Back to Journals » International Journal of Nanomedicine » Volume 19
Apelin-13-Loaded Macrophage Membrane-Encapsulated Nanoparticles for Targeted Ischemic Stroke Therapy via Inhibiting NLRP3 Inflammasome-Mediated Pyroptosis
Authors Ma CS, Ma YP, Han B, Duan WL, Meng SC, Bai M, Dong H, Zhang LY, Duan MY, Liu J , Deng AJ, He MT
Received 2 July 2024
Accepted for publication 3 September 2024
Published 7 September 2024 Volume 2024:19 Pages 9175—9193
DOI https://doi.org/10.2147/IJN.S475915
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Chang-Sheng Ma,1,2,* Ya-Ping Ma,1,3,* Bo Han,1,2,* Wan-Li Duan,1 Shu-Chen Meng,1 Min Bai,1 Hao Dong,1 Li-Ying Zhang,1 Meng-Yuan Duan,1,2 Jing Liu,1 Ai-Jun Deng,2 Mao-Tao He1,2
1Department of Basic Medical Sciences, Shandong Second Medical University, Weifang, Shandong, People’s Republic of China; 2Department of Ophthalmology, Affiliated Hospital of Shandong Second Medical University, Weifang, People’s Republic of China; 3Department of Pathology, The 942Hospital of the People’s Liberation Army Joint Logistic Support Force, Yinchuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mao-Tao He, Department of Basic Medical Sciences, Shandong Second Medical University, Weifang, Shandong, People’s Republic of China, Tel +86-18209617186, Fax +86-0536-3081201, Email [email protected] Ai-Jun Deng, Department of Ophthalmology, Affiliated Hospital of Shandong Second Medical University, Weifang, People’s Republic of China, Email [email protected]
Purpose: Ischemic stroke is a refractory disease wherein the reperfusion injury caused by sudden restoration of blood supply is the main cause of increased mortality and disability. However, current therapeutic strategies for the inflammatory response induced by cerebral ischemia-reperfusion (I/R) injury are unsatisfactory. This study aimed to develop a functional nanoparticle (MM/ANPs) comprising apelin-13 (APNs) encapsulated in macrophage membranes (MM) modified with distearoyl phosphatidylethanolamine-polyethylene glycol-RVG29 (DSPE-PEG-RVG29) to achieve targeted therapy against ischemic stroke.
Methods: MM were extracted from RAW264.7. PLGA was dissolved in dichloromethane, while Apelin-13 was dissolved in water, and CY5.5 was dissolved in dichloromethane. The precipitate was washed twice with ultrapure water and then resuspended in 10 mL to obtain an aqueous solution of PLGA nanoparticles. Subsequently, the cell membrane was evenly dispersed homogeneously and mixed with PLGA-COOH at a mass ratio of 1:1 for the hybrid ultrasound. DSPE-PEG-RVG29 was added and incubated for 1 h to obtain MM/ANPs.
Results: In this study, we developed a functional nanoparticle delivery system (MM/ANPs) that utilizes macrophage membranes coated with DSPE-PEG-RVG29 peptide to efficiently deliver Apelin-13 to inflammatory areas using ischemic stroke therapy. MM/ANPs effectively cross the blood-brain barrier and selectively accumulate in ischemic and inflamed areas. In a mouse I/R injury model, these nanoparticles significantly improved neurological scores and reduced infarct volume. Apelin-13 is gradually released from the MM/ANPs, inhibiting NLRP3 inflammasome assembly by enhancing sirtuin 3 (SIRT3) activity, which suppresses the inflammatory response and pyroptosis. The positive regulation of SIRT3 further inhibits the NLRP3-mediated inflammation, showing the clinical potential of these nanoparticles for ischemic stroke treatment. The biocompatibility and safety of MM/ANPs were confirmed through in vitro cytotoxicity tests, blood-brain barrier permeability tests, biosafety evaluations, and blood compatibility studies.
Conclusion: MM/ANPs offer a highly promising approach to achieve ischemic stroke-targeted therapy inhibiting NLRP3 inflammasome-mediated pyroptosis.
Keywords: apelin-13, cerebral ischemia-reperfusion injury, macrophage membrane, ischemic stroke therapy, pyroptosis
Graphical Abstract:

Introduction
Ischemic stroke is a significant global public health concern because of its high morbidity, mortality, and disability rates.1–3 Ischemic stroke occurs when the blood flow to the brain is blocked, leading to a lack of oxygen and nutrients. However, when blood flow is restored through reperfusion therapy, a new set of pathological processes known as ischemia-reperfusion (I/R) injury can occur.4 This phenomenon occurs due to the oxidative stress and inflammation that arise from the reintroduction of oxygen to the ischemic tissue.5,6 Despite extensive research, stroke has no ideal alternative treatments, highlighting the urgent need for effective and innovative therapeutic agents.
