Back to Journals » International Journal of Nanomedicine » Volume 20
Application of Multifunctional Metal Nanoparticles in the Treatment of Glioma
Authors Ren Y , Yang H, Xu D, Zhang Z, Gao S, Yu R
Received 7 September 2024
Accepted for publication 25 November 2024
Published 16 January 2025 Volume 2025:20 Pages 625—638
DOI https://doi.org/10.2147/IJN.S493565
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Yan Shen
Yanhong Ren,1,2,* Han Yang,3,* Duo Xu,4 Zhengkui Zhang,4 Shangfeng Gao,1,4 Rutong Yu1,4
1Department of Neurosurgery, The Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, 221002, People’s Republic of China; 2Beijing Institute of Brain Disorders, Capital Medical University, Beijing, 100069, People’s Republic of China; 3Department of Neurology, The First Hospital of Changsha, Changsha, 410008, People’s Republic of China; 4Institute of Nervous System Diseases, Xuzhou Medical University, Xuzhou, Jiangsu, 221002, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shangfeng Gao; Rutong Yu, Email [email protected]; [email protected]
Abstract: Glioma is the most common primary malignant brain tumor with a poor survival rate. It is characterized by diffuse and invasive growth and heterogeneity, which limits tumor identification and complete resection. Therefore, the precise detection and postoperative adjuvant therapy of gliomas have become increasingly important and urgent. Nanotechnology, with its excellent biocompatibility and controllable chemical properties, has attracted much attention in recent decades. Metal nanoparticles are widely used in the field of biomedical imaging and detection, and have shown promising applications in targeted drug delivery and therapy. The current review aims to systematically summarize the application of different types of metal nanoparticles in the treatment and detection of glioma. We also discussed the advantages and mechanisms of metal nanoparticles when used for glioma therapy, including chemotherapy, radiotherapy and photothermal therapy. We hope to promote the application of metallic nanoparticles in glioma diagnosis and treatment, moving towards clinical translation to benefit patients.
Keywords: metal nanoparticles, glioma, treatment
Introduction
Despite tremendous development in biomedical technology, malignant brain gliomas has been one of the most malignant and aggressive diseases, posing an extremely serious threat to human survival. Since the 21st century, the mortality rate of brain gliomas has risen significantly in the world.1 Most malignant brain gliomas are treated with maximum safe aggressive surgery followed by adjuvant therapy, eg chemotherapy and radiotherapy.2,3 Numerous studies are trying to overcome obstacles in the treatment and detection of glioma.4 However, the inherent aggressive growth of malignant glioma results in limited therapeutic efficacy.
Based on the molecular pathology of glioma, these tumors are classified as astrocytoma, oligodendroglioma or glioblastoma (GBM) according to WHO.5 GBM is the most aggressive form of glioma, its cell density and nuclear atypia are significantly increased, with multi nuclear fission and pathological mitosis, showing obvious endovascular hyperplasia and necrosis.6 After undergoing surgical resection, it is inevitable that there will be local residual tumor micro-infiltration and recurrence. In addition, radiotherapy resistance and chemotherapy resistance are prominent problems encountered in GBM treatment.7 Therefore, GBM remains one of the most difficult tumor to treat and the median survival time of GBM patients is less than 14.6 months.8,9 It is urgently needed to develop new methods to combat glioma and improve the life quality and survival time of patients.
Nanotechnology is an emerging field that has made huge advances in biomedicine and shown promising prospects in clinical trials for cancer treatment, detection, and precise targeted therapy (Table 1). Metal nanoparticles offer an unprecedented opportunity to revolutionize and invent novel detection methods for gliomas or to boost the effectiveness of therapy.10 They possess several unique properties, thereby establishing a prominent position in the field of drug delivery system. First, metal nanoparticle drugs not only exhibit improved absorption and enhanced bioavailability in vivo, but also offer potential advantages in pharmacokinetics, biocompatibility, and controlled degradability. Second, metal nanoparticles reach GBM sites through both passive and active targeting mechanisms. They can also accumulate at tumor sites through the enhanced permeability and retention (EPR) effect in passive targeting. Additionally, surface modifications and targeting ligands enable active targeting to specific tumor cells.11 Third, metal nanoparticles present an advantage in tumor target therapy. They not only provide improved specificity and increased sensitivity for medical imaging detection.12 All of these properties contribute to the future treatment and detection of glioma.
In this review, we will firstly introduce different types of metal nanomaterials, including gold nanoparticles, magnetic iron oxide nanoparticles and some metal-based quantum dots, illustrating advantages of nanoparticles in the treatment and detection of glioma. Secondly, we will reveal the capacity of metal nanoparticles in drug delivery, tumor target therapy and detection of malignant glioma. Finally, we will explore the mechanisms of metal nanoparticles in glioma imaging as well as the application of nanotechnology in radiotherapy, chemotherapy, and photothermal therapy.
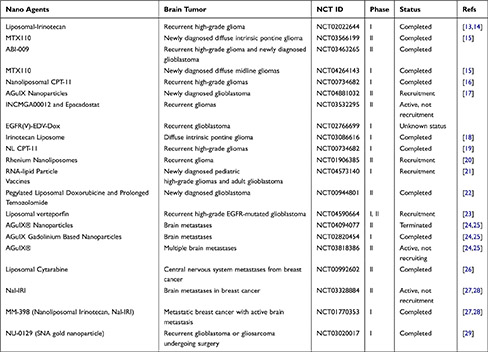 |
Table 1 Clinical Trials of Nanomaterials in Brain Cancers |
Different Types of Metal Nanoparticles in Glioma Therapy
Since liposomes were first used in the biomedical field, a large number of nanoparticles have been approved by the FDA for medical applications.13 Nanoparticles are defined as ultrafine particles, the size of which is in the range of 1–100 nm. These small size nanoparticles have a good ability to cross the Blood-Brain Barrier (BBB) without causing damage to normal tissues.14 Recently, metal nanomaterials are becoming increasingly important in the field of medical imaging and cancer treatment. The excellent biocompatibility and drug delivery capability of the metal nanomaterials make them to possess a promising future in the controlled drug release, cancer detection, drug delivery and tumor therapy. Actually, metal nanoparticles have been used in tumor diagnosis and treatment for decades.15 Since the 1990s, magnetic nanoparticles have been utilized for imaging detections and have been combined with various other contrast agents for the diagnosis of liver cancer.16 Many noble metal nanoparticles,17 such as gold and silver, are widely used in tumor radiotherapy, drug loaded chemotherapy, photothermal treatment and immunotherapy.18
Additionally, metal nanoparticles are involved in glioma resistance to therapies, with the PI3K/AKT, MAPK, and NF-κB signaling pathways contributing to this resistance.19 To overcome this resistance, current strategies focus on improving therapeutic efficacy and targeting the underlying resistance mechanisms, including selective inhibition of critical signaling pathways (eg PI3K/AKT, MAPK), combination with other treatment modalities,20,21 the use of functionalized nanoparticles for precise targeting,22,23 integration with gene therapy,24 and immune system modulation.25
In this chapter, we will not only introduce different types of metal nanoparticles, including noble metal nanoparticles, metal quantum dots, metal oxide nanoparticles, but also review their unusual advantages of nanotechnology in glioma treatment.
