Back to Journals » Drug Design, Development and Therapy » Volume 19
AMPK in Intestinal Health and Disease: A Multifaceted Therapeutic Target for Metabolic and Inflammatory Disorders
Authors Yibcharoenporn C , Muanprasat C, Moonwiriyakit A , Satitsri S , Pathomthongtaweechai N
Received 27 November 2024
Accepted for publication 4 April 2025
Published 21 April 2025 Volume 2025:19 Pages 3029—3058
DOI https://doi.org/10.2147/DDDT.S507489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Muzammal Hussain
Chamnan Yibcharoenporn,* Chatchai Muanprasat,* Aekkacha Moonwiriyakit, Saravut Satitsri, Nutthapoom Pathomthongtaweechai
Chakri Naruebodindra Medical Institute, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Samut Prakan, 10540, Thailand
*These authors contributed equally to this work
Correspondence: Nutthapoom Pathomthongtaweechai, Chakri Naruebodindra Medical Institute, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bang Phli, 111 Moo 14, Bang Pla, Bang Phli, Samut Prakan, 10540, Thailand, Tel +66984800365, Email [email protected]
Abstract: The intestines play essential roles in nutrient absorption and immune function and help maintain a protective barrier. Disruptions to its function can result in various diseases, including metabolic disorders, inflammation, and cancer. As a key regulator of cellular energy levels, 5’-adenosine monophosphate-activated protein kinase (AMPK) is essential for intestinal health. Beyond its established metabolic role, emerging evidence suggests that AMPK exerts profound effects on intestinal cell physiology, influencing cell proliferation and differentiation, inflammation, autophagy, barrier integrity, and smooth muscle contractility. Here, we explore the structure and regulation of AMPK, as well as its diverse roles in intestinal diseases and potential as a therapeutic target. Our findings reveal that AMPK is a multifaceted regulator of intestinal health, modulating various cellular processes and intestinal diseases. It plays a dual role in cancer, acting as both a tumor suppressor and promoter, and it regulates inflammatory pathways, autophagy, tight junction formation, and smooth muscle contractility. Both natural and synthetic AMPK activators offer promise as therapeutic agents. This review of AMPK’s mechanisms and activators offers valuable insights for developing novel therapies for intestinal disorders. Further research is needed to fully define AMPK’s roles and therapeutic potential.
Keywords: AMPK, AMPK activators, intestinal diseases, intestinal inflammation, intestinal cancer
Introduction
The intestinal tract is a pivotal site for various essential bodily functions, such as nutrient absorption, immune response modulation, and maintenance of the intestinal barrier. Pathological alterations to the intestines result in several intestinal diseases, including metabolic disorders, inflammatory conditions, and cancerous formations.1
Among the myriad signaling molecules that orchestrate intestinal cellular functions, 5’-adenosine monophosphate-activated protein kinase (AMPK), a highly conserved serine/threonine (Ser/Thr) kinase, is a central regulator and metabolic sensor that responds to changes in cellular energy status. Beyond its established role in cellular energy metabolism, emerging evidence suggests that AMPK exerts profound effects on intestinal cell physiology, influencing cell proliferation, differentiation, inflammatory responses, apoptosis, autophagy, epithelial barrier integrity, and smooth muscle contractility.2–4 These diverse effects highlight AMPK’s crucial role in regulating various intestinal functions, including nutrient absorption, ion transport, inflammation control, maintenance of gut barrier integrity, and intestinal mobility.2,3,5 Dysregulation of AMPK has been associated with the development of intestinal diseases.6 Indeed, AMPK signaling is disrupted in several intestinal disorders. Studies have shown that administering AMPK activators can protect against intestinal damage and toxicity.7 Conversely, models lacking AMPK subunits exhibit increased intestinal permeability and heightened susceptibility to colitis.3,7,8 Furthermore, AMPK’s protective effects extend to suppressing inflammation and reducing reactive oxygen species (ROS) production within the intestine.9
This comprehensive review explores the intricate regulatory mechanisms governing AMPK activation and its pathophysiological implications, while examining diverse exogenous AMPK activators. Moreover, we present growing evidence supporting AMPK’s potential as a therapeutic target across multiple pathological conditions, with particular emphasis on inflammation and cancer applications.
A Glance at AMPK
Structure of AMPK
AMPK is a heterotrimeric protein complex composed of three distinct subunits: the α, β, and γ subunits. The α subunit, the core of AMPK, contains a catalytic domain responsible for phosphorylating target substrates and flanking N-terminal kinase and C-terminal regulatory domains that facilitate phosphate transfer and modulate activity, respectively. The β subunit stabilizes the entire complex and potentially senses energy levels via its glycogen-binding domain. The γ subunit, with its four cystathionine β-synthase (CBS) domains, binds adenosine monophosphate (AMP)/adenosine triphosphate (ATP) to sense cellular energy levels and regulate the complex’s activity.4,10,11
The key activation site of the α subunit’s kinase domain is located at Thr172. Phosphorylation of Thr172 by upstream kinases, such as liver kinase B1 (LKB1) and Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) β, is crucial for AMPK activation.11,12 Notably, the α subunit has two isoforms: α1 and α2. The α1 isoform, which is linked to gene polymorphism rs10074991, is associated with a decreased risk of cancer susceptibility.13 The α2 isoform is important for neutrophil-mediated vascular repair and muscle differentiation, as it regulates genes that encode muscle creatinine kinase (MCK), pepsinogen C (PGC)-1α1, PGC-1α4, and cytochrome c.14,15 However, its phosphorylation is reduced during bladder ischemia.16
The β subunit acts as a scaffold, stabilizing the trimeric AMPK complex through interactions with the other subunits, and regulates glycogen metabolism via its glycogen-binding domain.4,10,11 This subunit has two isoforms, β1 and β2, which exhibit distinct tissue distribution patterns. For instance, the β1 isoform is crucial for both erythrocyte development and glucose homeostasis, and mice lacking the β1 isoform exhibit reduced kidney fibrosis, elevated blood sugar, and microcytic anemia with splenomegaly.17,18 In contrast, upregulation of the β2 isoform promotes adipogenesis.19 Interestingly, both the β1 and β2 isoforms determine gene expression during cardiac stem cell differentiation.20 It is also worth noting that myristoylation of the β subunit can suppress AMPK21 and that inhibition of this myristoylation can activate AMPK and prevent mice from developing high-fat diet-induced obesity and hepatic steatosis.22
The γ subunit of AMPK serves as a cellular energy sensor through its four CBS domains that bind adenine nucleotides, including AMP and ATP. This binding allows AMPK to detect changes in the AMP-to-ATP ratio, a key indicator of energy level. When energy is low (high AMP level), AMP binding allosterically activates AMPK via conformational changes in the γ subunit.10,23 Conversely, a high ATP level inhibits AMPK activation. Furthermore, prolyl isomerase 1 (Pin1) suppresses AMPK phosphorylation in the CBS domain.24 The γ subunit has three isoforms (γ1, γ2, and γ3) that have distinct functions. The γ1 isoform was found to regulate glucose metabolism in the liver and skeletal muscle in metformin-treated AMPKγ1H151R transgenic mice.25,26 Other studies have shown that the γ2 isoform is crucial for heart rate control and that the γ3 isoform regulates human lipoprotein metabolism.27,28
Regulation of AMPK
AMPK senses cellular energy depletion through its ability to detect rising levels of AMP and adenosine diphosphate (ADP). When energy stress occurs, the increase in cellular AMP results in AMP outcompeting ATP for binding to AMPK, and this binding triggers a conformational change in AMPK that activates its kinase.29 When there are high levels of ADP, it can bind to and activate AMPK via the same mechanism.12 Notably, ADP dominantly controls AMPK activation in skeletal muscle cells during exercise.30 Furthermore, AMP binding to the γ subunit of AMPK enhances AMPK activation through Thr172 phosphorylation by upstream kinases, such as LKB1 and CaMKKβ.10,11,23,31,32
Interestingly, when activated LKB1 contains mutations, it can still support cancer cell viability, proliferation, and metastasis.33 Additionally, the AMPK inhibitor BAY-3827 can increase phosphorylation at Thr172 without activating AMPK, indicating the presence of additional AMPK regulatory mechanisms.34 It has also been reported that protein phosphatase 2C (PP2C) and PH-domain leucine-rich repeat protein phosphatase 2 (PHLPP2) can deactivate AMPK by removing the phosphate group from Thr172, and this has been shown to induce various cellular processes, including cell death.35,36 Other studies have shown that AMPK can also be activated by high intracellular calcium levels, a hallmark of cellular stress, via CaMKKβ37 and that inhibition of CaMKKβ by STO-609 suppresses AMPK activation in LKB1-deficient cell lines.38 The CaMKKβ-dependent pathway that results in AMPK activation is described in detail elsewhere.39
It is important to note that AMPK can also be activated by other conditions. For example, low glucose or low oxygen (hypoxia) can activate AMPK as a cellular response to metabolic stress.40 Interestingly, hypoxia can trigger AMPK activation via a pathway involving reactive oxygen species (ROS)-mediated CaMKKβ activation.41 Collectively, AMPK regulation is shown in Figure 1.
Energy Regulation by AMPK
Acting as a cellular energy switch, AMPK regulates carbohydrate, lipid, and protein metabolism by catalyzing the phosphorylation of various key metabolic enzymes. In 1987, AMPK was first found to regulate fat and cholesterol production. AMPK has a direct effect on these processes by inhibiting acetyl coenzyme A carboxylase (ACC)1, which is crucial for fatty acid synthesis, and 3-hydroxy-4-methylglutaryl coenzyme A (HMG-CoA) reductase, the key enzyme in cholesterol production.42 Furthermore, AMPK promotes beta oxidation by inhibiting ACC2, an enzyme that suppresses this process. Additionally, AMPK inactivates sterol regulatory element-binding protein 1c (SREBP1c) and carbohydrate-responsive element-binding protein (ChREBP), two transcription factors that promote fat synthesis. These actions position AMPK as a promising target for treating fatty liver disease and dyslipidemia.43
Given that AMPK has been implicated in blood sugar regulation, it is being assessed as a target for treating type 2 diabetes mellitus (T2DM). Studies have shown that AMPK can inhibit gluconeogenesis and glycogen synthesis in the liver and that it can promote glucose uptake by skeletal muscle cells by enhancing glucose transporter (GLUT) 4 translocation and cell-surface expression.44,45 AMPK also enhances the translocation of GLUT1; however, more studies are necessary to confirm this phenomenon and its effects in living organisms.46
Under stress conditions, cells prioritize energy conservation by ceasing protein synthesis. AMPK triggers this process by terminating three key signaling pathways: translation initiation via suppressing mammalian target of rapamycin complex 1 (mTORC1), translation elongation by activating eukaryotic elongation factor 2 kinase (eEF2K), and ribosome biosynthesis through the inhibitory phosphorylation of Transcriptional initiation factor 1A (TIF-1A).47–49
Key Biological Activities of AMPK in Intestinal Diseases
The intestines are vital organs responsible for nutrient absorption and act as the first line of defense against external pathogens. Maintaining intestinal health is crucial for overall well-being, as their disruption can lead to various diseases, including IBD and colorectal cancer. AMPK plays a pivotal role in regulating cellular metabolism and stress responses. Emerging evidence suggests that AMPK plays a crucial role in maintaining intestinal health by exerting various biological activities.
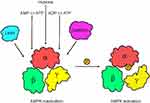
This section delves into the key biological activities of AMPK in intestinal health and disease, focusing on its roles as (i) a growth suppressor, (ii) an inflammation and stress modulator, (iii) an autophagy inducer, and (iv) a regulator of gut barrier function (Figure 2). By understanding these versatile functions of AMPK can pave the way for exploring its therapeutic potential in addressing various intestinal disorders and promoting gut health.
AMPK and Growth Suppression
AMPK is known to modulate the function and metabolism of tumor cells.50 It has been found to not only regulate diverse cellular responses pivotal to tumor cell growth, proliferation, and survival but also suppress tumor cells by regulating oncogenic metabolic processes and promoting cell-cycle arrest and pathways related to glucose, lipid, and protein biosynthesis.51 Given these findings, and the fact that AMPK has been shown to significantly arrest the cell cycle in a variety of tumor cell types (notably, melanoma and lung, colorectal, liver, and prostate cancer cells), it has potential as a therapeutic target in multiple types of cancer.52,53
In colorectal cancer (CRC), the third most common type of cancer, tumor cells perform aerobic glycolysis—also known as the Warburg effect—to promote tumor metastasis and remodel the tumor microenvironment.54,55 AMPK activation has been shown to counteract tumor progression by dampening the Warburg effect in tumor cells and inhibiting cell growth via the inactivation of the mammalian target of rapamycin (mTOR) signaling and suppression of hypoxia-inducible factor-1 alpha (HIF-1α), implicating the tumor-suppressor role of LKB1, the upstream AMPK kinase.56,57 In addition, relatively lower levels of phosphorylated AMPK have been found in patients with CRC.58 It should also be noted that pharmacological activation of AMPK with 5-aminoimidazole-4-carboxamide-ribonucleoside (AICAR) alone or in combination with chemotherapies has been reported to be cytotoxic and induce cell apoptosis in CRC cells, demonstrating the benefits of targeting AMPK in cancer therapy.51,59 In contrast, AMPK also reportedly promotes tumor cell survival and protects tumor cells against cellular stress via oncogenic phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) signaling.50,60 Treating CRC stem cells with the known AMPK inhibitor compound C has been shown to result in cell death.60 Hence, current evidence indicates that AMPK plays the dual roles of tumor promoters and tumor suppressors in tumorigenesis, depending on the context.
