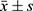Back to Journals » Infection and Drug Resistance » Volume 18
Application of Short Peptide Enteral Nutrition in Mechanically Ventilated Critically Ill Children with Severe Pneumonia and Its Impact on Patient Recovery
Authors Xu Z, Ding Y, Deng XM, Mao XX, Xia PF, Li DR, Lu YP
Received 25 October 2024
Accepted for publication 30 December 2024
Published 21 January 2025 Volume 2025:18 Pages 419—426
DOI https://doi.org/10.2147/IDR.S502880
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sandip Patil
Zhe Xu,1 Yi Ding,1 Xue-Mei Deng,1 Xiu-Xiu Mao,1 Ping-Fan Xia,1 Deng-Ran Li,1 Yong-Ping Lu2
1Department of Pediatrics, Guangyuan Central Hospital, Guangyuan, People’s Republic of China; 2Science & Technology Innovation Center, Guangyuan Central Hospital, Guangyuan, People’s Republic of China
Correspondence: Yong-Ping Lu, Science & Technology Innovation Center, Guangyuan Central Hospital, Guangyuan, People’s Republic of China, Email [email protected]
Objective: To explore the application of short-peptide enteral nutrition formulation in mechanically ventilated pediatric patients with severe pneumonia and its impact on rehabilitation outcomes, providing practical clinical evidence for the nutritional support strategy in critically ill pneumonia children.
Methods: This study retrospectively analyzed the clinical data of 90 neonatal pneumonia patients undergoing mechanical ventilation from May 2022 to December 2023. The patients were divided into an experimental group receiving short peptide enteral nutrition formulation via nasogastric tube and a control group receiving whole-protein enteral nutrition formulation via nasogastric tube. The nutritional risk was assessed using STRONGkids, and the nutritional status was analyzed through biochemical protein indicators. Additionally, mechanical ventilation time, hospitalization duration, incidence of ventilator-associated pneumonia (VAP), and disease outcomes were recorded and compared between the two groups.
Results: Both groups were assessed with medium to high nutritional risk, with no significant difference in the degree of nutritional risk (P > 0.05). After intervention, total protein, albumin, and prealbumin levels increased in both groups, with the experimental group showing significantly higher levels than the control group (P < 0.05). VAP predominantly occurred in the control group, with an incidence rate of 7% (3/45), showing a statistically significant difference between the two groups (P < 0.05). The experimental group exhibited significantly shorter Length of hospital stay and mechanical ventilation duration compared to the control group (P < 0.05). Moreover, there was no statistically significant difference in disease outcomes between the two groups (P > 0.05).
Conclusion: Short peptide enteral nutrition formulation contributes to improving the treatment outcomes of mechanically ventilated pneumonia patients, providing a therapeutic approach for the nutritional support of critically ill children requiring mechanical ventilation.
Keywords: short peptide enteral nutrition formulation, mechanical ventilation, pediatric patients with severe pneumonia, nutritional status
Introduction
Severe pneumonia is a common acute respiratory infection in children, characterized by a rapid onset, complex progression, and a high complication rate. In recent years, with the widespread use of antibiotics, the mortality rate has decreased, but the incidence of complications remains high, posing a threat to the life and health of children.1 Neonatal pneumonia accounts for 10% to 20% of the causes of neonatal death.2 The rapid and recurrent nature of severe pneumonia, often accompanied by clinical manifestations such as fever, sputum production, cough, shortness of breath, and pulmonary rales, makes its course challenging. As the condition worsens, the child’s digestive, nervous, and circulatory systems may be affected, seriously jeopardizing their life.3,4 Therefore, the timely and effective treatment of neonatal pneumonia is a focal point for clinical researchers.
Mechanical ventilation is an important therapeutic measure in the treatment of severe pneumonia, with a significant effect on improving clinical symptoms in children. However, it is essential to note that in actual clinical practice, some children may develop obstructive bronchiolitis as a complication, which could adversely affect treatment outcomes.5 Additionally, the nutritional status and related nutritional support issues of critically ill children are receiving increasing attention. Studies have found that during the hospitalization in the Pediatric Intensive Care Unit (PICU), nearly one-third of children fail to receive optimal nutritional support.6 The nutritional status of critically ill children is correlated with prognosis, and malnutrition is associated with increased duration of ventilator use, length of hospital stay, mortality rate, organ dysfunction, and the incidence of complications.7 Rational nutritional support plays a crucial role in the recovery process of the disease. Currently, Chinese guidelines prioritize enteral nutrition.8 However, critically ill children often experience varying degrees of gastrointestinal dysfunction, making the selection of suitable enteral nutrition formulations essential.