Noble metal nanoparticles
Noble metal nanoparticles include gold, silver, and platinum.26 These nanoparticles have good water dispersion and biocompatibility, they can effectively accumulate in the tumor site and have a long internal circulation time. Therefore, noble metal nanomaterials are considered as excellent CT contrast agent.27,28 For example, gold nanoparticles (Au NPs) have been widely studied and used in biomedical applications.29 Different shapes of gold nanostructures have been prepared, including gold spheres, gold rods, gold shells and gold cages.30 On the other hand, high Z metals are capable to deposit radiation energy and produce a series of secondary electrons, such as Compton electrons and Auger electrons. These electrons can either directly damage DNA of tumor cells or react with water, resulting in the generation of reactive oxygen species (ROS). This process may enhance the anti-tumor effect.31,32 Therefore, high Z metal nanoparticles can accumulate in the tumor area through the EPR effect, absorbing radiation energy, and sensitizing the effect of radiotherapy. Liu et al found that silver nanoparticles (Ag NPs) could enhance the radiotherapy efficacy in hypoxic glioma cells through promoting cell apoptosis and enhancing destructive autophagy.33 Nowadays, high Z metal nanoparticles have introduced a new therapeutic approach for GBM.34 Following chemical innovations with temozolomide, an alkylating agent, and subsequent biological innovations utilizing humanized IgG1 monoclonal antibody like bevacizumab, which targets all isoforms of VEGF-A, the first generation of nanoparticles has been developed. These nanoparticles aim at optimizing drug delivery and controlling the release rate of medications such as doxorubicin (Figure 1). Therefore, high Z metal nanoparticles have shown potential application value in the diagnosis and treatment of glioma.
 |
Figure 1 The development history of treating GBM with nanotechnology. Note: Reprinted from Pinel S, Thomas N, Boura C, Barberi-Heyob M. Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment. Adv Drug Deliv Rev. 2019;138:344–357.34 |
Metal oxide nanoparticles
Metal oxide nanoparticles and sulfide nanoparticles have higher chemical reactivity and unique physical properties due to its small size and large surface area. In general, metal oxide nanoparticles have the following advantages in the application of cancer treatments. First, the chemotherapy drugs, when loaded with metal oxides or their hybrid nanoparticles, such as Fe3O4 nanomaterials, have the potential to accumulate within the tumor core.35,36 This selectivity targets and kills tumor cells, with less side effects on normal tissue. Second, metal oxide nanoparticles can induce tumor cell apoptosis by generating ROS, which plays an important role in the damage of cancer cells. Third, metal oxide nanoparticles are significant for radiotherapy due to their enhanced action of sensitization and ionization. Owing to the advantages of metal oxide nanoparticles, their primary applications include magnetocaloric therapy, magnetic targeting therapy and magnetic resonance imaging. Photothermal therapy with metal nanoparticles can accelerate the speed of the blood circulation in tumor vessels and increase the amount of oxygen in the blood. Gu et al developed a new type of photosensitizer by combining photosensitive molecular porphyrin with iron oxide nanoparticles for photodynamic therapy and magnetocaloric therapy.37 This photosensitizer can improve the hypoxic microenvironment, and further enhance the sensitivity of photodynamic therapy, ultimately achieving the optimal therapeutic effect. In addition, magnetic oxide nanoparticles also have potential applications in molecularly targeted therapy. By applying a high gradient magnetic field at specific external locations, magnetic nanoparticle drugs can be concentrated within tumor tissue and can be released by altering certain parameter38,39 (Figure 2).
 |
Figure 2 The applications of Magnetic nanoparticle (MNP) for disease detection and therapy. Note: Reprinted from Materials Today, volume 31, Tong S, Zhu H, Bao G. Magnetic iron oxide nanoparticles for disease detection and therapy. 86–99, copyright 2019, with permission from Elsevier.39 |
Sulfide nanoparticle, another kind of metal oxide nanomaterial, possesses high absorption coefficient and strong X-ray attenuation ability. These characteristics make the material, such as Bi2S3 use in photothermal therapy. Liu et al reported a nanodrug based on Bi2S3 nanorods. The Bi2S3 nanorods have ideal photothermal effect and enhanced imaging contrast under multispectral photoacoustic tomography and X-ray computed tomography image navigation. This nanomaterial can accurately target the tumor after intravenous injection, effectively inhibiting tumor growth and metastasis. In addition, the Bi2S3 nanorods have good biocompatibility.41,42 Taken together, sulfide nanoparticle could simultaneously enable precise cancer therapy and diagnosis monitoring.
Metal quantum dots
Quantum dots (QDs), also known as semiconductor nanocrystals, are inorganic semiconductor nanoparticles with small radius or close to the radius of exciton Bohr.42 Due to their special optical properties, the surface of QDs is easy to be functionalized with different ligands. QDs have potential application prospects in drug modification, affinity ligands, and medical imaging detection43 (Figure 3). In particular, near infrared (NIR) fluorescent QDs can penetrate human tissues, and the fluorescence is rarely eliminated by metabolism in the human body, which makes QDs become a good in vivo imaging method.44 Gao et al designed a multifunctional QDs probe capable of simultaneously targeting tumor cells and capturing biological imaging in vivo. They conducted preliminary research on the application of QDs.45
 |
Figure 3 Quantum dots in drug delivery. (a) Schematic illustration of the nanovectors preparation protocol and their enzyme sensitive behavior. (b) Schematic illustration of the nanovectors delivering GEM to pancreatic cancer cells. Note: Reprinted from Matea CT, Mocan T, Tabaran F, et al. Quantum dots in imaging, drug delivery and sensor applications. Int j Nanomed. 2017;12:5421–5431.43, |
The Advantages of Metal Nanoparticles
Firstly, the surface of metal nanoparticles can be modified with different kinds of ligand molecules, such as small molecule compounds, polymers, and biological macromolecules. Under certain conditions, these molecules can be self-assembled or polymerized intelligently by changing hydrogen bonds, metal coordination, and electrostatic interaction. Polyethylene glycol, an amphiphilic molecule, is an ideal material for modifications. The hydrophobic end possesses a high affinity for nanoparticles, while the hydrophilic end can improve the water solubility of metal nanoparticles in liquid environment, thus reducing the toxicity of metal nanoparticles and improving the biocompatibility.47 Tian et al reported a design using polyethylene glycol-lysine-oleic acid to encapsulate magnetic nanoparticles, which is expected to serve as a magnetic resonance imaging (MRI) contrast agent and also for cancer therapy.48 Lai et al modified two groups of gold nanoparticles with chemical groups of o-nitrobenzyl alcohol and lipoic acid 4-aminomethylbenzylamine respectively. The photosensitive o-nitrobenzyl group was first photolyzed to an active nitrous intermediate under UV irradiation, which was then coupled with benzylamine-modified gold nanoparticles.52
Secondly, metal nanoparticles can be aggregated into tumor tissue through the EPR effect.49 Nanoparticles are cleared by lymphatic vessels in normal tissues, while in tumor tissues, these particles can reach the tumor sites due to leaky tumor vasculature and poor lymphatic drainage, known is the EPR effect.40 Yu et al prepared gold nanoparticles that were modified with DNA and protected by α-cyclodextrin to realize selective aggregation of gold nanoparticles in tumor lesions through the EPR effect.50 When the gold nanoparticles reach the tumor site, single stranded DNA is released from α-cyclodextrins, resulting in single chain complementary pairing.51
Thirdly, the surface of metal nanoparticles decorated with a variety of specific ligands is capable of recognizing tumor cells, thereby achieving active targeting function.46,53 Ag Seleci et al developed transferrin-modified magnetic iron oxide nanoparticles or QDs that allow for efficient dual imaging detection of glioma. These novel nanoparticles are not only beneficial for MRI, but also show great potential in active targeting effect.54 Xu et al encapsulated magnetic iron oxide nanoparticles, QDs and cilengitide (CGT) into liposomes, and this nanoparticle can actively target gliomas under the action of magnetic field and improve cellular uptake efficiency of drugs.55 In addition, the functionalized and chemically modified metal nanoparticles play a crucial role in minimizing direct contact between metals and normal cells, as well as biological tissues. This results in fewer side effects and enhanced biocompatibility.