AMPK and Inflammation and Stress
Inflammatory processes are typically initiated when stimuli are detected, such as pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs). Subsequently, intracellular inflammatory signaling pathways and molecules are activated, such as nuclear factor kappa B (NF-κB), mitogen-activated protein kinases (MAPKs), Janus kinase-signal transducers and activators of transcription (JAK-STAT), and inflammasomes, and these are extensively reviewed in the literature.61,62
Given its well-established role as a regulator of cellular energy and metabolic homeostasis, AMPK has garnered considerable attention in immunology regarding its potential applications in the treatment of inflammation and related diseases. Both pro-inflammatory and anti-inflammatory signals can influence AMPK activity.63 Compound C (an AMPK inhibitor) has been shown to suppress stimulator of interferon genes (STING)-mediated production of interferon (IFN)-β and tumor necrosis factor (TNF),64 as well as the production of type I IFN in response to double-stranded DNA (dsDNA)-dependent type I IFN induction.65 AMPK also plays a role in the interleukin (IL)-10-mediated anti-inflammatory process.66 AICAR (an AMPK activator) can reduce inflammation by preventing the nuclear translocation of the NF-κB p65 subunit.67 Additionally, AICAR inhibits the IL-6-mediated signal transducer and activator of transcription 3 (STAT3) phosphorylation in HepG2 cells.68 Treating mice with another AMPK activator, A-769662, before exposing them to lipopolysaccharide (LPS), reduces LPS-induced IL-6 secretion and lung injury.69 Notably, AICAR ameliorates 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced acute colitis in mice.70
Inflammasome is a cellular component involved in innate immunity that consists of multiprotein complexes activated by various stimuli. When activated, it releases pro-inflammatory cytokines (IL-1β and IL-18) and triggers a type of inflammation-related cell death called pyroptosis. Activation of the nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3 (NLRP3) inflammasome plays a significant role in colitis in mice, with deficiencies in inflammasome components exacerbating the disease in dextran sulfate sodium (DSS)-induced colitis models.71,72 AMPK has been found to regulate the activation of the NLRP3 inflammasome caused by different stimuli, including autophagy, mitochondria, endoplasmic reticulum (ER) stress, and sirtuin (SIRT)1.73–76 However, there is a need for further research in this area, as AMPK inhibition has been reported to cause both NLRP3 inflammasome activation and inhibition.77,78
Beyond its role in immune signaling, AMPK is crucial for immune cell function, especially in T cells and macrophages. These cells are key players in various inflammatory diseases, and their functioning affects disease outcomes. Here, we focus on one such inflammatory disease: colitis, a multifactorial disease with various etiologies, including infection, inflammation, autoimmunity, ischemia, and drug-induced factors. Colitis can partially be attributed to abnormal T-cell metabolism, with high glucose consumption worsening colitis in T cell transfer colitis models.79 AMPK has been shown to facilitate metabolic adaptation in T cells when nutrients are limited or altered80 and to be essential for T-cell expansion but not to influence the fate or differentiation of T cells in T cell-mediated chronic colitis.80 Interestingly, mice that lacked T cells and received AMPKα-deficient CD4+ T cells were found to develop less severe colitis.81 Mogrol, an active compound in some traditional Chinese medicines, was found to alleviate the severity of DSS-induced colitis in mice through AMPK activation.82
Metformin treatment reduces regulatory T cell (Treg) numbers, and AMPKα1-deficient Tregs have decreased suppressive function.83,84 AMPK enhances the suppressive function of Tregs by directly phosphorylating forkhead box P3 (FoxP3).84 However, the role that AMPK plays in Treg function remains unclear, as some studies have shown no significant differences in the suppressive function of Tregs and AMPK-deficient Tregs.80
The polarization of macrophages into the pro-inflammatory (M1) or anti-inflammatory (M2) phenotype significantly influences the development of various inflammatory diseases, including colitis.85 Similar to the situation in T cells, AMPK plays a pivotal role in regulating macrophage homeostasis and functions. AMPKα1-deficient macrophages fail to polarize into the M2 phenotype and exhibit impaired phagocytic activity in murine models of muscle regeneration.86 Furthermore, AMPKβ1-deficient mouse macrophages tend toward the M1 phenotype, with upregulation of pro-inflammatory cytokine mRNA (ie, TNF-α, IL-6, and IL-1β mRNA) after palmitate stimulation.87 Treating mouse bone marrow-derived macrophages (BMDMs) with metformin inhibits LPS-induced pro-inflammatory cytokine (TNF-α and IL-1β) secretion and promotes anti-inflammatory cytokine (IL-10) secretion.88
Neutrophils are essential for combating infections; however, they can also contribute to the pathogenesis of colitis. Neutrophil infiltration is common in ulcerative colitis (UC) and correlates with disease severity.89 Furthermore, neutrophil extracellular traps (NETs), which are released by neutrophils to combat pathogens, contribute to ongoing intestinal inflammation in UC.90 A novel highly potent AMPK activator, IM156, has been found to inhibit LPS-induced ROS production and NET formation in mouse neutrophils.91 In another study, it was found that AMPK regulates the neutrophil secretion of matrix metalloproteinases 8 (MMP-8), the zinc-dependent proteolytic enzymes that contribute to several pathological processes.92
A major challenge in translating preclinical AMPK findings to the clinic is the context-specificity of its effects, which vary depending on the inflammatory stimulus, cell type, disease model, and the specific AMPK modulator used. Conflicting data, particularly regarding NLRP3 inflammasome activation and Treg function, highlight the need for standardized protocols and rigorous analysis. While some AMPK-mediated inflammatory mechanisms are known, many remain unclear, hindering the development of targeted therapies. Furthermore, the interplay between AMPK and the gut microbiota, a key player in intestinal inflammation, requires further investigation, as it could significantly impact disease development and treatment.
AMPK and Autophagy
Autophagy serves as a cellular quality control system, ensuring that cellular homeostasis is maintained by degrading and recycling cellular components. It helps cells conserve energy for survival and provides protection against various stressors, particularly under glucose deprivation conditions.93–95 In non-starved cells, autophagy involves the selective degradation of damaged cellular components.96 Autophagy also plays a pivotal role in preserving intestinal balance, mediating the interplay between the gut microbiota and immune system and enhancing host defense against intestinal pathogens.
Patients with inflammatory bowel disease (IBD) exhibit defective autophagy, which is associated with mutation of the ATG16L1 gene and impaired intestinal barrier integrity.97
When the nutrient supply is sufficient, mTORC1 phosphorylates UNC-51-like kinase 1 (ULK1) at Ser757, which disrupts the AMPK–ULK1 complex and impedes autophagy. AMPK has been found to have dual, opposing effects on autophagy. Activating AMPK with AICAR, A769662, and metformin inhibits autophagy,98–102 whereas AMPK can phosphorylate and activate ULK1 at Ser317 and Ser777 to promote autophagy under glucose starvation.102,103
In CRC, autophagy can have either pro- or anti-tumorigenic effects, depending on the genetic mutation(s) present and the tumor type and stage.104–106 Autophagy can bolster tumor development by stabilizing the fate of cells and enabling cellular tolerance to environmental stress. Conversely, it can induce apoptosis and cell death in tumor cells, highlighting its implications for CRC treatment strategies and treatment resistance.107,108
In several studies, CRC patients were found to have higher levels of various proteins, such as microtubule-associated protein 1 light chain 3 beta (LC3B), Beclin 1 (BECN1), lysosome-associated membrane glycoprotein 3 (LAMP3), and autophagy-related proteins (eg, ATG5 and ATG7).105–107 It was also reported that suppression of ATG5 and ATG7 resulted in tumor-specific AMPK activation and consequent AMPK-induced cell cycle arrest and cell death, which suggests that these autophagy markers have pro-tumorigenic activity and are potential targets in CRC therapy.109–112 In addition, the level of ATG10 was found to be relevant to tumor metastasis.113 However, in other studies, BECN1, LC3B, and ATG5 loss correlated with poor prognosis in CRC patients.110 Furthermore, knocking down ATG5 or ATG7 has been shown to ameliorate apoptosis in CRC cells by suppressing autophagy, suggesting that autophagy has an anti-tumorigenic function.114,115 These findings imply that autophagy has dual effects on CRC development. To sum up, they highlight the importance of AMPK in autophagy-related diseases and underscore its potential as a therapeutic target. However, more research is needed to understand its mechanisms and develop effective intervention for IBD and CRC.
AMPK also plays a role in smooth muscle contractility, particularly in functional gastrointestinal disorders like diabetic gastroparesis (DGP). DGP is a common complication of diabetes mellitus, marked by impaired gastric motility, which is associated with oxidative stress and apoptosis of gastric smooth muscle cells (GSMCs), impairing gastric emptying.2 This GSMC apoptosis gradually increases in diabetes, driven by pathways such as PI3K-AKT-mTOR and AMPK-mTOR.116 Recent studies have focused on AMPK’s contribution in these effects, particularly under hyperglycemic conditions.117 Interestingly, AMPK activity exhibits a biphasic pattern in diabetes: decreasing in early stages but subsequently increasing as the disease progresses.118 This later increase in AMPK activity contributes to GSMC apoptosis by modulating key proteins, including p53 and Bcl-2.119 Furthermore, AMPK promotes a metabolic shift from mitochondrial respiration to glycolysis, leading to cellular dysfunction and reduced muscle contraction.2 In contrast to its detrimental role in DGP, AMPK can exert protective effects in cardiomyocytes, by maintaining cellular energy homeostasis and mitigating oxidative stress.120 However, within smooth muscle itself, AMPK’s actions can be complex. For instance, pro-inflammatory cytokines can induce hypercontractility in intestinal smooth muscle through an NF-κB/PKA pathway that inhibits AMPK and subsequently activates MLCK, ultimately increasing muscle contraction.121 This apparent paradox, where AMPK can be both detrimental and protective in smooth muscle, highlights the need for further research to fully elucidate AMPK’s precise role in DGP and to explore its potential as a therapeutic target.
AMPK and Gut Barrier Function
The gastrointestinal barrier, established by the epithelial lining, is part of the physical defense system that separates the body from the external environment. Tight junctions between epithelial cells regulate the paracellular transport of some nutrients and solutes across this barrier, while limiting the entry of noxious substances into the body. In this section, the involvement of AMPK in the regulation of paracellular permeability in the gut epithelium and in leakage-associated gastrointestinal diseases is discussed.
Tight junctions comprise a string of transmembrane molecules and dynamically aggregated cytoplasmic proteins. The transmembrane proteins that primarily contribute to epithelial integrity at cell–cell junctions include occludin, claudins, and junctional adhesion molecules (JAMs). These proteins bind to cytoplasmic anchoring proteins, such as zonula occludens-1 (ZO-1), cingulin, and afadin, which are connected to the cytoskeleton’s filamentous actins and microtubules.122
In 2006, the first evidence of AMPK’s involvement in the control of tight junctions was obtained using MDCK cells; it was found that AMPK can promote the reassembly of ZO-1 in the paracellular area.123 Since then, several studies have shown that activating AMPK, via LKB1 or CaMKKβ activation, can enhance tight junction assembly and gut epithelial barrier integrity.124–129 Recent research has identified several tight junction components as downstream targets of AMPK and two primary pathways that mediate this activity. First, the direct phosphorylation of tight junction proteins has been reported. AMPK-mediated phosphorylation of claudin-1 has been shown to prevent its degradation, and occludin binding was found to be promoted by AMPK-mediated phosphorylation of claudin-4.130,131 However, further studies are required to validate this activity in gut epithelial cells. Second, the phosphorylation of scaffold proteins has been reported. AMPK-mediated phosphorylation of cingulin has been demonstrated to improve the tight junction–cytoskeleton interaction by enhancing microtubule binding while reducing actin affinity.132,133 In addition, the actin-binding protein Girdin, which is crucial for maintaining apical polarity, can be directly phosphorylated by AMPK under energy deprivation conditions.134 Furthermore, AMPK indirectly regulates tight junction permeability through the phosphorylation of afadin and myosin light-chain kinase (MLCK), which are involved in F-actin remodeling.135,136 These mechanisms have primarily been observed in studies conducted with non-gut cells; however, these findings suggest that similar mechanisms occur in the gut. In short, AMPK promotes tight junction assembly and preserves tight junction integrity in response to cellular stress via the phosphorylation of claudin and tight junction scaffold proteins.
