Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
An Alternative Approach to Future Diagnosis: Can Generalized Anxiety Disorder Be Distinguished From Major Depressive Disorder, Healthy People by Differentially Expressed lncRNAs in Peripheral Blood
Received 10 December 2024
Accepted for publication 15 March 2025
Published 4 April 2025 Volume 2025:21 Pages 729—739
DOI https://doi.org/10.2147/NDT.S511375
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Lingming Kong,1 Liang Zhang2
1Treatment & Prevention Center for Mental Disorder, No. 904th Hospital, Changzhou, Jiangsu, 213003, People’s Republic of China; 2Psychiatry Department, The 5th People’s Hospital of Luoyang, Luoyang, Henan, 471027, People’s Republic of China
Correspondence: Lingming Kong, Treatment & Prevention Center for Mental Disorder, No. 904th Hospital, North Peace Road 55, Changzhou, Jiangsu, 213003, People’s Republic of China, Email [email protected]
Purpose: Symptomatic diagnosis combined with unclear pathological mechanism and diverse etiology may increase misdiagnosis risk for generalized anxiety disorder (GAD) in the clinical setting. This study aimed to confirm the diagnostic value of aberrantly expressed long non-coding RNAs (lncRNAs) for GAD.
Patients and Methods: Eighty sex- and age-matched patients with GAD and major depressive disorder (MDD) and healthy controls (HCs) were enrolled using a convenient sampling method. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to verify lncRNA expression levels in all participants, and a receiver operating characteristic (ROC) curve was used to test the accuracy of aberrantly expressed lncRNAs in differentiating health conditions.
Results: ΔCt values of ENST00000505825, NONHSAG017299, NONHSAT078768, NONHSAT029028, NONHSAT101077, NONHSAT031726, TCONS_l2_00010607 in GAD patients were less than in HCs (P< 0.05 or 0.01). The total severity score of HAMA-14 and somatic anxiety scores were negatively correlated with ΔCt values of ENST00000505825, NONHSAG017299, NONHSAT078768, NONHSAT029028, NONHSAT101077, NONHSAT031726, ENST00000505825, TCONS_l2_00010607, and NONHSAT131696, and psychic anxiety scores were negatively associated with the ΔCt value of ENST00000505825 (P< 0.05 or 0.01). The AUC of the combined ROC curve between patients with GAD and healthy people was 0.810, with sensitivity and specificity of 0.825 and 0.762, respectively (P< 0.05 or 0.01). The AUC of the combined ROC curve between patients with GAD and MDD patients was 0.938, with sensitivity and specificity of 0.850 and 0.900, respectively (P< 0.05 or 0.01).
Conclusion: ENST00000505825, NONHSAG017299, NONHSAT078768, NONHSAT029028, NONHSAT101077, NONHSAT031726, TCONS_l2_00010607, which may serve as biomarkers for the GAD diagnosis and differentiation between GAD and MDD, can improve the diagnostic accuracy and avoid misdiagnosis to a certain extent. It is also beneficial for personalized treatment of GAD.
Keywords: long non-coding RNAs, differential diagnosis, peripheral blood mononuclear cell, biomarker, receiver operating characteristic
Introduction
Generalized anxiety disorder (GAD) is a mental health condition characterized by excessive and uncontrollable worry about a wide range of life events or activities of daily living; it is often accompanied by physical symptoms (eg, muscle tension, muscle pain, cardiac dysrhythmia, restlessness, insomnia, respiratory distress, chest pain, and oral ulcers) and has been proven that GAD is associated with severe functional impairment and psychological distress. A Chinese national health and well-being survey found that the prevalence of undiagnosed/diagnosed GAD was 5.3% among the urban population, with only 0.5% of GAD respondents reporting a clinical diagnosis. A study that enrolled 29,709 children and adolescents aged 6–18 years old using the multistage random-cluster sampling method in Iran showed that the lifetime prevalence of GAD was 2.6%, and higher age, female sex, mother’s history of psychiatric hospitalization, mother’s low education level, and urban living were predictors of GAD. A meta-analysis of 32 studies showed that the prevalence of GAD among older adults (≥50 years) in low- and middle-income countries in Asia, South America, and Africa ranged from 0.2% to 32.2%.1–4 It is commonly considered that GAD is usually comorbid with or as a risk factor for hypertension, coronary heart disease, chronic pain, peptic ulcer disease, attention deficit and hyperactivity disorder,5–7 therefore, GAD may be strongly associated with low quality of life, serious loss of productivity and daily activity, and high consumption of medical resources.