The shorter active form of apelin, known as apelin-13 (APN), exhibits potent anti-oxidative, anti-apoptotic, and anti-inflammatory properties via its interaction with the APJ receptor.7–9 APN holds significance in diverse physiological processes, is present in many tissues and body fluids, and targets different cellular pathways. Studies have demonstrated the neuroprotective effect of APN on cerebral ischemia-reperfusion (I/R) injury and its ability to mitigate disruption of the blood–brain barrier (BBB).10 Studies have shown that APN prevents lipopolysaccharide-induced acute lung injury by inhibiting the NF-κB pathway and NLRP3 inflammasome activation.11 The APN/APJ system attenuates early brain injury by suppressing endoplasmic reticulum stress-associated thioredoxin-interacting protein/NLRP3 inflammasome activation and adenosine monophosphate-activated protein kinase-dependent oxidative stress after subarachnoid hemorrhage in rats.12 Nevertheless, the molecular weight of APN (1550.83 g/mol) is greater than that of other neurological drugs and thus cannot pass through the BBB.13 Studies have revealed that APN does not access an inflammation site through the BBB, thus failing to maximize its function in rescuing brain injury. Nanomaterial delivery systems can address some of these challenges, enhance APN’s targeting to inflammation sites in cerebral ischemic injury, and enhance its in vivo bioavailability.14,15
Macrophages play a vital role as cellular effectors in inflammation and tissue repair processes, and they possess an innate ability to migrate towards sites of inflammation.16,17 Macrophage membrane (MM)-camouflaged nanoparticles have been extensively studied.18,19 Due to the strong intrinsic affinity and targeted delivery efficiency of MM under various inflammatory conditions, they are a preferred camouflage object for many biomimetic nanomaterial delivery systems.20 Recent research has discovered that manganese dioxide nanoparticles, camouflaged as macrophages, exhibit neuroprotective effects in acute ischemic stroke. These nanoparticles effectively reduce oxidative stress and modulate the inflammatory microenvironment, thereby promoting the overall protection of neural tissue.21 Anghelache et al reported that MM-encapsulated nanoparticles are reliable targeted nanomedicine for treating atherosclerotic inflammation.22 RVG29, a brain-targeting peptide, can modify various carriers to carry bulky substances across the BBB by encapsulation, thereby promoting drug accumulation in the brain.23 RVG29 carries a peptide of RVG29 residues, is derived from the rabies virus, and can mimic the transient pathway of the rabies virus across the BBB.24 It binds specifically to the nicotinic acetylcholine receptor (nAChR), which is widely present on the surface of neurons and capillary endothelial cells, allowing RVG29 to cross the BBB efficiently through nAChR-mediated endocytosis.25 The application of RVG29 demonstrated that brain-targeted drug delivery and drug-carrier modification are safe strategies.26 RVG29-modified drug carriers act as trans-BBB drug delivery systems for neurological disorders and exhibit a safe and more selective pathway via nAChR-mediated transcytosis.27 RVG29, polyethylene glycol (PEG) with hydrophilic properties, and distearoyl phosphatidylethanolamine (DSPE) were coupled to synthesize DSPE-PEG-RVG29.
Given the advantages of MM for targeting an inflammation site and RVG29 for penetrating the BBB, the study hypothesized that DSPE-PEG-RVG29-targeted, MM-encapsulated APN-loaded poly-lactic-co-glycolic acid-carboxylic acid (PLGA-COOH) nanoparticles (MM/ANPs) could maintain local drug concentrations for targeted ischemic stroke therapy, thus inhibiting brain I/R injury. APN was added into PLGA-COOH to form an aqueous solution of APN-PLGA-COOH nanoparticles. PLGA has been approved by the US Food and Drug Administration and has good biodegradability and biocompatibility.28 The nanomaterials loaded with the PLGA-APN were camouflaged with MM, modifying the surface of MM with DSPE-PEG-RVG29. The neuroprotective effects of APN, the inflammatory chemotaxis of MM, and the brain injury-targeting effects of DSPE-PEG-RVG29 were integrated into a single strategy. We then intravenously administered these nanoparticles and evaluated their ability to cross the BBB and target sites of inflammation. In addition, we further evaluated the targeted delivery and release of APN with the aim of enriching APN delivery to the ischemic site. Finally, we explore the possible targets and pathways of MM/ANPs to achieve precise targeted therapy of ischemic stroke.
Materials and Methods
Materials
APN was purchased from MCE (Shanghai, China). PLGA-COOH, DSPE-PEG-RVG29, and CY5.5 were purchased from Ruixi Biological Technology (Xi’an, China). The RAW264.7 cells was purchased from the Haixing Biosciences (Jiangsu, China). Triphenyltetrazolium chloride (TTC) was purchased from Sigma-Aldrich (St. Louis, MO). MCC950 was purchased from MCE (Shanghai, China).
Preparation of MM/ANPs
PLGA was dissolved in dichloromethane, APN was dissolved in water, and CY5.5 was dissolved in dichloromethane. The three were mixed and sonicated for 1 min before adding polyvinyl alcohol aqueous solution and sonicated again for 1 min. The mixture was stirred for 1 h to remove the dichloromethane and centrifuged at 4000 rpm for 5–10 min to remove the supernatant. The precipitate was washed twice with ultrapure water and resuspended in 10 mL to obtain an aqueous solution of PLGA nanoparticles. Subsequently, the cell membrane was dispersed homogeneously and mixed with PLGA-CCOH at a mass ratio of 1:1 for the hybrid ultrasound. DSPE-PEG-RVG29 was added and incubated for 1 h to obtain MM/ANPs. Cy5.5 was linked to the surface of MM/ANPs for tracing. The nanoparticles were stored at −20°C for use.
MM Preparation
MM were extracted from RAW264.7 using a membrane protein extraction kit (Beyotime, Jiangsu, China). The kit instructions were strictly followed to obtain MM. The MM concentration was determined by the bicinchoninic acid (BCA) protein assay. The MM vesicles were stored at –80°C for subsequent use.
Nanoparticle Characterization
A transmission electron microscope (TEM; Hitachi, Tokyo, Japan) was utilized for observing the morphology of the MM/ANPs and determining their particle size, polydispersity index (PDI), and zeta potential. The particle size, PDI, and zeta potential measurements were conducted using a particle sizer and zeta potential analyzer (NanoBook 90Plus PALS; Brookhaven, Holtsville).
Drug-Loading Efficiency and Encapsulation Efficiency of the Nanoparticles
Fluorescence intensity and high-performance liquid chromatography were used to determine the quantity of APN in MM/ANPs. The drug-loading efficiency (LE) and encapsulation efficiency (EE) were calculated as shown in Equations 1 and 2:
In vitro Release of Nanoparticles
The release rate of APN from MM/ANPs was determined in vitro using the dialysis method. A 2-mg/mL solution of MM/ANPs was placed inside a disposable dialysis bag (molecular weight: 3.5 kDa; Thermo Fisher Scientific, Waltham, MA). The release medium used was phosphate-buffered saline (PBS) with a pH of 7.4. The dialysis bag was immersed in a funnel containing 10 mL PBS and turned with agitation at 37°C. Three sets of individual samples were analyzed per sample; 1 mL of the release medium was collected for analysis at different timepoints, followed by the addition of 1 mL of the release medium at 1, 2, 5, 7, 10, 25, and 50 h. We removed 1 mL at a time, replenished it with 1 mL of fresh release medium, and then determined the APN release profile.
