Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Airway Organoid Models as Pivotal Tools for Unraveling Molecular Mechanisms and Therapeutic Targets in Respiratory Diseases: A Literature Review
Authors Jiang SP, Lin BQ, Zhou XQ, Li MH, Feng ZC, Qin YY, Lin SQ, Zhou ZQ, Peng Y, Li L
Received 6 March 2025
Accepted for publication 8 June 2025
Published 24 June 2025 Volume 2025:21 Pages 975—986
DOI https://doi.org/10.2147/TCRM.S526727
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Shu-Ping Jiang,1,* Bing-Qi Lin,1,* Xing-Qiang Zhou,2,* Min-Hua Li,1,* Zhen-Cheng Feng,1 Yue-Ying Qin,1 Shi-Qi Lin,1 Zi-Qing Zhou,1 Yang Peng,1 Lian Li1
1State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China; 2Department of Otorhinolaryngology Head and Neck Surgery, Huizhou Central People’s Hospital, Huizhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lian Li, Email [email protected] Yang Peng, Email [email protected]
Abstract: Respiratory inflammatory and infectious diseases continue to impose a substantial global health burden, compounded by persistent gaps in understanding their pathogenic mechanisms and limited therapeutic advancements. To address these challenges, this review systematically analyzed literature from PubMed, Web of Science, and Scopus databases (2005– 2025) to evaluate the evolution and applications of airway organoid models in respiratory disease research. Key findings include: (1) the convergence of traditional culture techniques with advanced methodologies — including 3D matrix embedding, bioprinting and organoids-on-chips technologies — has enabled unprecedented recapitulation of human airway architecture and multicellular interactions; (2) these novel models provide unique insights into disease pathogenesis, host-microbe dynamics, and drug response variability; (3) the inherent capacity to maintain native cellular diversity and disease-associated phenotypes positions airway organoids as crucial platforms for personalized medicine approaches. Collectively, these advances establish airway organoids as transformative tools that bridge conventional in vitro models and clinical reality. Looking ahead, coupling organs-on-chips platforms with microgravity culture and single-cell lineage tracing will catalyze fundamental breakthroughs in respiratory disease research.
Keywords: airway organoids, respiratory diseases, precision treatment, technological progress
Introduction
Respiratory diseases impose a staggering global health burden, exemplified by chronic obstructive pulmonary disease (COPD), asthma, and infectious diseases, which collectively rank as the third leading cause of global mortality. These conditions emerge through complex interactions between genetic susceptibility and environmental triggers such as chronic smoking, air pollution, and respiratory pathogen exposure. Despite progress in clinical management, fundamental gaps persist in mapping the spatiotemporal dynamics of disease progression, particularly the molecular regulation of cellular crosstalk, immune-microenvironment remodeling, and host-pathogen interplay.1–3 Current experimental models, limited by their inability to mirror the multidimensional complexity of human airway pathophysiology, inadequately address these challenges. This critical unmet need drives the demand for next-generation platforms capable of achieving two complementary objectives: (1) The dissection of disease mechanisms across tissue hierarchies, cellular, and molecular; (2) the establishment of patient-specific frameworks for therapeutic development.
Airway organoid technologies, through their unique capacity to preserve native tissue architecture while enabling controlled microenvironmental modulation, now offer a transformative solution to these translational barriers.4 Organoids, derived from primary tissues, embryonic stem cells (ESCs), or induced pluripotent stem cells (iPSCs), demonstrate self-renewal and self-organization properties, closely recapitulating in vivo counterparts and faithfully replicating physiological and pathological conditions.5 The pioneering cultivation technologies of organoids were initially introduced in 2009 by Hans Clevers and his team, who successfully isolated and cultured mouse intestinal stem cells, as a result the formation of crypt and villus structures characteristic of the small intestine.6 Since then, organoids have progressively showcased substantial potential for a wide range of applications, Airway organoids technology emerged in 2015, technology provide a novel platform for facilitating a deeper understanding of the pathogenesis of pulmonary diseases, the development of novel treatment modalities, and drug screening.7,8
Recent years have witnessed a transformative paradigm shift in respiratory research, with airway organoid models emerging as a rapidly evolving frontier fueled by synergistic breakthroughs in stem cell biology and 3D tissue engineering. This convergence of interdisciplinary innovations has effectively circumvented longstanding limitations of traditional model systems, thereby catalyzing a new era of mechanistic discovery and therapeutic development in pulmonary medicine.
This review synthesized evidence through a systematic search of PubMed, Web of Science, and Scopus databases (2005–2025) using controlled vocabulary: Airway organoids, Respiratory diseases, Precision treatment, Technological Progress. Our review elucidates key technologies and recent progress in construction, while systematically analyzing applications in disease modeling, host-microbe interactions, and drug screening. Looking ahead, we propose a developmental roadmap leveraging microgravity culture and single-cell lineage tracing to achieve fundamental breakthroughs in respiratory disease research.