Symptomatology exerts a dominant effect on the diagnosis and differential diagnosis of mental diseases. Nevertheless, GAD symptoms, usually involving anxiety, somatization, and behavioral performance, are complex and diverse due to large inter-individual variability. For instance, heart rate variability (HRV), which reflects an individual’s ability to adapt to the environment, is a physiological marker for GAD; nevertheless, one study found that female gender was associated with higher perceived patient relationship anxiety and lower HRV in comparison to male, the gender male being associated with lower level of self-reported anxiety, these results confirmed that the female gender was associated with higher perceived patient-approach anxiety, lower parasympathetic activity, and a comparable sympathetic activity to the Male gender, thus fostering a possible vulnerability to excessive stress.8,9 Cultural difference is considered as another associated factor for GAD conceptualization and symptom presentation, and one study which showed Black/African American with high GAD symptoms tended to score lower on the Generalized Anxiety Disorder-7 than White/Caucasian, Hispanic participants with similar GAD symptoms, highlighted the urgent need for culturally sensitive GAD screening tools.10 Moreover, children are not fully developed in terms of verbal expression and introspective experience, it was found that GAD in childhood mainly manifested as somatic symptoms and behavioral problems, such as irritable bowel syndrome and increased aggression, nevertheless, their parents’ or legal guardians’ symptom reports in GAD diagnosis may be impacted by their attitude, belief and understanding about anxiety.11–13 To date, existing studies have demonstrated that the onset, severity, treatment efficacy, prognosis of GAD are associated with anxiety sensitivity, perceived social support, coping style, self-efficacy.14–16 Thus, symptomatic diagnosis combined with unclear pathological mechanism, complicated etiology may increase misdiagnosis risk of GAD.
GAD with comorbid major depressive disorder (MDD) is common in clinical practice, a national representative survey in India reported that the GAD prevalence was 0.57% and the most common comorbid psychiatric disorders of GAD was depression (15.8%); Incidence rate of mixed anxiety and depression disorder was 33% in patients with chronic musculoskeletal pain17,18 It was believed that environmental stress, high neuroticism, downregulated C-C motif chemokine ligand 2, and upregulated α2-macroglobulin and toll-like receptors-2 in serum were the risk factors for comorbidity of GAD and MDD. Furthermore, childhood abuse victims and women were also more susceptible to GAD and MDD comorbidity.19,20 These two frequently co-occurring disorders were associated with greater illness severity and worse treatment outcomes than with either condition alone.21 In addition, there was a bidirectional connection between GAD and MDD, which was mediated by stress reactivity, sleep quality and positive relation.22–24 At the same time, GAD and MDD have overlapping symptoms, including somatic symptoms, anxiety, insomnia, attention deficit, cognitive impairment, and social dysfunction. Antidepressants, commonly used in GAD treatment, are less effective than benzodiazepines for somatic symptoms,25–27 and these results highlight that definitive clinical diagnosis and personalized medication are still crucial obstacles in obtaining an ideal prognosis for GAD. The symptomatic diagnosis of GAD, affected by clinicians’ clinical experience, theoretical orientation, sociocultural value, and understanding of diagnostic criteria, may bring great difficulties in elucidating pathological mechanisms and improving treatment levels of GAD.3 Therefore, to confirm non-invasive, low-cost biomarkers with high specificity and sensitivity is a strong need for accurate diagnosis and precision therapy for GAD.
Although investigations have identified novel targets for diagnosing GAD and predicting treatment response through neuroimaging methods, heart rate variability, G protein-coupled estrogen receptor 1(GPER1), pro-inflammatory chemokines, and pharmacogenetics, these studies are often solitary, have not been replicated, and have several limitations. Benefiting from epigenetic research on the pathological process of human diseases, great progress has been made in finding the intermediator or bridge between genetic factors and environmental stimuli. Neuro-epigenetics is an incredibly new field of recent emerging research, long non-coding RNAs (lncRNAs), as key regulators with a length of >200 nucleotides and no protein-coding potential, could regulate gene expression in cellular homeostasis and participate in the pathological processes of diseases at epigenetic, transcriptional and post-transcriptional levels, it was found that lncRNAs, enriched in the brain with expression profiles of temporal-spatial specificity, extensively affecting the brain development, including self-renewal maintenance, cell fate decision, synapse plasticity, synaptogenesis and memory formation.28 Ten lncRNAs (ENST00000505825, NONHSAT103134, NONHSAG017299, NON-HSAT078768, NONHSAT029028, ENST00000505825, NonHSAT103134, Non-HSAG017299, NonHSAT029028, ENST00000505825, NONHSAT103134, NON-HSAT101077, NONHSAG049179, NONHSAT031726, TCONS_l2_00010607, NON-HSAT131696) with aberrant expression verified by chip screening in peripheral blood mononuclear cells (PBMCs), were correlated to GAD onset, symptom presentation, treatment efficacy.29,30 However, to date, no existing studies have reported the diagnostic value of aberrant expressed lncRNAs in GAD and healthy people or the differential diagnosis value between GAD and MDD.
