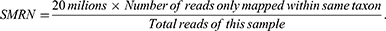Back to Journals » Infection and Drug Resistance » Volume 18
Application of Metagenomic Next-Generation Sequencing in HIV-Infected Patients with Bloodstream Infections
Authors Liu H, Xu T, Fu H, Dai B, Xie Y
Received 1 December 2024
Accepted for publication 15 April 2025
Published 8 May 2025 Volume 2025:18 Pages 2389—2399
DOI https://doi.org/10.2147/IDR.S509665
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Oliver Planz
Huiting Liu, Tianzhen Xu, Hongxin Fu, Bohao Dai, Yirui Xie
State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The Department of Infectious Diseases, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, 310003, People’s Republic of China
Correspondence: Yirui Xie, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The Department of Infectious Diseases, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, 310003, People’s Republic of China, Tel/Fax +86-571-87236416, Email [email protected]
Background: Bloodstream infections (BSI) are common complications in HIV-infected patients and are prone to septic shock and death. This study aimed to analyze the application of blood metagenomic next-generation sequencing (mNGS) in HIV-infected patients with BSI.
Methods: Fifty-four HIV-infected patients with suspected BSI were hospitalized at the First Affiliated Hospital of the Zhejiang University School of Medicine between August 2020 and June 2023. Blood mNGS and blood culture (BC) results were retrospectively reviewed and compared to the application value of BSI.
Results: The mNGS was more sensitive for detecting pathogens (82.4% versus 35.3%; P < 0.05), and when combining blood mNGS with culture results, the sensitivity increased to 88.2%. The detection rate of mNGS for blood-mixed infection was significantly higher than that of BC (P < 0.05). Among the positive results for fungi and bacteria detected by mNGS, 13.5% of the pathogenic microorganisms were consistent with the results of BC.
Conclusion: The mNGS combined with BC can improve pathogen detection sensitivity and the comprehensive identification of pathogenic microorganisms in HIV-infected patients with BSI.
Keywords: metagenomic next-generation sequencing, blood culture, bloodstream infections, HIV
Introduction
Bloodstream infections (BSI) are an important cause of morbidity and mortality worldwide.1 People with advanced human immunodeficiency virus (HIV) infection are more susceptible to developing BSI as their immune competence becomes severely compromised. In China, the prevalence of BSI among HIV-positive hospitalized patients is 9.38%, with 13.3% having a poor prognosis.2 HIV-infected patients have a higher risk of blood infection and mortality than HIV-seronegative hospitalized patients, thereby increasing the financial burden on patients to a certain extent.3,4 It is crucial to promptly diagnose and administer appropriate antibiotics for BSI, as this significantly reduces mortality and improves the quality of life of patients.5–9
HIV-infected patients are at a higher risk of developing various opportunistic infections than the general population. These individuals frequently present with atypical pathogens or polymicrobial infections that can be challenging to identify using conventional microbiological testing methods. Consequently, this complexity can adversely affect management and prognostic outcomes. Clinically, blood culture (BC) is an essential routine test in patients with suspected BSI. However, BC frequently requires a significant amount of time and inherently cannot detect viable but nonculturable (VBNC) bacteria, making prompt and precise diagnosis particularly challenging, especially in cases involving multiple or rare pathogens.
Metagenomic next-generation sequencing (mNGS) is a new and promising high-throughput sequencing technology that can unbiasedly and rapidly identify infections. The mNGS can overcome the limitations of BC testing and the discovery of new pathogenic microorganisms. The earlier mNGS sequencing is performed, the greater the likelihood of providing positive results and the earlier the disease can be effectively treated.10–12
The mNGS has been extensively applied in diagnosing and managing HIV-related conditions, including central nervous system and respiratory tract infections.13–16 Additionally, the application of mNGS may offer diagnostic benefits for HIV-infected patients with BSI. However, a paucity of empirical studies in this area substantiates its efficacy. This investigation aimed to systematically assess and compare the clinical utility of mNGS versus BC in HIV-infected patients with BSI.
