Back to Journals » International Journal of Nanomedicine » Volume 19
Application of Nanomaterials Targeting Immune Cells in the Treatment of Chronic Inflammation
Authors Ci Z, Wang H, Luo J, Wei C, Chen J, Wang D, Zhou Y
Received 12 October 2024
Accepted for publication 10 December 2024
Published 25 December 2024 Volume 2024:19 Pages 13925—13946
DOI https://doi.org/10.2147/IJN.S497590
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Zhen Ci,1,2,* Hanchi Wang,1,2,* Jiaxin Luo,1,2 Chuqiao Wei,1,2 Jingxia Chen,1,2 Dongyang Wang,2,3 Yanmin Zhou1,2
1Department of Oral Implantology, Hospital of Stomatology, Jilin University, Changchun, 130021, People’s Republic of China; 2Jilin Provincial Key Laboratory of Tooth Development and Bone Remodeling, Hospital of Stomatology, Jilin University, Changchun, 130021, People’s Republic of China; 3Department of Oral Biology, Hospital of Stomatology, Jilin University, Changchun, 130021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yanmin Zhou, Department of Oral Implantology, Hospital of Stomatology, Jilin University, Changchun, 130021, People’s Republic of China, Email [email protected] Dongyang Wang, Department of Oral Biology, Hospital of Stomatology, Jilin University, Changchun, 130021, People’s Republic of China, Email [email protected]
Abstract: Chronic inflammation is a common characteristic of all kinds of diseases, including autoimmune diseases, metabolic diseases, and tumors. It is distinguished by the presence of low concentrations of inflammatory factors stimulating the body for an extended period, resulting in a persistent state of infection. This condition is manifested by the aggregation and infiltration of mononuclear cells, lymphocytes, and other immune cells, leading to tissue hyperplasia and lesions. Although various anti-inflammatory medications, including glucocorticoids and non-steroidal anti-inflammatory drugs (NSAIDs), have shown strong therapeutic effects, they lack specificity and targeting ability, and require high dosages, which can lead to severe adverse reactions. Nanoparticle drug delivery mechanisms possess the capacity to minimize the effect on healthy cells or tissues due to their targeting capabilities and sustained drug release properties. However, most nanosystems can only target the inflammatory sites rather than specific types of immune cells, leaving room for further improvement in the therapeutic effects of nanomaterials. This article reviews the current research progress on the role of diverse immune cells in inflammation, focusing on the functions of neutrophils and macrophages during inflammation. It provides an overview of the domestic and international applications of nanomaterials in targeted therapy for inflammation, aiming to establish a conceptual foundation for the utilization of nanomaterials targeting immune cells in the treatment of chronic inflammation and offer new perspectives for the avoidance and management of inflammation.
Keywords: nanomaterials, periodontitis, chronic inflammation, target therapy, neutrophil, macrophage
Graphical Abstract:

Introduction
Inflammation is a protective reaction of the immune system to external stimuli, which maintains tissue homeostasis under various harmful conditions.1 It is an internal defense reaction induced by immune cells and cytokines, primarily triggered by specific pattern recognition receptors expressed on myeloid-derived cells, like neutrophils, monocytes, macrophages, and dendritic cells.2 The purpose of inflammation is to repair damaged tissues and eliminate various injurious factors, including pathogens. Any factor that leads to cell or tissue damage can potentially cause inflammation.3 The general process of inflammation is as follows: danger signals are released from damaged cells or pathogens, activating surrounding immune cells, which then release inflammatory mediators to recruit more immune cells to the inflammatory site. Subsequently, white blood cell migration occurs, resulting in the accumulation of neutrophils at the inflammatory site, releasing tumor necrosis factor-α (TNF) to increase vascular permeability. Monocytes release pro-inflammatory cytokines, further promoting the inflammatory response. Immune cells clear dead cells and inflammatory factors through phagocytosis or immune responses. Finally, anti-inflammatory cytokines, including interleukin-10 (IL-10), are discharged, inducing tissue regeneration, leading to the alleviation of the inflammatory response and eventual recovery. Inflammatory reactions can lead to temporary reductions in tissue function. When the inflammatory response is excessive or persistent, it promotes the development of numerous chronic ailments, leading to pathological changes and severe tissue damage, which may result in the progression of different chronic diseases, such as periodontitis, pneumonia, rheumatoid arthritis, cardiovascular diseases, malignant tumors, diabetes, chronic kidney disease, and autoimmune diseases.4,5 However, traditional anti-inflammatory drugs have issues such as poor permeability, rapid degradation, low bioavailability, and poor targeting ability, and may cause irreversible damage to normal tissues or organs. Therefore, there is a need to improve the targeted delivery of drugs and reduce their toxic side effects. Nanomaterials possess advantages such as large surface area, high drug loading capacity, strong drug stability, prolonged drug retention time at the inflammatory site, and reduced drug side effects. With the continuous development and innovation in the field of nanotechnology, nanomaterials have shown potential applications in multiple fields. Nanomaterials can not only improve bioavailability and enhance drug efficacy6 but also increase drug targeting by targeting specific immune cells, making them increasingly applied in the therapy of inflammation, infection and other diseases. The modulation of cytokine functions has been proposed to treat chronic inflammatory diseases, and immunotherapy that regulates host immunity has also shown promising application prospects in the treatment of infectious inflammation.7 Recently, many nanomaterials have been engineered to modulate the roles of immune cells and inflammation-associated cytokines to diminish inflammation, and these nanosystems have demonstrated remarkable therapeutic outcomes both in vitro and in vivo.
 |
Table 1 Macrophage-Targeted Nanomaterials |
Immune Cells in Inflammation
Innate immune cells, neutrophil (NET), macrophages, dendritic cells (DC), mast cells (MC), and adaptive immune cells, including thymus-derived lymphocytes (T cells), bone marrow-derived lymphocytes (B cells), and plasma cells, interact with tissues to regulate inflammatory responses and host immune modulation processes, influencing the development of inflammation. When pathogens invade human tissues, the innate immune response is activated.8 Innate immune cells produce cytokines and chemokines that directly regulate tissue metabolism: DCs generate cytokines and interact with immune cells, while NK cells express M-CSF and release histamine, platelet-activating factors, and other factors and mediators along with MCs, thereby modulating inflammatory responses.9 Similarly, adaptive immune cells produce various cytokines, chemokines, and interact with other immune cells, exerting direct or indirect effects on tissue metabolism: B cells secrete cytokines and interact with immune cells, while T cells tend to release more inflammatory factors and cytokines, such as IL-6, TNF-α, IL-1β, IL-17, and IL-22, interacting with other cells and mediating signaling pathways, including OPG/RANK/RANKL and TNF. Moreover, excessive innate immune responses lead to inflammation and tissue destruction, activating adaptive immune responses.10,11 Screening potential therapeutic targets can significantly improve the treatment of inflammation.12 To further enhance the specificity of nanoparticles, more and more stimuli-responsive nanomaterials are being studied and developed, and the acidic pH and elevated ROS levels in the inflammatory microenvironment are commonly used response factors for the construction of stimulus-responsive nanomaterials. Therefore, a deeper understanding of the mechanisms of immune cells in chronic inflammation facilitates the development of novel therapeutic approaches and drugs, providing new strategies and methods to treat and prevent chronic inflammation.
Neutrophil
Neutrophils, the most and primary white blood cells in the human body, are among the first cells to enter inflamed sites and serve as a crucial first line of defense against infectious challenges. They rapidly migrate into tissues, directly phagocytosing and eliminating pathogenic microorganisms and their products, playing a vital role in controlling infection and resolving inflammatory responses.13 Neutrophils exhibit a wide range of effector mechanisms to combat pathogens, including phagocytosis and the production of reactive oxygen species (ROS), proteases, and neutrophil extracellular traps (NETs). Furthermore, neutrophils possess various specific receptors, such as integrin αvβ1 and mannose receptors, which can quickly recognize and bind to cyclic arginine-glycine-aspartic acid peptides or mannose. Modifying drug-loaded nanoparticles with these specific ligands can increase drug concentrations at inflammatory sites by binding to specific receptors on neutrophils.14 Polymorphonuclear neutrophils express most types of Toll-like receptors, and the engagement of cell surface Toll-like receptors activates neutrophils and promotes antimicrobial functions, enabling the identification of a broad spectrum of pathogen-associated molecular patterns (PAMPs) and triggering responses to invading pathogens.15
Neutrophil Extracellular Traps (NETs)
In recent years, neutrophil extracellular traps (NETs) have been considered the culprit in neutrophil-mediated immunopathology.16 When exogenous pathogens invade or the body experiences certain inflammatory conditions, neutrophils release a fibrous network-like structure known as NETs,17 which are regarded as potential therapeutic targets. Figure 1 NETs are comprised of DNA, histones, and MPO, with histones and MPO exhibiting significant toxicity to epithelial cells.18 Brinkmann first described NETs as bactericidal traps that promote the elimination of extracellular bacteria.19 The process of NET formation, known as NETosis, is a unique form of cell death distinct from apoptosis as well as necrosis.20 NETs are a double-edged sword,21 with excessive production by neutrophils promoting the advancement of different aliments, such as atherosclerosis, rheumatoid arthritis, and cancer. Therefore, inhibiting NET formation or eliminating their excess may be a potential anti-inflammatory strategy.22–24 Research on NETs is crucial for understanding the function of the immune system and the mechanisms of inflammation and infection.
Neutrophils and Periodontitis
Periodontitis, one of the most widespread infectious inflammatory disorders within humans, is a chronic inflammatory condition triggered by local microbial communities and host immune responses, leading to periodontal tissue damage and even tooth loss.25 The disease is characterized by a dysregulated neutrophil response to specific bacterial species within the subgingival biofilm. Neutrophils are the primary inflammatory cells involved in periodontitis, and neutrophil infiltration is a major feature of periodontal lesions.26–28 Hirschfeld et al proposed that variations in neutrophil responses to different bacteria, such as quantitative deficiencies or functional abnormalities in neutrophils against various bacteria, may be the pathogenic mechanism leading to periodontal disease.29 As the most abundant immune cells, changes in neutrophil numbers or functions can exacerbate periodontal inflammation through multiple mechanisms, affecting periodontal immune homeostasis. On one hand, neutrophils participate in the progression of periodontitis,30 with continuous activation of neutrophils in periodontal tissues releasing large amounts of ROS, damaging periodontal structures. On the other hand, chronic inflammation causes alterations in bone marrow hematopoietic stem cells, with newly differentiated neutrophils exhibiting enhanced activity, further aggravating tissue destruction and worsening periodontitis.31 The research team led by Fang Fuchun identified a neutrophil subpopulation associated with NETs in gingival tissues using single-cell technology for the first time. They demonstrated the role of NETs in promoting gingival inflammation and alveolar bone resorption in severe periodontitis using a mouse model, suggesting the potential of targeting NETs for periodontitis treatment.32 Figure 2 Additionally, their experiments revealed the mechanism by which inflammatory gingival fibroblasts promote NET formation through MIF-CD74/CXCR4, providing new perspectives for the prevention and treatment of periodontitis.
