Back to Journals » Infection and Drug Resistance » Volume 18
Analysis of Clinical Characteristics and Severe Factors of 47 Cases of Psittacosis
Authors Zhou Q , Cai C, Chen M
Received 10 January 2025
Accepted for publication 27 May 2025
Published 3 June 2025 Volume 2025:18 Pages 2845—2853
DOI https://doi.org/10.2147/IDR.S515416
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Quan Zhou,1,2 Chunlin Cai,1 Minzhen Chen2
1Department of Hospital Infection Management, The Affiliated Changsha Hospital of Xiangya School of Medicine, Central South University, Changsha, Hunan, 410000, People’s Republic of China; 2Department of Infectious Diseases, The Affiliated Changsha Hospital of Xiangya School of Medicine, Central South University, Changsha, Hunan, 410000, People’s Republic of China
Correspondence: Minzhen Chen, Department of Infectious Diseases, The Affiliated Changsha Hospital of Xiangya School of Medicine, Central South University, Changsha, Hunan, 410000, People’s Republic of China, Email [email protected]
Purpose: Analyze the epidemiology, clinical manifestations, and imaging characteristics of 47 patients with psittacosis, combined with laboratory indicators, to explore influencing factors and early warning indicators of severe psittacosis.
Patients and Methods: The analysis was conducted on case data of patients with psittacosis admitted to The Affiliated Changsha Hospital of Xiangya School of Medicine, Central South University, from January 2021 to September 2024, comparing differences between non-severe and severe patients, analyzing the correlation and impact degree between laboratory indicators and severity of the illness, finding independent influencing factors for the progression of psittacosis patients to severe.
Results: The mean age of 47 patients was 61.53. 61.70% of patients were male. Most patients were retired and self-employed (23.40%). A clear history of exposure to poultry was observed in 36.17% of patients. These were mainly associated with hypertension (31.91%), cerebral infarction (10.64%), and Acquired Immune Deficiency Syndrome (10.64%). The median time from disease onset to hospitalization was 5 days. The median length of the hospital stay was 10 days. 85.11% of patients had fever. Chest CT features were mainly pulmonary lobe infectious lesions (93.62%). Compared with non-severe patients, severe patients had higher levels of aspartate aminotransferase, lactate dehydrogenase, D-dimer, C-reactive protein, and procalcitonin (P < 0.05). LDH, D-dimer, CRP, and PCT levels positively correlated with severe psittacosis (r > 0, P < 0.05). D-dimer level was an independent risk factor for the progression of psittacosis to severe disease (OR > 1, P < 0.05), with an AUC of 0.761. The optimal threshold was 2.50μg/mL, with 66.70% sensitivity and 78.80% specificity.
Conclusion: Forty-seven patients with psittacosis were mainly elderly males, with fever as the typical symptom. Chest CT revealed infectious lesions in the lung lobes. D-dimer level could be an independent factor for the early warning of severe psittacosis.
Keywords: psittacosis, d-dimer, severe illness, influencing factors, early warning
Introduction
Psittacosis is a global and underestimated animal borne disease, with increasing cases reported in many countries. There have been multiple outbreaks of psittacosis in China, with even fatalities. According to reports from Austria, Denmark, Germany, Sweden, and the Netherlands, there has been an abnormal and unexpected increase in cases of psittacosis from early 2023 to early 2024. Psittacosis is a zoonotic disease, also known as avian chlamydial, ornithosis, or parrot disease. It is caused by Chlamydia psittaci and is mainly transmitted from birds to humans.1 Chlamydia psittaci is a gram-negative bacterium that can cause atypical pneumonia when infecting the lungs. It can be transmitted to humans through direct contact with animals, avian secretions and excreta, and inhalation of feather dust.2 Mild patients with Chlamydia psittaci pneumonia mainly present with flu like symptoms such as fever, sore throat, and cough. Severe patients may develop severe pneumonia, myocarditis, endocarditis, renal insufficiency, encephalitis, and even death. Due to the lack of specificity in its clinical manifestations and the difficulty in detecting Chlamydia psittaci through routine testing, clinical diagnosis is difficult. The preferred treatment for Chlamydia psittaci pneumonia is doxycycline or minocycline, followed by macrocyclic esters. Quinolones also have good clinical efficacy.3 The latest research shows that Chlamydia psittaci has multiple transmission routes among humans, not only between infected individuals and contacts but also between mothers and children. Asymptomatic carriers can also transmit the pathogens. It has been reported that the mortality rates of psittacosis in the fetus and mother are 82.6% and 8.7%, respectively.4 In recent years, with the continuous update of diagnostic technology, metagenomic next-generation sequencing (mNGS) can detect Chlamydia psittaci quantitatively, rapidly, and accurately.5 This study summarizes the clinical manifestations and imaging results of 47 patients with psittacosis diagnosed by mNGS at The Affiliated Changsha Hospital of Xiangya School of Medicine, Central South University in the past 4 years, by comparing the differences in laboratory indicators between severe and non-severe patients and exploring the risk factors and warning indicators of severe patients to provide experience for early identification of severe patients with psittacosis.