Materials and Methods
Study Participants
In this study, data from 54 HIV-positive patients with suspected BSI who underwent blood mNGS at the First Affiliated Hospital of Zhejiang University between August 2020 and June 2023 were retrospectively analyzed. All HIV-positive patients (n = 54) underwent systematic and regular antiretroviral therapy. BSI is defined as positive BC in a patient exhibiting signs and symptoms of systemic infection. This infection may be attributable to an identifiable focus of infection or remains cryptogenic despite thorough investigation, and if a source is identified it may be secondary to a known source of infection.17 In certain instances, a diagnosis of BSI can be established even without a cultivable pathogen, provided that the patient demonstrates clinical signs of infection, responds positively to antimicrobial therapy, and has supportive laboratory findings. Comprehensive demographic data, medical history, laboratory test results, and mNGS findings were meticulously extracted from patients’ medical records during hospitalization.
All blood samples (n = 54) underwent mNGS and routine microbiological testing. The mNGS was performed at the Clinical Laboratory of the First Affiliated Hospital of Zhejiang University School of Medicine. The mNGS results for each sample included detected microbes and the number of microorganism-specific reads per 20 million sequenced reads (stringent mapped read number; SMRN). The SMRN was calculated as follows:
Full-Blood Cultures
Blood samples were collected from venous sites on both sides of the body, with a standard incubation period of 5–7 days; however, this could be adjusted depending on the suspected pathogen. The blood volume was approximately 5–10 mL per vial, and BCs were performed using a BACT/ALERT 3D system (bioMérieux, Inc., France) according to the standard operating procedures for clinical microbiology. Positive BC is defined as detecting a specific pathogen, such as a bacterium or fungus, in the blood, while negative BC is defined as the absence of any organism growing during the incubation period. A senior clinician made the decision on whether BC is contaminated.
mNGS Procedure and Bioinformatics Analysis
Whole blood samples (8–10 mL) were collected from each patient and stored in EDTA tubes at 4 °C. The samples were promptly sent to the mNGS laboratory for further analysis. Plasma was separated by centrifuging whole blood at 1600×g for 10 min at 4°C. DNA was extracted using the QIAamp DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany). Subsequently, DNA sequencing libraries were prepared via enzymatic DNA fragmentation, end repair, adapter ligation, and polymerase chain reaction (PCR) enrichment. Quality was assessed using an Agilent 2100 bioanalyzer (Agilent Technologies, Santa Clara, CA, United States), followed by sequencing using Illumina NextSeq 550 (Illumina, San Diego, CA, United States). Raw sequencing data were processed to remove adapter sequences and low-quality reads (read length <35 bp) using Fastp. Human host sequences were bioinformatically excluded by alignment to the GRCh38 reference genome with the Burrows-Wheeler Aligner. For identification, the remaining high-quality microbial reads were aligned with the NCBI RefSeq microbial genome database (ftp://ftp.ncbi.nlm.nih.gov/genomes/). The aligned data underwent rigorous bioinformatics analysis to identify potential pathogens, with detailed metrics, including read counts, coverage, and SMRN, provided for each suspected microorganism. If bacteria commonly present on the skin, including Staphylococcus epidermidis, Propionibacterium acnes, Clostridium spp., and Corynebacterium diphtheriae, or environmental bacteria, including Acinetobacter spp. and Bacillus spp., proliferated within the experimental process, they were classified as contaminants.
Criteria for a Positive mNGS Result
Common pathogenic microorganisms that may contaminate the skin and air of the blood sample were excluded if SMRN ≥ 3 for bacteria/fungi/DNA virus/Mycoplasma/Chlamydia, SMRN ≥ 1 for Mycobacterium tuberculosis/RNA virus, and SMRN ≥ 100 for parasites.18–20
For the final diagnostic conclusions, clinicians rely on their clinical experience and professional expertise to analyze the patient’s medical history and clinical data to determine the disease.