Excessive NET formation or clearance disorders have a causal relationship with periodontitis. Neutrophils recruit Th17 cells to periodontal tissues by releasing NETs and chemokines such as CCL2 and CCL20, indirectly exerting tissue-destructive effects through IL-17.16 After the rupture of the cell membrane within the extracellular space, NETs are released into the tissues, exerting antimicrobial effects and subsequently removed from the tissues. If NET removal fails, persistently high levels of NETs may cause damage to periodontal tissues.33 Non-surgical and surgical interventions have become traditional treatment modalities for periodontal therapy. Although adjunctive therapies (including antibiotics or supplements) accompany these therapies, their use is limited by antimicrobial resistance and partial effectiveness. Thus, novel approaches are required to regulate local inflammation in periodontal tissues as well as the host immune response.34 In the next few years, more research is required to improve immunotherapy treatment methods and continuously comprehend the risks as well as long-term effectiveness of new approaches in treating periodontitis.35
Neutrophils and Chronic Respiratory Diseases
In chronic obstructive pulmonary disease (COPD) patients, persistent NET formation has been observed, correlating with inflammation and disease severity. Research shows that neutrophils and NETs contribute to lung function decline by obstructing airways.36 Chronic lung infections are associated with high levels of neutrophil proteins and DNA, believed to be caused by NETs. In addition to causing direct damage, NETs provide pro-inflammatory stimuli to macrophages, promoting inflammatory responses in CF subjects. Wang et al found that GPR84 was highly upregulated in cells isolated from bronchoalveolar lavage fluid of LPS-induced mice.37 GPR84 blockade improved lung inflammation in mice by reducing neutrophil infiltration and oxidative stress. GPR84 can also induce neutrophil oxidant production by stimulating Lyn, AKT, and ERK1/2 activation. This study demonstrated the crucial role of GPR84 in neutrophil function and lung inflammation, suggesting GPR84 as a potential drug target for pneumonia.
Neutrophils and Chronic Liver Diseases
Nonalcoholic fatty liver disease (NAFLD) is presently the primary chronic liver disease, characterized by inflammation, hepatocyte injury, and fibrosis.38 NAFLD is the most frequent cause of liver disease-related mortality as well as morbidity. Nonalcoholic fatty liver includes nonalcoholic fatty liver steatosis and nonalcoholic steatohepatitis (NASH). Many studies have highlighted the role of neutrophils in NASH, with hepatic neutrophil infiltration promoting NASH development and circulating neutrophils correlating with its severity. Neutrophil infiltration in the liver promotes the development of nonalcoholic steatohepatitis.39,40 MPO-DNA levels, a marker of NETs, are elevated in NASH patients and mouse models.41 In mouse models, the development of steatosis is independent of NETs, suggesting that NET formation is a consequence of fatty liver accumulation. When NETosis is inhibited, inflammatory responses decrease, with reduced levels of macrophages and neutrophils in the liver. Therefore, regulating key targets that recruit neutrophils to the liver may slow disease progression.42
Neutrophils and Atherosclerosis
Atherosclerosis (AS) is a lipid-driven, multi-cell-mediated chronic inflammatory disease of blood vessels, primarily affecting large and medium-sized arteries. AS is featured by lipid accumulation in the arterial wall, immune cell infiltration, and the formation of a fibrous cap composed of smooth muscle cells and collagen.43 One mechanism by which neutrophils promote atherosclerosis is through the formation of NETs.44 Extracellular cholesterol crystals interact with neutrophils to induce NET release, and NETs stimulate macrophages to produce the precursor form of the inflammatory cytokine interleukin-1β (pro-IL-1β). Furthermore, cholesterol crystals bind to the cell surface protein CD36 on macrophages, activating inflammasomes (as shown in the figures 1-5), thereby promoting the maturation of endogenous IL-1β and upregulating another pro-inflammatory cytokine, IL-17, produced by.45 Consequently, macrophages present in atherosclerotic plaques possess inflammatory and plaque-destabilizing functions. Ultimately, this triggers thrombotic complications, interrupting arterial blood supply to downstream tissues, leading to myocardial infarction and stroke.
Neutrophils and Rheumatoid Arthritis (RA)
Rheumatoid arthritis (RA) is a systemic autoimmune disease featured by continuous synovial inflammation, leading to joint cartilage and bone damage. NETs serve as a kind of source of extracellular autoantigens, and citrullinated peptides generated by histone citrullination through PAD2 and PAD4 activity are overexpressed within neutrophils. It could even be detected within the synovium of RA patients.46 Activated neutrophils also express chemokines and chemokine receptors, promoting neutrophil migration and infiltration in RA joints.47 Furthermore, numerous studies have observed enhanced NET formation in RA patients, and the function of NETs in the pathogenesis of rheumatoid arthritis has been investigated.
Macrophages
Macrophages are core innate immune cells in the mortal body and are an essential part of the host immune response. They are also one of the most extensively studied immune cells, playing a important role in the pathogenesis of inflammation. Macrophages are vital in the initiation, maintenance, and resolution stages of inflammation. They can rapidly migrate and accumulate at inflammatory sites, where they exert dual roles in promoting disease progression and tissue repair.48 Under the induction of different factors, macrophages polarize into different functional phenotypes.
M1 and M2 macrophages participate in the destruction as well as repair phases of inflammatory tissues49 Figure 2. When the body encounters pathogenic microbial attacks, macrophages differentiate into M1 and M2 macrophages.50 M1 macrophages mainly participate in Th1-type immune responses and can produce a series of pro-inflammatory cytokines, including nitric oxide (NO), interleukin (IL)-1β, IL-2, IL-6, and other inflammatory factors, promoting Th1 activation. Increasing evidence suggests that inflammation is closely related to the increase in the M1/M2 ratio of polarized macrophages.51 CD64 is a high-affinity Fc-γ receptor, considered a marker of M1 macrophages, while CD163 and CD206 have been recognised as the primary markers of M2 macrophages. For example, in synovitis, macrophages expressing CD64 (Fc-γ receptor) play a key role and are considered a marker of macrophage activation.52,53 ROS are naturally occurring oxidants, and their production affects macrophage differentiation, promoting their transformation into the M1 phenotype. Under physiological conditions, antioxidants can effectively neutralize ROS, thus preventing ROS-mediated tissue damage.54
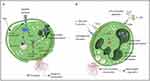 |
Figure 1 Mechanisms of NET formation. (A) PMA and other stimuli induce lytic-NET formation. Neutrophils are stimulated with PMA, resulting in the activation of NADPH oxidase via PKC and Raf-MEK-ERK signaling pathways, consequently generating ROS. Subsequently, PAD4 is activated and citrullinates arginine on histones, causing chromatin decondensation. MPO and NE are discharged from cytoplasmic azurophilic granules and then translocated to the nucleus, contributing to the unfolding of chromatin. The nuclear envelope subsequently disintegrates, discharging the chromatin into the cytosol, where it blends with cytosolic proteins. NE also cleaves GSDMD in the cytosol to its active form (GSDMD-NT), which, besides forming pores in the plasma membrane, also mediates pore formation in nuclear and granule membranes, enhancing the release of NE and other granular content. Finally, NETs are released, and the neutrophil undergoes cell death. (B) Nonlytic NET formation is induced by the recognition of stimuli via Toll-like receptor 2 (TLR2), TLR4, or complement receptors, independent of NAPDH oxidase activation. S. aureus and C. albicans activate TLR2 and complement receptors, respectively, while E. coli or LPS-activated platelets activate TLR4. Along with PAD4 activation and NE translocation to the nucleus, chromatin decondensation proceeds, and protein-decorated chromatin is expelled via vesicles without plasma membrane disruption. After the release of NETs, neutrophils remain alive for further functions. Reprinted from Blood, Vol 133/Edition 20, Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation, Page numbers 2178–2185, Copyright 2019, with permission from Elsevier.17 |
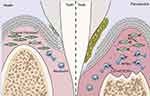 |
Figure 2 In inflamed gingival tissue, fibroblasts decreased while neutrophils increased; Fibroblasts induce excessive formation of NETS through MIFCD74/CXCR4 ligand receptor axis, thereby promoting the progression of periodontitis). Reprinted from Journal of Advanced Research, Qiu W, Guo R, Yu H, et al. Single-cell atlas of human gingiva unveils a NETs-related neutrophil subpopulation regulating periodontal immunity, Copyright 2024, with permission from Elsevier.32 |
Macrophages and Chronic Inflammation
Macrophages are regulators of immune activity and body homeostasis, adopting variable activation states as a function of time and environmental cues.55 In the pathogenesis of inflammatory lung diseases, macrophages promote the advancement as well as progression of acute or chronic inflammatory responses via secreting inflammatory cytokines/chemokines and activating transcription factors, such as in acute respiratory distress syndrome (ARDS) and chronic obstructive pulmonary disease (COPD). Eapen et al demonstrated that M2 phenotype macrophages predominate in the bronchoalveolar lavage (BALF) of COPD patients with increased cytokines, including IL-4, IL-13, IL-8, and IL-10.56 Osama et al showed that macrophage-induced eosinophilia is closely related to the severity of COPD.57 MCP-1 levels are elevated in sputum samples from COPD patients, and macrophages influence neutrophil recruitment by producing MCP-1, indicating that macrophages play a vital role in the development of COPD by inducing neutrophil influx.58 Macrophages are key coordinators in the pathogenesis of lung injury/acute respiratory distress syndrome, and regulating macrophage polarization can improve the prognosis of ALI/ARDS.59
In periodontitis, M1 and M2 macrophages participate in the destruction and repair stages of PD.49 Figure 3 M1 macrophages promote inflammation and activate osteoclasts by recruiting neutrophils to remove periodontal pathogens, leading to alveolar ridge resorption. M2 macrophages are involved in Th2-type immune responses60 (Figure 3), increasing the expression of IL-10 and chemokines, promoting Th2 activation, and exerting immunomodulatory effects on Th2 cells. They promote apoptosis and neutrophil recruitment to terminate the inflammatory process. M2 macrophages release various anti-inflammatory factors, including IL-4 and IL-10, secrete bone morphogenetic protein (BMP)-2, as well as vascular endothelial growth factor (VEGF) to participate in inflammation suppression and accelerate tissue healing, exerting anti-inflammatory and effect of angiogenesis, and activating osteoblasts to restore bone tissue.61,62 Additionally, M2 macrophages possess immunosuppressive, wound repair, and tumor-promoting functions.