The Application of Metal Nanoparticles in Glioma Treatments
Chemotherapy
Malignant brain tumors grow aggressively and rapidly infiltrate into surrounding tissues.56,57 The blurred boundary between malignant tissue and health tissue limits the effectiveness of surgery. Postoperative chemotherapy is a necessary adjuvant therapy to kill residual cancer cells. BBB is an important biological structure for the brain to resist harmful substances entering the brain tissue, such as bacteria, viruses and microorganisms. It is a structure between plasma and brain tissue composed of cerebrovascular endothelial cells, basement membrane, glial cells, pericytes and microglial cells. The tight connections between endothelial cells allow small molecules in plasma to enter the brain selectively. However, macromolecule chemotherapeutic, eg vincristine, can hardly cross the BBB. Moreover, conventional chemotherapeutic drugs also have bad influence on normal tissue and cause toxic side effects. Therefore, it is challenging for these drugs to accumulate in the tumor area due to the lack of specific targeting.
Owing to their unique physical and chemical properties, noble metal nanoparticles are designed as popular nano delivery system with targeting and responsive release abilities.58,59 Au NPs are readily adjustable in their particle size, and they not only have a larger specific surface area, but also offer flexibility for surface modification. For example, Au NPs show a strong affinity for the chemical reagent mercaptan, and these nanoparticles can be modified with various ligands such as polyethylene glycol (PEG) and oligonucleotides. Enhancing the time of blood circulation and the stability of nanoparticles in the blood is advantageous.60 Ruan et al designed a delivery system based on Au NPs. Au NPs were loaded with doxorubicin (DOX) by using an acid-reactive adaptor hydrazone. Angiopep-2, a specific ligand of low-density lipoprotein receptor associated protein-1 (LRP1), was used for nanoparticle functionalization. This nano delivery system can penetrate the BBB and target glioma cells, and thus realize precise chemotherapy. In vivo experiments showed that the modified nanoparticles can effectively prolong the median survival time of glioma-bearing mice. The Au NPs can also be decorated with PEG, allowing them to overcome the obstacle of BBB and enter the brain tumor area.61 Some Au NPs are coated with epidermal growth factor (EGF) peptides to kill cancer cells specifically. This type of nanoparticle protects EGF peptides from degrading in the blood and releases them into the slightly acidic microenvironment via the EPR effect, and also could minimize the toxic and side effects.62,63 The peptide sequence THRPPMWSPVWP, which interacts with transfer ferritin receptor of the microvascular endothelial cell, was conjugated with Au NPs. This conjugation enhances the therapeutic effect.64 Albertini et al injected Au NPs coated with arginine-glycine-aspartic acid-like peptide into the brain of mice. Two hours later, they observed that the number of peptide-modified nanoparticles aggregated in glioblastoma was 1.5 times higher than that of unmodified Au NPs.65 These data demonstrate the great potential of the Au NPs delivery system in the treatment of brain tumors.
Recent advances in magnetic nanomaterials have shown promising prospects for improving MRI and treating tumors. These nanoparticles, usually in a small nanoscale, are widely used in the biomedical field. Magnetic nanoparticles (MNPs) play an important role in the targeted therapy of brain cancer. One of the most useful MNPs is the Fe3O4 nanoparticles.66 Cole et al developed magnetic iron oxide nanoparticles modified with PEG. By applying an external magnetic field to the lesion area, these nanoparticles can help chemotherapy drugs accumulate in the tumor area.67 This approach prolongs the circulation duration of nanodrugs within the body, demonstrating the efficacy of MNPs. Wu et al synthesized superparamagnetic iron oxide nanoparticles (SPION) that were coated with a silicate and carbon shell. The hydrothermal synthesis of these ferrofluid composite nanoparticles demonstrated a significant ability to inhibit the migration of glioma cells.68 EGFR or mutant EGFRVIII was highly expressed in glioblastoma. Iron oxide nanoparticles coupled with the antibody against EGFR can effectively improve the targeting effect of chemotherapy drugs.69 Overall, MNPs offers a promising platform for innovative drug delivery system and for optimizing the targeting strategies in brain tumor treatments.
To date, various QDs have been used for surface modification of nanoparticles to improve their stability, payload capacity and cellular uptake efficiency. Wahab et al found that QDs can inhibit tumor growth, reduce the invasiveness of glioma, and decrease the stemness of glioblastoma cells.70 Mansur et al developed a ZnS QD biopolymer nanodrug system based on the method of host guest chemistry. The ZnS QDs were synthesized under normal pH physiological conditions, and then these QDs were chemically modified with carboxymethylcellulose (CMC) and polysaccharide. The chemotherapy drug DOX was electrostatically bound to the QDs. This newly formed supramolecular nanostructure can serve as an active fluorescent nanoprobe and a nano carrier for chemotherapy drugs, capable of controlling drug release and killing glioma cells.71 The further modification of QDs with targeted ligands can be used in the specific detection and treatment of glioma. Magnetic iron oxide nanoparticles and QDs modified with transferrin have be utilized for the treatment of glioma.54
Radiotherapy
Radiotherapy refers to the use of one or more types of ionizing radiation to treat malignant tumors and some benign diseases, and it is an important adjunct therapy for malignant glioma.72,73 Malignant glioblastoma shows invasive growth, tends to invade the lobes and deep structures of the brain, and even invades the contralateral hemisphere through the corpus callosum. Although radiation therapy can compensate for the weakness of surgical resection, there are still a large number of patients who can not avoid cancer recurrence and metastasis after radiation therapy.74 Moreover, it has some serious side effects because normal brain tissues can also be damaged by radiation. Therefore, researchers have been focusing on developing novel nanoparticles that are less harmful to healthy tissues and can boost the effectiveness of radiotherapy.