Statistical Analysis
The Statistical Package for the Social Sciences software (version 26.0) was used for data analysis. Kolmogorov–Smirnov test was used to test the normality of continuous variables. The enumeration data are presented as the mean ± standard deviation or median (interquartile range), while categorical data are expressed as frequencies and percentages. The chi-square test, Fisher exact test, or discrete variable McNemar test was applied to compare the differences between the results of mNGS test and blood culture. Statistical significance was set at P < 0.05.
Results
Clinical Characteristics
This study enrolled 54 patients with suspected BSI. The mean age of the patients was 41.8 ± 17.6 years, 87.0% were male (47/54), and most had underlying diseases (46/54). The top three clinical symptoms were fever (72.2%), cough (40.7%), and sputum expectoration (31.5%). The median duration of symptoms and hospitalization were 14 and 19 days, respectively. The median CD4+ T-lymphocyte count was 39 cells/μL. The mean C-reactive protein (CRP) level, an indicator of inflammation, was 54.4 mg/L, higher than the normal value. During hospitalization, 17 patients received glucocorticosteroids, and two required mechanical ventilation. Table 1 presents the results of routine blood tests, biochemical analyses, infection-related biomarkers, and lactate dehydrogenase levels.
 |
Table 1 Summary of the Clinical Characteristics |
Positivity Rate of mNGS and BC
The results of mNGS and BC were compared in 54 patients with suspected BSI. In this study, 43 (43/54, 79.6%) cases were positive for mNGS, and 18 (18/54, 33.3%) cases were positive for BC (Figure 1a). The detection rate of pathogens differed significantly between the two methods (79.6% versus 33.3%, P < 0.001). From mNGS results, 40 samples tested positive for viruses, 14 for bacteria, and 11 for fungi. For BC results, 14 samples tested positive for bacteria and 6 for fungi (Figure 1b). Additionally, regarding the infection pattern, mNGS results were primarily mono-viral infection (19, 44.2%), followed by a mixed infection of viruses and fungi (13, 30.2%), and viruses and bacteria (7, 16.3%, Figure 1c).
Pathogenic Spectrum Detected Using BC and mNGS
This study detected 22 unique pathogens using mNGS. When viruses were excluded, mNGS identified 10 bacterial and fungal species, including Pneumocystis jirovecii, the Mycobacterium tuberculosis complex, and Escherichia coli, which were not detected using BCs. Conversely, conventional BC detected 10 pathogenic microorganisms, six of which were not identified by mNGS, including Cryptococcus neoformans, Pseudomonas aeruginosa, and Oidium Coccidioides (Figure 2). Further analysis indicated that the combined use of mNGS and BC identified 28 pathogens in a cohort of 54 samples.
 |
Figure 2 The pathogen distribution was detected using two methods. Statistical significance was set at P < 0.05. |
Comparative Analysis of Clinical Diagnostic Values for BSI Using mNGS and BC
In this study, mNGS identified pathogens exclusively in 27 blood samples, whereas BC detected pathogens in only two samples. Both methods yielded positive results in 16 (21.9%) cases. Figure 3 reveals that the concordance between mNGS and BC was as follows: full concordance in 0 (0.0%) cases, partial concordance in 5 (9.3%) cases, and full discordance in 11 (20.4%) cases. Excluding viruses, mNGS and BC detected pathogens in 10 samples, with 13.5% (5/37) agreement for detecting bacteria and fungi (Figure 4). An analysis of the patients’ clinical data revealed 51 cases meeting the diagnostic criteria for BSI. Among these, 43 mNGS-positive samples were identified, with 42 demonstrating clinically significant results. Furthermore, 18 BC-positive cases were consistent with the diagnostic criteria. When integrated with the final clinical diagnoses, the sensitivity of mNGS (82.8%) was significantly higher than that of BC (35.3%; P < 0.001). When combining mNGS and BC results, the sensitivity was 88.2%, the specificity was 66.7%, and the positive predictive value (PPV) and negative predictive value (NPV) were 97.8% and 25.0%, respectively (Table 2).