The Emergence, Development and Cellular Sources of Airway Organoids
Cell cultures and animal models are widely utilized in the field of research on respiratory diseases, but they both possess inherent limitations.9 Traditional cell line cultures, typically conducted on two-dimensional (2D) surfaces, are commonly utilized in cell biology research and drug screening.10 However, these methods fail to adequately simulate the complex 3D environment in vivo, resulting in less reliable outcomes.11 As illustrated by Marta Kapałczyńska’s team, the bulk of cancer biology research leans heavily on 2D cell cultures, which possess constraints such as disrupted cell-extracellular matrix interactions and alterations in cell morphology, polarity, and division patterns, posing significant challenges in accurately mimicking the in vivo setting.12 While animal models have provided valuable insights into recapitulating key aspects of human airway disease pathophysiology, evolutionary divergence imposes fundamental biological constraints that limit their translational fidelity to human conditions. Moreover, the establishment and maintenance of animal models incur high costs, require longer experimental durations, and raise ethical concerns.9
As the cellular sources of airway organoids, stem cells were initially applied in the research to investigate fetal development processes, cellular assembly mechanisms, and the tumor formation.13 Recent research has deepened our understanding of airway adult stem cells(ASCs), which present new directions for the treatment of pulmonary diseases.14 ASCs are primarily derived from bronchoalveolar lavage fluid and surgically resected lung tissue.15,16 Importantly, nasal and nasopharyngeal epithelia also serve as alternative sources of ASCs. For example, the nasal epithelium harbors abundant basal cells capable of forming organoids in vitro, which have been widely used to model upper respiratory tract pathologies such as chronic sinusitis, allergic rhinitis, and viral infections.17 These stem cells possess self-renewal and multipotent differentiation capabilities, including basal stem cells, neuroendocrine stem cells, and bronchial-alveolar stem cells, which are distributed across different regions of the airways.18 In a specific nurturing environment, these stem cells undergo induced differentiation, they subsequently develop into tissues that exhibit structural and functional similarities to those of in vivo organs, thereby constituting the precursors to organoids.19 Additionally, airway organoids can be further categorized based on their anatomical origins into trachea ball organoids, bronchial organoids, and bronchoalveolar organoids, which have been widely applied in exploring the mechanisms underlying disease onset and progression.20
Progress of in vitro Functional Airway Models
In vitro functional airway models can be classified into four major categories based on their construction methods: (1) Air-liquid interface (ALI) cultures established through differentiated epithelial cell layers; (2) 3D organoids primarily embedded within matrix gels; (3) organoid models integrated with bioprinting technology; and (4) organoids-on-chips (OrgOCs) fabricated using organs-on-chips (OOCs) technologies (Figure 1). Each category demonstrates unique biological properties and plays indispensable roles across various research contexts in respiratory medicine, from mechanistic studies to translational applications.
ALI Culture Method
Serially passaged normal human bronchial epithelial cell monolayers were established on Transwell inserts via an ALI culture method.21 To mimic this unique physiological structure in an experimental setting, scientists have introduced the ALI culture technique. Compared to traditional submerged culture systems, ALI culture has demonstrated unique advantages in studying airway epithelial barrier functions.22
ALI primarily relies on a semi-permeable membrane to establish the interface between air and liquid, enabling airway epithelial cells (ECs) to proliferate, and differentiate. This model closely mimics the physiological environment of the airway in vivo in terms of both structure and function.23 (Figure 1A) Notably, it demonstrates functional electrophysiological characteristics of well-differentiated bronchial epithelial barriers.24 Figure 2A shows H&E staining and Immunofluorescence photos of ALI, these morphological characteristics can be harnessed to investigate normal biological processes, disease mechanisms, as well as to advance the development of novel therapeutic interventions.25
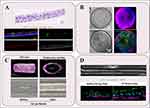 |
Figure 2 Morphological diagram of four different models in the study of pulmonary disease. (A) H&E staining (upper panel) and Immunofluorescence staining (lower panel) of ALI. Nuclear was label with DAPI (blue), goblet cells were label with MUC5AC (green), ciliated cells were label with Acetylated α-Tubulin (Ac-TUB) (red), and basal cells were marked by KRT5 (grey).Scale bars: 20 µm (upper panel), 50 µm (lower panel). (B) Bright field image and Immunofluorescence staining of 3D airway organoids in expansion medium (upper panel) and differentiation medium (lower panel). Nuclear was label with DAPI (blue), basal cells were marked by KRT5 (red, upper panel), ciliated cells were label with FOXJ1 (green, lower panel), and goblet cells were label with MUC5AC (red, lower panel). Scale bars:100 µm. (C) Confocal and brightfield images of a 3D bioprinting bronchioid model. Longitudinal and transverse slices of bronchioid printed by 3D bioprinting (upper panel), and brightfield images of bronchioids before and during air perfusion (lower panel). Scale bars: 100 µm (upper panel), 200 µm (lower panel). (Adapted with permission from Maurat E, Raasch K, Leipold AM, et al. A novel in vitro tubular model to recapitulate features of distal airways: the bronchioid. Eur Respir J. 2024;64(6):2400562.26) (D) Upper panel: Phase contrast images of bronchial epithelial cells and endothelium on an Airway Chip.Scale bars: channel width is 1 mm; bar, 2 mm. Lower panel: Representative immunofluorescence confocal microscopic images of vertical cross sections of healthy and CF Airway Chips, showing ciliated cells expressing β-Tubulin IV (green) and basal cells expressing CK5 (magenta) (Adapted from J Cyst Fibros, volume 21(4), Plebani R, Potla R, Soong M, et al. Modeling pulmonary cystic fibrosis in a human lung airway-on-a-chip. 606–615, Copyright 2022, with permission from Elsevier27). |
Weiling Xu et al advanced ALI models by designing an insert for a hippocampal analyzer capable of measuring the oxygen consumption rate and extracellular acidification rate of airway ECs.28 This innovation successfully assessed the oxidative metabolism and glycolysis of airway ECs, providing a novel approach to investigate the metabolic mechanisms underlying airway diseases and evaluate new therapeutic agents. Additionally, Mark E Becker et al developed an inverted ALI model that allows for real-time imaging to dynamically study the impact of mucociliary clearance in human airway epithelium cell infected with SARS-CoV-2.29
However, the ALI model is not devoid of limitations. Although culturing bronchial ECs at the ALI allows for the reconstruction of fully differentiated epithelia with functional cilia and facilitate accessibility to both the apical and basolateral sides, it lacks a 3D structural organization and cannot accurately replicate the gas flow dynamics associated with human breathing movement.30,31
3D Organoids (Matrigel Embedding Method)
3D organoids are commonly generated using Matrigel embedding methods, which allow cells to grow in 3D space and form organoid structures (Figure 1B). Alyssa J. Miller utilized 3D culture techniques with Matrigel to replicate the in vivo extracellular matrix environment.32 Matrigel serves as a physical scaffold that facilitates cellular self-organization into 3D airway-like tissue structures. When combined with optimized culture conditions, including a 5% CO2 atmosphere, maintenance at 37°C, and regular media supplementation with specific growth factors and nutrients, this system promotes the development of organoids displaying characteristic lung tissue architecture. The resulting structures recapitulate key pulmonary features, including alveolar formations and branching airway-like morphologies.15
Matrigel, the most widely used matrix in 3D culture, primarily composed of laminin, collagen IV, entactin, and heparan sulfate proteoglycans, which facilitates cell proliferation, differentiation, and self-organization, enabling the formation of 3D structures that closely resemble in vivo organs.33 In Matrigel culture, various growth factors and signal molecules can be added, such as Wnt signaling-related proteins (eg, R-spondin1, Wnt3A), fibroblast growth factors (eg, FGFs), and a range of small molecules (eg, TGF-beta/Smad inhibitors). These signal molecules possess the capacity to activate or inhibit intracellular signaling cascades, thereby enabling the differentiation of airway stem cells into diverse airway epithelial cell types, including ciliated cells and goblet cells. Consequently, they contribute to the formation of airway organoids that exhibit intricate structures and functional properties.