AMPK Activators
Natural Compounds
Given that AMPK plays a critical role in numerous bodily systems, researchers are actively exploring ways to stimulate its activity, particularly through the use of various natural compounds. These compounds are attractive due to their accessibility, potential for therapeutic development, and often lower toxicity compared to synthetic counterparts. Natural products, especially those used in traditional medicine, offer a rich source of bioactive compounds for managing various health conditions, including metabolic and inflammatory disorders. This is highlighted in several previous studies, which emphasizes the importance of bridging traditional knowledge with modern scientific research to unlock the therapeutic potential of these compounds.137,138 However, these studies also acknowledge the limitations of natural compounds, such as variability in composition and potency, and the need for further research to validate their efficacy and safety. This aligns with the growing interest in AMPK activators for treating conditions like diabetes, inflammation, cancer, and metabolic disorders. A list of natural AMPK activators can be found in Table 1.
 |
Table 1 AMPK Natural Activators |
Polyphenols, particularly flavonoids, are promising natural AMPK activators. Flavonoids have been extensively studied due to the potential benefits they offer to people with various health conditions, including cancer, inflammation, autophagy, diabetes, obesity, and fatty liver disease.185 The flavonoids epigallocatechin gallate (EGCG), kaempferol, quercetin, genistein, and naringenin are well-known examples of potent AMPK activators.185,186 EGCG, found abundantly in green tea extract, has been shown to suppress cancer cell proliferation by activating AMPK and subsequently inhibiting cyclooxygenase-2 (COX-2) in HT-29 colon cancer cells.139 Furthermore, EGCG can partially counteract insulin secretion by activating AMPK and mitigating the insulin-lowering effects of glutamate dehydrogenase in primary human myocytes.140 EGCG can also activate CaMKKβ and LKB1, the upstream kinases of AMPK, by increasing cytosolic calcium and phosphorylation, respectively.141–143 Kaempferol, a flavonol found in various plants (eg, grapes, kale, and strawberries), can activate AMPK, leading to enhanced glucose uptake in skeletal muscle, and induce autophagy by inhibiting mTOR in human hepatic cancer cells.145,146 Moreover, kaempferol can reduce lipid accumulation and attenuate nonalcoholic fatty liver disease (NAFLD) via SIRT1/AMPK signaling in an in vivo mouse model of T2DM.147 Quercetin, found in abundance in grapes, apples, and onions, has been shown to modulate the AMPK/SIRT1/NF-κB pathway, suppressing inflammation and oxidative stress in diabetic rats with atherosclerosis.148 In addition, quercetin can activate AMPK via LKB1, stimulating peroxisome proliferator-activated receptor (PPAR)α expression and decreasing lipid accumulation in the abdominal fat of broiler chickens.149 Similarly, genistein, a compound found in soy products, can activate AMPK through SIRT1 and LKB1, leading to autophagy and reduced lipid accumulation.152,153 Naringenin from citrus fruits, especially grapefruit, has been found to increase phosphorylation directly at AMPKα and CaMKKβ to alleviate NAFLD and regulate autophagy.154 Other polyphenols, including chalcone, curcumin, tannin, chlorogenic acid, and resveratrol, have also been demonstrated to activate AMPK, either directly or through its upstream kinases.155–159 Clinical trials have investigated several natural compounds from this group, including EGCG and Quercetin. While EGCG has been studied for its impact on cognition, mood, and brain function, its mechanism of action remains unclear.187 Quercetin’s anti-inflammatory and antioxidant properties have also been explored in clinical trials. One study, using a supplement combining quercetin with resveratrol, pterostilbene, Morin hydrate, δ-tocotrienol, riboflavin, and nicotinic acid, demonstrated a reduction in cardiovascular risk factors.150 Furthermore, both EGCG and quercetin have demonstrated a reduction in the risk of adverse outcomes for individuals with Inflammatory Bowel Disease.144,151
Glycosides, a class of compounds characterized by a sugar moiety linked to a non-sugar component, can effectively activate AMPK. Of particular note is the C-glycosides subgroup, which includes stilbene glycoside and flavonoid C-glycosides. Stilbene glycoside has demonstrated potential in mitigating neurodegenerative diseases and neuroinflammation by activating AMPK and modulating the SIRT3, PTEN-induced kinase 1 (PINK1)/Parkin, and Tet methylcytosine dioxygenase 2 (Tet2) signaling pathways.160,161,188 Flavonoid C-glycosides, such as baicalin, which is found in members of the Lamiaceae plant family, can mediate autophagy through AMPK activation and downstream pathways, such as the PGC-1α and mTOR signaling pathways.162,163 Additionally, other glycosides (eg, O-glycoside, stevioside, and ginsenoside) have been shown to activate AMPK and regulate fatty acid metabolism.164,168,189 Regarding the application of these compounds, stevioside, commonly found in stevia, is a prominent and widely recognized substance in this group. It is used as a sugar substitute and has been shown to reduce cardiovascular risk. Studies supplementing individuals with T2DM with stevioside for 24 weeks have demonstrated improved cardiometabolic risk in diabetic patients with an increase in total caloric intake and a decrease in BMI, waist circumference, waist-hip ratio, and fat mass index in the obese group.165,166 However, there is limited clinical research on its effects on gastrointestinal diseases, with most studies conducted on animals. For example, one study found that stevioside supplementation can alleviate lipopolysaccharide-induced intestinal mucosal damage through anti-inflammatory and antioxidant effects in broiler chickens.167
Alkaloids have also been shown to activate AMPK and thus have various biological effects. For example, hernandezine and thalidezine can induce autophagy, which indicates that they could potentially be utilized in cancer treatment.169,171 Additionally, it has been suggested that due to their AMPK-stimulating activity, hernandezine, berberine, and piperine could be used to reduce glucose for alleviating diabetes symptoms.170,172,176 Moreover, AMPK activation by alkaloids such as piperine and bouchardatine can contribute to reduce lipid metabolism, potentially alleviating obesity and metabolic disorders.177,179 Clinical trials investigating this group of compounds are still limited. However, some promising findings have emerged. For instance, piperine, found in black pepper, has been shown to reduce oxidative stress and inflammation in hemodialysis patients when combined with turmeric.178 Berberine, another compound in this group, has demonstrated cholesterol-lowering effects in patients with high cholesterol and can also reduce blood sugar levels in individuals with type 2 diabetes.173,174 In regards to intestinal health, a study in cats showed that berberine helped restore intestinal mucosal barrier function, suggesting a potential benefit for Inflammatory Bowel Disease (IBD).175
Oligosaccharides, including chitosan oligosaccharides (COS), fructooligosaccharides (FOS), and galactooligosaccharides (GOS), are prebiotics that can improve gut microecology and protect the gut barrier. COS, derived from chitin found in marine animal shells, have been shown to promote intestinal tight junctions by activating AMPK through the calcium-sensing receptor (CaSR)–phospholipase C (PLC)–inositol triphosphate (IP3) receptor pathway in T84 colon cancer cells.127,180 Similarly, FOS can strengthen the intestinal barrier by activating AMPK through the CaSR–PLC–CaMKKβ pathway.182 Additionally, GOS have been found to alleviate intestinal oxidative stress and dysfunction in LPS-challenged suckling piglets by activating AMPK and improving the expression of heme oxygenase-1 (HO-1) and nicotinamide adenine dinucleotide phosphate (NADPH).184 Many compounds in this group are commonly used in supplements, leading to a considerable number of clinical trials. For example, research on FOS has shown its impact on gut microbiota, which in turn affects various conditions like IBD.183,190 Similarly, chitosan oligosaccharide (COS) is widely used, particularly in supplements. Clinical trials have demonstrated that COS can modulate gut microbiota, leading to improved antioxidant activity and protection against coronary heart disease (CHD).181
While this is just a glimpse of the existing research on natural AMPK activators, it’s clear that these compounds hold significant promise. Studying them not only deepens our understanding of metabolic regulation but also opens doors to new AMPK-based therapies and interventions for a wide range of health issues.
Synthetic Compounds and Repositioned Drugs
In recent years, synthetic compounds and small molecules have emerged as powerful tools for modulating AMPK activity. By targeting specific sites on the AMPK protein, these molecules can activate AMPK, leading to a cascade of cellular responses. These compounds offer a precise and controlled approach to study AMPK signaling and its potential therapeutic applications, particularly in intestinal diseases such as IBD and colorectal cancer. A list of synthetic AMPK activators is provided in Table 2.
 |
Table 2 Synthetic Compounds and Repositioned Drugs |
Numerous synthetic compounds have been identified as AMPK activators. AICAR, a nucleotide analog, has been a cornerstone in AMPK research for decades. It is primarily known for its ability to activate AMPK by mimicking AMP, a key allosteric activator. However, AICAR’s biological effects extend beyond AMPK modulation.191 Several studies have demonstrated its potential in cancer therapy; it has been shown to induce differentiation of acute myeloid leukemia cells.228 Additionally, it activates large tumor suppressor kinase 1 and 2 (Lat1 and Lat2) in AMPK-double-knockout murine embryonic cells, suggesting a broader range of cellular targets.229 Despite its versatility, AICAR is still widely used as a positive control in AMPK-activation experiments, underscoring its reliability and significance in this field.230 From a clinical perspective, AICAR shows therapeutic potential by enhancing glucose transport and strengthening barrier function, while simultaneously reducing inflammatory cell infiltration.3,192
A-769662 directly activates AMPK through an allosteric mechanism, bypassing the need for changes in AMP or ADP levels or upstream kinases.193 With an EC50 of approximately 0.39 µM, it effectively stimulates AMPK activity.231 Studies have demonstrated that A-769662 has anti-inflammatory properties, showing that it reduces LPS-induced inflammation in the heart and lungs and inhibits IL-6 expression in inflammatory arthritis.232,233 Additionally, it can mitigate acute lung injury caused by sepsis through its AMPK-activating effect.234 A-769662 also exhibits AMPK-independent effects. It can inhibit glucose uptake in adipocytes, suppress insulin-induced nitric oxide synthesis and AKT phosphorylation in endothelial cells, and enhance intracellular calcium and ATP release from astrocytes.235–237 Collectively, A-769662 is a promising AMPK activator with a broader range of biological activities.
Compound 991, a benzimidazole derivative, is an even more potent AMPK activator than A-769662; it has an EC50 of approximately 0.09 µM and effectively activates AMPK through an allosteric mechanism.231 Compound 991 exhibits selectivity for the β1 isoform of AMPK, leading to its phosphorylation and activation. Notably, it demonstrates a stronger preference for the γ2 isoform compared to the γ1 and γ3 isoforms, although it can also activate the γ1 and γ3 isoforms in skeletal muscle cells.194 Furthermore, compound 991 has been shown to protect osteoblasts from dexamethasone-induced damage by activating AMPK.238 In summary, compound 991’s potency, selectivity, and ability to target various AMPK subunits make it a valuable tool for studying the mechanisms of AMPK in experimental settings.
PT-1 directly activates AMPK (independent of the AMP or ADP level) by binding to the γ1 isoform within the kinase domain.236 This interaction stimulates all three AMPK subunits. PT-1 has an EC50 of approximately 0.3 µM.239 While PT-1 stimulates all three γ isoforms, it has been observed to inhibit the respiratory chain in human cells, suggesting potential off-target effects.195
MK-8722 is a highly potent small-molecule AMPK activator that acts in an allosteric manner and targets the β1 isoform.194,238 MK-8722-mediated activation leads to increased AMPK phosphorylation and activity.196,197 Interestingly, MK-8722 exhibits anti-cancer properties. It suppresses the proliferation, migration, and invasion of pancreatic cancer cells and modulates lipid metabolism in epithelial ovarian cancer cells.240,241 Additionally, MK-8722 induces early-stage autophagy; therefore, it may play a role in cancer cell death.241
PF-06409577 is a potent and selective allosteric activator of AMPK that directly binds to AMPK and induces strong and sustained AMPK activation.196,197 Its EC50 for AMPK activation is approximately 7 nM, and it stimulates all three AMPK subunits.198,199 This molecule also inhibits renal cyst formation and cystic fibrosis transmembrane conductance regulator (CFTR) activity via the mTOR pathway.242
Additionally, drug repositioning has gained significant attention as a strategy to identify novel AMPK activators. By leveraging these approaches, researchers have discovered a diverse range of compounds that can activate AMPK. Repositioned drugs also are listed in Table 2.
Nonsteroidal anti-inflammatory drugs (NSAIDs) and salicylate are available as over-the-counter (OTC) analgesics and anti-inflammatory agents. Salicylic acid, an active metabolite of acetyl-salicylic acid (aspirin), has been documented to activate AMPK; hence, it could potentially be used to mitigate intestinal inflammation via multiple pathways.243–245 In addition, aspirin has been shown to inhibit breast cancer cell proliferation by AMPK-dependent mTORC1 inhibition and autophagy activation.203 Interestingly, the combination of metformin and salicylate promotes insulin sensitivity and inhibits lipogenesis in the liver via AMPK activation.200 Moreover, salicylate can suppress CRC metastasis via AMPK-mediated inhibition of c-MYC.246 Therefore, it has been shown that this well-known and popular drug (salicylate) can modulate the cell’s energy status by activating AMPK. Other NSAIDs, such as diclofenac and ibuprofen, reportedly activate AMPK in intestinal, liver, and neuronal cells.201,202 In addition, celecoxib halts chronic myelogenous leukemia (CML) cell proliferation and survival via AMPK/β-catenin/mTORC1/2 pathway.204
Metformin, a biguanide widely used as a first-line antidiabetic drug, activates AMPK through multiple mechanisms. Initially, it was thought to indirectly increase AMPK activity by increasing AMP levels and inhibiting mitochondrial respiration.205 However, recent studies have revealed more complex interactions. Metformin can directly modulate the AMPK heterotrimeric complex, promoting its phosphorylation by LKB1 and preventing its dephosphorylation by protein phosphatase.206,207 This activity contributes to metformin’s anti-inflammatory effects, mediated by the inhibition of NF-κB.208 Extending beyond its primary anti-hyperglycemic and anti-inflammatory functions, metformin has also been found to have metabolic, cardioprotective, neuroprotective, anti-cancer, and anti-microbial activity.208 It was recently demonstrated that at a low dose, metformin binds to the presenilin enhancer 2 (PEN2) subunit of γ-secretase to form a complex that inhibits vacuolar-type ATPase (V-ATPase) and AMPK activation, independent of AMP levels.209 In addition to its AMPK-activating effects, metformin has shown promise as a therapeutic agent for the treatment of a range of health conditions, including diabetes, inflammation, and liver disease, as well as for gut microbiota regulation.247–250 Like metformin, phenformin can activate AMPK by increasing the intracellular AMP level. However, its potential extends beyond glucose regulation.251 Studies have demonstrated that phenformin has anti-tumor effects, particularly in skin and breast cancers. For example, it enhanced keratinocyte differentiation in a mouse skin carcinogenesis model and suppressed pro-inflammatory cytokines in keratinocytes via downregulation of c-Myc expression.210,211 Moreover, phenformin has been shown to inhibit cell growth and promote apoptosis and autophagy in cholangiocarcinoma and breast cancer cells.212,213 In another study, it was found that oral phenformin therapy suppressed the progression of T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) by activating AMPK in a cell-autonomous manner.214 Despite its promising effects, phenformin’s clinical use is limited due to its association with lactic acidosis, a severe side effect.211 As a result, it remains primarily a research tool for studying AMPK activation.252 Thiazolidinedione (TZD) is an insulin-sensitizing agent used in the treatment of T2DM that binds to the nuclear receptor PPARγ in adipocytes and thus stimulates adipogenesis.253 This action enhances insulin sensitivity by upregulating the expression and release of adiponectin, an adipokine that activates AMPK.215 Two other members of the same class of drugs, rosiglitazone and pioglitazone, also trigger AMPK activation, and platelet aggregation is inhibited in these cases. Hence, AMPK is a potential therapeutic target due to this anti-platelet aggregation activity of rosiglitazone and pioglitazone.216 Finally, it has been revealed that dipeptidyl peptidase-4 (DPP4) inhibitors, the antidiabetic drugs that prevent the cleavage of incretin hormones by DPP4, increase the level of AMPK and suppress hepatic lipogenic gene expression in NAFLD models.217–219 Sitagliptin has been shown to inhibit adipose tissue inflammation and fatty liver disease by regulating adiponectin and AMPK levels.220 Saxagliptin has been found to improve endothelial aging by modulating the AMPK/SIRT1/nuclear factor erythroid 2-related factor 2 (Nrf2) pathway.221 Additionally, linagliptin has demonstrated efficacy in alleviating acetic acid-induced colitis through the regulation of the AMPK/SIRT/peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1α) and JAK2/STAT3 pathways.222
Bromocriptine, a dopamine D2 receptor agonist used in patients with prolactinomas, acromegaly, Parkinson’s disease, and hyperprolactinemia-associated conditions, has been shown to effectively control blood glucose levels and improve insulin resistance in individuals with T2DM.223 It lowers GLUT2 levels and postprandial insulin resistance, thereby relieving fatty liver conditions that involve AMPK activation.224 Additionally, bromocriptine has been found to reduce the phosphorylation of CaMKK2 and thus activate it and subsequently AMPK in a prostate cancer model.225
Fenofibrate, an agonist of PPARα, is prescribed for managing dyslipidemia and slowing the progression of atherosclerotic events in patients with T2DM.254,255 It reportedly activates AMPK and upregulates endothelial nitric oxide synthase (eNOS) in human umbilical vein endothelial cells (HUVECs).226
Pranlukast, a cysteinyl leukotriene receptor 1 (CysLT1) antagonist, is indicated for the treatment of asthma due to its capacity to inhibit chloride secretion in bronchial epithelial cells.256 In this example of drug repositioning, pranlukast has been shown to inhibit cyst progression in renal epithelial cells via activation of the CaMKKβ/AMPK/mTOR pathway.227
In conclusion, AMPK activators, including natural compounds, synthetic compounds, and repositioned drugs, hold great promise as therapeutic agents for various diseases, especially those affecting the gastrointestinal tract. By activating AMPK, these compounds can modulate cellular metabolism, reduce inflammation, and promote tissue repair. Further research is needed to fully elucidate the mechanisms of action of these compounds and to identify novel AMPK activators with improved efficacy and safety profiles. Ultimately, the development of effective AMPK activators could significantly impact the treatment of a wide range of diseases.
