Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 16
Assessment of Health-Related Quality of Life in Adults Living with HIV Attending Antiretroviral Clinics versus Traditional Healers’ Offices in Bukavu City, Democratic Republic of the Congo
Authors Kyambikwa Bisangamo C , El-Nimr NA, Kyamusugulwa PM, Wahdan IMH, Gad ZM
Received 29 July 2024
Accepted for publication 14 October 2024
Published 23 October 2024 Volume 2024:16 Pages 383—395
DOI https://doi.org/10.2147/HIV.S480879
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Célestin Kyambikwa Bisangamo,1 Nessrin Ahmed El-Nimr,2 Patrick Milabyo Kyamusugulwa,1 Iman Mohamed Helmy Wahdan,2 Zahira Metwally Gad2, †
1Department of Public Health, Bukavu High Institute of Medical Techniques (ISTM-Bukavu), Bukavu, Democratic Republic of the Congo; 2Department of Epidemiology, High Institute of Public Health, Alexandria University, Alexandria, Egypt
†Zahira Metwally Gad passed away on 19 December 2022
Correspondence: Célestin Kyambikwa Bisangamo, Email [email protected]; [email protected]
Background: The benefits of antiretroviral therapy (ART) for people living with HIV/AIDS (PLHIV) include immune system strengthening, viral load suppression, and improved health-related quality of life (HRQOL). This present study compares the HRQOL of PLHIV visiting ART clinics versus that of PLHIV attending traditional healers (THs)’ offices, assesses the adherence of PLHIV to ART, identifies possible predictors of nonadherence of PLHIV to ART and HRQOL, and estimates the proportion of patients with HIV referred by THs to health centers in Bukavu.
Patients and methods: Between February and June 2023, a cross-sectional comparative study was conducted on 150 adult PLHIV attending ART clinics and 150 adult PLHIV visiting THs’ offices in the 3 urban health zones of Bukavu. The World Health Organization Quality of Life questionnaire (WHOQOL-BREF) and a self-report questionnaire measuring ART adherence were used to collect the data. Regression models were used to identify the predictors of no adherence to ART and the HRQOL of PLHIV.
Results: Compared with those attending THs, PLHIV attending ART clinics had higher mean scores in all HRQOL domains. Approximately 84% of the participants were compliant with ART. The predictors associated with nonadherence to ART included illiterate participants [OR=23.3 (95% CI=1.23– 439.5), p=0.004] and divorced or separated participants [OR=10.3 (95% CI=1.12– 94.4), p=0.034]. The proportion of PLHIV referred to ART clinics by THs was only 10.7%.
Conclusion: PLHIV visiting ART clinics had a better HRQOL than did PLHIV attending THs’ offices. The rate of adherence to ART among PLHIV who attended ART clinics was high. It is recommended that PLHIV visiting THs be referred to ART clinics for improved HRQOL.
Keywords: HIV/AIDS infection, Health-related quality of life, Compliance, Antiretroviral medications
Background
Human immunodeficiency virus (HIV) infection is one of the most serious public health problems in the world and has high morbidity and mortality rates. Since the beginning of the epidemic, 85.6 million people were infected, and 40.4 million people were killed in 2022.1 Approximately 39 million individuals were living with HIV at the end of 2022, and 630,000 died from AIDS-related illnesses.2 Sub-Saharan Africa is the most severely affected region, with nearly one in every twenty-five adults (3.2%) living with HIV and accounting for more than two-thirds of the PLHIV worldwide.1 New HIV infections are particularly common in low- and middle-income countries (LMICs), such as the Democratic Republic of the Congo (DRC). In 2020, there were 510,000 PLHIV in the DRC, including approximately 20,000 new cases.3
Life expectancy for PLHIV has increased,4 and HIV-related morbidity and death have significantly decreased as a result of combined antiretroviral therapy (cART) and its adherence.5 Since the advent of safe and effective ART, HIV/AIDS infection has transformed from a fatal disease to a chronic disease that may be managed.6 When PLHIV receive ART, their viral load is suppressed,7 their immune system is strengthened,8 and their HRQOL is enhanced.9
In traditional African societies, THs play a multifaceted role in the community. They serve as guardians of religion and customs, offering guidance and counsel on matters pertaining to culture, psychology, social work, and healthcare.10 Approximately 80% of sub-Saharan Africans regularly benefit from TH health services due to their accessibility and acceptability.11 Some studies have shown that most PLHIV use traditional medicine (TM) alongside modern medicine.12,13 The practice of TM is governed by a ministerial decree in the DRC, where the Ministry of Health (MOH) has a National Program for the Promotion of Traditional Medicine and Medicinal Plants (PNMT/PM).14
When a health professional at one level of the health system lacks the resources to manage a clinical condition, he or she can refer a patient to a facility at a higher or better level to receive help in managing the client’s case or to receive better resources. Patient referrals are important because they guarantee that each patient receives the required specialized care, hence improving the quality of care.15
Health-related quality of life is multifaceted16 and can be considered a subjective evaluation that patients make of themselves based on their perception of the impact of the disease and/or its treatment on their well-being17 in the physical, spiritual, social, psychological, and environmental domains. HRQOL is a crucial metric that helps medical professionals understand patients’ perceived satisfaction and perception of disease.5,6 There is a complex relationship between HIV/AIDS incidence, ART, and HRQOL.18 The HRQOL of PLHIV on ART is essential for monitoring the impact of medication therapy on the progression of the disease.18,19
To our knowledge, no study has been conducted to compare the HRQOL of PLHIV attending ART clinics and PLHIV visiting THs’ offices in Bukavu. The current study will provide essential baseline information that will help health authorities design evidence-based interventions that are suitable for PLHIV in Bukavu.
