Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
Aripiprazole Lauroxil: Development and Evidence-Based Review of a Long-Acting Injectable Atypical Antipsychotic for the Treatment of Schizophrenia
Authors Citrome L , Correll CU , Cutler AJ, Dunbar M, Hoberg AR, Hopkinson C, Mattingly GW, McGrory JA , Rege B, Weiden PJ, McDonnell D
Received 16 October 2024
Accepted for publication 4 February 2025
Published 14 March 2025 Volume 2025:21 Pages 575—596
DOI https://doi.org/10.2147/NDT.S499367
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Taro Kishi
Long-Acting Injectable Atypical Antipsychotic in Schizophrenia – Video abstract [499367]
Views: 161
Leslie Citrome,1 Christoph U Correll,2– 6 Andrew J Cutler,7,8 Martin Dunbar,9 Amber R Hoberg,10 Craig Hopkinson,9 Gregory W Mattingly,11,12 James A McGrory,9 Bhaskar Rege,9 Peter J Weiden,13 David McDonnell14
1New York Medical College, Valhalla, NY, USA; 2Department of Psychiatry, The Zucker Hillside Hospital, Northwell Health, Glen Oaks, NY, USA; 3Department of Psychiatry and Molecular Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA; 4Center for Psychiatric Neuroscience, The Feinstein Institute for Medical Research, Northwell Health, New Hyde Park, NY, USA; 5Department of Child and Adolescent Psychiatry, Charité Universitätsmedizin Berlin, Berlin, Germany; 6German Center for Mental Health (DZPG), Partner Site Berlin, Berlin, Germany; 7Department of Psychiatry, SUNY Upstate Medical University, Syracuse, NY, USA; 8Neuroscience Education Institute, Lakewood Ranch, FL, USA; 9Alkermes, Inc, Waltham, MA, USA; 10WellMed Medical Management, South Texas Medical Center, San Antonio, TX, USA; 11Washington University School of Medicine, St. Louis, MO, USA; 12Midwest Research Group, St. Louis, MO, USA; 13Renaissance School of Medicine at Stony Brook University, Stony Brook, NY, USA; 14Alkermes Pharma Ireland Ltd, Dublin, Ireland
Abstract: This review article describes why and how aripiprazole was formulated as aripiprazole lauroxil (AL), an extended-release antipsychotic agent that is delivered via a long-acting injectable formulation, and the clinical trials investigating its use. AL was formulated as an inactive prodrug of aripiprazole using LinkeRx® technology to provide a prolonged-release antipsychotic with predictable dissolution over time. The resulting AL pharmacokinetic profile is characterized by a long half-life and little peak-to-trough aripiprazole concentration variability across dosing intervals of every 1 month, every 6 weeks, and every 2 months. The prodrug technology was further refined to develop an AL initiation formulation with a somewhat faster release of aripiprazole, eliminating the need for a 21-day oral aripiprazole supplementation period. With this initiation formulation, AL treatment can be started in 1 day. Key AL characteristics, including pharmacokinetic profile and efficacy, safety, and tolerability data, are presented. In addition to the efficacy and safety established in clinical trials of oral aripiprazole, a placebo-controlled 12-week pivotal study investigated AL 441 mg and 882 mg monthly regimens in patients with acutely exacerbated schizophrenia and provided efficacy and safety information that led to US Food and Drug Administration approval in 2015. Thereafter, studies established the long-term safety profile and durability of the AL treatment effect. The 25-week, active-controlled ALPINE study evaluated the feasibility and effectiveness of AL 1064 mg every 2 months, initiated using the 1-day AL initiation regimen, without further oral supplementation beyond day 1, in patients hospitalized for acutely exacerbated schizophrenia with subsequent transition to outpatient care. In short-term and long-term studies, AL was generally well tolerated at initiation and during acute and maintenance treatment. Pharmacokinetic, efficacy, and safety characteristics support the use of AL across inpatient and outpatient treatment settings.
Plain Language Summary: Treatment of schizophrenia with continuous antipsychotic medication has been shown to improve symptoms, daily functioning, and quality of life. One important option for antipsychotic treatment is a long-acting injectable medication. Antipsychotic injections can be administered weeks or months apart in place of daily oral medication. Aripiprazole lauroxil is a long-acting injectable antipsychotic for adults with schizophrenia that has several important features; it provides continuous, stable medication after monthly, every-6-week, or every-2-month injections, and multiple dose options are available (see video abstract). In addition, patients can start treatment with aripiprazole lauroxil using either a 1-day or 21-day initiation regimen with their first dose. The 1-day initiation regimen allows people with schizophrenia to start treatment during a doctor’s office visit or during a brief stay in the hospital. In short-term studies, aripiprazole lauroxil significantly reduced symptoms of acute schizophrenia. In longer-term studies, side effects were similar to those expected when using oral aripiprazole, and symptoms improved over extended durations of treatment lasting up to 3.5 years. Aripiprazole lauroxil’s safety and efficacy profile and multiple dosing and initiation options make it a treatment option for schizophrenia in inpatient and outpatient settings.
Keywords: dosing, pharmacokinetics, phase 3 clinical trials, safety, treatment efficacy, treatment initiation
Introduction
Long-acting injectable (LAI) antipsychotic medications have long been considered an option for long-term treatment of adults with schizophrenia.1 However, LAIs have been used primarily as a practical treatment method for addressing nonadherence to oral medication in patients with a history of poor adherence and relapse.2–7 Beyond that role, however, they can be used as a preferred antipsychotic formulation at any stage of schizophrenia treatment,8 in agreement with an evidence-based, patient-centered approach to the treatment of serious mental illness.1,9,10 LAIs offer reliable efficacy for the management of acute exacerbations of schizophrenia,8 reduce the risk of relapse and hospitalization,11–14 and provide consistent medication coverage during maintenance therapy.15 Moreover, LAI antipsychotic formulations have been associated with positive patient-reported outcomes, improved quality of life (QoL), and enhanced medication satisfaction.12,16–19 LAI antipsychotics can help strengthen the therapeutic alliance by supporting regular patient–clinician contact and removing the uncertainty regarding adherence to antipsychotic medication treatment.2,20
Several available atypical oral antipsychotics, including risperidone, paliperidone, olanzapine, and aripiprazole, are available as LAI formulations and are approved by the US Food and Drug Administration (FDA) for the treatment of schizophrenia in adults.21–27 When selecting an LAI antipsychotic, clinicians can match the unique features of current LAI options with the patient’s specific values and preferences.1 As with oral formulations, LAI antipsychotic selection is based in part on its efficacy and safety profile and the patient’s previous response to medications.1 Additional considerations for LAI choice include patient and physician familiarity with—and acceptance of—a particular option, pharmacokinetics and associated dosing considerations, site of administration, ease of use when preparing and injecting, and dosing interval, as well as other features inherent to particular formulations.3,28–30 In 2003, risperidone microspheres administered every 2 weeks became the first atypical LAI antipsychotic that received FDA approval.24 Since that time, additional LAIs that are differentiated according to active moiety and formulation characteristics have been developed, enabling tailored treatment approaches for individual patients.3,28,29
Aripiprazole lauroxil (AL [Aristada®, Alkermes, Inc., Waltham, MA]) was developed to offer an LAI treatment option differentiated from those available at the time of its FDA approval (2015).21 Aripiprazole was chosen for this new LAI formulation because of its known efficacy in treating symptoms of schizophrenia31–33 and its favorable safety and tolerability profile.34 AL was developed as a prodrug of aripiprazole, taking advantage of a proprietary technology to produce an LAI antipsychotic with specific pharmacokinetic characteristics,35 including having a long, slow, and predictable dissolution over time. In addition, the new prodrug formulation could be administered in a range of dosage strengths with 3 distinct injection interval options, could be placed in prefilled syringes,28,35,36 and had the potential to be further refined and developed as an initiation formulation, obviating the need for an extended period of oral aripiprazole supplementation at AL initiation.37
The aims of this article are to describe the development of AL and present the evidence base for its efficacy and safety in adults with schizophrenia from phase 3 and 4 clinical trials.