High atomic number (Z) metal nanoparticles have the ability to enhance ionization in their surroundings when exposed to ionizing radiation. Au NPs, which are high Z metal nanoparticles, have been shown to enhance the effect of radiotherapy in gliomas.75 Several mechanisms are involved in the sensitization effect of Au NPs in radiotherapy. Radiation can generate low-energy secondary electrons, trigger free radical effects, and cause indirect damage to DNA by assaulting its bases, demolishing ribose, and degrading oligonucleotide.76,77 In addition, radiation has the potential to harm biological membranes and proteins via lipid peroxidation. The Au NPs, characterized by high electron density, can effectively enhance the average cross section between tissue and radiation when they accumulate in tumor tissue to a certain extent. This accumulation improves the transfer and deposition of the physical dose of radiation during irradiation.
Au NPs are widely used in low dose radiotherapy because of their unique optical properties, good biocompatibility and excellent radiation sensitization. They can convert light energy into heat energy, showing a photothermal effect. This effect further enhances the efficacy of radiotherapy for gliomas. Kefayat et al designed a gold nanocluster modified with folic acid and bovine serum protein (BSA), named FA-AuNCs. Compared with traditional gold nanoparticles, these nanoparticles can specifically target C6 glioma cells and enhance the sensitivity of radiotherapy.78 In addition, gold nanoparticles can effectively overcome the obstacle of BBB.79 Ultra-small gold nanoparticles coated with gadolinium chelates (Au@DTDTPA-Gd) were injected intravenously into mice, allowing for accurate tumor location to be displayed via MRI. This technique can be beneficial for targeted radiotherapy. Studies have shown that it significantly extends the lifespan of tumor-bearing rats.80
Previous studies have demonstrated that Ag NPs possess a strong radio-sensitization ability when used in the treatment of U251 glioma cells.81 The mechanism involves promoting the apoptosis of tumor cells and increasing the level of autophagy. By employing Ag NPs, the radiation resistance of tumor tissue in hypoxic environment can be successfully decreased.33,82 Xu et al tested Ag NPs at concentration of one-tenth of the half maximal inhibitory concentration to target tumor cell, and found that Ag NPs, with a size of 20 nm, could significantly enhance the radiosensitivity of U251 glioma cells to X-rays without causing any toxic effects. These results indicate that Ag NPs also have the potential to be used in the sensitization of radiotherapy in gliomas.83
Magnetic iron oxide nanoparticles have also been used in the treatment of recurrent glioblastoma. Magnetic iron oxide nanoparticles were infused into glioblastomas by the neuro navigation technique, and the nanoparticles were then heated in an alternating magnetic field, producing thermal energy. In conjunction with stereotactic radiation techniques, the tumors were irradiated with a dose of 10 Gy per week. The results showed a significant extension in the overall survival of patients with recurrent glioblastoma.84 During the operation of fourteen cases of glioblastoma, aminosilane-coated iron oxide nanoparticles were injected into the tumor cavity under the guidance of 3D imaging technology, and then all patients received a single radiotherapy. The results showed that all patients had good tolerance to magnetic nanoparticles without any side effects. Using magnetic iron oxide nanoparticles for intracranial radiotherapy can further improve the sensitivity of radiotherapy.85 In addition, Grauer et al coated two layers of superparamagnetic iron oxide nanoparticles on the wall of the tumor cavity after resection of the tumor in recurrent glioblastoma patients. Supplemented with heat and radiation therapy, there was persistent necrosis near the tumor tissue in the nanoparticle aggregation area. Immunohistochemistry showed that the number of macrophages was increased, the expression of caspase-3 and heat shock protein 70 was up-regulated. Intratumoral hyperthermia combined with radiotherapy could cause obvious inflammatory responses around the tumor, which further stimulated the anti-tumor immune response.86 Overall, magnetic iron oxide nanoparticles play a significant role in enhancing immunoinflammation in the treatment of recurrent glioblastoma.
Photothermal Therapy
Photothermal therapy is a treatment method that utilizes materials with high optical energy conversion efficiency, combined with targeted recognition technology, to accumulate drugs near tumor tissues. Optical energy is converted into heat energy to kill cancer cells under certain conditions. Metal nanomaterials have emerged as excellent carriers for photothermal therapy, thanks to their distinctive optical properties, superior biological inertness, and local surface plasmon resonance (LSPR). Numerous metal materials, including gold and silver nanoparticles, generate a certain amount of heat upon light exposure, effectively killing tumor cells. Therefore, photothermal therapy is often combined with radiotherapy, chemotherapy, and surgery to improve anti-tumor effects.87 Ordinary gold nanoparticles possess a weak absorption capacity within the tissue penetrating NIR window, leading to the low efficiency of photothermal therapy. Wang et al discovered that fiber nanostructures assembled from positively charged spherical gold nanoparticles and negatively charged silk fibroin can improve the photothermal effect within the NIR window.88 They synthesized spiropyrans and imidazole polymer-functionalized nanoparticles by surface polymerization technology. Photo-responsive polymer gold nanoparticles were obtained by reducing gold ions to the surface of the nanoparticles. The surface of the gold nanoparticles can also be modified with biotin to specifically target brain tumor cells. The nanoparticles showed potential benefits for enhancing photothermal and photodynamic therapies. In addition, they can be combined with certain specific antibodies to create functional nanoprobes.89
Another metal nanomaterial, iron oxide, can also be used as thermos-sensitive nanoparticles for the treatment of tumors.47 Lu et al used Fe3O4@Au magnetic nanoparticles as the core shell, loaded with cetuximab to target glioma cells for photothermal therapy (Figure 4). These multifunctional nanomaterials can induce local plasma heating under the irradiation of NIR, which are less affected by the biological metabolism of tissues and cells. Moreover, metal oxide nanoparticles can also be utilized as photosensitizers in tumor photodynamic therapy. For example, the functionalized TiO2 nanoparticles can be used in photodynamic therapy, and it is non-toxic and harmless in the absence of light irradiation and has a long blood circulation time in the body. However, using ultraviolet or visible light to directly irradiate tissues to trigger TiO2 nanoparticles have some limitations. The tissue penetration depth of the light source at this wavelength is limited, and most tumor cells cannot be accurately targeted.90 NIR, with the wavelength of 700 nm to 1000 nm, possesses the ability to infiltrate tumor tissues, thereby enhancing the efficacy of photodynamic therapy. Shigeru et al synthesized TiO2-PEG nanoparticles by adsorbing PEG onto the surface of TiO2. The TiO2-PEG showed significantly inhibition effects on the growth of glioma cells.86 Photodynamic therapy using metal nanomaterials has increasingly emerged as a novel approach for the treatment of gliomas.