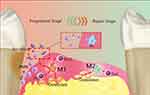 |
Figure 3 Polarized macrophages play a crucial role in the initiation and progression of Parkinson’s disease (PD). In PD, resident macrophages polarize into two primary phenotypes, M1 and M2, which respectively govern the inflammatory development and resolution phases. M1 macrophages are primarily proinflammatory and produce a series of proinflammatory factors, working in conjunction with Th1 cells, Th2 cells, and other cells. By collaborating with Th1-type immune cells, M1 macrophages can remove periodontal pathogenic microorganisms through the recruitment of PMNs. Simultaneously, M1 macrophages activate osteoclasts, leading to the absorption of the alveolar ridge. M2 macrophages primarily play an anti-inflammatory role and are mainly involved in immune interactions with Th2 cells. M2 macrophages terminate inflammatory development via accelerating the apoptosis for M1 macrophages and PMNs, perform tissue repair through various anti-inflammatory factors, and could activate osteoblasts to recover bone tissue. Reproduced from Sun X, Gao J, Meng X, Lu X, Zhang L, Chen R. Polarized Macrophages in Periodontitis: characteristics, Function, and Molecular Signaling. Front Immunol. 2021;12:763334.49 |
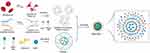 |
Figure 4 The preparation of LP, RLP and Effero‐RLP. The schematic of apoptotic RBC membrane preparation and the fusion with liposome particles. Reproduced from Han R, Ren Z, Wang Q, et al. Synthetic Biomimetic Liposomes Harness Efferocytosis Machinery for Highly Efficient Macrophages-Targeted Drug Delivery to Alleviate Inflammation. Adv Sci. 2024;11(29):e2308325.132 |
 |
Figure 5 Schematic illustration of Motor@M2M@SAM preparation and its mechanism for UC treatment. (A) Scheme depicting the fabrication process of Motor@M2M. (B) Scheme illustrating the fabrication process of Motor@M2M@SAM using microfluidic technology. (C) Mechanism for UC Treatment: Upon oral administration, SAM is disrupted as it enters the colon. Subsequently, Motor@M2M is released from the hydrogel into the colonic lumen. The propelling force of O2 bubbles, generated by the decomposition of local H2O2 in the inflammatory microenvironment, facilitates the penetration of Motor@M2M through the mucus layer. These nanomotors then target inflammatory colon cells through a macrophage-like function. They specifically interact with colon epithelial cells. Acting as decoys, Motor@M2M neutralizes inflammatory cytokines through receptor-ligand interactions and absorption. Ultimately, Motor@M2M exerts therapeutic effects against UC by scavenging ROS, reducing inflammation, reprogramming macrophages, repairing the epithelial barrier, and rebalancing the microbiota. Reproduced from Luo R, Liu J, Cheng Q, Shionoya M, Gao C, Wang R. Oral microsphere formulation of M2 macrophage-mimetic Janus nanomotor for targeted therapy of ulcerative colitis. Sci Adv. 2024;10(26):eado6798.63 |
Dendritic Cells (DCs)
The primary function of dendritic cells is to survey peripheral cells and present antigens to T cells. Exogenous stimuli activate DCs, leading to the secretion of cytokines and upregulation of surface costimulatory molecules.64 Mature DCs present antigens to naive CD4+ T cells,65 which differentiate into helper T cells (Th1, Th2, Th17) and regulatory T cells (Tregs).66 Interferon-γ (IFN-γ) and interleukin-12 (IL-12) secreted by dendritic cells induce Th1 cell formation in an inflammatory environment.67 Therapeutic approaches utilizing regulatory T cells (Tregs) to alleviate inflammatory tissue damage have been widely proposed. However, Tregs are unstable and may lose their function in an inflammatory environment, potentially converting into Th17 cells.68 Regulating the Treg/Th17 balance can significantly impact the pathogenesis of inflammation.69 Wen et al discovered that protein arginine methyltransferase 5 (PRMT5) attenuates activation and maturation by suppressing the expression of endotoxin-stimulated pro-inflammatory cytokines, ISGs, costimulatory molecules, and MHC. The inhibition of metabolic switching plays a key role in controlling activated dendritic cells, suggesting that PRMT5 is a promising therapeutic target for inflammation.70,71
Lymphocytes
Different subsets of lymphocytes also participate in the inflammatory process. Helper T cells (Th1, Th17) and B lymphocytes promote inflammatory responses, while regulatory T cells (Tregs) and B10 cells significantly suppress inflammatory reactions. Studies have shown that periodontitis primarily activates CD4+ T cell-polarized Th1, Th2, Th17, and Tregs, mediating immune responses through characteristic cytokines. The activation of Th cells is a pivotal factor in determining the progression of tissue damage, particularly the classical T cell subsets Th1, Th2, and Th17, which secrete various pro-inflammatory cytokines (IL-1β, IL-17).72
Therefore, in-depth exploration of the different roles of various immune cells at different stages of chronic inflammation is of great significance for the future clinical application of immunotherapy in the treatment of chronic inflammatory diseases.
Overview of Nanomaterials
Systemic administration is currently the most common method for treating many symptoms and diseases, and traditional inflammatory targeted therapies have shown promising therapeutic effects. However, most anti-inflammatory drugs are inevitably absorbed, have poor permeability through mucosal barriers, struggle to accurately reach the lesion site, have short retention times, and may even produce adverse side effects on the body, thus limiting their clinical application. Many anti-inflammatory drugs broadly suppress inflammation and are administered systemically at high dose concentrations, leading to various side effects, including immunodeficiency toxicity (eg, viral and bacterial infections) and systemic toxicity (eg, nephrotoxicity, hepatotoxicity).73 Therefore, numerous researchers have focused on designing and investigating drug carriers, wound dressings, and composite drug release systems, such as nanoparticles, exosomes, and biomimetic materials, to overcome the limitations of anti-inflammatory drugs.74,75 Nanomaterials are a type of multifunctional novel material with particle sizes ranging from 1 to 100 nm. The prominent features of nanomaterials are their unique size, shape, and surface properties that enable tissue penetration through passive or active targeting mechanisms. The emergence of nanotechnology has provided tremendous potential for overcoming biological barriers. Firstly, nanoparticles deliver drugs in a sustained and controlled manner, degrading and releasing drugs in response to specific environmental stimuli, thereby enhancing drug solubility, improving drug stability, reducing toxicity and drug degradation, ensuring stable cellular targeting and oral retention,76 and protecting drugs from pH effects and enzymatic degradation.77 Secondly, surface modification of nanoparticles can enhance their ability to target inflammatory microenvironments. Thirdly, the small size of nanocarriers allows them to accumulate in inflamed epithelial cells through the enhanced permeability and retention effect, more effectively targeting inflammatory tissues.78 The advantages of nanoscale delivery systems, such as biodegradability, biocompatibility, non-toxicity, and prolonged circulation, provide a platform for targeted therapy of inflammation.79 Nanoscale delivery systems have been utilized as a potential method for treating various diseases, including inflammation.80
Based on their chemical composition and structure, nanoparticle systems can be classified into organic nanoparticles, inorganic nanoparticles, and lipid-based nanoparticles. Organic nanoparticles, commonly referred to as polymeric nanoparticles, include synthetic and natural polymers. Nanoscale drug delivery systems with various structures have been widely used,81 including chitosan, poly(lactic-co-glycolic acid) (PLGA), nanohydrogels, liposomes, carbon nanoparticles, silica nanoparticles, and nanocomposites, all of which can serve as drug carriers and exhibit excellent drug loading and release capabilities,76,82 making them potential candidates for targeted drug delivery.83
Polymeric Nanoparticles
Polymeric nanoparticles (PNPs) are solid particles ranging in size from 10 to 1000 nanometers. Examples include poly(lactic-co-glycolic acid) (PLGA) nanoparticles,84 polycaprolactone (PCL), and hydrogel nanoparticles. Polyesters are the most commonly used polymers in nanosystems, containing ester functional groups in their polymer backbone, and their degradation can facilitate controlled drug release. Natural polymeric nanoparticles include polymers such as sodium alginate, chitosan, albumin, and gelatin.85 Moreover, with the rapid development of nanotechnology and materials science, there has been an increasing focus on developing safer and more effective hydrogels for treating severe inflammatory conditions, such as rheumatoid arthritis, osteoarthritis, periodontitis, and ulcerative colitis. Hydrogels can be classified into natural hydrogels (eg, chitosan, hyaluronic acid, sodium alginate, cellulose, gelatin) and hydrogels prepared from synthetic biomaterials (eg, poly(lactic acid) (PLA), polyacrylamide, poly(ethylene glycol) (PEG)). Nanohydrogels are nanoparticles composed of three-dimensional hydrogel materials, formed by crosslinked swellable polymer networks with high water retention capacity. Nanohydrogels are primarily composed of synthetic polymers, biopolymers, or combinations of chemically or physically crosslinked components.86
Nanostructured Lipid Carriers
Nanostructured lipid carriers (NLCs) are drug delivery systems that utilize solid lipids and liquid lipids as the core matrix. Compared to solid lipid nanoparticles and traditional drug delivery carriers, NLCs offer advantages including improved drug loading capacity, flexible drug release modulation, and improved stability. Moreover, NLCs exhibit good biocompatibility, low toxicity, increased solubility, reduced side effects, prolonged shelf life, and superior drug targeting and controlled release capabilities.87,88 In previous studies, NLCs have demonstrated effective therapeutic prospects through various administration routes, including pulmonary, local, nasal, ocular, and oral.89 Yang et al designed a rapidly monodispersed gelatin methacryloyl@liposome (GelMA@Lipo) hybrid microgel drug delivery platform by anchoring liposomes within a photocrosslinkable GelMA matrix. Compared to the control group, the liposomes were firmly fixed in the microgel through non-covalent interactions, enabling prolonged drug retention in the joints.
Classification of Inorganic Nanomaterials
Common inorganic nanomaterials include metal nanoparticles (Ag, Pd), metal oxide-based functional nanomaterials (ZnO, TiO2, MgO), carbon and graphene nanomaterials, and others. Compared to organic nanomaterials, inorganic nanomaterials possess unique physicochemical properties, such as optical, electrical, magnetic, ultrasonic, and catalytic properties, as well as controllable shape and size, which also offer tremendous advantages in anti-inflammatory therapy.90 Although inorganic nanomaterials have been widely used in biomedical applications, the extensive retention time of many functional inorganic nanomaterials in vivo increases the possibility of harmful toxicity.91 Therefore, further enhancement of their targeting ability and bioavailability is also required.92
Nanomaterials Targeting Specific Immune Cells
Although traditional nanoscale drug delivery systems have improved the therapeutic effects of drugs, nanoparticles carrying drugs with surfaces that bind to plasma proteins can activate immune responses and are easily cleared by the mononuclear phagocyte system, thereby reducing the bioavailability of the drugs.93 Moreover, most systems cannot precisely target the drug to the inflammatory tissue, and the drug is released into each type of cell at the inflammation site, further reducing the therapeutic effect.94,95 Therefore, additional studies are required to enhance the targeting ability of nanoparticles. For example, intravenous injection of mannosylated liposomes in rats with arthritis resulted in only partial drug delivery to the joints because liposomes are easily cleared by the reticuloendothelial system.96 In contrast, neutrophil-based drug delivery systems can avoid clearance by the reticuloendothelial system and enhance the ability of nanoparticles to target joints.97
In cancer treatment, the clinical progress of immunotherapy has been remarkable, and more clinical data have demonstrated that combining nanomedicines with immunotherapy can significantly improve the therapeutic effect.98 Nanoimmunotherapy is mainly achieved through three different approaches: nanomedicines are used to (1) target cancer cells, (2) target the tumor immune microenvironment, and (3) target the peripheral immune system. In the case of inflammation, the inflammatory microenvironment is formed by immune cells, inflammation-related enzymes, and inflammatory mediators. Similar to tumors, we can use immune cells or biomarkers to provide opportunities for precise targeting of anti-inflammatory drugs. Combined with the advantages of nanomedicines, such as precise localization of inflammatory tissues, overcoming barriers, enhancing interactions with epithelium, and reducing systemic adverse reactions, targeted delivery of nanomaterials to immune cells can be achieved for the treatment of chronic inflammation. The immunomodulatory effects of nanoparticles are one of the hotspots in nanomaterial research. Currently, many nanomaterials have been found to regulate the immune system, achieving significant progress in the fields of nanomedicine and immunotherapy.99
Macrophage-Targeted Nanomaterials
Macrophage Polarization
The main direction of macrophage polarization therapy is to induce macrophages to transform into the M2 type, thereby alleviating inflammation, promoting tissue repair, and achieving anti-inflammatory effects. The production of ROS is a major biological process in which stimulated macrophages participate in killing phagocytosed microorganisms.100 However, excessive ROS may drive macrophages to transform into the M1 type, exacerbating the development of inflammation.101 Sun et al constructed a cerium@ce6 multifunctional nanocomposite that regulates host immunity by downregulating M1 polarization and upregulating M2 polarization in macrophages. This nanocomposite can avoid periodontitis caused by high levels of ROS, prevent oxidative stress, and improve the regenerative potential of periodontal inflammatory tissues in animal models.102 Ni et al utilized gold nanoparticles (AuNPs) to induce macrophages to transform into the M2 type by regulating the production of inflammatory and regenerative cytokines, thereby improving the periodontal inflammatory microenvironment and modulating the inflammatory response in early periodontal tissues.103 Nanoparticles can protect drugs from the influence of pH and enzymatic degradation. By designing nanoparticles to respond to ROS, pH, or enzymatic reactions in the pathological microenvironment, drug release can be controlled.76 Hu et al prepared a mannosylated (Man) functionalized nanoscale metal-organic framework (MOF) to load Que for targeted therapy of myocardial infarction (MI). The nanomedicine Que@MOF/Man can reprogram macrophage polarization and neutralize ROS, thereby reducing oxidative stress and targeting the inflammatory infarcted heart.104 Man is a yeast polysaccharide containing D-mannose residues that can be recognized by mannose receptors. Mannose receptors are highly expressed on macrophages at inflammatory sites, promoting the inflammation-specific accumulation and targeted cellular internalization of Que@MOF/Man in macrophages. Galarraga et al exposed LPS-stimulated macrophages to extracted cranberry concentrate and found a significant decrease in M1 and a significant increase in M2, indicating that cranberry proanthocyanidins have effective anti-inflammatory effects in periodontal treatment.105 Wang et al introduced the antioxidant drug quercetin into nano-octahedral cerium to construct a nanocomposite (CeO2@QU), achieving synergistic regulation of host immunity in periodontal disease. The prepared nanocomposite not only effectively increased the M2/M1 ratio of macrophage polarization in inflammatory cell models but also promoted periodontal tissue regeneration by significantly decreasing the levels of pro-inflammatory cytokines while increasing the levels of anti-inflammatory cytokines, demonstrating therapeutic potential for local inflammation.106 Shi et al developed resveratrol-loaded liposomes (Lipo-RSV) that transformed macrophages from the M1 phenotype to the M2 phenotype by stimulating p-STAT3 and suppressing p-STAT1. Lipo-RSV elevated the mRNA levels of M2-associated markers (CD206, Arg-1, and Chil3) and reduced the mRNA levels of M1 macrophage markers (CD86, iNOS, and CCR7) in stimulated macrophages. Furthermore, Lipo-RSV could scavenge ROS, inhibit NF-κB signaling and inflammasomes, thus reducing pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, showing potential for treating periodontitis.107 Wali et al prepared dexamethasone-loaded ROS-responsive polymeric nanoparticles (PFTU@DEX NPs). Due to the ability of PFTU@DEX NPs to inhibit the expression of NLRP3, Caspase1, and IL-1β, they could effectively suppress inflammatory cells, ROS signaling pathways, and apoptosis, inducing macrophage phenotype polarization from M1 to M2, thus effectively alleviating acute lung injury.108 Ma et al developed folic acid-modified DNA origami nanostructures (FA-tDONs)109 by utilizing the inherent ROS and NO scavenging abilities of DNA molecules. FA-tDONs could effectively scavenge ROS and NO, actively target M1 macrophages at inflammatory sites, and promote M1-M2 transformation.