Future Perspectives and Conclusions
AMPK, a key regulator of cellular energy stores, is essential for intestinal health. Beyond its established metabolic role, emerging evidence suggests that AMPK exerts profound effects on intestinal cell physiology, influencing cell proliferation and differentiation, inflammation, autophagy, and barrier integrity. AMPK is activated by changes in the AMP-to-ATP ratio, which lead to conformational changes that activate the kinase and trigger various physiological activities. AMPK plays a dual role in intestinal diseases, acting as both a tumor suppressor and promoter. It can act as a tumor suppressor by regulating oncogenic metabolic processes and promoting cell cycle arrest. Conversely, it can act as a tumor promoter by activating oncogenic signaling. In the contexts of inflammation and autophagy, AMPK can influence both pro-inflammatory and anti-inflammatory signals, affecting cytokine production and inflammasome activation. Moreover, AMPK plays a crucial role in immune cell function, regulating T-cell metabolism and macrophage polarization. To maintain the integrity of the gut barrier, AMPK promotes the assembly of tight junctions and preserves their integrity by phosphorylating claudin and tight junction scaffold proteins. These actions are crucial for preventing leakage-associated gastrointestinal diseases. Research on the role of AMPK in gastrointestinal diseases remains limited. A key challenge is the context-dependent nature of AMPK’s activity, as it can function as both a tumor suppressor and a tumor promoter. A more thorough understanding of the specific conditions that determine its role in different tissues and disease states is crucial. Future studies should focus on identifying these contextual cues—including genetic mutations, microenvironmental factors, and upstream signaling pathways—to better predict and ultimately control AMPK’s effects. Furthermore, the precise mechanisms involved often remain unclear, likely because most experiments are conducted in vitro or in vivo, limiting the ability to observe the complex interplay of factors present in actual disease. Undiscovered crosstalk with other signaling pathways may also contribute to this complexity. Finally, the relatively short duration of some studies may not fully capture the long-term dynamics of chronic gastrointestinal diseases, highlighting the need for more longitudinal research.
Given the benefits associated with AMPK activation, numerous AMPK activators have been developed—both natural and synthetic. These activators have shown promising effects that can potentially be harnessed for the treatment of various health conditions, including diabetes, inflammation, cancer, and metabolic disorders. While the therapeutic potential of AMPK activation is promising, several challenges need to be overcome to fully realize its clinical applications. One major hurdle is AMPK’s widespread expression throughout the body, raising concerns about potential off-target effects. To address this, the development of tissue- or cell-specific AMPK modulators is crucial. This may involve exploring novel drug delivery systems, such as nanotechnology-based approaches, which offer enhanced bioavailability, targeted delivery, and controlled release of therapeutic agents.257 Additionally, a more comprehensive understanding of the complex molecular mechanisms underlying AMPK’s diverse actions is essential. This knowledge will enable the design of highly specific and effective AMPK-targeted therapies, minimizing the risk of unintended consequences. A critical, yet often overlooked, aspect is the pharmacokinetic (PK) and pharmacodynamic (PD) profiles of AMPK activators, especially those derived from natural compounds. Many natural AMPK activators lack comprehensive PK/PD data, hindering their clinical translation. Future studies must prioritize establishing the absorption, distribution, metabolism, and excretion (ADME) properties of these compounds, along with their dose-response relationships and duration of action. This includes determining optimal routes of administration, assessing bioavailability, and identifying potential drug-drug interactions. Preclinical studies should rigorously evaluate potential toxicities associated with long-term or excessive AMPK activation, optimizing dosage regimens and monitoring for adverse effects. In addition, anticipating and overcoming potential resistance mechanisms, particularly in cancer, will be essential for the long-term efficacy of AMPK-based therapies. Future investigations should explore combination therapies and strategies to prevent or reverse resistance development, ultimately maximizing the therapeutic benefit of AMPK activation. Finally, the development of new drugs, whether from novel compounds or through repurposing existing ones, is a complex and expensive process. It requires a rigorous approach that encompasses all stages of drug development, from in silico studies and in vitro experiments to in vivo testing and clinical trials. This comprehensive process demands significant financial investment and sustained effort to ensure the safety and efficacy of new therapies.
Acknowledgments
We thank Kristen Sadler, Ph.D., from Scribendi (www.scribendi.com) for editing a draft of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, or in all these areas; took part in drafting, revising or critically revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Mahidol University (Fundamental Fund: fiscal year 2023 by National Science Research and Innovation Fund (NSRF)).
Disclosure
The authors report no declarations of interest.
References
1. Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11(9):821–834. doi:10.1080/17474124.2017.1343143
2. Zhang MH, Fang XS, Guo JY, Jin Z. Effects of AMPK on apoptosis and energy metabolism of gastric smooth muscle cells in rats with diabetic gastroparesis. Cell Biochem Biophys. 2019;77(2):165–177. doi:10.1007/s12013-019-00870-9
3. Sun X, Yang Q, Rogers CJ, Du M, Zhu MJ. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017;24(5):819–831. doi:10.1038/cdd.2017.14
4. Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–1023. doi:10.1038/ncb2329
5. Sun X, Zhu MJ. AMP-activated protein kinase: a therapeutic target in intestinal diseases. Open Biol. 2017;7(8). doi:10.1098/rsob.170104
6. Wu Z, Xu C, Zheng T, et al. A critical role of AMP-activated protein kinase in regulating intestinal nutrient absorption, barrier function, and intestinal diseases. J Cell Physiol. 2022;237(10):3705–3716. doi:10.1002/jcp.30841
7. Olivier S, Diounou H, Pochard C, et al. Intestinal epithelial AMPK deficiency causes delayed colonic epithelial repair in DSS-induced colitis. Cells. 2022;11(4). doi:10.3390/cells11040590
8. Olivier S, Pochard C, Diounou H, et al. Deletion of intestinal epithelial AMP-activated protein kinase alters distal colon permeability but not glucose homeostasis. mol Metab. 2021;47:101183. doi:10.1016/j.molmet.2021.101183
9. King SJ, Bunz M, Chappell A, et al. AMPK mediates inhibition of electrolyte transport and NKCC1 activity by reactive oxygen species. Am J Physiol Gastrointest Liver Physiol. 2019;317(2):G171–G181. doi:10.1152/ajpgi.00317.2018
10. Hardie DG, Ashford ML. AMPK: regulating energy balance at the cellular and whole body levels. Physiology. 2014;29(2):99–107. doi:10.1152/physiol.00050.2013
11. Li J, Li S, Wang F, Xin F. Structural and biochemical insights into the allosteric activation mechanism of AMP-activated protein kinase. Chem Biol Drug Des. 2017;89(5):663–669. doi:10.1111/cbdd.12897
12. Xiao B, Sanders MJ, Underwood E, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472(7342):230–233. doi:10.1038/nature09932
13. Meng J, Fan X, Zhang M, Hao Z, Liang C. Do polymorphisms in protein kinase catalytic subunit alpha-1 gene associated with cancer susceptibility? a meta-analysis and systematic review. BMC Med Genet. 2018;19(1):189. doi:10.1186/s12881-018-0704-8
14. Abdel Malik R, Zippel N, Fromel T, et al. AMP-activated protein kinase alpha2 in neutrophils regulates vascular repair via hypoxia-inducible factor-1alpha and a network of proteins affecting metabolism and apoptosis. Circ Res. 2017;120(1):99–109. doi:10.1161/CIRCRESAHA.116.309937
15. Okamoto S, Asgar NF, Yokota S, Saito K, Minokoshi Y. Role of the alpha2 subunit of AMP-activated protein kinase and its nuclear localization in mitochondria and energy metabolism-related gene expressions in C2C12 cells. Metabolism. 2019;90:52–68. doi:10.1016/j.metabol.2018.10.003
16. Yang JH, Niu W, Li Y, Azadzoi KM. Impairment of AMPK-alpha2 augments detrusor contractions in bladder ischemia. Investig Clin Urol. 2021;62(5):600–609. doi:10.4111/icu.20210095
17. Cambridge EL, McIntyre Z, Clare S, et al. The AMP-activated protein kinase beta 1 subunit modulates erythrocyte integrity. Exp Hematol. 2017;45:64–68e5. doi:10.1016/j.exphem.2016.09.006
18. Choy SW, Fraser SA, Katerelos M, et al. Absence of the beta1 subunit of AMP-activated protein kinase reduces myofibroblast infiltration of the kidneys in early diabetes. Int J Exp Pathol. 2019;100(2):114–122. doi:10.1111/iep.12313
19. Katwan OJ, Alghamdi F, Almabrouk TA, et al. AMP-activated protein kinase complexes containing the beta2 regulatory subunit are up-regulated during and contribute to adipogenesis. Biochem J. 2019;476(12):1725–1740. doi:10.1042/BCJ20180714
20. Ziegler N, Bader E, Epanchintsev A, Margerie D, Kannt A, Schmoll D. AMPKbeta1 and AMPKbeta2 define an isoform-specific gene signature in human pluripotent stem cells, differentially mediating cardiac lineage specification. J Biol Chem. 2020;295(51):17659–17671. doi:10.1074/jbc.RA120.013990
21. Ali N, Ling N, Krishnamurthy S, et al. beta-subunit myristoylation functions as an energy sensor by modulating the dynamics of AMP-activated Protein Kinase. Sci Rep. 2016;6:39417. doi:10.1038/srep39417
22. Neopane K, Kozlov N, Negoita F, et al. Blocking AMPK beta1 myristoylation enhances AMPK activity and protects mice from high-fat diet-induced obesity and hepatic steatosis. Cell Rep. 2022;41(12):111862. doi:10.1016/j.celrep.2022.111862
23. Willows R, Navaratnam N, Lima A, Read J, Carling D. Effect of different gamma-subunit isoforms on the regulation of AMPK. Biochem J. 2017;474(10):1741–1754. doi:10.1042/BCJ20170046
24. Nakatsu Y, Iwashita M, Sakoda H, et al. Prolyl isomerase Pin1 negatively regulates AMP-activated protein kinase (AMPK) by associating with the CBS domain in the gamma subunit. J Biol Chem. 2015;290(40):24255–24266. doi:10.1074/jbc.M115.658559
25. Agius L, Ford BE, Chachra SS. The metformin mechanism on gluconeogenesis and AMPK activation: the metabolite perspective. Int J mol Sci. 2020;21(9). doi:10.3390/ijms21093240
26. Schonke M, Myers MG, Zierath JR, Bjornholm M. Skeletal muscle AMP-activated protein kinase gamma1(H151R) overexpression enhances whole body energy homeostasis and insulin sensitivity. Am J Physiol Endocrinol Metab. 2015;309(7):E679–90. doi:10.1152/ajpendo.00195.2015
27. Yavari A, Bellahcene M, Bucchi A, et al. Mammalian gamma2 AMPK regulates intrinsic heart rate. Nat Commun. 2017;8(1):1258. doi:10.1038/s41467-017-01342-5
28. Weyrich P, Machicao F, Staiger H, et al. Role of AMP-activated protein kinase gamma 3 genetic variability in glucose and lipid metabolism in non-diabetic whites. Diabetologia. 2007;50(10):2097–2106. doi:10.1007/s00125-007-0788-8
29. Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev mol Cell Biol. 2012;13(4):251–262. doi:10.1038/nrm3311
30. Coccimiglio IF, Clarke DC. ADP is the dominant controller of AMP-activated protein kinase activity dynamics in skeletal muscle during exercise. PLoS Comput Biol. 2020;16(7):e1008079. doi:10.1371/journal.pcbi.1008079
31. de Souza Almeida Matos AL, Oakhill JS, Moreira J, Loh K, Galic S, Scott JW. Allosteric regulation of AMP-activated protein kinase by adenylate nucleotides and small-molecule drugs. Biochem Soc Trans. 2019;47(2):733–741. doi:10.1042/BST20180625
32. Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9(8):563–575. doi:10.1038/nrc2676
33. Li TT, Zhu HB. LKB1 and cancer: the dual role of metabolic regulation. Biomed Pharmacother. 2020;132:110872. doi:10.1016/j.biopha.2020.110872
34. Hawley SA, Russell FM, Ross FA, Hardie DG. BAY-3827 and SBI-0206965: potent AMPK inhibitors that paradoxically increase Thr172 phosphorylation. Int J mol Sci. 2023;25(1). doi:10.3390/ijms25010453
35. Davies SP, Helps NR, Cohen PT, Hardie DG. 5’-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2C alpha and native bovine protein phosphatase-2AC. FEBS Lett. 1995;377(3):421–425. doi:10.1016/0014-5793(95)01368-7
36. Yan Y, Krecke KN, Bapat AS, et al. Phosphatase PHLPP2 regulates the cellular response to metabolic stress through AMPK. Cell Death Dis. 2021;12(10):904. doi:10.1038/s41419-021-04196-4
37. Fukumoto Y, Harada Y, Ohtsuka S, et al. Oligomerization of Ca(2+)/calmodulin-dependent protein kinase kinase. Biochem Biophys Res Commun. 2022;587:160–165. doi:10.1016/j.bbrc.2021.11.105
38. Ma Y, Yang F, Wang Y, et al. CaMKKbeta is involved in AMP-activated protein kinase activation by baicalin in LKB1 deficient cell lines. PLoS One. 2012;7(10):e47900. doi:10.1371/journal.pone.0047900
39. Najar MA, Rex DAB, Modi PK, et al. A complete map of the Calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2) signaling pathway. J Cell Commun Signal. 2021;15(2):283–290. doi:10.1007/s12079-020-00592-1
40. Dengler F. Activation of AMPK under hypoxia: many roads leading to Rome. Int J mol Sci. 2020;21(7). doi:10.3390/ijms21072428
41. Mungai PT, Waypa GB, Jairaman A, et al. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. mol Cell Biol. 2011;31(17):3531–3545. doi:10.1128/MCB.05124-11
42. Carling D, Zammit VA, Hardie DG. A common bicyclic protein kinase cascade inactivates the regulatory enzymes of fatty acid and cholesterol biosynthesis. FEBS Lett. 1987;223(2):217–222. doi:10.1016/0014-5793(87)80292-2
43. Steinberg GR, Hardie DG. New insights into activation and function of the AMPK. Nat Rev mol Cell Biol. 2023;24(4):255–272. doi:10.1038/s41580-022-00547-x
44. Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp mol Med. 2016;48(7):e245. doi:10.1038/emm.2016.81
45. Witczak CA, Sharoff CG, Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell mol Life Sci. 2008;65(23):3737–3755. doi:10.1007/s00018-008-8244-6
46. Wu N, Zheng B, Shaywitz A, et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. mol Cell. 2013;49(6):1167–1175. doi:10.1016/j.molcel.2013.01.035
47. Hsu CC, Peng D, Cai Z, Lin HK. AMPK signaling and its targeting in cancer progression and treatment. Semin Cancer Biol. 2022;85:52–68. doi:10.1016/j.semcancer.2021.04.006
48. Johanns M, Pyr Dit Ruys S, Houddane A, et al. Direct and indirect activation of eukaryotic elongation factor 2 kinase by AMP-activated protein kinase. Cell Signal. 2017;36:212–221. doi:10.1016/j.cellsig.2017.05.010
49. Hoppe S, Bierhoff H, Cado I, et al. AMP-activated protein kinase adapts rRNA synthesis to cellular energy supply. Proc Natl Acad Sci U S A. 2009;106(42):17781–17786. doi:10.1073/pnas.0909873106
50. Arkwright RT, Deshmukh R, Adapa N, et al. Lessons from nature: sources and strategies for developing AMPK activators for cancer chemotherapeutics. Anticancer Agents Med Chem. 2015;15(5):657–671. doi:10.2174/1871520615666141216145417
51. Keerthana CK, Rayginia TP, Shifana SC, et al. The role of AMPK in cancer metabolism and its impact on the immunomodulation of the tumor microenvironment. Front Immunol. 2023;14:1114582. doi:10.3389/fimmu.2023.1114582
52. Chen Z, Shen X, Shen F, et al. TAK1 activates AMPK-dependent cell death pathway in hydrogen peroxide-treated cardiomyocytes, inhibited by heat shock protein-70. mol Cell Biochem. 2013;377(1–2):35–44. doi:10.1007/s11010-013-1568-z
53. Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A. 2004;101(10):3329–3335. doi:10.1073/pnas.0308061100
54. Faubert B, Boily G, Izreig S, et al. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 2013;17(1):113–124. doi:10.1016/j.cmet.2012.12.001
55. Zhong X, He X, Wang Y, et al. Warburg effect in colorectal cancer: the emerging roles in tumor microenvironment and therapeutic implications. J Hematol Oncol. 2022;15(1):160. doi:10.1186/s13045-022-01358-5
56. Choi YK, Park KG. Metabolic roles of AMPK and metformin in cancer cells. mol Cells. 2013;36(4):279–287. doi:10.1007/s10059-013-0169-8
57. Zhan L, Su F, Li Q, et al. Phytochemicals targeting glycolysis in colorectal cancer therapy: effects and mechanisms of action. Front Pharmacol. 2023;14:1257450. doi:10.3389/fphar.2023.1257450
58. Khabaz MN, Abdelrahman AS, Al-Maghrabi JA. Expression of p-AMPK in colorectal cancer revealed substantial diverse survival patterns. Pak J Med Sci. 2019;35(3):685–690. doi:10.12669/pjms.35.3.159
59. Li W, Saud SM, Young MR, Chen G, Hua B. Targeting AMPK for cancer prevention and treatment. Oncotarget. 2015;6(10):7365–7378. doi:10.18632/oncotarget.3629
60. Guo B, Han X, Tkach D, Huang SG, Zhang D. AMPK promotes the survival of colorectal cancer stem cells. Animal Model Exp Med. 2018;1(2):134–142. doi:10.1002/ame2.12016
61. Soares CLR, Wilairatana P, Silva LR, et al. Biochemical aspects of the inflammatory process: a narrative review. Biomed Pharmacother. 2023;168:115764. doi:10.1016/j.biopha.2023.115764
62. Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–7218. doi:10.18632/oncotarget.23208
63. Sag D, Carling D, Stout RD, Suttles J. Adenosine 5’-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181(12):8633–8641. doi:10.4049/jimmunol.181.12.8633
64. Prantner D, Perkins DJ, Vogel SN. AMP-activated kinase (AMPK) promotes innate immunity and antiviral defense through modulation of stimulator of interferon genes (STING) signaling. J Biol Chem. 2017;292(1):292–304. doi:10.1074/jbc.M116.763268
65. Konno H, Konno K, Barber GN. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell. 2013;155(3):688–698. doi:10.1016/j.cell.2013.09.049
66. Zhu YP, Brown JR, Sag D, Zhang L, Suttles J. Adenosine 5’-monophosphate-activated protein kinase regulates IL-10-mediated anti-inflammatory signaling pathways in macrophages. J Immunol. 2015;194(2):584–594. doi:10.4049/jimmunol.1401024
67. Su RY, Chao Y, Chen TY, Huang DY, Lin WW. 5-Aminoimidazole-4-carboxamide riboside sensitizes TRAIL- and TNFalpha-induced cytotoxicity in colon cancer cells through AMP-activated protein kinase signaling. mol Cancer Ther. 2007;6(5):1562–1571. doi:10.1158/1535-7163.MCT-06-0800
68. Nerstedt A, Johansson A, Andersson CX, Cansby E, Smith U, Mahlapuu M. AMP-activated protein kinase inhibits IL-6-stimulated inflammatory response in human liver cells by suppressing phosphorylation of signal transducer and activator of transcription 3 (STAT3). Diabetologia. 2010;53(11):2406–2416. doi:10.1007/s00125-010-1856-z
69. Fan K, Lin L, Ai Q, et al. Lipopolysaccharide-induced dephosphorylation of AMPK-activated protein kinase potentiates inflammatory injury via repression of ULK1-dependent autophagy. Front Immunol. 2018;9:1464. doi:10.3389/fimmu.2018.01464
70. Bai A, Ma AG, Yong M, et al. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem Pharmacol. 2010;80(11):1708–1717. doi:10.1016/j.bcp.2010.08.009
71. Zhang J, Fu S, Sun S, Li Z, Guo B. Inflammasome activation has an important role in the development of spontaneous colitis. Mucosal Immunol. 2014;7(5):1139–1150. doi:10.1038/mi.2014.1
72. Allen IC, TeKippe EM, Woodford RM, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207(5):1045–1056. doi:10.1084/jem.20100050
73. Ma L, Li W, Zhang Y, et al. FLT4/VEGFR3 activates AMPK to coordinate glycometabolic reprogramming with autophagy and inflammasome activation for bacterial elimination. Autophagy. 2022;18(6):1385–1400. doi:10.1080/15548627.2021.1985338
74. Yang F, Qin Y, Wang Y, et al. Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Int J Biol Sci. 2019;15(5):1010–1019. doi:10.7150/ijbs.29680
75. Bae HR, Kim DH, Park MH, et al. beta-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget. 2016;7(41):66444–66454. doi:10.18632/oncotarget.12119
76. Price NL, Gomes AP, Ling AJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15(5):675–690. doi:10.1016/j.cmet.2012.04.003
77. Bullon P, Alcocer-Gomez E, Carrion AM, et al. AMPK phosphorylation modulates pain by activation of NLRP3 inflammasome. Antioxid Redox Signal. 2016;24(3):157–170. doi:10.1089/ars.2014.6120
78. Liu Y, He H, Fan L, et al. Compound C attenuates NLRP3 inflammasome despite AMPK knockdown in LPS plus palmitate-induced THP-1 cells. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(1):67–76. doi:10.1007/s00210-019-01712-4
79. Zhang D, Jin W, Wu R, et al. High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated tgf-beta cytokine activation. Immunity. 2019;51(4):671–681e5. doi:10.1016/j.immuni.2019.08.001
80. Mayer KA, Smole U, Zhu C, et al. The energy sensor AMPK orchestrates metabolic and translational adaptation in expanding T helper cells. FASEB J. 2021;35(4):e21217. doi:10.1096/fj.202001763RR
81. Blagih J, Coulombe F, Vincent EE, et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity. 2015;42(1):41–54. doi:10.1016/j.immuni.2014.12.030
82. Liang H, Cheng R, Wang J, et al. Mogrol, an aglycone of mogrosides, attenuates ulcerative colitis by promoting AMPK activation. Phytomedicine. 2021;81:153427. doi:10.1016/j.phymed.2020.153427
83. Michalek RD, Gerriets VA, Jacobs SR, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186(6):3299–3303. doi:10.4049/jimmunol.1003613
84. Zhu H, Liu Z, An J, Zhang M, Qiu Y, Zou MH. Activation of AMPKalpha1 is essential for regulatory T cell function and autoimmune liver disease prevention. Cell mol Immunol. 2021;18(12):2609–2617. doi:10.1038/s41423-021-00790-w
85. Zhang M, Li X, Zhang Q, Yang J, Liu G. Roles of macrophages on ulcerative colitis and colitis-associated colorectal cancer. Front Immunol. 2023;14:1103617. doi:10.3389/fimmu.2023.1103617
86. Mounier R, Theret M, Arnold L, et al. AMPKalpha1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013;18(2):251–264. doi:10.1016/j.cmet.2013.06.017
87. Galic S, Fullerton MD, Schertzer JD, et al. Hematopoietic AMPK beta1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J Clin Invest. 2011;121(12):4903–4915. doi:10.1172/JCI58577
88. Kelly B, Tannahill GM, Murphy MP, O’Neill LA. Metformin inhibits the production of reactive oxygen species from NADH:ubiquinone oxidoreductase to limit induction of interleukin-1beta (IL-1beta) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J Biol Chem. 2015;290(33):20348–20359. doi:10.1074/jbc.M115.662114
89. Bressenot A, Salleron J, Bastien C, Danese S, Boulagnon-Rombi C, Peyrin-Biroulet L. Comparing histological activity indexes in UC. Gut. 2015;64(9):1412–1418. doi:10.1136/gutjnl-2014-307477
90. Dinallo V, Marafini I, Di Fusco D, et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohn's Colitis. 2019;13(6):772–784. doi:10.1093/ecco-jcc/jjy215
91. Kang JH, Lee SK, Yun NJ, Kim YS, Song JJ, Bae YS. IM156, a new AMPK activator, protects against polymicrobial sepsis. J Cell mol Med. 2022;26(12):3378–3386. doi:10.1111/jcmm.17341
92. Ong CW, Elkington PT, Brilha S, et al. Neutrophil-derived MMP-8 drives AMPK-dependent matrix destruction in human pulmonary tuberculosis. PLoS Pathog. 2015;11(5):e1004917. doi:10.1371/journal.ppat.1004917
93. Klionsky DJ, Petroni G, Amaravadi RK, et al. Autophagy in major human diseases. EMBO J. 2021;40(19):e108863. doi:10.15252/embj.2021108863
94. Manzoor S, Muhammad JS, Maghazachi AA, Hamid Q. Autophagy: a versatile player in the progression of colorectal cancer and drug resistance. Front Oncol. 2022;12:924290. doi:10.3389/fonc.2022.924290
95. Morishita H, Mizushima N. Diverse cellular roles of autophagy. Annu Rev Cell Dev Biol. 2019;35:453–475. doi:10.1146/annurev-cellbio-100818-125300
