Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
An Event-Related Potential Study on Facial Recognition in Children with and without Autism Spectrum Disorders
Authors Deng JR, Tong M, Zhang XT, Lin ZP, Wang Z, Long J, Chen ZM
Received 15 January 2025
Accepted for publication 29 March 2025
Published 15 April 2025 Volume 2025:21 Pages 903—916
DOI https://doi.org/10.2147/NDT.S517704
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Jia-Rui Deng,1 Meiqinzi Tong,1 Xiao-Tong Zhang,1 Zhen-Ping Lin,1 Zhuo Wang,2 Jinyi Long,3 Zhuo-Ming Chen1
1Department of Rehabilitation Medicine, The First Affiliated Hospital of Jinan University, Guangzhou, Guangdong Province, People’s Republic of China; 2Wellness Technology Research Center, Hefei Intelligent Robot Institute, Hefei, Anhui Province, People’s Republic of China; 3College of Information Science and Technology, Jinan University, Guangzhou, Guangdong Province, People’s Republic of China
Correspondence: Zhuo-Ming Chen, Department of Rehabilitation Medicine, The First Affiliated Hospital of Jinan University, No. 613, West Huangpu Avenue, Tianhe District, Guangzhou, Guangdong Province, 510632, People’s Republic of China, Email [email protected] Jinyi Long, College of Information Science and Technology, Jinan University, Guangzhou, Guangdong Province, People’s Republic of China, Email [email protected]
Purpose: Facial recognition is very primary and important in individuals’ development and the event-related potential based on face recognition such as N170 is considered as the most potential objective marker of autism, the hot and difficult point of current research. We will explore the electrophysiological basis of facial recognition with autism and without autism. Given the link between facial recognition and social impairments, the core symptom of autism, it is also necessary to study the correlation between the P1 and N170 components and the severity of social functioning in autism.
Patients and Methods: In this study, autism and age-matched typically developing children were asked to examine photographs of faces, objects and butterflies and event-related potentials were recorded. The parents or caregivers of the participants were asked to fill out the Vineland Adaptive Behavior Scale. Finally, thirteen children with autism (6.60± 2.12years) and ten typically developing (6.65± 1.64years) children were included in the experiment.
Results: Children with autism showed slower P1 and N170 latencies than typically developing children. The N170 amplitude for faces was larger than that for objects. Considering age as a covariant, the results primarily remained unchanged and the effect size of age was significant for the P1 and N170 latencies. As for the correlation between ERPs and the severity of social impairment, there were some significant correlations between the P1 and N170 latencies and social functioning.
Conclusion: This result not only suggests the electrophysiological basis of facial recognition but also indicates that the P1 and N170 components could assist in the diagnosis and assessment of autism. Moreover, the results suggest that age should be considered in analyses of the P1 and N170 latencies. Due to a limited number of participants, conducting a multi-center and large-sample study in the future is necessary.
Keywords: ASD, N170, P1, EEG, age, social functioning
Introduction
Facial recognition develops beginning in the first year of life. Infants present a preference for their mother’s face within days after birth.1 Moreover, facial recognition plays a vital role in social functioning by providing the necessary emotional and cognitive information for social interactions. Therefore, facial recognition is a primary ability and important for individuals’ development. However, individuals with autism show atypical facial recognition.
Autism spectrum disorder (ASD) is a neurodegenerative disorder with core features such as restricted interests, stereotyped behaviors, and social and communicative impairments,2 which cause considerable burden to individuals, families and society. Some experts have put forward that ASD children do not pay attention to faces during sensitive developmental periods, which results in failures in facial recognition and related cortical specialization.3–5 This further contributes to social dysfunction, the core feature of ASD. Some studies have explored electrophysiological basis of facial recognition and atypical pattern in ASD individuals.
P1 is a positive evoked-related potential (ERP) that occurs at the occipital electrode and has a maximum amplitude of approximately 100 ms. P1 was thought to be the earliest endogenous visual ERP, reflecting early and rapid processing.6 Many studies have suggested that P1 reflects the neural response to the basic sensory input of visual perception6–8 and is influenced and modulated by selective attention.9,10 Studies investigating P1 in ASD have yielded mixed results, and some studies have indicated that faces elicit a faster P1 latency than objects in typically developing (TD) individuals.7,11–14
N170 is a negative ERP that is related to facial recognition, which has a maximum amplitude of approximately 170 ms at the occipito-temporal electrodes, with a shorter latency and larger amplitude in response to faces compared to objects.15 Some articles suggest that N170 can help distinguish ASD individuals from TD individuals because ASD individuals have a slower latency and smaller negative amplitude in response to faces than TD individuals do.16–19 However, some articles did not reproduce this result.20 In addition, we cannot help but raise the question of whether the N170 latency (N170L) or N170 amplitude (N170A) is a better objective marker of autism. McPartland et al reported that both children and adults with ASD presented a slower N170L than did age-matched TD individuals, but no significant difference in amplitude was detected.5,17 Some papers have shown that adult individuals with ASD have a slower latency and smaller amplitude than those in TD individuals.21,22
N170 has been reported to be potential not only to assist in the diagnosis of ASD but also to reflect the severity of ASD. A faster right hemisphere N170L was correlated with better performance on Benton facial recognition tests.17 N170L was also related to holistic face processing, with faster N170L indicating greater accuracy in the holistic task.23 In the same study, N170L was associated with social difficulties, as measured by the Child Behavior Checklist, with a faster N170L to upright faces indicating better social function. Considering the close connection between face recognition and social functioning, studies are needed to explore the correlation between N170 and the severity of social symptoms.