In summary, this study aimed to first prove the alteration of lncRNA expression levels in PBMCs of a larger GAD patient sample and, second, clarify the diagnostic value of lncRNAs in GAD and healthy people, and the differential diagnosis value for GAD and MDD by using a receiver operating characteristic (ROC) curve. This study provides new scientific data for accurate diagnosis and personalized treatment of GAD.
Materials and Methods
Participants
Study group. Eighty patients with GAD, 35 males and 45 females, aged 21–66 and 80 MDD patients, 36 males and 44 females, aged 21–59 years old, were continuously enrolled using a convenience sampling method from January 2022 to December 2023.
Diagnosis of GAD and MDD, from the Chinese Han population, were first diagnosed by two well-trained clinical psychiatrists according to criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V).31 Patients were included if they had no history of psychoactive substance abuse and blood transfusion within past 6 months and excluded if they used anxiolytic, antidepressants or had a history of neuropsychiatric disorder. Women who were pregnant or were breast feeding were excluded.
Control group. Eighty healthy volunteers, including 43 males and 37 females, aged 21–66 years, were recruited by convenience sampling from January 2022 to December 2023. The inclusion criteria were as follows: (1) Chinese Han nationality and (2) no previous disease or family history of GAD, MDD, or other mental disorders. Participants with severe neurological diseases or other physical diseases, including stroke, senile dementia, Parkinson’s disease, coronary atherosclerotic heart disease, kidney failure, and a history of drug abuse or blood transfusion in the past six months were excluded.
The Clinical Trial Registration Number is ChiCTR-OOC-16007994.
Clinical Measures
The Hamilton Anxiety Rating Scale-14 (HARS-14), which includes 14 items that were assigned to two dimensions of psychic anxiety and somatic anxiety, was used for the GAD assessment, the individual with a score of HARS-14 higher than 29 indicated severe anxiety according to Chinese norm.32 All GAD patients in this study met the severe anxiety criteria. The HARS-14 with a Cronbach’s α of 0.893, scored on a five-point Likert-type scale ranging 0–4.
Hamilton Depression Scale (HAMD-17) was employed for assessment of depression level in MDD patients, the individual with a score of HAMD higher than 24 indicated severe depression according to Chinese norm.32 All MDD patients in this study met the severe depression criteria. Cronbach’s α of HAMD-24, which scored on a five-point Likert-type scale ranging 0–4 or 0–2, was 0.812 in this study.
Real-Time Quantitative Reverse Transcription PCR (qRT–PCR)
Based on the literature,29,30 ENST00000505825, NONHSAT103134, NONHSAG017299, NON-HSAT078768, NONHSAT029028, NONHSAT101077, NONHSAG049179, NON-HSAT031726, TCONS_l2_00010607, and NONHSAT131696) were selected for further validation using qRT-PCR in PBMCs from 80 GAD patients, 80 with MDD patients and 80 healthy controls. Total RNAs were isolated from PBMCs using TRIzol reagent (Invitrogen®) for quantitative detection of lncRNAs. Complementary DNA was synthesized using the Reverse Transcription TaqMan RNA Reverse Transcription Kit (Applied Biosystems, Inc). According to the manufacturer’s protocol. Each RT reaction included 10 μL of total RNA, 3.0 μL TaqMan MicroRNA Assay, 4.16 μL nuclease free water, 0.19 μL RNase inhibitor, 1 μL Multiscribe Reverse Transcriptase, 0.15 μL dNTP, 1.5 μL 10×RT buffer, in a total volume of 15 μL. Reactions were performed under the following conditions: 30 min at 16°C, 30 min at 42°C, 5 min at 85°C, and 10 min at 4°C. Each sample was processed in duplicate for analysis and qRT-PCR was performed using an Applied Biosystems 7900HT Real-Time PCR System (Applied Biosystems, Inc.). Data were collected using SDS 2.3 software (Applied Biosystems, Inc.). Data were collected using the SDS 2.3 Software (Applied Biosystems, Inc.) and DataAssist v. 3.0. The expression levels of lncRNAs were calculated using the 2−ΔΔCt method after normalization to β-actin. This process was performed according to a standard detection program for lncRNA expression in PBMCs.33
Statistical Analysis
The ΔCt value, calculated as the threshold cycle difference between lncRNA and β-actin (ΔCt = CtlncRNA-Ctβ-actin), was used to represent the relative expression level of lncRNA; the higher ΔCt value indicated lncRNAs were down-regulated.33 The differences in ΔCt values between the case groups (GAD patients, MDD patients) and the control group were compared using the Mann–Whitney rank sum test. Spearman correlation was used to determine the relationship between lncRNA expression and anxiety symptoms. ROC curves for the GAD group and healthy controls, the GAD group, and the MDD group were established using ΔCt values of differentially expressed lncRNAs as test variables and health conditions (GAD or MDD patients and healthy controls) as the state variables. P < 0.05 (two-tailed) was considered statistically significant.
