Back to Journals » Research and Reports in Urology » Volume 16
An Evaluation of the Postvoid Residual Urine Volume in Acute Stroke Patients
Authors Yamashita K , Kudo M, Ando M, Ashida H, Shiseki T, Kubota S, Sakamoto T, Inui M
Received 29 May 2024
Accepted for publication 10 October 2024
Published 15 October 2024 Volume 2024:16 Pages 265—271
DOI https://doi.org/10.2147/RRU.S480444
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Guglielmo Mantica
Kaori Yamashita,1,2 Miyuki Kudo,2 Motoya Ando,2 Hikaru Ashida,2 Takahiro Shiseki,1,3 Satoshi Kubota,1 Tetsushi Sakamoto,1 Masashi Inui1,2
1Department of Urology, Tokyo Women’s Medical University Yachiyo Medical Center, Yachiyo, Japan; 2A Multidisciplinary Specialized Team, Tokyo Women’s Medical University Yachiyo Medical Center, Yachiyo, Japan; 3Department of Urology, Graduate School of Medicine, Chiba University, Chiba, Japan
Correspondence: Kaori Yamashita, Department of Urology, Tokyo Women’s Medical University Yachiyo Medical Center, 477-96, Owadashinden, Yachiyo-shi, Chiba, 276-8524, Japan, Tel +81 474506000, Fax +81 474587047, Email [email protected]
Introduction: We analyzed the time course of postvoid residual urine volume for patients with cerebrovascular diseases in the acute phase.
Materials and Methods: A multidisciplinary specialized team measured postvoid residual urine volume of 65 patients (31 patients with cerebral infarction and 34 patients with cerebral hemorrhage) from September 2021 to August 2023. If a patient’s postvoid residual urine volume was 100 mL or more, an indwelling urinary catheter was reinserted with or without medication, or clean intermittent catheterization was performed with or without medication. The multidisciplinary specialized team took repeated measurements of postvoid residual urine volume for a patient at every week’s round until the postvoid residual urine volume was < 100 mL. The cumulative incidence of the time interval between the onset of the cerebrovascular accident and the day of postvoid residual urine volume < 100 mL was calculated as 1 + the Kaplan–Meier estimator.
Results: In the Kaplan–Meier estimator, the median cumulative incidence of the period between the onset of a cerebrovascular accident and the day in which postvoid residual urine volume was < 100 mL was 16.5 days and 15.5 days for patients with cerebral infarction and cerebral hemorrhage, respectively. No significant difference existed between the two groups in the time interval from the onset of a cerebrovascular accident to the day in which postvoid residual urine volume was < 100 mL (The P value from the Log-rank test was 0.845). The time interval between the onset of a cerebrovascular accident and the day in which postvoid residual urine volume was < 100 mL was 75 days after the onset of both types of cerebrovascular accidents.
Conclusion: Postvoid residual urine volume of patients with cerebrovascular disease was expected to become < 100 mL within 75 days after the onset of the cerebrovascular accident.
Keywords: lower urinary tract symptom, multidisciplinary specialized team, stroke
Introduction
Lower urinary tract symptoms such as storage or voiding symptoms are common in patients with cerebrovascular diseases. The urinary tract symptoms depend on the phase of cerebrovascular disease1–8 or the type of cerebrovascular such as cerebral infarction (CI) or cerebral hemorrhage (CH).1–7 Patients with cerebrovascular disease in the acute phase have storage symptoms such as urinary retention or increased postvoid residual urine volume (PVR);1,2 therefore, urinary catheters are inserted in patients with cerebrovascular disease. In Japan, to avoid the unnecessary chronic use of indwelling urinary catheters, a multidisciplinary specialized team intervenes in patients who are suspected of having lower urinary tract symptoms. The multidisciplinary specialized team included medical doctors, nurses, physiotherapists, and occupational therapists. Our team members gathered once weekly to discuss lower urinary tract symptoms such as storage or voiding symptoms in patients with cerebrovascular diseases. Physiotherapists and occupational therapists help individuals maintain independence in toileting, nurses measured PVR9 of the patients and administered clean intermittent catheterization, and doctors prescribe medications. In our hospital, the team supports patients from various dimensions to improve their voiding function.