N170 has also been reported to be sensitive to configural information extraction supported by the inverted face effect.7,24 Inverted faces increase spatial structure recognition difficulties, so TD individuals exhibit longer N170L and greater negative amplitudes for inverted faces than for upright faces, which is referred to as the inverted face effect. There was no obvious inverted facial effect in the ASD group because of the spatial structure recognition dysfunction.17,25 However, other articles did not observe this phenomenon in their results.26 The inverted face effect was seen in some studies investigating P1.6,12,23,27
In addition, facial recognition is reported to involve right lateralization. For right-handed people, the left hemisphere of the brain is better at calculation, speaking, memory and so on, whereas the right hemisphere is better at music, art, and spatial processing. Therefore, it is not difficult to infer that facial recognition involves right lateralization, which has been tested in several studies using EEG and fMRI.17,19,22,28–30 While most TD individuals show activation of the right fusiform gyrus in response to facial stimuli, individuals with ASD show atypical hemisphere lateralization.17,31–33 However, Shen I. H. et al reported that there was no difference in the N170L between the two hemispheres in the TD group.13
These inconsistent findings may be driven by moderating factors, of which age is considered the most promising. With increasing age, N170L becomes shorter, and N170A becomes negatively larger. In a large sample study, the author conducted a cluster analysis to reveal the subgroups of ASD patients and reported that there was no significant difference in symptom severity, IQ, or sex across the clusters but that age significantly differed. Moreover, N170L significantly differed among the three clusters.34 These findings suggested that N170L was influenced by age. P1 has also been reported to be modulated by age.10,16,35
On this basis, we conducted behavioral and electrophysiological experiments on facial recognition to investigate the following: 1. the electrophysiological basis of facial recognition in ASD and TD individuals, including the differences in P1 and N170 between the ASD and TD groups as well as the presence of inverted facial effects and hemispherical lateralization, and 2. the correlation of P1 and N170 with the severity of social functioning in ASD patients, as well as the effect of age on P1 and N170, especially N170. We hypothesized that different patterns of face recognition may be observed between children with and without ASD, with ASD presenting the slower P1 and N170 latencies. Moreover, we predicted that N170L would be correlated with the severity of social functioning and with age increasing, the N170L would be faster.
Materials and Methods
Participants
Individuals with ASD were recruited from the rehabilitation department of the First Affiliated Hospital of Jinan University and were diagnosed by experienced psychiatrists according to the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria. In addition, included participants had a verbal IQ of 70 or more according to the Peabody Picture Vocabulary Test, which was strongly supported to be a proxy for verbal IQ in ASD studies.36 Participants with neurological disorders (epilepsy, head injury, severe sensorimotor impairment, schizophrenia, or dyslexia) and a family history of ASD were excluded from the TD group. For all participants, written consent was obtained from their guardian prior to the experiment.
Thirteen ASD individuals (age: M = 6.60 years; SD = 2.12) and ten TD individuals (age: M = 6.65 years; SD = 1.64) were ultimately recruited for our experiment as one ASD individual could not cooperate with EEG. Eleven participants in the ASD group and four participants in the TD group were boys. All the participants were right-handed according to the handedness scales of the Edinburgh Handedness Inventory. The study was approved by Scientific Research Ethics Committee of the First Affiliated Hospital of Jinan University (KY-2024-008). The research procedure can be seen in Figure 1(A).
 |
Figure 1 The research procedure. Abbreviation: VABS, Vineland Adaptive Behavior Scale. Notes: the research design picture(A) and the electrophysiological stimulation paradigm(B). This figure is created in BioRender. https://BioRender.com/fbv03x7. |
Task and Stimuli
Three categories of stimuli were used: upright faces, inverted faces and objects. For the upright face category, 10 photographs of unfamiliar actors (50% men) with neutral expressions were selected from the Karolinska Directed Emotional Faces database. These upright faces were turned upside down using Photoshop software for the inverted face category. For the object category, we selected 10 photographs of different chairs and 10 photographs of different houses. In addition, one butterfly photograph was used to maintain attention. All the photographs were black and white and were uniformly sized to 340×500 pixels by using Photoshop.
There were 2 blocks in total, with each block including 93 trials, which were presented in a random order with E-prime 3.0. Each photograph was presented 3 times in one block. One block included chair photographs as the object stimulus material, and the other block included house photographs. Each block, contained 30 trials for each category, and the butterfly stimulus was presented in 3 trials.
The participants sat approximately 60 cm from a screen in a quiet room with background lightning. As seen in Figure 1(B), at the beginning of a trial, a fixation cross was presented in the center of the screen for 500 ms, followed by a stimulus for 500 ms and finally a blank screen with a random duration of 1000–1200 ms. Following the previous literature,13,20,22 the participants were asked to press a button when they saw a photograph of a butterfly to maintain attention. The formal experiment began after the practice experiment was completed and the participants understood the rules.
