Back to Journals » Journal of Pain Research » Volume 18
Altered Brain Functional Connectivity and Neurometabolite Correlations in Orofacial Chronic Pain with Central Sensitization
Authors Terumitsu M , Takado Y, Fukuda KI, Kato E, Tanaka S, Tha KK
Received 22 November 2024
Accepted for publication 30 May 2025
Published 9 June 2025 Volume 2025:18 Pages 2849—2863
DOI https://doi.org/10.2147/JPR.S507893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr David Keith
Makoto Terumitsu,1,2 Yuhei Takado,3 Ken-Ichi Fukuda,2 Eisuke Kato,2 Sei Tanaka,4 Khin Khin Tha5
1Division of Dental Anesthesiology, School of Dentistry, Department of Human Biology and Pathophysiology, Health Sciences University of Hokkaido, Hokkaido, Japan; 2Division of Special Needs Dentistry and Orofacial Pain, Department of Oral Health and Clinical Science, Tokyo Dental College, Tokyo, Japan; 3Quantum Life Spin Group, Institute for Quantum Life Science, National Institutes for Quantum Science and Technology, Chiba, Japan; 4Department of Oral and Maxillofacial Surgery, Tokyo Dental College, Tokyo, Japan; 5Global Centre for Biomedical Science and Engineering, Faculty of Medicine, Hokkaido University, Hokkaido, Japan
Correspondence: Makoto Terumitsu, Division of Dental Anesthesiology, School of Dentistry, Department of Human Biology and Pathophysiology, Health Sciences University of Hokkaido, 1757 Kanazawa, Tobetsu-cho, Ishikari-gun, Hokkaido, 061-0293, Japan, Tel/Fax +81 133 23 1445, Email [email protected]
Purpose: Central sensitization (CS) is one of the causes of refractory chronic orofacial pain (COFP). Chronic pain reportedly alters resting-state functional connectivity (FC) in the brain. The salience network (SN) overlaps with brain regions involved in chronic pain. Moreover, excitatory and inhibitory neural activities can alter FC. This study investigated the correlations between FC of the SN and other neural circuits, neurometabolites in anterior cingulate cortex (ACC), and CS Inventory (CSI) scores.
Patients and Methods: Participants were 21 COFP patients and 21 healthy sex- and age-matched volunteers. We conducted a combined study of CSI scores, resting-state functional magnetic resonance imaging, and proton magnetic resonance spectroscopy of ACC.
Results: FC between the nucleus accumbens within the mesolimbic system and SN regions was significantly stronger in patients compared with that in controls. In patients, the results revealed positive correlations between CSI scores and FC in four pairs of regions: ACC-right putamen and ACC-right globus pallidus, left insula-right putamen, and right anterior supramarginal gyrus-right lateral prefrontal cortex. Regarding correlations between neurometabolites in ACC and FC between ACC and other cortical regions, the results revealed that bilateral ACC-anterior insula FC was negatively correlated with glutamate, glutamine, and glutathione. Additionally, aspartate, an N-methyl-D-aspartic acid receptor agonist, was negatively correlated with FC of ACC-right parietal cortex in the default mode network. Regarding FC of bilateral ACC-posterior parietal cortex in the frontoparietal network, FC on the right side was positively correlated with glutathione associated with excitatory neuronal activity, whereas that on the left side was negatively correlated with gamma-aminobutyric acid / total creatine associated with inhibitory neural activity.
Conclusion: Connectivity within the SN, and between the SN and the mesolimbic system and other networks, may be involved in CS in COFP. Neurometabolites in the ACC may modulate FC linked to the ACC.
Keywords: chronic pain, orofacial region, central sensitization inventory, resting-state fMRI, MR spectroscopy
Introduction
The incidence of orofacial pain is estimated to range from 16.1%1 to 33.2%,2 and the condition becomes chronic and refractory in many cases. A previous study reported that chronic orofacial pain (COFP) was present in 11.2% of older patients who visited dental hospitals.3 COFP can impair eating and sleep, induce anxiety, and reduce quality of life.4 Central sensitization (CS) in the nervous system is a possible cause of the transition from acute to chronic pain. CS is a neurophysiological mechanism defined as the amplification of neural signaling within the central nervous system that elicits pain hypersensitivity.5 This condition is clinically characterized by disproportionate pain, in which the severity of pain and functional disability are disproportionate to detectable tissue damage and pathophysiology, or which occurs regardless of peripheral lesions.6 Assessment of CS is critical for the diagnosis and treatment of COFP.
Functional connectivity (FC) in the central nervous system is used as an index for identifying CS and COFP, and can be measured using resting-state functional magnetic resonance imaging (rsfMRI). Regarding macro-scale brain networks,7 previous studies have reported variable findings regarding which networks are associated with COFP, and a triple network model involving the default mode network (DMN), salience network (SN), and central executive network has been proposed to underlie chronic pain.8 Among these networks, the SN is considered to modulate pain processing by facilitating the detection and response to nociceptive stimuli and interoception.9 The brain regions that comprise the SN overlap with the medial pathway that processes the emotional aspects of pain.10 The anterior cingulate cortex (ACC) and insular cortex (Ins) are included in the SN and act as key nodes, with many previous studies reporting that these two regions play important roles in the sensory processing and chronicity of pain.11 In one study, enhanced ACC-right Ins FC and ACC-secondary somatosensory cortex FC were reported to be correlated with pain intensity, depression, and anxiety ratings in patients with classic trigeminal neuralgia as a type of COFP.12 In another study, Ins-thalamus FC was increased in patients with medically refractory classic or idiopathic trigeminal neuralgia.13 Among these patients, non-responders to surgical treatment exhibited decreased ACC-amygdala and ACC-hippocampus FC. FC within the cortical regions of the SN and the rest of the brain may be deeply involved in the central nervous system mechanisms of COFP.
The SN also includes subcortical regions,14 which influence chronic pain. In a previous study, the ventrolateral periaqueductal gray and locus coeruleus were found to exhibit increased FC strength with higher brain regions such as the nucleus accumbens (Acb) and ACC in patients with chronic orofacial neuropathic pain following nerve injury.15 Specifically, the Acb is reported to be involved in the brain reward center as part of the mesolimbic network of the dopaminergic transmission system, which modulates the sensory aspects of nociception, the effects of analgesics, and the emotional symptoms of chronic pain.16 In addition, the Acb is the ventral extension of the striatum, the main input nucleus of the basal ganglia.17 Previous studies reported that stronger caudate nucleus-ACC FC was exhibited in chronic migraine patients,18 increased monthly migraine attack frequency was associated with increased caudate-Ins FC, and longer disease duration was correlated with increased Acb-ACC FC.19 These findings suggest that COFP and CS may involve changes in FC between the SN and the mesolimbic system.