Results
Between-Group Comparisons of Demographic Characteristics
As shown in Table 1, there were no significant differences in gender or age between the MDD patients and healthy controls (P>0.05).
 |
Table 1 Descriptive Statistics of Demographic Characteristics |
Comparison of lncRNA Expression Levels Between GAD Patients and Health Controls
The Mann–Whitney rank sum test between patients with GAD and healthy controls showed that the ΔCt values of ENST00000505825, NONHSAG017299, NONHSAT078768, NONHSAT029028, NONHSAT101077, NONHSAT031726, and TCONS_l2_00010607 were significantly lower in patients with GAD than in the control group (P<0.05 or 0.01) (Table 2).
 |
Table 2 Comparison of lncRNA Expression Levels Between GAD Patients and Health Controls (ΔCT Median, Mean Rank) |
Correlation Analysis Between Anxiety Symptoms and lncRNA Expression in GAD Patients
Spearman correlation analysis showed that the HARS-14 score and somatic anxiety were negatively correlated with the ΔCt values of ENST00000505825, NONHSAG017299, NONHSAT078768, NONHSAT029028, NONHSAT101077, NONHSAT031726, and TCONS_l2_00010607 NONHSAT131696, and psychic anxiety was negatively correlated with the ΔCt value of ENST00000505825 (P < 0.01) (Table 3).
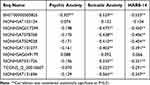 |
Table 3 Correlation Analysis Between Anxiety Symptom and lncRNA Expression in GAD Patients (r) |
ROC Curve for Differentially Expressed lncRNAs Between GAD Patients and Control Group
ROC curves were established using differentially expressed lncRNAs (ENST00000505825, NONHSAG017299, NONHSAT078768, NONHSAT029028, NONHSAT101077, NONHSAT031726, and TCONS_l2_00010607) as test variables and health conditions (GAD or healthy people) as state variables. The results suggested that the area under the curve (AUC) of the seven differentially expressed lncRNAs ranged from 0.600 to 0.730 (P<0.05 or 0.01), and the combined ROC curve of differentially expressed lncRNAs showed an AUC of 0.810 with a sensitivity and specificity of 0.825 and 0.762, respectively (Figure 1A, B and Table 4).
 |
Table 4 Results of ROC Curve About Aberrant Expression of lncRNAs in GAD Group and Healthy Controls |
ROC Curves for Differentially Expressed lncRNAs in GAD Patients and MDD Group
ROC curve analysis was used to test the accuracy of aberrantly expressed lncRNAs (ENST00000505825, NONHSAG017299, NONHSAT078768, NONHSAT029028, NONHSAT101077, NONHSAT031726, TCONS_l2_00010607) in differentiating health conditions between GAD and MDD; the results showed AUCs of seven differentially expressed lncRNAs ranging from 0.666 to 0.795 (P < 0.05 or 0.01) and the combined ROC curves of differentially expressed lncRNAs found that AUC was 0.938 with sensitivity and specificity of 0.850 and 0.900, respectively (see Figure 2A, B and Table 5).
 |
Table 5 Results of ROC Curve Analysis About Aberrant Expressed lncRNAs in GAD Patients and MDD Group |
Discussion
Symptom severity and functional impairment in patients with GAD are linked to cultural background, growth experience, coping strategies, and social support. It was reported that there is a bidirectional relationship between GAD and various physical or mental disorders. In addition, anxiety experience, with large tolerance variation among different population groups, was widespread. Therefore, it was difficult for GAD diagnosis and precision therapy, and it would be an urgent mission to identify biomarkers for GAD diagnosis and differential diagnosis.
