Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
Alterations in White Matter Structure in Sleep-Related Epilepsies: A TBSS-Based Diffusion Tensor Imaging Study
Authors Wang Z, Liu W, Feng X, Wang Q, Ning X, Xing W
Received 6 December 2024
Accepted for publication 15 May 2025
Published 30 May 2025 Volume 2025:21 Pages 1109—1118
DOI https://doi.org/10.2147/NDT.S508253
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Taro Kishi
Ziyun Wang,1,2,* Weiping Liu,3,* Ximei Feng,4 Qian Wang,1,2 Xun Ning,1,2 Wu Xing1,2
1Department of Radiology, Xiangya Hospital, Central South University, Changsha, People’s Republic of China; 2National Clinical Research Centre for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, People’s Republic of China; 3Department of Neurology, Xiangya Hospital, Central South University, Changsha, People’s Republic of China; 4Department of Radiology, Changsha Central Hospital, University of South China, Changsha, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wu Xing, Department of Radiology, Xiangya Hospital, Central South University, No. 87 Xiangya Road, Changsha, Hunan, 410008, People’s Republic of China, Email [email protected]
Purpose: The study aims to further investigate the relationship between the Sleep-related epilepsies (SRE) and alterations in the White Matter (WM) microstructure, as well as to explore the clinical value of diffusion tensor imaging (DTI) in patients with SRE.
Methods: DTI data were acquired for 52 patients with SRE and 74 Healthy Controls (HC), all of whom were matched for sex and age. The DTI indicators, including Fractional Anisotropy (FA), Mean Diffusivity (MD), Axial Diffusivity (AD) and Radial Diffusivity (RD), were compared between the two groups using Tract-Based Spatial Statistics (TBSS) to analyze alterations in WM microstructure. Altered WM was correlated with the age of onset and disease duration of SRE.
Results: Compared to the HC, FA decreased in the genu of the corpus callosum (GCC) and the left anterior corona radiata (ACR_L) in SRE. Conversely, the RD of the bilateral superior corona radiata (SCR), the anterior corona radiata (ACR), the GCC, and the body of the corpus callosum (BCC) increased. Furthermore, a negative correlation was identified between the increased RD values and age of onset in SRE.
Conclusion: The FA and RD values derived from DTI serve as neuroimaging markers to evaluate WM damage in SRE. These findings indicate that alterations in WM microstructure within the bilateral frontal lobes and corpus callosum may contribute to a potential pathophysiological mechanism underlying seizures in SRE. Furthermore, myelin damage appears to be more severe in early-onset SRE patients, highlighting the necessity for clinical vigilance regarding WM microstructural changes in young SRE patients.
Keywords: sleep-related epilepsies, diffusion tensor imaging, tract-based spatial statistics, white matter microstructure
Introduction
Epilepsy is a neurological disorder characterized by dysfunction of the central nervous system caused by recurrent, seizure-like abnormal discharge of neurons in the brain. There is a temporal imbalance in the episodes, which are mostly associated with circadian rhythms.1,2 Due to the close association between sleep and seizures, the American Academy of Sleep Medicine introduced the concept of Sleep-related epilepsies (SRE) in 2007. SRE refers to epilepsy syndromes in which seizures occur exclusively or predominantly during sleep (over 70% of the time).3 The causes of SRE include central nervous system (CNS) infections, focal cortical dysplasia (FCD), low-grade malignant tumors, soft brain foci, and cryptogenic factors.3,4 Among these, the cryptogenic SRE syndrome is characterized by an unknown clinical pathogenesis, with no lesions identifiable via Magnetic Resonance Imaging (MRI). This particular type of SRE poses significant challenges for clinical diagnosis and treatment. In addition, even in “pure sleep-related epilepsies”, there is a risk of seizures during waking hours, which will have a great impact on the patients’ life. Therefore, research on SRE is particularly important.