Specific Receptors
Compared to conventional nanosystems, active targeting nanocarriers that transfer cargo to disease sites with higher selectivity have shown advantages in treating inflammation, cancer, and other chronic diseases.110,111 Some active targeting nanoparticles have been developed to target macrophages by localizing to specific cell receptor surfaces in particular situations.112 Current approaches for targeting macrophages include targeting CD44, coupling folic acid, coupling mannose, microbial mimicry, and lactoferrin modification. Yang et al designed an injectable hydrogel (Gel/FA-PDA@Leon) based on mammalian collagen and peptides.113 Gel/FA-PDA@Leon can target and deliver Leon to M1 macrophages, reducing the secretion of pro-inflammatory cytokines by inhibiting the JAK2/STAT3 inflammatory signaling pathway. The hydrogel can also protect collagen cells from the effects of ferroptosis. Gel/FA-PDA@Leon hydrogel significantly inhibits the inflammatory response associated with rheumatoid arthritis (RA) and protects the integrity of joint structures, thereby promoting the recovery of joint function, making it effective for the treatment of RA. Feng Jie et al prepared a Mannose-PEG-PCL targeted nanomedicine that actively targets macrophage membrane receptors, increasing the endocytic uptake of nanoparticles by macrophages.114 The nanomedicine reshapes the synovial inflammatory microenvironment by activating the AKT/STAT6 pathway. Na et al developed a macrophage-based encapsulated hydrogel nanoformulation (MZ@PNM@GCP).115 MZ@PNM@GCP specifically blocks the binding of P.g. to immune cells through Toll-like receptor complex 2/1 (TLR2/1) targeting, preventing P.g. from disrupting the periodontal host immune response. Furthermore, FR2, a membrane protein overexpressed on inflammatory macrophages, can selectively recognize and internalize folic acid (FA)-modified nanodrug delivery systems into inflammatory macrophages.116 The Wang research group developed folic acid-modified genistein liposomes that target macrophages.106 These liposomes can regulate the TLR4/MyD88/NF-κB axis in macrophages and promote the osteogenic differentiation of PDLSCs. Sun et al constructed the M2M@PLGA/COX-siRNA delivery system, which has low toxicity and obvious targeting to the injured site, reducing inflammatory reactions and significantly improving tendon adhesion.117 Hyaluronic acid possesses unique biological properties that enable it to specifically combine with the overexpressed CD44 receptors on the surface of macrophages, endowing nanodrug delivery systems with the ability to target macrophages. Nanoparticles containing hyaluronic acid can be used to target macrophages for the treatment of inflammatory diseases.118
Nanomedicines selectively deliver drugs to the damaged regions of rheumatoid arthritis (RA) through passive targeting effects. The surfaces of nanomedicines are then modified with targeting ligands connected to specific receptors for active targeting of macrophages. 98 Jia et al constructed a pH-responsive dual-targeted nanodrug delivery system, RBA-NPs, loaded with targeting CD44 and folate receptors. The study found that intra-articular M1 macrophages were reprogrammed to M2 type through RBA. RBA-NPs can also drive M1-to-M2 phenotype conversion by downregulating glycolysis levels through blocking the ERK/HIF-1α/GLUT1 pathway. This nanocarrier effectively delivers RBA to inflammatory sites, significantly reducing inflammatory cytokine levels and promoting tissue repair, improving the efficiency of rheumatoid arthritis treatment. The study also identified a potential molecular target for regulating macrophage reverse reprogramming through energy metabolism.119 Feng Naibo et al designed and constructed a dual-responsive, macrophage-targeting nanocarrier loaded with small interfering RNA (si ERN1), effectively achieving targeted therapy for rheumatoid arthritis (RA). The nanocarrier demonstrated superior therapeutic effects, immune homeostasis regulation, and cartilage protection in collagen-induced arthritis (CIA) model mice.120 Tand et al developed an FA receptor-targeted GER nanocarrier, FA-NPs/GER. In vitro experiments confirmed that FA-NPs/GER could promote the transformation of M1 macrophages to M2 macrophages. In animal experiments, the drug selectively accumulated at inflammatory sites, significantly reducing inflammatory infiltration. Therefore, macrophage-targeted nanocarriers loaded with GER represent a safe and effective method for treating RA (Table 1).121
Natural Nanoparticles
In recent years, natural products have attracted growing interest as a potential therapy for various diseases because of their high therapeutic potential, low cost, and high safety.122–124 Natural products also possess antimicrobial, antioxidant, and anti-inflammatory properties. For example, plant-derived exosome-like nanoparticles (pELNs) are natural nanocarriers with sizes ranging from 50 to 500 nm. pELNs contain lipids, RNA, and other active molecules.122 pELNs can be derived from many plants and fruits, such as ginger, blueberries, and coconuts.125 ELNs derived from grapefruit, carrots, and ginger induce macrophages to express IL-10 and promote the activation of nuclear factor 2 (Nrf2) in macrophages, blocking the assembly of NLRP3 inflammasomes in macrophages.126 This suggests that G-ELNs are novel and effective drugs for blocking the assembly and activation of NLRP3 inflammasomes. NLRP3 inflammasomes regulate the release of interleukin-related inflammatory factors, and their activation promotes inflammation, making them a key factor in improving inflammatory responses. Ginseng-derived ELNs inhibit IL-4 and IL-13-induced M2-like polarization in macrophages and increase the secretion of M1-macrophage-related cytokines (including TNF-α, IL-12, and IL-6).127
pELNs are lipid bilayer membrane nanovesicles rich in lipids, proteins, RNA, and other active molecules. They have high bioavailability and low immunogenicity, making them relatively safe. pELNs have demonstrated the capability to penetrate mammalian cells and modulate cellular functions.122,128 Due to their wide availability, cost-effectiveness, and ease of acquisition, pELNs can serve as better alternatives to animal-derived exosomes (ADEs).129 pELNs not only have great potential in regulating immune function, inflammation, and tissue regeneration, but they also can act as drug carriers, cellular uptake in vivo and enhancing drug stability. Meng et al utilized exosome-like natural tea-derived carbon nanotubes to reduce ROS production, decrease the levels of pro-inflammatory cytokines TNF-α, IL-6, and IL-12, and significantly increase the levels of anti-inflammatory IL-10 secreted by macrophages, effectively preventing or reducing inflammation.130 pELNs can also regulate inflammatory responses by blocking the activation and release of NLRP3 inflammasomes. Liu et al found that ELNs derived from shiitake mushrooms (S-ELNs) can inhibit the activation of NLRP3 inflammasomes by preventing the formation of inflammasomes in primary macrophages. S-ELNs also inhibit pro-inflammatory cytokines such as IL-6, thus exhibiting good anti-inflammatory activity.131 Professor Lu Jiahong’s team prepared biomimetic nanoparticles (Effero-RLP) by fusing apoptotic red blood cell membranes with liposomes. The PPAR-γ agonist rosiglitazone was loaded into the biomimetic nanoparticles to regulate macrophage function and achieve anti-inflammatory effects. Experiments demonstrated that Effero-RLP has good macrophage targeting and efficient cellular uptake properties. Compared with other liposome carriers, Effero-RLP exhibited superior anti-inflammatory efficacy. In drug release experiments, Effero-RLP showed a relatively slow release rate. Therefore, Effero-RLP has great application potential in the treatment of inflammatory bowel disease132 Figure 4.
Neutrophil-Targeted Nanomaterials
Neutrophil Receptors
Typically activated neutrophils are considered to be primarily induced by Toll-like receptor (TLR) and interferon (IFN) γ signaling stimulation, which are enhanced during infection, stroke, and myocardial infarction. Naina et al demonstrated that the G protein-coupled receptor (GPCR) Mrgpra1 expressed by neutrophils drives anti-inflammatory neutrophils and inhibits activated neutrophils, serving as a negative regulator of neutrophil killing function.133 Mrgpra1-mediated signal transduction is driven by its ligand neuropeptide FF (NPFF), which determines the balance between pro-inflammatory and anti-inflammatory programming. Kang et al prepared neutrophil membrane-coated poly(lactic-co-glycolic acid) (PLGA) nanoparticles.134 Because of the high expression of adhesion molecules and chemokine receptors on neutrophils, the neutrophil membrane coating can endow nanocarriers with the ability to target synovitis and be recruited to the synovial fluid under the chemotactic effect of IL-8. Yang et al engineered a biomimetic ApoA-I mimetic peptide-modified neutrophil membrane-encapsulated F127 polymer (R4F-NM@F127)135 for targeted drug administration during rheumatoid arthritis (RA) therapy, effectively inhibiting synovial inflammation and reducing joint damage.