 |
Table 2 Comparison of the Diagnostic Performance of mNGS and BC in HIV-Infected Patients With BSI. PPV: Positive Predictive Value; NPV: Negative Predictive Value |
 |
Figure 4 Consistency of bacteria and fungi detected in blood using mNGS and BC. A Venn diagram displayed that the concordance rate between mNGS and BC for detecting bacteria and fungi was 13.5%. |
Routine BC was used to detect co-infections in three samples of bacterial and fungal species. In contrast, mNGS identified 31 cases of mixed infections (Figure 5a), demonstrating a significantly higher detection capability for co-infections than conventional BC (P < 0.05). Among the mixed infections detected by mNGS, 21 samples harbored two pathogens, while 11 contained at least three (Figure 5b). In the co-infection detected by mNGS, human herpesvirus 4 (HHV-4) and human herpesvirus 5 (HHV-5) were most susceptible to co-infection with other microorganisms, followed by Talaromyces marneffei and Pneumocystis jirovecii (Figure 6). In summary, mNGS offers valuable insights into diagnosing co-infections.
 |
Figure 5 (a) Comparison of the diagnostic performance of mNGS and BC for single and mixed infections. (b) The number of pathogens detected using mNGS. |
 |
Figure 6 Heatmap displaying the pathogen spectrum detected by mNGS. Orange and green represent positive, light pink represents negative, and 1 means SMRN≤1000 and 2 means SMRN > 1000 in the legend. |
Discussion
BSI is a severe systemic infectious disease caused by invasion of pathogenic microorganisms. BSI is a common complication in HIV-infected patients and is prone to septic shock and death.4,21–23 Timely and precise pathogen identification, coupled with the administration of appropriate antimicrobial therapy, is essential for enhancing clinical outcomes and disease prognosis.7,24–26 Therefore, this study conducted a retrospective assessment to compare the clinical utility of mNGS with traditional BC methods to detect pathogens in HIV-positive patients with BSI.
In this investigation, mNGS demonstrated superior sensitivity in pathogen detection compared to BC methods (82.4% versus 35.3%, P < 0.05). The combined application of mNGS and BC further enhanced the sensitivity to 88.2%, corroborating findings from earlier research.20,27–29 The heightened sensitivity attributed to mNGS is probably due to its ability to identify the genomic DNA of a broad spectrum of microorganisms, including minute quantities of non-viable or non-culturable pathogens, thereby minimizing the impact of antibiotic treatment on detection outcomes. Among 54 blood samples collected from HIV-infected patients with clinically suspected BSI, mNGS successfully identified pathogens in 43 (79.6%) samples. Conversely, BC yielded positive results for 18 (33.3%) samples. The suboptimal bacterial and fungal detection rate via BC is attributed to the stringent growth requirements of pathogens in culture, numerous interfering factors throughout the culturing process, and the employment of empirical antibiotics. In contrast, the positive BC rate post-antibiotic administration in the HIV-negative cohort ranged between 12% and 27.7%.30–32 Notably, the 33.3% BC detection rate observed in this study surpassed that reported for the HIV-negative population.
Multiple pathogenic BSI are frequently associated with a poorer prognosis than single-pathogen BSI. Studies have indicated that hospitalized patients with multiple pathogenic BSI have a mortality rate approximately twice as high as those with single microbial infections.33 Studies of BSI in immunocompromised populations have demonstrated that mNGS is also superior to BC for distinguishing between single- and mixed-pathogen infections,20,34 consistent with this result. However, BC identified three cases of mixed bacterial and fungal infections. The mNGS detected a broader range of viral and fungal co-infections, with HHV-4 and HHV-5 being the most frequent co-infecting agents. Notably, 13.5% of mNGS-detected bacterial and fungal infections were corroborated by BC, a higher concordance rate than observed in a previous HIV-negative cohort study.35 This increased agreement may be due to immunological perturbations caused by HIV infection, leading to patients presenting with multiple pathogenic infections or atypical clinical manifestations, frequently resulting in incomplete or partial coverage by empirical antibiotic therapy. Furthermore, the cumulative risk of drug-resistant pathogen infections in HIV-infected patients, particularly following multiple hospitalizations, contributes to the persistence of bloodborne pathogens and enhances the detection rate, thereby improving the consistency between mNGS and BC methods. Consequently, mNGS results complement BC findings, allowing for more comprehensive and targeted antibiotic selection based on the detected pathogens.