Within a 3D environment, ESCs can differentiate into airway ECs and other lung development-associated lineages, as demonstrated by Darrell N Kotton’s team, which induced iPSC differentiation into lung progenitor cell marker-expressing organoids using small molecules and enriched lung ECs through fluorescence reporter genes and cell surface marker sorting, ultimately constructing tracheal organoids.34 (Figure 2B) Additionally, Lian Li and colleagues constructed airway and alveolar organoids from ESCs by modulating signaling pathways and integrating CRISPR/Cas9 technology, providing a critical research framework for investigating the role of SRY-Box Transcription Factor 9 (SOX9) in human lung epithelial development.35
In terms of model advancements, increasing evidence suggests that 3D organoids offer significant advantages over traditional 2D cell methods and animal models.36,37 Traditional homogeneous epithelial organoids lack cellular diversity, but co-culture techniques, such as the metastatic model developed by David R. Jones’ team using lung adenocarcinoma patient-derived organoids and autologous peripheral blood mononuclear cells, preserve metastatic traits, enabling studies on tumor biology, drug efficacy, and immuno-stimulatory strategies, advancing lung adenocarcinoma treatment.38
In contrast to airway organoids derived from healthy epithelium, which prioritize physiological mimicry of mucociliary functions and barrier integrity, lung cancer organoids (LCOs) are predominantly established from tumor biopsies or circulating tumor cells, necessitating tailored niche factors (eg, R-spondin, Noggin) to sustain malignant clones.39 While airway models maintain structured differentiation (eg, basal-to-ciliated cell transitions), LCOs exhibit anarchic proliferation with genetic instability, mirroring tumor heterogeneity. Notably, co-culture innovations address LCOs’ limitations in immune-microenvironment recapitulation, bridging their utility in both basic tumor biology and precision oncology.40
However, embedding organoids in 3D Matrigel matrices possesses certain limitations. Firstly, the intricate composition of Matrigel may introduce batch-to-batch variability, potentially compromising the consistency of the culture. Secondly, the lack of dynamic controllability hinders the modification of culture conditions. Lastly, the substantial cost of Matrigel, coupled with its stringent storage demands, further contributes to the overall expenditure.
3D Bioprinting:Organoid-Like Model of Distal Airways
The 3D bio-printed bronchial model represents a cutting-edge technology for organoid construction.41 This process begins with the isolation of ESCs or the differentiated stem cells, which are then mixed with biomaterials such as collagen and sodium alginate to create bio-inks. Using 3D printing techniques, these cell-laden bio-inks are extruded or sprayed layer by layer according to bronchial 3D model data, allowing cells to proliferate and differentiate into bronchial structures that include lumens, wall layers, and matrix components. (Figure 1C) This approach effectively simulates physiological and pathological processes and provides a realistic in vitro platform for related disease research and therapeutic applications.42
Researchers have developed a so-called “bronchial model” by encapsulating human bronchial stem cells derived from clinical samples within tubular scaffolds made of alginate gel.26 (Figure 2C) Utilizing cell encapsulation technology, bronchial ECs are co-encapsulated with Matrigel in alginate-based tubular structures. Analysis of experimental data has shown that these bronchial organoids exhibit robust mucociliary and contractile functions, with advantages in recapitulating the characteristics and functions of distal airways. This model serves as a powerful tool for preclinical research, allowing for the assessment of clinical endpoints related to respiratory diseases. The 3D tissue model represents an advancement over existing technologies, aiming to overcome the limitations of 2D models and animal studies, while providing opportunities for drug development and the simulation of biological processes.43 Self-healing hydrogels have demonstrated significant potential in the field of 3D bioprinting.44 Building on this foundation, researchers have successfully developed a range of 3D tissue models encompassing both normal and diseased states, with the blood-air barrier models constructed via extrusion-based bioprinting techniques particularly drawing attention.45 To further enhance the realism of these models, researchers have ingeniously incorporated simulated respiratory motion, bringing these models functionally closer to actual physiological states, thereby laying a solid foundation for future biomedical research and clinical applications.26
While traditional bioprinting and assembly have advantages, complex tissue regeneration remains constrained.46 This technology faces two key shortcomings: limited achievable tube length, and the 3D printing of the final branches of the bronchial tree remains below the resolution limit of current bioprinting technologies. Achieving organ biomanufacturing and its clinical application necessitates collaboration across multiple disciplines. Currently, preclinical model structures have been developed and have been expanding to human-scale tissues.47 In this context, developing in vitro models with enhanced physiological fidelity to human systems represents a critical prerequisite for advancing this research field.