Animal Model
All animal procedures were performed in accordance with the NIH Guide for Care and Use of Laboratory Animals and was approved by the Animal Care and Use Committee of Shandong Second Medical University (approval no. 2021SDL481). Ethical guidelines were followed to ensure the humane treatment of animals, and the experiments complied with the Laboratory animals—General code of animal welfare. Male C57BL/6 mice weighing 20–25 g and aged 6–8 weeks were used in the study. These mice were obtained from the Animal Center of Shandong Second Medical University (Weifang, China) and were housed in a controlled environment with a 12-h light/dark cycle. They had free access to food and water throughout the experiment. Prior to the surgical procedures, the mice were randomly assigned to three groups: sham, middle cerebral artery occlusion and reperfusion (MCAO/R), and MM/ANPs experimental groups. The mice were anesthetized with 1.5% isoflurane and a midline incision was made at the neck. The right common external and internal carotid arteries were isolated sequentially. A silicone-coated nylon monofilament was carefully inserted into the internal carotid artery to induce occlusion. After 60 min of occlusion, the nylon monofilament was gently removed to restore blood supply, causing ischemia-reperfusion injury. The incision was then closed, and the mice were placed on an insulating blanket and closely monitored until they regained consciousness. In the MM/ANPs group, mice received an intravenous injection of a diluted MM/ANPs solution (making the dose of Apelin-13,100 ug/kg) immediately after the successful modeling of ischemia for 1 h. The MCAO/R and sham groups received an equal volume of saline (0.9% NaCl) injection instead.
Y-Maze Test
The Y-maze was used to test the spontaneous alternation phenomenon in mice. The Y-maze apparatus had three arms (10 × 40×16 cm) of equal size and length, angled 120° to each other, intersecting in a Y-shape. Before the start of the formal experiment, the mice were acclimatized to the Y-maze for 2 days; they were allowed to freely familiarize themselves with all arms for 10 min each day. During the testing phase, the mice were placed at the start of arm A and the gate of arm C was closed, allowing them to explore freely in arms A and B for only 10 min. After 10 min, the official test starts by opening the gate of the novel arm (arm C) and letting the mice explore freely in the three arms for 10 min. The entire experiment was conducted under dim light conditions (50 lx). The number of consecutive times the mice entered arms A, B, and C in a 10-min period is called an alternation. The total number of repetitions was recorded, and the alternation percentage was calculated.
Targeting Capability of MM/ANPs In vivo
Brain targeting of nanoparticles was assessed in the mice using an in vivo imaging system (IVIS) (PE IVIS Spectrum; Perkin Elmer, Waltham, MA). Briefly, sham and MCAO/R mice were injected with saline (0.9% NaCl) via the tail vein; MM/ANPs mice were injected with the same volume of MM/ANPs via the tail vein. Brain tissues were exposed to the IVIS for fluorescence measurements 1, 6, and 24 h after drug administration (n = 6 per group). The excitation and emission wavelengths were 680 and 710 nm, respectively.
Laser Speckle Contrast Imaging
A laser speckle contrast imaging (LSCI) system (RWD, Shenzhen, China) was used to evaluate cerebral blood flow changes. Mice were anesthetized with 2% isoflurane, and their heads were fixed on a brain stereotactic apparatus (RWD). The skull was exposed through a midline skin incision on the head. Cerebral blood flow was recorded continuously for 5 min for each mouse.
In vitro Hemolysis Test
Red blood cells isolated from mouse blood were used for hemolysis evaluation by diluting 1 mL of mouse blood with 1.25 mL of NaCl solution (0.9%, w/v) to obtain a whole blood sample; 0.1 mL was obtained and added to the MM/ANPs solution (5 mL, 2 mg/mL). A solution of red blood cells in 0.9% NaCl served as the negative control, while a solution of red blood cells in an equal volume of double-distilled water was used as the positive control. The mixtures were incubated at 37°C for 1 h and subsequently centrifuged at 3000 rpm for 10 min. The supernatant’s optical density (OD) was then measured at 540 nm using a microplate reader (Thermo Fisher Scientific Multiskan FC) to quantify the hemoglobin released from the lysed red blood cells. The hemolysis percentage was calculated based on the OD values measured for each group using Equation 3:
Hemolysis percentage = [(Sample OD – negative control OD)/(Positive control OD – negative control OD)] ×100% (3).
Cell Culture and Treatment Protocol
HT22 cells were purchased from Pricella (Wuhan, China). The BV2 and bEnd.3 cell lines were purchased from Haixing Biosciences (Jiangsu, China). They were cultured in Dulbecco’s modified Eagle medium (DMEM) (Gibco Laboratories, Waltham, MA) supplemented with 10% fetal bovine serum (Tianhang Biotechnology, Zhejiang, China), 1% penicillin, and streptomycin. The cells were cultured in a constant temperature, humidity, and aseptic incubator at 37°C with 90% room air and 10% CO2. The medium was replaced every 2–3 days. When the cell density reached approximately 80%, the cells were digested with 0.05% trypsin and seeded to other plates for subsequent experiments. To establish an oxygen–glucose deprivation model of HT22 cells, the HT22 cells were cultured in a hypoxic environment of 5% CO2, 0.5% O2, and 94.5% N2 in glucose-free DMEM (Biosharp, Beijing, China) for 8 h and then reoxygenated for 6 h in a normal environment after replacement with normal medium. Cells in the control group were cultured under normal conditions.
In vitro Safety Evaluation
The in vitro cytotoxicity of MM/ANPs was evaluated in HT22, BV2, and bEnd.3 cells using standard methyl thiazole tetrazolium (MTT) assays. Cells in the logarithmic growth phase were collected by digestion and seeded in 96 wells. After 24 h of normal incubation, the medium was replaced with fresh medium containing MM/ANPs (0.1, 0.5, 1, 3, and 5 µM) according to the group. Cell viability was tested using the MTT kit after 24 h of incubation. Absorbance was measured at 450 nm using a microplate reader.
Pathological Staining
Paraffin sections were routinely deparaffinized and hydrated for 0.6% hematoxylin and 1% eosin (H&E) staining (Solarbio, Beijing, China) or Nissl staining (Solarbio). The main organs of mice were removed, fixed with 4% paraformaldehyde, then embedded in paraffin for tissue sectioning. The sections were stained with H&E and observed under a microscope for pathological changes in each group of vital organs.