The pathogenesis of SRE remains unclear. In 1888, Hughlings-Jackson proposed that epilepsy is primarily a disease of the cerebral cortex. However, with the rapid development of functional imaging technology, the study of epilepsy diseases is no longer limited to the location and morphology of gray matter lesions, and the role of white matter structure and functional alterations in the generation and propagation of epilepsy is gradually being emphasized. White matter fiber tracts, as connecting channels between gray matter regions of the brain, carry complex information transmission to maintain normal brain function. In previous white matter studies on epilepsy, a Mendelian randomization study suggested a bidirectional causal relationship between epilepsy and microstructural changes in White Matter (WM), these changes have been observed in generalized epilepsy, focal epilepsy, and epilepsy of unknown origin,5,6 which may be accompanied by alterations in myelination, axonal integrity, and cellular composition.7,8 SRE predominantly manifests as a type of focal epilepsy,9 studies have suggested that SRE may be related to the enhancement of thalamocortical synchronization during sleep-wake transitions, facilitating the spread of epileptiform discharges and thereby triggering seizures.10–12 However, existing research indicates that SRE is associated with reduced functional connectivity in the bifrontal WM and microstructural damage in the thalamus-lesion WM.13,14 This suggests that damage to white matter structure exists in patients with different epilepsy syndromes, but that this alteration is variable across epilepsy syndromes and that the results are not consistent for study of the same type of epilepsy. As for SRE, to date, studies on white matter alterations are limited and have yielded inconsistent results. Based on the results of previous white matter-related studies in epilepsy, it is reasonable to hypothesize that evaluation of white matter microstructure in SRE patients with negative conventional MRI might be useful in searching for imaging markers of disease onset and progression in patients. Diffusion Tensor Imaging (DTI) is currently the only MRI technique that can noninvasively observe the morphology and microstructure of white matter fiber bundles in the living human brain, which can not only track the shape of fiber bundles, but also depict the details of brain white matter structure, quantitatively evaluate the microdiffusion of brain white matter fiber bundles, and realize the fine imaging of nerve fibers.15
Based on these, our study intends to use Tract-Based Spatial Statistics (TBSS), a fiber bundle-based spatial statistical analysis method, to investigate the differences in cerebral white matter regions between the SRE group and the Healthy Control (HC) group, and to statistically analyze the DTI parameters of the different white matter regions, in an attempt to search for the imaging markers of epileptic seizures in patients with SRE, and to provide more favorable imaging evidence for the elucidation of the pathogenesis of SRE.
Materials and Methods
Study Design
This study is a prospective, observational cohort study. Using convenience sampling, which is a non-probability sampling method, we prospectively collected data from 52 SRE patients diagnosed by a neurologist at Xiangya Hospital of Central South University. 74 HC were selected from the people who underwent physical examination at the same time in the physical examination center of our hospital. The sample size was determined based on findings from a previous study.16 The observed FA valuewas determined using a two-sample t-test, with the previously observed mean FA was 0.389 in the epilepsy group and 0.397 in the HC group, respectively, SD was assumed as 0.01, the number of subjects needed in each group to achieve 80% statistical power for a 5% significance level (two-sided test) was 14 subjects per group, considering a dropout ratio of about 20%. Ultimately, our sample sizes were 52 for the SRE group and 74 for the HC group, which exceed the calculated requirements. Written informed consent was obtained from all subjects prior to participation, for the participants younger than 18 years of age, written consent was also obtained from the parents or legal guardian, and the study received approval from the Medical Ethics Review Committee of Xiangya Hospital of Central South University.