EEN formulations can be categorized into three types based on their nitrogen sources: whole-protein, short-peptide, and amino-acid-based formulations. Whole-protein EEN formulations rely on intact proteins as the nitrogen source, which require digestion and absorption in the intestine.9 For patients with impaired intestinal function, this could increase the burden on the gut. Amino-acid-based formulations, on the other hand, have high osmolarity, which can lead to diarrhea and may result in essential fatty acid deficiency.10 Short-peptide EEN formulations, composed primarily of protein hydrolysates, can be absorbed directly by the intestine without digestion, thus avoiding additional stress on the gut. Studies have shown that the body primarily absorbs protein in the form of short peptides, with free amino acids being less efficient. Short peptides also serve as energy substrates for intestinal mucosal epithelial cells, promoting the development of intestinal mucosal tissue.11,12 Therefore, short-peptide EEN formulations are more suitable for repairing damaged intestinal mucosa.
This study provides a detailed analysis of the application of short-peptide enteral nutrition formulations in children with pneumonia caused by mechanical ventilation, aiming to improve the nutritional status and promote recovery. It explores the effectiveness of short-peptide enteral nutrition formulations in critically ill children with pneumonia, offering practical clinical evidence for the nutritional support strategy in critically ill children.
Materials and Methods
Clinical Patient Data
In this study, we selected the medical records of 90 neonatal pneumonia patients who underwent mechanical ventilation treatment between May 2022 and December 2023 as the research subjects. These patients met the diagnostic criteria for severe pneumonia.1 General clinical data, including age, gender, admission and discharge weight, length, oxygenation index, etc., were recorded. Inclusion criteria were as follows: ① Meeting the diagnostic criteria for severe pneumonia. ② Meeting the indications for mechanical ventilation. ③ Informed consent signed by the guardians. Exclusion criteria were: ① Severe hepatic or renal dysfunction. ② Concomitant digestive system surgical diseases such as gastrointestinal bleeding or intestinal obstruction. This study was approved by the Ethics Committee of Guangyuan Central Hospital. Informed consent was obtained from the guardians of all subjects. The study complies with the principles outlined in the Declaration of Helsinki.
Methods
All patients received mechanical ventilation treatment. The 90 patients were evenly divided into two groups based on the order of admission, with 45 patients in each group. The experimental group received short-peptide enteral nutrition formulations (Peptamen, Nestle) through a nasogastric tube, while the control group received whole-protein enteral nutrition formulations (Nutrison, Nestle) through a nasogastric tube. Parenteral nutrition was supplemented in the early stage of intervention, and no additional parenteral nutrition was provided later.
Within the first 48 hours of admission, all patients initiated nutritional support. Nasogastric tube feeding was performed every 3 hours, with an initial volume between 10–20 mL, gradually increased by 5–10 mL, and decreased or temporarily paused if intolerance occurred. If insufficient, “all-in-one” parenteral nutrition supplementation, initiated at 0.5–1.0 g/(kg•d) through slow intravenous infusion, was gradually increased to 3.0–3.5 g/(kg•d) following Chinese guidelines recommendations.13
Resting energy expenditure (REE) for each child was calculated using the Schofield formula as a basis for determining the minimum energy requirements. The nutritional support plan was dynamically monitored and adjusted according to the patient’s condition to gradually meet their physiological needs. Gastrointestinal tolerance in both groups was carefully observed and recorded, including abdominal distension, diarrhea, and gastrointestinal bleeding.
Clinical outcomes were recorded in detail, including mechanical ventilation time, length of PICU stay, and the occurrence of ventilator-associated pneumonia (VAP). The diagnosis criteria for VAP were referenced from the 2010 Pediatric Hospital-Acquired Pneumonia Management Program.3 Through these comprehensive and accurate data, a thorough assessment of the treatment outcomes was ensured, providing comprehensive attention to the patient’s condition.
Observational Indices
Primary Observational Indicators
Comparison of hospitalization time and mechanical ventilation time between the two groups. Evaluation of disease development results in the two groups, including the incidence of ventilator-associated pneumonia (VAP).