This study investigated the time course of PVR for patients with cerebrovascular diseases in the acute phase.
Materials and Methods
This study was conducted at Tokyo Women’s Medical University Yachiyo Medical Center (Yachiyo City, Chiba Prefecture, Japan). It was approved by the Institutional Review Board of Tokyo Women’s Medical University (registration no. 2023–0082). The patients’ informed consent for researchers to review their medical records was not required by the committee because of the retrospective of the study. However, we uploaded the information about the study to use the patients’ acute stroke data in our hospital’s homepage. The study and protocol were conducted in accordance with the Declaration of Helsinki guidelines. The patients’ data were extracted and stored on a password-protected shared drive, which could only be accessed by the personnel named in this study; therefore, patient confidentiality was protected. In this hospital-based study, we checked PVR of 65 patients with cerebrovascular disease who had been hospitalized in the Department of Neurosurgery Unit in Tokyo Women’s Medical University Yachiyo Medical Center (Tokyo, Japan) from September 2021 to August 2023. The exclusion criteria were (1) severe diabetes mellitus and (2) the presence of benign prostate hyperplasia with a volume >30 g. The 65 inpatients consisted of 31 patients with CI and 34 patients with CH. All 65 inpatients had an indwelling urinary catheter after the onset of cerebrovascular disease and had undergone intervention by the multidisciplinary specialized team. The indwelling urinary catheter was removed in the morning before each week’s afternoon round of the multidisciplinary specialized team. The multidisciplinary specialized team measured PVR by using a bladder volume ultrasound imaging device (Liliumα-200E; Lilium Otsuka Co., Ltd., Kanagawa, Japan) on 65 patients with cerebrovascular disease. If a patient’s PVR was <100 mL, an indwelling urinary catheter did not need to be reinserted. However, if a patient’s PVR was ≥100 mL, an indwelling urinary catheter needed to be reinserted with or without medications such as an alpha-blocker or distigmine bromide, or the patient needed to undergo clean intermittent catheterization with or without medications such as an alpha-blocker or distigmine bromide. The multidisciplinary specialized team performed repeated measurements of the patients’ PVR at each week’s round until PVR was <100 mL. We compared sex, age, the time interval between the onset of the cerebrovascular accident and the day of the first intervention by the multidisciplinary specialized team, PVR at the first intervention, and the choice of the medications for treating lower urinary tract symptoms of cerebrovascular diseases between the CI patient group and the CH patient group. We also compared the lesion localization in the patients.
The cumulative incidence of the time interval between the onset of a cerebrovascular accident and the day in which PVR was <100 mL was calculated as 1 + the Kaplan–Meier estimator. Statistical analysis was conducted by using JMP Pro 16 (SAS Institute Japan Co., Ltd., Minato-ku, Tokyo, Japan). Qualitative parameters were compared by using the χ2 test, and quantitative parameters were compared by using the unpaired two-sample t-test. The Log rank test was used in the Kaplan–Meier method. A value of P < 0.05 was significant.
Results
Sixty-five inpatients were included and consisted of 37 male patients and 28 female patients. Table 1 shows the clinical characteristics of patients with CI and CH. The median age of the patients was 79 years and 75.5 years in the CI group and the CH group, respectively. The median time interval between the onset of a cerebrovascular accident and the day of the first intervention by the multidisciplinary specialized team was 7 days and 9 days in the CI group and CH group, respectively. The median PVR at the first intervention was 300 mL and 365 mL in the CI group and in the CH group, respectively. We prescribed an α-blocker only to 19 (29%) of 65 patients, distigmine bromide only to 3 (5%) of 65 patients, and an α-blocker plus distigmine bromide to 9 (14%) of 65 patients. Fifty-two percent (34/65) of patients had no medications for the choice of the medications for treating lower urinary tract symptoms of cerebrovascular diseases. No significant differences existed in age, the time interval between the onset of a cerebrovascular accident and the day of the first examination by the multidisciplinary specialized team, PVR at the first intervention, and the choice of the medications for treating lower urinary tract symptoms of cerebrovascular diseases with CI and patients with CH. Table 2 shows the lesion localization among patients with CI and patients with CH. No significant differences existed between the two groups.