 |
Figure 4 Magnetic oxide nanoparticles accumulate in the tumor region under the action of a magnetic field, generating hyperthermia and toxic ROS when exposed to NIR laser, and thereby killing tumor cells. Note: Reprinted from Xue P, Yang R, Sun L, et al. Indocyanine Green-Conjugated Magnetic Prussian Blue Nanoparticles for Synchronous Photothermal/Photodynamic Tumor Therapy. Nanomicro Lett. 2018;10(4):74.47 |
Metal Nanoparticles for Enhancing Biocompatibility and Reducing Toxicity
The toxicity of metal nanoparticles in glioma cells has gained significant attention due to their potential in targeted cancer therapies, where they can selectively deliver drugs or therapeutic agents directly to tumor sites. However, concerns about their biocompatibility, long-term stability, and potential bioaccumulation in healthy tissues persist, raising important questions about the balance between therapeutic efficacy and safety.91 A deeper understanding of the mechanisms behind nanoparticle toxicity in glioma is essential for developing safer, more effective treatments that minimize adverse effects on normal cells and organs.
To reduce the toxicity of metal nanoparticles in glioma therapy, several solutions have been proposed. First, surface modification techniques are key to minimizing toxicity. By coating the nanoparticles with biocompatible materials such as PEG or chitosan, their stability can be improved, and interactions with normal cells can be reduced, thereby minimizing damage to healthy tissues.92,93 Second, controlling the size and morphology of the nanoparticles is an effective approach. Smaller nanoparticles can more easily penetrate the BBB, but excessively small particles may cause higher cytotoxicity.94 Therefore, optimizing the size and shape of the nanoparticles is crucial to balance therapeutic efficacy and safety.95 In addition, the development of targeted delivery systems allows metal nanoparticles to specifically target glioma cells, reducing the impact on surrounding normal tissues.96 Finally, adjusting the dosage and delivery timing can further minimize toxicity while enhancing therapeutic effects. The combination of these strategies provides a safer outlook for the clinical application of metal nanoparticles in glioma therapy.
Conclusions and Future Research
New treatment paradigm are urgently needed as the 5-year survival rate of GBM patients is still low. Recent advancements in nanomaterials have bought huge progress in the field of biology and medicine. In this paper, we summarized the properties of different types of metal nanomaterials, highlighting their advantages in the treatment and detection of gliomas. More importantly, we reviewed the potential applications of metal nanoparticles in glioma imaging, radiotherapy, chemotherapy, and photothermal therapy. Thanks to the excellent properties of metal nanoparticles, such as good electrical properties, optical properties, and magnetism, noble metal nanoparticles, metal oxide nanoparticles and metal quantum dots have been used to load chemotherapy drugs (eg, DOX, EGFR antibody), sensitize radiotherapy, and improve the photothermal effects in the treatment of gliomas. As shown in Table 1, a number of clinical trials are currently assessing the effectiveness and safety of nanodrugs in cancer treatment, detection, and precise targeted therapy. However, metal nanoparticles have not yet been utilized in clinical settings for the detection or treatment of gliomas. Nano-drugs face multiple challenges in entering clinical treatment for tumors, including complex preparation, high cost, poor stability, and significant toxic side effects. Ongoing research continues to push the boundaries of what’s possible, but translating these innovations into practical, safe, and effective therapies is a complex process.
To address current knowledge gaps in metal nanoparticle research, future studies should employ a range of methodologies. Longitudinal studies are crucial for assessing long-term effects, such as stability, toxicity, and tissue accumulation, while also helping to define safe dosage limits. In vivo models that closely replicate human physiology can provide valuable data on therapeutic efficacy and biocompatibility, highlighting interactions with biological systems and patterns of biodistribution. High-throughput screening can rapidly optimize nanoparticle formulations, refining key parameters like size, surface charge, and functionalization to maximize efficacy and minimize toxicity. Additionally, analyzing molecular pathways influenced by metal nanoparticles—such as those involved in inflammation and apoptosis—can help improve both design specificity and therapeutic outcomes. Advanced imaging techniques, such as real-time fluorescence imaging and MRI, can complement these efforts by enabling detailed tracking of biodistribution and cellular uptake. Together, these approaches offer a comprehensive framework for enhancing our understanding of metal nanoparticle behavior and guiding the safe, effective development of nanoparticle-based therapies in gliomas.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82072796 and No. 82072763) and the Social Development Project of Xuzhou (No. KC22242 and KC22103), Jiangsu Provincial Medical Key Discipline (No. ZDXK202228).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Wirsching H-G, Galanis E, Weller M. Glioblastoma. Handb Clin Neurol. 2016;134:381–397.
2. Zhao G, Jia J, Wang LS, et al. Local Delivery of Minocycline and Vorinostat Targets the Tumor Microenvironment to Inhibit the Recurrence of Glioma. Oncol Targets Ther. 2020;13:11397–11409. doi:10.2147/OTT.S273527
3. Gao X, Yue Q, Liu Z, et al. Guiding Brain-Tumor Surgery via Blood-Brain-Barrier-Permeable Gold Nanoprobes with Acid-Triggered MRI/SERRS Signals. Adv Mater. 2017;29. doi:10.1002/adma.201603917
4. Focusing on brain tumours and brain metastasis. Nat Rev Cancer. 2020;20:1. doi:10.1038/s41568-019-0232-7
5. Nayak L, Reardon DA. High-grade Gliomas. Continuum. 2017;23:1548–1563. doi:10.1212/CON.0000000000000554
6. Lapointe S, Perry A, Butowski NA. Butowski, Primary brain tumours in adults. Lancet. 2018;392:432–446. doi:10.1016/S0140-6736(18)30990-5
7. Carpentier AF. Neuro-oncology: the growing role of chemotherapy in glioma. Lancet Neurol. 2005;4:4–5. doi:10.1016/S1474-4422(04)00944-5
8. Aldape K, Brindle KM, Chesler L, et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019;16:509–520. doi:10.1038/s41571-019-0177-5
