Back to Journals » Journal of Pain Research » Volume 17
Assessing Gender Differences for Non-Predictable Breakthrough Cancer Pain Phenomenon: A Secondary Analysis from IOPS-MS Study
Authors Bimonte S , Di Gennaro P , Crispo A, Coluccia S, Luongo A , Amore A, Celentano E , Del Prato F, Schiavo D, Nocerino D, Cascella M , Cuomo A
Received 18 October 2023
Accepted for publication 9 May 2024
Published 5 September 2024 Volume 2024:17 Pages 2861—2871
DOI https://doi.org/10.2147/JPR.S445222
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amitabh Gulati
Sabrina Bimonte,1,* Piergiacomo Di Gennaro,2,* Anna Crispo,2 Sergio Coluccia,2 Assunta Luongo,2 Alfonso Amore,3,4 Egidio Celentano,2 Francesco Del Prato,1 Daniela Schiavo,1 Davide Nocerino,1 Marco Cascella,5 Arturo Cuomo1
1Division of Anesthesia and Pain Medicine, Istituto Nazionale Tumori, IRCCS Fondazione G. Pascale, Naples, Italy; 2Epidemiology and Biostatistics Unit, Istituto Nazionale Tumori, IRCCS Fondazione G. Pascale, Naples, Italy; 3Melanoma and Sarcoma Surgery Unit, Istituto Nazionale Tumori, IRCCS Fondazione G. Pascale, Naples, Italy; 4PhD School of Applied Medical-Surgical Sciences, University of Rome Tor Vergata, Rome, Italy; 5Department of Medicine, University of Salerno, Salerno, Italy
*These authors contributed equally to this work
Correspondence: Anna Crispo, Epidemiology and Biostatistics Unit, Istituto Nazionale Tumori, IRCCS Fondazione G. Pascale, Via Mariano Semmola 53, Naples, Italy, Tel +39 081 17770298, Email [email protected]
Purpose: Breakthrough cancer pain (BTcP) is a temporary exacerbation of pain that “breaks through” a phase of adequate pain control by an opioid-based therapy. The non-predictable BTcP (NP-BTcP) subtype occurs in the absence of any specific activity. Evidence showed that gender differences exist in pain response sensitivity and clinical pain risk. This analysis aimed to signify the gender differences for the NP-BTcP phenomenon.
Patients and Methods: This is a secondary analysis of the Italian Oncologic Pain multiSetting-Multicentric Survey (IOPS-MS), the largest study on BTcP. The subset of NP-BTcP cases for non-gender-specific cancer was considered. Univariable and multivariate analyses were conducted to identify gender differences for the NP-BTcP profile about its intensity, number of episodes per day, and type. A metastatic status-stratified analysis was performed to compare gender with the main clinical variables among the population with NP-BTcP.
Results: Males exhibited a higher occurrence of BTcP in the thorax region compared to females (15% vs 11%, respectively, p = 0.03). Males also had a higher onset of BTcP, a higher BTcP therapy dosage (33% vs 28%, p = 0.04, mean: 201 vs 186, p = 0.02) and a lower Karnofsky score (mean: 46.9 vs 49.2, p = 0.03) compared to females. Similar gender differences were found for metastatic patients in the BTcP site (14% vs 8.5%, respectively; p = 0.01), peak onset (33% vs 27%, p = 0.02), BTcP therapy dosage (199 vs 185, p=0.04), and Karnofsky score (mean 47.5 vs 50.4, p = 0.009). Phenotype 2 was more characterized by non-metastatic males (41% vs 23%, p = 0.020) while non-metastatic females presence was predominant among others.
Conclusion: In this study, gender differences according to site, onset and dosage of BTcP were found. The phenotype characterization of BTcP needs to be further investigated for a possible useful function in the management of cancer-related pain in non-metastatic patients.