A combination of methods is needed to assess CS. The CS Inventory (CSI) score is commonly used as a self-report questionnaire tool.20 In addition, several studies have combined rsfMRI with measurements of cortical neurometabolites using MR spectroscopy (MRS) in chronic pain patients, indicating correlations between FC and the levels of neurometabolites.21–23 Our previous MRS study revealed that levels of aspartate (Asp), an excitatory neuronal metabolite, and glutathione (GSH), which is associated with increased excitatory neuronal activity, are elevated in ACC in COFP patients.6 Additionally, we found a negative relationship between CSI scores and gamma-aminobutyric acid (GABA) / total creatine (tCr), and a positive relationship for glutamine in the ACC.6 Therefore, in the current study we hypothesized that neurometabolites associated with excitatory and inhibitory neuronal activity in the ACC, a key node of the SN, would affect FC linked to the ACC. In this study, we combined FC data from rsfMRI, CSI scores, and our previous MRS results in the ACC to examine CS in COFP patients.
Materials and Methods
Participants
We recruited 21 patients (19 women and two men) with COFP from the pain clinic of Tokyo Dental College at Suidobashi Hospital, Japan. The patients had spontaneous and persistent pain with widespread dysesthesia and/or static allodynia. Criteria for CS included disproportionate pain regardless of peripheral lesions24 as determined by imaging examinations such as computed tomography, conventional MRI, MR neurography (three-dimensional volume rendering MR neurography: 3DVR-MRN),25 and 3D anisotropy contrast on the basis of diffusion-weighted imaging.26 In addition, pain control was difficult, and patients were unresponsive or only temporarily responsive to conventional pain therapies such as various medications, nerve block anesthesia, and local anesthetic injection into myofascial trigger points, strongly suggesting CS. The patient group consisted of 20 patients from our previous MRS study6 plus one additional 68-year-old female patient. However, because of poor MRS data, only rsfMRI data were added to the additional patient data. Patients’ mean age was 53.1 ± 12.9 years (mean ± standard deviation [SD]). Pain duration ranged from 0.5 to 25 years, with a mean duration of 7.6 ± 8.1 years (mean ± SD).
The patients were classified into three groups according to the International Classification of Orofacial Pain categories:27 1) 4.1.2.3 post-traumatic trigeminal neuropathic pain (n = 13), 2) 2.1.2 chronic primary myofascial orofacial pain (n = 4), and 3) coexistence of 4.1.2.3 and 2.1.2 (n = 4).
A total of 21 sex- and age-matched healthy volunteers (19 women and two men) without pain, medication for central nervous system disorders, or cerebral nervous disease participated in the study as a control group. The mean age of the control group was 53.2 ± 12.9 years (mean ± SD), and there was no significant difference in age between the control and patient groups (p = 0.61, Student’s t-test).
Written informed consent was obtained from all participants. The cross-sectional study protocol was approved by the Ethics Committee of Tokyo Dental College (#923). The study complied with the Declaration of Helsinki.
Questionnaire Evaluation
The CSI was used as a self-report questionnaire tool.20 Patients completed the Japanese version of the CSI28 prior to MRI data acquisition. Scores were calculated using a five-point rating for 25 items in Part A of the CSI, and total scores ranged from 0 to 100. We were unable to obtain CSI scores for two patients.
Resting State Functional Magnetic Resonance Imaging
Image Acquisition
All experiments were performed using a 3.0 T Ingenia Philips MRI scanner (Philips Healthcare, Best, the Netherlands) with a 32-channel receive head coil and a multi-transmit body coil. Images for rsfMRI were obtained using a single-shot T2*-weighted echo-planar imaging sequence (repetition time: 1000 ms; echo time: 30 ms; flip angle: 70°; matrix: 64 × 64; field of view: 197×197 mm; 13 slices; slice thickness: 5.0 mm; slice gap: 2.5 mm; voxel size: 3.1 mm × 3.1 mm × 5.0 mm; 300 volumes; scan time: 5 min). The alignment for the fMRI images was at the anterior to posterior commissure line. During the scan, subjects were instructed to keep their eyes open, relax, and look at the ceiling of the MRI bore without moving or falling asleep. A high-resolution three-dimensional anatomical image was acquired using a T1-weighted gradient-echo pulse sequence (repetition time: 6.3 ms; echo time: 3.5 ms; flip angle: 8°; inversion time: 950 ms; matrix: 512 × 512; field of view: 256 mm × 256 mm; voxel size: 0.5 mm × 0.5 mm × 1.5 mm).
Functional Connectivity Analysis
The rsfMRI data were analyzed using SPM12 (Welcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm12/), CONN version 20.b (Functional Connectivity Toolbox; http://www.nitrc.org/projects/conn), and Matlab version 9.7 (R2019b; MathWorks, Natick, MA, USA). The parameters for analysis were the default values shown below. After discarding the first three images to eliminate any signal decay, preprocessing was performed, including realignment, slice-timing correction, outlier detection, co-registration to the anatomical image, segmentation of the anatomical image (gray matter [GM], white matter [WM], and cerebrospinal fluid [CSF]), normalization with the Montreal Neurological Institute standard brain, and smoothing with an 8-mm Gaussian kernel. After preprocessing, signal and motion artifacts (global signal z-value threshold ≥ 5; composite motion threshold ≥ 0.9 mm) were removed from the data using the CompCor strategy,29 and the data were band-pass filtered (0.008–0.09 hz) to reduce the influence of noise.
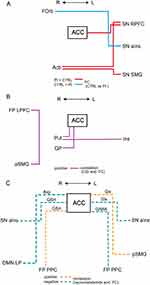
Subsequent region of interest (ROI)-to-ROI connectivity analyses were performed using ROIs that were part of CONN’s default networks and atlas ROIs. The ROIs were set in regions that constitute the SN in relation to chronic pain, including the ACC, Ins, rostral prefrontal cortex (RPFC), orbitofrontal cortex (FOrb), supramarginal gyrus (SMG), Acb, putamen (Put) and globus pallidus (GP), and that consist of frontoparietal network which overlaps with the SN, including the lateral prefrontal cortex (LPFC). A standard second-level general linear model analysis of FC MRI was used to compare the patient and control groups using parametric statistics on the basis of random field theory.30 Fisher-transformed correlation coefficients were calculated between each of the ROIs. The threshold for statistical significance was determined at p < 0.05 for the cluster-level false discovery rate (FDR).