Material and Methods
Case Source
Using retrospective analysis, we collected case data from patients with psittacosis admitted to The Affiliated Changsha Hospital of Xiangya School of Medicine, Central South University from January 2021 to September 2024. All diagnoses of patients were based on the “Consensus of Chinese Experts on Psittacosis Diagnosis and Treatment (2024)”.6 They had epidemiological history, clinical and imaging manifestations of psittacosis. All patients tested positive for Chlamydia psittaci nucleic acid in the bronchoalveolar lavage fluid or blood samples using mNGS. The BGI sequencing platform is used for mNGS. mNGS workflow includes two components: experimental manipulations (wet lab) and bioinformatic analysis (dry lab). The wet lab manipulations include sample preprocessing, nucleic acid extraction, library preparation, and sequencing. The dry lab bioinformatic analysis includes quality control of data, removal of human sequences and sequence alignment of sequences from microbial species. Patients with severe psittacosis are defined as the occurrence of respiratory failure, acute respiratory distress syndrome, and multiorgan system failures. Respiratory failure is defined as an oxygenation index <400 mmHg (1 mmHg = 0.133 kPa).6 This study excluded patients with incomplete case data.
Data Collection
The hospital information management system, laboratory information management system, and electronic medical record system collected the following observation indicators: age, sex, occupation, clinical manifestations, medical history, exposure history, onset-to-hospitalization time, length of hospital stay, white blood cell count (WBC), neutrophils (N), lymphocytes (L), erythrocyte sedimentation rate (ESR), fibrinogen (FIB), serum sodium, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase (CK), lactate dehydrogenase (LDH), D-dimer, C-reactive protein (CRP), procalcitonin (PCT), oxygen partial pressure, carbon dioxide partial pressure, and chest computed tomography (CT) results on the first day of admission.
Statistical Analysis
Statistical analyses were performed using SPSS 25.0. Count data are presented as n (%). The comparison of count data between the groups was conducted using the chi-squared test. Metric data are presented as mean ± standard deviation (SD). The comparison of metric data between groups was conducted using an independent sample t-test. Measurement data with skewed distribution are displayed as median and interquartile range [M (P25, P75)]. The Mann–Whitney U-test was used for intergroup comparisons. Adopting bivariate correlation analysis to investigate the correlation between laboratory indicators and the severity of the illness, r > 0 indicated a positive correlation, r < 0 indicated a negative correlation, r = 0 indicated complete independence, r = 1 indicated complete correlation. The closer | r | is to 1, the stronger the correlation. Using the condition (non-severe/severe) as the dependent variable and laboratory indicators as independent variables, a univariate binary logistic regression analysis was conducted to obtain the odds ratio (OR) and 95% confidence interval (CI), and to screen for independent prognostic factors. Receiver operating characteristic (ROC) curves were used to analyze the predictive power of laboratory indicators for severe psittacosis and calculate the area under the curve (AUC). P-value lower at 0.05 (P < 0.05) was considered for significant differences.
Results
Epidemiological Characteristics
The average age of the 47 patients with psittacosis was 61.53 years old, and the majority were male (61.70%). Retired personnel and freelancers account for 23.40%. A clear history of contact with poultry was observed in 36.17% of patients. The main comorbid diseases were hypertension (31.91%), cerebral infarction (10.64%), and AIDS (10.64%). The median time from disease onset to hospitalization was 5 days. The median length of the hospital stay was 10 days (Table 1).
 |
Table 1 Patient Population and Epidemiological Characteristics |
Clinical Manifestations
Most patients had fever (85.11%), followed by cough (46.81%), expectoration (19.15%), and headaches (12.77%) (Table 2).