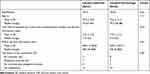 |
Table 1 Clinical Characteristics of Patients with Cerebral Infarction and Cerebral Hemorrhage |
 |
Table 2 Lesion Localization in Patients with Cerebral Infarction and Patients with Cerebral Hemorrhage |
Figure 1 presents the cumulative incidence of the time interval between the onset of a cerebrovascular accident and the day in which PVR became <100 mL. The median cumulative incidence of the interval was 16.5 days and 15.5 days in patients with CI and in patients with CH, respectively. The P value from the Log rank test was 0.845; thus, no significant difference existed between the two groups in the time interval from the onset of a cerebrovascular accident to the day when PVR was <100 mL. PVR of both patient groups became <100 mL at 75 days after the onset of the cerebrovascular disease.
 |
Figure 1 The cumulative incidence of the time interval between the onset of a cerebrovascular accident and the day in which postvoid residual urine volume was <100 mL. |
Discussion
Doshi et al4 reported that 20.9% of 140 patients with cerebrovascular disease during the acute phase had urinary retention, Prabhakar et al10 reported that 19.4% (6/31) of patients with CI had urinary retention. Urinary retention occurred in cerebrovascular disease patients during the acute phase; therefore urinary catheters may have been inserted in these patients. In Japan, to avoid the unnecessary use of indwelling urinary catheters during the acute phase, a multidisciplinary specialized team intervenes in patients who are suspected of having lower urinary tract symptoms. A multidisciplinary specialized team includes medical doctors, nurses, physiotherapists, and occupational therapists; assesses individuals` independence in toileting and urinary continence; and measures PVR of patients.9 When the patient’s PVR is decreased, the patient’s urinary catheter is removed. We investigated PVR during the acute phase in this study. Based on the Kaplan–Meier estimator, the median cumulative incidence of the period between the onset of a cerebrovascular accident and the day in which the PVR was <100 mL was 16.5 days and 15.5 days for patients with CI and CH, respectively. PVR of both patient groups became <100 mL within 75 days after the onset of a cerebrovascular accident. We speculated that (1) the urinary catheters of patients with cerebrovascular disease would be able to be removed within 75 days after the onset of cerebrovascular accidents and (2) the time course of PVR in the acute phase may be similar between patients with CI and patients with CH. To the best of our knowledge, this report is the first to investigate PVR of cerebrovascular diseases by using the Kaplan–Meier estimator.
Sumiya et al3 reported that patients with cerebrovascular disease in Japan are treated in three phases: acute phase, convalescent phase, and community-based phase. In their study, 20.9% (207/990) of post-stroke patients had their lower urinary tract symptom medications switched on transferring from an acute ward to a convalescence rehabilitation ward. Sumiya et al3 concluded that prescription drugs for voiding symptoms tended to increase during the acute care wards, while prescription drugs for storage symptoms tend to decrease during the acute care wards and increase in the convalescence rehabilitation wards. Therefore, lower urinary tract symptoms for patients with cerebrovascular disease are changeable and depend on the phase. We recommend clinicians measure PVR during the acute phase and during convalescent phase.