Correlation analysis was performed between each CSI score as a covariate and FC (ROI-to-ROI analysis) for the patients, using CONN at the significance level (cluster-wise FDR corrected p < 0.05). Each significant FC value and correlation coefficient were extracted. The normality test for the samples was evaluated with the Shapiro–Wilk test using SPSS (IBM SPSS Statistics version 29, IBM Corp, Armonk, NY, USA). The results revealed that all samples were normally distributed. Pearson’s correlation coefficients (r) were then calculated. Correlation coefficients were interpreted as follows: > 0.75, good to excellent relationship; 0.50 to 0.75, moderate to good relationship; 0.25 to 0.50, fair relationship; and 0.00 to 0.25, little or no relationship.31
Magnetic Resonance Spectroscopy
Single Voxel Spectroscopy
The MRS protocol included a single-voxel point-resolved spectroscopy (PRESS) sequence with chemical-shift selective water suppression. The MRS acquisition parameters were as follows: repetition time / echo time = 2000 / 35 ms, complex points for the spectral data = 1024, and total number of acquisitions = 128. The linewidth of the water spectrum in magnitude mode became less than 18 hz after shimming. The current study used the MRS data we obtained in our previous research.6
Voxel Location
Hydrogen 1 magnetic resonance spectroscopy voxels of interest (VOIs) were set in the ACC (20 mm × 30 mm × 20 mm).6 The VOIs in the ACC were positioned parallel to and above the corpus callosum, starting from the genu of the corpus callosum and extending 3 cm posteriorly, and covered left and right ACC32 in patients and controls.
MRS Data Processing and Measurement
MRS data were processed using a linear combination model (LCModel version 6.3, Stephen Provencher, Inc., Oakville, ON, Canada).33 Tissue composition within the VOI was calculated on the basis of the segmentation of 3D T1-weighted images using the Gannet3.0 open-source tool (https://www.gabamrs.com/gannet).34 Water concentrations used in the LCModel analysis were calculated on the basis of the volume fractions of WM, GM, and CSF. Metabolite concentrations were then divided by the WM and GM fractions to correct for CSF within the VOI, because metabolites are primarily present in the WM and GM.35 Water scaling and eddy current correction were performed, and metabolites were fitted in the chemical shift range (0.2–4.2 ppm).
We measured glutamate + glutamine (Glx), aspartate (Asp) / tCr, GABA / tCr, and glutathione (GSH). The tCr (Cr + PCr) was used for normalization because tCr is widely used as an internal reference in human studies.36
Metabolites processed by LCModel with Cramer-Rao lower bounds < 25% for GSH and 15% for the others were analyzed. Mean signal-to-noise ratio (SNR) ± SD values for patients and controls were 27.35 ± 6.67 and 31.29 ± 2.97 in the ACC, respectively. Mean full-width at half maximum ± SD values for the patients and controls were 0.033 ± 0.010 ppm and 0.037 ± 0.006 ppm in the ACC, respectively. No significant differences in full-width at half maximum in the region were observed between patients and controls (F[1, 78] = 2.99, p = 0.088, two-way analysis of variance [ANOVA]). Patients’ data showed lower SNR compared with controls’ data in the ACC (F[1, 78] = 3.99, p = 0.049, Tukey’s honestly significant difference test for post hoc test: p = 0.014 [ACC]). Statistical analyses were performed using Statistica version 13 (TIBCO Software Inc. CA, USA).
Correlation Analysis Between Neurometabolites and FC
Correlation analysis was performed between each neurometabolite level as a covariate and FC setting a seed in ACC for the patients, using CONN at the significance level (cluster-wise FDR corrected p < 0.05). Each significant FC value and correlation coefficient was extracted for the patients, and the FC value and correlation coefficient in each FC that was significant in patients were also extracted for controls. Spearman correlation analysis was used to determine statistical significance in non-normally distributed data (ie, Asp / tCr and ACC-right lateral parietal FC in the DMN in patients, Glx and ACC-left posterior SMG FC and ACC-left anterior Ins (aIns) FC in both groups, and GABA / tCr and ACC-left posterior parietal cortex (PPC) FC in controls). The rest of the data, which exhibited a normal distribution, were evaluated using Pearson’s correlation analyses.
Because of the exploratory nature of this study, correction for multiple comparisons was not performed.
Results
Compared with the control group, patients with COFP exhibited significantly stronger FC of ACC-left RPFC in the SN, left RPFC-right Acb, and right Acb-left SMG in the SN. In contrast, the patient group exhibited significantly weaker FC of left aIns in the SN-right FOrb (Figure 1, Figure 2A, and Table 1).
 |
Table 1 ROI-ROIs in Patients Vs Normal Controls |
In patients, positive correlations were found between CSI scores and FC in four pairs of regions (Figures 2B and (3A–D, and Table 2): ACC-right Put (Figure 3A), ACC-right GP (Figure 3B), left Ins-right Put (Figure 3C), and right anterior SMG-lateral prefrontal cortex (LPFC) in the frontoparietal network (Figure 3D).
 |
Table 2 Functional Connectivity with Significant Correlation with Central Sensitization Inventory Score |
Regarding the relationships between neurometabolites and FC (Figure 2C Figure 4A–F, and Table 3), Asp / tCr was negatively correlated with FC of ACC-right lateral parietal in the DMN in patients (Figure 4A). GSH was positively correlated with FC of ACC-right PPC in the FP network (Figure 4B), and negatively correlated with FC of ACC-right aIns in the SN (Figure 4C). Glx was positively correlated with FC of ACC-left posterior SMG (Figure 4D) and negatively correlated with FC of ACC-left aIns in the SN (Figure 4E). GABA / tCr was negatively correlated with FC of ACC-left PPC in the FP network (Figure 4F). In controls, there were no significant correlations in the same six combinations.
 |
Table 3 Neurometabolite and Functional Connectivity with Significant Correlations |
Discussion
FC in patients significantly differed from that of controls in SN regions, and between the Acb and SN regions. CSI scores were positively correlated with FC within the SN and between the SN and the mesolimbic system and FP network. Neurometabolites in ACC were correlated with FC between ACC and other regions within the SN and the other DMN and FP networks.