BBB Assessment in vitro
We used the Transwell system to study the permeability of MM/ANPs through the BBB in vitro. The bEnd.3 cells were seeded in the upper chambers (0.4 μm; Corning, Corning, NY) with 3×105 cells and incubated for 5 days until the upper compartment cells grew and fused into a monolayer. Free MM/ANPs were added to the upper compartment for 12 h, and the lower-chamber DMEM was collected and assessed for fluorescent expression using the IVIS.
Western Blotting
After euthanizing the mice with 1.5% isoflurane anesthesia, the right infarcted brain tissue was collected, lysed with RIPA buffer (1:10, RIPA Lysis Buffer, Solarbio, Beijing, China), and thoroughly homogenized. Protein concentration was determined using the BCA method. Antibody labeling at the end of gel electrophoresis of each group of proteins. The primary antibodies used were specific for NLRP3, N-terminus of gasdermin D (GSDMD-N), Caspase-1, Cleaved caspase-1, ASC, interleukin IL-1β, and pro-IL-1β (all 1:1000; CST, Boston, MA, USA). In addition, IL-18, pro-IL-18 (both 1:1000; Affinity, Jiangsu, China), and APJ (1:1000; Proteintech, Wuhan, China) were also used. Anti-tubulin (1:4000; Proteintech) was used as a reference standard for protein loading. We then incubated the protein strip in the secondary antibody dilution at room temperature and analyzed using an Amersham ImageQuant 800 system (Cytiva, Marlborough, MA).
Immunofluorescence Staining
The mice were sacrificed under heavy anesthesia 24 h after MCAO/R, and the tissues were perfused, collected, fixed, dehydrated, and sliced according to conventional methods. The coronal sections were boiled in an all-purpose powerful antigen retrieval solution (Beyotime) and then blocked with 10% goat serum for 20 min. The sections were incubated overnight at 4°C with primary antibodies caspase-1 and ASC (ABclonal, Wuhan, China), GSDMD (Proteintech), and IL-1β (Affinity). The membranes were incubated with a secondary antibody (Proteintech). We then incubated the sections with the corresponding secondary antibodies for 1 h at 37°C, after which the sections were incubated with 4′,6-diamidino-2-phenylindole (DAPI) for 10 min at room temperature. Images were captured at 400× magnification.
Blood Test and Enzyme-Linked Immunosorbent Assay
The collected mouse sera were subjected to blood biochemical analysis using a biochemical analyzer (BS-240VET; Mindray, Shenzhen, China). The IL-18, IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α) levels in mouse serum were measured using enzyme-linked immunosorbent assay (ELISA) kits (J&L Biological, Shanghai, China).
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 9.0 (GraphPad, USA). Independent two-sample t-tests and one-way ANOVA were used for group comparisons. When ANOVA indicated significance, Tukey’s post hoc test was applied for pairwise comparisons. Statistical significance was defined as, *p < 0.05, **p < 0.01, and ***p < 0.001, ****p < 0.0001. Data are presented as mean ± standard deviation (SD).
Results
Rational Design and in situ Synthesis of Nanoparticle Preparation and Characterization of MM/ANPs
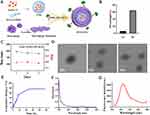
This study rationally designed and synthesized MM-coated nanoparticles consisting mainly of PLGA-COOH and DSPE-PEG-RVG29 to enhance BBB penetration by APN for targeting in ischemic stroke (Graphical Abstract).
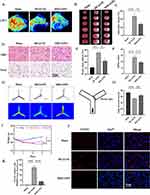
MM/ANPs were constructed sequentially by (1) preparation of APN, (2) isolation of MM, (3) camouflage of APN with MM (Supplementary Figure 1), and (4) targeting peptides DSPE-PEG-RVG29 to MM (Figure 1A). A nanoprecipitation method was used to prepare the MM/ANPs. The drug LE of APN was 6.2%, and the EE was 64% (Figure 1B), showing that APN was effectively encapsulated into the nanoparticles. The hydrodynamic diameter of the MM/ANPs was 187 nm. Zeta potential analysis showed a PDI of 0.19 and a zeta potential of –24.02 mV for the MM/ANPs (Figure 1C).
The cloaking of APN with MM was confirmed through TEM. As shown in Figure 1D, the MM/ANPs presented a clear core–shell structure. Drug-release experiments showed small leakage of APN; MM/ANPs sustainably release encapsulated APN in PBS (pH 7.4) at 37°C. The long-term, stable release of APN from MM/ANPs suggests that they are suitable for sustained release (Figure 1E). Additionally, the samples were further characterized using ultraviolet-visible spectroscopy (UV-Vis) and fluorescence spectroscopy. The changes in the absorption peak positions and intensities in the UV-Vis plots indicated the sample composition varied little during the reaction, and the samples had good stability (Figure 1F). The fluorescence excitation and emission peaks were at 673 nm and 712 nm, respectively, with the greatest visible peak around the fluorescence wavelength of 710 nm, indicating successful CY5.5 wrapping (Figure 1G). These results confirm the successful synthesis of MM/ANPs.
Vitro Cytotoxicity and BBB Permeability
The in vitro cytotoxicity of the MM-coated nanoparticles and their ability to penetrate the BBB were evaluated. The cytotoxicity of MM/ANPs in HT22, BV2, and bEnd.3 cells were examined using the MTT assay. The MM/ANPs were diluted to different concentrations with DMEM and then co-incubated with the above three cell types for 24 h. No significant alterations in cell viability were observed when compared to the control group (Figure 2A-C). The MM/ANPs exhibited better cytocompatibility. To study the penetration capability of MM/ANPs into the BBB, a Transwell model was used to simulate the in vitro BBB (Figure 2D). As shown in Figure 2E, MM/ANPs crossed the BBB in vitro. Based on this result, normal C57BL/6 mice were imaged using the IVIS at different timepoints after injecting MM/ANPs into the tail vein; fluorescence was visible in the mice brains, demonstrating that nanoparticles can be transmitted through the BBB in vivo (Figure 2F). These results suggest that MM-coated nanoparticles can pass through the endothelial cells of the BBB, laying the groundwork for subsequent in vivo experiments.
