Back to Journals » Infection and Drug Resistance » Volume 18
An Approach to, and Tool for, Predicting the Time Course of an Infectious Disease Outbreak as a Function of Behavioral Interventions
Authors Abebe HT , Siraj A, Berhane K, Siraj D, Van Breukelen GJ
Received 15 December 2024
Accepted for publication 14 May 2025
Published 30 May 2025 Volume 2025:18 Pages 2717—2729
DOI https://doi.org/10.2147/IDR.S501934
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Haftom Temesgen Abebe,1,2 Amir Siraj,3 Kiros Berhane,4 Dawd Siraj,5 Gerard JP Van Breukelen6
1Department of Biostatistics, Mekelle University College of Health Sciences, Mekelle, Tigray, Ethiopia; 2Laboratory Interdisciplinary Statistical Data Analysis, Mekelle University College of Health Sciences, Mekelle, Tigray, Ethiopia; 3Department of Biological Sciences, University of Notre Dame, Notre Dame, IN, USA; 4Department of Biostatistics, Columbia University, New York, NY, USA; 5Division of Infectious Diseases, University of Wisconsin-Madison, Madison, WI, USA; 6Department of Methodology and Statistics, School CAPHRI Care and Public Health Research Institute and Graduate School of Psychology and Neuroscience, University of Maastricht, Maastricht, The Netherlands
Correspondence: Haftom Temesgen Abebe, Department of Biostatistics, Mekelle University College of Health Sciences, P.O. Box 1871, Mekelle, Tigray, Ethiopia, Email [email protected]
Introduction: In the early twenty-first century, humanity faced life-threatening infectious diseases, primarily caused by zoonotic pathogens, such as Ebola, Influenza, Middle East Respiratory Syndrome Coronavirus and Severe Acute Respiratory Syndrome Coronavirus. Early warnings based on epidemic models are crucial for preventing and controlling outbreaks. However, most decisions rely on expert opinions rather than robust epidemic model outputs. Implementing epidemic models can be challenging without a strong background in statistical modeling.
Methods: This paper presents a simple, user-friendly tool in MATLAB to predict the time course of an infectious disease outbreak using various modified epidemic models. These models incorporate key non-pharmaceutical interventions to limit transmission within communities. Additionally, we introduce improved epidemiological model structures for outbreak control and prevention. To demonstrate the application of our interactive program and discuss user decision-making, we provide an example using key parameters from recent studies. The simulation model was run with varying scenarios, adjusting the effectiveness and coverage of social distancing, hand washing, and face mask usage.
Results: Without any intervention, 99.6% of the population could be infected within 100 days. Combining social distancing with 80% coverage of face masks and hand washing reduced transmission and death by 99.96% and 99.98%, respectively, compared to no preventive measures.
Conclusion: This interactive computer program aids epidemiologists, public health experts, and decision-makers in understanding and predicting infectious transmission. It is also valuable for generating rapid reports on infectious diseases and outbreak responses where time is a critical. Furthermore, it enhances collaboration between public health stakeholders and modeling professionals, aiming to optimize disease prevention and control strategies during outbreaks or epidemics.
Plain Language Summary: Early warning to prevent and control outbreaks or epidemics of infectious diseases often rely on expert opinion rather than robust epidemiological model outputs. Predicting the time course of an infectious disease outbreak is a challenging task. This interactive computer program predicts the time course of an infectious disease outbreak using various improved epidemiological model structures under different scenarios of non-pharmaceutical interventions that can limit transmission within a community. Combining and maintaining social distancing with 80% coverage of face masks and hand washing reduced transmission by 99.96% and the death rate by 99.98% compared to the scenario of no preventive measures. This tool helps epidemiologists, public health experts, and decision-makers better understand and predict infectious transmission. It will enhance the collaboration between public health stakeholders and modeling professionals with the objective of optimizing disease prevention and control strategies during infectious disease outbreaks or epidemics.
Keywords: Infectious disease, COVID-19, epidemic models, non-pharmaceutical interventions
Introduction
Infectious diseases are among the leading causes of morbidity and mortality, worldwide. Starting early twenty-first century, humans have suffered from several serious infectious diseases of animal origin, namely Ebola, Influenza, Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), and Middle East Respiratory Syndrome Coronavirus (MERS-CoV).1 For instance, in December 2019, the SARS-CoV-2 was identified first in Wuhan, China causing fever, cough and severe respiratory syndrome in human being.2,3
Early warning is essential for preventing and controlling outbreaks or epidemics of infectious diseases. However, many decisions rely on subjective expert opinions. To prevent, control, and make early warnings, statistical methods are indispensable for establishing appropriate models, ranging from early warnings to controlling infectious diseases. Such statistical models are also acknowledged by the WHO as they play a key role in evidence-based decisions.4 In this regard, different epidemic models have been proposed to study the spread of infectious disease outbreaks.5–7 Epidemic models are powerful tools used to assess the dynamics of infectious diseases and design possible control measures in a timely manner. Recently, different non-pharmaceutical intervention approaches have been implemented to reduce the spread of coronavirus (Covid-19).8–12 The model projections of such approaches are subjective and often change because epidemic models rely on assumptions about human behavior, such as the frequency of social contact.