ERPs
We used an elastic cap with 64 Ag-AgCl electrodes placed according to the 10–20 international system. The impedance of each electrode was less than 45 KΩ, and the sampling rate was 500 hz. Electrophysiological data were recorded using Curry 8 (NeuroScan, Compumedics). The EEG signals were amplified and filtered using a bioamplifier (SynAmps, Compumedics, USA).
We used MATLAB 2023b software to analyze the EEG data, which were filtered at 0.1--30 hz. Cz was used as the reference electrode and average reference was used for re-reference during the acquisition, with the butterfly stimulus being not analyzed. The EEG data were segmented into 600 ms epochs, from 100 ms before the stimulus to 500 ms after the stimulus. The prestimulus interval was used to correct for baseline shifts. Each epoch was visually inspected to remove bad epochs and epochs were rejected for inclusion in the analysis for the following reasons: an amplitude greater than the absolute value of 150 μV; a ≥140 μV drift in a moving average of 80 ms (eye blinks); and a ≥55 μV drift with the same other parameters (eye movements).22,37 Channels with artifacts on more than 50% of the trials were defined as bad channels and interpolated using MATLAB. EEG data with fewer than 50% of the trials for stimuli or with more than 20 bad channels were excluded from the final analysis. There was no significant difference in acceptance rate of the trials between the two groups (ASD:88.44%; TD:94.45%).
With reference to previous studies, we selected the P7/P8 and PO7/PO8 electrodes (N170) and the O1/O2 electrodes (P1) for the recordings and a time window of 150–340 ms (N170) and 80–210 ms (P1) for peak detection. N170A was defined as the maximum negative peak across the specified electrodes in the specified time window, and N170L was defined as the latency to N170A. P1 was the maximum positive peak at the O1 (left) and O2 (right) electrodes around the time window. The data averaged across the P7/PO7 electrodes represented the N170 of the left hemisphere, and those averaged across the P8/PO8 electrodes represented the N170 of the right hemisphere, which were extracted for each participant for each stimulus category.
Behavior Data
The Vineland Adaptive Behavior Scale (VABS), the first standardized adaptive behavior scale, is often used to evaluate adaptive functioning in communication, daily living skills, socialization skills and motor skills. The reliability of Vineland-3 Comprehensive Interview Form was from 0.70 to 0.81, while the reliability of Internal Consistency was from 0.90 to 0.98. The validity is determined by investigating the relationships with other scales that measure the same construct. The Vineland-II scores were compared to the scores of the Adaptive Behavior Assessment System-Second Edition with the correlation coefficient of 0. 70. In a research including 106 parents/caregivers of individuals diagnosed with neurodevelopmental disorders, the concordance correlation coefficient estimates all exceeded 0.70 between the Vineland-II and the Vineland-3 for each subscale and domain.38 A study, including 684 autistic individuals, provided Vineland special population norms in ASD individuals and highlighted the significance of employing norms to evaluate ASD individuals.39 Recently, the VABS and its revised edition is widely used in ASD individuals40 and the social impairments in ASD have been well documented using the VABS.41
We used the Vineland-3 Comprehensive Level Parent/Caregiver Forms, which include 11 subscales in four domains: communication, daily living, social skills and relationships, and physical activity. In our experiment, we focused on the social functioning of individuals with ASD and asked parents or caregivers of participants with ASD to complete the social skills and relationships domain, which consists of three subscales (relating to others, playing and using leisure time and adapting). On the day of the EEG experiment, we explained the scoring criteria to the parents or caregivers and asked them to complete the scale. However, two ASD individuals’ parent failed to fill out the VABS in spite of the children completed an EEG. Therefore, eleven ASD individuals participated in an analysis to explore the correlations between P1 and N170 latencies and the severity of social functioning. The average V-scores of the VABS social domain in ASD individuals were as follow: the “relating to others” subscale (mean:10.09, SD: 2.59), the “playing and using leisure time” subscale (mean:11.18, SD: 2.75) and the “adapting” subscale (mean:10.91, SD: 3.05).
Statistics
We conducted a mixed three-way analysis of variance (ANOVA) for N170 and P1, with a between-subject factor (group: ASD vs TD), and two within-subject factors (hemisphere: left vs right; stimulus: upright faces vs inverted faces vs objects). Post hoc pairwise comparisons were performed between the same categories, and we used the Bonferroni correction for multiple comparisons. Correlation analyses were conducted between the behavioral data and EEG data.
Results
ERP Results
P1
P1 latency: As seen in Figure 2(A), There was a significant main effect of group (F= 5.853, p=0.017) and stimuli (F= 5.862, p=0.004), with TD individuals presenting a shorter P1 latency than ASD children did and upright faces elicit a shorter latency than inverted faces and objects (upright faces: 144.96 ms; inverted faces: 155.48 ms; objects: 157.70ms). Post hoc pairwise comparisons and a Bonferroni correction suggested that objects and inverted faces presented slower P1 latency values than did upright faces (upright faces vs objects: p=0.005; upright faces vs inverted faces: p=0.027). However, there were no significant differences between hemispheres. When age was used as a covariate, the effect size of group (F= 6.942, p=0.009) and stimuli (F= 6.178, p=0.003) became more obvious, and age significantly influenced the P1 latency (F=8.170, p=0.005). In addition, no interaction effect was found.