Nutritional Risk Scoring: To comprehensively understand the nutritional status of the children, the STRONGkids nutritional risk screening tool14 was used to score all patients for nutritional risk, with detailed records. The scoring included four aspects to assess the nutritional status of children. A score of 0 indicated low nutritional risk, requiring no nutritional intervention. Scores of 1–3 indicated moderate nutritional risk, necessitating nutritional intervention with weight monitoring and weekly reassessment. Scores of 4 and above indicated high nutritional risk, requiring consultation with a nutritionist.
Assessment of Critical Illness Severity in Children: To accurately assess the severity of the children’s condition, the Pediatric Critical Illness Score (PCIS)15 was used to score all patients, with detailed records. Based on the scoring results, patients were categorized into non-critical group (PCIS > 80 points), critical group (70 points ≤ PCIS ≤ 80 points), and extremely critical group (PCIS ≤ 70 points).
Secondary Observational Indicators
Fasting venous blood samples (5 mL) were collected from both groups before the intervention and on the 8th day post-intervention. The samples were centrifuged at 3000 r/min for 10 minutes, and serum nutritional indicators were analyzed using a fully automated biochemical analyzer (Model: BK-280, Manufacturer: Jinan Chengteng Biotechnology Co., Ltd.) and enzyme-linked immunosorbent assay (ELISA). The primary indicators assessed included total protein (TP), albumin (ALB), and prealbumin (PA).
Statistical Analysis
Data were processed using GraphPad Prism 9 software and statistically analyzed using SPSS 20.0 software. For normally distributed quantitative data, the mean ± SD deviation (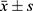 ) was used, and an independent sample t-test was employed for between-group comparisons. Count data were expressed as counts and percentages (%), and a chi-square test was used for between-group comparisons. The significance level was set at P < 0.05, indicating statistical significance when differences were observed.
) was used, and an independent sample t-test was employed for between-group comparisons. Count data were expressed as counts and percentages (%), and a chi-square test was used for between-group comparisons. The significance level was set at P < 0.05, indicating statistical significance when differences were observed.
Results
Baseline Data Comparison
There were no significant differences in general clinical data between the two groups of patients (P > 0.05), as shown in Table 1.
 |
Table 1 General Clinical Data of Children with Severe Pneumonia |
Assessment of Nutritional Risk Level in Children
There was no significant difference in the degree of nutritional risk between the two groups (P > 0.05), as detailed in Table 2.
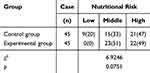 |
Table 2 Nutritional Risk Assessment of Children in Two Groups [Case/ (%)] |
Comparison of Serum Nutritional Indicators Between the Two Groups Before and After the Intervention
Before the intervention, there were no significant differences in albumin (ALB), prealbumin (PA), and total protein (TP) levels between the two groups (P > 0.05). After the intervention, the levels of ALB, PA, and TP increased significantly in both groups, with the experimental group exhibiting significantly higher levels than the control group (P < 0.05). Detailed data are presented in Figure 1. However, given that these markers can be influenced by inflammatory and acute stress responses, their interpretation should be considered in conjunction with clinical outcomes such as mechanical ventilation time and length of stay.
Comparison of Ventilator-Associated Pneumonia (VAP) Incidence, Mechanical Ventilation Time, and Length of Hospital Stay Between Two Groups
The incidence of VAP was mainly observed in the control group, with an incidence rate of 7% (3/45), and the difference between the two groups was statistically significant (P < 0.05). The length of hospital stay and mechanical ventilation time were significantly shorter in the experimental group compared to the control group. Detailed data can be found in Table 3.
 |
Table 3 Mechanical Ventilation Time and Length of Hospital Stay in Two Groups ( |
Comparison of Disease Development Results Between Two Groups
The results of disease development in both groups showed an overall effective rate of 95%, with a mortality rate of 2%. In terms of disease progression, there were no statistically significant differences between the two groups (P > 0.05). See details in Table 4.
 |
Table 4 Comparison of Disease Development Results Between Two Groups |
Discussion
Nutritional deficiencies in mechanically ventilated children can impair immune function and delay recovery. Malnutrition may lead to muscle atrophy, reduced respiratory muscle tension, weakened contractility and endurance, respiratory muscle fatigue, and difficulties in weaning from mechanical ventilation, ultimately affecting treatment efficacy.16 Appropriate nutritional support is therefore crucial and has become an integral part of comprehensive care for critically ill children. The incidence of malnutrition and nutritional risk in mechanically ventilated children is relatively high. Domestic studies have reported a significant correlation between mechanical ventilation and the occurrence of malnutrition. Univariate analyses also suggest that malnutrition is significantly associated with prolonged PICU stays.14,17
Among the 90 critically ill pneumonia patients included in this study, three cases of ventilator-associated pneumonia (VAP) were reported, all in the control group. These cases exhibited gastrointestinal intolerance, which may be related to bacterial translocation in the gastrointestinal tract. However, further clinical studies are needed to confirm this association. Literature has shown that early mechanical ventilation in pneumonia can significantly improve disease progression.15 In this study, the experimental group had significantly shorter hospital stays and mechanical ventilation durations compared to the control group, with statistical significance.