Keywords: non-predictable breakthrough cancer pain, gender cancer pain, cluster analysis
Introduction
Cancer pain is a debilitating condition and has severe effects on patients’ quality of life.1,2 The breakthrough cancer pain (BTcP) phenomenon represents a clinical peculiarity of cancer, which is characterized by a temporary exacerbation of pain that “breaks through” a phase of adequate control by an opioid-based therapy.3,4 This symptom can affect up to 70% of patients with cancer.5,6 Notably, two types of BTcP have been recognized: predictable BTcP (P-BTcP) and non-predictable BTcP (NP-BTcP) that have different features according to pathophysiology, clinical and therapeutic involvements.7,8 The P-BTcP is subdivided into three subgroups: the volitional BTcP (caused by a voluntary act), the non-volitional subtype (provoked by an involuntary act), and the procedural pain. The NP-BTcP occurs in the absence of any specific activity, which is also called idiopathic or spontaneous BTcP.9,10
Shreds of evidence demonstrate that men and women differ in their responses to pain. Specifically, women showed both greater sensitivity to pain and higher clinical risk than men; as reported by Bartley et al, many biopsychosocial mechanisms contribute to these sex differences in pain, including gender roles, sex hormones, endogenous opioid function and genetic factors.11 In this paper, we described the results of a secondary analysis based on the Italian Oncologic Pain multiSetting-Multicentric Survey (IOPS-MS) focusing on gender differences regarding non-gender-specific cancer (No-GSC) from the NP-BTcP subset.
Materials and Methods
Details from IOPS-MS Dataset
Details concerning the enrollment of patients and all recorded variables have been previously described.7 Briefly, the IOPS-MS study was a multicenter survey that involved 5 palliative care units (PCU), 7 oncology centers (ONC), and 9 outpatient pain clinics (OPC). The study was proposed by an expert group of 27 Italian centers representative of different settings of cancer pain and 21 centers agreed to participate. The primary aim of this study was to characterize BTcP in a large number of patients performed in different settings and to assess possible factors influencing its development. The secondary aim was to gather information about the diagnosis and management of BTcP as well as patient satisfaction with the treatment.7
The study protocol was approved by the local Ethical Committee, and informed consent was obtained from all patients enrolled in the study.
Inclusion criteria were: age greater than 18 years; diagnosis of cancer at any stage; well-controlled and stable background pain with an intensity ≤4 (on a 0–10 numerical rating scale, NRS); the presence of BTcP episode of moderate–severe intensity clearly distinguished from background pain. Exclusion criteria were as follows: no cancer diagnosis; unstable and/or uncontrolled background pain (>4/10); no relevant peaks in pain intensity (<5/10); poor collaboration or refusal to participate. Considering that in spontaneous BTcP more than 3–4 episodes per day usually indicate uncontrolled background pain (requiring a careful optimization of basal pain) we adopted the cut-off of 4 episodes per day.8 Among all recorded variables from the original study, some variables were selected for this secondary analysis including age, gender, setting, BTcP site, therapies and dosages, onset, type of BTcP pain, Karnofsky performance status scale and type of physician . The study was observational and pharmaceutical therapies were used according to local policy, without following strict protocols. In our previous study, a multiple correspondence analysis (MCA) and a hierarchical clustering principal components analysis (HCPC)12 were adopted to interpret the BTcP phenomenon on the original IOPS-MS dataset that included 2790 (69.6%) patients with NP-BTcP. The four clusters (phenotypes) represent a classification of patients based on BTcP status, defined by variables of BTcP intensity, number of episodes and type. Phenotypes were from P1 to P4 with the best group (P1) versus the worst one (P4) and with the same features.7,12
Briefly, P1 was characterized by older age (≥75 years), slower onset (>10 min), gastrointestinal as primary tumor, and greater propensity to be treated in the context of the palliative care setting. On the contrary, the main features of phenotype 4 were as follows: younger age (<55 years) and rapid onset; furthermore, it most frequently concerns inpatients affected by lung cancer. Moreover, regarding the NP-BTcP therapy, the worst phenotype (P4) was mainly managed with rapid-onset opioids (ROOs); on the contrary, in phenotype 1 many patients were treated with oral, subcutaneous, or intravenous morphine. Moreover, the number of patients who did not receive therapy (ROOs, morphine and other therapy) decreased from P1 to P4.