The current study aimed to compare the HRQOL of PLHIV who visited ART clinics versus those who visited THs’ offices. Additionally, the study sought to evaluate the adherence of PLHIV to ART, identify potential predictors of PLHIV nonadherence to ART and to their HRQOL, and estimate the proportion of PLHIV who were referred to health centers in Bukavu by THs.
Methods
Study Design and Setting
A comparative cross-sectional study employing quantitative approach was carried out between February and June 2023 among HIV-positive patients attending ART clinics and PLHIV visiting THs’ offices.
The study was conducted in Bukavu city, the capital city of the South Kivu Province in the eastern DRC. Bukavu city is subdivided into three health zones: the Ibanda Health Zone, the Kadutu Health Zone, and the Bagira Health Zone.
Study Participants and Sampling Procedure
The target population consisted of adult patients with HIV (≥18 years old) who had been diagnosed at ART clinics for at least a year and adult PLHIV (≥18 years old) who visited THs.
In the Bukavu health zones, there are 30 ART clinics and 71 THs’ offices recognized by the PNMT/PM in South Kivu. The sample size was calculated using the StatCalc TM function in Epi Info® 7.4 software. Since the proportion of PLHIV who have a poor HRQOL is unknown in our setting, this proportion was set at 0.5 (ie, 50%), which provides a conservative estimate of the variance.20 Based on the assumptions that PLHIV should experience poor HRQOL 50% of the time, an odds ratio of 2, two-sided confidence levels of 95%, a power of 80%,21 a ratio of unexposed/exposed of 1, a prevalence ratio of 1.3, and a percentage of outcome in the exposed group of 66.7%, the Fleiss with continuity correction formula produced a sample size of 296 patients with HIV, which was rounded to 300 (150 patients with HIV per group).
To select PLHIV from the ART clinics, a three-stage sampling technique was used. The first stage is the health zones. The city of Bukavu has three health zones, and all three zones were included in the study. In the second stage, we have the ART clinics. A list of all ART clinics was obtained from the provincial office of the National AIDS Control Program (PNLS) and used as a sampling frame. There are 10 ART clinics in each health zone. Five ART clinics were randomly selected in each health zone using a simple random sampling technique. A total of 15 ART clinics were selected. And in the third stage, we selected HIV-positive patients. In each ART clinic, we consecutively selected 10 hIV-positive patients using convenience sampling. A total of 150 PLHIV were recruited.
All THs’ offices (71) were visited for the recruitment of PLHIV. Some patients visiting THs were known to have HIV, and they visited THs in search of possible treatment. On the other hand, others come to THs for treatment because of poisoning or bewitchment. In this case, THs require them to take an HIV test in a hospital or health center before treatment. Only HIV-positive patients were selected for this study. HIV-positive patients visiting the THs were consecutively recruited until the desired sample size was reached (150 PLHIV were selected).
Data Collection
A predesigned structured interviewer-assisted questionnaire was used to collect the following data from PLHIV: personal and demographic characteristics (age, sex, marital status, level of education, religious affiliation, ethnicity, occupation, area of residence, family size, income, and insurance status); clinical characteristics (signs and symptoms); reasons for using traditional medicine; and other health providers consulted and sources of the referral of HIV-positive patients to clinical facilities.
The French version of the World Health Organization Quality of Life questionnaire (WHOQOL-BREF) was used to collect data about the QOL of PLHIV. The WHOQOL-BREF provides a valid and reliable alternative to the lengthier WHOQOL-100.22 The tool has 26 items and four domains, namely:
- The physical health domain (7 items) included activities of daily living, dependence on medicinal substances and medical aids, energy and fatigue, mobility, pain and discomfort, sleep and rest, and work capacity.
- Psychological health (6 items): bodily image and appearance, negative feelings, positive feelings, self-esteem, spirituality/religion/personal beliefs, thinking, learning, memory, and concentration.
- Social relationships (3 items): personal relationships, social support, and sexual activity.
- Environmental health (8 items): financial resources, freedom, physical safety and security, health, and social care: accessibility and quality, home environment, opportunities for acquiring new information and skills, participation in and opportunities for recreation/leisure activities, physical environment (pollution/noise/traffic/climate), and transport.