P1 amplitude: There was a significant main effect of hemisphere (F=7.568, p=0.007), and the P1 amplitude in the left hemisphere was smaller than that in the right hemisphere. There was a tendency that TD individuals presented a larger P1 amplitude than did ASD individuals (F= 3.227, p=0.075). No significant effect of stimuli was seen. When age was used as a covariate, the effect size of group became smaller (F= 2.836, p=0.095) and the effect size of hemisphere was more obvious (F= 8.037, p=0.005), and age demonstrated an influence on the P1 amplitude. However, there was no significant effect of stimuli, with objects eliciting a larger amplitude (p= 0.355). No interaction effect was found.
N170
N170L: As seen in Figure 2(B), there was a significant main effect of group (F= 10.297, p=0.002). TD individuals presented a shorter N170L than did ASD individuals, as expected, but there was no significant difference in stimuli. In addition, there was a tendency that the right N170L was shorter than the left N170L (left: 225.13 ms, right:216.23 ms; F=2.523, p=0.115). When age was used as a covariate, the effect size became more significant (group: F= 12.751, p=0.000; stimuli: F=2.782, p=0.098), and age had a significant influence on N170L (F= 14.614; p=0.000). However, there was no interaction effect.
N170A: As seen in Figure 2(C), there was an obvious significant effect of stimuli (F=15.106, p=0.000) across all groups and hemispheres. Post hoc pairwise comparisons and a Bonferroni correction suggested that both upright and inverted faces presented greater N170A values than did objects. In addition, N170A in the left hemisphere was greater than N170A in the right hemisphere (F=10.559, p=0.001). When age was used as a covariate, there was no significant change in the results, and age did not significantly affect the effect. The details of average latency and amplitude measures were shown in Table 1. Grand average waveforms in ASD and TD groups across all stimuli and hemispheres can be seen in Figure 3(A) and (B), and grand average waveforms for stimuli across groups and hemispheres can be seen in Figure 3(C).
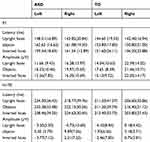 |
Table 1 Mean and SD of Average Latency and Amplitude |
Correlation Between P1 and N170 and Social Function
P1
As seen Figure 4, the P1 latency in the bilateral hemispheres in response to faces was negatively associated with the “playing and using leisure time” V-scores (left: upright faces: r=−0.777, p=0.005; inverted faces: r=−0.787, p=0.004; objects: r=−0.627, p=0.039; right: upright faces: r= −0.835, p=0.001; inverted faces: r= −0.557, p=0.075). Moreover, the P1 latency for inverted faces in the bilateral hemisphere was negatively associated with the V-scores of the “relating to others” subscale (left: r= −0.598, p=0.052; right: r= −0.658, p=0.028). The P1 latency for upright faces in left hemisphere was correlated to the “adapting” V-scores (left: r= −0.708, p=0.015). There were no significant correlations of the P1 amplitude.
N170
Interestingly, there were no significant correlations between N170A and the social skills and relationships domain scores (relating to others, playing and using leisure time and adapting) of the Vineland-3 Comprehensive Level Parent/Caregiver Forms.
For N170L, there was also no significant correlation between N170L in the left hemisphere and the social skills or relationships domain score. The results suggested that N170L in the right hemisphere for objects was correlated with the V-scores of the “relating to others” subscale (r= −0.782, p=0.004) and the “playing and using leisure time” subscale (r= −0.678, p=0.022) and “adapting” subscale (r= −0.568, p=0.069). There was also a significant correlation between N170L in the right hemisphere for faces and the V-scores of the “playing and using leisure time” subscale (upright faces: r= −0.530, p=0.094; inverted faces: r=−0.601, p=0.050), which indicated that individuals with ASD who had a shorter N170L in the right hemisphere might exhibit better social functions. The details can be seen in Figure 5.
Discussion
Our data suggest different patterns of facial recognition on the basis of the electrophysiological results in ASD and TD individuals. ASD children presented a slower P1 latency and N170 latency than TD children did.