Participant Selection and Clinical Data Collection
SRE patients were included in the research upon fulfilment of all of the following criteria, based on the diagnostic criteria of the International League Against Epilepsy (ILAE) in 201717 and Standard procedures for the diagnostic pathway of sleep-related epilepsies.18 The inclusion criteria were as follows: (1) Meet the 2017 ILAE epilepsy diagnostic criteria and procedures of SRE in 2020; (2) More than 75% of the seizures occurred during sleep, based on the medical history; (3) Without significant lesions in routine brain sequences of MRI; (4) Right-handed; (5) Aged between 16 and 60 years old; (6) had no physical diseases or history of psychiatric or neurological diseases leading to epilepsy. The exclusion criteria were as follows: (1) consumption of alcohol, excessive coffee intake, and the use of anti-epileptic drugs (AEDs) that may affect sleep in the past two weeks. (2) head motion exceeding ±2 mm or ± 2°. (3) MRI examination not including the full brain. For all the participants, we collected fundamental clinical information, which included age, gender, education level, age of seizure onset, duration of the disease, family history of epilepsy, past medical history (including birth history, history of febrile convulsions, encephalitis, surgical history, etc). Data were gathered from patients’ epilepsy diaries as well as comprehensive descriptions provided by the patients and their families to ensure enrollment accuracy of SRE.
MRI Data Acquisition
All subjects underwent the scanning on the Siemens Prisma 3T magnetic resonance scanner with the standard 32 channel head coil. The subjects took a supine position, staying relaxed and calm. DTI parameters of scanning sequence were as follows: TR=5400ms; TE=72ms; FOV=220×220mm; Slice thickness=1.6mm; The scanning time was 9 minutes and 18 seconds. 3D-T1WI scanning parameters were as follows: TR=2110ms; TE=3.18ms; FOV=233×233mm; Slice thickness=0.73mm; The scanning time was 4 minutes and 38 seconds.
MRI Data Processing
After DTI data were collected and saved, the specific processing steps were as follows:(1) first, we used dcm2nii toolkit to convert DICOM to NIFTI format, deleted the data with artifacts, unqualified gradient files, and unqualified b-value files; (2) original data pre-processing: head motion eddy current correction, gradient direction correction, and extracting b0, b1000 data to obtain brain tissue mask were performed mainly in FSL (http://www.fmrib.ox.ac.uk/fsl/); (3) all FA images were registered to the FMRIB58_FA template in MNI space using nonlinear method; (4) created an average FA image skeleton based on the data of this study, and projected the FA data of all subjects onto the average FA skeleton to obtain the FA skeleton of each subject; (5) MD, AD and RD data were calculated based on the average FA skeleton.
TBSS Analysis
Firstly, WM-DTI metrics (FA, MD, AD, RD) in the white matter skeleton were extracted for each participant. Secondly, in the FMRIB software library (http://www.fmrib.ox.ac.uk/fsl/), two-sample t-tests were then performed to compare these indexes between the SRE and HC groups, with age and sex included as covariates in the analysis. Subsequently, voxel-by-voxel statistics were conducted in TBSS using a permutation-based inference tool, ‘randomize’, which is part of FSL, to perform nonparametric statistical thresholding. In this study, voxel group comparisons were executed between the SRE and HC groups using a nonparametric two-sample t-test while controlling for the effects of age and sex. The mean FA skeleton served as a mask, thresholded at a mean FA value of 0.2. The threshold of significant between-group differences was established at P<0.05 (multiple comparisons corrected for familywise error rate (FWE)) using the Threshold-Free Cluster Enhancement (TFCE) option in the ‘Randomized’ permutation test tool in FSL. Then, between-group comparisons were conducted for MD, AD, and RD images.
Statistical Analysis
Statistical analyses were conducted by using SPSS (version 26.0, IBM SPSS) to compare the general data between the SRE group and the normal control group, with a significance level set at P < 0.05. The Shapiro–Wilk test was employed to assess the normality of continuous variables. An independent samples t-test was utilized for age comparisons, while the chi-square test was applied to examine differences in gender and education level. Additionally, a linear regression model was employed to analyze the correlation between DTI parameters and age of onset, treating age and gender as covariates, a significant correlation was defined as P < 0.05.