Previous research suggests that malnutrition may lead to longer hospital stays, increased ICU stay duration, higher infection rates, and ICU readmission rates. It may also contribute to multi-organ failure and increased in-hospital mortality risk.18 A study by Gonzalez et al19 found a high incidence of malnutrition in mechanically ventilated children in the PICU, with 88.8% of children experiencing insufficient feeding during mechanical ventilation. Most critically ill children fail to meet their nutritional needs during hospitalization. The same study reported that increasing energy intake from 33% to 66% of the prescribed target could reduce mortality in critically ill children. Similarly, Heffernan et al20 found that increasing energy supply significantly shortened hospital and mechanical ventilation durations, and the percentage of target energy supply was negatively correlated with mortality. In summary, the significantly shorter hospital stay and mechanical ventilation duration observed in the experimental group indicate that short-peptide nutritional supplements positively impact these outcomes.
Studies have shown that the more severe the child’s illness, the greater the nutritional risk.21 In this study, using the STRONGkids nutritional risk assessment, it was found that there was no significant difference in the degree of moderate to high nutritional risk between the two groups. Further analysis of biochemical protein indicators in critically ill children showed that the levels of total protein, albumin, and prealbumin in both groups increased after intervention, with a more significant increase in the experimental group. This suggests that short peptides can be absorbed by critically ill pneumonia children even when gastrointestinal function is impaired, promoting the recovery of intestinal tissue without increasing the gastrointestinal burden and resulting in better tolerance.22 It is well known that pneumonia children who develop pneumonia due to mechanical ventilation often have severe inflammation, and their bodies are in an acute stress state, which may lead to weakened or damaged gastrointestinal function. The use of short-peptide nutritional supplements is more beneficial for the recovery of intestinal mucosa in critically ill children.
Conclusion
This study found that short-peptide enteral nutrition support improved recovery in critically ill pediatric pneumonia patients, with shorter mechanical ventilation time and hospital stay compared to the control group. Although biochemical markers such as ALB, PA, and TP improved, the main clinical benefit was a faster recovery. No significant differences in disease outcomes or overall effectiveness were observed between the groups. These findings suggest that short-peptide nutrition support can enhance clinical recovery by reducing mechanical ventilation time and hospital stay. However, given the small sample size and single-center nature of the study, further multicenter trials with larger samples are needed to confirm these results.
Abbreviations
PCIS, Pediatric Critical Illness Score; PICU, Pediatric Intensive Care Unit; REE, Resting energy expenditure; VAP, ventilator-associated pneumonia.
Funding
This study was supported by Medical Research Program of Sichuan Province (S21100).
Disclosure
The authors state that there are no conflicts of interest to disclose for this work.
References
1. Celik NB, Tanyildiz M, Yetimakman F, et al. Comparison of high flow oxygen therapy versus noninvasive mechanical ventilation for successful weaning from invasive ventilation in children: an observational study. Medicine. 2022;101(39):e30889. doi:10.1097/MD.0000000000030889
2. Chen R, Liu Y, Dang H. Definition, risk factors, and outcome analysis of prolonged mechanical ventilation in children. Pediatr Pulmonol. 2024;59(10):2507–2516. doi:10.1002/ppul.27054
3. Greene C, Nian H, Zhu Y, et al. Associations between comorbidity-related functional limitations and pneumonia outcomes. J Hosp Med. 2022;17(7):527–533. doi:10.1002/jhm.12904
4. Ludovichetti FS, Zuccon A, Positello P, et al. Preventive oral hygiene and ventilator-associated pneumonia in paediatric intensive care unit. Eur J Paediatr Dent. 2022;23(4):298–302. doi:10.23804/ejpd.2022.23.04.09
5. Neves CC, Fiamenghi VI, Fontela PS, et al. Continuous clonidine infusion: an alternative for children on mechanical ventilation. Rev Assoc Med Bras. 2022;68(7):xxx. doi:10.1590/1806-9282.20220166
6. Slubowski D, Ruttan T. High-flow nasal cannula and noninvasive ventilation in pediatric emergency medicine. Pediatr Emerg Med Pract. 2020;17(8):1–24.