The P1 latency was different between ASD and TD children, with ASD children presenting a longer P1 latency. Although some studies have not reported differences between these two groups,16,17,22 Neuhaus E. et al showed that the P1 latency was faster in individuals without ASD compared to individuals with ASD in a group with an average age of 11.3 years.23 O’Connor K. et al also reported that ASD adult individuals showed P1 latency delays for all expressions compared to controls.21 It is worth noting that several articles have found differences in P1 regarding the stimulus, with faces eliciting a shorter P1 latency compared to inverted faces and objects.7,11,12 Shen I.H. et al found that the P1 latency for toys was slower than those for eyes, mouths and faces.13 Our data also suggested that faces elicited a shorter latency than objects and inverted faces, which indicates that P1 might be modulated by the social nature of the stimulus. As for the P1 amplitude, there was no significant differences between ASD and TD children, regardless of whether age was used as a covariate. We observed that the P1 amplitude did not vary as significantly as the P1 latency did between the two groups, which is consistent with the findings of many other studies.12,14
N170 could distinguish ASD individuals from TD individuals, with longer N170L and smaller negative N170A in the ASD group.42–46 This finding is consistent with the results of several other studies. McPartland J. C. et al reported that N170L for faces in TD individuals was significantly faster than that in ASD children.47 Tavares P. P. et al reported that there was a main effect of group on both N170L and N170A, with a shorter N170L and larger N170A in the TD group for photograph stimuli.19 There seems to be no doubt that N170L can assist in the diagnosis of ASD. Our results suggested that comparing to N170A, the difference of N170L between ASD and TD children was significant. Moreover, in another meta-analysis we conducted, we found differences between the ASD and TD groups only in N170L. In addition, Webb S. J. et al identified a group of ASD children who scored low on facial memory, cognitive and language skills using age-adjusted N170L.48
There was no significant effect of the stimulus, hemisphere or their interaction on N170L in our results, whereas there were some effects of the hemisphere and stimulus on N170A. It is worth noting that N170L suggests a trend of right hemisphere lateralization. However, the N170A indicated a negative larger amplitude in the left hemisphere, while the P1 amplitude suggests a larger positive amplitude in the right hemisphere. Although the previous studies had mixed results on stimuli and hemispheres, the main effect of stimulus, that is, that N170A in response to faces was greater than that in response to objects, was relatively consistent across all the studies. N170 is thought to be sensitive to categories and stimuli.5,13,19,47 Some studies have also suggested that N170L may be less specific to faces than N170A,49,50 which our data confirmed, whereas N170L is believed to be more suitable for assisting in the diagnosis of ASD. As for the less than obvious differences between ASD and TD individuals, one study explained that many experimental tasks do not require attention to faces, and differentiation of the N170 amplitudes between ASD and TD individuals are more prominent when the tasks are more face-specific.51
We also explored whether N170 could reflect the severity of ASD symptoms. In our analyses, no significant correlation between N170A and level of social functioning was found, but some significant associations between N170L and social functioning were discovered. The N170L in the right hemisphere for objects was correlated with the V-scores of the “relating to others” subscale, the “playing and using leisure time” subscale and the “adapting” subscale, whereas the N170L in the right hemisphere for upright faces and inverted faces was correlated with the V-scores of the “playing and using leisure time” subscale. Our results clearly indicate that N170L is associated with the severity of social clinical symptoms, with a faster N170L indicating better socialization in ASD individuals. In terms of its correlation with social clinical symptoms, N170L is also more suitable for evaluating social functioning in individuals with ASD. In a multicenter, large-sample randomized study, the author conducted behavioral and EEG measurements in a group of individuals aged 6–36 years, and reported that faster N170L at baseline was associated with greater improvements in the play and leisure time subdomain V-scores between baseline and the first follow-up visit.34 This finding also suggested that faster N170L implies better performance and prognosis in social functioning. In addition, Key A. P. et al reported that N170L was related to clinical symptoms.49 A longer N170L to photographs of houses was associated with higher Autism Diagnostic Observation Schedule scores, whereas a faster latency was associated with higher IQ scores and better NEPSY Memory for Faces subtest score. This finding was consistent with our results that N170L was associated with clinical symptoms and that a shorter N170L implied better function. Finally, Since the N170L indicated a tendency of right hemisphere lateralization and the right N170L was correlated with the severity of social functions in ASD individuals, the lateralization of face recognition is needed to be deeply explored.
The P1 latency was also correlated with the severity of ASD symptoms, with some significant associations with the social skills and relationships domain scores, especially the “playing and using leisure time” subscale. In a study on the differences between twins with and without ASD, the authors also found that stronger social skills and fewer social difficulties were associated with faster P1 and N170 responses to upright faces, whereas there was no correlation between the P1 amplitude and social skills. The authors explained that because the amplitude reflects neural effort to some extent, this result may suggest that successful social functioning depends more on the speed of facial processing than on neural effort.23
Our results revealed that there was basically no inverted face effect in either the ASD or TD groups, except upright faces elicited a shorter P1 latency than inverted faces did (Bonferroni correction: p=0.027). There was a main effect of stimulus on N170A, with post hoc analysis showing that N170A in response to objects was less negative than that in response to upright faces and inverted faces. Although there were no differences in N170A between upright faces and inverted faces, N170A in response to upright faces (−1.96 µV) was greater than that in response to inverted faces (−0.81 µV) across all groups and hemispheres. Neuhaus E. et al also reported that N170A was more negative for upright faces than inverted faces.23 The inverted face effect is related to spatial configuration information. Daphne Maurer distinguished three types of spatial configuration processing: first-order processing, which defines the face (ie, two eyes above the nose and mouth), holistic processing (combining features to form a whole), and second-order processing (ie, the spacing between features).52 The control group and ASD individuals presented similar P1 and N170 results when Mooney faces were used as stimuli.26 Tavares P. P. et al reported that the ASD and TD groups also presented similar results, but both groups exhibited the inverted face effect.19 These results support the idea of a partial-based processing strategy in which individuals with ASD have the ability to process first-order information configurations in the same way as TD individuals.