A group comparison of FC between patients and controls showed significant differences in FC between four pairs of regions. First, FC between ACC and left RPFC in SN was stronger in patients. The RPFC corresponds approximately to Brodmann area 10 and is one of the brain regions comprising the SN.14 Its projections are broad and reciprocal, and the cellular properties of neurons in RPFC are well suited to integrate their inputs.37 Previous studies have reported that the RPFC and ACC were co-activated or co-deactivated for various pain stimuli. These two regions were co-activated when a painful thermal stimulus was applied to patients with sympathetically mediated chronic pain38 and to normal volunteers,39 and during electrically induced tooth pain in normal volunteers.40 Additionally, a positron emission tomography study reported that the RPFC and ACC were co-deactivated when painful stimuli were applied to patients with chronic central pain after lateral medullary infarction.41 Co-deactivation was observed following both painful and tactile stimuli.42 Moreover, several studies indicated that gray matter volume decreased in both RPFC and ACC in chronic pain patients.43–45 Although the role of the RPFC in pain processing is currently unclear, this region is thought to be involved in various forms of pain-related attention, consciousness, modulation, memory, and emotion because of its connections to broad cortical regions and other large-scale brain networks, and its diverse functions.46
The RPFC is connected to various subcortical regions, including the striatum.47 The right Acb, which is part of the ventral striatum, showed increased FC with left RPFC and left SMG in patients in the current study. A previous study reported that Acb-FOrb FC was associated with medication-overuse headaches.48 Additionally, elevated FC between Acb and the prefrontal cortex (PFC) has been shown to predict persistence and chronicity of pain in patients with chronic low back pain.49,50 Acb and PFC are part of the mesolimbic system, which is composed of dopaminergic neurons. This circuit plays an important role in the perception and regulation of chronic pain.51 Acb receives direct afferent input from the contralateral trigeminal nucleus,52 and is bilaterally connected to SMG and the inferior parietal lobule (IPL; BA 40). Furthermore, a previous study reported that Acb-SMG FC exhibited a negative value in normal volunteers.53 This finding was consistent with the present results, while the mean value of FC was positive in patients. Although the negative relationship between these regions has not been well elucidated, it appears to occur between functional systems with apparently opposite goals or functions.54 The IPL has been reported to be generally engaged in selective attention, action observation, and imitating emotions,55 and the SMG is involved in the ventral attention network.56 Furthermore, Acb-SMG FC is reported to be decreased in anhedonia with major depression.57 Changes in FC between these areas suggest that the modulation of attention and negative reward to pain and the control of emotional cognition may be involved in COFP.
Finally, FC of the right FOrb-left aIns in SN was lower in patients compared with that in controls in the current study. The FOrb, together with the Ins and ACC, forms the paralimbic system and is part of the SN.14 The Ins is highly interconnected with the FOrb (BA12).58 The FOrb is adjacent to RPFC and also communicates with the somatosensory cortex and striatum, integrating sensation, emotion, learning, and motivation through the reward system.59 FC of FOrb-Ins was reported to be negatively correlated with pain relief from positive reward in a previous study.60 Additionally, FC of the FOrb-striatum was found to be reduced in patients with dental phobia.61 In patients with chronic pain caused by irritable bowel syndrome, thicker gray matter in the FOrb and Ins was found to be correlated with pain inhibition and pain duration.62 Additionally, FC between these regions has been reported to induce autonomic responses.63 Therefore, we speculate that the reduced FC observed in this study may affect various aspects of pain-related emotions, cognition, and even behavior and autonomic responses.
The current results revealed that CSI scores were significantly correlated with FC between four pairs of brain regions. CSI scores were positively correlated with FC of ACC-right Put and ACC-right GP, and FC of left Ins-right Put. The Put is part of the ventral striatum and is located adjacent to the Acb in the dorsal striatum. The GP receives input from the striatum and outputs to other cortical and subcortical regions. Both of these areas are part of the mesolimbic dopaminergic circuitry and are involved in reward-aversion processing64 and pain processing, such as the sensory-discriminative, emotional/affective, and cognitive dimensions of pain and pain modulation.65 The Put is consistently activated during acute and chronic pain conditions and is affected by analgesic administration.65 Previous studies have reported that the GP is activated in response to cold and mechanical allodynia in chronic trigeminal neuralgia pain,66 and during catastrophizing in fibromyalgia.67 Decreased FC of ACC-Put and ACC-GP was reported in dental phobia patients.61 Moreover, pain modulation by neurometabolites or analgesics occurs in the basal ganglia. Pain stimuli elicit significant dopamine release in the Put.68 The activation of dopamine D2 neurotransmission was positively associated with individual variation in subjective ratings of sensory and affective qualities of pain.68 In contrast, decreased dopamine in the Put was reported in patients with burning mouth syndrome, suggesting that reduced dopaminergic inhibition causes chronic pain.69 Additionally, the basal ganglia contain high levels of endogenous opioids, and high binding of opioid receptors. A previous study reported that oxycodone reduced ACC-bilateral Put FC.70 Microinjections of morphine into bilateral GP were found to induce analgesia.71 Inhibiting kappa-opioid receptor in ACC decreased FC of ACC-ventral GP.72 Because the output of the basal ganglia is inhibitory, we speculate that the CS of COFP involves an inhibitory effect on the connections between the basal ganglia and the limbic system and cortex.
In the current study, FC of right anterior SMG-right LPFC in the SN was positively correlated with CSI scores. The FC of left LPFC-IPL has been reported to be positively correlated with pain intensity in chronic postherpetic neuralgia.73 FC between SMG and PFC exhibits laterality; compared with the left SMG, the right temporoparietal junction of the SMG (including BA 40) was reported to be more strongly connected with the salience and ventral attention network as well as other regions implicated in salience/pain-related processing.74 The right-lateralized salience/ventral attention network is considered to encode the prolonged salience of pain.75 In addition, the mesencephalic-basal ganglia closed loops are reported to contribute to implicitly addressing and modulating selective attention to prioritized stimuli.76 These findings suggest that FC that is correlated with CSI scores may enhance CS by acting on pain salience/attention networks involving the frontoparietal cortices and the mesolimbic circuit. CS is closely related to category 7 in the International Classification of Orofacial Pain (Psychosocial assessment of patients with orofacial pain), and was evaluated using various questionnaires such as for pain- and function-related constructs, and psychosocial constructs. The FCs correlating to CSI score may indicate brain function associated with this category.
Significant correlations were found between four neurometabolites within ACC and FC in six pairs of regions, setting ACC as a seed. Excitatory neurometabolites include agonists of the N-methyl-D-aspartate (NMDA) receptor, Asp and Glx. GSH, a metabolite of glutamine (Glu) and a major intracellular antioxidant, is approximately twice as abundant in glial cells as in neurons.77 The fundamental role of GSH is to detoxify reactive oxygen species, which is critical for maintaining the normal function of the human brain.78 Excessive stimulation of the NMDA receptor and an influx of calcium ions causes the production of excess free radicals and oxidative stress, leading to CS and cellular apoptosis.79 Thus, GSH may reflect increased excitatory nerve activity.
