y, Hu X, et al. Codelivery of adavosertib and olaparib by tumor-targeting nanoparticles for augmented efficacy and reduced toxicity. Acta Biomater. 2023;157:428–441. doi:10.1016/j.actbio.2022.12.021
78. Liang X, Yang Y, Huang C, et al. cRGD-targeted heparin nanoparticles for effective dual drug treatment of cisplatin-resistant ovarian cancer. J Control Release. 2023;356:691–701. doi:10.1016/j.jconrel.2023.03.017
79. Han X, Gong C, Yang Q, Zheng K, Wang Z, Zhang W. Biomimetic nano-drug delivery system: an emerging platform for promoting tumor treatment. Int J Nanomed. 2024;19:571–608. doi:10.2147/IJN.S442877
80. Liu X, Cao Y, Wang S, Liu J, Hao H. Extracellular vesicles: powerful candidates in nano-drug delivery systems. Drug Deliv Transl Res. 2024;14(2):295–311. doi:10.1007/s13346-023-01411-x
81. Li J, Zeng H, Li L, Yang Q, He L, Dong M. Advanced generation therapeutics: biomimetic nanodelivery system for tumor immunotherapy. ACS Nano. 2023;17(24):24593–24618. doi:10.1021/acsnano.3c10212
82. Li H, Wang Z, Chen Z, et al. Disrupting tumour vasculature and recruitment of aPDL1-loaded platelets control tumour metastasis. Nat Commun. 2021;12(1):2773. doi:10.1038/s41467-021-22674-3
83. Cong M, Tan S, Li S, et al. Technology insight: plant-derived vesicles-How far from the clinical biotherapeutics and therapeutic drug carriers? Adv Drug Deliv Rev. 2022;182:114108. doi:10.1016/j.addr.2021.114108
84. Xiao Q, Zhao W, Wu C, et al. Lemon-derived extracellular vesicles nanodrugs enable to efficiently overcome cancer multidrug resistance by endocytosis-triggered energy dissipation and energy production reduction. Adv Sci. 2022;9(20):e2105274. doi:10.1002/advs.202105274
85. Liu S, Wu X, Chandra S, et al. Extracellular vesicles: emerging tools as therapeutic agent carriers. Acta Pharm Sin B. 2022;12(10):3822–3842. doi:10.1016/j.apsb.2022.05.002
86. Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16(7):748–759. doi:10.1038/s41565-021-00931-2
87. Wang Q, Zhuang X, Mu J, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4(1):1867. doi:10.1038/ncomms2886
88. Zhuang X, Teng Y, Samykutty A, et al. Grapefruit-derived nanovectors delivering therapeutic miR17 through an intranasal route inhibit brain tumor progression. Mol Ther. 2016;24(1):96–105. doi:10.1038/mt.2015.188
89. Zhang Y, Dong L, Liu L, Wu Z, Pan D, Liu L. Recent advances of stimuli-responsive polysaccharide hydrogels in delivery systems: a review. J Agric Food Chem. 2022;70(21):6300–6316. doi:10.1021/acs.jafc.2c01080
90. El-Husseiny HM, Mady EA, Hamabe L, et al. Smart/stimuli-responsive hydrogels: cutting-edge platforms for tissue engineering and other biomedical applications. Mater Today Bio. 2022;13:100186. doi:10.1016/j.mtbio.2021.100186
91. Singh D, Sharma Y, Dheer D, Shankar R. Stimuli responsiveness of recent biomacromolecular systems (concept to market): a review. Int J Biol Macromol. 2024;261(Pt 2):129901. doi:10.1016/j.ijbiomac.2024.129901
92. Franco MS, Gomes ER, Roque MC, Oliveira MC. Triggered drug release from liposomes: exploiting the outer and inner tumor environment. Front Oncol. 2021;11:623760. doi:10.3389/fonc.2021.623760
93. Koltai T. The Ph paradigm in cancer. Eur J Clin Nutr. 2020;74(Suppl 1):14–19. doi:10.1038/s41430-020-0684-6
94. Li Y, Angelova A, Hu F, et al. pH responsiveness of hexosomes and cubosomes for combined delivery of brucea javanica oil and doxorubicin. Langmuir. 2019;35(45):14532–14542. doi:10.1021/acs.langmuir.9b02257
95. Garcia MC, Calderon-Montano JM, Rueda M, et al. pH-temperature dual-sensitive nucleolipid-containing stealth liposomes anchored with PEGylated AuNPs for triggering delivery of doxorubicin. Int J Pharm. 2022;619:121691. doi:10.1016/j.ijpharm.2022.121691
96. Wlodarczyk MT, Dragulska SA, Chen Y, et al. Pt(II)-PLGA hybrid in a ph-responsive nanoparticle system targeting ovarian cancer. Pharmaceutics. 2023;15(2):607. doi:10.3390/pharmaceutics15020607
97. Yadav S, Ramesh K, Reddy OS, et al. Redox-responsive comparison of diselenide and disulfide core-cross-linked micelles for drug delivery application. Pharmaceutics. 2023;15(4):1159. doi:10.3390/pharmaceutics15041159