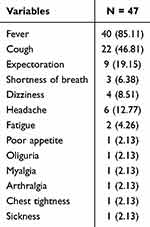 |
Table 2 Clinical Manifestations of Patients [N (%)] |
Chest CT Characteristics
Most patients had infectious lung lobe lesions (93.62%), followed by pleural effusion (42.55%), and pulmonary consolidation (29.79%) (Table 3).
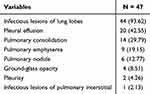 |
Table 3 Chest CT Characteristics of Patients [N (%)] |
Laboratory Tests
Compared to non-severe patients, severe patients had higher levels of AST, LDH, D-dimer, CRP, and PCT (P < 0.05), while there were no statistically significant differences in the levels of WBC, N, L, ESR, FIB, serum sodium, ALT, CK, oxygen pressure, or carbon dioxide pressure (P > 0.05). Moreover, the levels of serum sodium, oxygen pressure, and carbon dioxide pressure were lower than the normal range, while ESR, FIB, ALT, AST, LDH, D-dimer, CRP, and PCT levels were higher than the normal range. Additionally, the average absolute value of L in severe patients was lower than the normal range (Table 4).
 |
Table 4 Comparison of Laboratory Tests Between Non Severe and Severe Patients |
Correlation Analysis Between Laboratory Indicators and Severity of the Illness
LDH, D-dimer, CRP, and PCT levels were positively correlated with severe psittacosis (r > 0, P < 0.05). However, the correlation between the levels of CRP, PCT, LDH and the severity of the disease is poor (0.2 ≤ |r| < 0.4, P < 0.05) (Table 5).
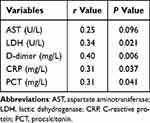 |
Table 5 Correlation Analysis Between Laboratory Indicators and Severity of the Illness |
Independent Influencing Factors of Severe Psittacosis
D-dimer level was an independent risk factor for the progression of severe psittacosis (OR > 1, P < 0.05) (Table 6).
 |
Table 6 Single Factor Binary Logistic Regression Analysis of Laboratory Indicators and Early Warning of Severe Psittacosis |
Predictive Value of D-Dimer for Severe Psittacosis
The ROC curve was used to evaluate the warning efficacy of D-dimer for severe psittacosis, with an area under the curve of 0.761 (P < 0.01). When the D-dimer cut-off value was 2.50, the sensitivity and specificity for predicting severe psittacosis were 66.70% and 78.80%, respectively (Table 7 and Figure 1).
 |
Table 7 ROC Curve of D-Dimer for Predicting the Severity of Psittacosis |
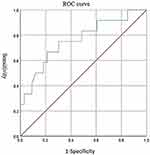 |
Figure 1 ROC curve analysis of diagnostic efficacy of D-dimer. |
Discussion
Chlamydia psittaci is an obligate intracellular gram-negative bacterium that primarily infects birds and causes zoonotic infections. Humans are usually infected by inhaling aerosols from the excrement of infected birds.7 The clinical manifestations of psittacosis are similar to influenza, including headache, fever, muscle pain, cough, and shortness of breath.8 Our study confirmed this feature, with fever being the most prominent clinical symptom for patients. The patient’s body temperature is usually above 39 °C, with the highest reaching 41 °C.
With early identification and appropriate antibiotic treatment, psittacosis rarely leads to death. However, because of the frequent use of cell culture and serological testing before 2019, which requires high standards from laboratories and operators, and the lack of diagnostic timeliness in serological testing, it was difficult to determine the type of chlamydia, making diagnosis extremely challenging. This led to the worsening of the patient’s illness, developing into explosive severe pneumonia and multiple organ failure.9 With the popularization of mNGS in clinical practice, there have been increasing reports of cases of psittacosis. There have been a series of case reports on psittacosis pneumonia; however, the sample size was relatively small. In this study, we collected case data from 47 patients with psittacosis over the past 4 years. By expanding the sample size, we observed the clinical characteristics and analyzed the risk factors for severe illness, thus providing a basis for the early identification of severe patients.