Results
Demographic and Clinical Characteristics
This study was divided into two groups, including SRE group and HC group. Among them, TBSS analysis included 52 patients (29 males and 23 females) with SRE, mean age 30.02 years, SD ± 13.58, range 16-59; 74 healthy controls (35 males and 39 females), mean age 31.95 years, SD ± 9.76, range 18-59. Mean duration of epilepsy 8.56 years, SD ± 7.86; Mean age of seizure onset 22.84 years, SD ± 11.65. See Table 1 for demographic information and clinical data of the two groups after statistical analysis.
 |
Table 1 Demographic and Clinical Characteristics of SRE Group and HC Group |
TBSS and Correlated Analysis Results
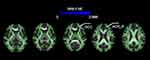
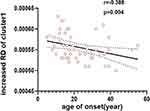
Compared with the HC group, the FA values of the SRE group in the genu of the corpus callosum (GCC) and the left anterior corona radiata (ACR_L) decreased (p=0.04, TFCE correction, cluster-related statistical information see Table 2 and Figure 1, Figure 2). The RD values of the SRE group increased mainly in the genu of the corpus callosum (GCC), body of the corpus callosum (BCC), bilateral anterior corona radiata (ACR), and superior corona radiata (SCR) (p=0.023/0.05, TFCE correction, cluster-related statistical information see Table 3 and Figure 3, Figure 4). In addition, after TFCE correction, no significant difference was found in MD and AD values of SRE group. The age of seizure onset was negatively correlated with the RD of the ACR_L (r= −0.388, p=0.004, correlated analysis information see Figure 5). However, the correlation was not observed with the other DTI parameters.
 |
Table 2 Brain Areas with Decreased FA in SRE Group Compared with HC Group |
 |
Table 3 Brain Areas with Increased RD in SRE Group Compared with HC Group |
 |
Figure 2 The violin plot shows mean FA of cluster 1(cluster 1 refers to GCC and ACR _ L) with statistical significance between the SRE group and HC group (P<0.05, FWE corrected for multiple comparisons); Table 2 supports detailed information. Abbreviations: SRE, Sleep-related epilepsies; HC, Healthy control; GCC, Genu of the corpus callosum; ACR, Anterior corona radiata; BCC, Body of the corpus callosum; SCR, Superior corona radiata. |
 |
Figure 4 The violin plot shows mean RD of two clusters (cluster1: ACR, BCC, SCR; cluster2: BCC) with statistical significance between the SRE group and HC group (P<0.05, FWE corrected for multiple comparisons); Table 3 supports detailed information. |
Discussion
In this study, we examined the alteration of WM structure in SRE patients by using TBSS based on fiber bundle tracing. The key findings were as follows: (i) compared with the control group, the FA values of SRE patients were significantly decreased mainly in the GCC and theACR_L; (ii) RD increased, corresponding to the brain regions including the bilateral SCR, the ACR, the GCC and the BCC; no significant difference was detected in MD and AD values of the SRE group; (iii) the age of seizure onset was negatively correlated with the increased RD.
In previous studies, researchers have taken pains to describe the changes of WM microstructure of various epilepsy syndromes, in order to answer questions related to cognition, behavior and epilepsy prognosis of clinical epilepsy patients.19,20 In fact, most patients with epilepsy show decreased FA values and increased MD values in different WM regions, but this change varies across in different epilepsy syndromes, indicating that WM structure damage is widespread in patients with different epilepsy syndromes, but there is also disease heterogeneity. Our study showed decreased FA values and increased RD values in SRE patients. These findings were consistent with previous studies. However, no significant changes were found in AD and MD across brain regions.