In the absence of pharmaceutical interventions, such as a safe and effective human vaccine, efforts have focused on implementing non-pharmaceutical interventions. These include social distancing, face mask usage, hand washing, quarantine of suspected cases, and hospitalization of confirmed cases. Strict social-distancing measures have been the primary tool for curbing the spread of the pandemic. Face masks have long been utilized to limit the spread of respiratory diseases within the population. Several countries have employed combinations of non-pharmaceutical interventions to reduce the spread of SARS-CoV-2 transmission.13,14
Epidemic models are usually difficult to implement by researchers and decision-makers without a strong background in statistical modeling. Therefore, because of the increasing need to estimate the incidence and assess the effects of interventions with various appropriate warning models in a single environment, this paper provides a simple user-friendly tool for predicting the incidence of infectious disease outbreaks using various improved epidemic models. The interactive computer program includes the main non-pharmaceutical interventions implemented to limit infectious transmission in a community (ie, social distancing, hand washing, and use of face masks). This tool helps users to produce plots of new projected cases and cumulative cases of infections over a period of time, as well as of the projected cumulative number of deaths and recoveries occurring over a period of time, as a function of several possible interventions. A manual is presented in the Appendix with descriptions running in a MATLAB environment. Although the manual can be considered part of the manuscript, it can be consulted independently.
The improved epidemic models are described as follows. Thereafter, an example was used to demonstrate the software application and the different decisions that users must make when estimating the incidence. Finally, a discussion and conclusions are presented.
Epidemic Models
The most common infectious disease forecast and warning models are susceptible–infectious–removed (SIR), susceptible–infectious–susceptible (SIS), and susceptible–infectious–recovery–susceptible (SIRS). Currently, the COVID-19 outbreak has been widely modeled using the classical SIR model to analyze the epidemic situation. The SIR model, initially introduced by Kermack and McKendrick,15 is a compartmental model used to predict disease outcomes when individuals obtain permanent immunity after an infection. This model divides the population into three subpopulations: individuals who are not infected (susceptible S), infected individuals with symptoms that can transmit the disease (I), and removed individuals (R) containing recovered and dead people. However, the SIR model is inappropriate for diseases with a latent stage (exposed individuals) such as COVID-19. This model can be extended to each of the following five extras, resulting in 32 possible models (Figure 1):
 |
Figure 1 The various improved SIR models. Interv.: Intervention. Only models in the rounded bigger rectangles are discussed in this paper. |
Extra 1: Death (SIDR).
Extra 2: Re-infection (SIRS).
Extra 3: Exposed but not yet infectious (SEIR).
Extra 4: Quarantine (SIQR).
Extra 5: Interventions (SIR with Interventions).
Improved SIR Models
In the next section, we introduce improved SIR models that incorporate the main non-pharmaceutical interventions implemented to limit COVID-19 transmission (ie, social distancing, hand washing, and use of face masks). Figure 1 shows the various improved SIR models. First, we describe the general epidemic model that contains all the extras in it. Thereafter, we present sub-models that include the extra death (D) and one of the other extra’s, which can be obtained from the general model by setting certain parameters to zero.
SEIQDRS Model
In this section, we introduce an improved SIR model that contains all the extras in it (see Figure 1), including the main non-pharmaceutical interventions that have been implemented to limit COVID-19 transmission in a community setting (ie, social distancing, hand washing, and use of face masks). The SEIQDRS model is an extensive model used to predict infectious disease dynamics by subdividing the total population size (N) at period t into six mutually exclusive compartments: susceptible (S(t)), exposed (E(t)), infectious (I(t)), quarantined (Q(t)), death (D(t)), and recovered (R(t)). Furthermore, this model allows individuals to acquire immunity only for a certain period after infection (ie, recovered individuals gain no permanent immunity and can be re-infected by the disease in the future). It also considers that infected individuals are not instantly infectious; hence, the exposed segment apart from the infectious one. Moreover, this model distinguishes quarantined/isolated infected individuals from non-isolated ones. In this model, we assumed that isolated infected individuals are removed from the population and cannot infect new individuals while they remain isolated. The SEIQDRS model with the main non-pharmaceutical interventions implemented to limit COVID-19 transmission in a community setting (ie, social distancing, hand washing, and use of face masks) is shown in Figure 2.