9. Knisely J, Schulder M. Radiation plus Chemotherapy in Low-Grade Glioma. New Engl J Med. 2016;375:490.
10. Chen JJ, Zhu YF, Wu CT, et al. Nanoplatform-based cascade engineering for cancer therapy. Chem Soc Rev. 2020;49:9057–9094. doi:10.1039/d0cs00607f
11. Li Y, Baiyang L, Leran B, et al. Reduction-responsive PEtOz-SS-PCL micelle with tailored size to overcome blood–brain barrier and enhance doxorubicin antiglioma effect. Drug Delivery. 2017;24:1782–1790. doi:10.1080/10717544.2017.1402218
12. Li Y, Zhang H. Nanoparticle-Based Drug Delivery Systems for Enhanced Tumor-Targeting Treatment. J Biomed Nanotech. 2019;15:1–27. doi:10.1166/jbn.2019.2670
13. Barenholz Y. Doxil® — the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160:117–134. doi:10.1016/j.jconrel.2012.03.020
14. Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi:10.1021/nn900002m
15. Sharma H, Mishra PK, Talegaonkar S, et al. Metal nanoparticles: a theranostic nanotool against cancer. Drug Discovery Today. 2015;20:1143–1151. doi:10.1016/j.drudis.2015.05.009
16. Gallo J, Long NJ, Aboagye EO. Magnetic nanoparticles as contrast agents in the diagnosis and treatment of cancer. Chem Soc Rev. 2013;42:7816. doi:10.1039/c3cs60149h
17. Butterworth KT, Wyer JA, Brennan-Fournet M, et al. Variation of Strand Break Yield for Plasmid DNA Irradiated with High-ZMetal Nanoparticles. Radiation Rese. 2008;170:381–387. doi:10.1667/RR1320.1
18. Yaqoob, R SB, Adnan R, Rameez Khan M, et al. Gold, Silver, and Palladium Nanoparticles: a Chemical Tool for Biomedical Applications. Front Chem. 2020;(8).
19. Pedrosa RC, Felipe KB, Wilhelm Filho D. Editorial: oncogenic PI3KT/Akt/mTOR pathway alterations, ROS homeostasis, targeted cancer therapy and drug resistance. Front Oncol. 2024;14:1372376. doi:10.3389/fonc.2024.1372376
20. Ismail M, Wang Y, Li Y, et al. Stimuli-Responsive Polymeric Nanocarriers Accelerate On-Demand Drug Release to Combat Glioblastoma. Biomacromolecules. 2024;25:6250–6282. doi:10.1021/acs.biomac.4c00722
21. Ismail M, Yang W, Li Y, et al. Targeted liposomes for combined delivery of artesunate and temozolomide to resistant glioblastoma. Biomaterials. 2022;287:121608. doi:10.1016/j.biomaterials.2022.121608
22. Rehman FU, Liu Y, Yang Q, et al. Heme Oxygenase-1 targeting exosomes for temozolomide resistant glioblastoma synergistic therapy. J Control Release. 2022;345:696–708. doi:10.1016/j.jconrel.2022.03.036
23. Muhammad P, Hanif S, Li JY, et al. Carbon dots supported single Fe atom nanozyme for drug-resistant glioblastoma therapy by activating autophagy-lysosome pathway. Nano Today. 45(101530).
24. Yu Y, Wang A, Wang S, et al. Efficacy of Temozolomide-Conjugated Gold Nanoparticle Photothermal Therapy of Drug-Resistant Glioblastoma and Its Mechanism Study. Mol Pharmaceut. 2022;19:1219–1229. doi:10.1021/acs.molpharmaceut.2c00083
25. Yan J, Hanif S, Zhang D, et al. Arsenic Prodrug-Mediated Tumor Microenvironment Modulation Platform for Synergetic Glioblastoma Therapy. ACS Appl Mater Interfaces. 2022;14:36487–36502. doi:10.1021/acsami.2c12076
26. Zhang C, Yan L, Gu Z, et al. Strategies based on metal-based nanoparticles for hypoxic-tumor radiotherapy. Chem. Sci. 2019;10:6932–6943. doi:10.1039/C9SC02107H
27. Popovtzer R, Agrawal A, Kotov NA, et al. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008;8:4593–4596. doi:10.1021/nl8029114
28. Meir R, Shamalov K, Betzer O, et al. Nanomedicine for Cancer Immunotherapy: tracking Cancer-Specific T-Cells in Vivo with Gold Nanoparticles and CT Imaging. ACS Nano. 2015;9:6363–6372. doi:10.1021/acsnano.5b01939
29. Zhao Z, Xu H, Li S, et al. Hypoxic Radiosensitizer-Lipid Coated Gold Nanoparticles Enhance the Effects of Radiation Therapy on Tumor Growth. J Biomed Nanotech. 2019;15:1982–1993. doi:10.1166/jbn.2019.2830
30. Hu M, Chen J, Li Z-Y, et al.Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem Soc Rev. 2006;(35):1084. doi:10.1039/b517615h
31. Xie Y, Han Y, Zhang X, et al. Application of New Radiosensitizer Based on Nano-Biotechnology in the Treatment of Glioma. Front Oncol. 2021;(11).
32. Song G, Cheng L, Chao Y, et al. Emerging Nanotechnology and Advanced Materials for Cancer Radiation Therapy. Adv. Mater. 2017;29. doi:10.1002/adma.201700996
33. Liu Z, Tan H, Zhang X, et al. Enhancement of radiotherapy efficacy by silver nanoparticles in hypoxic glioma cells. Artif Cells Nanomed Biotech. 2018;46:922–930. doi:10.1080/21691401.2018.1518912
34. Pinel S, Thomas N, Boura C, et al. Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment. Adv Drug Delivery Rev. 2019;138:344–357. doi:10.1016/j.addr.2018.10.013
35. Heidari Majd M, Barar J, Asgari D, et al. Targeted fluoromagnetic nanoparticles for imaging of breast cancer mcf-7 cells. Adv Pharm Bull. 2013;3:189–195. doi:10.5681/apb.2013.031
36. Gupta R, Sharma D. Biofunctionalization of magnetite nanoparticles with stevioside: effect on the size and thermal behaviour for use in hyperthermia applications. Int j Hyperthermia. 2019;36:301–311. doi:10.1080/02656736.2019.1565787
37. Gu H, Xu K, Yang Z, et al. Synthesis and cellular uptake of porphyrin decorated iron oxide nanoparticles—a potential candidate for bimodal anticancer therapy. Chem Commun. 2005. 4270. doi:10.1039/b507779f
38. Wang C, Hsu C-H, Li Z, et al. Effective heating of magnetic nanoparticle aggregates for in vivo nano-theranostic hyperthermia. Int j Nanomed. 2017;12:6273–6287. doi:10.2147/IJN.S141072
39. Tong S, Zhu H, Bao G. Magnetic iron oxide nanoparticles for disease detection and therapy. Mater Today. 2019;31:86–99. doi:10.1016/j.mattod.2019.06.003
40. Kalyane D, Raval N, Maheshwari R, et al. Employment of enhanced permeability and retention effect (EPR): nanoparticle-based precision tools for targeting of therapeutic and diagnostic agent in cancer. Mater Sci Eng C Mater Biol Appl. 2019;98:1252–1276. doi:10.1016/j.msec.2019.01.066
41. Liu J, Zheng X, Yan L, et al. Bismuth sulfide nanorods as a precision nanomedicine for in vivo multimodal imaging-guided photothermal therapy of tumor. ACS Nano. 2015;9:696–707. doi:10.1021/nn506137n
42. Cui L, Li -C-C, Tang B, et al. Advances in the integration of quantum dots with various nanomaterials for biomedical and environmental applications. Analyst. 2018;143:2469–2478. doi:10.1039/C8AN00222C
43. Matea CT, Mocan T, Tabaran F, et al. Quantum dots in imaging, drug delivery and sensor applications. Int j Nanomed. 2017;12:5421–5431. doi:10.2147/IJN.S138624