The average age of patients in our study was 61.53 years, with male patients accounting for 61.70%, which was similar to the studies of Tianyun Shi,10 Anbing Zhang,11 and Yubo Huang.12 Because the majority of patients were elderly, most of them had comorbidities of hypertension and cerebral infarction. Mycoplasma psittaci infection is not uncommon in AIDS patients, which is one of the newly reported pathogens of AIDS patients complicated with lung infection in recent years.13 Retired individuals and freelancers were more susceptible to psittacosis, as they may had more opportunities to come into contact with pet birds in pet stores and chickens, ducks, and pigeons in agricultural markets.14 The history of exposure to poultry is an important basis for diagnosing psittacosis,6 but only one-third of patients clearly indicate a history of exposure. According to research,15 Chlamydia psittaci can survive at least 72 h at temperatures below 56°C and for several months in dry bird feces. It is possible that most patients are exposed to indirect environments such as accidental contact with contaminated bird secretions or excrement, which can lead to infection. The median time from disease onset to hospitalization was 5 days. The median length of the hospital stay was 10 days. There were similar to the statistics of Weizhong Jin.16 Our study found that chest CT scans of patients often present with infectious lesions in the lung lobes, pleural effusion, and consolidation, which was similar to reports by Ying Gao,17 Juan Jiang,18 and Xiang Chen.19 The occurrence of pleural effusion in psittacosis pneumonia may be more common than lung infections caused by other pathogens,19 suggesting that healthcare workers need to consider the possibility of Chlamydia psittaci infection when discovering patients with such characteristics.
The laboratory test results showed that unlike pneumonia caused by other bacteria, WBC and N counts were within the normal range, which was consistent with the research of Yannick Vande Weygaerde20 and helps clinical workers to exclude other traditional pneumonia bacterial infections. Although there was no significant difference in the average absolute value of L in severe patients compared with non-severe patients, it was lower than the normal range, which may be due to the weakened immune system in severe patients.21 Some patients had type I respiratory failure, with oxygen and carbon dioxide partial pressures below the normal range. The levels of ESR, CRP, PCT, and FIB were also associated with infection. ESR is used to reflect the aggregation of FIB and immunoglobulin in the body and can increase the inflammatory response after 2 days.22 CRP is synthesized by liver cells and is an acute phase response protein that rapidly rises after tissue damage and under the action of inflammatory factors. PCT is a protein produced and secreted by thyroid C cells, and consists of calcitonin, an N-terminal disabled fragment, and calcitonin. It significantly increases after acute or chronic pneumonia. FIB is present in human plasma and is commonly used to test coagulation function. When the body experiences inflammatory reactions, circulatory disorders, and high viscosity, it affects coagulation function and causes changes in coagulation index levels.23 The levels of ALT, AST, and LDH rise indicate liver and myocardial damage. ALT is mainly present in the cytoplasm of liver cells, whereas AST is mainly present in myocardial cells, followed by the liver cell mitochondria. When the liver is damaged, the permeability of the liver cell membrane increases, large amounts of ALT and AST are released into the liver cells.24 LDH mainly exists in myocardial cells. Once myocardial cells are damaged, AST and LDH enter the bloodstream.25 The levels of CRP, PCT, AST, and LDH in severe patients increase significantly, which is similar to the results of the study by Shi.10 Our experiment found that the levels of CRP, PCT, and LDH were positively correlated with the severity of the disease. However, these correlations are relatively low. This may be related to the fact that CRP and PCT mainly reflect bacterial infections, and not all patients have myocardial damage. The level of serum sodium was below the normal range, which was consistent with the statistical results of Cheng Ying Kong26 and Xiang Chen.19 It is worth noting that this study found that the level of D-dimer in psittacosis patients was higher than the normal range. D-dimer increase was more pronounced in severe patients than in non-severe patients and positively correlated with the severity of the condition, which has rarely been reported before. D-dimer is released after the degradation of cross-linked fibrin polymer, reflecting the level of fibrin polymer in the plasma, which is commonly used to assess coagulation function. When the levels of inflammatory mediators rise in patients, the coagulation system is activated, forming microthrombi, resulting in the level of D-dimer increased.27 We through binary logistic regression analysis and ROC curve found that D-dimer was an independent risk factor for the progression of severe psittacosis in patients. When D-dimer ≥ 2.50 mg/L, it has high sensitivity and specificity in predicting the severity of psittacosis, indicating that the hypercoagulable state of blood in patients with psittacosis deserves high attention from physicians.