From the secondary analysis based on IOPS-MS data, the subset of NP-BTcP cases for non-gender-specific cancer (Non-GSC) was considered (n = 1871). Patients not in BTcP therapy, with diagnosis of multiple tumors or with rare tumors, and Non-GSC patients in hormone therapy were excluded from the analysis (n = 304).
Statistical Analysis
An univariable analysis was performed to compare gender within the population with NP-BTcP. Statistical tests for comparing means (Wilcoxon rank-sum test) and distributions (Chi-square and Fisher-exact tests) were performed to observe the main differences between males and females. A multivariable analysis according to metastatic status was performed to compare gender within the population with unpredictable pain, in particular, adjusted logistic multivariable models were implemented to assess associations between gender and selected variables. Odds ratios (ORs) and test results were reported with a 95% confidence level for significance. Analyses were computed with R software version 4.2.1.
Results
The gender-based differences and sample characteristics of patients with BTcP are shown in Table 1. Male patients experienced BTcP more frequently in the thorax and less frequently in the abdomen compared to female patients (15% vs 11% and 21% vs 28%, respectively, p = 0.03). Male patients also had a higher onset of BTcP (33% vs 28%, p = 0.04) and a higher BTcP therapy dosage (mean: 201 vs 186, p = 0.02). Males were more likely to receive treatment in a clinic or day hospital, while females were more likely to receive treatment in a hospice or home setting (44% vs 37%; 22% vs 16%, respectively, p = 0.002). Additionally, male patients had a lower Karnofsky score (mean: 46.9 vs 49.2, p = 0.03). Other BTcP main elements were not found statistically significant. Results described above were mostly confirmed in the metastatic subgroup (Table 2, right): the abdomen region was the main BTcP site for females with metastasis, while thoracic pain was found as more common among male metastatic patients (29% vs 22%; 14% vs 8.5%, respectively; p = 0.01); the peak onset time (≤10 min vs >10 min) remained higher for metastatic males (33% vs 27%, p = 0.02); BTcP therapy dosage was meanly lower for female (185 vs 199, p = 0.04). Results in care setting were confirmed in metastatic patients (p =0.003) as males showed a lower Karnofsky score (mean: 47.5 vs 50.4, p =0.009). Among non-metastatic group, P2 was more characterized by males (41% vs 23%, p = 0.02) while females were predominant among others. Although not reaching the significance (p = 0.06), males were less prone to undergo any adverse reactions than females among metastatic cancer patients.
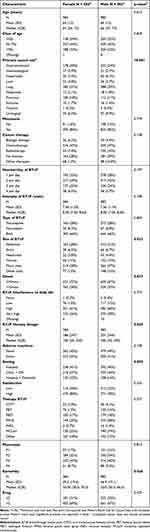 |
Table 1 Non-GSC Cancer Patients: Main Characteristics by Gender (Male vs Female) |
 |
Table 2 Metastatic vs Non-Metastatic Tumors by Gender (Male vs Female) in Non-GSC Cancer Patients |
Finally, Table 3 shows logistic multivariable regression for gender stratified by metastatic status. In the non-metastatic subgroup, male cancer patients were more likely to be treated with OTFT (OR = 7.86, 95% CI = [1.34, 68.9]) and with FPNS (OR = 4.45, 95% CI = [1.45, 14.2]). Phenotype was associated with gender: male cancer patients were more likely to belong to P2 (OR = 3.05, 95% CI = [1.03, 9.23], p = 0.03). Setting was found as barely associated with gender (p = 0.07).