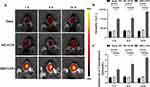
Targeting Ability of MM/ANPs in Cerebral I/R Injury
The neuroprotective effects of nanoparticles in mice were investigated using an IVIS to confirm the targeting of nanoparticles in the mouse brain. The fluorescent dye CY5.5 was encapsulated during nanoparticle preparation to facilitate nanoparticle tracking in mice. First, an MCAO/R mouse model was prepared by blocking the middle cerebral artery.29 MM/ANPs were injected into the tail vein of the MM/ANPs groups after MCAO/R; an equal amount of saline (0.9% NaCl) was injected into the tail vein of the sham group and MCAO/R group. In vivo imaging of brain tissue was performed using the IVIS at 1, 6, and 24 h post-injection. As shown in Figure 3A, no fluorescence was observed in the sham and MCAO/R groups at any time. In contrast, the MM/ANPs group showed obvious fluorescence enrichment concentrated on the ischemic side of the brain (Figure 3B,C). This result indicates that nanoparticles of MM-encapsulated brain-targeting peptides can penetrate the damaged BBB to achieve targeted therapeutic effects.
MM/ANPs Alleviate Cerebral I/R Injury
In vivo experiments were designed using the MCAO/R model, prepared after all mice were divided randomly into three groups. Cerebral blood flow was monitored with LSCI after preparation to evaluate the success of the model preparation (Figure 4A). The cerebral infarct volume was assessed by TTC staining (Figure 4B). As shown in Figure 4C, the cerebral infarct volume was significantly reduced in the MM/ANPs group compared to the MCAO/R group. The therapeutic effects of MM/ANPs were also investigated using H&E staining and Nissl staining. Notably, cells in the ischemic region of the MCAO/R group showed significant changes in cell morphology: cell volume shrank and cells were sparsely distributed. In contrast, the cell morphology of the MM/ANPs and sham groups were similar. The Nissl staining showed that the number of Nissl bodies was significantly reduced and lighter in color in the MCAO/R group; rescue by MM/ANPs increased the number and volume of Nissl bodies, consistent with previous findings (Figure 4D). At 24 h reperfusion after MCAO, the degree of brain edema in the mice was examined using the wet–dry method. The brain water content was significantly greater in the MCAO/R group than in the sham group; however, a significant improvement was found in the MM/ANPs group (Figure 4E). The neurological deficit score showed significant improvement in the MM/ANPs group (Figure 4F). In the Y-maze test, the number of alternations between the three arms was significantly decreased in the MCAO/R group compared to the sham group. Compared to the MCAO/R group, mice in the MM/ANPs group showed significant improvement in exploration and cognitive abilities. These results indicated that the spatial learning and memory abilities of the MM/ANPs group were significantly improved, and the degree of neurological deficits was decreased (Figure 4G and H). After 3 days of reperfusion, the decreasing of bodyweight in mice compared with MCAO/R group was attenuated with additional MM/ANPs treatment (Figure 4I). The co-staining assay of the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) and DAPI co-staining assay in brain sections of MCAO mice revealed that brain tissue apoptosis was significant in the MCAO/R group, with a significantly higher number of apoptosis-positive cells. In contrast, significantly fewer apoptotic cells were observed in the MM/ANPs group compared with the MCAO/R group (Figure 4J,K). This finding suggests that treatment with MM/ANPs effectively prevented the onset of apoptosis and inhibited neuronal damage, thereby reducing further damage induced by stroke. These results indicate that MM/ANPs have protective efficacy in the MCAO/R mouse model.
MM/ANPs Inhibit Inflammation and Pyroptosis Induced by Cerebral I/R Injury
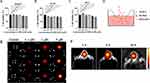
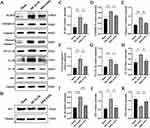
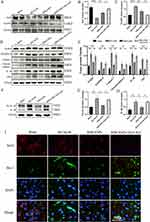
Ischemic stroke occurs with a series of inflammatory responses.30,31 MCAO/R can lead to the assembly and activation of the NLRP3 inflammasome.32,33 Therefore, the changes in NLRP3 and its downstream inflammasome proteins and pyroptosis markers were investigated in mouse brain tissues. Western blotting showed that MCAO/R significantly activated NLRP3, the junction protein ASC, and the pyroptosis markers caspase-1 and GSDMD-N and upregulated the release of inflammatory factors. MM/ANPs significantly reversed the upregulation of each protein in the NLRP3 pathway; IL-1β and IL-18, which are associated with pyroptosis, were significantly downregulated. In the MM/ANPs group, the GSDMD-N, a major effector of pyroptosis, was significantly downregulated (Figure 5A and C–J). Thus, NLRP3-induced pyroptosis was determined to be a major cell death pathway regulated by MM/ANPs in brain I/R injury. The investigation of the changes in the protein levels of APJ, the receptor for APN, showed that it was significantly downregulated in the MCAO/R group compared to the sham group; however, rescue of MM/ANPs dramatically upregulated the APJ levels (Figure 5B and K). These results suggest that MM/ANPs can reach inflammation sites in MCAO/R mice and release APN and that this salvage effect is achieved by APN binding to the increased levels of APJ.
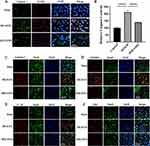
TUNEL and caspase-1 double-staining suggested that the number of caspase-1-positive cells in the penumbra area increased 1 day after MCAO/R compared to that in the sham group and that MM/ANPs treatment significantly inhibited further apoptosis and caspase-1 activation in neuronal cells (Figure 6A). Caspase-1 activity in HT-22 cells was significantly elevated in the OGD/R group, and MM/ANPs rescue inhibited the activation of caspase-1 (Figure 6B). Cerebral I/R injury promotes the assembly and activation of NLRP3, triggering pyroptosis.34 The therapeutic mechanism of MM/ANPs was investigated using immunofluorescence staining for NeuN/caspase-1, NeuN/GSDMD, ASC/caspase-1, and NeuN/IL-1β colocalization in the penumbra area. Similar to the Western blot results, the APN-loaded nanoparticles significantly reduced caspase-1, GSDMD, ASC, and IL-1β-positive cells in the penumbra area (Figure 6C–F).