Development of Aripiprazole Lauroxil
Aripiprazole
Aripiprazole is an atypical antipsychotic with partial agonist activity at serotonin 5-HT1A receptors and antagonist activity at 5-HT2A receptors.38–41 It also has partial agonist activity at dopamine D2 receptors, differentiating it from earlier antipsychotics.40–43 Oral aripiprazole has a relatively long elimination half-life (approximately 75 hours),44 with steady state achieved by day 14.45 It has an established history of efficacy in treating schizophrenia.31–33 Treatment with oral aripiprazole is associated with a low risk of late-emerging adverse events (AEs) during long-term continuous exposure46,47 and with few dose-related AEs.34 Akathisia is among the most common AEs experienced by patients treated with aripiprazole;48,49 it generally occurs early in treatment and is mild or moderate in severity.48,50 Rates of other drug-induced movement disorders with aripiprazole are similar to those with other atypical antipsychotics.33,48 Aripiprazole has minimal or modest effects on metabolic parameters and weight gain33,51 and is not associated with elevated prolactin plasma concentrations.43
Prodrug Technology
Development of an LAI formulation of aripiprazole that would be an advance in drug-delivery technology required optimizing its pharmacokinetic profile so that it would enter the bloodstream gradually and consistently over weeks to months after injection.35 This advance was achieved via development of a prodrug of aripiprazole.35 Prodrugs are inactive agents that are converted by physiologic processes into the desired active agent. Use of a prodrug can result in improved pharmacokinetics, which can increase a medication’s effective half-life from hours to days or weeks, thus lengthening the interval during which plasma drug concentrations remain at a relevant level for clinical efficacy.15 AL was formulated as a prodrug of aripiprazole in which a fatty acid (lauric acid) “tail” is attached to the active aripiprazole molecule,52 forming stable crystalline particles in an aqueous suspension that can be injected into muscle tissue.35–37,52 The tail itself is attached reversibly using a proprietary linker technology (LinkeRx®, Alkermes, Inc., Waltham, MA).52,53 Once AL is administered, the fatty acid tail is cleaved from the linker by esterases, and then the linker dissociates from the aripiprazole molecule52,53 via hydrolysis (Figure 1), completing the 2-step bioconversion and releasing aripiprazole molecules into the bloodstream.52–54
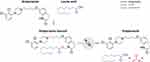 |
Figure 1 Aripiprazole lauroxil is a prodrug of aripiprazole.a Notes: aThe lauroxil tail (fatty acid chain; blue) is reversibly attached to aripiprazole (black) via a linker (red) using LinkeRx technology.35 After the prodrug in aqueous solution is injected, the fatty acid tail is cleaved from the linker by esterase activity, and then the linker dissociates from the active aripiprazole molecule52,53 by hydrolysis.52,53 The release of active aripiprazole is governed by the size of its crystalline particles, not by the rate of enzymatic conversion of the prodrug into active aripiprazole.52 |
The solubility and dissolution rates of AL, however, are further governed by the size of its crystalline particles, not just by the rate of enzymatic conversion of the prodrug into active aripiprazole.52 Larger particles dissolve more slowly than smaller particles because of their lower surface-to-volume ratio (Figure 2).55 The micrometer-sized particles of AL facilitate controlled and extended release over time as the prodrug molecules within the particles are initially protected from bioconversion, while only the outermost molecules are exposed to esterases and thus convert to active aripiprazole.52 The AL particle size allows for dosing intervals that can occur monthly, every 6 weeks, or every 2 months.52 At the time of its initial approval, AL treatment was initiated using 21 days of oral aripiprazole supplementation to increase plasma aripiprazole concentrations to relevant levels more rapidly during the initial 6 weeks of treatment.37
 |
Figure 2 Kinetics of ALNCD and aripiprazole lauroxil.a Abbreviation: ALNCD, aripiprazole lauroxil NanoCrystal Dispersion. Notes: Adapted from Jain R, Meyer J, Wehr A, Rege B, von Moltke L, Weiden PJ. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectrums. 2020;25(3):323–330. Copyright © 2019, Cambridge University Press. Creative Commons CC BY license.37 aSchematic of plasma aripiprazole levels over time of the nanomolar particle-sized ALNCD versus the micromolar particle-sized aripiprazole lauroxil. Plasma levels are based on model simulations of aripiprazole levels achieved after administration of ALNCD or aripiprazole lauroxil. |
To reduce the need for an extended period of oral aripiprazole supplementation at initiation, a second formulation of the AL prodrug molecule was developed (Aristada Initio®; Alkermes, Inc., Waltham, MA) with pharmacokinetics suited to deliver relevant plasma aripiprazole concentrations more rapidly after injection.37,56 This product was formulated using a NanoCrystal Dispersion technology to generate crystals smaller than those present in the maintenance AL formulation, enabling faster dissolution.52,57 The smaller nanometer-sized crystals in the AL NanoCrystal Dispersion formulation (ALNCD) dissolve faster than the larger micrometer-sized crystals in AL, resulting in a shorter delay in achieving relevant plasma concentrations (Figure 2).37 The short remaining lag time for reaching relevant aripiprazole concentrations after a 675 mg ALNCD injection can be addressed with a single 30 mg oral dose of aripiprazole administered on the same day as ALNCD for a 1-day initiation regimen.37 The AL maintenance dose (which can be any available dose level) can be administered the same day as the 1-day initiation regimen or up to 10 days later.21 Thus, on day 1, patients can receive an injection of AL, ALNCD, and a single dose of oral aripiprazole. The plasma aripiprazole levels of the 3 components separately and together are illustrated in Figure 3.
 |
Figure 3 Pharmacokinetics of aripiprazole lauroxil and 1-day initiation regimen components.a Abbreviations: AL, aripiprazole lauroxil; ALNCD, aripiprazole lauroxil NanoCrystal Dispersion. Notes: Adapted from Jain R, Meyer J, Wehr A, Rege B, von Moltke L, Weiden PJ. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectrums. 2020;25(3):323–330. Copyright © 2019, Cambridge University Press. Creative Commons CC BY license.37 Plasma concentrations based on model simulations of aripiprazole levels after administration of ALNCD, AL 1064 mg, one 30 mg dose of oral aripiprazole, or ALNCD + AL 1064 mg + 30 mg oral aripiprazole. Concentrations for oral aripiprazole are based on observed data. |
Pharmacokinetic Profile of Aripiprazole Lauroxil
The terminal half-life of AL ranges from approximately 54 to 57 days across dosage strengths (Supplementary Table 1),58,59 compared with approximately 75 hours for oral aripiprazole.44 ALNCD was designed to have a half-life of approximately 15 days, substantially shorter than that of AL. Figure 4 shows the slow rise in plasma aripiprazole concentrations after administration of the AL 441 mg monthly, AL 882 mg every-6-week, and AL 1064 mg every-2-month regimens (started without ALNCD or supplemental oral aripiprazole) and the extended aripiprazole exposure that occurs after an AL injection at steady state.60 As a consequence of this long, slow change in aripiprazole concentrations, little variation is observed across the dosing interval.35 The modest peak-to-trough ratio (1.1 for monthly regimens; 1.3 for 882 mg every 6 weeks35) indicates that AL dissolution results in consistent aripiprazole concentrations over the injection interval, with little variability around the average concentration at steady state.35 By comparison, a long-acting injectable aripiprazole formulated without a prodrug technology (aripiprazole monohydrate)61 has a shorter mean half-life (300 mg, 29.9 days; 400 mg, 46.5 days) and a greater peak-to-trough ratio (300 mg, 1.7; 400 mg, 1.5) for monthly dosing intervals.62,63
 |
Figure 4 Plasma aripiprazole concentrations with multiple-dose exposure to aripiprazole lauroxil.a Abbreviation: qxwk, every x weeks. Notes: Adapted with permission from Hard ML, Mills RJ, Sadler BM, Wehr AY, Weiden PJ, von Moltke L. Pharmacokinetic Profile of a 2-Month Dose Regimen of Aripiprazole Lauroxil: A Phase I Study and a Population Pharmacokinetic Model. CNS Drugs. 2017 Jul;31(7):617–624. © The Author(s) 2017. Creative Commons Attribution-NonCommercial 4.0 International License.60 aAripiprazole lauroxil was administered in a phase 1 study using 3 dosing regimens; no initiation regimen was used in this study. |
Aripiprazole Lauroxil Initiation and Dosing Options
The pharmacokinetic characteristics of the AL prodrug formulation leads to tailored options that may facilitate individualized treatment for adult patients with schizophrenia (as described in the video abstract). AL’s dissolution rate enabled the development of multiple dosing regimens with a range of injection intervals, including the first FDA-approved every-2-month LAI antipsychotic regimen.60 Altogether, 5 AL dosing regimens are available: AL 441 mg monthly, 662 mg monthly, and 882 mg monthly; AL 882 mg every 6 weeks; and AL 1064 mg every 2 months.21 Each of these dosing regimens provides predictable plasma aripiprazole levels that are generally stable over the dosing interval, whether 4, 6, or 8 weeks in duration.28,35,60
Aripiprazole dose exposures resulting from the 5 AL regimens fall within the range of approved oral aripiprazole dosages, from 10 to 20 mg or higher per day.21,44 Among the AL regimens, the 441 mg monthly regimen results in the lowest possible dose exposure, equivalent to oral aripiprazole 10 mg/day, while the AL 882 mg monthly regimen yields the highest possible dose exposure (Figure 5), equivalent to oral aripiprazole 20 mg or higher per day. The remaining 3 AL regimen options provide an intermediate dose exposure (equivalent to oral aripiprazole 15 mg/day), each based on a different dosing interval:35,60 AL 662 mg monthly, AL 882 mg every 6 weeks, and AL 1064 mg every 2 months. The range of AL dose-level and injection-interval options allows clinicians to select a regimen suited to an individual’s treatment needs64,65 and facilitates dose conversion when switching to LAI treatment with AL.21 The availability of higher dose strengths also allows AL to be used when co-prescribed with medications that act as a CYP 3A4 inducer, as described in the AL FDA prescribing information.21,66
A 12-week pivotal AL study (NCT01469039) established the efficacy of the 441 mg and 882 mg monthly regimens versus placebo67 and thus the lower and upper relevant plasma aripiprazole concentrations, respectively (ie, concentrations that were associated with clinical efficacy for those 2 doses).28 Based on data from a phase 1 study and population pharmacokinetic modelling, AL 662 mg monthly, 882 mg every-6-week, and 1064 mg every-2-month regimens each produced plasma aripiprazole concentrations that were intermediate between those upper and lower concentrations28,60,68 (Figure 5). Consequently, clinicians can select an AL regimen that yields lower, intermediate, or higher plasma aripiprazole concentrations,28 depending on patient needs and history of response to antipsychotic treatment. Further, the 3 regimens yielding comparable intermediate plasma aripiprazole concentrations over 3 different dosing interval options—monthly, every 6 weeks, and every 2 months—enable flexibility and personalization of the dosing interval for those patients prescribed an intermediate regimen28 (Figure 5).