Bacteria in the VBNC state are incapable of proliferating on standard culture media while maintaining cellular viability and preserving virulence factors. These VBNC bacteria can regain culturability under specific environmental stimuli. Notably, this survival strategy has been documented across diverse bacterial taxa, including clinically significant human pathogens that may enter the VBNC state under stress conditions.36,37 In our study, some bacteria—including the Mycobacterium tuberculosis complex, known to enter the VBNC state under certain conditions—were detected exclusively by mNGS.38 While VBNC bacteria are rare in the blood of healthy individuals, HIV-infected individuals with immune deficiencies and frequent antibiotic use may have a higher likelihood of harboring VBNC bacteria.39,40 These bacteria may persist and be potentially pathogenic, but their role in bloodstream infections is still unclear, and no standard exists to determine whether they can cause such infections. mNGS, which does not rely on culturing and is unaffected by antibiotics, can detect VBNC bacteria, providing a broader pathogen profile and reducing the risk of missed diagnoses. However, mNGS cannot distinguish between proliferating live bacteria and VBNC bacteria. Notably, our study did not directly validate the VBNC state through bacterial activity assays. Future research could integrate mNGS with bacterial viability tests and multi-omics approaches, such as metabolomics, proteomics, and transcriptomics, to further investigate the pathogenic potential of VBNC bacteria in immunocompromised patients. This will help investigate the immune evasion, resuscitation mechanisms, and antibiotic effects on VBNC bacteria.
Regarding pathogen detection, such as in BC, mNGS technology can detect bacteria, fungi, viruses, and parasites. However, statistical analysis did not reveal a significant difference in bacteria and fungi detection rates between mNGS and BC methods. The mNGS demonstrated a clear advantage by identifying a broader spectrum and diversity of microorganisms. Certain pathogens, including Mycobacterium tuberculosis, can be detected using mNGS. Mycobacterium tuberculosis bacteremia is particularly concerning, as it has a propensity to evolve into septic shock and is a predominant cause of mortality among HIV-positive patients.41,42 Conventional diagnostic techniques frequently fall short in the timely diagnosis of Mycobacterium tuberculosis, leading to diagnostic delays.43,44 The mNGS is an important assay for diagnosing Mycobacterium tuberculosis bacteremia.
This study has several limitations. First, this was a retrospective analysis and lacked the support of data from a multicenter, large-sample cohort. The high cost of mNGS limits its clinical use, especially in resource-limited areas and among financially constrained patients, which also limited our study’s sample size. Advances in mNGS technology are expected to reduce costs, improving accessibility. Second, most patients were treated with antibiotics before undergoing mNGS or culture, which may have affected the sensitivity of both methods. Finally, BSI diagnosis was explained by the subjective judgment of the senior clinician, leading to subjective bias. And reliable instruments and skilled personnel remain essential for accurate results. Currently, there are no universally accepted diagnostic criteria for BSI caused by HHV5 and HHV4. In this study, clinical significance was determined based on the expertise and clinical judgment of physicians, who evaluated patient histories, PCR, and other conventional diagnostic methods, and the response to antiviral therapy to validate mNGS results. This approach aimed to enhance the reliability of clinical predictions.
In conclusion, this study indicates that mNGS exhibits superior sensitivity to BC in detecting pathogens in HIV-infected patients with BSI. The BC with mNGS can further enhance the sensitivity. The combined application of blood mNGS and BC facilitates a more comprehensive identification of pathogenic microorganisms in HIV-infected patients with BSI.