We summarized the comparison of lower urinary tract symptoms between patients with CI and patients with CH in Table 3. We concluded in this study that, in the acute phase, the rate of patients whose PVR was >100 mL at the first intervention by our multidisciplinary specialized team was 83.9% (26/31 patients) and 79.4% (27/34 patients) in patients with CI and CH, respectively. Therefore, both groups of patients had voiding symptoms. Lucke-Wold et al2 reported that patients with CI had detrusor overactivity, leading to urinary retention, and patients with CH had increased bladder residual volume; therefore, both of these cerebrovascular disease patient groups had voiding symptoms in the acute phase. However, Han et al7 in their urodynamic study reported that, in the chronic phase, ischemic patients had detrusor overactivity (70.7%) or detrusor underactivity (29.3%), and hemorrhagic patients had detrusor overactivity (34.6%) or detrusor underactivity (65.4%). Pizzi et al8 reported that 48% (30/63) of patients with CI had detrusor overactivity, 16% (10/63) of patients had detrusor overactivity with impaired contractility, and 6% (4/63) of patients had detrusor underactivity. Cai et al5 and Itoh et al6 reported that CI and CH patients both tended to have storage symptoms such as overactive bladder and urinary incontinence. Therefore, lower urinary tract symptom of cerebrovascular disease in the chronic phase tends to vary from patient to patient.
 |
Table 3 The Difference in Lower Urinary Tract Symptoms Between Patients with Cerebral Infarction and Patients with Cerebral Hemorrhage |
With regard to the relationship between lower urinary tract symptoms and lesion localization of CI and CH, Lucke-Wold et al2 reported that the pontine micturition center and frontal lobe urinary control center were important regulators of urinary function. Pontine micturition center damage causes an inability to void and increases PVR, whereas frontal lobe damage causes urinary incontinence and increased urinary frequency in the acute phase. Therefore, cerebral disease of the frontal lobe may be suspected in cases of overactive bladder. Our study included three patients with CI and five patients with CH who had frontal lobe damage. At the first intervention by our multidisciplinary specialized team, the mean PVR of the three patients with CI of the frontal lobe was 312 mL (range; 116–700 mL) and the mean PVR of the five patients with CH in the frontal lobe was 664 mL (range: 420–700 mL). We believed that cerebrovascular disease of the frontal lobe increased PVR in the acute phase.
With regard to medicine for patients whose PVR was ≥100 mL, we prescribed alpha-blockers and/or distigmine bromide. Bayrak et al11 reported that alpha-blockers and cholinesterase inhibitors such as distigmine bromide are useful for treating underactive bladder and concluded that the combination of alpha-blockers and distigmine (5 mg) could be administered effectively and reliably. Yamanishi et al12 reported a significant decrease in PVR with alpha-blockers and cholinergic agent combination treatment, compared with the decrease in PVR with alpha-blocker treatment alone or with cholinergic drug treatment alone.
Our multidisciplinary specialized team regularly measured PVR. When a patient’s PVR was <100 mL, an indwelling urinary catheter did not need to be reinserted, it might be expected to be useful in avoiding the unnecessary chronic use of indwelling urinary catheters. Gopinath et al13 reported that a multidisciplinary team in urogynecology provides outstanding service to patients and allows the members to learn and support each other, thereby providing a positive working environment and richer work experience. Balachandran et al14 report the potential need for further debate and a randomized trial to assess whether involving a multidisciplinary team in managing urinary incontinence is cost-effective. Investigating the benefit and the cost of a multidisciplinary team for patients who have lower urinary tract symptom might be needed in the future.
Our study has several limitations. First, the study was based on retrospective information and the evidence level was lower than that of prospective studies. Second, our hospital is a University Hospital for patients in the acute phase and convalescent phase. When patients were in the community-based phase, following the acute phase and convalescent phase, they needed to be transferred to another Hospital. We investigated PVR from the acute phase to the convalescent phase only; therefore, assessing the patients long-term was problematic. Third, the number of patients was small. In the future, we hope to collect more patient data with regard to PVR. Fourth, our study group had three patients with diabetes and five patients with benign prostate hyperplasia. Diabetes and benign prostate hyperplasia are risk factors for an increased PVR. However, the patients’ diabetes was not severe and the volume of benign prostate hyperplasia was <30 g. Therefore, we believed these factors had little influence on PVR. Fifth, we did not measure PVR and did not evaluate lower urinary tract function in precerebrovascular disease because all patients did not visit our department of urology before the incident of cerebrovascular disease. Sixth, we could not unify the type of medication or catheter, we prescribed an alpha-blocker and/or distigmine bromide, and we decided on intermittent or long-term catheter use but treatment choice depended on the decision of each physician and patient’s condition.