First, this study found that GAD patients had lower ΔCt values of ENST00000505825, NONHSAG017299, NONHSAT078768, NONHSAT029028, NONHSAT101077, NONHSAT031726, TCONS_l2_00010607 and GAD symptoms of somatic anxiety, psychic anxiety strongly associated with ΔCt values of ENST00000505825, NONHSAG017299, NONHSAT078768, NONHSAT029028, NONHSAT101077, NONHSAT031726, TCONS_l2_00010607 NONHSAT131696. These results indicated that lncRNA expression levels in PBMCs of patients with GAD were upregulated, and the more severe the GAD symptoms, the higher the lncRNA expression level. Anxiety experiences are common in daily life, and moderate anxiety levels are conducive to improving activity efficiency and adaptive ability. However, severe symptoms in patients with GAD induce distinct mental grief and functional impairment. Some individuals with individual traits are susceptible to GAD, and previous studies found that GAD patients experienced more childhood abuse with severe attachment anxiety, higher neuroticism, less extroversion, and had attention bias to environmental threats with attention fixation and escape or shifting difficulty; however, the visual search and further cognitive processes were slowed down for neutral or positive stimuli,34–36 so GAD patients may experience chronic stress for a long time before disease onset. In the absence of a single environmental or genetic theory that could perfectly elucidate the pathological process of GAD, the mediators between environmental stimuli and heredity have attracted great concern in recent years, chronic unpredictable mild stress during maternal pregnancy could lead to elevated activity in the hypothalamo-pituitary-adrenal (HPA) axis and elevated serum levels of IL-6 and TNF-α in the offspring, suggesting that their physiological arousal state and Gng3-related PI3K/Akt/NF-κB pathway activation affect the Th17/Treg balance and further induce aberrantly expressed mRNAs via the lncRNA-mRNA co-expression network,37,38 That is, chronic stress can regulate mRNA expression by the Gng3 signaling pathway in the immune system, and lncRNA expression is upregulated in GAD patients through the lncRNA-mRNA co-expression network. The increased expression level of lncRNA is also a protective response to nervous system inflammation.39 lncRNA was considered as critical regulator of gene expression and gene regulatory network in the brain, aberrant expressed lncRNAs would promote the expression of GAD susceptibility genes and initiate neurophysiological processes such as immune-biochemical response, HPA axis activation, neuroplasticity, stress response, and negative attention bias.40–44 It is ultimately associated with the onset, progression, and severity of GAD.
AUC of ROC curve exceeded 0.700 indicated differentiation degree was statistically significant, the larger the AUC value, the higher the prediction accuracy.45 In this study, using seven differentially expressed lncRNAs in GAD patients as test variables, health conditions (GAD vs healthy controls, GAD vs MDD) as state variables, this study preliminarily verified that the combined ROC curve of seven lncRNAs differentially expressed in GAD patients was effective to distinguish GAD from MDD or healthy people. lncRNAs, which are widely expressed in the brain, can regulate numerous gene expression and functional pathways, and lncRNAs with spatiotemporal and tissue specificity play a major role in the process of tissue differentiation and development and may present special expression profiles in brain tissue.46,47 In this study, lncRNAs may be specifically expressed in GAD-related brain regions that could modulate GAD-specific regulatory pathways or networks. Brain samples provide direct evidence of lncRNA dysregulation but are not readily accessible. In recent years, investigations of the pathophysiology and potential biomarkers of neuropsychiatric disease have increasingly relied upon blood-based expression profiling of lncRNAs for gene expression was similar in whole blood, and alterations in peripheral biomarkers may mirror pathological processes in the brain,48 additionally, the peripheral tissue samples can be obtained from living subjects with minimal invasion, and thus it was widely used in basis research field and clinical practice. The aberrantly expressed lncRNAs in PBMCs of patients with GAD may mirror the GAD-specific expression profile in the brain tissue; therefore, the combined curve of seven lncRNAs differentially expressed in patients with GAD could provide efficacious diagnostic and differential diagnostic values for GAD.
This study proved that differential expression of lncRNAs in peripheral blood can be used as a potential biomarker for clinical diagnosis of GAD, which can improve diagnostic accuracy and avoid misdiagnosis to a certain extent. It is also beneficial to personalized treatment for GAD, in clinical practice, benzodiazepines and antidepressants may singly or combined be used in the GAD treatment, existing studies reported benzodiazepines were superior to antidepressants in the somatic symptoms of GAD and there no significant difference between benzodiazepines and antidepressants in treatment efficacy on psychic anxiety symptoms (Beyer et al, 2024). The present study found that ENST00000505825, NONHSAG017299, NONHSAT078768, NONHSAT029028, NONHSAT101077, NONHSAT031726, and TCONS_l2_00010607 NONHSAT131696 were significantly associated with somatic anxiety symptoms only. Therapeutic drugs can be selected according to the lncRNA expression level in patients with GAD to improve treatment efficacy and prognosis. Peripheral blood collection is minimally invasive, highly efficient, and inexpensive, which is conducive to improving patient compliance. It is also believed that precise diagnosis and personalized treatment could indirectly reduce substance abuse, drug dependence, aggressive behavior, sexual promiscuity, sexually transmitted disease (STD), etc.49–51
This study has the following limitations need to note and future research directions: (1) The sample size is small, and it is necessary to conduct a multicenter, double-blind, randomized controlled study in the future to further verify the validity and reliability of the results in this study. (2) The lncRNA expression profile in peripheral blood and diagnostic value for GAD are preliminarily confirmed in this study. However, the regulatory pathways and networks of lncRNAs in the pathological process of GAD remain unclear and need to be further explored. (3) Based on full verification of the results in this study, a detection kit for differentially expressed lncRNAs in GAD can be prepared and applied to clinical diagnosis and efficacy evaluation in the future. Overall, these efforts can serve to identify genetic and epigenetic factors that may ultimately improve treatment response and reduce the socioeconomic burden of GAD.