98. Guo X, Cheng Y, Zhao X, Luo Y, Chen J, Yuan WE. Advances in redox-responsive drug delivery systems of tumor microenvironment. J Nanobiotechnology. 2018;16(1):74. doi:10.1186/s12951-018-0398-2
99. Zou L, Liu X, Li J, et al. Redox-sensitive carrier-free nanoparticles self-assembled by disulfide-linked paclitaxel-tetramethylpyrazine conjugate for combination cancer chemotherapy. Theranostics. 2021;11(9):4171–4186. doi:10.7150/thno.42260
100. Qu H, Yang J, Li S, et al. Programmed-response cross-linked nanocarrier for multidrug-resistant ovarian cancer treatment. J Control Release. 2023;357:274–286. doi:10.1016/j.jconrel.2023.03.031
101. Baghbani F, Moztarzadeh F. Bypassing multidrug resistant ovarian cancer using ultrasound responsive doxorubicin/curcumin co-deliver alginate nanodroplets. Colloids Surf B Biointerfaces. 2017;153:132–140. doi:10.1016/j.colsurfb.2017.01.051
102. Cheng R, Meng F, Deng C, Klok HA, Zhong Z. Dual and multi-stimuli responsive polymeric nanoparticles for programmed site-specific drug delivery. Biomaterials. 2013;34(14):3647–3657. doi:10.1016/j.biomaterials.2013.01.084
103. Bai S, Ma X, Shi X, et al. Smart unimolecular micelle-based polyprodrug with dual-redox stimuli response for tumor microenvironment: enhanced in vivo delivery efficiency and tumor penetration. ACS Appl Mater Interfaces. 2019;11(39):36130–36140. doi:10.1021/acsami.9b13214
104. Chang D, Ma Y, Xu X, Xie J, Ju S. Stimuli-responsive polymeric nanoplatforms for cancer therapy. Front Bioeng Biotechnol. 2021;9:707319. doi:10.3389/fbioe.2021.707319
105. Fathi M, Barar J, Erfan-Niya H, Omidi Y. Methotrexate-conjugated chitosan-grafted pH- and thermo-responsive magnetic nanoparticles for targeted therapy of ovarian cancer. Int J Biol Macromol. 2020;154:1175–1184. doi:10.1016/j.ijbiomac.2019.10.272
106. Hahn SK, Park JK, Tomimatsu T, Shimoboji T. Synthesis and degradation test of hyaluronic acid hydrogels. Int J Biol Macromol. 2007;40(4):374–380. doi:10.1016/j.ijbiomac.2006.09.019
107. Nair SK, Basu S, Sen B, et al. Colloidal gels with tunable mechanomorphology regulate endothelial morphogenesis. Sci Rep. 2019;9(1):1072. doi:10.1038/s41598-018-37788-w
108. Correa S, Grosskopf AK, Lopez Hernandez H, et al. Translational applications of hydrogels. Chem Rev. 2021;121(18):11385–11457. doi:10.1021/acs.chemrev.0c01177
109. Ho TC, Chang CC, Chan HP, et al. Hydrogels: properties and applications in biomedicine. Molecules. 2022;27(9):2902. doi:10.3390/molecules27092902
110. Zhu H, Zheng J, Oh XY, et al. Nanoarchitecture-integrated hydrogel systems toward therapeutic applications. ACS Nano. 2023;17(9):7953–7978. doi:10.1021/acsnano.2c12448
111. Suneetha M, Zo S, Choi SM, Han SS. Antibacterial, biocompatible, hemostatic, and tissue adhesive hydrogels based on fungal-derived carboxymethyl chitosan-reduced graphene oxide-polydopamine for wound healing applications. Int J Biol Macromol. 2023;241:124641. doi:10.1016/j.ijbiomac.2023.124641
112. Liu Y, Dong T, Chen Y, et al. Biodegradable and cytocompatible hydrogel coating with antibacterial activity for the prevention of implant-associated infection. ACS Appl Mater Interfaces. 2023;15(9):11507–11519. doi:10.1021/acsami.2c20401
113. Kasinski A, Zielinska-Pisklak M, Oledzka E, Sobczak M. Smart Hydrogels - Synthetic Stimuli-Responsive Antitumor Drug Release Systems. Int J Nanomed. 2020;15:4541–4572. doi:10.2147/IJN.S248987
114. Li X, Xu X, Xu M, Geng Z, Ji P, Liu Y. Hydrogel systems for targeted cancer therapy. Front Bioeng Biotechnol. 2023;11:1140436. doi:10.3389/fbioe.2023.1140436
115. Ikeda M, Tanida T, Yoshii T, et al. Installing logic-gate responses to a variety of biological substances in supramolecular hydrogel-enzyme hybrids. Nat Chem. 2014;6(6):511–518. doi:10.1038/nchem.1937
116. Wang H, Chen Y, Wei R, et al. Synergistic chemoimmunotherapy augmentation via sequential nanocomposite hydrogel-mediated reprogramming of cancer-associated fibroblasts in osteosarcoma. Adv Mater. 2023;36(15):e2309591. doi:10.1002/adma.202309591