7. Tsou PY, Hayden LP. Obstructive sleep apnea is associated with use of assisted ventilation among children with bronchopulmonary dysplasia hospitalized with respiratory illness: a nationwide inpatient cohort. Sleep Med. 2023;109:181–189. doi:10.1016/j.sleep.2023.06.030
8. Yakame K, Shoji T, Kanazawa T, et al. Severe neonatal COVID-19 pneumonia requiring mechanical ventilation. Pediatr Int. 2022;64(1):e14677. doi:10.1111/ped.14677
9. Pang XF, Dai X-Y, Zhao L-J, et al. Short-peptide-based enteral nutrition affects rats MDP translocation and protects against gut-lung injury via the PepT1-NOD2-beclin-1 pathway in vivo. Mol Biol Rep. 2024;51(1):891. doi:10.1007/s11033-024-09759-0
10. Lim DW, Diané A, Muto M, et al. Differential effects on intestinal adaptation following exogenous glucagon-like peptide 2 therapy with and without enteral nutrition in neonatal short bowel syndrome [Formula: see text]. JPEN J Parenter Enteral Nutr. 2017;41(2):156–170. doi:10.1177/0148607116665812
11. Chen S, Bie R, Lai Y, et al. Trends and development in enteral nutrition application for ventilator associated pneumonia: a scientometric research study (1996-2018). Front Pharmacol. 2019;10:246. doi:10.3389/fphar.2019.00246
12. Doley J. Enteral Nutrition Overview. Nutrients. 2022;14(11):2180. doi:10.3390/nu14112180
13. Kocoshis SA, Merritt RJ, Hill S, et al. Safety and efficacy of teduglutide in pediatric patients with intestinal failure due to short bowel syndrome: a 24-week, phase III study. JPEN J Parenter Enteral Nutr. 2020;44(4):621–631. doi:10.1002/jpen.1690
14. Zhao X, Yan J, Wu B, et al. Sleep cycle in children with severe acute bronchopneumonia during mechanical ventilation at different depths of sedation. BMC Pediatr. 2022;22(1):589. doi:10.1186/s12887-022-03658-8
15. Elmahdi A, Eisa M, Omer E. Aspiration pneumonia in enteral feeding: a review on risks and prevention. Nutr Clin Pract. 2023;38(6):1247–1252. doi:10.1002/ncp.11020
16. Yang S, Lu S, Guo Y, et al. A comparative study of general and severe mycoplasma pneumoniae pneumonia in children. BMC Infect Dis. 2024;24(1):449. doi:10.1186/s12879-024-09340-x
17. Zhang J, Qu D, Ren X, et al. [High frequency oscillatory ventilation and conventional mechanical ventilation in the treatment of infants with severe respiratory syncytial virus pneumonia: a randomized controlled trial]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33(4):455–459. doi:10.3760/cma.j.cn121430-20201106-00706 Dutch
18. Fuentes Padilla P, Martínez G, Vernooij RW, et al. Early enteral nutrition (within 48 hours) versus delayed enteral nutrition (after 48 hours) with or without supplemental parenteral nutrition in critically ill adults. Cochrane Database Syst Rev. 2019;2019(10). doi:10.1002/14651858.CD012340.pub2
19. Gonzalez JT, Dirks ML, Holwerda AM, et al. Intermittent versus continuous enteral nutrition attenuates increases in insulin and leptin during short-term bed rest. Eur J Appl Physiol. 2020;120(9):2083–2094. doi:10.1007/s00421-020-04431-4
20. Heffernan AJ, Talekar C, Henain M, et al. Comparison of continuous versus intermittent enteral feeding in critically ill patients: a systematic review and meta-analysis. Crit Care. 2022;26(1):325. doi:10.1186/s13054-022-04140-8
21. Hermans AJH, Laarhuis BI, Kouw IWK, et al. Current insights in ICU nutrition: tailored nutrition. Curr Opin Crit Care. 2023;29(2):101–107. doi:10.1097/MCC.0000000000001016
22. Huang L, Li G, Zhou B, et al. Clinical effects of total protein and short peptide enteral nutrition during recovery after radical gastrectomy. Asia Pac J Clin Nutr. 2020;29(2):239–244. doi:10.6133/apjcn.202007_29(2).0005
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.