There is no doubt that age affects P1 and N170 indices, especially P1 latency and N170L.53,54 When age was used as a covariate, its effect on P1 latency and N170L was extremely significant in our study. In a large-sample study with an average age of 8 years, Webb et al reported that the mean N170L for upright faces was estimated to be 0.018 ms faster and the P1 latency for upright faces was estimated to be 0.0056 ms faster for one day after six months of follow-up.55 In 2006, Webb conducted another study on a group of 3–4-year-olds and reported that N170’s precursor component, N290. O’Connor K. et al reported that TD individuals in the adult group presented a shorter N170L than did those in the child group.21 N170L clearly shortens with increasing age, but we do not know exactly the pattern of change in N170L. Several studies have found different brain network connectivity in children, adolescents and adults, which could explain the effect of age.56–58 These results all indicated that the effect of age must be emphasized in the analysis of N170L. Although N170L in the adult group was faster than that in the child group, the difference in N170L was more obvious between the ASD and TD groups in adults but not in children.21,54 There are several explanations for this result. As a developmental disorder, ASD individuals lag farther behind TD individuals in adulthoods than in childhood, and the child group received more treatments, resulting in a smaller difference.
Conclusion
Our results explored the electrophysiological basis of facial recognition and reported P1 and N170 can distinguish ASD individuals from TD individuals. While N170A is more specific to categories such as faces, N170L is more suitable as an objective marker of ASD. P1 latency and N170L may be correlated with the severity of social symptoms and affected by age. Therefore, age- adjusted p1 latency and N170L has great potential for clinical applications in ASD, such as diagnosis and assessment.
However, no significant inverted effects of N170 were found in our results. Considering that the ASD group and the TD group presented similar results, ASD individuals may have the ability to perform first-order processing, such as holistic facial processing, in the same way as TD individuals. Of course, it is necessary to conduct multi- center and large- sample studies and discuss the influence of sex ratio for further exploration. Moreover, considering that ASD is closely related to genetics, exploring the correlation between N170 and genetic indicators may be very meaningful.
Ethics Approval and Informed Consent
All procedures were approved by Scientific Research Ethics Committee of the First Affiliated Hospital of Jinan University (KY-2024-008). The parents or legal guardians were informed about the purpose of the study and written informed consent was obtained from them. Our study adheres to the principles of the Declaration of Helsinki.
Acknowledgments
The work was supported by Key Realm R&D Program of Guangdong Province [grant numbers 2019B030335001]and National Key R&D Program of China [grant numbers 2020YFC2005700].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Fagan JF. Infants’ recognition memory for faces. J Experil Child Psychol. 1972;14(3):453–476. doi:10.1016/0022-0965(72)90065-3
2. American Psychiatric Association D, Association AP. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Vol. 5. Washington, DC: American psychiatric association; 2013.
3. Behrmann M, Thomas C, Humphreys K. Seeing it differently: visual processing in autism. Trends Cognitive Sci. 2006;10(6):258–264. doi:10.1016/j.tics.2006.05.001
4. Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Developmental Neuropsychol. 2005;27(3):403–424. doi:10.1207/s15326942dn2703_6
5. McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. J Child Psychol Psychiat Allied Discipline. 2004;45(7):1235–1245. doi:10.1111/j.1469-7610.2004.00318.x
6. Taylor MJ. Non-spatial attentional effects on P1. Clin Neurophysiol. 2002;113(12):1903–1908. doi:10.1016/S1388-2457(02)00309-7
7. Itier RJ, Taylor MJ. N170 or N1? Spatiotemporal differences between object and face processing using ERPs. Cerebral Cortex. 2004;14(2):132–142.
8. Liu J, Harris A, Kanwisher N. Stages of processing in face perception: an MEG study. Nat Neurosci. 2002;5(9):910–916. doi:10.1038/nn909
9. Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philos Trans R Soc London, Ser B. 1998;353(1373):1257–1270. doi:10.1098/rstb.1998.0281
10. Mangun GR. Neural mechanisms of visual selective attention. Psychophysiology. 1995;32(1):4–18. doi:10.1111/j.1469-8986.1995.tb03400.x
11. Itier RJ, Taylor MJ. Inversion and contrast polarity reversal affect both encoding and recognition processes of unfamiliar faces: a repetition study using ERPs. NeuroImage. 2002;15(2):353–372.