OrgOCs
The concept of OrgOCs was first introduced in 2019 in Science, which characterized this platform as an “advanced iteration” of conventional OOCs systems.48 Emerging from accelerated innovations in microphysiological engineering, OrgOCs capitalize on breakthroughs in OOCs technology to synergistically combine organoid self-organization principles with precise microenvironmental control. This hybrid approach achieves quasi-physiological tissue architectures through dynamic regulation of biochemical and biophysical cues, thereby orchestrating multipotent stem cell self-organization and guided organoid maturation.49 (Figure 1D)
In comparison to airway organoids derived from ASCs, OrgOCs can replicate the respiratory motion, thereby more accurately mimicking the functional characteristics of lung tissue found in vivo.27 (Figure 2D) Currently, OrgOCs have gained widespread application across diverse fields, including drug development, disease modeling, and precision medicine.50 Kambez H Benam et al demonstrated that an airway-on-a-chip device composed from COPD patient epithelial cells can reconstitute goblet cell hyperplasia, ciliary dysfunction, cytokine hypersecretion, infection-based exacerbation, and smoke-induced pathologies (ie, oxidative stress).51,52
However, the complexity and high cost of OrgOCs technology, along with issues such as inadequate model integrity and representativeness, challenging experimental manipulations and data analysis, and suboptimal long-term stability and reproducibility, pose limitations on its broader and more efficient application.
Applications of Airway Organoids
The applications of airway organoids in disease research encompass disease modeling, host-microbe interactions, and drug screening. In recent years, organoids have gained increasing attention in the study of respiratory diseases. For instance, COPD organoids exhibit distinct pathological features and uncover potential therapeutic targets;53 Cystic Fibrosis (CF) organoids successfully replicate CFTR defects, thereby contributing to advancements in therapy development;54 furthermore, lung cancer organoids mirror tumor heterogeneity, enabling the facilitation of targeted therapies, immunotherapies, and personalized medicine approaches.55
Application of Disease Models
The rapid development of organoid research is becoming increasingly pivotal in the field of respiratory disease studies. Organoids contribute to elucidating the cellular foundations of diseases such as COPD, CF, asthma, and airway tumors. Furthermore, by establishing coculture systems, they enable the analysis of intercellular interactions and allow for the tracking of disease dynamics.20 For instance, Louisa L. Y. Chan and colleagues established organoids from healthy and COPD patients, revealing disease characteristics and the effects of SARS-CoV-2 infection.53,56 In exploring the relationship between COPD and SARS-CoV-2, researchers constructed an ALI model to simulate the status of peripheral lung distal airway cells in COPD, observing the changes in expression and functionality of angiotensin-converting enzyme 2 and their influence on susceptibility to SARS-CoV-2 infection.57
ALI and airway organoids enable the study of CF and asthma by replicating disease-specific pathophysiology, providing insights into cellular dysfunction, immune responses, and therapeutic strategies. In the study of hereditary airway diseases like CF, the ALI culture method can be employed to cultivate patient-derived airway organoids to observe cellular differentiation abnormalities in the disease context. For instance, airway ECs from CF patients exhibit chloride channel dysfunction, and organoids cultured via ALI can mimic this pathological state for investigating its underlying mechanisms. Airway organoids can emulate CFTR protein dysfunction, offering valuable insights into strategies aimed at enhancing CFTR function and facilitating gene repair.54 Regarding asthma, airway organoids serve as models to replicate the pathophysiological processes of the disease, showcasing epithelial damage repair, alterations in mucus production, and inflammatory responses.58 Furthermore, airway organoids facilitate the investigation of immune responses and mechanisms underlying airway remodeling, thereby contributing to a deeper understanding of disease pathogenesis.59
In the realm of airway tumors, organoids assist in studying tumorigenesis mechanisms, identifying diagnostic biomarkers and prognostic assessment models, developing precision treatment strategies, and exploring novel therapeutic options.60 In addition, single-cell analyses can also be utilized to characterize tumor cell subpopulations.61
Host-Microbe Interaction
In the field of microbiome-host interactions, organoid technology is increasingly recognized as a versatile and powerful tool, with expanding applications that are becoming more comprehensive and sophisticated, particularly in viral research for investigating infection mechanisms, transmission pathways, and host immune responses. Yang Peng and colleagues developed ALI to simulate virus-host interactions, allowing for drug efficacy evaluation against SARS-CoV-2 Omicron, which offers therapeutic strategies for COVID-19 and sets a precedent for organoid use in studying other viral diseases.62 (Figure 2B) Jie Zhou and Guoyong Yuan ‘s research team successfully established a continuous culture of human rhinovirus type C using human airway organoids, elucidating the interaction mechanisms between the virus and its host.63 Additionally, Shanshan Zhao and colleagues established human adenovirus infection models using airway and alveolar organoids, elucidating the high pathogenicity mechanisms of human adenovirus type 55, highlighting the advantages of organoids in studying viral airway diseases by more accurately simulating virus replication and infection processes in human airways compared to species-limited animal models.64