This study has some limitations. Firstly, the study cases were sourced from a single center, with the sample source limited. Secondly, the inter group comparison in this study did not take into account the confounding factors caused by comorbidities. It may have some impact on the logistic regression and ROC curve results. Last, the severity of pneumonia, such as the pneumonia severity index and CURB-65 score, has not yet been evaluated in detail. In the future, we will improve the analysis to provide a more theoretical basis for early identification and intervention in patients with severe psittacosis.
Conclusion
Our study analyzed the population characteristics, epidemiological history, clinical manifestations, laboratory test results, and imaging results of patients with psittacosis. It is believed that psittacosis is common in elderly males, with the majority of retirees and freelancers. History of contact with poultry is an important diagnostic criterion. The main clinical symptoms are fever, cough, sputum production, and headaches. Chest CT revealed infectious lesions in the lung lobes, pleural effusion, and pulmonary consolidation. Moreover, LDH, D-dimer, CRP, and PCT levels positively correlated with disease severity. However, the correlation between the levels of CRP, PCT, LDH and the severity of the disease is relatively low. In addition, D-dimer level could be an independent risk factor for the progression of psittacosis to severe illness.
Data Sharing Statement
The datasets for this study are available from the corresponding authors on reasonable request.
Ethics Approval and Consent to Participate
This study was conducted in accordance with all relevant tenets of the Declaration of Helsinki and approved by the Medical Ethics Committee of the First Hospital of Changsha (China) (Approval Number: 2024 Ethical Rapid Review [Clinical Article] No. 24) without the need. Written informed consent was not required. Data were extracted from routine clinical records and transferred to an anonymized, password-protected database that was securely maintained and did not contain personally identifiable information.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research is funded by Changsha Municipal Key Discipline Construction Project Changcaishezhi [2023] No. 95.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dembek ZF, Mothershead JL, Owens AN, et al. Psittacosis: an Underappreciated and Often Undiagnosed Disease. Pathogens. 2023;12(9):1165. doi:10.3390/pathogens12091165
2. Rybarczyk J, Versteele C, Lernout T, et al. Human psittacosis: a review with emphasis on surveillance in Belgium. Acta Clin Belg. 2020;75(1):42–48. doi:10.1080/17843286.2019.1590889
3. Saxena S, Ranjan Sahoo N, et al. Confronting and Addressing the Presence of Psittacosis in Europe. Infect Disord Drug Targets. 2025;25(4):e18715265322768. doi:10.2174/0118715265322768240807102042
4. Wang L, Lin C, Qi Y. Gestational psittacosis causes severe pneumonia and miscarriage: a case report and literature review. Radiol Case Rep. 2023;18(5):1959–1962. doi:10.1016/j.radcr.2023.02.034
5. Li X, Xiao T, Hu P, et al. Clinical, radiological and pathological characteristics of moderate to fulminant psittacosis pneumonia. PLoS One. 2022;17(7):e0270896. doi:10.1371/journal.pone.0270896
6. Clinical Microbiology Laboratory Special Committee of Chinese Hospital Association. infectious diseases Group of Laboratory Medicine Branch of Chinese Geriatrics Society Chinese expert consensus on the diagnosis and treatment of psittacosis [J]. Chin J Clin Infect Dis. 2024;17(03):191–204.