96. Larabi A, Barnich N, Nguyen HTT. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy. 2020;16(1):38–51. doi:10.1080/15548627.2019.1635384
97. Kabat AM, Harrison OJ, Riffelmacher T, et al. The autophagy gene Atg16l1 differentially regulates Treg and TH2 cells to control intestinal inflammation. Elife. 2016;5:e12444. doi:10.7554/eLife.12444
98. Meley D, Bauvy C, Houben-Weerts JH, et al. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem. 2006;281(46):34870–34879. doi:10.1074/jbc.M605488200
99. Nwadike C, Williamson LE, Gallagher LE, Guan JL, Chan EYW. AMPK inhibits ULK1-dependent autophagosome formation and lysosomal acidification via distinct mechanisms. mol Cell Biol. 2018;38(10). doi:10.1128/MCB.00023-18
100. Samari HR, Seglen PO. Inhibition of hepatocytic autophagy by adenosine, aminoimidazole-4-carboxamide riboside, and N6-mercaptopurine riboside. Evidence for involvement of amp-activated protein kinase. J Biol Chem. 1998;273(37):23758–23763. doi:10.1074/jbc.273.37.23758
101. Benito-Cuesta I, Ordonez-Gutierrez L, Wandosell F. AMPK activation does not enhance autophagy in neurons in contrast to MTORC1 inhibition: different impact on beta-amyloid clearance. Autophagy. 2021;17(3):656–671. doi:10.1080/15548627.2020.1728095
102. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–141. doi:10.1038/ncb2152
103. Egan DF, Shackelford DB, Mihaylova MM, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331(6016):456–461. doi:10.1126/science.1196371
104. Alves S, Castro L, Fernandes MS, et al. Colorectal cancer-related mutant KRAS alleles function as positive regulators of autophagy. Oncotarget. 2015;6(31):30787–30802. doi:10.18632/oncotarget.5021
105. Dong Y, Wu Y, Zhao GL, Ye ZY, Xing CG, Yang XD. Inhibition of autophagy by 3-MA promotes hypoxia-induced apoptosis in human colorectal cancer cells. Eur Rev Med Pharmacol Sci. 2019;23(3):1047–1054. doi:10.26355/eurrev_201902_16992
106. Singh SS, Vats S, Chia AY, et al. Dual role of autophagy in hallmarks of cancer. Oncogene. 2018;37(9):1142–1158. doi:10.1038/s41388-017-0046-6
107. Burada F, Nicoli ER, Ciurea ME, Uscatu DC, Ioana M, Gheonea DI. Autophagy in colorectal cancer: an important switch from physiology to pathology. World J Gastrointest Oncol. 2015;7(11):271–284. doi:10.4251/wjgo.v7.i11.271
108. Rybstein MD, Bravo-San Pedro JM, Kroemer G, Galluzzi L. The autophagic network and cancer. Nat Cell Biol. 2018;20(3):243–251. doi:10.1038/s41556-018-0042-2
109. Devenport SN, Singhal R, Radyk MD, et al. Colorectal cancer cells utilize autophagy to maintain mitochondrial metabolism for cell proliferation under nutrient stress. JCI Insight. 2021;6(14). doi:10.1172/jci.insight.138835
110. Koukourakis MI, Giatromanolaki A, Sivridis E, Pitiakoudis M, Gatter KC, Harris AL. Beclin 1 over- and underexpression in colorectal cancer: distinct patterns relate to prognosis and tumour hypoxia. Br J Cancer. 2010;103(8):1209–1214. doi:10.1038/sj.bjc.6605904
111. Levy J, Cacheux W, Bara MA, et al. Intestinal inhibition of Atg7 prevents tumour initiation through a microbiome-influenced immune response and suppresses tumour growth. Nat Cell Biol. 2015;17(8):1062–1073. doi:10.1038/ncb3206
112. Zheng HY, Zhang XY, Wang XF, Sun BC. Autophagy enhances the aggressiveness of human colorectal cancer cells and their ability to adapt to apoptotic stimulus. Cancer Biol Med. 2012;9(2):105–110. doi:10.3969/j.issn.2095-3941.2012.02.004
113. Jo YK, Kim SC, Park IJ, et al. Increased expression of ATG10 in colorectal cancer is associated with lymphovascular invasion and lymph node metastasis. PLoS One. 2012;7(12):e52705. doi:10.1371/journal.pone.0052705
114. Choi JH, Cho YS, Ko YH, Hong SU, Park JH, Lee MA. Absence of autophagy-related proteins expression is associated with poor prognosis in patients with colorectal adenocarcinoma. Gastroenterol Res Pract. 2014;2014:179586. doi:10.1155/2014/179586
115. Song C, Xu W, Wu H, et al. Photodynamic therapy induces autophagy-mediated cell death in human colorectal cancer cells via activation of the ROS/JNK signaling pathway. Cell Death Dis. 2020;11(10):938. doi:10.1038/s41419-020-03136-y
116. Zhang MH, Jiang JZ, Cai YL, Piao LH, Jin Z. Significance of dynamic changes in gastric smooth muscle cell apoptosis, PI3K-AKT-mTOR and AMPK-mTOR signaling in a rat model of diabetic gastroparesis. mol Med Rep. 2017;16(2):1530–1536. doi:10.3892/mmr.2017.6764
117. Zhang XZ, Sun Y, Zhang MH, Jin Z. Insulin-like growth factor-1 inhibits apoptosis of rat gastric smooth muscle cells under high glucose condition via adenosine monophosphate-activated protein kinase (AMPK) pathway. Folia Histochem Cytobiol. 2022;60(1):74–88. doi:10.5603/FHC.a2022.0005
118. Song B, Zhang G, Bao Y, Zhang M. Involvement of oxidative stress-AMPK-Cx43-NLRP3 pathway in extracellular matrix remodeling of gastric smooth muscle cells in rats with diabetic gastroparesis. Cell Stress Chaperones. 2024;29(3):440–455. doi:10.1016/j.cstres.2024.04.005
119. Zhang XZ, Zhang MH, Fang XS, Cui XS, Jin Z. Mechanism of AMPK-mediated apoptosis of rat gastric smooth muscle cells under high glucose condition. Biosci Rep. 2019;39(12). doi:10.1042/BSR20192504
120. Xu Q, Si LY. Protective effects of AMP-activated protein kinase in the cardiovascular system. J Cell mol Med. 2010;14(11):2604–2613. doi:10.1111/j.1582-4934.2010.01179.x
121. Nalli AD, Kumar DP, Mahavadi S, et al. Hypercontractility of intestinal longitudinal smooth muscle induced by cytokines is mediated by the nuclear factor-kappaB/AMP-activated kinase/myosin light chain kinase pathway. J Pharmacol Exp Ther. 2014;350(1):89–98. doi:10.1124/jpet.113.212522
122. Moonwiriyakit A, Pathomthongtaweechai N, Steinhagen PR, Chantawichitwong P, Satianrapapong W, Pongkorpsakol P. Tight junctions: from molecules to gastrointestinal diseases. Tissue Barriers. 2023;11(2):2077620. doi:10.1080/21688370.2022.2077620
123. Zhang L, Li J, Young LH, Caplan MJ. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci U S A. 2006;103(46):17272–17277. doi:10.1073/pnas.0608531103
124. Pongkorpsakol P, Buasakdi C, Chantivas T, Chatsudthipong V, Muanprasat C. An agonist of a zinc-sensing receptor GPR39 enhances tight junction assembly in intestinal epithelial cells via an AMPK-dependent mechanism. Eur J Pharmacol. 2019;842:306–313. doi:10.1016/j.ejphar.2018.10.038
125. Rowart P, Erpicum P, Krzesinski JM, Sebbagh M, Jouret F. Mesenchymal stromal cells accelerate epithelial tight junction assembly via the AMP-activated protein kinase pathway, independently of liver kinase B1. Stem Cells Int. 2017;2017:9717353. doi:10.1155/2017/9717353
126. Wang B, Wu Z, Ji Y, Sun K, Dai Z, Wu G. L-glutamine enhances tight junction integrity by activating CaMK kinase 2-AMP-activated protein kinase signaling in intestinal porcine epithelial cells. J Nutr. 2016;146(3):501–508. doi:10.3945/jn.115.224857
127. Muanprasat C, Wongkrasant P, Satitsri S, et al. Activation of AMPK by chitosan oligosaccharide in intestinal epithelial cells: mechanism of action and potential applications in intestinal disorders. Biochem Pharmacol. 2015;96(3):225–236. doi:10.1016/j.bcp.2015.05.016
128. Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139(9):1619–1625. doi:10.3945/jn.109.104638
129. Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci U S A. 2007;104(3):819–822. doi:10.1073/pnas.0610157104
130. Shiomi R, Shigetomi K, Inai T, Sakai M, Ikenouchi J. CaMKII regulates the strength of the epithelial barrier. Sci Rep. 2015;5:13262. doi:10.1038/srep13262
131. Xiang RL, Mei M, Cong X, et al. Claudin-4 is required for AMPK-modulated paracellular permeability in submandibular gland cells. J mol Cell Biol. 2014;6(6):486–497. doi:10.1093/jmcb/mju048
132. Yano T, Matsui T, Tamura A, Uji M, Tsukita S. The association of microtubules with tight junctions is promoted by cingulin phosphorylation by AMPK. J Cell Biol. 2013;203(4):605–614. doi:10.1083/jcb.201304194
133. Yano T, Torisawa T, Oiwa K, Tsukita S. AMPK-dependent phosphorylation of cingulin reversibly regulates its binding to actin filaments and microtubules. Sci Rep. 2018;8(1):15550. doi:10.1038/s41598-018-33418-7
134. Ghosh P. The stress polarity pathway: AMPK ‘GIV’-es protection against metabolic insults. Aging. 2017;9(2):303–314. doi:10.18632/aging.101179
135. Zhang L, Jouret F, Rinehart J, et al. AMP-activated protein kinase (AMPK) activation and glycogen synthase kinase-3beta (GSK-3beta) inhibition induce Ca2+-independent deposition of tight junction components at the plasma membrane. J Biol Chem. 2011;286(19):16879–16890. doi:10.1074/jbc.M110.186932
136. Miao W, Wu X, Wang K, et al. Sodium butyrate promotes reassembly of tight junctions in Caco-2 monolayers involving inhibition of MLCK/MLC2 pathway and phosphorylation of PKCbeta2. Int J mol Sci. 2016;17(10). doi:10.3390/ijms17101696
137. Ahikiriza AA, Bukke SPN, Yadesa TM, et al. The potential of natural products in metabolic disease management: a thorough exploration of the case of Uganda. Endocr Metab Immune Disord Drug Targets. 2025. doi:10.2174/0118715303320109241014064209
138. Atanasov AG, Zotchev SB, Dirsch VM, et al. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021;20(3):200–216. doi:10.1038/s41573-020-00114-z
139. Hwang JT, Ha J, Park IJ, et al. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007;247(1):115–121. doi:10.1016/j.canlet.2006.03.030
140. Pournourmohammadi S, Grimaldi M, Stridh MH, et al. Epigallocatechin-3-gallate (EGCG) activates AMPK through the inhibition of glutamate dehydrogenase in muscle and pancreatic ss-cells: a potential beneficial effect in the pre-diabetic state? Int J Biochem Cell Biol. 2017;88:220–225. doi:10.1016/j.biocel.2017.01.012
141. Kim H-S, Montana V, Jang H-J, Parpura V, J-a K. Epigallocatechin gallate (EGCG) stimulates autophagy in vascular endothelial cells: a potential role for reducing lipid accumulation*. J Biol Chem. 2013;288(31):22693–22705. doi:10.1074/jbc.M113.477505
142. Murase T, Misawa K, Haramizu S, Hase T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochem Pharmacol. 2009;78(1):78–84. doi:10.1016/j.bcp.2009.03.021
143. Kim H-S, Quon M, Kim J-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; Lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014;2:187–195. doi:10.1016/j.redox.2013.12.022
144. Dryden GW, Lam A, Beatty K, Qazzaz HH, McClain CJ. A pilot study to evaluate the safety and efficacy of an oral dose of (-)-epigallocatechin-3-gallate-rich polyphenon E in patients with mild to moderate ulcerative colitis. Inflamm Bowel Dis. 2013;19(9):1904–1912. doi:10.1097/MIB.0b013e31828f5198
145. Huang WW, Tsai SC, Peng SF, et al. Kaempferol induces autophagy through AMPK and AKT signaling molecules and causes G2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int J Oncol. 2013;42(6):2069–2077. doi:10.3892/ijo.2013.1909
146. Moore WT, Luo J, Liu D. Kaempferol improves glucose uptake in skeletal muscle via an AMPK-dependent mechanism. Food Science and Human Wellness. 2023;12(6):2087–2094. doi:10.1016/j.fshw.2023.03.028
147. Li N, Yin L, Shang J, et al. Kaempferol attenuates nonalcoholic fatty liver disease in type 2 diabetic mice via the Sirt1/AMPK signaling pathway. Biomed Pharmacother. 2023;165:115113. doi:10.1016/j.biopha.2023.115113
148. Zhang F, Feng J, Zhang J, Kang X, Qian D. Quercetin modulates AMPK/SIRT1/NF-kappaB signaling to inhibit inflammatory/oxidative stress responses in diabetic high fat diet-induced atherosclerosis in the rat carotid artery. Exp Ther Med. 2020;20(6):280. doi:10.3892/etm.2020.9410
149. Wang M, Wang B, Wang S, et al. Effect of quercetin on lipids metabolism through modulating the gut microbial and AMPK/PPAR signaling pathway in broilers. Front Cell Dev Biol. 2021;9:616219. doi:10.3389/fcell.2021.616219