Organoids also exhibit pertinent applications in mimicking bacterial infections within the respiratory tract. For instance, Pseudomonas aeruginosa is a significant pathogen in chronic lung infections, particularly in individuals with CF and COPD.65,66 Through the study of pneumonia-associated bacterial infections utilizing airway organoids, researchers can gain a deeper understanding of the colonization and dissemination patterns of bacteria within respiratory ECs, thereby identifying potential targets for the development of antibacterial therapies.20 Pseudomonas aeruginosa biofilms exhibit higher antibiotic tolerance and immune resistance than planktonic forms, complicating eradication and promoting chronic infections.67 There is an urgent need for novel therapeutic approaches to combat Pseudomonas aeruginosa, given its increasing resistance to antibiotics and its significant role in chronic infections. The team led by Philipp Grubwieser utilized 3D organoids to simulate the initial stages of Pseudomonas aeruginosa respiratory infections.68 The experimental results demonstrated that following epithelial barrier penetration by Pseudomonas aeruginosa, robust bacterial proliferation occurred within the epithelial compartment. This infectious challenge triggered a potent innate immune defense mechanism, characterized by significant upregulation of critical genes within the nitric oxide biosynthesis pathway, including inducible nitric oxide synthase (iNOS/NOS2) and associated reactive nitrogen species (RNS)-generating enzymes. The activation of inducible nitric oxide synthase in airway organoids exposed the bacteria to nitrosative stress, effectively inhibiting the proliferation of pathogens within the epithelium.6
However, the selective pressures and adaptive strategies that govern Pseudomonas aeruginosa growth dynamics and antibiotic resistance evolution within the human pulmonary niche remain poorly characterized. Future research employing human lung models holds promise for exploring the adaptive compromises faced by Pseudomonas aeruginosa during colonization of mucosal surfaces and its adaptation to antibiotic pressures. While these lung models mimic numerous aspects of the human airway, they do not fully capture the intricacies of in vivo human infections, particularly in terms of immune responses and interactions with another lung microbiota.
Drug Screening
Airway organoids increase the likelihood of successful clinical translation for drugs identified through organoid-based screening systems. By mimicking key aspects of the human airway, such as its architecture, cell types, and interactions, airway organoids provide a more physiologically relevant context for drug testing and personalized medicine.69 Researchers have the capacity to test a vast array of pharmaceutical compounds on airway organoids in order to pinpoint potential drugs that could enhance airway function, alleviate inflammation, or arrest disease progression. Organoids function as an outstanding platform for drug development, facilitating the screening and assessment of medications’ impacts on critical parameters, such as ion transport and mucus secretion.39 Additionally, they enable the elucidation of therapeutic efficacy and the investigation of the mechanisms underlying drug action, thereby advancing our understanding of how drugs interact with human airway tissues and guiding the rational design of more effective therapies.70
While organoid-based high-throughput drug screening offers patient-specific insights, batch-to-batch variability in Matrigel remains a critical bottleneck. Recent advance in synthetic hydrogels (eg, PeptiGels) and suspension cultures (eg, AggreWell™) can be adapted for the culture of human organoids.71 Coupled with microfluidic platforms that standardize drug delivery, these innovations may bridge the gap between preclinical models and clinical predictability.72
In personalized medicine, airway organoids can be customized and analyzed based on individual patient profiles, facilitating patient stratification and precision treatment. Genomic sequencing of these organoids enables the identification of disease subtypes and prognosis prediction, facilitating real-time treatment response monitoring, dynamic therapeutic evaluation, and timely adjustment of treatment strategies. This patient-specific approach offers crucial support and provides powerful tools for advancing research, treatment strategies, and the practice of precision medicine in CF and other respiratory diseases.73
In cancer therapy, tumor organoids derived from patient tumor tissues maintain the heterogeneity and individual characteristics of the tumors. By testing a range of anticancer drugs on these personalized tumor organoids, researchers can swiftly determine the most effective treatment regimen for each patient. Specifically, in lung cancer patients, tumor organoids can mirror the specific genetic mutations, cellular composition, and microenvironment features of the tumors.74 During drug screening, researchers can precisely select drug combinations that demonstrate high cytotoxicity against the patient’s tumor cells while minimizing side effects, thereby circumventing the “one-size-fits-all” paradigm of traditional therapies.75 This approach enhances treatment success rates and patient survival. Furthermore, by assessing changes in organoid cell viability, inflammatory factors, and mucus secretion, researchers can screen for potential drugs and simultaneously utilize these organoids to evaluate drug efficacy tailored to individual patients, ultimately achieving personalized medical care.76
Challenges and Prospects of Airway Organoids
Airway organoids are currently hampered by various limitations. In terms of culture techniques, their cultivation systems are complex and unstable, with differences in methods and reagents used across laboratories affecting the reproducibility of results and impeding the development of standardization. Regarding physiological relevance, airway organoids predominantly emphasize airway ECs and lack the representation of other crucial cell types, such as smooth muscle cells and fibroblasts, along with their interactions. This limitation makes it difficult to depict the comprehensive spectrum of complex respiratory diseases that involve multicellular interactions. In terms of data interpretation and application, the extensive datasets generated from techniques like single-cell sequencing pose substantial challenges in processing and interpretation.
Despite these challenges, airway organoids possess considerable potential to address existing medical dilemmas, offering promising prospects for the future. Serving as a high-throughput drug screening platform, they facilitate the identification of potential pharmaceuticals for diseases such as COPD and enable personalized drug susceptibility testing. Airway organoids can also validate the feasibility of gene editing technologies, such as CRISPR-Cas9, for the repair of genetic mutations, optimize gene delivery strategies, and support immune cell therapies. Furthermore, they provide an avenue for the investigation of drug mechanisms, aiding in the discovery of novel drug targets and furnishing a foundation for innovative drug design aimed at tackling respiratory diseases.