Ethics Approval and Informed Consent
This study conforms to the ethical norms of the 1975 helsinki Declaration. The research protocol was approved by the Institutional Review Committee of the First Affiliated Hospital of Zhejiang University (approval number: 2022-936). All participants in this study provided written informed consent prior to the collection of whole blood samples. All data used for analysis were anonymized.
Acknowledgments
We thank all participants in this study.
Funding
This study was funded by the National Key Research and Development Program of China (2022YFC2304500). These funding agencies have no role in research design, data collection, analysis, interpretation, or manuscript writing.
Disclosure
The authors reported no conflicts of interest.
References
1. Becker JU, Theodosis C, Jacob ST, Wira CR, Groce NE. Surviving sepsis in low-income and middle-income countries: new directions for care and research. Lancet Infect Dis. 2009;9(9):577–582. doi:10.1016/S1473-3099(09)70135-5
2. Qi T, Zhang R, Shen Y, et al. Etiology and clinical features of 229 cases of bloodstream infection among Chinese HIV/AIDS patients: a retrospective cross-sectional study. Eur J Clin Microbiol Infect Dis. 2016;35(11):1767–1770. doi:10.1007/s10096-016-2724-7
3. De Matos A, Lopes SB, Serra JE, Ferreira E, da Cunha JS. Mortality predictive factors of people living with human immunodeficiency virus and bloodstream infection. Inter J Infect Dis. 2021;110:195–203. doi:10.1016/j.ijid.2021.06.032
4. Chaka W, Berger C, Huo S, et al. Presentation and outcome of suspected sepsis in a high-HIV burden, high antiretroviral coverage setting. Inter J Infect Dis. 2020;96:276–283. doi:10.1016/j.ijid.2020.04.004
5. Gaieski DF, Mikkelsen ME, Band RA, et al. Impact of time to antibiotics on survival in patients with severe sepsis or septic shock in whom early goal-directed therapy was initiated in the emergency department. Crit Care Med. 2010;38(4):1045–1053. doi:10.1097/CCM.0b013e3181cc4824
6. Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49(9):3640–3645. doi:10.1128/AAC.49.9.3640-3645.2005
7. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi:10.1097/01.CCM.0000217961.75225.E9
8. Kang CI, Kim SH, Kim HB, et al. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clinl Infect Dis. 2003;37(6):745–751. doi:10.1086/377200
9. Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clinl Infect Dis. 2003;36(11):1418–1423. doi:10.1086/375057
10. Geng S, Mei Q, Zhu C, et al. Metagenomic next-generation sequencing technology for detection of pathogens in blood of critically ill patients. Inter J Infect Dis. 2021;103:81–87. doi:10.1016/j.ijid.2020.11.166
11. Qin C, Zhang S, Zhao Y, Ding X, Yang F, Zhao Y. Diagnostic value of metagenomic next-generation sequencing in sepsis and bloodstream infection. Front Cell Infect Microbiol. 2023;13:1117987. doi:10.3389/fcimb.2023.1117987
12. Zuo YH, Wu YX, Hu WP, et al. The Clinical Impact of Metagenomic Next-Generation Sequencing (mNGS) Test in Hospitalized Patients with Suspected Sepsis: a Multicenter Prospective Study. Diagnostics. 2023;13(2):323. doi:10.3390/diagnostics13020323
13. Zhu Y, Zhao W, Yang X, et al. Metagenomic next-generation sequencing for identification of central nervous system pathogens in HIV-infected patients. Front Microbiol. 2022;13:1055996. doi:10.3389/fmicb.2022.1055996