The Mrgpra1-NPFF axis mediates the counter-regulation of interferon (IFN) γ-mediated neutrophil polarization during acute lung infection, suggesting that it may balance excessive neutrophil responses. Therefore, intrinsic pathways within neutrophils determine their cell fate, function, and the extent of infection. Albumin NPs manufactured using organic solvents can specifically bind to FcγRIII receptors on activated neutrophils,136 and albumin NPs can be absorbed by activated neutrophils in the blood, mediating the delivery of nanotherapeutic drugs in inflammation or tumors. This provides numerous opportunities for the rational design and engineering of targeted drug delivery to activated neutrophils. The NLRP3 inflammasome is a protein complex, it can help the body resist pathogen invasion under physiological conditions, but its overactivation may lead to excessive release of inflammatory mediators and overactivation of inflammatory cells, disrupting the immune balance within tissues and ultimately leading to various inflammatory diseases, including periodontitis. The NLRP3 inflammasome can be involved within the activation regarding neutrophils, macrophages, osteoclasts (OCs), and human periodontal ligament fibroblasts (HPLFs) and may contribute to the progression of various inflammatory as well as autoimmune conditions. Therefore, targeted therapy to regulate the function of the NLRP3 inflammasome provides a new approach for the adjuvant treatment of periodontitis.137 The MCC950 targeted therapeutic strategy has high specificity and can significantly reduce the levels of the pro-inflammatory cytokine IL-1β without affecting other types of inflammasomes and their corresponding inflammatory factor expression.138,139
Targeted Regulation of NETs for Inflammation Treatment
The balance of NETs is crucial for maintaining health and homeostasis in the body. NETs have become potential therapeutic targets for inflammatory and autoimmune diseases. The excessive production of NETs and the degradation of their components can be inhibited by drugs, thereby achieving targeted treatment of inflammation.140 Hu et al designed anti-inflammatory nanoparticles based on a luminol-conjugated α-cyclodextrin material (LaCD).141 The results showed that LaCD NPs could effectively inhibit neutrophil-mediated aortic aneurysm inflammation by attenuating the structure of NETs and suppressing NET-mediated pro-inflammatory events. This study demonstrated the effectiveness and potential mechanism of anti-NETosis nanotherapy for the targeted treatment of abdominal aortic aneurysms and provided a promising reference for the precise treatment of other inflammatory diseases. Chen et al designed NET-like structures using DNA and ZnO nanoparticles.142 In the anti-inflammatory assay, ZnO/DNA-HCl NG significantly inhibited the expression of TNF-α, IL-6, iNOS, and COX-2 in LPS-stimulated Raw264.7 cells. Furthermore, ZnO/DNA-HCl NG significantly alleviated the clinical symptoms of LPS-induced peritonitis in mice. Hu et al found that LaCD NPs could effectively attenuate the formation of neutrophil extracellular traps (NETs), thereby inhibiting NET-mediated pro-inflammatory events and NETosis-related inflammatory progression, demonstrating the effectiveness and potential mechanism of anti-NETosis nanotherapy for targeted treatment of inflammatory diseases.143 Liu et al prepared RGD-modified bovine serum albumin (BSA) nanoparticles (CBR NPs). These nanoparticles could selectively target inflammatory neutrophils (INEs) in circulation and induce INE apoptosis. Simultaneously, they were able to suppress the activation of NETs through the NF-κB pathway and block the release process of NETs, thereby inhibiting the infiltration of circulating neutrophils (INEs) into inflamed joints and reducing tissue damage by suppressing NET release.143 Zhou et al modified the surface of Prussian blue nanoparticles (PB NPs) with a neutrophil elastase (NE)-binding peptide to target activated neutrophils. These NET-targeted nanoparticles exhibited effective treatment for antiphospholipid antibody-mediated thrombosis during pregnancy.144
Biomembrane Targeting
Nanoparticles, while being the ideal drug carriers, can enhance targeting to a certain degree. However, the straightforward biofunctionalization of nanoparticles still encounters significant challenges within the complex intercellular environment. The clinical application of nanoparticles also presents some issues; once synthesized, nanoparticles are engulfed by immune cells of the immune system, triggering immune responses and toxic effects. Some nanomaterials may inherently possess biological toxicity, such as cardiotoxicity and cytotoxicity, and they can also negatively impact the environment, thus raising concerns about their safety. It is imperative to further investigate the toxicity and biocompatibility of nanomaterials. Consequently, researchers have been inspired by the bionics strategy to coat nanomedicine with a biofilm, which not only reduces rejection reactions and other adverse effects but also enhances targeting capabilities and mitigates toxic reactions.Cell membrane-mimicking nanomaterials have emerged in the fields of disease diagnosis and targeted drug delivery, becoming a new therapeutic strategy.145
Nanomaterials modified with cell membrane derivatives retain antigens and cell membrane structures, enabling them to acquire the unique functions of the original cells, such as active targeting, long-term blood circulation, and immune escape.146 Therefore, this biomimetic strategy demonstrates long-term circulation of nanoparticles that are difficult for the immune system to recognize. By coating nanoparticles with cell membranes, various functional proteins can be modified on the surface to form biomimetic nanoparticles, providing multiple pathways for nanoparticles to participate in physiological and pathological processes. The constructed cell membrane-coated nanoparticles retain the various characteristics and inherent targeting capabilities of the core nanoparticles. These advantages make these materials show application potential in inflammation treatment. Drug delivery systems specifically targeting the inflammatory microenvironment have been developed. Currently, various biomembrane nanocarriers have been developed, mainly including macrophage membranes, neutrophil membranes, extracellular vesicles, and hybrid cell membranes.146 The most prominent are macrophage and neutrophil-targeting nanoparticles and their derived biomimetic nanoparticles.146
Macrophage Cell Membrane
The macrophage membrane inherits the surface protein spectrum and biointerface characteristics of the source cells, protecting synthetic nanoparticles from being engulfed by immune cells and accurately recognizing antigens to target inflamed tissues.147 Luo et al prepared alginate microspheres (Motor@M2M@SAM) combined with Janus nanomotors and M2 macrophage membranes for targeted treatment of ulcerative colitis63 Figure 5. Zhao et al utilized the characteristics of the CCL2/CCR2 chemokine axis to recruit macrophages and prepared macrophage membrane-coated nanoparticles, which showed significant accumulation in breast cancer lung metastasis.148 Ma et al used mouse macrophage membranes to cover nanogelatin and ChS, constructing an “egg yolk-shell” structured artificial M2 macrophage for the treatment of osteoarthritis.149 Compared with nanohydrogels without cell membrane coating, the artificial M2 macrophages exhibited significant adhesion and accumulation on the surface of inflamed cartilage and synovium, producing a notable anti-inflammatory effect. Gao et al reported a biomimetic drug delivery system of macrophage membrane-coated ROS-responsive nanoparticles. The macrophage membrane not only prevented the clearance of NPs from the endothelial system and assisted NPs in entering inflamed tissues but also isolated pro-inflammatory cytokines and inhibited local inflammation, improving the therapeutic effect on atherosclerosis. This experiment demonstrated that cell membrane-coated drug delivery methods might be more suitable for treating inflammatory diseases than live cell methods.150 Although macrophage membrane-camouflaged nanoparticles are currently in the embryonic stage, there is still great potential and challenges in exploring their translational models in clinical settings.
Neutrophil Cell Membrane
Recently, some nanomedicines have been used to alleviate rheumatoid arthritis (RA) by targeting functional cells modified with regulatory ligands. Other nanoparticles disguised with membranes or extracellular vesicles (EVs) of these functional cells have been employed to target and attack lesions for RA treatment.151 Yu et al coated self-assembled PEGylated L-arginine nanoparticles with inflammatory neutrophil membranes to construct a neutrophil membrane-based biomimetic nanoplatform, NM-LANPs@Ru.152 In vivo studies on a mouse osteoarthritis model showed that the biomimetic nanoplatform could specifically target inflammatory sites through dual-modal imaging, exhibiting higher penetration depth compared to non-membrane-coated nanomaterials. Yang Ni et al developed an R4 peptide-modified neutrophil membrane-coated biomimetic nanomedicine (R4F-NM@F127-Cel) with a clear targeting ability for SR-B1+ cells. By targeting the synovial membrane through the SR-B receptor, it effectively inhibited synovial inflammation and improved joint damage, providing a promising strategy for the clinical treatment of RA.153 Wang et al developed a biomimetic neutrophil-like aggregation-induced emission (AIE) nanorobot (CM@AIE NPs). The neutrophil membrane on the surface enabled CM@AIE NPs to mimic source cells and interact with immune regulatory molecules. Additionally, the excellent photothermal properties of AIE allowed precise localization of inflammatory sites and exerted the anti-inflammatory effect of nanoparticles, minimizing damage to surrounding normal tissues.154 Zang et al found that neutrophil membrane-coated nanoparticles (NM-NPs) could prevent the infiltration of neutrophils and macrophages to inflammatory sites by capturing chemokines and blocking adhesion to inflammatory endothelial cells. NM-NPs showed a significant anti-inflammatory effect in vivo on endotoxin-induced inflammatory liver injury without drug loading. This experimental study revealed the anti-inflammatory effects and mechanisms of NM-NPs in the absence of drug loading, providing new insights and evidence for developing safer and more effective targeted drug delivery systems.155
Conclusion
In chronic inflammatory diseases such as pneumonia, periodontitis and rheumatoid arthritis, controlling the progression of inflammation is a key step in treating the disease. In recent years, nanodrug delivery systems have received widespread attention due to their different functions of immune cells and extracellular matrix factors, which are highly expressed in the inflammatory microenvironment. With the increased understanding and comprehension of various aspects of inflammation, researchers have developed various nanotargeted drug delivery systems. The unique physicochemical and targeting properties of nanodrug delivery systems have created a favorable platform for drug delivery to treat inflammation. In this review, we briefly outlined the relationship between inflammation and immune cells, and reviewed nanotreatment strategies to provide insights for future advances in inflammation treatment and the design of immune-targeted nanodrug delivery systems. Overall, immune-targeted nanomaterials have shown great potential in anti-inflammation, but the greater challenge lies in clinically evaluating the risks and benefits of candidate drugs. Inducing neutrophil apoptosis, inhibiting neutrophil extracellular traps, or targeting neutrophils for drug delivery are therapeutic strategies that can help alleviate inflammation and cure diseases, but relevant research is still in the exploratory stage, and clinical application has a long way to go. Therefore, we need more scientific research to continuously improve the development of new nanotreatment strategies. Immune-targeted nanomaterials will soon provide new opportunities for inflammation treatment, thereby reducing the suffering of patients and the medical burden on society.
Advanced nanomaterials have broad application prospects and can be applied to more fields in the future, exhibiting more excellent properties. With in-depth research on the pathogenesis of various inflammations, new understandings and discoveries are constantly emerging. These new insights not only provide new targets for anti-inflammatory drugs but also offer new ideas for the development of treatment methods, which will bring better treatment options for patients. We can not only start from the perspective of immune cells and immune factors but also delve into relevant inflammatory signaling pathways (NF-κB, MAPK, Akt, Jak/Stat, etc) to understand the role of related pathways in the disease and find effective targets for inflammation. With the deepening understanding of chronic inflammation mechanisms and the discovery of new targets, I believe that the research and development of anti-inflammatory drugs will achieve more innovations and breakthroughs. Although many specific targeted nanomaterials are relatively new, targeted anti-inflammatory nanomaterials will have a bright future in advancing the field of nanomedicine, enabling new applications to become possible.
Data Sharing Statement
No data was used for the research described in the article.
Acknowledgments
We would like to thank all the participants who participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Provincial Science and Technology Program of Jilin (No.JCSZ2023481-5),the Natural Science Foundation of Jilin Province (No. YDZJ202201ZYTS047)and the Jilin University Norman Bethune Program (No. 2023B30).