117. Chen Q, Li Y, Zhou S, et al. Sequentially sustained release of anticarcinogens for postsurgical chemoimmunotherapy. J Control Release. 2022;350:803–814. doi:10.1016/j.jconrel.2022.09.006
118. Normanno N, De Luca A, Bianco C, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366(1):2–16. doi:10.1016/j.gene.2005.10.018
119. Siwak DR, Carey M, Hennessy BT, et al. Targeting the epidermal growth factor receptor in epithelial ovarian cancer: current knowledge and future challenges. J Oncol. 2010;2010:568938. doi:10.1155/2010/568938
120. Xi X, Lei F, Gao K, et al. Ligand-installed polymeric nanocarriers for combination chemotherapy of EGFR-positive ovarian cancer. J Control Release. 2023;360:872–887. doi:10.1016/j.jconrel.2023.07.033
121. Liang JL, Jin XK, Luo GF, et al. Immunostimulant hydrogel-guided tumor microenvironment reprogramming to efficiently potentiate macrophage-mediated cellular phagocytosis for systemic cancer immunotherapy. ACS Nano. 2023;17(17):17217–17232. doi:10.1021/acsnano.3c05093
122. Cheng F, Su T, Zhou S, et al. Single-dose injectable nanovaccine-in-hydrogel for robust immunotherapy of large tumors with abscopal effect. Sci Adv. 2023;9(28):eade6257. doi:10.1126/sciadv.ade6257
123. Liu J, Yang TY, Dai LQ, et al. Intravesical chemotherapy synergize with an immune adjuvant by a thermo-sensitive hydrogel system for bladder cancer. Bioact Mater. 2024;31:315–332. doi:10.1016/j.bioactmat.2023.08.013
124. Li Z, Xu W, Yang J, et al. A tumor microenvironments-adapted polypeptide hydrogel/nanogel composite boosts antitumor molecularly targeted inhibition and immunoactivation. Adv Mater. 2022;34(21):e2200449. doi:10.1002/adma.202200449
125. Chen W, Shi K, Liu J, et al. Sustained co-delivery of 5-fluorouracil and cis-platinum via biodegradable thermo-sensitive hydrogel for intraoperative synergistic combination chemotherapy of gastric cancer. Bioact Mater. 2023;23:1–15. doi:10.1016/j.bioactmat.2022.10.004
126. Gu J, Zhao G, Yu J, et al. Injectable pH-responsive hydrogel for combinatorial chemoimmunotherapy tailored to the tumor microenvironment. J Nanobiotechnology. 2022;20(1):372. doi:10.1186/s12951-022-01561-z
127. Gong Y, Chen W, Chen X, et al. An injectable epigenetic autophagic modulatory hydrogel for boosting umbilical cord blood NK cell therapy prevents postsurgical relapse of triple-negative breast cancer. Adv Sci. 2022;9(23):e2201271. doi:10.1002/advs.202201271
128. Fu W, Li X, Li Y, et al. A programmable releasing versatile hydrogel platform boosts systemic immune responses via sculpting tumor immunogenicity and reversing tolerogenic dendritic cells. Biomaterials. 2024;305:122444. doi:10.1016/j.biomaterials.2023.122444
129. Dai X, Meng J, Deng S, et al. Targeting CAMKII to reprogram tumor-associated macrophages and inhibit tumor cells for cancer immunotherapy with an injectable hybrid peptide hydrogel. Theranostics. 2020;10(7):3049–3063. doi:10.7150/thno.42385
130. Hua Y, Yin H, Liu X, et al. Salt-inducible kinase 2-triggered release of its inhibitor from hydrogel to suppress ovarian cancer metastasis. Adv Sci. 2022;9(22):e2202260. doi:10.1002/advs.202202260
131. Miranda F, Mannion D, Liu S, et al. Salt-inducible kinase 2 couples ovarian cancer cell metabolism with survival at the adipocyte-rich metastatic niche. Cancer Cell. 2016;30(2):273–289. doi:10.1016/j.ccell.2016.06.020
132. Gao T, Zhang X, Zhao J, et al. SIK2 promotes reprogramming of glucose metabolism through PI3K/AKT/HIF-1alpha pathway and Drp1-mediated mitochondrial fission in ovarian cancer. Cancer Lett. 2020;469:89–101. doi:10.1016/j.canlet.2019.10.029
133. Wu Y, Chang X, Yang G, et al. A physiologically responsive nanocomposite hydrogel for treatment of head and neck squamous cell carcinoma via proteolysis-targeting chimeras enhanced immunotherapy. Adv Mater. 2023;35(12):e2210787. doi:10.1002/adma.202210787
134. Wang B, Chen J, Caserto JS, Wang X, Ma M. An in situ hydrogel-mediated chemo-immunometabolic cancer therapy. Nat Commun. 2022;13(1):3821. doi:10.1038/s41467-022-31579-8
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