Items are rated on a five-point Likert scale, with 1 indicating a low score and five indicating a high score. In most questions, options 1 and 5 represent the lowest and highest values, respectively. However, for questions where a higher score did not indicate a better HRQOL, the responses were first reversed and subsequently calculated. The score for each domain was calculated by adding up the total points for the questions in each domain, dividing the total value by the number of questions, and then multiplying the result by four. The score for each domain ranged from 4 to 20, with a score of 4 indicating the worst condition and a score of 20 representing the best condition in the domain. The total HRQOL was calculated based on the 26-item questionnaire. The scores were then linearly transformed to a 0–100 scale, with a higher score representing better HRQOL. Cronbach’s alpha was used to check the reliability of the WHOQOL-BREF questionnaire, with a value greater than 0.7 indicating high reliability.
Finally, we used the validated French version of a self-assessment questionnaire23 to assess adherence to antiretroviral treatment among PLHIV attending ART clinics. This version comprises nine main questions. First, patients were asked to name their antiretroviral medication and the daily dosage. A chart with a picture and the name of each available antiretroviral on the market was provided to help respondents recall the name of their antiretroviral medication. Subsequently, they reported the number of antiretroviral pills missed on the preceding and penultimate days. Then, three questions were used as aid-recall tools for situations that might have hampered regular adherence to medication during the preceding seven days. Two questions were used to assess nonadherence during the preceding seven days. Respondents were asked to indicate how many times they missed taking one or more of their antiretroviral pills during the preceding seven days and then to translate this information into the total number of antiretroviral pills missed during this period of time. The last three questions referred to the preceding 30 days as a time frame.
The original version of the questionnaire was in French and was used because the language of the participants was French. The clarity of the questionnaire was tested beforehand on a group of 20 patients from the target population. PLHIV were considered nonadherent if they reported forgetting to take their antiretroviral treatment pills at least once in the week prior to the survey. Conversely, patients who took all their antiretroviral treatment pills during the same period were considered adherent.
Data Management and Analysis
The completeness of the data was checked during the data collection process. The data were entered into Kobocollect, cleaned, and coded in Microsoft Excel. Epi Info version 3.5.1 and the Statistical Package for the Social Sciences (SPSS) version 16 were used to analyze the data.
The mean and standard deviation (SD) or median and interquartile range (IQR) were used to summarize quantitative variables, depending upon the distribution of the data. Categorical variables were summarized using the frequency and percentage of subjects in each category.
The T-test was used to compare means. Bivariate comparisons of categorical variables were assessed using Pearson’s chi-square (X2) test. Whenever X2 was not valid, Fisher’s exact test was used for 2*2 tables. A multivariate logistic regression model was used to identify the significant predictors of nonadherence to ART as the dependent variable. The independent variables were sociodemographic characteristics such as age, sex, education level, religion, tribe, place of residence and training in taking care of HIV infection. Multivariate linear regression was used to identify the predictors of HRQOL as the dependent variable. Sociodemographic factors such as age, sex, education level, religion, tribe, place of residence, occupation, number of family members, monthly income in US dollars, and health insurance status were the independent variables. The correlation coefficient was used to verify the existence of a link between the HRQOL domains. The significance level was set at a p value of less than 0.05.
Ethical Considerations
The study was conducted in accordance with International Guidelines for Research Ethics. Approval was obtained from the Ethics Committee of the High Institute of Public Health, Alexandria University, Egypt (IRB N°: 00013692). All study participants provided informed written consent following the explanation of the purpose and benefits of the research. Anonymity and confidentiality were maintained and guaranteed.
To rationalize the study execution, the necessary administrative and preparatory communications with the health structures’ authorities were completed. The competent authority of the South Kivu Provincial Health Division (N°003/CD/DPS-SK/2019 and N°002/CD-DPS-SK/2022) and the Provincial Directorate of the National Health Ethics Committee (CNES/SK: 001–4125/001-113/2019) authorized the conduction of the study.
Results
Distribution of PLHIV Attending ART Clinics and Those Visiting THs’ Offices According to Their Personal and Socio-Demographic Characteristics
The mean age of the participants attending THs’ offices was 41.3 ± 11.4 years, and that of participants attending ART clinics was 42.9 ± 13.1 years. Female participants were predominant in both groups (64.7% vs 35.3% among PLHIV visiting THs’ offices and 72.7% vs 27.3% among PLHIV attending ART clinics). Married participants represented the majority of individuals in both groups (52.0% among PLHIV visiting THs’ offices and 45.5% among PLHIV attending ART clinics). The highest percentage of patients visiting the THs’ offices had completed secondary education (46.7%), followed by those with university education (30.7%), while most patients attending ART clinics had secondary or primary education (49.3% and 24.0%, respectively). Most of those visiting THs’ offices had a household size of more than six individuals (45.3%) and a monthly income of less than $100 (62.7%). On the other hand, those attending ART clinics had a household size of three to six individuals and a monthly income of less than $100. The participants whose healthcare expenditures came from their mutual health insurance predominated in both groups (55.4% and 45.4%, respectively). These results are presented in Supplementary Table 1.