Conclusions
PVR increases in cerebrovascular disease in acute phase. PVR of the patients with cerebrovascular disease was expected to become <100 mL within 75 days after the onset of the cerebrovascular accident.
Abbreviations
CI, cerebral infarction; CH, cerebral hemorrhage; PVR, postvoid residual urine volume.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The patient’s informed consent to review the medical record was not required by the committee due to the retrospective of the study. The study and protocol were in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors declare that this study has not received any fundings.
Disclosure
The authors have no conflict of interest directly relevant to the content of this article.
References
1. Uemura T, Ohta H, Yokota A, Yarimizu S, Nishizawa S. Urinary retention associated with stroke. J UOEH. 2016;38(4):263–269. doi:10.7888/juoeh.38.263
2. Lucke-Wold B, Vaziri S, Scott K, Busl K. Urinary dysfunction in acute brain injury: a narrative review. Clin Neurol Neurosurg. 2020;189:105614. doi:10.1016/j.clineuro.2019.105614
3. Sumiya K, Shogenji M, Ikenaga Y, et al. Association between switching prescribed drugs for lower urinary tract symptoms and independent of urination in post-stroke patients: a retrospective cohort study. J Stroke Cerebrovasc Dis. 2023;32(12):107419. doi:10.1016/j.jstrokecerebrovasdis.2023.107419
4. Doshi VS, Say JH, Young SH-Y, Doraisamy P. Complications in stroke patients: a study carried out at the rehabilitation medicine service, Changi General Hospital. Singapore Med J. 2003;44(12):643–652.
5. Cai T, Wang J, Wang L, Wang J, Guo L. Prevalence and risk factors of urinary incontinence for post-stroke inpatients in Southern China. Neurourol urodyn. 2015;34(3):231–235. doi:10.1002/nau.22551
6. Itoh Y, Yamada S, Konoeda F, et al. Burden of overactive bladder symptom on quality of life in stroke patients. Neurourol urodyn. 2013;32(5):428–434. doi:10.1002/nau.22336
7. Han K-S, Heo SH, Lee S-J, Jeon SH, Yoo KH. Comparison of urodynamics between ischemic and hemorrhagic stroke patients; can we suggest the category of urinary dysfunction in patients with cerebrovascular accident according to type of stroke? Neurourol urodyn. 2010;29(3):387–390. doi:10.1002/nau.20708
8. Pizzi A, Falsiki C, Martini M, Rossetti MA, Verdesca S, Tosto A. Urinary incontinence after ischemic stroke: clinical and urodynamic studies. Neurourol urodyn. 2014;33(4):420–425. doi:10.1002/nau.22420
9. Nishii H. A review of aging and the lower urinary tract: the future of urology. Int Neurourol J. 2021;25(4):273–284. doi:10.5213/inj.2142042.021
10. Prabhakar AT, Ahmed ASI, Nair AV, et al. Neural correlates of urinary retention in lateral medullary. Int Neurourol J. 2019;23(3):205–210. doi:10.5213/inj.1836256.128
11. Bayrak O, Dmochowski RR. Underactive bladder: a review of the current treatment concepts. Turk J Urol. 2019;45(6):401–409. doi:10.5152/tud.2019.37659
12. Yamanishi T, Yasuda K, Kamai T, et al. Combination of a cholinergic drug and an alpha-blocker is more effective than monotherapy for the treatment of voiding difficulty in patients with underactive detrusor. Int J Urol. 2004;11(2):88–96. doi:10.1111/j.1442-2042.2004.00753.x
13. Gopinath D, Jha S. Multidisciplinary team meetings in urogynecology. Int Urogynecol J. 2015;26(8):1221–1227. doi:10.1007/s00192-015-2662-4
14. Balachandran A, Duckett J. What is the role of the multidisciplinary team in the management of urinary incontinence. Int Urogynecol J. 2015;26(6):791–793. doi:10.1007/s00192-014-2579-3
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