Looking ahead, through the integration of cutting-edge innovations such as OrgOCs, microgravity culture environments, and single-cell lineage tracing techniques, we are optimistic that research in the field of airway organoids will unveil unprecedented significant discoveries in the study of human respiratory diseases.
Acknowledgments
We acknowledge the First Affiliated Hospital of Guangzhou Medical University for providing representative clinical samples used to generate the schematic diagram in Figure 2A and B.
Funding
This work was supported by National Natural Science Foundation of China (82100024, 82301273), R&D Program of Guangzhou Laboratory (GZNL2023A02002), The grant of State Key Laboratory of Respiratory Disease (SKLRD-0P-202306), the Plan on enhancing scientific research in GMU (02-412-2302-2176XM), Noncommunicable Chronic Diseases-National Science and Technology Major Project (No. 2024ZD052400) and Guangzhou Science and Technology Project (No. 2023A04J0570).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Purev E, Bahmed K, Kosmider B. Alveolar organoids in lung disease modeling. Biomolecules. 2024;14(1):115. doi:10.3390/biom14010115
2. Liu T, Zhou C, Shao Y, et al. Construction and application of in vitro alveolar models based on 3D printing technology. Chin J Mech Eng Addit Manuf Front. 2022;1(2):100025. doi:10.1016/j.cjmeam.2022.100025
3. Calvert BA, Ryan Firth AL. Application of iPSC to modelling of respiratory diseases. Adv Exp Med Biol. 2020;1237:1–16. doi:10.1007/5584_2019_430
4. van der Vaart J, Clevers H. Airway organoids as models of human disease. J Intern Med. 2021;289(5):604–613. doi:10.1111/joim.13075
5. Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345(6194):1247125. doi:10.1126/science.1247125
6. Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. doi:10.1038/nature07935
7. Dye BR, Hill DR, Ferguson MAH, et al. In vitro generation of human pluripotent stem cell derived lung organoids. eLife. 2015;4:e05098. doi:10.7554/eLife.05098
8. Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020;21(10):571–584. doi:10.1038/s41580-020-0259-3
9. Shanks N, Greek R, Greek J. Are animal models predictive for humans? Philosophy Ethics Human Med. 2009;4(1):2. doi:10.1186/1747-5341-4-2
10. Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. ASSAY Drug Dev Technol. 2014;12(4):207. doi:10.1089/adt.2014.573
11. Tian L, Gao J, Garcia IM, Chen HJ, Castaldi A, Chen YW. Human pluripotent stem cell-derived lung organoids: potential applications in development and disease modeling. WIREs Developl Biol. 2021;10(6):e399. doi:10.1002/wdev.399
12. Kapałczyńska M, Kolenda T, Przybyła W, et al. 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch Med Sci AMS. 2018;14(4):910–919. doi:10.5114/aoms.2016.63743
13. Slack Jonathan MW. “stem cell”. Encyclopedia Britannica. 2025. Available from: https://www.britannica.com/science/stem-cell.
14. Snoeck HW. Modeling human lung development and disease using pluripotent stem cells. Dev Camb Engl. 2015;142(1):13–16. doi:10.1242/dev.115469
15. Sachs N, Papaspyropoulos A, Zomer-van Ommen DD, et al. Long-term expanding human airway organoids for disease modeling. EMBO J. 2019;38(4):e100300. doi:10.15252/embj.2018100300
16. Koga T, Soh J, Hamada A, et al. Clinical relevance of patient-derived organoid of surgically resected lung cancer as an In vitro model for biomarker and drug testing. JTO Clin Res Rep. 2023;4(9):100554. doi:10.1016/j.jtocrr.2023.100554
17. Liu J, Zhang Y, Yu Y. Establishment of nasal and olfactory epithelium organoids for unveiling mechanism of tissue regeneration and pathogenesis of nasal diseases. Cell Mol Life Sci. 2025;82(1):33. doi:10.1007/s00018-024-05557-w
18. Randell SH. Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(8):718–725. doi:10.1513/pats.200605-117SF
19. Huch M, Koo BK. Modeling mouse and human development using organoid cultures. Dev Camb Engl. 2015;142(18):3113–3125. doi:10.1242/dev.118570
20. Kühl L, Graichen P, von Daacke N, et al. Human lung organoids-a novel experimental and precision medicine approach. Cells. 2023;12(16):2067. doi:10.3390/cells12162067
21. Zavala DC, Rossi NP, Bedell GN. Bronchial brush biopsy. A valuable diagnostic technique in the presurgical evaluation of indeterminate lung densities. Ann Thorac Surg. 1972;13(6):519–528. doi:10.1016/s0003-4975(10)65168-5
22. Randell SH, Fulcher ML, O’Neal W, Olsen JC. Primary epithelial cell models for cystic fibrosis research. In: Amaral MD, Kunzelmann K editors. Cystic Fibrosis, Methods in Molecular Biology. Humana Press; 2011:285–310. doi:10.1007/978-1-61779-120-8_18.