A comparison of the sociodemographic characteristics of the PLHIV attending ART clinics with those visiting THs’ offices revealed that PLHIV attending THs’ offices were more likely to be separated and widowed and had a low level of education, a household size ≤ six years and a low monthly income; additionally, their source of healthcare expenditure was the family.(Supplementary Table 1)
Distribution of PLHIV Visiting THs’ Offices and Those Attending ART Clinics According to Their HRQOL
The results of the HRQOL comparison between patients with HIV/AIDS visiting THs’ offices and those attending ART clinics are presented in Table 1. Patients attending ART clinics had a better HRQOL than did those attending THs’ offices in all HRQOL domains; physical (11.8 ± 2.5 versus 7.99 ± 2.3), psychological (10.9 ± 2.8 versus 7.96 ± 2.4), social (14.4 ± 3.1 versus 7.43 ± 2.0) and environmental aspects (10.4 ± 2.2 versus 7.43 ± 2.0). The same was true for overall HRQOL (11.0 ± 2.2 versus 8.24 ± 1.8). The differences in overall HRQOL and in different domains between HIV-positive patients attending THs’ offices and those attending ART clinics were statistically significant. (p ˂ 0.05).
 |
Table 1 Distribution of PLHIV Visiting THs’ Offices and Those Attending ART Clinics According to Their HRQOL (Bukavu, 2023) |
Multiple Linear Regression Analysis of the Predictors of HRQOL in PLHIV Visiting THs’ Offices and Those Attending ART Clinics
The findings of multiple linear regression analysis of predictors of HRQOL in patients with HIV/AIDS are summarized in Table 2. Place of residence was the only significant predictor of the overall HRQOL of patients who visited THs’ offices (p < 0.05). The model correctly classified 15% of the changes in HRQOL. For patients attending ART clinics, adherence to antiretroviral therapy was the only significant predictor of HRQOL (p < 0.05). Of the changes in HRQOL, 12.3% were correctly categorized by the model.
 |
Table 2 Results of the Multiple Linear Regression Analysis of the Predictors of HRQOL in PLHIV Visiting THs’ Offices and Those Attending ART Clinics (Bukavu, 2023) |
Distribution of PLHIV Attending ART Clinics Referred by THs and Their Adherence to ART
The referral rate of patients with HIV/AIDS to ART clinics by THs is presented in Figure 1. Only 10.7% of patients attending the ART clinics were referred by THs. The data related to adherence to ART therapy among PLHIV attending ART clinics are presented in Figure 2. In total, 83.9% of the participants were compliant, and 16.1% were noncompliant. These adherence and nonadherence percentages have been calculated from the results presented in Supplementary Table 2.
 |
Figure 1 PLHIV attending ART clinics referred by THs. |
 |
Figure 2 ART adherence of PLHIV attending ART clinics. |
Distribution of PLHIV Attending ART Clinics According to Their Personal and Sociodemographic Characteristics and Their Adherence to ART
Table 3 summarizes the results of the bivariate analysis of the personal and demographic characteristics of PLHIV attending ART clinics according to their adherence to ART. The following sociodemographic characteristics were significantly associated with nonadherence to ART: illiterate participants [OR=23.3 (95% CI= 1.23–439.5), p=0.004] and divorced or living separated participants [OR=10.3 (95% CI=1.12–94.4), p=0.034].
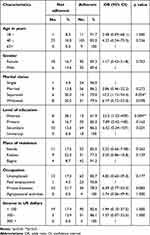 |
Table 3 Distribution of PLHIV Attending ART Clinics According to Their Personal and Sociodemographic Characteristics and Their Adherence to ART (Bukavu, 2023) |
Independent Predictors of Nonadherence in PLHIV Attending ART Clinics
Table 4 presents the results of the multivariate analysis of independent predictors of nonadherence using the logistic regression model. Only the level of education (being illiterate) was significantly associated with nonadherence to ART [adjusted OR=2.07 (95% CI=1.13–3.78), p=0.018].
 |
Table 4 Results of the Logistic Regression Analysis of the Independent Predictors of Nonadherence in PLHIV Attending ART Clinics (Bukavu, 2023) |
Relationships Between the HRQOL of Patients Attending ART Clinics and Their Adherence to ART
Higher mean HRQOL scores in all domains were observed among PLHIV who were adherent to ART than among those who were not adherent to treatment (Table 5). The overall HRQOL also showed similar results (11.2 ± 2.13 versus 9.99 ± 2.53). A significant relationship was observed between treatment adherence and the overall HRQOL score and between the physical domain and environmental domain scores of HRQOL (p ˂ 0.05).