 |
Figure 5 Steady-state plasma aripiprazole concentrations for the 5 aripiprazole lauroxil dosing regimens.a Abbreviation: AL, aripiprazole lauroxil. Notes: Adapted with permission from Hard ML, Mills RJ, Sadler BM, Wehr AY, Weiden PJ, von Moltke L. Pharmacokinetic Profile of a 2-Month Dose Regimen of Aripiprazole Lauroxil: A Phase I Study and a Population Pharmacokinetic Model. CNS Drugs. 2017;31(7):617-624. Creative Commons CC BY license.60 aPanel (A) Median simulated steady-state plasma aripiprazole concentrations for weeks 24–56 after initiation of each AL regimen using 21-day oral aripiprazole supplementation. Panel (B) Average simulated steady-state aripiprazole concentrations for the same AL regimens calculated for the injection interval starting at week 48 (arrow in panel [A]). The AL 441 mg monthly and 882 mg monthly regimens provide plasma aripiprazole exposure in the lower (equivalent to oral aripiprazole 10 mg/day) and higher (equivalent to oral aripiprazole 20 mg or higher per day) ranges, respectively, of aripiprazole concentrations associated with efficacy. The AL 662 mg monthly, 882 mg every-6-week, and 1064 mg every-2-month regimens provide intermediate-range aripiprazole concentrations (equivalent to oral aripiprazole 15 mg/day). Boxes, 25th to 75th percentiles; whiskers, 10th and 90th percentiles. |
Since the development of ALNCD, AL treatment can be initiated using either of 2 strategies: the 1-day regimen of one ALNCD injection and a single 30 mg oral dose of aripiprazole, or a 21-day regimen of oral aripiprazole supplementation.37,52,56 The 2 initiation strategies, while providing comparable aripiprazole exposures (Figure 6),56 are suitable in different settings of care and allow for flexibility to align with patients’ clinical needs. Oral supplementation for starting LAI treatment may be preferable in certain circumstances;6,37 for example, patients who are stabilized on oral aripiprazole can continue treatment with oral aripiprazole for 21 days when starting AL.21 Initiating AL treatment with ALNCD should be avoided in patients requiring dosage adjustments due to drug interactions.21 Alternatively, the 1-day regimen can be used to initiate AL treatment in one office visit or during an inpatient stay;69 once administered, along with 30 mg of oral aripiprazole, initiation dosing is complete and continuous plasma aripiprazole exposure is ensured from day 1.56 Further, the ALNCD injection (and the AL 441 mg dose) can be administered via either the deltoid or gluteal muscle,21,70 which both patients and clinicians consider valuable options.3,71,72
Importantly, the prolonged-release characteristics of AL are advantageous if doses are missed or delayed.35 A population pharmacokinetic analysis indicated that when AL concentrations are at steady state, delays in the scheduled injection interval of up to 4 weeks resulted in minimal decreases in median aripiprazole plasma concentrations; injection delays of 4 and 6 weeks resulted in 10% and 25% decreases in median aripiprazole plasma concentrations, respectively, for each AL dose assessed.35 These findings are the basis of guidance on missed doses, according to which AL dosing can be resumed without the use of an initiation regimen up to 6 weeks after a 441 mg dose, up to 8 weeks after a 662 mg or 882 mg dose, or up to 10 weeks after a 1064 mg dose.21 After longer gaps (6–7 weeks for 441 mg, 8–12 weeks for 662 mg or 882 mg, and 10–12 weeks for 1064 mg), AL can be reinitiated using either 7 days of oral aripiprazole supplementation or ALNCD alone without oral supplementation.21,35
Aripiprazole Lauroxil Outcomes
The efficacy, safety, and tolerability of AL have been assessed in multiple phase 3 or Phase 4 clinical studies for the treatment of adults with schizophrenia.46,47,67,69,73 Study characteristics are summarized in Table 1; additional details associated with each study are discussed within the respective publications cited.
 |
Table 1 Summary of Phase 3 and 4 Studies of Aripiprazole Lauroxil in Patients with Schizophrenia |
The 12-week pivotal study examined outcomes in patients with an acute exacerbation of schizophrenia treated with AL 441 mg or 882 mg monthly, initiated with the 21-day regimen, versus placebo.67 Safety and tolerability of the AL 441 mg or 882 mg monthly regimens were also examined in 3 open-label studies: a 52-week safety study46 (NCT01626456) with a 1- to 2.5-year extension47 (NCT01895452) and a 6-month open-label study of patients switched from another LAI antipsychotic73 (NCT02634320).
Following the FDA approval of the AL monthly and every-6-weeks regimens, the AL 1064 mg every-2-month regimen and ALNCD received FDA approval on the basis of pharmacokinetic and safety studies in which they were comparable with the AL monthly regimens and 21-day oral supplementation, respectively.56,60,74 The ALPINE (Aripiprazole Lauroxil and Paliperidone palmitate: INitiation Effectiveness; NCT03345979) study provided additional efficacy data supporting the use of the ALNCD initiation regimen and the AL 1064 mg every-2-month regimen in patients with acute schizophrenia without any oral aripiprazole dosing beyond day 1. ALPINE was a 25-week effectiveness and safety study assessing AL 1064 mg every 2 months using the ALNCD initiation regimen or paliperidone palmitate 156 mg monthly, included as an active control with known efficacy,75–77 in adult patients hospitalized for acute schizophrenia through the transition to outpatient treatment and during continued care in the community setting.69
Efficacy for Acute Symptoms
AL significantly reduced acute symptoms of schizophrenia on the primary endpoint in both of the phase 3 double-blind studies (Table 2). In the placebo-controlled 12-week pivotal study, change from baseline in Positive and Negative Syndrome Scale78 (PANSS) total score was statistically significant versus placebo at week 12,67 and comparable efficacies were observed for the 2 dose groups (AL 441 mg every 4 weeks and AL 882 mg every 4 weeks; Figure 7). Cohen’s d for the pooled AL doses was 0.61 (95% CI: 0.44–0.79); number needed to treat based on ≥30% improvement from baseline in PANSS total score at day 85 was 6 (95% CI: 5–11).79 In exploratory post hoc subgroup analyses, significant improvements in PANSS total score were observed in the AL 441 mg and 882 mg dose groups versus placebo regardless of sex and across patient age groups, including in those under 30 years of age.80 AL 441 mg and 882 mg also were associated with efficacy in patients with the most severe symptoms (PANSS total score >92) at baseline.81
 |
Figure 7 Efficacy results from the phase 3 aripiprazole lauroxil 12-week pivotal study. Abbreviations: AL, aripiprazole lauroxil; LOCF, last observation carried forward; NNT, number needed to treat; PANSS, Positive and Negative Syndrome Scale; q4wk, every 4 weeks. Notes: Adapted with permission from Meltzer HY, Risinger R, Nasrallah HA, Du Y, Zummo J, Corey L, Bose A, Stankovic S, Silverman BL, Ehrich EW. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015 Aug;76(8):1085–90. © Copyright 2015 Physicians Postgraduate Press, Inc.67 aChange from baseline in PANSS total score during 12 weeks of double-blind treatment with AL 441 mg or 882 mg monthly, initiated using the 21-day regimen, versus placebo for acutely exacerbated schizophrenia (LOCF). NNT versus placebo was calculated based on a ≥30% reduction from baseline PANSS total score at day 85.79 *P=0.004, **P<0.001 versus placebo. |
 |
Table 2 Summary of Efficacy Findings From Phase 3 and 4 Studies of Aripiprazole Lauroxil |
Secondary efficacy analysis findings were consistent with those in the primary 12-week pivotal study results (Table 2). AL was associated with significant improvement in illness severity based on Clinical Global Impression–Severity (CGI-S) and Clinical Global Impression–Improvement (CGI-I) scores at week 12 in the 12-week pivotal study.67,82
Results from ALPINE supported the efficacy of both the 1-day ALNCD plus 30 mg oral aripiprazole initiation regimen and treatment over 25 weeks with AL 1064 mg administered every 2 months.69 In ALPINE, treatment with AL was associated with a significant reduction in PANSS total score versus baseline at the week 4 primary endpoint (Figure 8).69 Significant improvements from baseline in PANSS total score were also observed after transition to outpatient care at the 9- and 25-week key secondary endpoints. Statistically significant reductions from baseline in CGI-S88 and in PANSS Positive, Negative, and General Psychopathology subscale scores at week 25 were observed with AL treatment.85 Statistically significant reductions were observed also during active control treatment with paliperidone palmitate;69,85 the study was not powered to test between-group differences.69
 |
Figure 8 Efficacy results from the phase 3 ALPINE study. Abbreviations: AL, aripiprazole lauroxil; ALPINE, Aripiprazole Lauroxil and Paliperidone palmitate: INitiation Effectiveness; BL, baseline; PANSS, Positive and Negative Syndrome Scale; PP, paliperidone palmitate. Notes: Adapted with permission from Weiden PJ, Claxton A, Kunovac J, Walling DP, Du Y, Yao B, Yagoda S, Bidollari I, Keane E, Cash E. Efficacy and Safety of a 2-Month Formulation of Aripiprazole Lauroxil With 1-Day Initiation in Patients Hospitalized for Acute Schizophrenia Transitioned to Outpatient Care: Phase 3, Randomized, Double-Blind, Active-Control ALPINE Study. J Clin Psychiatry. 2020 May 19;81(3):19m13207. © Copyright 2020 Physicians Postgraduate Press, Inc.69 aMean (95% CI) change in PANSS total score from baseline to week 4 (primary endpoint, red box) and weeks 9 and 25 (secondary endpoints, gray boxes) during 25 weeks of double-blind treatment with aripiprazole lauroxil 1064 mg every 2 months, initiated using the 1-day regimen; paliperidone palmitate 156 mg monthly was included as an active control. ALPINE was not powered for between-group comparison with paliperidone palmitate. |
Durability of Long-Term Aripiprazole Lauroxil Therapy