44. Zamberlan F, Turyanska L, Patanè A, et al. Stable DHLA-PEG capped PbS quantum dots: from synthesis to near-infrared biomedical imaging. J Mater Chem B. 2018;6:550–555. doi:10.1039/C7TB02912H
45. Gao X, Cui Y, Levenson RM, et al. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–976. doi:10.1038/nbt994
46. Li S, Xu Q, Zhao L, et al. Angiopep-2 Modified Cationic Lipid-Poly-Lactic-Co-Glycolic Acid Delivery Temozolomide and DNA Repair Inhibitor Dbait to Achieve Synergetic Chemo-Radiotherapy Against Glioma. J Nanosci Nanotechnol. 2019;19:7539–7545. doi:10.1166/jnn.2019.16775
47. Xue P, Yang R, Sun L, et al. Indocyanine Green-Conjugated Magnetic Prussian Blue Nanoparticles for Synchronous Photothermal/Photodynamic Tumor Therapy. Nanomicro Lett. 2018;10:74. doi:10.1007/s40820-018-0227-z
48. Tian J, Yan C, Liu K, et al. Paclitaxel-Loaded Magnetic Nanoparticles: synthesis, Characterization, and Application in Targeting. J Pharm Sci. 2017;106:2115–2122. doi:10.1016/j.xphs.2017.04.023
49. Liu H, Xie Y, Zhang Y, et al. Development of a hypoxia-triggered and hypoxic radiosensitized liposome as a doxorubicin carrier to promote synergetic chemo-/radio-therapy for glioma. Biomaterials. 2017;121:130–143. doi:10.1016/j.biomaterials.2017.01.001
50. Liu H, Li Y, Mozhi A, et al. SiRNA-phospholipid conjugates for gene and drug delivery in cancer treatment. Biomaterials. 2014;35:6519–6533. doi:10.1016/j.biomaterials.2014.04.033
51. Yu Z, Wang M, Pan W, et al. Tumor microenvironment-triggered fabrication of gold nanomachines for tumor-specific photoacoustic imaging and photothermal therapy. Chem. Sci. 2017;8:4896–4903. doi:10.1039/C7SC00700K
52. Lai J, Xu Y, Mu X, et al. Light-triggered covalent assembly of gold nanoparticles in aqueous solution. Chem Commun. 2011;47:3822–3824. doi:10.1039/c0cc03361h
53. Ye C, Pan B, Xu H, et al. Co-delivery of GOLPH3 siRNA and gefitinib by cationic lipid-PLGA nanoparticles improves EGFR-targeted therapy for glioma. J Mol Med. 2019;97:1575–1588. doi:10.1007/s00109-019-01843-4
54. Ag Seleci D, Maurer V, Barlas FB, et al. Transferrin-Decorated Niosomes with Integrated InP/ZnS Quantum Dots and Magnetic Iron Oxide Nanoparticles: dual Targeting and Imaging of Glioma. Int J Mol Sci. 2021;22:4556. doi:10.3390/ijms22094556
55. Xu H-L, Yang -J-J, ZhuGe D-L, et al. Glioma-Targeted Delivery of a Theranostic Liposome Integrated with Quantum Dots, Superparamagnetic Iron Oxide, and Cilengitide for Dual-Imaging Guiding Cancer Surgery. Adv Healthc Mater. 2018;7:e1701130. doi:10.1002/adhm.201701130
56. Liu H, Qiao C, Yang J, et al. Self-assembling doxorubicin-prodrug nanoparticles as siRNA drug delivery system for cancer treatment: in vitro and in vivo. J Mater Chem B. 2014;2:5910–5924. doi:10.1039/C4TB00814F
57. Shi H, Sun S, Xu H, et al. Combined Delivery of Temozolomide and siPLK1 Using Targeted Nanoparticles to Enhance Temozolomide Sensitivity in Glioma. Int j Nanomed. 2020;15:3347–3362. doi:10.2147/IJN.S243878
58. Mao H, Xie Y, Ju H, et al. Design of Tumor Microenvironment-Responsive Drug-Drug Micelle for Cancer Radiochemotherapy. ACS Appl Mater Interfaces. 2018;10:33923–33935. doi:10.1021/acsami.8b11159
59. Xu H, Han Y, Zhao G, et al. Hypoxia-Responsive Lipid-Polymer Nanoparticle-Combined Imaging-Guided Surgery and Multitherapy Strategies for Glioma. ACS Appl Mater Interfaces. 2020;12:52319–52328. doi:10.1021/acsami.0c12971
60. Hyafil F, Cornily J-C, Feig JE, et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007;13:636–641. doi:10.1038/nm1571
61. Ruan S, Yuan M, Zhang L, et al. Tumor microenvironment sensitive doxorubicin delivery and release to glioma using angiopep-2 decorated gold nanoparticles. Biomaterials. 2015;37:425–435. doi:10.1016/j.biomaterials.2014.10.007
62. Cheng Y, Dai Q, Morshed RA, et al. Blood-brain barrier permeable gold nanoparticles: an efficient delivery platform for enhanced malignant glioma therapy and imaging. Small. 2014;10:5137–5150. doi:10.1002/smll.201400654
63. Feng Q, Shen Y, Fu Y, et al. Self-Assembly of Gold Nanoparticles Shows Microenvironment-Mediated Dynamic Switching and Enhanced Brain Tumor Targeting. Theranostics. 2017;7:1875–1889. doi:10.7150/thno.18985
64. Prades R, Guerrero S, Araya E, et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials. 2012;33:7194–7205. doi:10.1016/j.biomaterials.2012.06.063
65. Albertini B, Mathieu V, Iraci N, et al. Tumor Targeting by Peptide-Decorated Gold Nanoparticles. Mol Pharmaceut. 2019;16:2430–2444. doi:10.1021/acs.molpharmaceut.9b00047
66. Zhu L, Zhou Z, Mao H, et al. Magnetic nanoparticles for precision oncology: theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine. 2017;12:73–87. doi:10.2217/nnm-2016-0316
67. Cole AJ, David AE, Wang J, et al. Magnetic brain tumor targeting and biodistribution of long-circulating PEG-modified, cross-linked starch-coated iron oxide nanoparticles. Biomaterials. 2011;32:6291–6301. doi:10.1016/j.biomaterials.2011.05.024
68. Wu VM, Huynh E, Tang S, et al. Brain and bone cancer targeting by a ferrofluid composed of superparamagnetic iron-oxide/silica/carbon nanoparticles (earthicles. Acta Biomater. 2019;88:422–447. doi:10.1016/j.actbio.2019.01.064
69. Hadjipanayis CG, Machaidze R, Kaluzova M, et al. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70:6303–6312. doi:10.1158/0008-5472.CAN-10-1022
70. Wahab R, Kaushik N, Khan F, et al. Gold quantum dots impair the tumorigenic potential of glioma stem-like cells via β-catenin downregulation in vitro. Int j Nanomed. 2019;14:1131–1148. doi:10.2147/IJN.S195333
71. Mansur AAP, Caires AJ, Carvalho SM, et al. Dual-functional supramolecular nanohybrids of quantum dot/biopolymer/chemotherapeutic drug for bioimaging and killing brain cancer cells in vitro. Colloids Surf B Biointerf. 2019;184:110507. doi:10.1016/j.colsurfb.2019.110507
72. Hua L, Wang Z, Zhao L, et al. Hypoxia-responsive lipid-poly-(hypoxic radiosensitized polyprodrug) nanoparticles for glioma chemo- and radiotherapy. Theranostics. 2018;8:5088–5105. doi:10.7150/thno.26225
73. Zong Z, Hua L, Wang Z, et al. Self-assembled angiopep-2 modified lipid-poly (hypoxic radiosensitized polyprodrug) nanoparticles delivery TMZ for glioma synergistic TMZ and RT therapy. Drug Delivery. 2019;26:34–44. doi:10.1080/10717544.2018.1534897
74. Liu H, Cai Y. Development of a Hypoxic Radiosensitizer-Prodrug Liposome Delivery DNA Repair Inhibitor Dbait Combination with Radiotherapy for Glioma Therapy. Adv Healthc Mater. 2017;(6).