 |
Table 5 Relationships Between the HRQOL of Patients Attending ART Clinics and Their Adherence to ART (Bukavu, 2023) |
Discussion
The current study found that PLHIV attending ART clinics had better HRQOL compared to PLHIV visiting THs’ offices, and the residence of PLHIV attending THs’ offices was the only significant predictor of poor HRQOL. Approximately 84% of PLHIV attending ART clinics were adherent to ART, and the predictive factors associated with nonadherence were illiteracy and being divorced or separated. The proportion of PLHIV referred by THs to ART clinics was only 10.7%. This study is the first in Bukavu to compare the HRQOL of PLHIV who visit ART clinics versus PLHIV who visit THs’ offices. The number of HIV cases increased in 2022,3 so it is relevant to understand what influences HRQOL to tailor better health and social care services and improve the HRQOL of PLHIV. Because current HIV/AIDS treatment strategies allow patients to live longer, HRQOL has emerged as a key indicator of health outcomes, and improving HRQOL is a key objective.7
The present study showed that PLHIV attending ART clinics had a greater overall HRQOL than did PLHIV visiting THs’ offices. The same was true for all domains of HRQOL, with PLHIV attending ART clinics having a better HRQOL than PLHIV visiting THs. The greater HRQOL of PLHIV attending ART clinics than of PLHIV visiting THs’ offices could be explained by the fact that the majority (83.9%) of PLHIV attending clinics were adherent to ART. Numerous studies have demonstrated a strong correlation between ART adherence and the HRQOL of PLHIV. Patients with low/moderate ART adherence were 60% less likely than patients with high adherence to have a high overall HRQOL score, according to a study performed in Ethiopia (2020).7 Similar findings were also reported by other studies conducted in Tunisia24 and Ethiopia,25 which suggest that adherence to antiretroviral treatment is associated with a greater HRQOL for PLHIV. The reasons for the low HRQoL of PLHIV attending THs may be due to the lack of clinical, immunological and virological follow-up, as well as poor management of opportunistic infections. A regimen lowers the viral load and enhances patients’ clinical status, both of which have an impact on HRQOL.26–28
In the present study, multiple linear regression analysis revealed that only the place of residence of PLHIV visiting THs was a significant predictor of their HRQOL. These results were different from those obtained in Brazilian and Italian studies.9,29 Increased education can help people deal with HIV more effectively, increase patient awareness of illness, and ultimately improve HRQOL.30,31 Age causes a decline in HRQOL, but these findings are different from those from Brazil and the United States.9,32
Adherence to ART, defined as the patient’s ability to follow the medication regimen and dietary restrictions, must be 70–90% to effectively suppress the viral load and reduce the risk of transmitting HIV to another person. Poor adherence can also lead to higher hospitalization rates and lost productivity.33,34
The issue of nonadherence is far more serious, especially for individuals who are HIV/AIDS positive. In addition to having an impact on nonadherent people, poor patient adherence can also have a broad societal impact. Adherence is necessary for viral suppression, decreased infection, minimized opportunistic infections, and decreased resistance to antiretroviral medications.35–37 The results of the present study showed a therapeutic nonadherence rate of 16.1% among PLHIV attending ART clinics. This low rate of nonadherence could be explained by the therapeutic education and awareness that PLHIV received in ART clinics about the advantages of adherence and the disadvantages of nonadherence. The rate of nonadherence found in this study was lower than that reported in studies carried out in Gabon, Ethiopia and Cameroon,38–40 and higher than that reported in Madagascar (2023).41 The current findings also differed from those observed in certain towns in the DRC.42,43 This variability in nonadherence rates could be explained by differences in the populations studied on the one hand and by the method used to assess compliance on the other hand.
Regarding the relationship between adherence to ART by PLHIV and their HRQOL, the present study showed that good adherence to ART increased HRQOL. This may be explained by the fact that adherence to treatment is the most important factor in determining the success of ART and long-term viral suppression.44 In other words, adherence prevents the virus from multiplying, which reduces the risk of mutation and resistance to HIV, thus strengthening the immune system. A previous literature review and studies conducted in South Africa,45 Brazil,46 and Ethiopia7,47,48 revealed that ART adherence improves HRQOL. In addition, a study from Colombia showed that nonadherence to combined ART was associated with lower HRQOL.49
According to our bivariate analysis, being separated and having a low level of education were determinants of nonadherence and therefore of poor therapeutic outcomes. Only a low level of education remained significantly associated with nonadherence to ART according to multivariate analysis via the logistic regression model. Having a low level of education as a predictor of nonadherence was not consistent with the results obtained by other authors in most of the studies that explored this issue.39,40,43,50 In itself, level of education does not encourage people to take their treatment as planned. However, when a patient and doctor agree on the best course of treatment, a shared decision-making process is followed, and the level of education helps patients adhere to their treatment.51
Strengths and Limitations
The strength of this study lies in its evaluation of the HRQOL of PLHIV attending ART clinics versus those attending THs’ offices, which provides a broader perspective on the need for integrated preventive care strategies to improve the health of PLHIV. The multicenter nature of the study provides a diverse picture. Although it included a moderate number of participants, it represents a relatively generalizable population of PLHIV in the city of Bukavu.
The study also has certain limitations related to the lack of assessment of treatment adherence among PLHIV who visit THs, as well as the lack of assessment of the clinical, immunological, and virological staging of the participants. In addition, we did not specify the level of HIV care provided by the THs.
Conclusion
The HRQOL of PLHIV visiting THs is lower than that of PLHIV attending ART clinics. The latter had high scores in all HRQOL domains. Adherence to ART by PLHIV attending ART clinics was high. Adherence to ART improves the HRQOL of PLHIV. People living with HIV are encouraged to go to ART clinics for better care and to improve their HRQOL. The implementation of community HIV awareness, information and education activities, as well as future research into the prevention and management of HIV in this population, could improve the HRQOL of PLHIV.