 |
Figure 2 Model flow for the SEIQDRS model with interventions. |
The improved model consists of the following system of differential equations:
where θ is the incubation rate, which describes the rate at which the exposed individuals develop symptoms (the transition rate from exposed to infectious class); δ is the rate of quarantine; κ is the rate at which infected individuals die if not quarantined; and π is the rate at which the infected non-quarantined individuals recover. Parameter β is the rate of recovery among quarantined individuals. Parameter γ is the death rate among infected quarantined individuals and ρ is the fixed rate at which recovered individuals return to the susceptible group. Finally, susceptible (S) individuals become exposed to the virus at time t at rate Λ, a parameter known as the force of infection rate, is defined as.
where α is the effective transmission rate from susceptible to exposed (probability of infection per contact), Λ depends on the prevalence of infection (I(t)/N) and the probability of transmission parameter (α), N is the total population size (=S(t)+E(t)+I(t)+Q(t)+D(t)+R(t)), t is time measurement (eg, day, week, month), 0 < φ ≤ 1 is the effectiveness of social distance, 0 ≤ µ ≤ 1 is the coverage of social distance (ie, the amount of use of social distancing by the community), 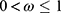 is the effectiveness of face masks,
is the effectiveness of face masks,  is the coverage of face mask usage,
is the coverage of face mask usage, 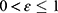 is the effectiveness of hand washing, and
is the effectiveness of hand washing, and  is the coverage of hand washing. Social distancing minimizes the rate of contact between individuals, hand washing prevents the spread of infectious diseases, while face-mask use reduces the chance of infection given contact, all leading to a lower force of infection. If
is the coverage of hand washing. Social distancing minimizes the rate of contact between individuals, hand washing prevents the spread of infectious diseases, while face-mask use reduces the chance of infection given contact, all leading to a lower force of infection. If  ,
,  or
or  (so if at least one intervention is very effective and has good coverage), the force of infection drops strongly; that is, Λ goes to 0. Conversely, if all three terms go to zero (none of the interventions are effective and implemented with good coverage), Λ goes to
(so if at least one intervention is very effective and has good coverage), the force of infection drops strongly; that is, Λ goes to 0. Conversely, if all three terms go to zero (none of the interventions are effective and implemented with good coverage), Λ goes to  , and this model reduces to the SEIQDRS model without interventions.
, and this model reduces to the SEIQDRS model without interventions.
SIQDR Model
Here, we introduce an improved SIR model that includes Extra 1 and 4 (Figure 1). This model, shown in Figure 3, assumes that there is no latent period for the onset of infectiousness. Infected individuals are instantly infectious and can obtain permanent immunity after the infection. The SIQDR model reflects the effect of quarantine/isolation of infected individuals on the time course of infections, in comparison with the SIDR model. The SIQDR model comprises five compartments: susceptible (S), infected (I), quarantined (Q), death (D), and recovered (R). This model can be obtained by setting θ=1 and ρ=0 in the SEIQDRS model (Table 1).
 |
Table 1 The Different Sub-Models, Which Can Be Obtained From the General SEIQDRS Model by Setting Certain Parameters to Zero |
 |
Figure 3 Model flow diagram for the SIQDR model. |
By setting Λ = 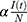 , this model reduces to the SIQDR model without interventions.
, this model reduces to the SIQDR model without interventions.
SEIDR Model
This is an improved SIR model that includes extras 1 and 3 (Figure 1), and does not distinguish quarantined/isolated infected individuals from non-isolated ones. This model, shown in Figure 4, can be used for diseases when recovered individuals acquire permanent immunity and there is a latent period prior to the start of infectiousness. The SEIDR model can be obtained from the SEIQDRS model by setting ρ=0 and δ=0 (see Table 1).
 |
Figure 4 The SEIDR model flow chart. |
SIDRS Model
This model contains the extras 1 and 2 and assumes (a) the absence of quarantine (δ=0) or of any effect of quarantine (κ = γ) and π = β), (b) all exposed persons are infectious (θ=1), and (c) individuals do not acquire permanent immunity after recovery (ρ > 0). The SIDRS model consists of four compartments (Figure 5): susceptible (S), infectious (I), death (D), and recovered (R).
 |
Figure 5 The SIDRS model flow chart. |
SIDR Model
The SIDR model can be obtained by setting ρ=0, θ=1, and δ=0 in the SEIQDRS model and can be used when individuals (a) obtain permanent immunity after infection, (b) there is no latency between exposure and infectiousness, and (c) there is no quarantine or no effect of quarantine. The scheme is illustrated in Figure 6.
 |
Figure 6 The SIDR model flow chart. |
The choice ρ=0, θ=1, δ=0, and κ= 0 in equations 1–6 gives the classic SIR model.