Longer-term, open-label AL treatment was associated with continued therapeutic efficacy over treatment durations of up to 3.5 years (Figure 9).47,86 Patients who completed the 12-week pivotal study and continued into the 52-week safety study had sustained improvements in PANSS total and CGI-S scores over 1 year of continuous AL exposure.86 In a post hoc analysis of 112 weeks of continuous data (including the 52-week study and long-term open-label extension), symptoms (PANSS total score) and severity of illness (CGI-S) improved over the course of the analysis period. Consistent with findings for oral aripiprazole for schizophrenia,89 no dose effect between groups was observed (Table 2).47 In addition, clinically stable patients with schizophrenia (n=51) who initiated AL because of lack of efficacy or tolerability concerns with their previous LAI antipsychotic achieved statistically significant improvement in CGI-S (Figure 9) and Brief Psychiatric Rating Scale90 scores in the 6 months after the switch.73
 |
Figure 9 Efficacy results for phase 3, open-label aripiprazole lauroxil studies.a,b Abbreviations: AL, aripiprazole lauroxil; ALPINE, Aripiprazole Lauroxil and Paliperidone palmitate: INitiation Effectiveness; CGI-S, Clinical Global Impressions–Severity; LAI, long-acting injectable; LS, least squares; MMRM, mixed-effects model for repeated measurement; PANSS, Positive and Negative Syndrome Scale; qxwk, every x weeks. Notes: aPanel (A) Therapeutic durability of AL 441 mg or 882 mg monthly in a post hoc analysis of data from the 12-week pivotal study67 and a 52-week open-label safety study.85 Change from baseline in PANSS total score (MMRM) is shown for patients who had at least 1 PANSS/CGI-S assessment after drug administration in the extension study. Adapted with permission from McEvoy JP, Risinger R, Mykhnyak S, Du Y, Liu CC, Stanford AD, Weiden PJ. Durability of Therapeutic Response With Long-Term Aripiprazole Lauroxil Treatment Following Successful Resolution of an Acute Episode of Schizophrenia. J Clin Psychiatry. 2017 Sep-Oct;78(8):1103–1109. © Copyright 2017 Physicians Postgraduate Press, Inc.85 bPanel (B) Change from baseline in CGI-S scores (MMRM) during 6-month open-label treatment with AL 441, 662, or 882 mg q4wk (flexible dosing) or AL 882 mg q6wk in 50 patients switched from paliperidone palmitate (1 patient who switched from risperidone LAI was excluded); mean (SD) CGI-S score at baseline: 3.9 (0.6). Adapted with permission from Miller BJ, Claxton A, Du Y, Weiden PJ, Potkin SG. Switching patients with schizophrenia from paliperidone palmitate to aripiprazole lauroxil: a 6-month, prospective, open-label study. Schizophr Res. Feb 7 2019;208:44–48. © 2019 The Authors. Published by Elsevier B.V. Creative Commons CC-BY-NC-ND license.73 cP values reported for change in mean PANSS total score from week 0 to week 64. dA separate analysis comparing changes in mean PANSS total score from week 12 (beginning of extension study) to week 64 showed significant reductions in PANSS total score (P<0.0001). eIndicated weeks denote assessment time points. *P<0.05 versus baseline. |
Exploratory Efficacy and Patient- and Caregiver-Reported Outcomes
In secondary and post hoc analyses, 12-week pivotal study patients administered the AL 441 mg or 882 mg monthly regimen had significantly higher rates of response (≥30% improvement from baseline in PANSS total score) compared with patients administered placebo and significantly greater improvements from baseline versus placebo on PANSS Positive, Negative, and General Psychopathology subscale scores, as well as PANSS 5-factor scores (positive symptoms, negative symptoms, disorganized thought, uncontrolled hostility/excitement, and anxiety/depression91) at day 85 (Table 2).82 Treatment with the AL 441 mg or 882 mg monthly regimen was also associated with significant improvements in social functioning, based on PANSS Prosocial Subscale scores, and in amelioration of agitation/hostility with reductions in PANSS excited component and hostility item scores compared with placebo at day 85.83,84 Functional outcomes were also assessed in the 12-week pivotal study using the Personal and Social Performance (PSP) scale,92 and significant improvements were observed versus placebo in the PSP total score and disturbing and aggressive behavior domain score at day 85.83,84
Treatment with AL had a positive effect on functional and health-related QoL outcomes in additional short- and longer-term assessments (Table 2). In ALPINE, patients reported stable QoL on the Quality of Life Enjoyment and Satisfaction Questionnaire–Short Form93 through the outpatient period (weeks 5, 13, and 25).85 The majority of ALPINE patients remained satisfied or very satisfied with AL throughout treatment, and their caregivers reported an improvement in burden of care from baseline to weeks 9 and 25.85 Active control treatment (paliperidone palmitate) also was associated with improvements in patient and caregiver outcomes.85 Significant improvement in mental health–related QoL was observed over 124 weeks in the combined 52-week and long-term extension studies87 based on SF-36v2 health Survey94 mental component summary scores.
Safety and Tolerability
The safety and tolerability profile of AL was consistent with that of the oral aripiprazole formulation in inpatients and outpatients with an acute exacerbation of schizophrenia,67,69 in clinically stable patients with schizophrenia initiating AL after a switch from another LAI,73 and during long-term continuous exposure47 (Table 3).47,67,69,73 The prodrug technology would not be likely to influence the safety or tolerability of AL. Lauric acid (the fatty acid tail) is a major component of coconut and palm kernel oils,95 and the linker molecule is hydrolyzed to a normal metabolic intermediary in fatty acid metabolism.52,96 Indeed, no new or unexpected safety findings emerged during long-term AL safety studies, either overall or with respect to AEs of clinical interest that may occur during atypical antipsychotic treatment.46,47 Among patients switching from oral antipsychotics, the safety profile of AL did not appear to be influenced by previous treatment.97
 |
Table 3 Summary of Key Safety Findings From Aripiprazole Lauroxil Phase 3 and 4 Studies |
In the 12-week pivotal study, 14/207 (6.8%) patients assigned to AL 441 mg every 4 weeks and 6/208 (2.9%) patients assigned to AL 882 mg every 4 weeks discontinued treatment because of AEs, compared with 36/207 (17.4%) patients assigned to placebo.67 The higher rate in the placebo arm was attributable primarily to exacerbations of schizophrenia.67 Rates of discontinuation due to AEs during up to 180 weeks of continuous open-label treatment were 2.7% (3/110) and 8.4% (31/368) for the AL 441 mg and 882 mg monthly regimens, respectively.47 The most common AEs leading to discontinuation in the combined long-term studies were exacerbation/worsening of schizophrenia (14/368 [2.9%]), exacerbation of psychotic disorder (2/368 [0.4%]), and akathisia (2/368 [0.4%]).47 Among patients assigned to AL 1064 mg every 8 weeks in ALPINE, 10/99 (10.1%) discontinued because of AEs, the most common of which were exacerbation/worsening of schizophrenia (5 [5.1%]), injection site pain (2 [2.0%]; 1 associated with placebo injection), and psychotic disorders (2 [2.0%]).69
Akathisia was the only AE reported by greater than 5% of and at least twice the rate observed with placebo in patients administered AL 441 mg every 4 weeks or AL 882 mg every 4 weeks in the 12-week pivotal study (Table 3).67 As observed with oral aripiprazole,50 akathisia was generally reported early in AL treatment; first occurrences of akathisia were reported mostly within 4 weeks of AL initiation in the 12-week pivotal study and in ALPINE,67,69 and higher rates were not observed with longer-term exposure.47,69 Few patients discontinued AL treatment because of akathisia (2/478 during up to 180 weeks of open-label AL treatment; 0/99 AL-treated patients in the 25-week ALPINE study [2/101 paliperidone palmitate–treated patients in ALPINE]).47,69 Other drug-induced movement disorder AEs (dystonia, dyskinesia, Parkinson-like events) were reported in 9 (9%) patients in ALPINE (paliperidone palmitate: 12%); of those 9 AL-treated patients, 8 (8%) reported the AEs during the first 4 weeks of treatment.98 No drug-induced movement disorder AEs were reported by >2% of patients in the 12-week pivotal study.67 Among 478 patients who received up to 1 year of open-label AL exposure, 18 (3.8%) patients reported AEs of akathisia, and 45 (9.4%) patients reported any drug-induced movement disorder AEs (dyskinesia, dystonia, Parkinson-like events, restlessness, or akathisia), including those who reported akathisia.46
With extended antipsychotic exposure, the potential for clinically significant weight gain is a particular concern.99–104 Two to 3% of patients treated with AL in the 12-week pivotal study and 9% of those who received AL in the 25-week ALPINE study reported weight gain as an AE (Table 3).67,69 Figure 10 presents change in weight over time in ALPINE study patients. In an analysis of metabolic and endocrine profiles of patients enrolled in the 52-week safety study, AL (441 mg or 882 mg every 4 weeks) was associated with mean (SD) weight change of +0.8 (5.9) kg (body mass index: +0.3 [2.0]); 18.4% of patients had a ≥7% increase in body weight from baseline at any assessment.46 Among patients with up to 3.5 years of AL treatment in the combined 52-week safety and long-term extension studies, 6.1% reported an AE of weight gain.47 Patients in those combined long-term studies gained a mean of 1.2 (6.6) kg from baseline to last assessment; no discontinuations because of weight gain were reported.47
 |