In the current study, ACC-right aIns FC and ACC-left aIns FC were negatively correlated with Glx and GSH, respectively. A previous study reported that Glu in dorsal ACC was associated with connectivity strength in dorsal ACC and aIns in the patients with schizophrenia.80 In another study, Glu levels in the ACC were reported to be reduced by oxycodone and tapentadol administration, whereas they remained unchanged in the insular cortex.81 Furthermore, oxycodone has been demonstrated to decrease ACC-aIns FC.81 Thus, changes in excitatory neural activity of ACC and the decrease in ACC-aIns FC suggest an inhibitory effect on pain.
Generally, activity in the SN is anticorrelated with that in the DMN.82 In a previous study, the anticorrelation of FC between the two networks was found to be reduced in chronic pain patients compared with that in controls,83 and was correlated with Glu level in ACC.84 Additionally, less anticorrelated FC and connectivity between the SN and DMN were reported to be related to pain severity and disease-related symptoms in chronic pain.83 Asp, an excitatory neurometabolite in ACC, may be involved in cross-network FC.
In the current study, Glx in ACC was positively correlated with FC between ACC and left posterior SMG. Although some previous studies have reported that Glu in ACC is elevated in patients with chronic pain,84 other studies reported that it is decreased in these patients.85 In one study measuring Glu in ACC in normal subjects 24 hours after administration of S-/racemic ketamine, a non-competitive NMDA receptor antagonist, Glu was found to be elevated and FC of ACC-left IPL was decreased.86 Although differences in the experimental conditions between that study and the present study make direct comparison difficult, the findings suggest that Glx levels in ACC and FC of ACC-left SMG are linked. Furthermore, another study reported that FC between these regions increased with improvement in mindfulness scores when mindfulness-based stress reduction was used to relieve pain and anxiety.87 Additionally, the correlation between the FC and Glx showed an inverse relationship between the patient and control groups in the current findings, suggesting that this effect is specific to chronic pain.
In the current study, FC of ACC-right PPC in the FP network was positively correlated with GSH, whereas ACC-left PPC in the FP network was negatively correlated with GABA. If GSH reflects increased excitatory neural activity, these findings suggest that FC may be modulated by excitatory and inhibitory nerves, respectively. The right IPL and ACC were previously reported to be associated with anticipation of pain,88 while patients with both rheumatoid arthritis and fibromyalgia were found to exhibit stronger FC of left IPL-ACC.89 The present study suggests that differences in neurometabolites within ACC may influence the laterality of ACC-parietal lobes FC.
The current study involved several limitations that should be considered. Chronic pain is more common in women than in men,90 and most of the subjects in the current study sample were women. In our pain clinic, the vast majority of patients with COFP are women. We asked patients to participate in the study according to our patient selection criteria, which led to this sex difference. Although a random sampling method from a sample population was not performed, this difference may reflect the sex ratio of the COFP patient population. However, the small sample size in the current study makes it difficult to examine sex differences from these results, and this is a topic for future research. GABA / tCr was used in the analysis to quantify GABA because the PRESS method of MRS was used to measure neurometabolites. A Mescher Garwood Point Resolved Spectroscopy (MEGA)-PRESS method should be used to accurately measure direct GABA concentration in future studies. Additionally, we only measured neurometabolites in ACC and calculated the FC of correlated brain regions using ACC as a seed. Neurometabolites in other brain regions that are correlated with ACC should also be measured whenever possible. Correlation coefficients greater than 0.45 and significant FC values were reported in the results, and p-values for correlations were not calculated for multiple comparisons among significant combinations. Therefore, our correlation analyses between neurometabolites and FC values should be considered exploratory. Consequently, further refinement of the data acquisition methods and statistical analysis in future studies may provide additional insight.
Conclusion
A study combining CSI scores, rsfMRI, and MRS was performed in patients with COFP. Significant increases in FC in the patient group were found in ACC-left RPFC in the SN, and Acb in the mesolimbic system-left RPFC and mesolimbic system-left SMG in the SN. In the patient group, CSI scores were positively correlated with FC in four pairs of regions: right LPFC-right anterior SMG, ACC-right GP, and ACC-right Put in the mesolimbic system, and left Ins-right Put.
Regarding the correlations between neurometabolites within ACC and FC of ACC and other cortical regions, bilateral FC of ACC-aIns exhibited negative correlations with Glx and GSH, which are associated with excitatory neuronal activity. Additionally, the results revealed a negative correlation between Asp and FC of ACC-right parietal cortex in the DMN, and in FC of bilateral ACC-PPC in the FP network, in which FC on the right side was positively correlated with GSH, whereas FC on the left side was negatively correlated with GABA / tCr.
Neurocognitive and social cognitive dysfunction and organic brain deficits have been reported to be represented by unique brain network localization.91 The current results suggested that FC within the SN that exhibited changes in OFP patients occurred in the frontal cortex, ACC, Ins, SMG, and subcortical regions. Additionally, FC between ACC and Ins to the basal ganglia, and FC where the FP network and SN overlap, were identified as regions in which CS, one of the factors of chronic pain, is encoded. Neurometabolites within ACC, particularly those associated with excitatory and inhibitory neural activity, may be involved in altering FC related to COFP.
Patients with COFP are more likely to exhibit complications involving psychosocial disorders such as anxiety, depression, and catastrophizing. COFP can also be classified as a somatic symptom disorder with predominant pain in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).92 A combination of questionnaires on psychosocial factors is typically utilized for assessing somatic symptom disorders. A recent study on COFP reported that the CSI used in the current experiment was strongly correlated with many other questionnaires that assess these factors, such as the Hospital Anxiety and Depression Scale (HADS) and the Pain Catastrophizing Scale (PCS).93 A comprehensive biopsychosocial perspective and approach to the diagnosis and treatment of COFP is essential. Achieving this goal requires an understanding of the alteration of neural networks in relation to psychosocial factors, and the current study provides useful insights into the mechanisms involved. Furthermore, the orofacial region has multiple and important functions, ranging from life-sustaining feeding and swallowing to interpersonal communication. Therefore, multidimensional aspects, complexity and unclear pathology are often present in COFP. This makes diagnosis difficult and can sometimes lead to unnecessary invasive treatment by dentists. If cases are not diagnosed and treated appropriately, 20% of patients with acute orofacial pain may progress to refractory COFP.92 The accumulation of data using a combination of FC, neurometabolites, and questionnaire methods will provide healthcare providers with greater insight into the central nervous mechanisms of COFP, and constitutes important evidence underpinning the diagnosis, treatment, and prevention of chronicity.