Example
As an illustration of the application and to discuss the different decisions that the user has to make when predicting the prevalence of an infectious disease as a function of time since the outbreak, we consider the SEIDR model and input parameter values from recent studies in Table 2. This model is used when individuals can obtain permanent immunity after infection and assumes that (a) there is a latent period prior to the start of infectiousness and the model assumes that there is no quarantining (δ=0) or no effect of quarantining (κ = γ and π= β).
 |
Table 2 Parameters and Assumptions Used to Run the Modeling Scenarios for SEIDR Model with Interventions (the Non-Pharmaceutical Interventions) |
Modeling Scenarios
We considered the following eight scenarios to assess their potential impact on the COVID-19 pandemic:
Scenario-1: No social distancing, No face mask usage, No hand washing.
Scenario-2: No social distancing, No face mask usage, 80% hand washing.
Scenario-3: No social distancing, 80% face mask usage, No hand washing.
Scenario-4: No social distancing, 80% face mask usage, 80% hand washing.
Scenario-5: Social distancing, No face mask usage, No hand washing.
Scenario-6: Social distancing, No face mask usage, 80% hand washing.
Scenario-7: Social distancing, 80% face mask usage, No hand washing.
Scenario-8: Social distancing, 80% face mask usage, 80% hand washing.
The SEIDR model is used for the eight scenarios described above. Based on a population of 300,000 residents, model projections are given for the daily number of infections (Figure 7) and cumulative number of infections (Figure 8) over time. For Scenario-1, where no interventions (No Social distance, No Face mask and No hand washing) are taken at all, the epidemic is projected to reach a peak after 61 days, with an estimated number of new infections of 85,288 individuals (28.4% of the population can be infected) (Figure 7), and the total cumulative number of infections reached in a 100 days is 298,887 (99.6% of the population can be infected) (Figure 8). The total number of deaths is 8,908 at 100 days (Figure 7).
Figure 7 Continued. Figure 8 Continued.

On the other hand, an intervention that combines social distancing, 80% coverage in the use of face masks, and 80% coverage of hand washing (Scenario-8) leads to an estimated number of new infections of 18 individuals over 100 days. The total number of deaths is two in 100 days, which is a dramatic decrease of 99.98% compared with the scenario of no interventions (Figure 7). The total cumulative number of infections under Scenario 8 is 105 (0.035% of the population infected) over 100 days (Figure 8).
Discussion
Early warning is a crucial measure during infectious disease outbreaks, essential for controlling and preventing outbreaks and epidemics. Accurate and reliable predictions of infectious disease dynamics are invaluable to public health organizations when planning interventions to decrease or prevent disease transmission. Epidemic models are powerful tools for assessing the dynamics of infectious diseases and effects of possible control methods in a timely manner. However, implementing these models is often challenging without a strong background in statistical modeling. Consequently, most decisions are based on expert opinions rather than robust epidemiological model outputs.
To address this, we present a user-friendly tool designed to predict the time course of an infectious disease outbreak based on behavioral interventions, using various improved epidemic models applicable to disease prevention and control. Recent literature was reviewed to identify the key parameters relevant to illustrating the program. In a hypothetical example, combining social distancing with 80% coverage of face masks and hand washing reduced transmission by 99.96% and the death rate by 99.98% compared to the scenario of no preventive measures. Our results demonstrate that non-pharmaceutical interventions (social distancing, hand washing, and face mask usage) not only lower the force of infection and death rates, but also reduce the accumulation of cases and delay the peak transmission time. This enables hospitals and healthcare systems to better manage severe and critical cases. Our findings support recent data that indicates non-pharmaceutical interventions are beneficial against SARS-CoV-2 epidemics.12,17–19
This interactive computer program will help epidemiologists, public health experts, and decision-makers in better understanding and predicting the time course of infectious disease outbreaks and the effects of preventive measures on infectious disease outbreaks. It is also helpful for generating rapid reports and predictions in outbreak situations where a fast response is required. Moreover, it will improve collaboration between public health and modeling professionals, with the objective of optimizing disease prevention and control strategies. The computer program EpiModeling_Infectious runs in MATLAB and can be obtained upon request from the corresponding author, with potential future availability via the internet. Our current models do not differentiate between symptomatic and asymptomatic infections. Future extensions of the epidemic models and software could include separate considerations for symptomatic and asymptomatic infections.