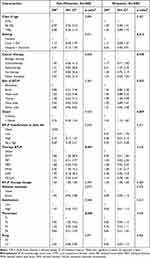 |
Table 3 Logistic Regression Models for Gender (Male vs Female) and by Metastatic Status in Non-GSC Cancer Patients |
In Metastatic group, males were 35% less likely to be taken into care by hospice or domicile than females (OR = 0.65, 95% CI = [0.46, 0.91], p = 0.01), were more prone to suffer from thorax BTcP (OR = 1.99, 95% CI = [1.30, 3.09]), underwent radiotherapy (OR = 2.31, 95% CI = [1.22, 4.42]) and experimented a higher onset (OR = 1.43, 95% CI = [1.10, 1.87]).
Discussion
BTcP represents a clinical peculiarity of cancer pain characterized by distinct pathogenic mechanisms underlying its subtypes.13–20 Given the complexity of the NP-BTcP subtype, it is crucial to enhance its characterization for more effective therapy guidance, to ameliorate patients’ quality of life and satisfaction.7,19,20 To our knowledge, this is the first study that investigates gender differences in the NP-BTcP phenomenon.
Regarding our results, we found that female patients showed more frequent BTcP in the abdomen region and less in the thorax compared to male patients; moreover, male patients also had a higher onset of BTcP and BTcP therapy dosage. Additionally, female patients had a higher Karnofsky score. Such findings were confirmed among metastatic group where abdomen BTcP site was more frequent for females and the thorax region occurred more frequently among males; moreover, the peak onset remained lower for females such as the BTcP therapy dosage, among the metastatic group, too.
The multivariable analysis demonstrates that P2 was mainly characterized by non-metastatic male patients, while the other phenotypes were greater for non-metastatic females. The role of a phenotype characterization of BTcP needs to be further investigated for a possible useful function in the management of cancer-related pain in patients with non-metastatic disease, taking into account gender differences and other key variables. Moreover, as reported by previous analyses,7,18,20 several key points should be considered in the pharmacological management of BTcP to improve patients’ quality of life.
Conclusion
Our analysis sheds light on gender differences in the NP-BTcP phenomenon, particularly in its intensity, onset and treatment profile. Female patients exhibited distinct patterns, experiencing more frequent BTcP in the abdomen region, with a shorter onset and lower BTcP therapy dosage compared to male patients. Moreover, females tended to have a higher Karnofsky score, indicating potentially better functional status. These findings were consistent across both non-metastatic and metastatic subgroups, underscoring the relevance of gender-specific considerations in pain management strategies. Additionally, the analysis of BTcP phenotypes revealed differential distributions between genders. Consequently, there is a need for further investigation into the role of phenotype characterization in guiding personalized pain management approaches, especially for non-metastatic patients. Recognizing and addressing gender disparities in NP-BTcP can contribute to more effective and tailored interventions, ultimately improving the quality of life for individuals suffering from cancer-related pain.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (PROTOCOLLO DI STUDIO IOPS-MS) Policlinico Tor Vergata (REGISTRO SPERIMENTAZIONI n.21/13) Roma, 20 February 2013.
Data Sharing Statement
All data are available in the manuscript and at link DOI: “10.5281/zenodo.7821879”.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent was obtained from the patient(s) to publish this paper.