Acknowledgments
The authors would like to thank the participants in this study. The authors are also grateful to Takashi Namiki for technical assistance in MR data collection, MR staff at Ichikawa general hospital of the Tokyo Dental College and the Advanced Imaging Center Yaesu Clinic. We thank Benjamin Knight, MSc. from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP 18K09821 and 22K10130.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Horst OV, Cunha-Cruz J, Zhou L, Manning W, Mancl L, DeRouen TA. Prevalence of pain in the orofacial regions in patients visiting general dentists in the Northwest Practice-based REsearch Collaborative in Evidence-based DENTistry research network. J Am Dent Assoc. 2015;146(10):721–728e723. doi:10.1016/j.adaj.2015.04.001
2. de Melo Junior PC, Aroucha J, Arnaud M, et al. Prevalence of TMD and level of chronic pain in a group of Brazilian adolescents. PLoS One. 2019;14(2):e0205874. doi:10.1371/journal.pone.0205874
3. Derafshi R, Rezazadeh F, Ghapanchi J, Basandeh Sharif D, Farzin M. Prevalence of chronic orofacial pain in elderly patients referred to shiraz dental school From 2005 to 2017. Anesth Pain Med. 2019;9(6):e91182. doi:10.5812/aapm.91182
4. Ananthan S, Benoliel R. Chronic orofacial pain. J Neural Transm. 2020;127(4):575–588. doi:10.1007/s00702-020-02157-3
5. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. doi:10.1016/j.pain.2010.09.030
6. Terumitsu M, Takado Y, Fukuda KI, Kato E, Tanaka S. Neurometabolite levels and relevance to central sensitization in chronic orofacial pain patients: a magnetic resonance spectroscopy study. J Pain Res. 2022;15:1421–1432. doi:10.2147/JPR.S362793
7. Uddin LQ, Yeo BTT, Spreng RN. Towards a universal taxonomy of macro-scale functional human brain networks. Brain Topogr. 2019;32(6):926–942. doi:10.1007/s10548-019-00744-6
8. De Ridder D, Vanneste S, Smith M, Adhia D. Pain and the triple network model. Front Neurol. 2022;13:757241. doi:10.3389/fneur.2022.757241
9. Iannetti GD, Mouraux A. From the neuromatrix to the pain matrix (and back). Exp Brain Res. 2010;205(1):1–12. doi:10.1007/s00221-010-2340-1
10. De Ridder D, Adhia D, Vanneste S. The anatomy of pain and suffering in the brain and its clinical implications. Neurosci Biobehav Rev. 2021;130:125–146. doi:10.1016/j.neubiorev.2021.08.013
11. Lee JA, Chen Q, Zhuo M. Synaptic plasticity in the pain-related cingulate and insular cortex. Biomedicines. 2022;10(11):2745. doi:10.3390/biomedicines10112745
12. Wang Y, Cao DY, Remeniuk B, Krimmel S, Seminowicz DA, Zhang M. Altered brain structure and function associated with sensory and affective components of classic trigeminal neuralgia. Pain. 2017;158(8):1561–1570. doi:10.1097/j.pain.0000000000000951
13. Danyluk H, Lang S, Monchi O, Sankar T. Pre-operative limbic system functional connectivity distinguishes responders from non-responders to surgical treatment for trigeminal neuralgia. Front Neurol. 2021;12:716500. doi:10.3389/fneur.2021.716500
14. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi:10.1523/JNEUROSCI.5587-06.2007
15. Mills EP, Di Pietro F, Alshelh Z, et al. Brainstem pain-control circuitry connectivity in chronic neuropathic pain. J Neurosci. 2018;38(2):465–473. doi:10.1523/JNEUROSCI.1647-17.2017
16. Mitsi V, Zachariou V. Modulation of pain, nociception, and analgesia by the brain reward center. Neuroscience. 2016;338:81–92. doi:10.1016/j.neuroscience.2016.05.017
17. Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl). 2007;191(3):521–550. doi:10.1007/s00213-006-0510-4
18. Krebs K, Rorden C, Androulakis XM. Resting state functional connectivity after sphenopalatine ganglion blocks in chronic migraine with medication overuse headache: a pilot longitudinal fMRI study. Headache. 2018;58(5):732–743. doi:10.1111/head.13318
19. Yuan K, Zhao L, Cheng P, et al. Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. J Pain. 2013;14(8):836–844. doi:10.1016/j.jpain.2013.02.010
20. Mayer TG, Neblett R, Cohen H, et al. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012;12(4):276–285. doi:10.1111/j.1533-2500.2011.00493.x
21. Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119(6):1453–1464. doi:10.1097/ALN.0000000000000017
22. As-Sanie S, Kim J, Schmidt-Wilcke T, et al. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J Pain. 2016;17(1):1–13. doi:10.1016/j.jpain.2015.09.008
23. Baumbach P, Meissner W, Reichenbach JR, Gussew A. Functional connectivity and neurotransmitter impairments of the salience brain network in chronic low back pain patients: a combined resting-state functional magnetic resonance imaging and 1 H-MRS study. Pain. 2022;163(12):2337–2347. doi:10.1097/j.pain.0000000000002626
24. Nijs J, Torres-Cueco R, van Wilgen CP, et al. Applying modern pain neuroscience in clinical practice: criteria for the classification of central sensitization pain. Pain Physician. 2014;17(5):447–457. doi:10.36076/ppj.2014/17/447
25. Terumitsu M, Seo K, Matsuzawa H, Yamazaki M, Kwee IL, Nakada T. Morphologic evaluation of the inferior alveolar nerve in patients with sensory disorders by high-resolution 3D volume rendering magnetic resonance neurography on a 3.0-T system. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111(1):95–102. doi:10.1016/j.tripleo.2010.09.002
26. Terumitsu M, Matsuzawa H, Seo K, et al. High-contrast high-resolution imaging of posttraumatic mandibular nerve by 3DAC-PROPELLER magnetic resonance imaging: correlation with the severity of sensory disturbance. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(1):85–94. doi:10.1016/j.oooo.2017.02.017
27. Benoliel R, May A, Svensson P, et al. International classification of orofacial pain, 1st edition (ICOP). Cephalalgia. 2020;40(2):129–221. doi:10.1177/0333102419893823
28. Tanaka K, Nishigami T, Mibu A, et al. Validation of the Japanese version of the Central Sensitization Inventory in patients with musculoskeletal disorders. PLoS One. 2017;12(12):e0188719. doi:10.1371/journal.pone.0188719
29. Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi:10.1016/j.neuroimage.2007.04.042
30. Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4(1):58–73. doi:10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O
31. Wm PLG. Foundations of Clinical Research: Applications to Practice, 3rd Ed. Upper Saddle River, NJ: Pearson Prentice Hall; 2009.