Figure 10 Weight change in ALPINE.a Abbreviations: AL, aripiprazole lauroxil; ALPINE, Aripiprazole Lauroxil and Paliperidone palmitate: INitiation Effectiveness; PP, paliperidone palmitate; qxwk, every x weeks. Notes: Reprinted with permission from Citrome L, Yagoda S, Bidollari I, Wang M. Safety and Tolerability of Starting Aripiprazole Lauroxil With Aripiprazole Lauroxil NanoCrystal Dispersion in 1 Day Followed by Aripiprazole Lauroxil Every 2 Months Using Paliperidone Palmitate Monthly as an Active Control in Patients With Schizophrenia: A Post Hoc Analysis of a Randomized Controlled Trial. J Clin Psychiatry. 2024 Feb 28;85(1):23m15095. © Copyright 2024 Physicians Postgraduate Press, Inc.98 aWeight change during 25 weeks of double-blind treatment with aripiprazole lauroxil 1064 mg every 2 months, initiated using the 1-day regimen; paliperidone palmitate 156 mg monthly was included as an active control. ALPINE was not powered for between-group comparison with paliperidone palmitate. |
No clinically meaningful changes were observed for any glycemic-control or lipid parameters in the 52-week safety study.46 Consistent with the known effects of aripiprazole,105 little change in plasma prolactin concentrations was observed with AL treatment in male and female patients in ALPINE (Figure 11).98 Mean (SD) prolactin concentrations decreased from baseline to the last assessment in the 52-week study (males: −8.7 [14.7] ng/mL; females: −14.9 [43.4] ng/mL)46 and in the combined long-term studies (males: −6.7 [16.5] ng/mL; females: −16.5 [45.2] ng/mL).47 In a post hoc analysis of a 6-month open-label switching study where 50 patients were switched from prior treatment with paliperidone palmitate to AL, mean (SD) serum prolactin reductions of 23.0 (11.7) ng/mL for males and 49.8 (54.1) ng/mL for females were observed in patients who had data after 6 months of AL treatment (n=32); proportions of patients with elevated prolactin levels fell from 90.0% at baseline to 6.3% at month 6.106
 |
Figure 11 Change in plasma prolactin concentrations in ALPINE.a Abbreviations: AL, aripiprazole lauroxil; ALPINE, Aripiprazole Lauroxil and Paliperidone palmitate: INitiation Effectiveness; PP, paliperidone palmitate; qxwk, every x weeks. Notes: Reprinted with permission from Citrome L, Yagoda S, Bidollari I, Wang M. Safety and Tolerability of Starting Aripiprazole Lauroxil With Aripiprazole Lauroxil NanoCrystal Dispersion in 1 Day Followed by Aripiprazole Lauroxil Every 2 Months Using Paliperidone Palmitate Monthly as an Active Control in Patients With Schizophrenia: A Post Hoc Analysis of a Randomized Controlled Trial. J Clin Psychiatry. 2024 Feb 28;85(1):23m15095. © Copyright 2024 Physicians Postgraduate Press, Inc.98 aChange in plasma prolactin concentrations during 25 weeks of double-blind treatment with aripiprazole lauroxil 1064 mg every 2 months, initiated using the 1-day regimen; paliperidone palmitate 156 mg monthly was included as an active control. ALPINE was not powered for between-group comparison with paliperidone palmitate. |
Incidence of injection site pain was generally low in phase 3 or phase 4 AL clinical studies, with fewer than 5% of patients reporting AEs of injection site pain in the 12-week pivotal study, in patients switched from other LAIs, and in the combined long-term studies (Table 3).47,67,73 Injection site pain was reported in 17.2% of AL-treated patients in ALPINE, which included an additional injection (ALNCD) at initiation and placebo injections to maintain the blind (ALPINE patients received twice as many intramuscular injections as would be administered in clinical practice, including both deltoid and gluteal injections on the first and eighth days of treatment).69 Injection site pain occurred most commonly early in treatment, associated with the ALNCD and/or the first AL injection.67,69,107 Few AEs of injection site pain were reported after week 4.67,69
Overall, the long-term safety and tolerability profile of AL is consistent with that of oral aripiprazole and with short-term observations from the 12-week pivotal study.46,47 During up to 3.5 years of continuous open-label treatment with AL 441 mg or 882 mg monthly regimens in the 52-week safety study and long-term extension, no clinically meaningful findings in physical examinations, vital signs, laboratory tests, or electrocardiography values were observed.46,47 Further, primary and post hoc analyses from the ALPINE study indicated that the initiation of AL using a 1-day initiation regimen (ALNCD plus a single 30 mg oral dose of aripiprazole) was not associated with the emergence of additional safety risks compared with the known risks of oral aripiprazole.69,98
Real‑World Outcomes and Costs
Real-world data on the use of AL in clinical practice are increasingly available, and healthcare utilization and costs associated with AL have now been examined.108 In a retrospective analysis of Medicaid claims data (April 2015 to December 2017) for patients with a diagnosis code for schizophrenia, there was no significant change in overall all-cause total costs from the 6-month period before first claim for AL (baseline) to the 6-month follow-up period (Figure 12).108 A significant increase in pharmacy costs from baseline to follow-up was offset by reductions in inpatient utilization and costs and mental health–related emergency department utilization. Mean number of all-cause inpatient admissions per patient decreased significantly between the baseline and follow-up periods.108 The greatest reductions in inpatient costs, as well as in all-cause and mental health–related inpatient admissions and emergency department visits, were observed among patients who had been prescribed an oral antipsychotic (rather than an LAI or no antipsychotic) before starting AL.108 Reductions in inpatient and mental health–related emergency department utilization are consistent with the evidence that LAIs are effective in preventing psychotic relapse and hospitalization.11–14
 |
Figure 12 Real‑world all-cause costs during treatment with aripiprazole lauroxil.a Abbreviations: LAI, long-acting injectable antipsychotic; USD, US dollars. Notes: Reprinted with permission from Lauriello J, Weiden PJ, Gleeson CD, Shah A, Boulanger L, Jariwala-Parikh K, Hedgeman E, O’Sullivan AK. Real-World Outcomes and Costs Following 6 Months of Treatment with the Long-Acting Injectable (LAI) Aripiprazole Lauroxil for the Treatment of Schizophrenia. CNS Drugs. 2021 Oct;35(10):1123–1135. © The Author(s) 2021. Creative Commons Attribution-NonCommercial 4.0 International License.108 aMean differences in all-cause healthcare costs were calculated for the 6-month period before (baseline) versus after (follow-up) initiation of treatment with aripiprazole lauroxil. *P<0.05 versus baseline period. |
Conclusions
AL was developed using a prodrug technology for slow dissolution to provide an LAI antipsychotic with several dosage strengths (lower, intermediate, and higher, equivalent to oral aripiprazole doses of 10, 15, and 20 mg or higher per day, respectively) for use over a range of dosing intervals (4, 6, or 8 weeks) to meet individualized patient medical and quality-of-care needs. The placebo-controlled 12-week pivotal study, the 25-week active-controlled ALPINE study, and the additional open-label studies67,69,82,85 provide safety and efficacy data on the use of AL for acutely exacerbated schizophrenia in adults, and as an ongoing maintenance treatment during inpatient-to-outpatient transitions, after a switch from another LAI antipsychotic, and for long-term maintenance treatment. Furthermore, based on the ALPINE study results, AL can be initiated in 1 day (ALNCD + 30 mg oral aripiprazole)70 without any oral supplementation beyond day 1 and this first every-2-month LAI formulation of aripiprazole provides effective maintenance treatment over time.
Abbreviations
AE, adverse event; AL, aripiprazole lauroxil; ALNCD, AL NanoCrystal Dispersion formulation; ALPINE, Aripiprazole Lauroxil and Paliperidone palmitate: INitiation Effectiveness; CGI-I, Clinical Global Impression–Improvement; CGI-S, Clinical Global Impression–Severity; FDA, US Food and Drug Administration; LAI, long-acting injectable; PANSS, Positive and Negative Syndrome Scale; PSP, Personal and Social Performance; QoL, quality of life.
Data Sharing Statement
The data used in the preparation of this manuscript are proprietary to Alkermes, Inc. Alkermes, Inc., is committed to public sharing of data in accordance with applicable regulations and laws, and requests can be submitted to the corresponding author.
Funding
This article was sponsored by Alkermes, Inc. (Waltham, MA, USA). Medical writing and editorial support were provided by Kathleen M. Dorries, PhD, John H. Simmons, MD, and Noud van Helmond, MD, PhD, of Peloton Advantage, LLC, an OPEN Health company, and funded by Alkermes, Inc. The authors thank Mark S. Todtenkopf, PhD, of Alkermes, Inc., who assisted in the preparation and proofreading of the manuscript. Dr Todtenkopf is an employee of Alkermes, Inc., and may also own stock.
Disclosure
LC serves as consultant to AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, Biogen, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Cerevel, Clinilabs, COMPASS, Delpor, Eisai, Enteris BioPharma, HLS Therapeutics, Idorsia, INmune Bio, Impel, Intra-Cellular Therapies, Janssen, Karuna, Lundbeck, Luye, Lyndra, MapLight, Marvin, MedAvante-ProPhase, Merck, Mitsubishi-Tanabe Pharma, Neumora, Neurocrine, Neurelis, Noema, Novartis, Noven, Otsuka, Ovid, Praxis, Recordati, Relmada, Reviva, Sage, Sumitomo/Sunovion, Supernus, Teva, University of Arizona, Vanda, Wells Fargo, and one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research; speaker for AbbVie/Allergan, Acadia, Alkermes, Angelini, Axsome, BioXcel, Eisai, Idorsia, Intra-Cellular Therapies, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Recordati, Sage, Sunovion, Takeda, and Teva and CME activities organized by medical education companies such as Medscape, NACCME, NEI, Vindico, and universities and professional organizations/societies; owns stocks (small number of shares of common stock) in Bristol-Myers Squibb, Eli Lilly, J&J, Merck, and Pfizer, purchased >10 years ago; owns stock options in Reviva; and receives royalties/publishing income from Taylor & Francis (editor-in-chief, Current Medical Research and Opinion, 2022–date), Wiley (editor-in-chief, International Journal of Clinical Practice, through end 2019), UpToDate (reviewer), Springer Healthcare (book), and Elsevier (topic editor, Psychiatry, Clinical Therapeutics).