Abbreviations
PLHIV, People living with HIV; ART, Antiretroviral Therapy; HRQOL, Health-Related Quality of Life; HIV/AIDS, Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome.
Authors’ Information
We would like to report the death of the Professor ZMG, which occurred sometime after the study protocol and data collection tools had been developed.
Data Sharing Statement
There is unrestricted access to all the information. All relevant data are included in the paper and additional files.
Ethics Approval and Consent to Participate
The current study complies with the Declaration of Helsinki. It was authorized by the Ethics Committee of the High Institute of Public Health, Alexandria University, Egypt (IRB N°: 00013692), the Provincial Directorate of the National Health Ethics Committee (CNES/SK: 001-4125/001-113/2019), and the Provincial Health Division of South Kivu (N°003/CD/DPS-SK/2019 and N°002/CD-DPS-SK/2022). All the procedures were followed in accordance with the international Guidelines of Research Ethics.
Acknowledgments
We thank PLHIV for their cooperation in the data collection phase of this study. We would like to express our gratitude to the data clerk and medical professionals for their invaluable assistance with the data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation. They took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. With the exception of the late ZMG, who contributed to the creation of the data collection instruments and the study protocol, may she rest in peace.
Funding
The authors did not receive any specific grant for this research.
Disclosure
The authors declare that they have no conflicting interests. This paper has been uploaded to Research Square as a preprint: https://www.researchsquare.com/article/rs-3943678/v1
References
1. World Health Organization. HIV: global situation and trends. Fact sheet. Geneva: UNAIDS. Available from: https://www.who.int/data/gho/data/themes/hiv-aids.
2. World Health Organization. HIV and AIDS statistics – fact sheet. Geneva: UNAIDS. Available from: https://www.who.int/news-room/fact-sheets/detail/hiv-aids.
3. UNAIDS. Country factsheets: democratic Republic of the Congo. Fact sheet. Geneva. Available from: https://www.unaids.org/en/regionscountries/countries/democraticrepublicofthecongo.
4. Trickey A, Sabin CA, Burkholder G, et al. Life expectancy after 2015 of adults with HIV on long-term antiretroviral therapy in Europe and North America: a collaborative analysis of cohort studies. Lancet HIV. 2023;10(5):e295–e307. doi:10.1016/S2352-3018(23)00028-0
5. Nyongesa MK, Mwangi P, Wanjala SW, et al. Correlates of health-related quality of life among adults receiving combination antiretroviral therapy in coastal Kenya. Health Qual Life Outcomes. 2020;18(1):1–14. doi:10.1186/s12955-020-01421-0
6. Ahmed A, Saqlain M, Bashir N, et al. Health-related quality of life and its predictors among adults living with HIV/AIDS and receiving antiretroviral therapy in Pakistan. Qual Life Res. 2021;30(6):1653–1664. doi:10.1007/s11136-021-02771-y
7. Desta A, Biru TT, Kefale AT. Health related quality of life of people receiving highly active antiretroviral therapy in Southwest Ethiopia. PLoS One. 2020;15(8):e0237013. doi:10.1371/journal.pone.0237013
8. Ahmed M, Mital D, Abubaker NE, et al. Bone health in people living with HIV/AIDS: an update of where we are and potential future strategies. Microorganisms. 2023;11(3):789. doi:10.3390/microorganisms11030789
9. Castro R, De Boni RB, Luz PM, et al. Health-related quality of life assessment among people living with HIV in Rio de Janeiro, Brazil: a cross-sectional study. Qual Life Res. 2019;28:1035–1045. doi:10.1007/s11136-018-2044-8
10. Mokgobi MG. Understanding traditional African healing. Afr J Phys Health Edu Recreat Dance. 2014;20(sup–2):24–34.
11. Hooft A, Nabukalu D, Mwanga-Amumpaire J, Gardiner MA, Sundararajan R. Factors motivating traditional healer versus biomedical facility use for the treatment of pediatric febrile illness: results from a qualitative study in Southwestern Uganda. Am J Trop Med Hyg. 2020;103(1):501–507. doi:10.4269/ajtmh.19-0897
12. Adejumo OA, Malee KM, Ryscavage P, Hunter SJ, Taiwo BO. Contemporary issues on the epidemiology and antiretroviral adherence of HIV-infected adolescents in sub Saharan Africa: a narrative review. J Int AIDS Soc. 2015;18(1):20–49. doi:10.7448/IAS.18.1.20049
13. Gail H, Tarryn B, Oluwaseyi A, Denver D, Oluchi MDG. An ethnobotanical survey of medicinal plants used by traditional health practitioners to manage HIV and its related opportunistic infections in Mpoza, Eastern Cape Province, South Africa. J Ethnopharmacol. 2015;171:109–115. doi:10.1016/j.jep.2015.05.029
14. Ministère de la Santé Publique. Arrêté n°1250/CAB/MIN/S/CJ/KIZ/62/2002 du 25/10/2002 portant organisation de l’exercice de la médecine traditionnelle. MSP. 2002;8. French.