Decreased FA in SRE
For patients with SRE, the results demonstrated a decreased FA in the GCC and the ACR_L, indicating a disruption in the integrity of WM nerve fibers. An increase in FA suggests that nerve fibers are more consistently oriented, which indicates a more intact and compact structure, thereby enhancing the efficient transmission of nerve signals. High FA values are typically associated with the health status of nerve fiber bundles.7 It has been established that thalamocortical circuits serve as central regulators of sleep-wake activity21 and are responsible for driving physiological sleep transients that play a crucial role in the development of SRE.22 A consistent TBSSstudy suggests that reduced FA values in the thalamic-lesion region pathway may elucidate the underlying pathophysiology of SRE in patients with type II FCD.13 Additionally, multishell diffusion magnetic resonance fiber bundle imaging revealed reduced FA values in the right frontal lobe of a patient with sleep-related hypermotor epilepsy, and these alterations were consistent with neurological localization, serving as a potential lesion for seizure disorders in this patient.23 The corona radiata refers to the radial distribution of fiber bundles between the inner capsule and the cerebral cortex, in which the anterior and upper corona radiata mainly project information from other brain regions to the prefrontal cortex. Therefore, the damage to the anterior and superior radiating crowns means that the prefrontal cortex’s ability to receive information from other brain regions is weakened.In a study of TBSS in patients with Parkinson’s disease (PD),24 it was found that WM damage to the ACR and corpus callosum may lead to the disconnection of the cortical hypothalamic-basal nucleus-prefrontal circuit, resulting in difficulties in information transmission and cognitive impairment. Therefore, We speculate that seizures in patients with SRE may result from a disconnection in thalamo-prefrontal neural signaling that disrupts sleep, subsequently inducing seizures. The disconnection may involve the corpus callosum. The corpus callosum, located in the center of the brain, is the largest connecting fiber bundle in the brain, and is also an important structural basis for connecting the bilateral frontal, parietal, temporal and occipital lobe.25 Previous studies have shown that the microstructure of the WM of the corpus callosum in epileptic patients is widespread damaged, suggesting that epileptic activity is not confined to a certain area of the brain or one side of the cerebral hemisphere, but can be spread through extensive WM fiber connections.26,27 Clinically, corpus callosotomy uses this mechanism to alleviate seizures in refractory epilepsy. The results of our study show that the injuries of the corpus callosum in SRE patients are mainly concentrated in the genu and the anterior body regions, which are the main connecting fibers connecting the bilateral frontal lobes, suggesting that the epileptiform discharges of epilepsy in SRE patients may be more spread in the bilateral frontal lobe areas through the anterior part of the corpus callosum. These findings reinforce the understanding that bilateral frontal WM changes in SRE are significant pathophysiological mechanisms for epileptogenesis.
Increased RD in SRE
In addition, the results of this study show that the patients with SRE have increased RD in bilateral frontal lobe and the corpus callosum limitedly. Increased RD values represent loss of WM myelin and axonal damage. Interestingly, only RD was found in this study, whitch may be due to the fact that, compared to MD and AD, RD is sensitive to pathological changes in myelin.28 Compared with other common epilepsy syndromes, such as temporal lobe epilepsy, which exhibits bilateral WM changes in extensive brain regions,26,29 the range of WM damage in the SRE patients in our study was more limited. The reason may be related to the low frequency of SRE itself, effective drug control and mostly focal epilepsy. A resting-state fMRI study indicated that patients with newly diagnosed focal epilepsy exhibited some WM dysfunction which was reversed following pharmacological treatment.30 Therefore, we thought that the limited bilateral prefrontal regions may be caused by drugs. This finding suggests the drug’s control effect is excellent in this benign form of focal epilepsy, and underscores the importance of pharmacological management in treating patients with SRE. MD and AD values of the brain regions of these patients have not changed significantly. In general, the change of MD value does not directly reflect the integrity of WM fiber bundles. The increase in MD value more represents the decrease of local cell density, increase of membrane permeability and expansion of extracellular space caused by cell lysis and death.31 The increase or decrease of AD value reflects more acute and chronic injury of axon. There is no significant change in MD and AD values in this study. The reason may be that SRE patients have less seizure frequency and mild symptoms. When the WM fibers are damaged by seizures, the glycoprotein contained in the mature outer myelin sheath alleviates the neuroexcitotoxicity caused by abnormal discharge to a certain extent32 and plays a certain protective role in the axons of the inner layer and even the entire nerve fiber, which is manifested as an increase in RD value, while AD value and MD value do not change significantly. Therefore, the changes in DTI parameters in the local brain area of SRE patients (mainly manifested by the decreased FA and increased RD) may reflect the changes of WM microstructure in the brain area and the damage of myelin sheath, but are not significantly involved in cell disintegration, necrosis and axonal injury.