150. Qureshi AA, Khan DA, Mahjabeen W, Papasian CJ, Qureshi N. Suppression of nitric oxide production and cardiovascular risk factors in healthy seniors and hypercholesterolemic subjects by a combination of polyphenols and vitamins. J Clin Exp Cardiolog. 2012;S5:8. doi:10.4172/2155-9880.S5-008
151. Wang T, Lu SY, Dan L, et al. Higher dietary quercetin intake is associated with lower risk of adverse outcomes among individuals with inflammatory bowel disease in a prospective cohort study. J Nutr. 2024;154(6):1861–1868. doi:10.1016/j.tjnut.2024.04.025
152. Lee KY, Kim J-R, Choi HC. Genistein-induced LKB1–AMPK activation inhibits senescence of VSMC through autophagy induction. Vascular Pharmacol. 2016;81:75–82. doi:10.1016/j.vph.2016.02.007
153. Jiang Z, Wang H, Yang Y, Yao Y, Ma H. Genistein activated SIRT1-AMPK signaling pathway mediated by ERbeta-FOXO1-Nampt to reduce fat accumulation in chicken hepatocytes. Life Sci. 2023;312:121259. doi:10.1016/j.lfs.2022.121259
154. Yang Y, Wu Y, Zou J, et al. Naringenin attenuates non-alcoholic fatty liver disease by enhancing energy expenditure and regulating autophagy via AMPK. Front Pharmacol. 2021;12:687095. doi:10.3389/fphar.2021.687095
155. Yibcharoenporn C, Chusuth P, Jakakul C, Rungrotmongkol T, Chavasiri W, Muanprasat C. Discovery of a novel chalcone derivative inhibiting CFTR chloride channel via AMPK activation and its anti-diarrheal application. J Pharmacol Sci. 2019;140(3):273–283. doi:10.1016/j.jphs.2019.07.012
156. Xiao K, Jiang J, Guan C, et al. Curcumin induces autophagy via activating the AMPK signaling pathway in lung adenocarcinoma cells. J Pharmacol Sci. 2013;123(2):102–109. doi:10.1254/jphs.13085FP
157. Kwon HY, Kim JH, Kim B, Srivastava SK, Kim SH. Regulation of SIRT1/AMPK axis is critically involved in gallotannin-induced senescence and impaired autophagy leading to cell death in hepatocellular carcinoma cells. Arch Toxicol. 2018;92(1):241–257. doi:10.1007/s00204-017-2021-y
158. Lukitasari M, Nugroho DA, Widodo N. Chlorogenic acid: the conceivable chemosensitizer leading to cancer growth suppression. J Evid Based Integr Med. 2018;23:2515690X18789628. doi:10.1177/2515690X18789628
159. Lan F, Weikel KA, Cacicedo JM, Ido Y. Resveratrol-induced AMP-activated protein kinase activation is cell-type dependent: lessons from basic research for clinical application. Nutrients. 2017;9(7). doi:10.3390/nu9070751
160. Gao Y, Li J, Li J, et al. Tetrahydroxy stilbene glycoside alleviated inflammatory damage by mitophagy via AMPK related PINK1/Parkin signaling pathway. Biochem Pharmacol. 2020;177:113997. doi:10.1016/j.bcp.2020.113997
161. Li Y, Hu K, Liang M, et al. Stilbene glycoside upregulates SIRT3/AMPK to promotes neuronal mitochondrial autophagy and inhibit apoptosis in ischemic stroke. Adv Clin Exp Med. 2021;30(2):139–146. doi:10.17219/acem/130608
162. Jin X, Zhu L, Lu S, et al. Baicalin ameliorates CUMS-induced depression-like behaviors through activating AMPK/PGC-1alpha pathway and enhancing NIX-mediated mitophagy in mice. Eur J Pharmacol. 2023;938:175435. doi:10.1016/j.ejphar.2022.175435
163. Zhang JA, Luan C, Huang D, Ju M, Chen K, Gu H. Induction of autophagy by baicalin through the AMPK-mTOR pathway protects human skin fibroblasts from Ultraviolet B radiation-induced apoptosis. Drug Des Devel Ther. 2020;14:417–428. doi:10.2147/DDDT.S228047
164. Park M, Baek H, Han JY, Lee HJ. Stevioside enhances the anti-adipogenic effect and beta-oxidation by activating AMPK in 3T3-L1 cells and epididymal adipose tissues of db/db mice. Cells. 2022;11(7). doi:10.3390/cells11071076
165. Rashad NM, Abdelsamad MAE, Amer AM, Sitohy MZ, Mousa MM. The impact of stevioside supplementation on glycemic control and lipid profile in patients with type 2 diabetes: a controlled clinical trial. Egyptian J Internal Med. 2019;31(1):22–30. doi:10.4103/ejim.ejim_68_18
166. Onakpoya IJ, Heneghan CJ. Effect of the natural sweetener, steviol glycoside, on cardiovascular risk factors: a systematic review and meta-analysis of randomised clinical trials. Eur J Prev Cardiol. 2015;22(12):1575–1587. doi:10.1177/2047487314560663
167. Jiang J, Qi L, Lv Z, Jin S, Wei X, Shi F. Dietary stevioside supplementation alleviates lipopolysaccharide-induced intestinal mucosal damage through anti-inflammatory and antioxidant effects in broiler chickens. Antioxidants. 2019;8(12):575.
168. Shen L, Xiong Y, Wang DQ, et al. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J Lipid Res. 2013;54(5):1430–1438. doi:10.1194/jlr.M035907
169. Law BY, Mok SW, Chan WK, et al. Hernandezine, a novel AMPK activator induces autophagic cell death in drug-resistant cancers. Oncotarget. 2016;7(7):8090–8104. doi:10.18632/oncotarget.6980
170. Bai J, Zhang S, Cao J, et al. Hernandezine, a natural herbal alkaloid, ameliorates type 2 diabetes by activating AMPK in two mouse models. Phytomedicine. 2022;105:154366. doi:10.1016/j.phymed.2022.154366
171. Law BYK, Gordillo-Martinez F, Qu YQ, et al. Thalidezine, a novel AMPK activator, eliminates apoptosis-resistant cancer cells through energy-mediated autophagic cell death. Oncotarget. 2017;8(18):30077–30091. doi:10.18632/oncotarget.15616
172. Yin J, Ye J, Jia W. Effects and mechanisms of berberine in diabetes treatment. Acta Pharmaceutica Sinica B. 2012;2(4):327–334. doi:10.1016/j.apsb.2012.06.003
173. Kong W, Wei J, Abidi P, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10(12):1344–1351. doi:10.1038/nm1135
174. Zhang Y, Gu Y, Ren H, et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020;11(1):5015. doi:10.1038/s41467-020-18414-8
175. Cao J, Chen M, Xu R, Guo M. Therapeutic mechanisms of berberine to improve the intestinal barrier function via modulating gut microbiota, TLR4/NF-κ B/MTORC pathway and autophagy in cats. Front Microbiol. 2022;13:961885. doi:10.3389/fmicb.2022.961885
176. Maeda A, Shirao T, Shirasaya D, et al. Piperine promotes glucose uptake through ROS-dependent activation of the CAMKK/AMPK signaling pathway in skeletal muscle. mol Nutr Food Res. 2018;62(11):e1800086. doi:10.1002/mnfr.201800086
177. Kim KJ, Lee MS, Jo K, Hwang JK. Piperidine alkaloids from Piper retrofractum Vahl. protect against high-fat diet-induced obesity by regulating lipid metabolism and activating AMP-activated protein kinase. Biochem Biophys Res Commun. 2011;411(1):219–225. doi:10.1016/j.bbrc.2011.06.153
178. Freitas e Silva-Santana NC, Rodrigues HCN, Pereira Martins TF, et al. Turmeric supplementation with piperine is more effective than turmeric alone in attenuating oxidative stress and inflammation in hemodialysis patients: a randomized, double-blind clinical trial. Free Radic Biol Med. 2022;193:648–655. doi:10.1016/j.freeradbiomed.2022.11.008
179. Rao Y, Yu H, Gao L, et al. Natural alkaloid bouchardatine ameliorates metabolic disorders in high-fat diet-fed mice by stimulating the sirtuin 1/liver kinase B-1/AMPK axis. Br J Pharmacol. 2017;174(15):2457–2470. doi:10.1111/bph.13855
180. Mehmood T, Pichyangkura R, Muanprasat C. Chitosan oligosaccharide prevents afatinib-induced barrier disruption and chloride secretion through modulation of AMPK, PI3K/AKT, and ERK signaling in T84 cells. Polymers. 2022;14:4255. doi:10.3390/polym14204255
181. Jiang T, Xing X, Zhang L, Liu Z, Zhao J, Liu X. Chitosan oligosaccharides show protective effects in coronary heart disease by improving antioxidant capacity via the increase in intestinal probiotics. Oxid Med Cell Longev. 2019;2019:1–11. doi:10.1155/2019/7658052
182. Wongkrasant P, Pongkorpsakol P, Ariyadamrongkwan J, et al. A prebiotic fructo-oligosaccharide promotes tight junction assembly in intestinal epithelial cells via an AMPK-dependent pathway. Biomed Pharmacother. 2020;129:110415. doi:10.1016/j.biopha.2020.110415
183. Azpiroz F, Dubray C, Bernalier-Donadille A, et al. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: a randomized, double blind, placebo controlled study. Neurogastroenterol Motil. 2017;29(2). doi:10.1111/nmo.12911
184. Tian S, Wang J, Gao R, Wang J, Zhu W. Early-life galacto-oligosaccharides supplementation alleviates the small intestinal oxidative stress and dysfunction of lipopolysaccharide-challenged suckling piglets. J Anim Sci Biotechnol. 2022;13(1):70. doi:10.1186/s40104-022-00711-5
185. Yong Y, Shin SY, Jung Y, et al. Flavonoids activating adenosine monophosphate-activated protein kinase. J Korean Soc Appl Biol Chem. 2015;58(1):13–19. doi:10.1007/s13765-015-0003-4
186. Noor HB, Mou NA, Salem L, et al. Anti-inflammatory property of AMP-activated protein kinase. Antiinflamm Antiallergy Agents Med Chem. 2020;19(1):2–41. doi:10.2174/1871523018666190830100022
187. Mancini E, Beglinger C, Drewe J, Zanchi D, Lang UE, Borgwardt S. Green tea effects on cognition, mood and human brain function: a systematic review. Phytomedicine. 2017;34:26–37. doi:10.1016/j.phymed.2017.07.008
188. Miao BB, Gao D, Hao JP, et al. Tetrahydroxy stilbene glucoside alters neurogenesis and neuroinflammation to ameliorate radiation-associated cognitive disability via AMPK/Tet2. Int Immunopharmacol. 2022;110:108928. doi:10.1016/j.intimp.2022.108928
189. Guo H, Liu G, Zhong R, Wang Y, Wang D, Xia M. Cyanidin-3-O-beta-glucoside regulates fatty acid metabolism via an AMP-activated protein kinase-dependent signaling pathway in human HepG2 cells. Lipids Health Dis. 2012;11:10. doi:10.1186/1476-511X-11-10
190. Bouhnik Y, Raskine L, Simoneau G, Paineau D, Bornet F. The capacity of short-chain fructo-oligosaccharides to stimulate faecal bifidobacteria: a dose-response relationship study in healthy humans. Nutr J. 2006;5:8. doi:10.1186/1475-2891-5-8
191. Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229(2):558–565. doi:10.1111/j.1432-1033.1995.tb20498.x
192. Hawley SA, Ross FA, Chevtzoff C, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11(6):554–565. doi:10.1016/j.cmet.2010.04.001
193. Goransson O, McBride A, Hawley SA, et al. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007;282(45):32549–32560. doi:10.1074/jbc.M706536200
194. Bultot L, Jensen TE, Lai YC, et al. Benzimidazole derivative small-molecule 991 enhances AMPK activity and glucose uptake induced by AICAR or contraction in skeletal muscle. Am J Physiol Endocrinol Metab. 2016;311(4):E706–E719. doi:10.1152/ajpendo.00237.2016
195. Jensen TE, Ross FA, Kleinert M, et al. PT-1 selectively activates AMPK-gamma1 complexes in mouse skeletal muscle, but activates all three gamma subunit complexes in cultured human cells by inhibiting the respiratory chain. Biochem J. 2015;467(3):461–472. doi:10.1042/BJ20141142
196. Feng D, Biftu T, Romero FA, et al. Discovery of MK-8722: a systemic, direct pan-activator of AMP-activated protein kinase. ACS Med Chem Lett. 2018;9(1):39–44. doi:10.1021/acsmedchemlett.7b00417
197. Myers RW, Guan HP, Ehrhart J, et al. Systemic pan-AMPK activator MK-8722 improves glucose homeostasis but induces cardiac hypertrophy. Science. 2017;357(6350):507–511. doi:10.1126/science.aah5582
198. Cameron KO, Kung DW, Kalgutkar AS, et al. Discovery and preclinical characterization of 6-Chloro-5-[4-(1-hydroxycyclobutyl)phenyl]-1H-indole-3-carboxylic Acid (PF-06409577), a direct activator of adenosine monophosphate-activated protein kinase (AMPK), for the potential treatment of diabetic nephropathy. J Med Chem. 2016;59(17):8068–8081. doi:10.1021/acs.jmedchem.6b00866
199. Huang T, Sun J, Zhou S, Gao J, Liu Y. Identification of direct activator of adenosine monophosphate-activated protein kinase (AMPK) by structure-based virtual screening and molecular docking approach. Int J mol Sci. 2017;18(7). doi:10.3390/ijms18071408
200. Ford RJ, Fullerton MD, Pinkosky SL, et al. Metformin and salicylate synergistically activate liver AMPK, inhibit lipogenesis and improve insulin sensitivity. Biochem J. 2015;468(1):125–132. doi:10.1042/BJ20150125
201. Pongkorpsakol P, Pathomthongtaweechai N, Srimanote P, Soodvilai S, Chatsudthipong V, Muanprasat C. Inhibition of cAMP-activated intestinal chloride secretion by diclofenac: cellular mechanism and potential application in cholera. PLoS Negl Trop Dis. 2014;8(9):e3119. doi:10.1371/journal.pntd.0003119