CUC has been a consultant and/or advisor to or has received honoraria from AbbVie, Acadia, Adcock Ingram, Alkermes, Allergan, Angelini, Aristo, Biogen, Boehringer-Ingelheim, Bristol-Meyers Squibb, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Delpor, Denovo, Eli Lilly, Gedeon Richter, Hikma, Holmusk, Intra-Cellular Therapies, Jamjoom Pharma, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MapLight, MedinCell, MedLink, Merck, MindPax, Mitsubishi Tanabe Pharma, Mylan, Neumora Therapeutics, Neurelis, Neurocrine, Newron, Noven, Novo Nordisk, Otsuka, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Sage, Saladax, Sanofi, Seqirus, SK Life Science, Sumitomo Pharma America, Sunovion, Sun Pharma, Supernus, Tabuk, Takeda, Teva, Terran, Tolmar, Vertex, Viatris, and Xenon Pharmaceuticals; has provided expert testimony for Janssen, Lundbeck, and Otsuka; has served on a data safety monitoring board for Compass Pathways, Denovo, Intra-Cellular Therapies, Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva; has received grant support from Boehringer Ingelheim, Janssen, and Takeda; has received royalties from UpToDate; and is a stock option holder of Cardio Diagnostics, Küleon Biosciences, LB Pharma, MedLink, MindPax, Quantic, and Terran.
AJC has been a consultant to AbbVie, Acadia, Alfasigma, Alkermes, Anavex Life Sciences, Axsome, Biogen, Biohaven, BioXcel, Boehringer Ingelheim, Brii Biosciences, Bristol Myers Squibb, Cerevel, Chase Therapeutics, Cognitive Research, Corium, Delpor, Evolution Research Group, 4M Therapeutics, Intra-Cellular Therapies, Ironshore Pharmaceuticals, Janssen/J&J, Jazz Pharma, Karuna, LivaNova, Lundbeck, Luye Pharma, MapLight Therapeutics, MedAvante-ProPhase, Mentavi Health, Neumora, Neurocrine, Neuroscience Education Institute, NeuroSigma, Noven, Otsuka, PaxMedica, PureTech Health, Relmada, Reviva, Sage Therapeutics, Sumitomo Pharma America, Sunovion, Supernus, Teva, Thynk, Tris Pharma, Vanda Pharmaceuticals, VistaGen, and VivoSense; is on the speakers’ bureau and has received honoraria from AbbVie, Acadia, Alfasigma, Alkermes, Axsome, BioXcel, Corium, Intra-Cellular Therapies, Ironshore Pharmaceuticals, Janssen/J&J, Lundbeck, Neurocrine, Noven, Otsuka, Sunovion, Supernus, Teva, Tris Pharma, and Vanda Pharmaceuticals; is on data safety monitoring boards for AbbVie, Acadia, Alfasigma, Alkermes, Axsome, BioXcel, Corium, Intra-Cellular Therapies, Ironshore Pharmaceuticals, Janssen/J&J, Lundbeck, Neurocrine, Noven, Otsuka, Sunovion, Supernus, Teva, Tris Pharma, and Vanda Pharmaceuticals; is the chief medical officer of the Neuroscience Education Institute; holds stock options for 4M Therapeutics and Relmada; and receives no royalties.
MD, CH, JAM, BR, and DM are or were employees of Alkermes, Inc., and may own stock/options in the company.
ARH has served on advisory boards for Acadia, Alkermes, Biogen, BioXcel, Intra-Cellular, and Teva and on speaker’s bureaus for Acadia, Alkermes, Axsome, BioXcel, Bristol Myers Squibb, Intra-Cellular, Neurocrine, and Teva.
GWM has served as a consultant for AbbVie, Alkermes, Axsome, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Corium, Eisai, Ironshore, Intra-Cellular, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Redax, Roche, Sage, Sirona, Sunovion, Supernus, Takeda, and Teva; received speaker fees from AbbVie, Alkermes, Axsome, Biogen, Bristol Myer Squibb, Corium, Intra-Cellular, Ironshore, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Sunovion, Supernus, Takeda, and Tris Pharma; and conducted research for AbbVie, Akili, Alkermes, Axsome, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Compass, Emalex, Idorsia, Janssen, Karuna, Lumos Labs, Medgenics, Neurocrine, NLS-1 Pharma AG, Otsuka, Redax, Relmada, Roche, Sage, Sirtsei, Sunovion, Supernus, Takeda, and Teva.
PJW is a former employee of Alkermes and has been a consultant for Alkermes, Lyndra, MapLight, and Teva.
The authors report no other conflicts of interest in this work.
References
1. American Psychiatric Association. The American psychiatric association practice guideline for the treatment of patients with schizophrenia. American Psychiatric Association. 2021.
2. Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry. 2003;64(11):1308–1315. doi:10.4088/JCP.v64n1105
3. Geerts P, Martinez G, Schreiner A. Attitudes towards the administration of long-acting antipsychotics: a survey of physicians and nurses. BMC Psychiatry. 2013;13:58. doi:10.1186/1471-244x-13-58
4. Haddad PM, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat Outcome Meas. 2014;5:43–62. doi:10.2147/prom.s42735
5. Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216–226. doi:10.1002/wps.20060
6. Højlund M, Correll CU. Switching to long-acting injectable antipsychotics: pharmacological considerations and practical approaches. Expert Opin Pharmacother. 2023;24(13):1463–1489. doi:10.1080/14656566.2023.2228686
7. Citrome L, Belcher E, Stacy S, Suett M, Mychaskiw M, Salinas GD. Management of schizophrenia with long-acting injectable antipsychotic medications: an assessment of the educational needs of clinicians. Neuropsychiatr Dis Treat. 2022;18:111–123. doi:10.2147/ndt.s326299
8. Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1–24. doi:10.4088/JCP.15032su1
9. Substance Abuse and Mental Health Services Administration. SAMHSA’s working definition of recovery: 10 guiding principles of recovery. Substance Abuse and Mental Health Services Administration. Available from: https://www.drugsandalcohol.ie/16678/1/SAMHSA_recovery_definition.pdf.
10. Cohen AN, Gorrindo T. New tools for implementing evidence-based care for serious mental illness. Focus. 2020;18(4):432–435. doi:10.1176/appi.focus.20200023
11. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686–693. doi:10.1001/jamapsychiatry.2017.1322
12. Kishimoto T, Hagi K, Kurokawa S, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021;8(5):387–404. doi:10.1016/s2215-0366(21)00039-0
13. Lähteenvuo M, Tanskanen A, Taipale H, et al. Real-world effectiveness of pharmacologic treatments for the prevention of rehospitalization in a Finnish nationwide cohort of patients with bipolar disorder. JAMA psychiatry2018;75(4):347–355. doi:10.1001/jamapsychiatry.2017.4711
14. Mathews M, Gopal S, Singh A, et al. Comparison of relapse prevention with 3 different paliperidone formulations in patients with schizophrenia continuing versus discontinuing active antipsychotic treatment: a post-hoc analysis of 3 similarly designed randomized studies. Neuropsychiatr Dis Treat. 2020;16:1533–1542. doi:10.2147/ndt.s221242
15. Milz R, Benson C, Knight K, et al. The effect of longer dosing intervals for long-acting injectable antipsychotics on outcomes in schizophrenia. Neuropsychiatr Dis Treat. 2023;19:531–545. doi:10.2147/ndt.s395383
16. Lindenmayer J-P, Jarboe K, Bossie CA, Zhu Y, Mehnert A, Lasser R. Minimal injection site pain and high patient satisfaction during treatment with long-acting risperidone. Int Clin Psychopharmacol. 2005;20(4):213–221. doi:10.1097/00004850-200507000-00004
17. Kaplan G, Casoy J, Zummo J. Impact of long-acting injectable antipsychotics on medication adherence and clinical, functional, and economic outcomes of schizophrenia. Patient Prefer Adherence. 2013;7:1171–1180. doi:10.2147/PPA.S53795
18. Lin D, Thompson-Leduc P, Ghelerter I, et al. Real-world evidence of the clinical and economic impact of long-acting injectable versus oral antipsychotics among patients with schizophrenia in the United States: a systematic review and meta-analysis. CNS Drugs. 2021;35(5):469–481. doi:10.1007/s40263-021-00815-y
19. Fu AZ, Pesa JA, Lakey S, Benson C. Healthcare resource utilization and costs before and after long-acting injectable antipsychotic initiation in commercially insured young adults with schizophrenia. BMC Psychiatry. 2022;22(1):250. doi:10.1186/s12888-022-03895-2
20. Correll CU, Lauriello J. Using long-acting injectable antipsychotics to enhance the potential for recovery in schizophrenia. J Clin Psychiatry. 2020;81(4):MS19053AH5C. doi:10.4088/jcp.ms19053ah5c