Immunofluorescence staining was performed to detect microglia in ischemic brain tissue.35,36 Compared with the sham group, the number of microglia was significantly increased on the ipsilateral side of MCAO/R mice; MM/ANPs significantly reduced pro-inflammatory microglial activation (Figure 7A). This phenomenon was verified by an ELISA with mouse sera. Pro-inflammatory cytokines, including TNF-α and interleukins IL-1β, IL-18, and IL-6, were significantly upregulated 24 h after I/R injury in the MCAO/R group. In contrast, the pro-inflammatory cytokines in the MM/ANP-treated group showed different degrees of downregulation and were similar to those in the sham group (Figure 7B–E). Mitochondrial ultrastructure was observed using TEM. Mitochondrial morphology and integrity were normal in the sham group; in contrast, marked mitochondrial swelling, low matrix electron density, and absence of mitochondrial cristae was observed in the MCAO/R group. However, MM/ANPs treatment restored the mitochondrial cristae and matrix electron density and thus improved mitochondrial morphology (Figure 7F). These results verified that treatment with MM/ANPs could manifest neuroprotective effects in treating ischemic stroke by reducing NLRP3 inflammasome pathway activation.
MM/ANPs Positively Regulate SIRT3 to Inhibit NLRP3-Mediated Inflammatory Responses
We hypothesized that MM/ANPs may inhibit NLRP3-mediated inflammatory response by regulating SIRT3. Western blot results confirmed that SIRT3 levels decreased in the MCAO/R group compared to the sham group, but treatment with MM/ANPs significantly upregulated SIRT3 levels. Additionally, the results showed that the NLRP3 inhibitor (MCC950) did not affect the protective effects of MM/ANPs after MCAO/R, suggesting that SIRT3 acts upstream of the NLRP3 signaling pathway (Figure 8A–C).
To further verify whether MM/ANPs exert their inhibitory effects on the NLRP3 pathway through SIRT3, we used transgenic Sirt3-knockout mice to further argue this point. As shown by Western blot, MM/ANPs significantly inhibited the activation of NLRP3 in the brain tissue of MCAO/R mice, but MM/ANPs did not inhibit the activation of inflammasome in Sirt3-knockout mice after MCAO/R injury, and the difference was statistically significant (Figure 8D–H). Meanwhile, immunofluorescence also demonstrated that the number of SIRT3-positive cells was significantly reduced in the MCAO/R group compared with the sham group, but treatment with MM/ANPs significantly increased the number of SIRT3-positive cells (Figure 8I). This again suggests that MM/ANPs can inhibit the activation of inflammasome in cerebral I/R injury and that the inhibitory effect is exerted at least through SIRT3.
Biosafety Assessment and Blood Compatibility Studies
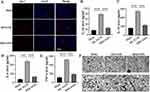
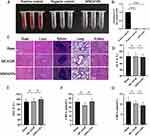
To assess biosafety and explore the potential side effects of MM/ANPs nanoparticle treatment, an in vitro hemolysis test was performed with MM/ANPs. Hemocompatibility is an important safety indicator for biomaterials, especially those in direct contact with blood.37,38 As shown in Figure 9A and 9B, MM/ANPs showed no obvious hemolysis in the blood in vitro; their OD values were not significantly different from those of the negative control group, confirming good biocompatibility. The mice’s heart, liver, spleen, lung, and kidney were collected 24 h after drug injection for tissue sectioning and H&E staining. No significant change was found in the organs of the different treatment groups, indicating that MM/ANPs did not cause significant damage to the organs, as shown in Figure 9C. Blood samples were collected for serum chemistry analyses related to liver and kidney function. The levels of aspartate aminotransferase, alanine aminotransferase, urea nitrogen, and creatinine were normal in all groups, suggesting that liver and kidney function were not affected by MM/ANPs treatment (Figure 9D–G). Overall, no significant side effect or immunotoxicity was observed after treatment with MM/ANPs, suggesting that MM/ANPs are safe for treating ischemic stroke in mice.
Discussion
Ischemic stroke, a leading cause of disability and mortality globally, is primarily driven by cerebral I/R injury, characterized by a cascade of pathological events post-blood flow restoration.39 The rapid reinstatement of circulation, while essential for tissue salvage, paradoxically initiates oxidative stress, inflammatory response, and cellular death mechanisms, notably pyroptosis. Current therapeutic modalities, primarily thrombolytic agents, offer limited windows of opportunity and fail to address the ensuing inflammatory onslaught, underlining the imperative for novel intervention strategies. The application of targeted nanoparticles in the treatment of ischemic stroke has shown significant therapeutic potential. A recent study successfully developed multifunctional ORD@SHp@ANG nanoparticles that effectively scavenge ROS, protect neurons, and reduce cerebral infarction in ischemic stroke models.40 Another recent study developed a ceria nanoenzymes synergistic drug-carrying nanosystem targeting mitochondria, providing a promising strategy for ischemic stroke treatment.41 The study introduces a novel therapeutic strategy using functional nanoparticles, specifically MM/ANPs, modified with DSPE-PEG-RVG29. These nanoparticles are engineered to traverse the BBB efficiently, targeting inflammation-inflicted sites in the brain. The design leverages the natural tendency of MM for inflammatory chemotaxis and the enhanced endocytic capabilities provided by DSPE-PEG-RVG29 modification, facilitating targeted delivery to the ischemic brain areas.
These nanoparticles ingeniously exploit the inherent inflammatory chemotaxis of MM and are refined with DSPE-PEG-RVG29 to enhance BBB penetration and site-specific drug delivery.42,43 APN, a SIRT3 modulator, is pivotal in mitigating the NLRP3 inflammasome-mediated inflammatory cascade and pyroptotic cell death.44 Once at the target site, the controlled release of APN from MM/ANPs plays a crucial role in modulating the pathological process. APN, through its influence on the SIRT3 pathway, inhibits the assembly and activation of the NLRP3 inflammasome, thus attenuating the inflammatory response and pyroptosis. These molecular effects are pivotal in preventing the cascade of cellular events that lead to extensive neuronal damage and loss. The strategic encapsulation within MM not only ensures a sustained release but also accentuates the therapeutic potency of APN within the neural microenvironment. We have shown the proficiency of MM/ANPs in traversing the BBB, congregating at injury epicenters, and sequentially discharging APN to attenuate inflammatory and pyroptotic sequelae. In mouse models of I/R injury, these nanoparticles have demonstrated a significant amelioration in neural functionality and a decrement in infarct volume, indicative of their potential to transform stroke outcome paradigms. These findings underscore the therapeutic potential of this nanoparticle system in specifically targeting and ameliorating the effects of ischemic stroke.