202. King TS, Russe OQ, Moser CV, et al. AMP-activated protein kinase is activated by non-steroidal anti-inflammatory drugs. Eur J Pharmacol. 2015;762:299–305. doi:10.1016/j.ejphar.2015.06.001
203. Henry WS, Laszewski T, Tsang T, et al. Aspirin SUPPRESSES Growth in PI3K-mutant breast cancer by activating AMPK and inhibiting mTORC1 signaling. Cancer Res. 2017;77(3):790–801. doi:10.1158/0008-5472.CAN-16-2400
204. Riva B, De Dominici M, Gnemmi I, et al. Celecoxib inhibits proliferation and survival of chronic myelogeous leukemia (CML) cells via AMPK-dependent regulation of beta-catenin and mTORC1/2. Oncotarget. 2016;7(49):81555–81570. doi:10.18632/oncotarget.13146
205. Fontaine E. Metformin-induced mitochondrial complex I inhibition: facts, uncertainties, and consequences. Front Endocrinol. 2018;9:753. doi:10.3389/fendo.2018.00753
206. Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108(8):1167–1174. doi:10.1172/JCI13505
207. Meng S, Cao J, He Q, et al. Metformin activates AMP-activated protein kinase by promoting formation of the alphabetagamma heterotrimeric complex. J Biol Chem. 2015;290(6):3793–3802. doi:10.1074/jbc.M114.604421
208. Goel S, Singh R, Singh V, et al. Metformin: activation of 5’ AMP-activated protein kinase and its emerging potential beyond anti-hyperglycemic action. Front Genet. 2022;13:1022739. doi:10.3389/fgene.2022.1022739
209. Ma T, Tian X, Zhang B, et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature. 2022;603(7899):159–165. doi:10.1038/s41586-022-04431-8
210. Zhou Q, Kim SH, Perez-Lorenzo R, et al. Phenformin promotes keratinocyte differentiation via the calcineurin/NFAT pathway. J Invest Dermatol. 2021;141(1):152–163. doi:10.1016/j.jid.2020.05.114
211. Liu G, Li D, Zhang L, et al. Phenformin down-regulates c-myc expression to suppress the expression of pro-inflammatory cytokines in keratinocytes. Cells. 2022;11(15). doi:10.3390/cells11152429
212. Hu S, Ouyang Q, Cheng Q, et al. Phenformin inhibits cell proliferation and induces cell apoptosis and autophagy in cholangiocarcinoma. mol Med Rep. 2018;17(4):6028–6032. doi:10.3892/mmr.2018.8573
213. Liu Z, Ren L, Liu C, Xia T, Zha X, Wang S. Phenformin induces cell cycle change, apoptosis, and mesenchymal-epithelial transition and regulates the AMPK/mTOR/p70s6k and MAPK/ERK pathways in breast cancer cells. PLoS One. 2015;10(6):e0131207. doi:10.1371/journal.pone.0131207
214. Vara-Ciruelos D, Dandapani M, Russell FM, et al. Phenformin, but not metformin, delays development of T cell acute lymphoblastic leukemia/lymphoma via cell-autonomous AMPK activation. Cell Rep. 2019;27(3):690–698e4. doi:10.1016/j.celrep.2019.03.067
215. LeBrasseur NK, Kelly M, Tsao TS, et al. Thiazolidinediones can rapidly activate AMP-activated protein kinase in mammalian tissues. Am J Physiol Endocrinol Metab. 2006;291(1):E175–81. doi:10.1152/ajpendo.00453.2005
216. Liu Y, Park JM, Chang KH, Huh HJ, Lee K, Lee MY. AMP-activated protein kinase mediates the antiplatelet effects of the thiazolidinediones rosiglitazone and pioglitazone. mol Pharmacol. 2016;89(2):313–321. doi:10.1124/mol.115.102004
217. Nakamura K, Fukunishi S, Yokohama K, et al. A long-lasting dipeptidyl peptidase-4 inhibitor, teneligliptin, as a preventive drug for the development of hepatic steatosis in high-fructose diet-fed ob/ob mice. Int J mol Med. 2017;39(4):969–983. doi:10.3892/ijmm.2017.2899
218. Ohyama T, Sato K, Yamazaki Y, et al. MK-0626, a selective DPP-4 inhibitor, attenuates hepatic steatosis in ob/ob mice. World J Gastroenterol. 2014;20(43):16227–16235. doi:10.3748/wjg.v20.i43.16227
219. Ideta T, Shirakami Y, Miyazaki T, et al. The dipeptidyl peptidase-4 inhibitor teneligliptin attenuates hepatic lipogenesis via AMPK activation in non-alcoholic fatty liver disease model mice. Int J mol Sci. 2015;16(12):29207–29218. doi:10.3390/ijms161226156
220. Prakash S, Rai U, Kosuru R, Tiwari V, Singh S. Amelioration of diet-induced metabolic syndrome and fatty liver with sitagliptin via regulation of adipose tissue inflammation and hepatic Adiponectin/AMPK levels in mice. Biochimie. 2020;168:198–209. doi:10.1016/j.biochi.2019.11.005
221. Chen Z, Yu J, Fu M, et al. Dipeptidyl peptidase-4 inhibition improves endothelial senescence by activating AMPK/SIRT1/Nrf2 signaling pathway. Biochem Pharmacol. 2020;177:113951. doi:10.1016/j.bcp.2020.113951
222. El-Ghannam MS, Saad MA, Nassar NN, El-Yamany MF, El-Bahy AAZ. Linagliptin ameliorates acetic acid-induced colitis via modulating AMPK/SIRT1/PGC-1alpha and JAK2/STAT3 signaling pathway in rats. Toxicol Appl Pharmacol. 2022;438:115906. doi:10.1016/j.taap.2022.115906
223. Kvernmo T, Hartter S, Burger E. A review of the receptor-binding and pharmacokinetic properties of dopamine agonists. Clin Ther. 2006;28(8):1065–1078. doi:10.1016/j.clinthera.2006.08.004
224. Tavares G, Marques D, Barra C, et al. Dopamine D2 receptor agonist, bromocriptine, remodels adipose tissue dopaminergic signalling and upregulates catabolic pathways, improving metabolic profile in type 2 diabetes. mol Metab. 2021;51:101241. doi:10.1016/j.molmet.2021.101241
225. Bai L, Li X, Yang Y, et al. Bromocriptine monotherapy overcomes prostate cancer chemoresistance in preclinical models. Transl Oncol. 2023;34:101707. doi:10.1016/j.tranon.2023.101707
226. Murakami H, Murakami R, Kambe F, et al. Fenofibrate activates AMPK and increases eNOS phosphorylation in HUVEC. Biochem Biophys Res Commun. 2006;341(4):973–978. doi:10.1016/j.bbrc.2006.01.052
227. Pathomthongtaweechai N, Soodvilai S, Chatsudthipong V, Muanprasat C. Pranlukast inhibits renal epithelial cyst progression via activation of AMP-activated protein kinase. Eur J Pharmacol. 2014;724:67–76. doi:10.1016/j.ejphar.2013.12.013
228. Dembitz V, Lalic H, Kodvanj I, et al. 5-aminoimidazole-4-carboxamide ribonucleoside induces differentiation in a subset of primary acute myeloid leukemia blasts. BMC Cancer. 2020;20(1):1090. doi:10.1186/s12885-020-07533-6
229. Philippe C, Pinson B, Dompierre J, et al. AICAR antiproliferative properties involve the AMPK-independent activation of the tumor suppressors LATS 1 and 2. Neoplasia. 2018;20(6):555–562. doi:10.1016/j.neo.2018.03.006
230. Visnjic D, Lalic H, Dembitz V, Tomic B, Smoljo T. AICAr, a widely used AMPK activator with important AMPK-independent effects: a systematic review. Cells. 2021;10(5). doi:10.3390/cells10051095
231. Xiao B, Sanders MJ, Carmena D, et al. Structural basis of AMPK regulation by small molecule activators. Nat Commun. 2013;4:3017. doi:10.1038/ncomms4017
232. Rameshrad M, Soraya H, Maleki-Dizaji N, Vaez H, Garjani A. A-769662, a direct AMPK activator, attenuates lipopolysaccharide-induced acute heart and lung inflammation in rats. mol Med Rep. 2016;13(3):2843–2849. doi:10.3892/mmr.2016.4821
233. Guma M, Wang Y, Viollet B, Liu-Bryan R. AMPK activation by A-769662 controls IL-6 expression in inflammatory arthritis. PLoS One. 2015;10(10):e0140452. doi:10.1371/journal.pone.0140452
234. Kitzmiller L, Ledford JR, Hake PW, O’Connor M, Piraino G, Zingarelli B. Activation of AMP-activated protein kinase by A769662 ameliorates sepsis-induced acute lung injury in adult Mice. Shock. 2019;52(5):540–549. doi:10.1097/SHK.0000000000001303
235. Kopietz F, Alshuweishi Y, Bijland S, et al. A-769662 inhibits adipocyte glucose uptake in an AMPK-independent manner. Biochem J. 2021;478(3):633–646. doi:10.1042/BCJ20200659
236. Strembitska A, Mancini SJ, Gamwell JM, Palmer TM, Baillie GS, Salt IP. A769662 inhibits insulin-stimulated Akt activation in human macrovascular endothelial cells independent of AMP-activated protein kinase. Int J mol Sci. 2018;19(12). doi:10.3390/ijms19123886
237. Vlachaki Walker JM, Robb JL, Cruz AM, et al. AMP-activated protein kinase (AMPK) activator A-769662 increases intracellular calcium and ATP release from astrocytes in an AMPK-independent manner. Diabetes Obes Metab. 2017;19(7):997–1005. doi:10.1111/dom.12912
238. Xu YY, Chen FL, Ji F, Fei HD, Xie Y, Wang SG. Activation of AMP-activated protein kinase by compound 991 protects osteoblasts from dexamethasone. Biochem Biophys Res Commun. 2018;495(1):1014–1021. doi:10.1016/j.bbrc.2017.11.132
239. Pang T, Zhang ZS, Gu M, et al. Small molecule antagonizes autoinhibition and activates AMP-activated protein kinase in cells. J Biol Chem. 2008;283(23):16051–16060. doi:10.1074/jbc.M710114200
240. Wang C, Huang B, Sun L, et al. MK8722, an AMPK activator, inhibiting carcinoma proliferation, invasion and migration in human pancreatic cancer cells. Biomed Pharmacother. 2021;144:112325. doi:10.1016/j.biopha.2021.112325
241. Wang L, Zhu H, Shi Z, et al. MK8722 initiates early-stage autophagy while inhibiting late-stage autophagy via FASN-dependent reprogramming of lipid metabolism. Theranostics. 2024;14(1):75–95. doi:10.7150/thno.83051
242. Su L, Yuan H, Zhang H, et al. PF-06409577 inhibits renal cyst progression by concurrently inhibiting the mTOR pathway and CFTR channel activity. FEBS Open Bio. 2022;12(10):1761–1770. doi:10.1002/2211-5463.13459
243. Hawley SA, Fullerton MD, Ross FA, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336(6083):918–922. doi:10.1126/science.1215327
244. Serizawa Y, Oshima R, Yoshida M, et al. Salicylate acutely stimulates 5’-AMP-activated protein kinase and insulin-independent glucose transport in rat skeletal muscles. Biochem Biophys Res Commun. 2014;453(1):81–85. doi:10.1016/j.bbrc.2014.09.066
245. Banskota S, Wang H, Kwon YH, et al. Salicylates ameliorate intestinal inflammation by activating macrophage AMPK. Inflamm Bowel Dis. 2021;27(6):914–926. doi:10.1093/ibd/izaa305
246. Liu C, Rokavec M, Huang Z, Hermeking H. Salicylate induces AMPK and inhibits c-MYC to activate a NRF2/ARE/miR-34a/b/c cascade resulting in suppression of colorectal cancer metastasis. Cell Death Dis. 2023;14(10):707. doi:10.1038/s41419-023-06226-9
247. Chang W, Li W, Li P. The anti-diabetic effects of metformin are mediated by regulating long non-coding RNA. Front Pharmacol. 2023;14:1256705. doi:10.3389/fphar.2023.1256705
248. Lin H, Ao H, Guo G, Liu M. The role and mechanism of metformin in inflammatory diseases. J Inflamm Res. 2023;16:5545–5564. doi:10.2147/JIR.S436147
249. Ruan G, Wu F, Shi D, Sun H, Wang F, Xu C. Metformin: update on mechanisms of action on liver diseases. Front Nutr. 2023;10:1327814. doi:10.3389/fnut.2023.1327814
250. Shivaprakash P, Beeraka NM, Madhunapantula SRV, Nikolenko VN, Basalingappa KM. Metformin effects on SHIP2, AMPKs and gut microbiota: recent updates on pharmacology. Curr Med Chem. 2024. doi:10.2174/0109298673289342240213040144
251. Zhang L, He H, Balschi JA. Metformin and phenformin activate AMP-activated protein kinase in the heart by increasing cytosolic AMP concentration. Am J Physiol Heart Circ Physiol. 2007;293(1):H457–66. doi:10.1152/ajpheart.00002.2007
252. McGuinness ME, Talbert RL. Phenformin-induced lactic acidosis: a forgotten adverse drug reaction. Ann Pharmacother. 1993;27(10):1183–1187. doi:10.1177/106002809302701004
253. Hauner H. The mode of action of thiazolidinediones. Diabetes Metab Res Rev. 2002;18(Suppl 2):S10–5. doi:10.1002/dmrr.249
254. Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation. 1998;98(19):2088–2093. doi:10.1161/01.cir.98.19.2088
255. Diabetes Atherosclerosis Intervention Study Investigators. Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the diabetes atherosclerosis intervention study, a randomised study. Lancet. 2001;357(9260):905–910.
256. Keam SJ, Lyseng-Williamson KA, Goa KL. Pranlukast: a review of its use in the management of asthma. Drugs. 2003;63(10):991–1019. doi:10.2165/00003495-200363100-00005
257. Sarma K, Thalluri C, Reddy J. Nanofibers in drug delivery systems: a comprehensive scientific review of recent approaches. Int J Pharm Invest. 2024;14:633–646. doi:10.5530/ijpi.14.3.75
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.