21. Aristada [Package Insert]. Alkermes, Inc.; 2023.
22. Abilify Maintena [Package Insert]. Otsuka America Pharmaceutical; 2020.
23. Invega Sustenna [package insert]. Janssen Pharmaceuticals. 2024.
24. Risperdal [package insert]. Janssen Pharmaceuticals. 2022.
25. Zyprexa Relprevv [Package Insert]. CHEPLAPHARM; 2023.
26. Faden J, Ramirez C, Martinez V, Citrome L. An overview of the currently available and emerging long-acting formulations of risperidone for schizophrenia and bipolar disorder. Expert Rev Neurother. 2024;24(8):761–771. doi:10.1080/14737175.2024.2370349
27. Markowicz-Piasecka M, Kubisiak M, Asendrych-Wicik K, et al. Long-acting injectable antipsychotics—a review on formulation and in vitro dissolution. Pharmaceutics. 2023;16(1):28. doi:10.3390/pharmaceutics16010028
28. Sommi RW, Rege B, Wehr A, Faldu S, Du Y, Weiden PJ. Aripiprazole lauroxil dosing regimens: understanding dosage strengths and injection intervals. CNS Spectr. 2022;27(3):262–267. doi:10.1017/s1092852920002072
29. Citrome L. Long-acting injectable antipsychotics: what, when, and how. CNS Spectr. 2021;26(2):118–129. doi:10.1017/S1092852921000249
30. Citrome L Long-acting injectable antipsychotics: what, when, and how—addendum. CNS Spectr. 2021;26(2):184. doi:10.1017/s1092852921000456
31. Citrome L. A systematic review of meta-analyses of the efficacy of oral atypical antipsychotics for the treatment of adult patients with schizophrenia. Expert Opin Pharmacother. 2012;13(11):1545–1573. doi:10.1517/14656566.2011.626769
32. Khanna P, Suo T, Komossa K, et al. Aripiprazole versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2014;2014(1):CD006569. doi:10.1002/14651858.CD006569.pub5
33. Ribeiro ELA, de Mendonça Lima T, Vieira MEB, Storpirtis S, Aguiar PM. Efficacy and safety of aripiprazole for the treatment of schizophrenia: an overview of systematic reviews. Eur J Clin Pharmacol. 2018;74(10):1215–1233. doi:10.1007/s00228-018-2498-1
34. Preda A, Shapiro BB. A safety evaluation of aripiprazole in the treatment of schizophrenia. Expert Opin Drug Saf. 2020;19(12):1529–1538. doi:10.1080/14740338.2020.1832990
35. Hard ML, Mills RJ, Sadler BM, Turncliff RZ, Citrome L. Aripiprazole lauroxil: pharmacokinetic profile of this long-acting injectable antipsychotic in persons with schizophrenia. J Clin Psychopharmacol. 2017;37(3):289–295. doi:10.1097/jcp.0000000000000691
36. Farwick S, Hickey MB, Jacobs G, Faldu S, Vandiver J, Weiden PJ. Best practices for aripiprazole lauroxil administration: from formulation development to injection technique. J Psychiatr Pract. 2019;25(2):82–90. doi:10.1097/PRA.0000000000000376
37. Jain R, Meyer J, Wehr A, Rege B, Von Moltke L, Weiden PJ. Size matters: the importance of particle size in a newly developed injectable formulation for the treatment of schizophrenia. CNS Spectr. 2020;25(3):323–330. doi:10.1017/S1092852919000816
38. Jordan S, Koprivica V, Chen R, Tottori K, Kikuchi T, Altar CA. The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor. Eur J Pharmacol. 2002;441(3):137–140. doi:10.1016/s0014-2999(02)01532-7
39. Croxtall JD. Aripiprazole: a review of its use in the management of schizophrenia in adults. CNS Drugs. 2012;26(2):155–183. doi:10.2165/11208400-000000000-00000
40. Citrome L. The ABC’s of dopamine receptor partial agonists—aripiprazole, brexpiprazole and cariprazine: the 15-min challenge to sort these agents out. Int J Clin Pract. 2015;69(11):1211–1220. doi:10.1111/ijcp.12752
41. Casey AB, Canal CE. Classics in chemical neuroscience: aripiprazole. ACS Chem Neurosci. 2017;8(6):1135–1146. doi:10.1021/acschemneuro.7b00087
42. Burris KD, Molski TF, Xu C, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther. 2002;302(1):381–389. doi:10.1124/jpet.102.033175
43. Goodnick PJ, Jerry JM. Aripiprazole: profile on efficacy and safety. Expert Opin Pharmacother. 2002;3(12):1773–1781. doi:10.1517/14656566.3.12.1773
44. Abilify [Package Insert]. Otsuka America Pharmaceuticals; 2024.
45. Mallikaarjun S, Salazar DE, Bramer SL. Pharmacokinetics, tolerability, and safety of aripiprazole following multiple oral dosing in normal healthy volunteers. J Clin Pharmacol. 2004;44(2):179–187. doi:10.1177/0091270003261901
46. Nasrallah HA, Aquila R, Du Y, Stanford AD, Claxton A, Weiden PJ. Long-term safety and tolerability of aripiprazole lauroxil in patients with schizophrenia. CNS Spectr. 2019;24(4):395–403. doi:10.1017/s1092852918001104
47. Lauriello J, Claxton A, Du Y, Weiden PJ. Beyond 52-week long-term safety: long-term outcomes of aripiprazole lauroxil for patients with schizophrenia continuing in an extension study. J Clin Psychiatry. 2020;81(5):19m12835. doi:10.4088/JCP.19m12835
48. Marder SR, McQuade RD, Stock E, et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res. 2003;61(2–3):123–136. doi:10.1016/s0920-9964(03)00050-1
49. Thomas JE, Caballero J, Harrington CA. The incidence of akathisia in the treatment of schizophrenia with aripiprazole, asenapine and lurasidone: a meta-analysis. Curr Neuropharmacol. 2015;13(5):681–691. doi:10.2174/1570159X13666150115220221
50. Kane JM, Barnes TR, Correll CU, et al. Evaluation of akathisia in patients with schizophrenia, schizoaffective disorder, or bipolar I disorder: a post hoc analysis of pooled data from short- and long-term aripiprazole trials. J Psychopharmacol. 2010;24(7):1019–1029. doi:10.1177/0269881109348157
51. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123(2–3):225–233. doi:10.1016/j.schres.2010.07.012
52. Ehret MJ, Davis E, Luttrell SE, Clark C. Aripiprazole lauroxil nanocrystal dispersion technology (Aristada Initio). Clin Schizophr Relat Psychoses. 2018;12(2):92–96. doi:10.3371/csrp.Ehda071918
53. Remenar JF. Making the leap from daily oral dosing to long-acting injectables: lessons from the antipsychotics. Mol Pharm. 2014;11(6):1739–1749. doi:10.1021/mp500070m
54. Rohde M, Mørk N, Håkansson AE, et al. Biological conversion of aripiprazole lauroxil—an N-acyloxymethyl aripiprazole prodrug. Results Pharma Sci. 2014;4:19–25. doi:10.1016/j.rinphs.2014.04.002
55. Nagarwal RC, Kumar R, Dhanawat M, Das N, Pandit JK. Nanocrystal technology in the delivery of poorly soluble drugs: an overview. Curr Drug Deliv. 2011;8(4):398–406. doi:10.2174/156720111795767988
56. Hard ML, Wehr AY, Du Y, Weiden PJ, Walling D, von Moltke L. Pharmacokinetic evaluation of a 1-day treatment initiation option for starting long-acting aripiprazole lauroxil for schizophrenia. J Clin Psychopharmacol. 2018;38(5):435–441. doi:10.1097/jcp.0000000000000921
57. Merisko-Liversidge E, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18(2):113–120. doi:10.1016/s0928-0987(02)00251-8
58. Risinger R, Hard M, Weiden PJ. A phase-1 study comparing pharmacokinetic and safety profiles of three different dose intervals of aripiprazole lauroxil. Psychopharmacol Bull. 2017;47(3):26–34.
59. Hard ML, Wehr A, von Moltke L, et al. Pharmacokinetics and safety of deltoid or gluteal injection of aripiprazole lauroxil nanocrystal dispersion used for initiation of the long-acting antipsychotic aripiprazole lauroxil. Ther Adv Psychopharmacol. 2019;9:1–9. doi:10.1177/2045125319859964
60. Hard ML, Mills RJ, Sadler BM, Wehr AY, Weiden PJ, von Moltke L. Pharmacokinetic profile of a 2-month dose regimen of aripiprazole lauroxil: a phase I study and a population pharmacokinetic model. CNS Drugs. 2017;31(7):617–624. doi:10.1007/s40263-017-0447-7
61. Kane JM, Sanchez R, Perry PP, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(5):617–624. doi:10.4088/JCP.11m07530
62. Mallikaarjun S, Kane JM, Bricmont P, et al. Pharmacokinetics, tolerability and safety of aripiprazole once-monthly in adult schizophrenia: an open-label, parallel-arm, multiple-dose study. Schizophr Res. 2013;150(1):281–288. doi:10.1016/j.schres.2013.06.041
63. Correll CU, Kim E, Sliwa JK, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. 2021;35(1):39–59. doi:10.1007/s40263-020-00779-5
64. Sparshatt A, Taylor D, Patel MX, Kapur S. A systematic review of aripiprazole—dose, plasma concentration, receptor occupancy, and response: implications for therapeutic drug monitoring. J Clin Psychiatry. 2010;71(11):1447–1456. doi:10.4088/JCP.09r05060gre
65. Hart XM, Hiemke C, Eichentopf L, et al. Therapeutic reference range for aripiprazole in schizophrenia revised: a systematic review and metaanalysis. Psychopharmacology. 2022;239(11):3377–3391. doi:10.1007/s00213-022-06233-2
66. Citrome L. Aripiprazole long-acting injectable formulations for schizophrenia: aripiprazole monohydrate and aripiprazole lauroxil. Expert Rev Clin Pharmacol. 2016;9(2):169–186. doi:10.1586/17512433.2016.1121809
67. Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085–1090. doi:10.4088/JCP.14m09741
68. Weiden PJ, Du Y, von Moltke L, et al. Pharmacokinetics, safety, and tolerability of a 2-month dose interval regimen of the long-acting injectable antipsychotic aripiprazole lauroxil: results from a 44-week phase i study. CNS Drugs. 2020;34(9):961–972. doi:10.1007/s40263-020-00745-1
69. Weiden PJ, Claxton A, Kunovac J, et al. Efficacy and safety of a 2-month formulation of aripiprazole lauroxil with 1-day initiation in patients hospitalized for acute schizophrenia transitioned to outpatient care: phase 3, randomized, double-blind, active control ALPINE study. J Clin Psychiatry. 2020;81(3):19m13207. doi:10.4088/JCP.19m13207