23. Lin H, Li H, Cho HJ, et al. Air-liquid interface (ALI) culture of human bronchial epithelial cell monolayers as an in vitro model for airway drug transport studies. J Pharm Sci. 2007;96(2):341–350. doi:10.1002/jps.20803
24. Ootani A, Li X, Sangiorgi E, et al. Sustained in vitro intestinal epithelial culture within a wnt-dependent stem cell niche. Nat Med. 2009;15(6):701–706. doi:10.1038/nm.1951
25. Pezzulo AA, Starner TD, Scheetz TE, et al. The air-liquid interface and use of primary cell cultures are important to recapitulate the transcriptional profile of in vivo airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2011;300(1):L25–31. doi:10.1152/ajplung.00256.2010
26. Maurat E, Raasch K, Leipold AM, et al. A novel in vitro tubular model to recapitulate features of distal airways: the bronchioid. Eur Respir J. 2024;64(6):2400562. doi:10.1183/13993003.00562-2024
27. Plebani R, Potla R, Soong M, et al. Modeling pulmonary cystic fibrosis in a human lung airway-on-a-chip. J Cyst Fibros. 2022;21(4):606–615. doi:10.1016/j.jcf.2021.10.004
28. Xu W, Janocha AJ, Leahy RA, et al. A novel method for pulmonary research: assessment of bioenergetic function at the air-liquid interface. Redox Biol. 2014;2:513–519. doi:10.1016/j.redox.2014.01.004
29. Becker ME, Martin-Sancho L, Simons LM, et al. Live imaging of airway epithelium reveals that mucociliary clearance modulates SARS-CoV-2 spread. Nat Commun. 2024;15(1):9480. doi:10.1038/s41467-024-53791-4
30. Jorissen M, Van der schueren B, Van den Berghe H, Cassiman JJ. Contribution of in vitro culture methods for respiratory epithelial cells to the study of the physiology of the respiratory tract. Eur Respir J. 1991;4(2):210–217. doi:10.1183/09031936.93.04020210
31. Adler KB, Cheng PW, Kim KC. Characterization of Guinea pig tracheal epithelial cells maintained in biphasic organotypic culture: cellular composition and biochemical analysis of released glycoconjugates. Am J Respir Cell Mol Biol. 1990;2(2):145–154. doi:10.1165/ajrcmb/2.2.145
32. Miller AJ, Dye BR, Ferrer-Torres D, et al. Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc. 2019;14(2):518–540. doi:10.1038/s41596-018-0104-8
33. Aisenbrey EA, Murphy WL. Synthetic alternatives to Matrigel. Nat Rev Mater. 2020;5(7):539–551. doi:10.1038/s41578-020-0199-8
34. McCauley KB, Hawkins F, Serra M, Thomas DC, Jacob A, Kotton DN. Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of wnt signaling. Cell Stem Cell. 2017;20(6):844–857.e6. doi:10.1016/j.stem.2017.03.001
35. Li L, Feng J, Zhao S, Rong Z, Lin Y. SOX9 inactivation affects the proliferation and differentiation of human lung organoids. Stem Cell Res Ther. 2021;12(1):343. doi:10.1186/s13287-021-02422-6
36. Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7(3):211–224. doi:10.1038/nrm1858
37. Yamada KM, Cukierman E. Modeling tissue morphogenesis and cancer in 3D. Cell. 2007;130(4):601–610. doi:10.1016/j.cell.2007.08.006
38. Liu Y, Lankadasari M, Rosiene J, et al. Modeling lung adenocarcinoma metastases using patient-derived organoids. Cell Rep Med. 2024;5(10):101777. doi:10.1016/j.xcrm.2024.101777
39. Kim M, Mun H, Sung CO, et al. Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat Commun. 2019;10(1):3991. doi:10.1038/s41467-019-11867-6
40. Dijkstra KK, Cattaneo CM, Weeber F, et al. Generation of tumor-reactive T cells by Co-culture of peripheral blood lymphocytes and tumor organoids. Cell. 2018;174(6):1586–1598.e12. doi:10.1016/j.cell.2018.07.009
41. Dabaghi M, Carpio MB, Moran-Mirabal JM, Hirota JA. 3D (bio)printing of lungs: past, present, and future. Eur Respir J. 2023;61(1):2200417. doi:10.1183/13993003.00417-2022
42. De Santis MM, Bölükbas DA, Lindstedt S, Wagner DE. How to build a lung: latest advances and emerging themes in lung bioengineering. Eur Respir J. 2018;52(1):1601355. doi:10.1183/13993003.01355-2016
43. Konar D, Devarasetty M, Yildiz DV, Atala A, Murphy SV. Lung-on-a-chip technologies for disease modeling and drug development. Biomed Eng Comput Biol. 2016;7(Suppl 1):17–27. doi:10.4137/BECB.S34252
44. Cohen DL, Malone E, Lipson H, Bonassar LJ. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006;12(5):1325–1335. doi:10.1089/ten.2006.12.1325
45. Horváth L, Umehara Y, Jud C, Blank F, Petri-Fink A, Rothen-Rutishauser B. Engineering an in vitro air-blood barrier by 3D bioprinting. Sci Rep. 2015;5(1):7974. doi:10.1038/srep07974
46. Moroni L, Boland T, Burdick JA, et al. Biofabrication: a guide to technology and terminology. Trends Biotechnol. 2018;36(4):384–402. doi:10.1016/j.tibtech.2017.10.015
47. Moroni L, Burdick JA, Highley C, et al. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat Rev Mater. 2018;3(5):21–37. doi:10.1038/s41578-018-0006-y
48. Park SE, Georgescu A, Huh D. Organoids-on-a-chip. Sci. 2019;364(6444):960–965. doi:10.1126/science.aaw7894
49. Wang Y, Qin J. Advances in human organoids-on-chips in biomedical research. Life Med. 2023;2(1):lnad007. doi:10.1093/lifemedi/lnad007
50. Zhao Y, Landau S, Okhovatian S, et al. Integrating organoids and organ-on-a-chip devices. Nat Rev Bioeng. 2024;2(7):588–608. doi:10.1038/s44222-024-00207-z