Data Sharing Statement
An interactive computer MATLAB program can be freely obtained from the corresponding author upon request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Bai Z, Gong Y, Tian X, Cao Y, Liu W, Li J. The rapid assessment and early warning models for COVID-19. Virol Sin. 2020;35:272–279. doi:10.1007/s12250-020-00219-0
2. Sadeghi Dousari A, Taati Moghadam M, Satarzadeh N. COVID-19 (Coronavirus Disease 2019): a new coronavirus disease. Infect Drug Resist. 2020;13:2819–2828. PMID: 32848431; PMCID: PMC7429403. doi:10.2147/IDR.S259279
3. Chauhan S. Comprehensive review of coronavirus disease 2019 (COVID-19). Biomed J. 2020;43(4):334–340. PMID: 32788071; PMCID: PMC7263230. doi:10.1016/j.bj.2020.05.023
4. Morgan O. How decision-makers can use quantitative approaches to guide outbreak responses. Philos Trans R Soc Lond B Biol Sci. 2019;374(1776):20180365. PMID: 31104605; PMCID: PMC6558558. doi:10.1098/rstb.2018.0365
5. Loli Piccolomini E, Zama F, Villaverde AF. Monitoring Italian COVID-19 spread by a forced SEIRD model. PLoS One. 2020;15(8):e0237417. PMID: 32760133; PMCID: PMC7410324. doi:10.1371/journal.pone.0237417
6. Osemwinyen AC, Diakhaby A. Mathematical Modelling of the Transmission Dynamics of Ebola Virus. Appl Comput Math. 2015;4(4):313–320.doi:10.11648/j.acm.20150404.19
7. Zhou T, Xianbin L, Houqiang L. Prediction of new coronavirus infection based on a modified SEIR model. doi:10.1101/2020.03.03.20030858
8. Eikenberry SE, Mancuso M, Iboi E, et al. To mask or not to mask: modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infect Dis Model. 2020;5:293–308. PMID: 32355904; PMCID: PMC7186508. doi:10.1016/j.idm.2020.04.001
9. Ngonghala CN, Iboi E, Eikenberry S, et al. Mathematical assessment of the impact of non-pharmaceutical interventions on curtailing the 2019 novel Coronavirus. Math Biosci. 2020;325:108364. PMID: 32360770; PMCID: PMC7252217. doi:10.1016/j.mbs.2020.108364
10. Raherinirina A, Fontaine R, Tsilefa SF, Rivo AR. Mathematical model and non-pharmaceutical control of the coronavirus 2019 disease in Madagascar. Open J Model Sim. 2021;9:259–274.
11. Giordano G, Blanchini F, Bruno R, et al. Modeling the COVID-19 epidemic and implementation of population-wide interventions in Italy. Nature Medicine. 2020;26:855–860. doi:10.1038/s41591-020-0883-7
12. Prem K, Liu Y, Russell TW. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: a modelling study. Lancet Public Health. 2020;5:e261–70. doi:10.1016/S2468-2667(20)30073-6
13. Daniel FG, Agnar H, Hakon J, et al. Spread of SARS-CoV-2 in the Icelandic population. New England Journal of Medicine. 2020;382:2302–2315. doi:10.1056/NEJMoa2006100
14. Hsiang S, Allen D, Annan-Phan S, et al. The effect of large-scale anti-contagion policies on the COVID-19 pandemic. Nature. 2020;586:262–267. doi:10.1038/s41586-020-2404-8
15. Kermack WO, McKendrick AG. Contributions to the mathematical theory of epidemics. Bull Math Biol. 1991;53:33–55.
16. Linton NM, Kobayashi T, Yang Y, et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med. 2020;9(2):538. PMID: 32079150; PMCID: PMC7074197. doi:10.3390/jcm9020538
17. Amir S, Alemayehu W, Kiros B, et al. Early estimates of COVID-19 infectious in small, medium and large population clusters. BMJ Global Health. 2020;5:e003055. doi:10.1136/bmjgh-2020-003055
18. Feng S, Shen C, Xia N, Song W, Fan M, Cowling BJ. Rational use of face masks in the COVID-19 pandemic. Lancet Respir Med. 2020;8:434–436. doi:10.1016/S2213-2600(20)30134-X
19. Singh R, Adhikari R. Age-structured impact of social distancing on the COVID-19 epidemic in India. Available from: https://arxiv.org/abs/2003.12055.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.