12. Taylor MJ, Edmonds GE, McCarthy G, Allison T. Eyes first! Eye processing develops before face processing in children. Neuroreport. 2001;12(8):1671–1676. doi:10.1097/00001756-200106130-00031
13. Shen IH, Lin SC, Wu YY, Chen CL. An event-related potential study on the perception and the recognition of face, facial features, and objects in children with autism spectrum disorders. Perceptual and Motor Skills. 2017;124(1):145–165. doi:10.1177/0031512516681694
14. Kuefner D, de Heering A, Jacques C, Palmero-Soler E, Rossion B. Early visually evoked electrophysiological responses over the human brain (P1, N170) show stable patterns of face-sensitivity from 4 years to adulthood. Front Human Neurosci. 2010;3:67. doi:10.3389/neuro.09.067.2009
15. Churches O, Baron-Cohen S, Ring H. The psychophysiology of narrower face processing in autism spectrum conditions. Neuroreport. 2012;23(6):395–399. doi:10.1097/WNR.0b013e3283525bc8
16. Hileman CM, Henderson H, Mundy P, Newell L, Jaime M. Developmental and individual differences on the P1 and N170 ERP components in children with and without autism. Developmental Neuropsychol. 2011;36(2):214–236. doi:10.1080/87565641.2010.549870
17. McPartland JC, Wu J, Bailey CA, Mayes LC, Schultz RT, Klin A. Atypical neural specialization for social percepts in autism spectrum disorder. Social Neuroscience. 2011;6(5–6):436–451. doi:10.1080/17470919.2011.586880
18. O’Connor K, Hamm JP, Kirk IJ. Neurophysiological responses to face, facial regions and objects in adults with Asperger’s syndrome: an ERP investigation. InterJ Psychophysiol. 2007;63(3):283–293. doi:10.1016/j.ijpsycho.2006.12.001
19. Tavares PP, Mouga SS, Oliveira GG, Castelo-Branco M. Preserved face inversion effects in adults with autism spectrum disorder: an event-related potential study. Neuroreport. 2016;27(8):587–592. doi:10.1097/WNR.0000000000000576
20. Gunji A, Inagaki M, Inoue Y, Takeshima Y, Kaga M. Event-related potentials of self-face recognition in children with pervasive developmental disorders. Brain Dev. 2009;31(2):139–147. doi:10.1016/j.braindev.2008.04.011
21. O’Connor K, Hamm JP, Kirk IJ. The neurophysiological correlates of face processing in adults and children with Asperger’s syndrome. Brain and Cognition. 2005;59(1):82–95. doi:10.1016/j.bandc.2005.05.004
22. Apicella F, Sicca F, Federico RR, Campatelli G, Muratori F. Fusiform gyrus responses to neutral and emotional faces in children with autism spectrum disorders: a high density ERP study. 2013;251:162.
23. Neuhaus E, Kresse A, Faja S, Bernier RA, Webb SJ. Face processing among twins with and without autism: social correlates and twin concordance. Soc Cognit Affective Neurosci. 2015;11(1):44–54. doi:10.1093/scan/nsv085
24. Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. J Cognitive Neurosci. 1996;8(6):551–565. doi:10.1162/jocn.1996.8.6.551
25. Langdell T. Recognition of faces: an approach to the study of autism. J Child Psychol Psychiat Allied Discipline. 1978;19(3):255–268. doi:10.1111/j.1469-7610.1978.tb00468.x
26. Naumann S, Senftleben U, Santhosh M, McPartland J, Webb SJ. Neurophysiological correlates of holistic face processing in adolescents with and without autism spectrum disorder. J Neurodevelop Disord. 2018;10(1):27. doi:10.1186/s11689-018-9244-y
27. Taylor MJ, Batty M, Itier RJ. The faces of development: a review of early face processing over childhood. J Cognitive Neurosci. 2004;16(8):1426–1442. doi:10.1162/0898929042304732
28. Clark VP, Maisog JM, Haxby JV. fMRI study of face perception and memory using random stimulus sequences. J Neurophysiol. 1998;79(6):3257–3265. doi:10.1152/jn.1998.79.6.3257
29. Frässle S, Paulus FM, Krach S, Jansen A. Test-retest reliability of effective connectivity in the face perception network. Human Brain Mapp. 2016;37(2):730–744. doi:10.1002/hbm.23061
30. Rossion B, Joyce CA, Cottrell GW, Tarr MJ. Early lateralization and orientation tuning for face, word, and object processing in the visual cortex. NeuroImage. 2003;20(3):1609–1624. doi:10.1016/j.neuroimage.2003.07.010
31. Ji Y, Liu J, Zhu XQ, et al. Atypical N170 lateralization of face and word recognition in Chinese children with autism spectrum disorder. J Neurolinguistics. 2019;52.