51. Benam KH, Villenave R, Lucchesi C, et al. Small airway-on-a-chip enables analysis of human lung inflammation and drug responses in vitro. Nat Methods. 2016;13(2):151–157. doi:10.1038/nmeth.3697
52. Benam KH, Novak R, Nawroth J, et al. Matched-comparative modeling of normal and diseased human airway responses using a microengineered breathing lung chip. Cell Syst. 2016;3(5):456–466.e4. doi:10.1016/j.cels.2016.10.003
53. Chan LLY, Anderson DE, Cheng HS, et al. The establishment of COPD organoids to study host-pathogen interaction reveals enhanced viral fitness of SARS-CoV-2 in bronchi. Nat Commun. 2022;13(1):7635. doi:10.1038/s41467-022-35253-x
54. Bulcaen M, Kortleven P, Liu RB, et al. Prime editing functionally corrects cystic fibrosis-causing CFTR mutations in human organoids and airway epithelial cells. Cell Rep Med. 2024;5(5):101544. doi:10.1016/j.xcrm.2024.101544
55. Zhang Y, Hu Q, Pei Y, et al. A patient-specific lung cancer assembloid model with heterogeneous tumor microenvironments. Nat Commun. 2024;15(1):3382. doi:10.1038/s41467-024-47737-z
56. Stoleriu MG, Ansari M, Strunz M, et al. COPD basal cells are primed towards secretory to multiciliated cell imbalance driving increased resilience to environmental stressors. Thorax. 2024;79(6):524–537. doi:10.1136/thorax-2022-219958
57. Peng Y, Wang ZN, Chen SY, et al. Angiotensin-converting enzyme 2 in peripheral lung club cells modulates the susceptibility to SARS-CoV-2 in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2022;322(5):L712–L721. doi:10.1152/ajplung.00305.2021
58. Russell RJ, Boulet LP, Brightling CE, et al. The airway epithelium: an orchestrator of inflammation, a key structural barrier and a therapeutic target in severe asthma. Eur Respir J. 2024;63(4):2301397. doi:10.1183/13993003.01397-2023
59. Zhou Y, Duan Q, Yang D. In vitro human cell-based models to study airway remodeling in asthma. Biomed Pharmacother. 2023;159:114218. doi:10.1016/j.biopha.2023.114218
60. Drost J, Karthaus WR, Gao D, et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat Protoc. 2016;11(2):347–358. doi:10.1038/nprot.2016.006
61. Wu AR, Neff NF, Kalisky T, et al. Quantitative assessment of single-cell RNA-sequencing methods. Nat Methods. 2014;11(1):41–46. doi:10.1038/nmeth.2694
62. Peng Y, ying CS, ni WZ, et al. Dicoumarol is an effective post-exposure prophylactic for SARS-CoV-2 Omicron infection in human airway epithelium. Sig Transduct Target Ther. 2023;8(1):242. doi:10.1038/s41392-023-01511-7
63. Li C, Yu Y, Wan Z, et al. Human respiratory organoids sustained reproducible propagation of human rhinovirus C and elucidation of virus-host interaction. Nat Commun. 2024;15(1):10772. doi:10.1038/s41467-024-55076-2
64. Zhao S, Wu X, Tan Z, et al. Generation of human embryonic stem cell-derived lung organoids for modeling infection and replication differences between human adenovirus types 3 and 55 and evaluating potential antiviral drugs. J Virol. 2023;97(5):e00209–23. doi:10.1128/jvi.00209-23
65. Rossi E, La Rosa R, Bartell JA, et al. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nat Rev Microbiol. 2021;19(5):331–342. doi:10.1038/s41579-020-00477-5
66. Cendra MDM, Torrents E. Pseudomonas aeruginosa biofilms and their partners in crime. Biotechnol Adv. 2021;49(2):107734. doi:10.1016/j.biotechadv.2021.107734
67. Lebeaux D, Ghigo JM, Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev MMBR. 2014;78(3):510–543. doi:10.1128/MMBR.00013-14
68. Grubwieser P, Böck N, Soto EK, et al. Human airway epithelium controls pseudomonas aeruginosa infection via inducible nitric oxide synthase. Front Immunol. 2024;15:1508727. doi:10.3389/fimmu.2024.1508727
69. Chen JH, Chu XP, Zhang JT, et al. Genomic characteristics and drug screening among organoids derived from non-small cell lung cancer patients. Thorac Cancer. 2020;11(8):2279–2290. doi:10.1111/1759-7714.13542
70. Yang W, Li Y, Shi F, Liu H. Human lung organoid: models for respiratory biology and diseases. Dev Biol. 2023;494:26–34. doi:10.1016/j.ydbio.2022.12.001
71. Gjorevski N, Sachs N, Manfrin A, et al. Designer matrices for intestinal stem cell and organoid culture. Nature. 2016;539(7630):560–564. doi:10.1038/nature20168
72. Razian G, Yu Y, Ungrin M. Production of large numbers of size-controlled tumor spheroids using microwell plates. J Vis Exp. 2013;(81):e50665. doi:10.3791/50665
73. Berkers G, van Mourik P, Vonk AM, et al. Rectal organoids enable personalized treatment of cystic fibrosis. Cell Rep. 2019;26(7):1701–1708.e3. doi:10.1016/j.celrep.2019.01.068
74. Tiriac H, Belleau P, Engle DD, et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov. 2018;8(9):1112–1129. doi:10.1158/2159-8290.CD-18-0349
75. Yao Y, Xu X, Yang L, et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell. 2020;26(1):17–26.e6. doi:10.1016/j.stem.2019.10.010
76. Singh S, Dutta J, Ray A, Karmakar A, Mabalirajan U. Airway epithelium: a neglected but crucial cell type in asthma pathobiology. Diagn. 2023;13(4):808. doi:10.3390/diagnostics13040808
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