15. Seyed Nezhad M, Ahmadi B, Akbari Sari A. Factors affecting the successful implementation of the referral system: a scoping review. J Family Med Prim Care. 2021;10(12):4364–4375. doi:10.4103/jfmpc.jfmpc_514_21
16. Cooper V, Clatworthy J, Harding R, Whetham J. Measuring quality of life among people living with HIV: a systematic review of reviews. Health Qual Life Outcomes. 2017;15(1):1–20. doi:10.1186/s12955-017-0778-6
17. Whoqol Group T, Group TW. The World Health Organization quality of life assessment (WHOQOL): development and general psychometric properties. Soc Sci Med. 1998;46(12):1569–1585. doi:10.1016/S0277-9536(98)00009-4
18. Emuren L, Welles S, Macalino G, et al. Predictors of health-related quality of life among military HIV-infected individuals. Qual Life Res. 2020;29(7):1855–1869. doi:10.1007/s11136-020-02441-5
19. Kall M, Marcellin F, Harding R, Lazarus JV, Carrieri P. Patient-reported outcomes to enhance person-centered HIV care. Lancet HIV. 2020;7(1):e59–e68. doi:10.1016/S2352-3018(19)30345-5
20. Australian Bureau of Statistics. Sample Size Calculator Help. Available from: https://www.abs.gov.au/websitedbs/D3310114.nsf/Home/Sample+Size+Calculator+Help?.
21. Naing L, Nordin RB, Abdul Rahman H, Naing YT. Sample size calculation for prevalence studies using Scalex and ScalaR calculators. BMC Med Res Methodol. 2022;22(209):1–8. doi:10.1186/s12874-022-01694
22. World Health Organization. WHOQOL: measuring quality of life. Available from: https://www.who.int/tools/whoQoL.
23. Godin G, Gagné C, Naccache H. Validation of a self-report questionnaire assessing adherence to antiretroviral medication. AIDS Patient Care STDS. 2004;17(7):325–332. doi:10.1089/108729103322231268
24. Berrezouga L, Kooli I, Marrakchi W, Harzallah G, Chakroun M. Quality of life of people living with HIV on antiretroviral therapy: a cross-sectional study in Monastir, Tunisia. HIV/AIDS Res Palliat Care. 2023;15:671–682. doi:10.2147/HIV.S430376
25. Mohammed SA, Yitafr MG, Workneh BD, Hailu AD. Health-related quality of life and associated factors among people living with human immunodeficiency virus on highly active antiretroviral therapy in North East Ethiopia: cross-sectional study. PLoS One. 2021;16(3):e0247777. doi:10.1371/journal.pone.0247777
26. Molla AA, Gelagay AA, Mekonnen HS, Teshome DF. Adherence to antiretroviral therapy and associated factors among HIV positive adults attending care and treatment in University Of Gondar Referral Hospital, Northwest Ethiopia. BMC Infect Dis. 2018;18(1):1–8. doi:10.1186/s12879-018-3176-8
27. Abadiga M, Hasen T, Mosisa G, Abdisa E. Adherence to antiretroviral therapy and associated factors among human immunodeficiency virus positive patients accessing treatment at Nekemte referral hospital, west Ethiopia, 2019. PLoS One. 2020;15(5):e0232703. doi:10.1371/journal.pone.0232703
28. Zhang S, Rust G, Cardarelli K, Felizzola J, Fransua M, Stringer HG Jr. Adherence to highly active antiretroviral therapy impact on clinical and economic outcomes for medicaid enrollees with HIV and hepatitis C coinfection. AIDS Care. 2016;27(7):829–835. doi:10.1080/09540121.2015.1021745
29. Venturini A, Cenderello G, Di Biagio A, et al. Quality of life in an Italian cohort of people living with HIV in the era of combined antiretroviral therapy (Evidence from IANUA study-investigation on antiretroviral therapy). AIDS Care. 2017;29(11):1373–1377. doi:10.1080/09540121.2017.1286286
30. Nobre N, Pereira M, Roine RP, Sutinen J, Sintonen H. HIV-related self-stigma and health-related quality of life of people living with HIV in Finland. J Assoc Nurses AIDS Care. 2018;29(2):254–265. doi:10.1016/j.jana.2017.08.006
31. Rzeszutek M, Gruszczyńska E, Firląg-Burkacka E. Coping profiles and subjective well-being among people living with HIV: less intensive coping corresponds with better well-being. Qual Life Res. 2017;26(10):2805–2814. doi:10.1007/s11136-017-1612-7
32. Emuren L, Welles S, Evans AA, et al. Health-related quality of life among military HIV patients on antiretroviral therapy. PLoS One. 2017;12(6):e0178953. doi:10.1371/journal.pone.0178953
33. Ortego C, Huedo-Medina TB, Vejo J, Llorca FJ. Adherence to highly active antiretroviral therapy in Spain. A meta-analysis. Gac Sanit. 2011;25(4):282–289. doi:10.1016/j.gaceta.2010.10.016
34. Nachega JB, Uthman OA, Peltzer K, et al. Association between antiretroviral therapy adherence and employment status: systematic review and meta-analysis. Bull World Health Organ. 2015;93(1):29–41. doi:10.2471/BLT.14.138149
35. Sangeda RZ, Mosha F, Aboud S, et al. Predictors of nonadherence to antiretroviral therapy at an urban HIV care and treatment center in Tanzania. Drug Healthc Patient Saf. 2018;10:79–88. doi:10.2147/DHPS.S143178
36. Soares RDCA, Brito AMD, Lima K, Lapa TM. Adherence to antiretroviral therapy among people living with HIV/AIDS in northeastern Brazil: a cross-sectional study. Sao Paulo Med J. 2020;137(6):479–485. doi:10.1590/1516-3180.2019.0212170919
37. Ontario HIV Treatment Network. Impact of nonadherence to antiretroviral therapy (ART) on population-level health outcomes. Rapid response service. Available from: https://www.ohtn.on.ca/wp-content/uploads/2021/07/RR158_nonadherence.pdf.