Conclusion
In summary, this study found that seven lncRNAs (ENST00000505825, NON-HSAG017299, NONHSAT078768, NONHSAT029028, NONHSAT101077, NON-HSAT031726, TCONS_l2_00010607) in peripheral blood of GAD patients were upregulated and the combined ROC curve of these dysregulated lncRNAs could significantly distinguished GAD from MDD patients and health people. The upregulated lncRNAs in PBMCs of patients with GAD may serve as diagnostic biomarkers for their particular property of stability.
Abbreviations
GAD, generalized anxiety disorder; MDD, major depressive disorder; HARS-14, Hamilton Anxiety Rating Scale-14; PBMCs, peripheral blood mononuclear cells; lncRNAs, long non-coding RNAs; ROC, receiver operating characteristic; AUC, area under the curve; qRT–PCR, real-time quantitative reverse transcription PCR.
Data Sharing Statement
The dataset used in the current study is available from the corresponding author upon reasonable request.
Ethics Statement
This study was approved by the ethics review committee of the No. 904th Hospital. All participants provided written informed consent to participate in this study. All the procedures in this study complied with the Declaration of Helsinki.
Acknowledgments
We thank all participants and their families for their cooperation in this study and Prof. Zhang LY for his critical reading and constructive comments.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yu W, Singh SS, Calhoun S, et al. Generalized anxiety disorder in urban China: prevalence, awareness, and disease burden. J Affect Disord. 2018;234:89–96. doi:10.1016/j.jad.2018.02.012
2. Mohammadi MR, Pourdehghan P, Mostafavi SA, et al. Generalized anxiety disorder: prevalence, predictors, and comorbidity in children and adolescents. J Anxiety Disord. 2020;73:102234. doi:10.1016/j.janxdis.2020.102234
3. Edwards N, Walker S, Paddick SM, et al. Prevalence of depression and anxiety in older people in low- and middle- income countries in Africa, Asia and South America: a systematic review and meta-analysis. J Affect Disord. 2023;325:656–674. doi:10.1016/j.jad.2023.01.068
4. Xu WQ, Tan WY, Li XL, et al. Prevalence and correlates of depressive and anxiety symptoms among adults in Guangdong province of China: a population-based study. J Affect Disord. 2022;308:535–544. doi:10.1016/j.jad.2022.04.089
5. Meng J, Zhang T, Hao T, et al. Functional and structural abnormalities in the pain network of generalized anxiety disorder patients with pain symptoms. Neuroscience. 2024;543:28–36. doi:10.1016/j.neuroscience.2024.02.006
6. Bawish BM, Rabab MA, Gohari ST, et al. Promising effect of Geranium robertianum L. leaves and Aloe vera gel powder on Aspirin®-induced gastric ulcers in Wistar rats: anxiolytic behavioural effect, antioxidant activity, and protective pathways. Inflammopharmacology. 2023;31:3183–3201. doi:10.1007/s10787-023-01205-0
7. Zhang Y, Feng Y, Liu L, et al. Abnormal prefrontal cortical activation during the GO/NOGO and verbal fluency tasks in adult patients with comorbid generalized anxiety disorder and attention-deficit/hyperactivity disorder: an fNIRS study. J Psychiatr Res. 2024;172:281–290. doi:10.1016/j.jpsychires.2024.02.053
8. Wang Z, Luo Y, Zhang Y, et al. Heart rate variability in generalized anxiety disorder, major depressive disorder and panic disorder: a network meta-analysis and systematic review. J Affect Disord. 2023;330:259–266. doi:10.1016/j.jad.2023.03.018
9. Queirolo L, Bacci C, Roccon A, et al. Anxiety in a regular day of work: a 24 hour psychophysiological investigation in young dentists with gender comparison. Front Psychol. 2023;14:1045974. doi:10.3389/fpsyg.2023.1045974
10. Parkerson HA, Thibodeau MA, Brandt CP, et al. Cultural-based biases of the GAD-7. J Anxiety Disord. 2015;31:38–42. doi:10.1016/j.janxdis.2015.01.005
11. Császár-Nagy N, Bókkon I. Hypnotherapy and IBS: implicit, long-term stress memory in the ENS? Heliyon. 2022;9:e12751. doi:10.1016/j.heliyon.2022.e12751
12. Mallott MA, Stryker JST, Schmidt NB. Paranoia and social anxiety: predicting aggressive behavior. Behav Ther. 2024;55:825–838. doi:10.1016/j.beth.2023.12.003