14. Xie Y, Dai B, Zhou X, et al. Diagnostic Value of Metagenomic Next-Generation Sequencing for Multi-Pathogenic Pneumonia in HIV-Infected Patients. Infect Drug Resist. 2023;16:607–618. doi:10.2147/IDR.S394265
15. Niu J, Wang J, Jia P, Zhang M, Wei E. Clinical features and diagnostic value of metagenomic next -generation sequencing in five cases of non-HIV related Pneumocystis jirovecii pneumonia in children. Front Cell Infect Microbiol. 2023;13:1132472. doi:10.3389/fcimb.2023.1132472
16. Yan L, Sun W, Lu Z, Fan L. Metagenomic Next-Generation Sequencing (mNGS) in cerebrospinal fluid for rapid diagnosis of Tuberculosis meningitis in HIV-negative population. Inter J Infect Dis. 2020;96:270–275. doi:10.1016/j.ijid.2020.04.048
17. Timsit JF, Ruppé E, Barbier F, Tabah A, Bassetti M. Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med. 2020;46(2):266–284. doi:10.1007/s00134-020-05950-6
18. Qin H, Peng J, Liu L, et al. A Retrospective Paired Comparison Between Untargeted Next Generation Sequencing and Conventional Microbiology Tests With Wisely Chosen Metagenomic Sequencing Positive Criteria. Front Med. 2021;8:686247. doi:10.3389/fmed.2021.686247
19. Miao Q, Ma Y, Wang Q, et al. Microbiological Diagnostic Performance of Metagenomic Next-generation Sequencing When Applied to Clinical Practice. Clin Infect Dis. 2018;67(suppl_2):S231–s40. doi:10.1093/cid/ciy693
20. Liu Q, Liu X, Hu B, et al. Diagnostic performance and clinical impact of blood metagenomic next-generation sequencing in ICU patients suspected monomicrobial and polymicrobial bloodstream infections. Front Cell Infect Microbiol. 2023;13:1192931. doi:10.3389/fcimb.2023.1192931
21. Lewis JM, Feasey NA, Rylance J. Aetiology and outcomes of sepsis in adults in sub-Saharan Africa: a systematic review and meta-analysis. Critical Care. 2019;23(1):212. doi:10.1186/s13054-019-2501-y
22. Japiassú AM, Amâncio RT, Mesquita EC, et al. Sepsis is a major determinant of outcome in critically ill HIV/AIDS patients. Critical Care. 2010;14(4):R152. doi:10.1186/cc9221
23. Huson MA, Stolp SM, van der Poll T, Grobusch MP. Community-acquired bacterial bloodstream infections in HIV-infected patients: a systematic review. Clinl Infect Dis. 2014;58(1):79–92. doi:10.1093/cid/cit596
24. Hechtman RK, Kipnis P, Cano J, Seelye S, Liu VX, Prescott HC. Heterogeneity of Benefit from Earlier Time-to-Antibiotics for Sepsis. Am J Respir Crit Care Med. 2024;209(7):852–860. doi:10.1164/rccm.202310-1800OC
25. Hu W, Chen H, Wang H, et al. Identifying high-risk phenotypes and associated harms of delayed time-to-antibiotics in patients with ICU onset sepsis: a retrospective cohort study. J Crit Care. 2023;74:154221. doi:10.1016/j.jcrc.2022.154221
26. Arulappen AL, Danial M, Ng LW, Teoh JC. The Impact of Antibiotics Administration on Mortality for Time in Sepsis and Septic Shock Patients including Possible Reasons for Delayed Administration in Malaysia. Antibiotics. 2022;11(9):1202. doi:10.3390/antibiotics11091202
27. Duan H, Li X, Mei A, et al. The diagnostic value of metagenomic next⁃generation sequencing in infectious diseases. BMC Infect Dis. 2021;21(1):62. doi:10.1186/s12879-020-05746-5
28. Wu C, Yu X, Gai W, et al. Diagnostic value of plasma and blood cells metagenomic next-generation sequencing in patients with sepsis. Biochem Biophys Res Commun. 2023;683:149079. doi:10.1016/j.bbrc.2023.10.011