38. Collinet O Facteurs associés à une mauvaise observance thérapeutique chez les patients vivant avec le VIH à l’hôpital Albert Schweitzer au Gabon en 2019. Sciences du Vivant [q-bio]. 2019; ffdumas–02401977. Available from: https://dumas.ccsd.cnrs.fr/dumas-02401977.
39. Nigusso FT, Mavhandu-Mudzusi AH. Magnitude of nonadherence to antiretroviral therapy and associated factors among adult people living with HIV/AIDS in Benishangul-Gumuz Regional State, Ethiopia. PeerJ. 2020;8:e8558. doi:10.7717/peerj.8558
40. Lantche MW, Fokam J, Cheudjui AJN, et al. Factors associated with nonadherence to antiretroviral therapy among HIV-infected adolescents aged 15-19 years: a snapshot from the Mother and Child Center in Yaounde, Cameroon. Pan Afr Med J. 2021;39(154):1–13.
41. Andriamamonjisoa J, Andriananja V, Andrianarivony R, et al. Facteurs associés à la nonobservance thérapeutique des personnes vivant avec le VIH sous antirétroviraux. Med Mal Infect. 2023;2(2):Suppl148–9. French. doi:10.1016/j.mmifmc.2023.03.343
42. Izizag BB, Situakibanza H, Kiazayawoko F, et al. Déterminants de la non-observance au traitement antirétroviral chez l’adulte à Kinshasa. Pan Afr Med J. 2020;37(157):1–15. French.
43. Mandro CN, Tibamwenda YB, Mosomo TK, Wolyec ST, Kibendelwa ZT, Wembonyama SO. Déterminants de la non observance du traitement antirétroviral chez les personnes vivant avec le VIH dans les villes de Bunia et Goma: une étude transversale analytique. J Med Public Health Policy. 2023;3(1):34–42. French.
44. Legesse TA, Reta MA. Adherence to antiretroviral therapy and associated factors among people living with HIV/AIDS in Hara town and its surroundings, North-Eastern Ethiopia: a cross-sectional study. Ethiop J Health Sci. 2019;29(3):299–308. doi:10.4314/ejhs.v29i3.2
45. Rajesh VV, Johanna CM, Brian G, Gous AGS. Relationship between adherence and health-related quality of life among HIV-patients in South Africa: findings and implications. J AIDS HIV Res. 2018;10(8):121–132. doi:10.5897/JAHR2018.0478
46. Cunha GHD, Ramalho AKL, Fontenele MSM, Dantas MB, Fechine FV, Abreu WCD. Quality of life and adherence to antiretroviral therapy in people living with HIV in the Ceará, Brazil. AIDS Care. 2023;1–14. doi:10.1080/09540121.2023.2275035
47. Surur AS, Teni FS, Wale W, Ayalew Y, Tesfaye B. Health related quality of life of HIV/AIDS patients on highly active anti-retroviral therapy at a university referral hospital in Ethiopia. BMC Health Serv Res. 2017;17(737):1–8. doi:10.1186/s12913-017-2714-1
48. Girma D, Dejene H, Geleta LA, et al. Health related quality of life of HIV-positive women on ART follow-up in north Shewa zone public hospitals, central Ethiopia: evidence from a cross-sectional study. Heliyon. 2023;9(2):e13318. doi:10.1016/j.heliyon.2023.e13318
49. Narváez M, Lins-Kusterer L, Valdelamar-Jiménez J, Brites C. Quality of life and antiretroviral therapy adherence: a cross-sectional study in Colombia. AIDS Res Hum Retroviruses. 2022;38(8):660–669. doi:10.1089/aid.2021.0233
50. Usman SA, Shehu A, Ajumobi O, et al. Predictors of nonadherence to antiretroviral therapy among HIV patients in secondary health care facilities in Kano State-Nigeria: a case‒control study. Pan Afr Med J. 2019;32:3):1–4. doi:10.11604/pamj.supp.2019.32.1.13746
51. Driever EM, Brand PL. Education makes people take their medication: myth or maxim? Breathe. 2020;16(1):1–8. doi:10.1183/20734735.0338-2019
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