Although the initial results are promising, translating these findings into clinical practice involves overcoming several challenges. The specificity and efficiency of targeting, long-term stability and release kinetics of APN, potential immunogenicity of the nanoparticle components, and the scalability of nanoparticle synthesis are critical factors that need to be addressed. Furthermore, exploring the modulatory effects of APN on other neuroprotective pathways could unveil additional therapeutic dimensions. Additionally, long-term safety and efficacy studies, pharmacokinetics, and pharmacodynamics assessments are essential to ensure that these nanoparticles can be safely and effectively used in human patients.
Conclusion
In this study, we developed a functional nanoparticle (MM/ANPs) delivery system using MM wrapped with DSPE-PEG-RVG29 peptide to efficiently deliver APN to inflammatory regions for treatment of ischemic stroke. MM/ANPs actively cross the BBB and target in ischemic–inflammatory regions. This APN-based nanoscale delivery system coated with MM offers a highly promising approach to achieve ischemic stroke-targeted therapy inhibiting NLRP3 inflammasome-mediated pyroptosis. The functional nanoparticles strategy in this study provides the possibility for precise targeted treatment of ischemic stroke.
Funding
This work was supported by the National Natural Science Foundation of China (82101410), the Medicine and Science Innovation Plan Project of Shandong Second Medical University (no. 2021BKQ009), and a Graduate Student Research Grant from Shandong Second Medical University. We would like to thank Editage for English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yu ZL, Qin B, Ma ZY, et al. Emerging bioinspired artificial woods. Adv materials. 2021;33(28):e2001086. doi:10.1002/adma.202001086
2. Gao J, Cupolillo A, Nappini S, et al. Surface reconstruction, oxidation mechanism, and stability of Cd3As2. Adv Funct Mater. 2019;29(26):1900965. doi:10.1002/adfm.201900965
3. Feigin VL, Brainin M, Norrving B, et al. World Stroke Organization (WSO): global stroke fact sheet 2022. Int J Stroke. 2022;17(1):18–29. doi:10.1177/17474930211065917
4. Zhang Q, Jia M, Wang Y, Wang Q, Wu J. Cell death mechanisms in cerebral ischemia-reperfusion injury. Neurochemical Res. 2022;47(12):3525–3542. doi:10.1007/s11064-022-03697-8
5. Jurcau A, Simion A. Neuroinflammation in cerebral ischemia and ischemia/reperfusion injuries: from pathophysiology to therapeutic strategies. Int J Mol Sci. 2021;23(1). doi:10.3390/ijms23010014
6. Hu J, Nan D, Lu Y, et al. Microcirculation no-reflow phenomenon after acute ischemic stroke. Eur Neurol. 2023;86(2):85–94. doi:10.1159/000528250
7. Zeng GG, Tang SS, Jiang WL, Yu J, Nie GY, Tang CK. Apelin-13: a protective role in vascular diseases. Curr Prob Cardiol. 2024;49(1 Pt B):102088. doi:10.1016/j.cpcardiol.2023.102088
8. Li J, Chen Z, Chen J, Yu Y. The beneficial roles of apelin-13/APJ system in cerebral ischemia: pathogenesis and therapeutic strategies. Front Pharmacol. 2022;13:903151. doi:10.3389/fphar.2022.903151
9. Zhang Y, Jiang W, Sun W, et al. Neuroprotective roles of apelin-13 in neurological diseases. Neurochemical Res. 2023;48(6):1648–1662. doi:10.1007/s11064-023-03869-0
10. Zhang R, Wu F, Cheng B, Wang C, Bai B, Chen J. Apelin-13 prevents the effects of oxygen-glucose deprivation/reperfusion on Bend.3 cells by inhibiting AKT-mTOR signaling. Experimental Biol Med. 2023;248(2):146–156. doi:10.1177/15353702221139186
11. Zhang H, Chen S, Zeng M, et al. Apelin-13 administration protects against LPS-induced acute lung injury by inhibiting NF-κB pathway and NLRP3 inflammasome activation. Cell Physiol Biochem. 2018;49(5):1918–1932. doi:10.1159/000493653
12. Xu W, Li T, Gao L, et al. Apelin-13/APJ system attenuates early brain injury via suppression of endoplasmic reticulum stress-associated TXNIP/NLRP3 inflammasome activation and oxidative stress in a AMPK-dependent manner after subarachnoid hemorrhage in rats. J Neuroinflammation. 2019;16(1):247. doi:10.1186/s12974-019-1620-3
13. Pardridge WM. Drug transport across the blood-brain barrier. J Cerebral Blood Flow Metabol. 2012;32(11):1959–1972. doi:10.1038/jcbfm.2012.126
14. Liao J, Gong L, Xu Q, et al. Revolutionizing neurocare: biomimetic nanodelivery via cell membranes. Adv materials. 2024;36(26):e2402445. doi:10.1002/adma.202402445
15. Liao J, Fan L, Li Y, et al. Recent advances in biomimetic nanodelivery systems: new brain-targeting strategies. J Controlled Release. 2023;358:439–464. doi:10.1016/j.jconrel.2023.05.009
16. Jones GE. Cellular signaling in macrophage migration and chemotaxis. J Leukocyte Biol. 2000;68(5):593–602. doi:10.1189/jlb.68.5.593
17. Lopes J, Lopes D, Pereira-Silva M, et al. Macrophage cell membrane-cloaked nanoplatforms for biomedical applications. Small Methods. 2022;6(8):e2200289. doi:10.1002/smtd.202200289
18. Zou L, Zhang Y, Cheraga N, et al. M2 macrophage membrane-camouflaged Fe(3) O(4) -Cy7 nanoparticles with reduced immunogenicity for targeted NIR/Mr imaging of atherosclerosis. Small. 2024;20(8):e2304110. doi:10.1002/smll.202304110