Increased RD Associated with an Earlier Age of Onset in SRE
Elevated RD values are negatively correlated with the age of onset in patients with SRE. In healthy populations, there is an upward trend in RD values with increasing age.33 However, in patients with epilepsy, WM impairment is associated with the age at seizure onset, being more severe when seizures occur at a younger age.5 Furthermore, previous studies have indicated that in the early-onset epilepsy group, the timing of seizures is the primary cause of WM abnormalities, whereas in late-onset epilepsy, it plays a secondary role in inducing alterations in WM.34 Consistent with these findings, myelin damage in the frontal lobe and corpus callosum of SRE patients is more pronounced at a younger age. As previously mentioned, medication control is effective in patients with SRE; therefore, it is crucial to provide sufficient clinical attention to young patients with SRE through early diagnosis and timely treatment. On the one hand, for SRE patients, both AD and MD remain unchanged and are not specific to pathological changes in axons or myelin sheaths, complicating the interpretation of results, on the other hand, compared to FA values, we observed more extensive WM damage in RD, suggesting that RD may serve as the most sensitive biomarker for detecting early WM microstructural changes in SRE; this highlights its potential significance value for early clinical diagnosis.
Currently, the exploration of the pathological mechanisms underlying SRE is still in its early stages. Research concerning the onset, progression, and the interplay between SRE and sleep remains incomplete. Our study encountered several limitations, including a relatively small patient sample, reliance on a single analytical method, and the constraints of a single-center study. Furthermore, patients’ heterogeneity and variations in therapeutic agents may have influenced the results. Therefore, to further elucidate the pathophysiological mechanisms of SRE, we plan to incorporate the clinical characteristics of epilepsy patients, electroencephalography, and advanced multimodal functional magnetic resonance imaging analysis methods in future research. In addition, the thalamus will be taken into consideration. This approach aims to investigate the pathogenesis of the disease from multiple perspectives, including structure, function, and metabolism, and to facilitate advancements in clinical diagnosis and treatment.
Conclusion
Our research indicates subtle damage to the WM in the bifrontal lobe and corpus callosum in patients with SRE. This suggests that limited alterations in WM integrity and damage to myelin sheaths may mediate abnormalities in neural signaling, subsequently affecting the patient’s sleep and potentially inducing epilepsy. These findings may contribute to exploring the pathophysiological mechanisms in SRE. Furthermore, the observation that myelin damage was more severe in early-onset SRE highlights concerns regarding brain structural alterations in younger patients, with RD may serve as a sensitive biomarker for detecting early WM damage.
Data Sharing Statement
Data will be made available on request.
Ethics Approval and Informed Consent
Our research was supported by the Medical Ethics Committee of Xiangya Hospital Central South University.
The authors declare that the work described has been carried out in accordance with the Declaration of Helsinki of the World Medical Association revised in 2013 for experiments involving humans.
Acknowledgments
We express sincere thanks to all the participants that devoted themselves to the study, including Weiping Liu, Ximei Feng, Qian Wang, Xun Ning and Wu Xing.