7. Yao W, Chen X, Wu Z, et al. A cluster of Psittacosis cases in Lishui, Zhejiang Province, China, in 2021. Front Cell Infect Microbiol. 2022;12:1044984. doi:10.3389/fcimb.2022.1044984
8. Shi Y, Chen J, Shi X, et al. A case of chlamydia psittaci caused severe pneumonia and meningitis diagnosed by metagenome next-generation sequencing and clinical analysis: a case report and literature review. BMC Infect Dis. 2021;21(1):621. doi:10.1186/s12879-021-06205-5
9. Duan Z, Gao Y, Liu B, et al. The Application Value of Metagenomic and Whole-Genome Capture Next-Generation Sequencing in the Diagnosis and Epidemiological Analysis of Psittacosis. Front Cell Infect Microbiol. 2022;12:872899. doi:10.3389/fcimb.2022.872899
10. Shi T, Yu Y, Shen Y, et al. Serum C-reactive protein to albumin ratio as a potential risk indicator of pneumonia caused by Chlamydia psittaci: a multicenter retrospective study. Front Cell Infect Microbiol. 2024;14:1371625. doi:10.3389/fcimb.2024.1371625
11. Zhang A, Xia X, Yuan X, et al. Clinical characteristics of 14 cases of severe Chlamydia psittaci pneumonia diagnosed by metagenomic next-generation sequencing: a case series. Medicine. 2022;101(24):e29238. doi:10.1097/MD.0000000000029238
12. Huang Y, Zheng W, Gan W, et al. Chlamydia psittaci pneumonia: a clinical analysis of 12 patients. Ann Transl Med. 2023;11(3):144. doi:10.21037/atm-22-6624
13. de Perio MA, Kobayashi M, Wortham JM. Occupational Respiratory Infections. Clin Chest Med. 2020;41(4):739–751. doi:10.1016/j.ccm.2020.08.003
14. Huang W, Wang F, Cai Q, et al. Epidemiological and clinical characteristics of psittacosis among cases with complicated or atypical pulmonary infection using metagenomic next-generation sequencing: a multi-center observational study in China. Ann Clin Microbiol Antimicrob. 2023;22(1):80. doi:10.1186/s12941-023-00631-w
15. Tang J, Tan W, Luo L, et al. Application of Metagenomic Next-Generation Sequencing in the Diagnosis of Pneumonia Caused by Chlamydia psittaci. Microbiol Spectr. 2022;10(4):e0238421. doi:10.1128/spectrum.02384-21
16. Jin W, Liang R, Tian X, et al. Clinical features of psittacosis in 46 Chinese patients. Enferm Infecc Microbiol Clin. 2023;41(9):545–548. doi:10.1016/j.eimc.2022.05.012
17. Gao Y, Wu Y, Xu D, et al. Chlamydia psittaci pneumonia in Wuxi, China: retrospective analysis of 55 cases and predictors of severe disease. Front Med. 2023;10:1150746. doi:10.3389/fmed.2023.1150746
18. Jiang J, Yang W, Wu Y, et al. Metagenomic next-generation sequencing for identifying pathogens in patients with rheumatic diseases and diffuse pulmonary lesions: a retrospective diagnostic study. Front Cell Infect Microbiol. 2022;12:963611. doi:10.3389/fcimb.2022.963611
19. Chen X, Cao K, Wei Y, et al. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection. 2020;48(4):535–542. doi:10.1007/s15010-020-01429-0
20. Vande Weygaerde Y, Versteele C, Thijs E, et al. An unusual presentation of a case of human psittacosis. Respir Med Case Rep. 2018;23:138–142. doi:10.1016/j.rmcr.2018.01.010
21. Wu Y, Xu X, Liu Y, et al. Case Report: clinical analysis of a cluster outbreak of chlamydia psittaci pneumonia. Front Cell Infect Microbiol. 2023;13:1214297. doi:10.3389/fcimb.2023.1214297
22. Dai J, Lian X, Mo J, et al. Case report: a clinical case study of six patients with Chlamydia psittaci pneumonia. Front Cell Infect Microbiol. 2023;13:1084882. doi:10.3389/fcimb.2023.1084882
23. Deng H, Shi Y, Xie M, et al. Diagnosis and treatment experience of Chlamydia psittaci pneumonia: a multicenter retrospective study in China. BMC Infect Dis. 2024;24(1):1333. doi:10.1186/s12879-024-10198-2
24. Xiao Q, Shen W, Zou Y, et al. Sixteen cases of severe pneumonia caused by Chlamydia psittaci in South China investigated via metagenomic next-generation sequencing. J Med Microbiol. 2021;70(11). doi:10.1099/jmm.0.001456.
25. Yuan L, Chen Q, Zhu XY, et al. Evaluation of clinical characteristics and risk factors associated with Chlamydia psittaci infection based on metagenomic next-generation sequencing. BMC Microbiol. 2024;24(1):86. doi:10.1186/s12866-024-03236-1
26. Kong CY, Zhu J, Lu JJ, et al. Clinical characteristics of Chlamydia psittaci pneumonia. Chin Med J. 2021;134(3):353–355. doi:10.1097/CM9.0000000000001313
27. Balato G, De Franco C, Balboni F, et al. The role of D-dimer in periprosthetic joint infection: a systematic review and meta-analysis. Diagnosis. 2021;9(1):3–10. doi:10.1515/dx-2021-0032
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