Disclosure
All authors declared no conflicts of interest in this work.
References
1. He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules. 2015;20(5):9183–9213. doi:10.3390/molecules20059183
2. Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2018;9(6):7204–7218. doi:10.18632/oncotarget.23208
3. Henson PM. Dampening inflammation. Nat Immunol. 2005;6(12):1179–1181. doi:10.1038/ni1205-1179
4. Nasef NA, Mehta S, Ferguson LR. Susceptibility to chronic inflammation: an update. Arch Toxicol. 2017;91(3):1131–1141. doi:10.1007/s00204-016-1914-5
5. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi:10.1016/j.cell.2010.01.025
6. Thiagarajan V, Alex SA, Seenivasan R, Chandrasekaran N, Mukherjee A. Interactive effects of micro/nanoplastics and nanomaterials/pharmaceuticals: their ecotoxicological consequences in the aquatic systems. Aquat Toxicol. 2021;232:105747. doi:10.1016/j.aquatox.2021.105747
7. Wallis RS, O’Garra A, Sher A, Wack A. Host-directed immunotherapy of viral and bacterial infections: past, present and future. Nat Rev Immunol. 2023;23(2):121–133. doi:10.1038/s41577-022-00734-z
8. Galli SJ, Gaudenzio N, Tsai M. Mast Cells in Inflammation and Disease: recent Progress and Ongoing Concerns. Annu Rev Immunol. 2020;38:49–77. doi:10.1146/annurev-immunol-071719-094903
9. Palucka K, Coussens LM, O’Shaughnessy J. Dendritic cells, inflammation, and breast cancer. Cancer J. 2013;19(6):511–516. doi:10.1097/PPO.0000000000000007
10. Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28(10):429–436. doi:10.1016/j.it.2007.08.004
11. Xu XW, Liu X, Shi C, Sun HC. Roles of Immune Cells and Mechanisms of Immune Responses in Periodontitis. Chin J Dent Res. 2021;24(4):219–230. doi:10.3290/j.cjdr.b2440547
12. Yamanishi Y, Karasuyama H. Basophils and mast cells in immunity and inflammation. Semin Immunopathol. 2016;38(5):535–537. doi:10.1007/s00281-016-0582-0
13. Allen LH, Criss AK. Cell intrinsic functions of neutrophils and their manipulation by pathogens. Curr Opin Immunol. 2019;60:124–129. doi:10.1016/j.coi.2019.05.004
14. Gajbhiye KR, Gajbhiye V, Siddiqui IA, Gajbhiye JM. cRGD functionalised nanocarriers for targeted delivery of bioactives. J Drug Target. 2019;27(2):111–124. doi:10.1080/1061186X.2018.1473409
15. Prince LR, Whyte MK, Sabroe I, Parker LC. The role of TLRs in neutrophil activation. Curr Opin Pharmacol. 2011;11(4):397–403. doi:10.1016/j.coph.2011.06.007
16. Kim TS, Silva LM, Theofilou VI, et al. Neutrophil extracellular traps and extracellular histones potentiate IL-17 inflammation in periodontitis. J Exp Med. 2023;220:9. doi:10.1084/jem.20221751
17. Castanheira FVS, Kubes P. Neutrophils and NETs in modulating acute and chronic inflammation. Blood. 2019;133(20):2178–2185. doi:10.1182/blood-2018-11-844530
18. Wei Z, Wang J, Wang Y, et al. Effects of Neutrophil Extracellular Traps on Bovine Mammary Epithelial Cells in vitro. Front Immunol. 2019;10:1003. doi:10.3389/fimmu.2019.01003
19. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi:10.1126/science.1092385
20. Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25(3):486–541. doi:10.1038/s41418-017-0012-4
21. Bissenova S, Ellis D, Mathieu C, Gysemans C. Neutrophils in autoimmunity: when the hero becomes the villain. Clin Exp Immunol. 2022;210(2):128–140. doi:10.1093/cei/uxac093
22. Zhao Z, Pan Z, Zhang S, et al. Neutrophil extracellular traps: a novel target for the treatment of stroke. Pharmacol Ther. 2023;241:108328. doi:10.1016/j.pharmthera.2022.108328
23. Pan W, Xin Q, Xu J, et al. IgD enhances the release of neutrophil extracellular traps (NETs) via FcδR in rheumatoid arthritis patients. Int Immunopharmacol. 2023;114:109484. doi:10.1016/j.intimp.2022.109484
24. Cacciotto C, Alberti A. Eating the Enemy: mycoplasma Strategies to Evade Neutrophil Extracellular Traps (NETs) Promoting Bacterial Nucleotides Uptake and Inflammatory Damage. Int J Mol Sci. 2022;23:23. doi:10.3390/ijms232315030
25. Peres MA, Macpherson LMD, Weyant RJ, et al. Oral diseases: a global public health challenge. Lancet. 2019;394(10194):249–260. doi:10.1016/S0140-6736(19)31146-8
26. Williams DW, Greenwell-Wild T, Brenchley L, et al. Human oral mucosa cell atlas reveals a stromal-neutrophil axis regulating tissue immunity. Cell. 2021;184(15):4090–4104.e15. doi:10.1016/j.cell.2021.05.013
27. White PC, Chicca IJ, Cooper PR, Milward MR, Chapple IL. Neutrophil Extracellular Traps in Periodontitis: a Web of Intrigue. J Dent Res. 2016;95(1):26–34. doi:10.1177/0022034515609097
28. Kim TS, Moutsopoulos NM. Neutrophils and neutrophil extracellular traps in oral health and disease. Exp Mol Med. 2024;56(5):1055–1065. doi:10.1038/s12276-024-01219-w
29. Hirschfeld J, White PC, Milward MR, Cooper PR, Chapple ILC, McCormick B. Modulation of Neutrophil Extracellular Trap and Reactive Oxygen Species Release by Periodontal Bacteria. Infect Immun. 2017;85:12. doi:10.1128/IAI.00297-17
30. Li X, Wang H, Yu X, et al. Maladaptive innate immune training of myelopoiesis links inflammatory comorbidities. Cell. 2022;185(10):1709–1727.e18. doi:10.1016/j.cell.2022.03.043
31. Dutzan N, Kajikawa T, Abusleme L, et al. A dysbiotic microbiome triggers T(H)17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med. 2018;10:463. doi:10.1126/scitranslmed.aat0797
32. Qiu W, Guo R, Yu H, et al. Single-cell atlas of human gingiva unveils a NETs-related neutrophil subpopulation regulating periodontal immunity. J Adv Res. 2024. doi:10.1016/j.jare.2024.07.028
33. Delgado-Rizo V, Martínez-Guzmán MA, Iñiguez-Gutierrez L, García-Orozco A, Alvarado-Navarro A, Fafutis-Morris M. Neutrophil Extracellular Traps and Its Implications in Inflammation: an Overview. Front Immunol. 2017;8:81. doi:10.3389/fimmu.2017.00081
34. Kim WJ, Soh Y, Heo SM. Recent Advances of Therapeutic Targets for the Treatment of Periodontal Disease. Biomol Ther. 2021;29(3):263–267. doi:10.4062/biomolther.2021.001
35. Yang B, Pang X, Li Z, Chen Z, Wang Y. Immunomodulation in the Treatment of Periodontitis: progress and Perspectives. Front Immunol. 2021;12:781378. doi:10.3389/fimmu.2021.781378
36. Grabcanovic-Musija F, Obermayer A, Stoiber W, et al. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir Res. 2015;16(1):59. doi:10.1186/s12931-015-0221-7
37. Wang SW, Zhang Q, Lu D, et al. GPR84 regulates pulmonary inflammation by modulating neutrophil functions. Acta Pharmacol Sin. 2023;44(8):1665–1675. doi:10.1038/s41401-023-01080-z
38. Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24(7):908–922. doi:10.1038/s41591-018-0104-9
39. Luci C, Bourinet M, Leclère PS, Anty R, Gual P. Chronic Inflammation in Non-Alcoholic Steatohepatitis: molecular Mechanisms and Therapeutic Strategies. Front Endocrinol. 2020;11:597648. doi:10.3389/fendo.2020.597648
40. Antonucci L, Porcu C, Timperi E, Santini SJ, Iannucci G, Balsano C. Circulating Neutrophils of Nonalcoholic Steatohepatitis Patients Show an Activated Phenotype and Suppress T Lymphocytes Activity. J Immunol Res. 2020;2020:4570219. doi:10.1155/2020/4570219
41. Du J, Zhang J, Chen X, et al. Neutrophil extracellular traps induced by pro-inflammatory cytokines enhance procoagulant activity in NASH patients. Clin Res Hepatol Gastroenterol. 2022;46(1):101697. doi:10.1016/j.clinre.2021.101697
42. van der Windt DJ, Sud V, Zhang H, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68(4):1347–1360. doi:10.1002/hep.29914
43. Soehnlein O, Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov. 2021;20(8):589–610. doi:10.1038/s41573-021-00198-1
44. Schumski A, Ortega-Gómez A, Wichapong K, et al. Endotoxinemia Accelerates Atherosclerosis Through Electrostatic Charge-Mediated Monocyte Adhesion. Circulation. 2021;143(3):254–266. doi:10.1161/CIRCULATIONAHA.120.046677
45. Sheedy FJ, Grebe A, Rayner KJ, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14(8):812–820. doi:10.1038/ni.2639
46. Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. 2013;5(178):178ra40. doi:10.1126/scitranslmed.3005580
47. Talbot J, Bianchini FJ, Nascimento DC, et al. CCR2 Expression in Neutrophils Plays a Critical Role in Their Migration Into the Joints in Rheumatoid Arthritis. Arthritis Rheumatol. 2015;67(7):1751–1759. doi:10.1002/art.39117
48. Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targ Inflamm All. 2005;4(3):281–286. doi:10.2174/1568010054022024
49. Sun X, Gao J, Meng X, Lu X, Zhang L, Chen R. Polarized Macrophages in Periodontitis: characteristics, Function, and Molecular Signaling. Front Immunol. 2021;12:763334. doi:10.3389/fimmu.2021.763334
50. Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi:10.1016/j.immuni.2014.06.008
51. Zhou LN, Bi CS, Gao LN, et al. Macrophage polarization in human gingival tissue in response to periodontal disease. Oral Dis. 2019;25(1):265–273. doi:10.1111/odi.12983
52. van der Veen TA, de Groot LES, Melgert BN. The different faces of the macrophage in asthma. Curr Opin Pulm Med. 2020;26(1):62–68. doi:10.1097/MCP.0000000000000647
53. Theeuwes WF, Di Ceglie I, Dorst DN, et al. CD64 as novel molecular imaging marker for the characterization of synovitis in rheumatoid arthritis. Arthritis Res Ther. 2023;25(1):158. doi:10.1186/s13075-023-03147-y
54. Sczepanik FSC, Grossi ML, Casati M, et al. Periodontitis is an inflammatory disease of oxidative stress: we should treat it that way. Periodontol. 2000;84(1):45–68. doi:10.1111/prd.12342
55. Li C, Xu MM, Wang K, Adler AJ, Vella AT, Zhou B. Macrophage polarization and meta-inflammation. Transl Res. 2018;191:29–44. doi:10.1016/j.trsl.2017.10.004
56. Eapen MS, Hansbro PM, McAlinden K, et al. Abnormal M1/M2 macrophage phenotype profiles in the small airway wall and lumen in smokers and chronic obstructive pulmonary disease (COPD). Sci Rep. 2017;7(1):13392. doi:10.1038/s41598-017-13888-x
57. Eltboli O, Bafadhel M, Hollins F, et al. COPD exacerbation severity and frequency is associated with impaired macrophage efferocytosis of eosinophils. BMC Pulm Med. 2014;14:112. doi:10.1186/1471-2466-14-112
58. Henrot P, Prevel R, Berger P, Dupin I. Chemokines in COPD: from Implication to Therapeutic Use. Int J Mol Sci. 2019;20(11):2785. doi:10.3390/ijms20112785
59. Chen X, Tang J, Shuai W, Meng J, Feng J, Han Z. Macrophage polarization and its role in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Inflamm Res. 2020;69(9):883–895. doi:10.1007/s00011-020-01378-2
60. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166–6173. doi:10.4049/jimmunol.164.12.6166
61. Garaicoa-Pazmino C, Fretwurst T, Squarize CH, et al. Characterization of macrophage polarization in periodontal disease. J Clin Periodontol. 2019;46(8):830–839. doi:10.1111/jcpe.13156
62. Wang M, Xie J, Wang C, Zhong D, Xie L, Fang H. Immunomodulatory Properties of Stem Cells in Periodontitis: current Status and Future Prospective. Stem Cells Int. 2020;2020:9836518. doi:10.1155/2020/9836518
63. Luo R, Liu J, Cheng Q, Shionoya M, Gao C, Wang R. Oral microsphere formulation of M2 macrophage-mimetic Janus nanomotor for targeted therapy of ulcerative colitis. Sci Adv. 2024;10(26):eado6798. doi:10.1126/sciadv.ado6798
64. Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi:10.1146/annurev.immunol.18.1.767
65. Su X, Zhang J, Qin X. CD40 up-regulation on dendritic cells correlates with Th17/Treg imbalance in chronic periodontitis in young population. Innate Immun. 2020;26(6):482–489. doi:10.1177/1753425920917731
66. Zou J, Zeng Z, Xie W, Zeng Z. Immunotherapy with regulatory T and B cells in periodontitis. Int Immunopharmacol. 2022;109:108797. doi:10.1016/j.intimp.2022.108797
67. Arun KV, Talwar A, Kumar TS. T-helper cells in the etiopathogenesis of periodontal disease: a mini review. J Indian Soc Periodontol. 2011;15(1):4–10. doi:10.4103/0972-124X.82255
68. Sakaguchi S, Vignali DAA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nat Rev Immunol. 2013;13(6):461–467. doi:10.1038/nri3464
69. Rajendran M, Looney S, Singh N, et al. Systemic Antibiotic Therapy Reduces Circulating Inflammatory Dendritic Cells and Treg-Th17 Plasticity in Periodontitis. J Immunol. 2019;202(9):2690–2699. doi:10.4049/jimmunol.1900046
70. Mi W, Qiao S, Zhang X, Wu D, Zhou L, Lai H. PRMT5 inhibition modulates murine dendritic cells activation by inhibiting the metabolism switch: a new therapeutic target in periodontitis. Ann Transl Med. 2021;9(9):755. doi:10.21037/atm-20-7362
71. Meghil MM, Ghaly M, Cutler CW. A Tale of Two Fimbriae: how Invasion of Dendritic Cells by Porphyromonas gingivalis Disrupts DC Maturation and Depolarizes the T-Cell-Mediated Immune Response. Pathogens. 2022;11:3. doi:10.3390/pathogens11030328
72. Yang J, Zhu Y, Duan D, et al. Enhanced activity of macrophage M1/M2 phenotypes in periodontitis. Arch Oral Biol. 2018;96:234–242. doi:10.1016/j.archoralbio.2017.03.006
73. Kwiatkowski AJ, Stewart JM, Cho JJ, Avram D, Keselowsky BG. Nano and Microparticle Emerging Strategies for Treatment of Autoimmune Diseases: multiple Sclerosis and Type 1 Diabetes. Adv Healthc Mater. 2020;9(11):e2000164. doi:10.1002/adhm.202000164
74. Affabris E, Mangino G, Romeo G. Introduction to the special issue: extracellular vesicles and biological signal delivery in the inflammatory microenvironment. Cytokine Growth Factor Rev. 2020;51:10–11. doi:10.1016/j.cytogfr.2020.01.002
75. Alderton G, Scanlon ST. Inflammation. Science. 2021;374(6571):1068–1069. doi:10.1126/science.abn1721
76. Li J, Wang Y, Tang M, et al. New insights into nanotherapeutics for periodontitis: a triple concerto of antimicrobial activity, immunomodulation and periodontium regeneration. J Nanobiotech. 2024;22(1):19. doi:10.1186/s12951-023-02261-y
77. Wang L, Li Y, Ren M, et al. pH and lipase-responsive nanocarrier-mediated dual drug delivery system to treat periodontitis in diabetic rats. Bioact Mater. 2022;18:254–266. doi:10.1016/j.bioactmat.2022.02.008
78. Nunes R, Neves JD, Sarmento B. Nanoparticles for the regulation of intestinal inflammation: opportunities and challenges. Nanomed. 2019;14(19):2631–2644. doi:10.2217/nnm-2019-0191
79. Howard M, Zern BJ, Anselmo AC, Shuvaev VV, Mitragotri S, Muzykantov V. Vascular targeting of nanocarriers: perplexing aspects of the seemingly straightforward paradigm. ACS Nano. 2014;8(5):4100–4132. doi:10.1021/nn500136z
80. Zięba M, Chaber P, Duale K, et al. Polymeric Carriers for Delivery Systems in the Treatment of Chronic Periodontal Disease. Polymers. 2020;12:7. doi:10.3390/polym12071574
81. Ren H, He Y, Liang J, et al. Role of Liposome Size, Surface Charge, and PEGylation on Rheumatoid Arthritis Targeting Therapy. ACS Appl Mater Interfaces. 2019;11(22):20304–20315. doi:10.1021/acsami.8b22693
82. Kumaar NR, Nair SC. Nanomaterials: an intra-periodontal pocket drug-delivery system for periodontitis. Ther Deliv. 2023;14(3):227–249. doi:10.4155/tde-2023-0001
83. Da Silva CG, Rueda F, Löwik CW, Ossendorp F, Cruz LJ. Combinatorial prospects of nano-targeted chemoimmunotherapy. Biomaterials. 2016;83:308–320. doi:10.1016/j.biomaterials.2016.01.006
84. Gao L, Li J, Song T. Poly lactic-co-glycolic acid-based nanoparticles as delivery systems for enhanced cancer immunotherapy. Front Chem. 2022;10:973666. doi:10.3389/fchem.2022.973666
85. Zhang Z, Tsai PC, Ramezanli T, Michniak-Kohn BB. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip Rev Nanomed Nanobiotech. 2013;5(3):205–218. doi:10.1002/wnan.1211
86. Soni KS, Desale SS, Bronich TK. Nanogels: an overview of properties, biomedical applications and obstacles to clinical translation. J Control Release. 2016;240:109–126. doi:10.1016/j.jconrel.2015.11.009
87. Fang CL, Al-Suwayeh SA, Fang JY. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat Nanotechnol. 2013;7(1):41–55. doi:10.2174/187221013804484827
88. Dobreva M, Stefanov S, Andonova V. Natural Lipids as Structural Components of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Topical Delivery. Curr Pharm Des. 2020;26(36):4524–4535. doi:10.2174/1381612826666200514221649
89. Khosa A, Reddi S, Saha RN. Nanostructured lipid carriers for site-specific drug delivery. Biomed Pharmacother. 2018;103:598–613. doi:10.1016/j.biopha.2018.04.055
90. Bianchi E, Vigani B, Viseras C, Ferrari F, Rossi S, Sandri G. Inorganic Nanomaterials in Tissue Engineering. Pharmaceutics. 2022;14:6. doi:10.3390/pharmaceutics14061127
91. Yang G, Phua SZF, Bindra AK, Zhao Y. Degradability and Clearance of Inorganic Nanoparticles for Biomedical Applications. Adv Mater. 2019;31(10):e1805730. doi:10.1002/adma.201805730
92. Wei Y, Quan L, Zhou C, Zhan Q. Factors relating to the biodistribution & clearance of nanoparticles & their effects on in vivo application. Nanomed. 2018;13(12):1495–1512. doi:10.2217/nnm-2018-0040
93. Li W, Su Z, Hao M, Ju C, Zhang C. Cytopharmaceuticals: an emerging paradigm for drug delivery. J Control Release. 2020;328:313–324. doi:10.1016/j.jconrel.2020.08.063
94. Jin K, Luo Z, Zhang B, Pang Z. Biomimetic nanoparticles for inflammation targeting. Acta Pharm Sin B. 2018;8(1):23–33. doi:10.1016/j.apsb.2017.12.002
95. Ejigah V, Owoseni O, Bataille-Backer P, Ogundipe OD, Fisusi FA, Adesina SK. Approaches to Improve Macromolecule and Nanoparticle Accumulation in the Tumor Microenvironment by the Enhanced Permeability and Retention Effect. Polymers. 2022;14:13. doi:10.3390/polym14132601
96. Neog MK, Rasool M. Targeted delivery of p-coumaric acid encapsulated mannosylated liposomes to the synovial macrophages inhibits osteoclast formation and bone resorption in the rheumatoid arthritis animal model. Eur J Pharm Biopharm. 2018;133:162–175. doi:10.1016/j.ejpb.2018.10.010
97. Vijayan V, Uthaman S, Park IK. Cell Membrane Coated Nanoparticles: an Emerging Biomimetic Nanoplatform for Targeted Bioimaging and Therapy. Adv Exp Med Biol. 2018;1064:45–59.
98. Shi Y, Lammers T. Combining Nanomedicine and Immunotherapy. Acc Chem Res. 2019;52(6):1543–1554. doi:10.1021/acs.accounts.9b00148
99. Azevedo S, Costa-Almeida R, Santos SG, Magalhães FD, Pinto AM. Advances in carbon nanomaterials for immunotherapy. Appl Mater Today. 2022;27.
100. Figueiredo RDA, Ortega AC, González Maldonado LA, et al. Perillyl alcohol has antibacterial effects and reduces ROS production in macrophages. J Appl Oral Sci. 2020;28:e20190519. doi:10.1590/1678-7757-2019-0519
101. Kim J, Kim HY, Song SY, et al. Synergistic Oxygen Generation and Reactive Oxygen Species Scavenging by Manganese Ferrite/Ceria Co-decorated Nanoparticles for Rheumatoid Arthritis Treatment. ACS Nano. 2019;13(3):3206–3217. doi:10.1021/acsnano.8b08785
102. Sun Y, Sun X, Li X, et al. A versatile nanocomposite based on nanoceria for antibacterial enhancement and protection from aPDT-aggravated inflammation via modulation of macrophage polarization. Biomaterials. 2021;268:120614. doi:10.1016/j.biomaterials.2020.120614
103. Ni C, Zhou J, Kong N, et al. Gold nanoparticles modulate the crosstalk between macrophages and periodontal ligament cells for periodontitis treatment. Biomaterials. 2019;206:115–132. doi:10.1016/j.biomaterials.2019.03.039
104. Hu D, Li R, Li Y, et al. Inflammation-Targeted Nanomedicines Alleviate Oxidative Stress and Reprogram Macrophages Polarization for Myocardial Infarction Treatment. Adv Sci. 2024;11(21):e2308910. doi:10.1002/advs.202308910
105. Galarraga-Vinueza ME, Dohle E, Ramanauskaite A, et al. Anti-inflammatory and macrophage polarization effects of Cranberry Proanthocyanidins (PACs) for periodontal and peri-implant disease therapy. J Periodontal Res. 2020;55(6):821–829. doi:10.1111/jre.12773
106. Wang Y, Li C, Wan Y, et al. Quercetin-Loaded Ceria Nanocomposite Potentiate Dual-Directional Immunoregulation via Macrophage Polarization against Periodontal Inflammation. Small. 2021;17(41):e2101505. doi:10.1002/smll.202101505
107. Shi J, Zhang Y, Zhang X, et al. Remodeling immune microenvironment in periodontitis using resveratrol liposomes as an antibiotic-free therapeutic strategy. J Nanobiotech. 2021;19(1):429. doi:10.1186/s12951-021-01175-x
108. Muhammad W, Zhu J, Zhai Z, et al. ROS-responsive polymer nanoparticles with enhanced loading of dexamethasone effectively modulate the lung injury microenvironment. Acta Biomater. 2022;148:258–270. doi:10.1016/j.actbio.2022.06.024
109. Ma Y, Lu Z, Jia B, et al. DNA Origami as a Nanomedicine for Targeted Rheumatoid Arthritis Therapy through Reactive Oxygen Species and Nitric Oxide Scavenging. ACS Nano. 2022;16(8):12520–12531. doi:10.1021/acsnano.2c03991
110. Sun X, Dong S, Li X, et al. Delivery of siRNA using folate receptor-targeted pH-sensitive polymeric nanoparticles for rheumatoid arthritis therapy. Nanomedicine. 2019;20:102017. doi:10.1016/j.nano.2019.102017
111. Yu C, Li X, Hou Y, et al. Hyaluronic Acid Coated Acid-Sensitive Nanoparticles for Targeted Therapy of Adjuvant-Induced Arthritis in Rats. Molecules. 2019;24:1.