29. Li X, Liang S, Zhang D, He M, Zhang H. The clinical application of metagenomic next-generation sequencing in sepsis of immunocompromised patients. Front Cell Infect Microbiol. 2023;13:1170687. doi:10.3389/fcimb.2023.1170687
30. Cheng MP, Stenstrom R, Paquette K, et al. Blood Culture Results Before and After Antimicrobial Administration in Patients With Severe Manifestations of Sepsis: a Diagnostic Study. Ann Internal Med. 2019;171(8):547–554. doi:10.7326/M19-1696
31. Scheer CS, Fuchs C, Gründling M, et al. Impact of antibiotic administration on blood culture positivity at the beginning of sepsis: a prospective clinical cohort study. Clin Microbiol Infect. 2019;25(3):326–331. doi:10.1016/j.cmi.2018.05.016
32. Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clinl Infect Dis. 2001;32(11):1651–1655. doi:10.1086/320527
33. Lin JN, Lai CH, Chen YH, et al. Characteristics and outcomes of polymicrobial bloodstream infections in the emergency department: a matched case-control study. Acad Emergency Med. 2010;17(10):1072–1079. doi:10.1111/j.1553-2712.2010.00871.x
34. Chien JY, Yu CJ, Hsueh PR. Utility of Metagenomic Next-Generation Sequencing for Etiological Diagnosis of Patients with Sepsis in Intensive Care Units. Microbiology Spectrum. 2022;10(4):e0074622. doi:10.1128/spectrum.00746-22
35. Zhou Y, Shi W, Wen Y, Mao E, Ni T. Comparison of pathogen detection consistency between metagenomic next-generation sequencing and blood culture in patients with suspected bloodstream infection. Sci Rep. 2023;13(1):9460. doi:10.1038/s41598-023-36681-5
36. Lleò MM, Benedetti D, Tafi MC, Signoretto C, Canepari P. Inhibition of the resuscitation from the viable but non-culturable state in Enterococcus faecalis. Environ Microbiol. 2007;9(9):2313–2320. doi:10.1111/j.1462-2920.2007.01345.x
37. Oliver JD. Recent findings on the viable but nonculturable state in pathogenic bacteria. FEMS Microbiol Rev. 2010;34(4):415–425. doi:10.1111/j.1574-6976.2009.00200.x
38. Oliver JD. The viable but nonculturable state in bacteria. J Microbiol. 2005;43:93–100.
39. Ramamurthy T, Ghosh A, Pazhani GP, Shinoda S. Current Perspectives on Viable but Non-Culturable (VBNC) Pathogenic Bacteria. Front Public Health. 2014;2:103. doi:10.3389/fpubh.2014.00103
40. Fleischmann S, Robben C, Alter T, Rossmanith P, Mester P. How to Evaluate Non-Growing Cells-Current Strategies for Determining Antimicrobial Resistance of VBNC Bacteria. Antibiotics. 2021;10(2):115. doi:10.3390/antibiotics10020115
41. Feasey NA, Banada PP, Howson W, et al. Evaluation of Xpert MTB/RIF for detection of tuberculosis from blood samples of HIV-infected adults confirms Mycobacterium tuberculosis bacteremia as an indicator of poor prognosis. J Clin Microbiol. 2013;51(7):2311–2316. doi:10.1128/JCM.00330-13
42. Jacob ST, Pavlinac PB, Nakiyingi L, et al. Mycobacterium tuberculosis bacteremia in a cohort of hiv-infected patients hospitalized with severe sepsis in Uganda–high frequency, low clinical suspicion [corrected] and derivation of a clinical prediction score. PLoS One. 2013;8(8):e70305. doi:10.1371/journal.pone.0070305
43. Munseri PJ, Talbot EA, Bakari M, Matee M, Teixeira JP, von Reyn CF. The bacteraemia of disseminated tuberculosis among HIV-infected patients with prolonged fever in Tanzania. Scand J Infect Dis. 2011;43(9):696–701. doi:10.3109/00365548.2011.577802
44. Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29(15):1987–2002. doi:10.1097/QAD.0000000000000802
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.