75. Choi J, Kim G, Cho SB, et al. Radiosensitizing high-Z metal nanoparticles for enhanced radiotherapy of glioblastoma multiforme. J Nanobiotechnology. 2020;18:122. doi:10.1186/s12951-020-00684-5
76. Tang Y, Huang L, Huang L, et al. In vitro cytotoxicity of gold nanorods in A549 cells. Environ Toxicol Pharmacol. 2015;39:871–878. doi:10.1016/j.etap.2015.02.003
77. Mateo D, Morales P, Ávalos A, et al. Oxidative stress contributes to gold nanoparticle-induced cytotoxicity in human tumor cells. Toxicol Mech Methods. 2014;24:161–172. doi:10.3109/15376516.2013.869783
78. Kefayat A, Ghahremani F, Motaghi H, et al. Ultra-small but ultra-effective: folic acid-targeted gold nanoclusters for enhancement of intracranial glioma tumors’ radiation therapy efficacy. Nanomedicine. 2019;16:173–184. doi:10.1016/j.nano.2018.12.007
79. Gao N, Sun H, Dong K, et al. Gold-nanoparticle-based multifunctional amyloid-β inhibitor against Alzheimer’s disease. Chemistry. 2015;21:829–835. doi:10.1002/chem.201404562
80. Miladi I, Alric C, Dufort S, et al. The In Vivo Radiosensitizing Effect of Gold Nanoparticles Based MRI Contrast Agents. Small. 2014;10:1116–1124. doi:10.1002/smll.201302303
81. Liu P, Jin H, Guo Z, et al. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. Int j Nanomed. 2016;11:5003–5014. doi:10.2147/IJN.S115473
82. Zhang X, Liu Z, Lou Z, et al. Radiosensitivity enhancement of Fe3O4@Ag nanoparticles on human glioblastoma cells. Artif Cells Nanomed Biotech. 2018;46:975–984. doi:10.1080/21691401.2018.1439843
83. Xu R, Ma J, Sun X, et al. Ag nanoparticles sensitize IR-induced killing of cancer cells. Cell Res. 2009;19:1031–1034. doi:10.1038/cr.2009.89
84. Maier-Hauff K, Ulrich F, Nestler D, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103:317–324. doi:10.1007/s11060-010-0389-0
85. Maier-Hauff K, Rothe R, Scholz R, et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: results of a feasibility study on patients with glioblastoma multiforme. J Neurooncol. 2007;81:53–60. doi:10.1007/s11060-006-9195-0
86. Grauer O, Jaber M, Hess K, et al. Combined intracavitary thermotherapy with iron oxide nanoparticles and radiotherapy as local treatment modality in recurrent glioblastoma patients. J Neurooncol. 2019;141:83–94. doi:10.1007/s11060-018-03005-x
87. Li X, Lovell JF, Yoon J, et al. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17:657–674. doi:10.1038/s41571-020-0410-2
88. Yin J, Xie M, Wang J, et al. Gold-Nanoparticle-Mediated Assembly of High-Order DNA Nano-Architectures. Small. 2022;18:e2200824. doi:10.1002/smll.202200824
89. Zhi D, Yang T, O’Hagan J, et al. Photothermal therapy. J Control Release. 2020;325:52–71. doi:10.1016/j.jconrel.2020.06.032
90. Loosli F, Le Coustumer P, Stoll S. TiO2 nanoparticles aggregation and disaggregation in presence of alginate and Suwannee River humic acids. pH and concentration effects on nanoparticle stability. Water Res. 2013;47:6052–6063. doi:10.1016/j.watres.2013.07.021
91. Medici S, Peana M, Pelucelli A, et al. An updated overview on metal nanoparticles toxicity. Semin Cancer Biol. 2021;76:17–26. doi:10.1016/j.semcancer.2021.06.020
92. Djurišić AB, Leung YH, Ng AMC, et al. Toxicity of metal oxide nanoparticles: mechanisms, characterization, and avoiding experimental artefacts. Small. 2015;11:26–44. doi:10.1002/smll.201303947
93. Li B, Chen X, Qiu W, et al. Synchronous Disintegration of Ferroptosis Defense Axis via Engineered Exosome-Conjugated Magnetic Nanoparticles for Glioblastoma Therapy. Adv Sci. 2022;9:e2105451. doi:10.1002/advs.202105451
94. Karathanasis E, Ghaghada KB. Crossing the barrier: treatment of brain tumors using nanochain particles. Wiley Interdiscip Rev Nanomed Nanobiotech. 2016;8:678–695. doi:10.1002/wnan.1387
95. Vikram SK, Ali J, Ali J, et al. Potential of Nanocarrier-Associated Approaches for Better Therapeutic Intervention in the Management of Glioblastoma. Assay Drug Dev Technol. 2024;22:73–85. doi:10.1089/adt.2023.073
96. Naveed M, Mahmood S, Aziz T, et al. Green-synthesis of silver nanoparticles AgNPs from Podocarpus macrophyllus for targeting GBM and LGG brain cancers via NOTCH2 gene interactions. Sci Rep. 2024;14:25489. doi:10.1038/s41598-024-75820-4
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