70. Aristada Initio [package insert]. Alkermes, Inc.; 2023.
71. Kamei H, Homma Y, Takeuchi I, et al. Acceptance of the deltoid muscle injection of aripiprazole long-acting injectable in the patients with schizophrenia. Clin Psychopharmacol Neurosci. 2020;18(1):49–57. doi:10.9758/cpn.2020.18.1.49
72. Blackwood C, Sanga P, Nuamah I, et al. Patients’ preference for long-acting injectable versus oral antipsychotics in schizophrenia: results from the patient-reported medication preference questionnaire. Patient Prefer Adherence. 2020;14:1093–1102. doi:10.2147/ppa.s251812
73. Miller BJ, Claxton A, Du Y, Weiden PJ, Potkin SG. Switching patients with schizophrenia from paliperidone palmitate to aripiprazole lauroxil: a 6-month, prospective, open-label study. Schizophr Res. 2019;208:44–48. doi:10.1016/j.schres.2019.01.038
74. Hard ML, Wehr AY, Sadler BM, Mills RJ, von Moltke L. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461–469. doi:10.1007/s13318-018-0488-4
75. Pandina G, Lane R, Gopal S, et al. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):218–226. doi:10.1016/j.pnpbp.2010.11.008
76. Bossie CA, Sliwa JK, Ma YW, Fu DJ, Alphs L. Onset of efficacy and tolerability following the initiation dosing of long-acting paliperidone palmitate: post-hoc analyses of a randomized, double-blind clinical trial. BMC Psychiatry. 2011;11:79. doi:10.1186/1471-244X-11-79
77. Fu DJ, Bossie CA, Kern SJ, Ma YW, Alphs L. Paliperidone palmitate versus risperidone long-acting injection in markedly-to-severely ill schizophrenia subjects: onset of efficacy with recommended initiation regimens. Clin Schizophr Relat Psychoses. 2014;8(2):101–9,109A. doi:10.3371/CSRP.FUBO.022213
78. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi:10.1093/schbul/13.2.261
79. Citrome L, Du Y, Weiden PJ. Assessing effectiveness of aripiprazole lauroxil vs placebo for the treatment of schizophrenia using number needed to treat and number needed to harm. Neuropsychiatr Dis Treat. 2019;15:2639–2646. doi:10.2147/NDT.S207910
80. Targum SD, Risinger R, Du Y, Pendergrass JC, Jamal HH, Silverman BL. Effect of patient age on treatment response in a study of the acute exacerbation of psychosis in schizophrenia. Schizophr Res. 2017;179:64–69. doi:10.1016/j.schres.2016.09.034
81. Potkin SG, Risinger R, Du Y, et al. Efficacy and safety of aripiprazole lauroxil in schizophrenic patients presenting with severe psychotic symptoms during an acute exacerbation. Schizophr Res. 2017;190:115–120. doi:10.1016/j.schres.2017.03.003
82. Citrome L, Risinger R, Cutler AJ, et al. Effect of aripiprazole lauroxil in patients with acute schizophrenia as assessed by the positive and negative syndrome scale-supportive analyses from a phase 3 study. CNS Spectr. 2018;23(4):284–290. doi:10.1017/s1092852917000396
83. Correll CU, Stanford AD, Claxton A, Du Y, Weiden PJ. Social and functional outcomes with two doses of aripiprazole lauroxil vs placebo in patients with schizophrenia: a post-hoc analysis of a 12-week phase 3 efficacy study. Psychiatry Res Apr. 2019;274:176–181. doi:10.1016/j.psychres.2019.02.021
84. Citrome L, Du Y, Risinger R, et al. Effect of aripiprazole lauroxil on agitation and hostility in patients with schizophrenia. Int Clin Psychopharmacol. 2016;31(2):69–75. doi:10.1097/YIC.0000000000000106
85. Nasrallah HA, Weiden PJ, Walling DP, et al. Aripiprazole lauroxil 2-month formulation with 1-day initiation in patients hospitalized for an acute exacerbation of schizophrenia: exploratory efficacy and patient-reported outcomes in the randomized controlled ALPINE study. BMC Psychiatry. 2021;21(1):492. doi:10.1186/s12888-021-03420-x
86. McEvoy JP, Risinger R, Mykhnyak S, et al. Durability of therapeutic response with long-term aripiprazole lauroxil treatment following successful resolution of an acute episode of schizophrenia. J Clin Psychiatry. 2017;78(8):1103–1109. doi:10.4088/JCP.17m11625
87. McEvoy JP, Weiden PJ, Lysaker PH, Sun X, O’Sullivan AK. Long-term effect of aripiprazole lauroxil on health-related quality of life in patients with schizophrenia. BMC Psychiatry. 2021;21(1):164. doi:10.1186/s12888-021-03124-2
88. Guy W. CGI Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. US Department of Health, Education, and Welfare, National Institute of Mental Health; 1976:217–222.
89. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63(9):763–771. doi:10.4088/JCP.v63n0903
90. Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10(3):799–812. doi:10.2466/pr0.1962.10.3.799
91. Marder SR, Davis JM, Chouinard G. The effects of risperidone on the five dimensions of schizophrenia derived by factor analysis: combined results of the North American trials. J Clin Psychiatry. 1997;58(12):538–546. doi:10.4088/jcp.v58n1205
92. Patrick DL, Burns T, Morosini P, et al. Reliability, validity and ability to detect change of the clinician-rated personal and social performance scale in patients with acute symptoms of schizophrenia. Curr Med Res Opin. 2009;25(2):325–338. doi:10.1185/03007990802611919
93. Endicott J, Nee J, Harrison W, Blumenthal R. Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–326.
94. Ware JE Jr, Gandek B. Overview of the SF-36 health survey and the International Quality of Life Assessment (IQOLA) project. J Clin Epidemiol. 1998;51(11):903–912. doi:10.1016/s0895-4356(98)00081-x
95. Beare-Rogers J, Dieffenbacker A, Holm AJV. Lexicon of lipid nutrition. Pure Appl Chem. 2001;73(4):685–744. doi:10.1351/pac200173040685
96. World Health Organization. WHO Guidelines for Indoor Air Quality: Selected Pollutants. World Health Organization, Regional Office for Europe; 2010.
97. Weiden PJ, Du Y, Liu CC, Stanford AD. Switching stable patients with schizophrenia from their oral antipsychotics to aripiprazole lauroxil: a post hoc safety analysis of the initial 12-week crossover period. CNS Spectr. 2019;24(4):419–425. doi:10.1017/s1092852918000986
98. Citrome L, Yagoda S, Bidollari I, Wang M. Safety and tolerability of starting aripiprazole lauroxil with aripiprazole lauroxil NanoCrystal Dispersion in 1 day followed by aripiprazole lauroxil every 2 months using paliperidone palmitate monthly as an active control in patients with schizophrenia: a post hoc analysis of a randomized controlled trial. J Clin Psychiatry. 2024;85(1):23m15095. doi:10.4088/JCP.23m15095
99. Consensus development conference on antipsychotic drugs and obesity and diabetes. J Clin Psychiatry. 2004;65(2):267–272. doi:10.4088/JCP.v65n0219
100. Velligan DI, Sajatovic M, Hatch A, Kramata P, Docherty JP. Why do psychiatric patients stop antipsychotic medication? A systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449–468. doi:10.2147/ppa.s124658
101. Doane MJ, Sajatovic M, Weiden PJ, et al. Antipsychotic treatment experiences of people with schizophrenia: patient perspectives from an online survey. Patient Prefer Adherence. 2020;14:2043–2054. doi:10.2147/ppa.s270020
102. DiBonaventura M, Gabriel S, Dupclay L, Gupta S, Kim E. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12:20. doi:10.1186/1471-244x-12-20
103. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi:10.1016/s0140-6736(13)60733-3
104. Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. doi:10.1016/s2215-0366(19)30416-x
105. El-Sayeh HG, Morganti C. Aripiprazole for schizophrenia. Cochrane Database Syst Rev. 2006;2006(2):CD004578. doi:10.1002/14651858.CD004578.pub3
106. Kelly DL, Claxton A, Bidollari I, Du Y. Analysis of prolactin and sexual side effects in patients with schizophrenia who switched from paliperidone palmitate to aripiprazole lauroxil. Psychiatry Res. 2021;302:114030. doi:10.1016/j.psychres.2021.114030
107. Sommi RW, Saklad SR, Weiden PJ, Still D, Wang M, Yagoda S. Initiating aripiprazole lauroxil: post hoc analysis of safety and tolerability of 1-day and 21-day regimens. J Clin Psychiatry. 2024;85(3):23m15132. doi:10.4088/JCP.23m15132
108. Lauriello J, Weiden PJ, Gleeson CD, et al. Real-world outcomes and costs following 6 months of treatment with the long-acting injectable (LAI) aripiprazole lauroxil for the treatment of schizophrenia. CNS Drugs. 2021;35(10):1123–1135. doi:10.1007/s40263-021-00849-2
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