32. Pierce K, Müller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain J Neurol. 2001;124(Pt 10):2059–2073. doi:10.1093/brain/124.10.2059
33. Schultz RT, Gauthier I, Klin A, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57(4):331–340. doi:10.1001/archpsyc.57.4.331
34. Mason L, Moessnang C, Chatham C, et al. Stratifying the autistic phenotype using electrophysiological indices of social perception. Sci Trans Med. 2022;14(658):eabf8987. doi:10.1126/scitranslmed.abf8987
35. Key AP, Dove GO, Maguire MJ. Linking brainwaves to the brain: an ERP primer. Developmental Neuropsychol. 2005;27(2):183–215. doi:10.1207/s15326942dn2702_1
36. Krasileva KE, Sanders SJ, Bal VH. Peabody picture vocabulary test: proxy for verbal IQ in genetic studies of autism spectrum disorder. J Autism Develop Disord. 2017;47(4):1073–1085. doi:10.1007/s10803-017-3030-7
37. Faja S, Webb SJ, Jones E, et al. The effects of face expertise training on the behavioral performance and brain activity of adults with high functioning autism spectrum disorders. J Autism Develop Disord. 2012;42(2):278–293. doi:10.1007/s10803-011-1243-8
38. Farmer C, Adedipe D, Bal VH, Chlebowski C, Thurm A. Concordance of the Vineland adaptive behavior scales, second and third editions. J Intellectl Disab Res. 2020;64(1):18–26. doi:10.1111/jir.12691
39. Carter AS, Volkmar FR, Sparrow SS, et al. The Vineland adaptive behavior scales: supplementary norms for individuals with autism. J Autism Develop Disord. 1998;28(4):287–302. doi:10.1023/a:1026056518470
40. Klin A, Saulnier CA, Sparrow SS, Cicchetti DV, Volkmar FR, Lord C. Social and communication abilities and disabilities in higher functioning individuals with autism spectrum disorders: the Vineland and the ADOS. J Autism Develop Disord. 2007;37(4):748–759. doi:10.1007/s10803-006-0229-4
41. Cicchetti DV, Carter AS, Gray SAO. Vineland adaptive behavior scales. In: Volkmar FR, editor. Encyclopedia of Autism Spectrum Disorders. New York: Springer New York; 2013:3281–3284.
42. Chen B, Jiang L, Lu G, et al. Altered dynamic network interactions in children with ASD during face recognition revealed by time-varying EEG networks. Cerebral Cortex. 2023;33(22):11170–11180.
43. Luyster RJ, Bick J, Westerlund A, Nelson CA. Testing the effects of expression, intensity and age on emotional face processing in ASD. Neuropsychologia. 2019;126:128–137. doi:10.1016/j.neuropsychologia.2017.06.023
44. Parker TC, Crowley MJ, Naples AJ, et al. The N170 event-related potential reflects delayed neural response to faces when visual attention is directed to the eyes in youths with ASD. Autism Res. 2021;14(7):1347–1356. doi:10.1002/aur.2505
45. Richards JE, Guy MW, Hogan AL, Roberts JE. Neural correlates of face processing among preschoolers with fragile X syndrome, autism spectrum disorder, autism siblings, and typical development. Autism Res. 2024;17(1):89–108. doi:10.1002/aur.3045
46. Tye C, Battaglia M, Bertoletti E, et al. Altered neurophysiological responses to emotional faces discriminate children with ASD, ADHD and ASD+ADHD. Biological Psychology. 2014;103:125–134. doi:10.1016/j.biopsycho.2014.08.013
47. McPartland J, Perszyk D, Crowley M, Naples A, Mayes L. Attention to faces and brain responses in autism. Clin Transl Sci. 2011;4(2):134.
48. Webb SJ, Naples AJ, Levin AR, et al. The autism biomarkers consortium for clinical trials: initial evaluation of a battery of candidate EEG biomarkers. Am J Psychiatry. 2022;appiajp21050485.
49. Key AP, Corbett BA. The unfulfilled promise of the N170 as a social biomarker. Biol Psych. 2020;5(3):342–353. doi:10.1016/j.bpsc.2019.08.011
50. Vettori S, Jacques C, Boets B, Rossion B. Can the N170 be used as an electrophysiological biomarker indexing face processing difficulties in autism spectrum disorder? Biol Psych. 2019;4(3):321–323.
51. Levy EJ, Foss-Feig J, Isenstein EL, et al. Electrophysiological studies of reception of facial communication in autism spectrum disorder and schizophrenia. Rev J Autism Dev Disord. 2022;9(4):521–554.
52. Maurer D, Grand RL, Mondloch CJ. The many faces of configural processing. Trends Cognitive Sci. 2002;6(6):255–260. doi:10.1016/s1364-6613(02)01903-4
53. Vettori S, Dzhelyova M, Van der Donck S, et al. Reduced neural sensitivity to rapid individual face discrimination in autism spectrum disorder. NeuroImage Clin. 2019;21:101613.
54. Kang E, Keifer CM, Levy EJ, Foss-Feig JH, McPartland JC, Lerner MD. Atypicality of the N170 event-related potential in autism spectrum disorder: a meta-analysis. Biol Psych. 2018;3(8):657–666. doi:10.1016/j.bpsc.2017.11.003
55. Webb SJ, Emerman I, Sugar C, et al. Identifying age based maturation in the ERP response to faces in children with autism: implications for developing biomarkers for use in clinical trials. Front Psychiatry. 2022;13:841236. doi:10.3389/fpsyt.2022.841236
56. Rashid B, Blanken LME, Muetzel RL, et al. Connectivity dynamics in typical development and its relationship to autistic traits and autism spectrum disorder. Human Brain Mapp. 2018;39(8):3127–3142.
57. Lawrence KE, Hernandez LM, Bookheimer SY, Dapretto M. Atypical longitudinal development of functional connectivity in adolescents with autism spectrum disorder. Autism Res. 2019;12(1):53–65. doi:10.1002/aur.1971
58. Haghighat H, Mirzarezaee M, Araabi BN, Khadem A. Functional networks abnormalities in autism spectrum disorder: age-related hypo and hyper connectivity. Brain Topography. 2021;34(3):306–322.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