112. Yang M, Feng X, Ding J, Chang F, Chen X. Nanotherapeutics relieve rheumatoid arthritis. J Control Release. 2017;252:108–124. doi:10.1016/j.jconrel.2017.02.032
113. Yang R, Yan L, Xu T, et al. Injectable bioadhesive hydrogel as a local nanomedicine depot for targeted regulation of inflammation and ferroptosis in rheumatoid arthritis. Biomaterials. 2024;311:122706. doi:10.1016/j.biomaterials.2024.122706
114. Jie F. Role and Mechanism of Macrophage-Targeted Curcumin Nanodrug Microenvironment Remodeling in vitro Master. Chengdu University of Traditional Chinese Medicine; 2023.
115. Yan N, Xu J, Liu G, et al. Penetrating Macrophage-Based Nanoformulation for Periodontitis Treatment. ACS Nano. 2022;16(11):18253–18265. doi:10.1021/acsnano.2c05923
116. Low PS, Henne WA, Doorneweerd DD. Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases. Acc Chem Res. 2008;41(1):120–129. doi:10.1021/ar7000815
117. Sun J, Ju F, Jin J, et al. M2 Macrophage Membrane-Mediated Biomimetic-Nanoparticle Carrying COX-siRNA Targeted Delivery for Prevention of Tendon Adhesions by Inhibiting Inflammation. Small. 2023;19(33):e2300326. doi:10.1002/smll.202300326
118. Lokeshwar VB, Mirza S, Jordan A. Targeting hyaluronic acid family for cancer chemoprevention and therapy. Adv Cancer Res. 2014;123:35–65.
119. Jia N, Gao Y, Li M, et al. Metabolic reprogramming of proinflammatory macrophages by target delivered roburic acid effectively ameliorates rheumatoid arthritis symptoms. Signal Transduct Target Ther. 2023;8(1):280. doi:10.1038/s41392-023-01499-0
120. Feng Naibo. The therapeutic effect and mechanism of biresponsive targeted nanomaterial loaded siERN1 in autoimmune inflammatory diseases. PhD, Chongqing Medical University, 2023.
121. Tan T, Huang Q, Chu W, et al. Delivery of germacrone (GER) using macrophages-targeted polymeric nanoparticles and its application in rheumatoid arthritis. Drug Deliv. 2022;29(1):692–701. doi:10.1080/10717544.2022.2044936
122. Zhang Z, Yu Y, Zhu G, et al. The Emerging Role of Plant-Derived Exosomes-Like Nanoparticles in Immune Regulation and Periodontitis Treatment. Front Immunol. 2022;13:896745. doi:10.3389/fimmu.2022.896745
123. Yuan H, Ma Q, Ye L, Piao G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules. 2016;21:5. doi:10.3390/molecules21050559
124. Isola G, Galarraga‐Vinueza ME, Dohle E. Current Evidence of Natural Agents in Oral and Periodontal Health. Nutrients. 2020;12:2. doi:10.3390/nu12020585
125. Xiao J, Feng S, Wang X, et al. Identification of exosome-like nanoparticle-derived microRNAs from 11 edible fruits and vegetables. PeerJ. 2018;6:e5186. doi:10.7717/peerj.5186
126. Chen X, Zhou Y, Yu J. Exosome-like Nanoparticles from Ginger Rhizomes Inhibited NLRP3 Inflammasome Activation. Mol Pharm. 2019;16(6):2690–2699. doi:10.1021/acs.molpharmaceut.9b00246
127. Cao M, Yan H, Han X, et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunother Cancer. 2019;7(1):326. doi:10.1186/s40425-019-0817-4
128. Bai C, Liu J, Zhang X, et al. Research status and challenges of plant-derived exosome-like nanoparticles. Biomed Pharmacother. 2024;174:116543. doi:10.1016/j.biopha.2024.116543
129. Yi Q, Xu Z, Thakur A, et al. Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol Res. 2023;190:106733. doi:10.1016/j.phrs.2023.106733
130. Zu M, Xie D, Canup BSB, et al. ‘Green’ nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials. 2021;279:121178. doi:10.1016/j.biomaterials.2021.121178
131. Liu B, Lu Y, Chen X, et al. Protective Role of Shiitake Mushroom-Derived Exosome-Like Nanoparticles in D-Galactosamine and Lipopolysaccharide-Induced Acute Liver Injury in Mice. Nutrients. 2020;12:2.
132. Han R, Ren Z, Wang Q, et al. Synthetic Biomimetic Liposomes Harness Efferocytosis Machinery for Highly Efficient Macrophages-Targeted Drug Delivery to Alleviate Inflammation. Adv Sci. 2024;11(29):e2308325. doi:10.1002/advs.202308325
133. Gour N, Yong HM, Magesh A, et al. A GPCR-neuropeptide axis dampens hyperactive neutrophils by promoting an alternative-like polarization during bacterial infection. Immunity. 2024;57(2):333–348.e6. doi:10.1016/j.immuni.2024.01.003
134. Kang T, Zhu Q, Wei D, et al. Nanoparticles Coated with Neutrophil Membranes Can Effectively Treat Cancer Metastasis. ACS Nano. 2017;11(2):1397–1411. doi:10.1021/acsnano.6b06477
135. Yang N, Li M, Wu L, et al. Peptide-anchored neutrophil membrane-coated biomimetic nanodrug for targeted treatment of rheumatoid arthritis. J Nanobiotech. 2023;21(1):13. doi:10.1186/s12951-023-01773-x
136. Wang Z, Li J, Cho J, Malik AB. Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils. Nat Nanotechnol. 2014;9(3):204–210. doi:10.1038/nnano.2014.17
137. Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17(9):688. doi:10.1038/nrd.2018.149
138. Liu S, Du J, Li D, et al. Oxidative stress induced pyroptosis leads to osteogenic dysfunction of MG63 cells. J Mol Histol. 2020;51(3):221–232. doi:10.1007/s10735-020-09874-9
139. Bakhshi S, Shamsi S. MCC950 in the treatment of NLRP3-mediated inflammatory diseases: latest evidence and therapeutic outcomes. Int Immunopharmacol. 2022;106:108595. doi:10.1016/j.intimp.2022.108595
140. Yang H, Marion TN, Liu Y, et al. Nanomaterial Exposure Induced Neutrophil Extracellular Traps: a New Target in Inflammation and Innate Immunity. J Immunol Res. 2019;2019:3560180. doi:10.1155/2019/3560180
141. Hu K, Zhong L, Lin W, et al. Pathogenesis-Guided Rational Engineering of Nanotherapies for the Targeted Treatment of Abdominal Aortic Aneurysm by Inhibiting Neutrophilic Inflammation. ACS Nano. 2024;18(8):6650–6672. doi:10.1021/acsnano.4c00120
142. Chen Y-F, Chiou Y-H, Chen Y-C, Jiang Y-S, Lee T-Y, Jan J-S. ZnO-loaded DNA nanogels as neutrophil extracellular trap-like structures in the treatment of mouse peritonitis. Mater Sci Eng C Mater Biol Appl. 2021;131:112484. doi:10.1016/j.msec.2021.112484
143. Liu S, Liu M, Xiu J, et al. Celastrol-loaded bovine serum albumin nanoparticles target inflamed neutrophils for improved rheumatoid arthritis therapy. Acta Biomater. 2024;174:345–357. doi:10.1016/j.actbio.2023.11.028
144. Zhou Y, Xu L, Jin P, et al. NET-targeted nanoparticles for antithrombotic therapy in pregnancy. iScience. 2024;27(5):109823. doi:10.1016/j.isci.2024.109823
145. Hu CM, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108(27):10980–10985. doi:10.1073/pnas.1106634108
146. Wang H, Liu Y, He R, et al. Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery. Biomater Sci. 2020;8(2):552–568. doi:10.1039/C9BM01392J
147. Wu Y, Wan S, Yang S, et al. Macrophage cell membrane-based nanoparticles: a new promising biomimetic platform for targeted delivery and treatment. J Nanobiotech. 2022;20(1):542. doi:10.1186/s12951-022-01746-6
148. Zhao H, Li L, Zhang J, et al. C–C Chemokine Ligand 2 (CCL2) Recruits Macrophage-Membrane-Camouflaged Hollow Bismuth Selenide Nanoparticles To Facilitate Photothermal Sensitivity and Inhibit Lung Metastasis of Breast Cancer. ACS Appl Mater Interfaces. 2018;10(37):31124–31135. doi:10.1021/acsami.8b11645
149. Ma Y, Yang H, Zong X, et al. Artificial M2 macrophages for disease-modifying osteoarthritis therapeutics. Biomaterials. 2021;274:120865. doi:10.1016/j.biomaterials.2021.120865
150. Gao C, Huang Q, Liu C, et al. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat Commun. 2020;11(1):2622. doi:10.1038/s41467-020-16439-7
151. Deng Y, Zheng H, Li B, et al. Nanomedicines targeting activated immune cells and effector cells for rheumatoid arthritis treatment. J Control Release. 2024;371:498–515. doi:10.1016/j.jconrel.2024.06.010
152. Yu Q, Huang Y, Chen X, et al. A neutrophil cell membrane-biomimetic nanoplatform based on L-arginine nanoparticles for early osteoarthritis diagnosis and nitric oxide therapy. Nanoscale. 2022;14(32):11619–11634. doi:10.1039/D2NR02601E
153. Yang N. Effect and mechanism of targeted treatment of RA by biomimetic nanomaterials coated with polypeptide modified neutrophil cell membrane. Master. 2023.
154. Wang W, Gao Y, Zhang M, Li Y, Tang BZ. Neutrophil-like Biomimic AIE Nanoparticles with High-Efficiency Inflammatory Cytokine Targeting Enable Precise Photothermal Therapy and Alleviation of Inflammation. ACS Nano. 2023;17(8):7394–7405. doi:10.1021/acsnano.2c11762
155. Zhang Q, Hu C, Feng J, et al. Anti-inflammatory mechanisms of neutrophil membrane-coated nanoparticles without drug loading. J Control Release. 2024;369:12–24. doi:10.1016/j.jconrel.2024.03.030
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