13. Wecht S, Hendrixson M, Radović A. A mixed method investigation of parent-adolescent communication about mental health. J Adolesc Health. 2024;75:904–911. doi:10.1016/j.jadohealth.2024.07.012
14. Metts AV, Roy-Byrne P, Stein MB, et al. Reciprocal and indirect effects among intervention, perceived social support, and anxiety sensitivity within a randomized controlled trial for anxiety disorders. Behav Ther. 2024;55:80–92. doi:10.1016/j.beth.2023.05.008
15. Feng Q, Li Y, Liu C, et al. Functional connectivity mediating passive coping style and perceived stress in predicting anxiety. J Affect Disord. 2023;340:828–834. doi:10.1016/j.jad.2023.08.079
16. Mudra S, Göbel A, Barkmann C, et al. The longitudinal course of pregnancy-related anxiety in parous and nulliparous women and its association with symptoms of social and generalized anxiety. J Affect Disord. 2020;260:111–118. doi:10.1016/j.jad.2019.08.033
17. Jayasankar P, Suhas S, Nirisha LP, et al. Current prevalence and determinants of generalized anxiety disorder from a nationally representative, population-based survey of India. Indian J Psychiatry. 2023;65:1244–1248. doi:10.4103/indianjpsychiatry.indianjpsychiatry_824_23
18. Duque RH, Andrade CVC, Campos VR, et al. Cross-sectional study of psychiatric disorders in patients with chronic musculoskeletal pain and individuals without pain. Adv Rheumatol. 2024;64:40. doi:10.1186/s42358-024-00375-x
19. Wang X, Lin J, Liu Q, et al. Major depressive disorder comorbid with general anxiety disorder: associations among neuroticism, adult stress, and the inflammatory index. J Psychiatr Res. 2022;148:307–314. doi:10.1016/j.jpsychires.2022.02.013
20. Su Y, Li M, Meng X. Symptom patterns in the co-occurrence of depressive and generalized anxiety symptoms: a network analysis of a Canadian nationally representative sample. J Affect Disord. 2024;351:888–894. doi:10.1016/j.jad.2024.01.266
21. Bobo WV, Grossardt BR, Virani S, et al. Association of depression and anxiety with the accumulation of chronic conditions. JAMA Netw Open. 2022;5:e229817. doi:10.1001/jamanetworkopen.2022.9817
22. Barber KE, Zainal NH, Newman MG. Positive relations mediate the bidirectional connections between depression and anxiety symptoms. J Affect Disord. 2023;324:387–394. doi:10.1016/j.jad.2022.12.082
23. Nguyen VV, Zainal NH, Newman MG. Why sleep is key: poor sleep quality is a mechanism for the bidirectional relationship between major depressive disorder and generalized anxiety disorder across 18 years. J Anxiety Disord. 2022;90. doi:10.1016/j.janxdis.2022.102601
24. Barber KE, Zainal NH, Newman MG. The mediating effect of stress reactivity in the 18-year bidirectional relationship between generalized anxiety and depression severity. J Affect Disord. 2023;325:502–512. doi:10.1016/j.jad.2023.01.041
25. Yu Y, Zhang T, Li Q, et al. Distinction in the function and microstructure of white matter between major depressive disorder and generalized anxiety disorder. J Affect Disord. 2025;374:55–62. doi:10.1016/j.jad.2025.01.018
26. Luo W, Luo L, Wang Q, et al. Disorder-specific impaired neurocognitive function in major depression and generalized anxiety disorder. J Affect Disord. 2022;318:123–129. doi:10.1016/j.jad.2022.08.129
27. Beyer C, Currin CB, Williams T, et al. Meta-analysis of the comparative efficacy of benzodiazepines and antidepressants for psychic versus somatic symptoms of generalized anxiety disorder. Compr Psychiatry. 2024;132. doi:10.1016/j.comppsych.2024.152479
28. Hosseini E, Bagheri-Hosseinabadi Z, De Toma I, et al. The importance of long non-coding RNAs in neuropsychiatric disorders. Mol Aspects Med. 2019;70:127–140. doi:10.1016/j.mam.2019.07.004
29. Zhang JY, Niu W, Kong LM, et al. Correlations of prognosis of generalized anxiety disorder with lncRNA expression level and psychosocial factors. J Prev Med Chin PLA. 2018;36:469–473.
30. Kong LM, Zhu XL, Niu W, et al. The alteration of lncRNA expression in peripheral blood mononuclear cells and the effects of anti-anxiety treatment on lncRNA expression of generalized anxiety disorder patients. Chin J Behav Med Brain Sci. 2019;28:870–874.