Funding
Our research was funded by Hunan Provincial Natural Science Fund (2023JJ30954).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Quigg M, Pavlova MK, Buchanan GF. Proceedings of the sleep and epilepsy workshop: introduction. Epilepsy Curr. 2021;21(3):15357597211004551. doi:10.1177/15357597211004551
2. Pavlova MK, Ng M, Allen RM, et al. Proceedings of the sleep and epilepsy workgroup: section 2 comorbidities: sleep related comorbidities of epilepsy. Epilepsy Curr. 2021;21(3):15357597211004549. doi:10.1177/15357597211004549
3. Nobili L, Cardinale F, Magliola U, et al. Taylor’s focal cortical dysplasia increases the risk of sleep-related epilepsy. Epilepsia. 2009;50(12):2599–2604. doi:10.1111/j.1528-1167.2009.02169.x
4. Tsiptsios DI, Howard RS, Koutroumanidis MA. Electroencephalographic assessment of patients with epileptic seizures. Expert Rev Neurother. 2010;10(12):1869–1886. doi:10.1586/ern.10.175
5. Sayin U, Hutchinson E, Meyerand ME, Sutula T. Age-dependent long-term structural and functional effects of early-life seizures: evidence for a hippocampal critical period influencing plasticity in adulthood. Neuroscience. 2015;288:120–134. doi:10.1016/j.neuroscience.2014.12.017
6. Xie Z, Chen Z, Jiang Y, et al. Causal relationships between epilepsy and the microstructure of the white matter: a Mendelian randomization study. Medicine 2024;103(44):e40090. doi:10.1097/MD.0000000000040090
7. Li K, Li H, Wang J, et al. Causal relationship between sleep traits and risk of epilepsy: a Mendelian randomization study. Epilepsy Behav. 2025;165:110310. doi:10.1016/j.yebeh.2025.110310
8. Deleo F, Thom M, Concha L, Bernasconi A, Bernhardt BC, Bernasconi N. Histological and MRI markers of white matter damage in focal epilepsy. Epilepsy Res. 2018;140:29–38. doi:10.1016/j.eplepsyres.2017.11.010
9. Kumar J, Solaiman A, Mahakkanukrauh P, Mohamed R, Das S. Sleep related epilepsy and pharmacotherapy: an insight. Front Pharmacol. 2018;9:1088. doi:10.3389/fphar.2018.01088
10. Schiller K, von Ellenrieder N, Avigdor T, et al. Focal epilepsy impacts rapid eye movement sleep microstructure. Sleep. 2023;46(2). doi:10.1093/sleep/zsac250.
11. Frauscher B, von Ellenrieder N, Ferrari-Marinho T, Avoli M, Dubeau F, Gotman J. Facilitation of epileptic activity during sleep is mediated by high amplitude slow waves. Brain. 2015;138(Pt 6):1629–1641. doi:10.1093/brain/awv073
12. Liu WK, Kothare S, Jain S. Sleep and Epilepsy. Semin Ped Neurol. 2023;48:101087. doi:10.1016/j.spen.2023.101087
13. Jin B, Zhang Z, Wang C, et al. Focal thalamocortical circuit abnormalities in sleep related epilepsy caused by focal cortical dysplasia type II. Seizure Europ J Epilepsy. 2022;99:153–158. doi:10.1016/j.seizure.2022.05.019
14. Zhou C, Qiu C, Pan C, et al. Brain changes in sleep-related hypermotor epilepsy observed from wakefulness and N2 sleep: a matched case-control study. Clin Neurophysiol. 2025;171:31–37. doi:10.1016/j.clinph.2024.12.020
15. De Vito A, Mankad K, Pujar S, Chari A, Ippolito D, D’Arco F. Narrative review of epilepsy: getting the most out of your neuroimaging. Transl Pediatr. 2021;10(4):1078–1099. doi:10.21037/tp-20-261
16. Liu Z, Xu Y, An J, et al. Altered brain white matter integrity in temporal lobe epilepsy: a TBSS study. J Neuroimaging. 2015;25(3):460–464. doi:10.1111/jon.12154
17. Fisher RS, Cross JH, D’Souza C, et al. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58(4):531–542. doi:10.1111/epi.13671