19. Gu C, Geng X, Wu Y, et al. Engineered macrophage membrane-coated nanoparticles with enhanced CCR2 expression promote spinal cord injury repair by suppressing neuroinflammation and neuronal death. Small. 2024;20(10):e2305659. doi:10.1002/smll.202305659
20. Wu M, Ping H, Wang K, et al. Oral delivery of pectin-chitosan hydrogels entrapping macrophage-targeted curcumin-loaded liposomes for the treatment of ulcerative colitis. Int J Pharm. 2023;647:123510. doi:10.1016/j.ijpharm.2023.123510
21. Li C, Zhao Z, Luo Y, et al. Macrophage-disguised manganese dioxide nanoparticles for neuroprotection by reducing oxidative stress and modulating inflammatory microenvironment in acute ischemic stroke. Adv Sci. 2021;8(20):e2101526. doi:10.1002/advs.202101526
22. Anghelache M, Voicu G, Deleanu M, et al. Biomimetic nanocarriers of pro-resolving lipid mediators for resolution of inflammation in atherosclerosis. Adv Healthcare Mater. 2024;13(3):e2302238. doi:10.1002/adhm.202302238
23. Yang J, Wang P, Jiang X, et al. A nanotherapy of octanoic acid ameliorates cardiac arrest/cardiopulmonary resuscitation-induced brain injury Via Rvg29- And neutrophil membrane-mediated injury relay targeting. ACS Nano. 2023;17(4):3528–3548. doi:10.1021/acsnano.2c09931
24. Ji F, Xu L, Long K, et al. Rabies virus glycoprotein 29 (RVG29) promotes CAR-T immunotherapy for glioma. Translationl Res. 2023;259:1–12. doi:10.1016/j.trsl.2023.03.003
25. Liu Y, Huang R, Han L, et al. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials. 2009;30(25):4195–4202. doi:10.1016/j.biomaterials.2009.02.051
26. Han Y, Gao C, Wang H, et al. Macrophage membrane-coated nanocarriers Co-Modified by RVG29 and TPP improve brain neuronal mitochondria-targeting and therapeutic efficacy in Alzheimer’s disease mice. Bioact Mater. 2021;6(2):529–542. doi:10.1016/j.bioactmat.2020.08.017
27. Hao R, Sun B, Yang L, Ma C, Li S. RVG29-modified microRNA-loaded nanoparticles improve ischemic brain injury by nasal delivery. Drug Delivery. 2020;27(1):772–781. doi:10.1080/10717544.2020.1760960
28. Sadat Tabatabaei Mirakabad F, Nejati-Koshki K, Akbarzadeh A, et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pacific J Cancer Prevent. 2014;15(2):517–535. doi:10.7314/APJCP.2014.15.2.517
29. Chiang T, Messing RO, Chou WH. Mouse model of middle cerebral artery occlusion. J Visualiz Experim. 2011;(48). doi:10.3791/2761-v
30. Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16(1):142. doi:10.1186/s12974-019-1516-2
31. Jiang M, Yin P, Bai X, Yang L, Zhang J, Xu S. Proinflammatory and anti-inflammatory genes in stroke pathogenesis. Curr Pharm Des. 2020;26(34):4220–4233. doi:10.2174/1381612826666200701212859
32. Ran Y, Su W, Gao F, et al. Curcumin ameliorates white matter injury after ischemic stroke by inhibiting microglia/macrophage pyroptosis through NF- κB suppression and NLRP3 inflammasome inhibition. Oxid Med Cell Longev. 2021;2021(1):1552127. doi:10.1155/2021/1552127
33. Zhu H, Jian Z, Zhong Y, et al. Janus kinase inhibition ameliorates ischemic stroke injury and neuroinflammation through reducing NLRP3 inflammasome activation via JAK2/STAT3 pathway inhibition. Front Immunol. 2021;12:714943. doi:10.3389/fimmu.2021.714943
34. Ge Y, Wang L, Wang C, et al. CX3CL1 inhibits NLRP3 inflammasome-induced microglial pyroptosis and improves neuronal function in mice with experimentally-induced ischemic stroke. Life Sci. 2022;300:120564. doi:10.1016/j.lfs.2022.120564
35. Coll RC, Schroder K, Pelegrín P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci. 2022;43(8):653–668. doi:10.1016/j.tips.2022.04.003
36. Wei X, Xie F, Zhou X, et al. Role of pyroptosis in inflammation and cancer. Cell. Mol. Immunol. 2022;19(9):971–992.
37. Shim G, Kim D, Kim J, Suh MS, Kim YK, Oh YK. Bacteriomimetic poly-γ-glutamic acid surface coating for hemocompatibility and safety of nanomaterials. Nanotoxicology. 2017;11(6):762–770. doi:10.1080/17435390.2017.1353155
38. Leszczak V, Smith BS, Popat KC. Hemocompatibility of polymeric nanostructured surfaces. J Biomater Sci Poly Ed. 2013;24(13):1529–1548. doi:10.1080/09205063.2013.777228
39. Campbell BCV, De Silva DA, Macleod MR, et al. Ischaemic stroke. Nature Rev Dis Primers. 2019;5(1):70. doi:10.1038/s41572-019-0118-8
40. Li Y, Liao J, Xiong L, et al. Stepwise targeted strategies for improving neurological function by inhibiting oxidative stress levels and inflammation following ischemic stroke. J Controlled Release. 2024;368:607–622. doi:10.1016/j.jconrel.2024.02.039
41. Liao J, Li Y, Fan L, et al. Bioactive ceria nanoenzymes target mitochondria in reperfusion injury to treat ischemic stroke. ACS nano. 2024; 18(7): 5510–5529
42. Liu Y, Luo J, Liu Y, et al. Brain-targeted biomimetic nanodecoys with neuroprotective effects for precise therapy of Parkinson’s disease. ACS Cent Sci. 2022;8(9):1336–1349. doi:10.1021/acscentsci.2c00741
43. Ouyang Q, Liu K, Zhu Q, et al. Brain-penetration and neuron-targeting DNA nanoflowers co-delivering miR-124 and rutin for synergistic therapy of Alzheimer’s disease. Small. 2022;18(14):e2107534. doi:10.1002/smll.202107534
44. Xin Q, Cheng B, Pan Y, et al. Neuroprotective effects of apelin-13 on experimental ischemic stroke through suppression of inflammation. Peptides. 2015;63:55–62. doi:10.1016/j.peptides.2014.09.016
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.