31. American Psychiatry Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5).
32. Zhang ZJ. Handbook of Behavioral Medicine Scale. China Med Multimedia Press; 2005.
33. Cui X, Sun X, Niu W, et al. Long non-coding RNA: potential diagnostic and therapeutic biomarker for major depressive disorder. Med Sci Monit. 2016;22:5240–5248. doi:10.12659/MSM.899372
34. Santos MA, Jardim GB, Ranjbar S, et al. Childhood maltreatment and late-life generalized anxiety disorder: are personality and attachment characteristics mediators? J Affect Disord Rep. 2023;12. doi:10.1016/j.jadr.2023.100514
35. Nejati V, Khalaji S, Goodarzi H, et al. The role of ventromedial and dorsolateral prefrontal cortex in attention and interpretation biases in individuals with general anxiety disorder (GAD): a tDCS study. J Psychiatr Res. 2021;144:269–277. doi:10.1016/j.jpsychires.2021.10.034
36. Hallion LS, Tolin DF, Diefenbach GJ. Enhanced cognitive control over task-irrelevant emotional distractors in generalized anxiety disorder versus obsessive-compulsive disorder. J Anxiety Disord. 2019;64:71–78. doi:10.1016/j.janxdis.2019.02.004
37. Possamai-Della T, Cararo JH, Aguiar-Geraldo JM, et al. Prenatal stress induces long-term behavioral sex-dependent changes in rats offspring: the role of the HPA axis and epigenetics. Mol Neurobiol. 2023;60:5013–5033. doi:10.1007/s12035-023-03348-1
38. Li Y, Yao G, Wang R, et al. Maternal immune activation mediated prenatal chronic stress induces Th17/Treg cell imbalance may relate to the PI3K/Akt/NF-κB signaling pathway in offspring rats. Int Immunopharmacol. 2024;126. doi:10.1016/j.intimp.2023.111308
39. Carvalho LB, Dos Santos Sanna PL, Dos Santos Afonso CC, et al. MicroRNA biogenesis machinery activation and lncRNA and REST overexpression MicroRNA biogenesis machinery activation and lncRNA and REST overexpression as neuroprotective responses to fight inflammation in the hippocampus as neuroprotective responses to fight inflammation in the hippocampus. J Neuroimmunol. 2023;382. doi:10.1016/j.jneuroim.2023.578149
40. Teng P, Li Y, Ku L, et al. The human lncRNA GOMAFU suppresses neuronal interferon response pathways affected in neuropsychiatric diseases. Brain Behav Immun. 2023;112:175–187. doi:10.1016/j.bbi.2023.06.009
41. Chen C, Ding P, Yan W, et al. Pharmacological roles of lncRNAs in diabetic retinopathy with a focus on oxidative stress and inflammation. Biochem Pharmacol. 2023;214:115643. doi:10.1016/j.bcp.2023.115643
42. Morelli NR, Maes M, Bonifacio KL, et al. Increased nitro-oxidative toxicity in association with metabolic syndrome, atherogenicity and insulin resistance in patients with affective disorders. J Affect Disord. 2021;294:410–419. doi:10.1016/j.jad.2021.07.057
43. Hallock HL, Quillian HM, Maynard KR, et al. Molecularly defined hippocampal inputs regulate population dynamics in the prelimbic cortex to suppress context fear memory retrieval. Biol Psychiatry. 2020;88:554–565. doi:10.1016/j.biopsych.2020.04.014
44. Xie C, Xue C, Li Y, et al. The characteristics of event-related potentials in generalized anxiety disorder: a systematic review and meta-analysis. J Psychiatr Res. 2025;181:470–483. doi:10.1016/j.jpsychires.2024.12.016
45. Yao Y, Zhang H, Liu H, et al. CT-based radiomics predicts CD38 expression and indirectly reflects clinical prognosis in epithelial ovarian cancer. Heliyon. 2024;10:e32910. doi:10.1016/j.heliyon.2024.e32910
46. Fort V, Khelifi G, Hussein SMI. Long non-coding RNAs and transposable elements: a functional relationship. Biochim Biophys Acta Mol Cell Res. 2021;1868:118837. doi:10.1016/j.bbamcr.2020.118837
47. Beylerli O, Shi H, Begliarzade S, et al. MiRNAs as new potential biomarkers and therapeutic targets in brain metastasis. Noncoding RNA Res. 2024;9:678–686. doi:10.1016/j.ncrna.2024.02.014
48. Zhang C, Tang L, Zhang Y, et al. Febuxostat, a xanthine oxidase inhibitor, regulated long noncoding RNAs and protected the brain after intracerebral hemorrhage. Neuroreport. 2023;34:703–712. doi:10.1097/WNR.0000000000001945
49. Mathur A, Neema S, Sahu R, et al. Anxiety, depression and harmful use of alcohol in severe chronic plaque psoriasis: a cross-sectional study. Med J Armed Forces India. 2023;79(4):464–469. doi:10.1016/j.mjafi.2020.10.014
50. Nakhaei MRS, Noorbala AA, Haghighi AS, et al. Neuropsychiatric symptoms in the psychiatric counseling of patients admitted with COVID-19 infection. Psychiatry Res. 2022;317. doi:10.1016/j.psychres.2022.114855
51. Zhang R, Qiao S, Aggarwal A, et al. Impact of enacted stigma on mental health, substance use, and HIV-related behaviors among sexual minority men in Zambia. Arch Psychiatr Nurs. 2024;48:51–58. doi:10.1016/j.apnu.2024.01.004
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.