18. Nobili L, de Weerd A, Rubboli G, et al. Standard procedures for the diagnostic pathway of sleep-related epilepsies and comorbid sleep disorders: a European Academy of neurology, European against epilepsy-Europe consensus review. J Sleep Res. 2020;29(6):e13184. doi:10.1111/jsr.13184
19. Kraus D, Farah R, Fischer H, et al. Altered white matter organization and its correlations with executive functioning among adolescents with epilepsy. Eur J Paediatr Neurol. 2023;46:82–88. doi:10.1016/j.ejpn.2023.07.004
20. Wang G, Song Y, Su J, et al. Altered cerebellar-motor loop in benign adult familial myoclonic epilepsy type 1: the structural basis of cortical tremor. Epilepsia. 2022;63(12):3192–3203. doi:10.1111/epi.17430
21. Mak-McCully RA, Rolland M, Sargsyan A, et al. Coordination of cortical and thalamic activity during non-REM sleep in humans. Nat Commun. 2017;8(1):15499. doi:10.1038/ncomms15499
22. Beenhakker MP, Huguenard JR. Neurons that fire together also conspire together: is normal sleep circuitry hijacked to generate epilepsy? Neuron. 2009;62(5):612–632. doi:10.1016/j.neuron.2009.05.015
23. Tchopev ZN, Yeh PH, Morgan GW, et al. Acquired sleep-related hypermotor epilepsy with disrupted white matter tracts assessed by multishell diffusion magnetic resonance imaging. Front Neurol. 2018;9:6. doi:10.3389/fneur.2018.00006
24. Gorges M, Müller HP, Liepelt-Scarfone I, et al. Structural brain signature of cognitive decline in Parkinson’s disease: DTI-based evidence from the LANDSCAPE study. Therape Adv Neurolog Disor. 2019;12:1756286419843447. doi:10.1177/1756286419843447
25. Goldstein A, Covington BP, Mahabadi N, Mesfin FB. Neuroanatomy, corpus callosum. 2017.
26. Mao LY, Cai Y, Zhang QQ, Luo WY, Ding J, Wang X. The association between frontotemporal connecting fibers and seizure severity in patients with temporal lobe epilepsy. Zhonghua Yi Xue Za Zhi. 2024;104(21):1994–1997. doi:10.3760/cma.j.cn112137-20230919-00495
27. Brodovskaya A, Batabyal T, Shiono S, Sun H, Kapur J. Distinct roles of rodent thalamus and corpus callosum in seizure generalization. Ann Neurol. 2022;91(5):682–696. doi:10.1002/ana.26338
28. Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi:10.1006/nimg.2002.1267
29. Chen J, Ngo A, Rodríguez-Cruces R, et al. A worldwide enigma study on epilepsy-related gray and white matter compromise across the adult lifespan. bioRxiv. 2024.
30. Jiang Y, Song L, Li X, et al. Dysfunctional white-matter networks in medicated and unmedicated benign epilepsy with centrotemporal spikes. Hum Brain Mapp. 2019;40(10):3113–3124. doi:10.1002/hbm.24584
31. Harsan LA, Poulet P, Guignard B, et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res. 2006;83(3):392–402. doi:10.1002/jnr.20742
32. Tsuda K, Tsuji T, Ishida T, et al. Widespread abnormalities in white matter integrity and their relationship with duration of illness in temporal lobe epilepsy. Epilepsia Open. 2018;3(2):247–254. doi:10.1002/epi4.12222
33. Inano S, Takao H, Hayashi N, Abe O, Ohtomo K. Effects of age and gender on white matter integrity. AJNR Am J Neuroradiol. 2011;32(11):2103–2109. doi:10.3174/ajnr.A2785
34. Nagy SA, Horváth R, Perlaki G, et al. Age at onset and seizure frequency affect white matter diffusion coefficient in patients with mesial temporal lobe epilepsy. Epilepsy Behav. 2016;61:14–20. doi:10.1016/j.yebeh.2016.04.019
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.