32. Ito T, Tanaka-Mizuno S, Iwashita N, et al. Proton magnetic resonance spectroscopy assessment of metabolite status of the anterior cingulate cortex in chronic pain patients and healthy controls. J Pain Res. 2017;10:287–293. doi:10.2147/JPR.S123403
33. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. doi:10.1002/mrm.1910300604
34. Harris AD, Puts NA, Edden RA. Tissue correction for GABA-edited MRS: considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging. 2015;42(5):1431–1440. doi:10.1002/jmri.24903
35. Xin L, Mekle R, Fournier M, et al. Genetic polymorphism associated prefrontal glutathione and its coupling with brain glutamate and peripheral redox status in early psychosis. Schizophr Bull. 2016;42(5):1185–1196. doi:10.1093/schbul/sbw038
36. Wilson M, Andronesi O, Barker PB, et al. Methodological consensus on clinical proton MRS of the brain: review and recommendations. Magn Reson Med. 2019;82(2):527–550. doi:10.1002/mrm.27742
37. Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004;5(3):184–194. doi:10.1038/nrn1343
38. Apkarian AV, Thomas PS, Krauss BR, Szeverenyi NM. Prefrontal cortical hyperactivity in patients with sympathetically mediated chronic pain. Neurosci Lett. 2001;311(3):193–197. doi:10.1016/S0304-3940(01)02122-X
39. Coghill RC, McHaffie JG, Yen YF. Neural correlates of interindividual differences in the subjective experience of pain. Proc Natl Acad Sci U S A. 2003;100(14):8538–8542. doi:10.1073/pnas.1430684100
40. Jantsch HHF, Kemppainen P, Ringler R, Handwerker HO, Forster C. Cortical representation of experimental tooth pain in humans. Pain. 2005;118(3):390–399. doi:10.1016/j.pain.2005.09.017
41. Peyron R, Garcia-Larrea L, Gregoire MC, et al. Allodynia after lateral-medullary (Wallenberg) infarct. A PET study. Brain. 1998;121(Pt 2):345–356. doi:10.1093/brain/121.2.345
42. Lui F, Duzzi D, Corradini M, Serafini M, Baraldi P, Porro CA. Touch or pain? Spatio-temporal patterns of cortical fMRI activity following brief mechanical stimuli. Pain. 2008;138(2):362–374. doi:10.1016/j.pain.2008.01.010
43. Rocca MA, Ceccarelli A, Falini A, et al. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37(7):1765–1770. doi:10.1161/01.STR.0000226589.00599.4d
44. Kim JH, Suh SI, Seol HY, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008;28(6):598–604. doi:10.1111/j.1468-2982.2008.01550.x
45. Fritz HC, McAuley JH, Wittfeld K, et al. Chronic back pain is associated with decreased prefrontal and anterior insular gray matter: results from a population-based cohort study. J Pain. 2016;17(1):111–118. doi:10.1016/j.jpain.2015.10.003
46. Peng K, Steele SC, Becerra L, Borsook D. Brodmann area 10: collating, integrating and high level processing of nociception and pain. Prog Neurobiol. 2018;161:1–22. doi:10.1016/j.pneurobio.2017.11.004
47. Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10(3):206–219. doi:10.1093/cercor/10.3.206
48. Torta DM, Costa T, Luda E, et al. Nucleus accumbens functional connectivity discriminates medication-overuse headache. Neuroimage Clin. 2016;11:686–693. doi:10.1016/j.nicl.2016.05.007
49. Baliki MN, Petre B, Torbey S, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15(8):1117–1119. doi:10.1038/nn.3153
50. Apkarian AV, Baliki MN, Farmer MA. Predicting transition to chronic pain. Curr Opin Neurol. 2013;26(4):360–367. doi:10.1097/WCO.0b013e32836336ad
51. Serafini RA, Pryce KD, Zachariou V. The mesolimbic dopamine system in chronic pain and associated affective comorbidities. Biol Psychiatry. 2020;87(1):64–73. doi:10.1016/j.biopsych.2019.10.018
52. Burstein R, Giesler GJ Jr. Retrograde labeling of neurons in spinal cord that project directly to nucleus accumbens or the septal nuclei in the rat. Brain Res. 1989;497(1):149–154. doi:10.1016/0006-8993(89)90981-5
53. Di Martino A, Scheres A, Margulies DS, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18(12):2735–2747. doi:10.1093/cercor/bhn041
54. Parente F, Frascarelli M, Mirigliani A, Di Fabio F, Biondi M, Colosimo A. Negative functional brain networks. Brain Imaging Behav. 2018;12(2):467–476. doi:10.1007/s11682-017-9715-x
55. Zhao Y, Zhang L, Rutgen M, Sladky R, Lamm C. Neural dynamics between anterior insular cortex and right supramarginal gyrus dissociate genuine affect sharing from perceptual saliency of pretended pain. Elife. 2021;10:e69994.
56. Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. 2011;34:569–599. doi:10.1146/annurev-neuro-061010-113731
57. Liu R, Wang Y, Chen X, Zhang Z, Xiao L, Zhou Y. Anhedonia correlates with functional connectivity of the nucleus accumbens subregions in patients with major depressive disorder. Neuroimage Clin. 2021;30:102599. doi:10.1016/j.nicl.2021.102599
58. Mesulam MM, Mufson EJ. Insula of the old world monkey. III: efferent cortical output and comments on function. J Comp Neurol. 1982;212(1):38–52. doi:10.1002/cne.902120104
59. Rolls ET. Emotion, motivation, decision-making, the orbitofrontal cortex, anterior cingulate cortex, and the amygdala. Brain Struct Funct. 2023;228(5):1201–1257. doi:10.1007/s00429-023-02644-9
60. Becker S, Gandhi W, Pomares F, Wager TD, Schweinhardt P. Orbitofrontal cortex mediates pain inhibition by monetary reward. Soc Cogn Affect Neurosci. 2017;12(4):651–661. doi:10.1093/scan/nsw173
61. Scharmuller W, Wabnegger A, Schienle A. Functional brain connectivity during fear of pain: a comparison between dental phobics and controls. Brain Connect. 2015;5(3):187–191. doi:10.1089/brain.2014.0297
62. Piche M, Chen JI, Roy M, Poitras P, Bouin M, Rainville P. Thicker posterior insula is associated with disease duration in women with irritable bowel syndrome (IBS) whereas thicker orbitofrontal cortex predicts reduced pain inhibition in both IBS patients and controls. J Pain. 2013;14(10):1217–1226. doi:10.1016/j.jpain.2013.05.009
63. Quadt L, Critchley H, Nagai Y. Cognition, emotion, and the central autonomic network. Auton Neurosci. 2022;238:102948. doi:10.1016/j.autneu.2022.102948
64. Richard JM, Castro DC, Difeliceantonio AG, Robinson MJ, Berridge KC. Mapping brain circuits of reward and motivation: in the footsteps of Ann Kelley. Neurosci Biobehav Rev. 2013;37(9 Pt A):1919–1931. doi:10.1016/j.neubiorev.2012.12.008
65. Borsook D, Upadhyay J, Chudler EH, Becerra L. A key role of the basal ganglia in pain and analgesia--insights gained through human functional imaging. Mol Pain. 2010;6:27. doi:10.1186/1744-8069-6-27
66. Becerra L, Morris S, Bazes S, et al. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26(42):10646–10657. doi:10.1523/JNEUROSCI.2305-06.2006
67. Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127(Pt 4):835–843. doi:10.1093/brain/awh098
68. Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26(42):10789–10795. doi:10.1523/JNEUROSCI.2577-06.2006
69. Jaaskelainen SK, Rinne JO, Forssell H, et al. Role of the dopaminergic system in chronic pain -- a fluorodopa-PET study. Pain. 2001;90(3):257–260. doi:10.1016/S0304-3959(00)00409-7
70. Baumgartner U, Buchholz HG, Bellosevich A, et al. High opiate receptor binding potential in the human lateral pain system. Neuroimage. 2006;30(3):692–699. doi:10.1016/j.neuroimage.2005.10.033
71. Anagnostakis Y, Zis V, Spyraki C. Analgesia induced by morphine injected into the pallidum. Behav Brain Res. 1992;48(2):135–143. doi:10.1016/S0166-4328(05)80149-4
72. Peeters LM, Hinz R, Detrez JR, et al. Chemogenetic silencing of neurons in the mouse anterior cingulate area modulates neuronal activity and functional connectivity. Neuroimage. 2020;220:117088. doi:10.1016/j.neuroimage.2020.117088
73. Li J, Huang X, Sang K, Bodner M, Ma K, Dong XW. Modulation of prefrontal connectivity in postherpetic neuralgia patients with chronic pain: a resting-state functional magnetic resonance-imaging study. J Pain Res. 2018;11:2131–2144. doi:10.2147/JPR.S166571
74. Kucyi A, Hodaie M, Davis KD. Lateralization in intrinsic functional connectivity of the temporoparietal junction with salience- and attention-related brain networks. J Neurophysiol. 2012;108(12):3382–3392. doi:10.1152/jn.00674.2012
75. Shulman GL, Astafiev SV, Franke D, et al. Interaction of stimulus-driven reorienting and expectation in ventral and dorsal frontoparietal and basal ganglia-cortical networks. J Neurosci. 2009;29(14):4392–4407. doi:10.1523/JNEUROSCI.5609-08.2009
76. Esposito M, Palermo S, Nahi YC, Tamietto M, Celeghin A. Implicit selective attention: the role of the mesencephalic-basal ganglia system. Curr Neuropharmacol. 2024;22(9):1497–1512. doi:10.2174/1570159X21666230831163052
77. Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82(4):1213–1223. doi:10.1016/S0306-4522(97)00347-3
78. Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62(6):649–671. doi:10.1016/s0301-0082(99)00060-x
79. Xu B, Xu ZF, Deng Y, Liu W, Yang HB, Wei YG. Protective effects of MK-801 on methylmercury-induced neuronal injury in rat cerebral cortex: involvement of oxidative stress and glutamate metabolism dysfunction. Toxicology. 2012;300(3):112–120. doi:10.1016/j.tox.2012.06.006
80. Limongi R, Jeon P, Mackinley M, et al. Glutamate and dysconnection in the salience network: neurochemical, effective connectivity, and computational evidence in schizophrenia. Biol Psychiatry. 2020;88(3):273–281. doi:10.1016/j.biopsych.2020.01.021
81. Hansen TM, Frokjaer JB, Mark EB, Drewes AM. Tapentadol and oxycodone reduce cingulate glutamate in healthy volunteers. Br J Clin Pharmacol. 2022;88(3):1358–1364. doi:10.1111/bcp.15050
82. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi:10.1073/pnas.0504136102
83. Hemington KS, Wu Q, Kucyi A, Inman RD, Davis KD. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct Funct. 2016;221(8):4203–4219. doi:10.1007/s00429-015-1161-1
84. Kong N, Gao C, Xu M, Gao X. Changes in the anterior cingulate cortex in Crohn’s disease: a neuroimaging perspective. Brain Behav. 2021;11(3):e02003. doi:10.1002/brb3.2003
85. Zhao X, Xu M, Jorgenson K, Kong J. Neurochemical changes in patients with chronic low back pain detected by proton magnetic resonance spectroscopy: a systematic review. Neuroimage Clin. 2017;13:33–38. doi:10.1016/j.nicl.2016.11.006
86. Danyeli LV, Sen ZD, Colic L, et al. Association of the delayed changes in glutamate levels and functional connectivity with the immediate network effects of S-ketamine. Transl Psychiatry. 2023;13(1):60. doi:10.1038/s41398-023-02346-0
87. Vishnubhotla RV, Radhakrishnan R, Kveraga K, et al. Advanced meditation alters resting-state brain network connectivity correlating with improved mindfulness. Front Psychol. 2021;12:745344. doi:10.3389/fpsyg.2021.745344
88. Kong J, Jensen K, Loiotile R, et al. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain. 2013;154(3):459–467. doi:10.1016/j.pain.2012.12.004
89. Kaplan CM, Schrepf A, Ichesco E, et al. Association of inflammation with pronociceptive brain connections in rheumatoid arthritis patients with concomitant fibromyalgia. Arthritis Rheumatol. 2020;72(1):41–46. doi:10.1002/art.41069
90. Osborn NR, Davis KC. Sex and gender differences in pain. Int Rev Neurobiol. 2022;164:277–307.
91. Cheng Y, Cai H, Liu S, et al. Brain network localization of gray matter atrophy and neurocognitive and social cognitive dysfunction in schizophrenia. Biol Psychiatry. 2025;97(2):148–156. doi:10.1016/j.biopsych.2024.07.021
92. Canfora F, Ottaviani G, Calabria E, et al. Advancements in understanding and classifying chronic orofacial pain: key insights from biopsychosocial models and international classifications (ICHD-3, ICD-11, ICOP). Biomedicines. 2023;(12):3266. doi:10.3390/biomedicines11123266
93. Salbego RS, Conti PCR, Soares FFC, et al. Central sensitization inventory is associated with psychological functioning but not with psychophysical assessment of pain amplification. Eur J Pain. 2025;29(2):e4713. doi:10.1002/ejp.4713
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.