Acknowledgments
The authors thank all study participants for their involvement in the study. The authors thank Maria Cristina Romano for data curation and the IOPS-MS group for their work in the original study. The authors thank the Italian Government, Ministry of Health, Ricerca Corrente 2022 L4/4.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The original study was sponsored by Molteni Farmaceutici, Italy. Data were independently analyzed and managed by authors. The funders had no role in the design of these secondary analyses or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Deandrea S, Corli O, Consonni D, Villani W, Greco MT, Apolone G. Prevalence of breakthrough cancer pain: a systematic review and a pooled analysis of published literature. J Pain Sympt Manage. 2014;47(1):57–76. doi:10.1016/j.jpainsymman.2013.02.015
2. van den Beuken-van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, Tjan-Heijnen VCG, Janssen DJA. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Sympt Manage. 2016;51(6):1070–1090.e9. doi:10.1016/j.jpainsymman.2015.12.340
3. Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain. 2019;160(1):19–27. doi:10.1097/j.pain.0000000000001384
4. Bennett MI, Kaasa S, Barke A, et al. The IASP classification of chronic pain for ICD-11: chronic cancer-related pain. Pain. 2019;160(1):38–44. doi:10.1097/j.pain.0000000000001363
5. Mercadante S, Portenoy RK. Breakthrough cancer pain: twenty-five years of study. Pain. 2016;157(12):2657–2663. doi:10.1097/j.pain.0000000000000721
6. Davies AN, Elsner F, Filbet MJ, et al. Breakthrough cancer pain (BTcP) management: a review of international and national guidelines. BMJ Support Palliat Care. 2018;8(3):241–249. doi:10.1136/bmjspcare-2017-001467
7. Mercadante S, Lazzari M, Reale C, et al. Italian oncological pain survey (IOPS): a multicentre Italian study of breakthrough pain performed in different settings. Clin J Pain. 2015;31(3):214–221. doi:10.1097/AJP.0000000000000161
8. Mercadante S. Breakthrough pain in cancer patients: prevalence, mechanisms and treatment options. Curr Opin Anaesthesiolo. 2015;28(5):559–564. doi:10.1097/ACO.0000000000000224
9. Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G. The management of cancer-related breakthrough pain: recommendations of a task group of the science committee of the association for palliative medicine of Great Britain and Ireland. Eur J Pain. 2009;13(4):331–338. doi:10.1016/j.ejpain.2008.06.014
10. Davies AN. The management of breakthrough cancer pain. Br J Nurs. 2011;20(13):803–807. doi:10.12968/bjon.2011.20.13.803
11. Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52–58. PMID: 23794645; PMCID: PMC3690315. doi:10.1093/bja/aet127
12. Cascella M, Crispo A, Esposito G, et al. Multidimensional statistical technique for interpreting the spontaneous breakthrough cancer pain phenomenon. A secondary analysis from the IOPS-MS study. Cancers. 2021;13(16):4018. doi:10.3390/cancers13164018
13. Narayana A, Katz N, Shillington AC, et al. National breakthrough pain study: prevalence, characteristics, and associations with health outcomes. Pain. 2015;156(2):252–259. doi:10.1097/01.j.pain.0000460305.41078.7d
14. Husson F, Josse J, Pagès J. Principal component methods hierarchical clustering partitional clustering: why would we need to choose for visualizing data?; 2010.
15. Lê S, Josse J, Husson F. FactoMineR: an R package for multivariate analysis. J Stat Soft. 2008;25(1). doi:10.18637/jss.v025.i01
16. Caraceni A, Shkodra M. Cancer pain assessment and classification. Cancers. 2019;11(4):510. doi:10.3390/cancers11040510
17. Løhre ET, Thronæs M, Klepstad P. Breakthrough cancer pain in 2020. Curr Opin Support Palliat Care. 2020;14(2):94–99. doi:10.1097/SPC.0000000000000494
18. Cuomo A, Bimonte S, Forte CA, Botti G, Cascella M. Multimodal approaches and tailored therapies for pain management: the trolley analgesic model. J Pain Res. 2019;12:711–714. doi:10.2147/JPR.S178910
19. Pantano F, Manca P, Armento G, et al. Breakthrough cancer pain clinical features and differential opioids response: a machine learning approach in patients with cancer from the IOPS-MS study. JCO Precis Oncol. 2020;4:
20. Mazzotta M, Filetti M, Piras M, Mercadante S, Marchetti P, Giusti R. Patients’ satisfaction with breakthrough cancer pain therapy: a secondary analysis of IOPS-MS study. Cancer Manag Res. 2022;14:1237–1245. doi:10.2147/CMAR.S353036
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

