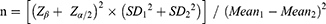Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Assessment of the Impact of Dexmedetomidine on Myocardial Injury in TAVI Patients: A Retrospective Cohort Study Utilizing PSM-DID
Authors Song Y, Zhang J, Xu H, Gui C, Cheng H, Chen Y, Wang S
Received 21 November 2024
Accepted for publication 15 April 2025
Published 1 May 2025 Volume 2025:21 Pages 583—592
DOI https://doi.org/10.2147/TCRM.S507439
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Yang Song,1,* Jin Zhang,2,* Huiping Xu,2 Chaoqun Gui,2 Hao Cheng,1 Yongquan Chen,1 Shaolin Wang2
1Department of Anaesthesiology, The First Affiliated Hospital of Wannan Medical College, Wuhu, 241001, People’s Republic of China; 2Department of Anaesthesiology, The Second People’s Hospital of Wuhu, Wuhu, 241001, People’s Republic of China
*These authors contributed equally to this study
Correspondence: Yongquan Chen, Department of Anaesthesiology, The First Affiliated Hospital of Wannan Medical College, No. 2 Zheshan West Road, Wuhu City, Anhui Province, 241001, People’s Republic of China, Email [email protected] Shaolin Wang, Department of Anaesthesiology, The Second People’s Hospital of Wuhu, No. 6 Duchun Road, Wuhu City, Anhui Province, 241000, People’s Republic of China, Email [email protected]
Background: Transcatheter Aortic Valve Implantation (TAVI) is a minimally invasive procedure for treating severe aortic valve diseases but can lead to perioperative myocardial damage (PMD). Dexmedetomidine (DEX), an α 2-adrenergic receptor agonist, has shown potential to reduce myocardial injury in other cardiac procedures. This effect is attributed to its anti-inflammatory properties, which help reduce the inflammatory response associated with myocardial damage, and its antioxidant properties, which combat oxidative stress that contributes to cell injury. But its effectiveness during TAVI remains unclear.
Objective: To assess the impact of DEX on myocardial injury in patients undergoing TAVI under general anesthesia.
Methods: A retrospective cohort study of 159 patients (after exclusions) who underwent TAVI from January 2022 to August 2024. Patients were divided into DEX and control groups. Primary outcomes were peak levels of cardiac troponin I and CK-MB within 48 hours postoperatively. Secondary outcomes included IL-6, PCT, and NT-proBNP levels. Propensity score matching (PSM) and Differences-in-Differences (DID) method were used for analysis.
Results: After PSM, the DEX group exhibited significantly lower peak values of troponin I (P < 0.001) and CK-MB (P < 0.001) compared to the control group, indicating reduced myocardial injury. No significant differences were observed in IL-6, PCT, and NT-proBNP levels between the groups. The DID analysis suggested a negative correlation between DEX use and major adverse postoperative events, highlighting DEX as a potential protective factor.
Conclusion: Dexmedetomidine administration during TAVI was associated with reduced levels of myocardial injury markers, indicating a potential cardioprotective role. By reducing myocardial injury, DEX may contribute to improved perioperative outcomes, including a decreased risk of major adverse postoperative events. These results highlight the potential clinical utility of DEX in the perioperative management of TAVI patients, suggesting that its inclusion in anesthetic protocols could enhance patient care and recovery.
Keywords: dexmedetomidine, transcatheter aortic valve replacement, myocardial ischemia, inflammation, propensity score
Introduction
Transcatheter Aortic Valve Implantation (TAVI) is a minimally invasive procedure used to treat severe aortic valve diseases.1,2 Despite its advantages, TAVI can lead to perioperative myocardial injury (PMI), which is associated with adverse outcomes.3 Myocardial injury in TAVI can be attributed to several factors, including hemodynamic instability, the use of contrast media, and mechanical stress imposed by the procedure. Hemodynamic fluctuations during TAVI, such as rapid ventricular pacing and balloon valvuloplasty, can cause myocardial ischemia and reperfusion injury.4 The use of contrast media for procedural guidance may contribute to renal dysfunction, which is a known risk factor for myocardial injury.5 Additionally, the mechanical stress from valve deployment and the potential for coronary emboli can lead to myocardial necrosis, particularly in the apical region when using the transapical approach.6
Dexmedetomidine (DEX), an α2-adrenergic receptor agonist, has shown promising results in myocardial protection during cardiopulmonary bypass valve replacement surgeries. A study by Altınkaya et al investigated the preventive effect of DEX on postoperative delirium and atrial fibrillation after cardiac surgery, highlighting its potential benefits.7 Another study by Yuan et al found that DEX can provide safe and effective adjuvant analgesia for patients undergoing cardiac surgery without adverse hemodynamic effects.8 These findings suggest that DEX may have a protective effect in cardiac surgery. Research has demonstrated that DEX reduces myocardial ischemia-reperfusion injury through various mechanisms, including its anti-inflammatory, antioxidant, and anti-apoptotic effects, as well as its ability to regulate energy metabolism.9–11 For instance, studies have reported that DEX significantly reduces peak levels of cardiac troponin I and CK-MB in patients undergoing cardiac surgery, indicating a reduction in myocardial injury.10,11 However, its effectiveness in reducing postoperative myocardial injury specifically in TAVI patients—who do not undergo cardiopulmonary bypass—remains uncertain.
The limitations of prior research include a lack of detailed investigation into the pathophysiological mechanisms of myocardial injury in TAVI and the effectiveness of DEX in this context. Therefore, this study aimed to investigate whether DEX administration during TAVI can reduce the risk of postoperative myocardial injury in patients undergoing general anesthesia. By addressing this gap, we aim to provide insights into the potential cardioprotective role of DEX in the context of TAVI, contributing to improved perioperative management and patient outcomes.
Methods
Study Design
This retrospective cohort study was conducted at the First Affiliated Hospital of Wannan Medical College. Patients who underwent TAVI from January 2022 to August 2024 were included. The study was approved by the hospital’s Ethics Committee, and informed consent was obtained from all participants. To account for potential differences in myocardial injury due to varying surgical approaches, patients undergoing non-femoral artery procedures were excluded from this study. Data collection was performed with rigorous quality control measures. We defined strict inclusion and exclusion criteria, such as excluding patients who died within 24 hours postoperatively, underwent a change in surgical procedure, required ECMO, or were lost to follow-up. Patient demographic information, perioperative blood test results, and anesthetic management details were extracted from the electronic medical records at our medical center. Patients were categorized into two groups based on whether they received dexmedetomidine during their TAVI procedure (DEX group) or not (control group). This grouping was based on historical medication records as documented in the electronic medical system. For the propensity score matching (PSM) process, we used a matching ratio of 1:2, resulting in 36 patients in the DEX group and 66 patients in the control group for final analysis.
Sample Size Calculation and Power Analysis
Given the retrospective nature of this study, the sample size was determined by the number of eligible patients who underwent TAVI during the specified study period. A total of 175 patients were initially identified, with 156 meeting the final inclusion criteria after applying the exclusion criteria (Figure 1).
To evaluate the adequacy of our sample size, a post-hoc power analysis was conducted using the mean difference in peak troponin I levels between the DEX and control groups, considering the variability observed in our study population. Calculations were performed using the following formula for comparing two independent means:
Z β corresponds to the desired power (0.84 corresponds to 80% power),
Z α/2 corresponds to the significance level (1.96 for α=0.05),
SD represents the standard deviations of the two groups,
Mean represents the mean difference in outcome measures between the two groups.
The calculated n is equal to about 42. Given our 36 patients in Group D and 66 in Group S post-PSM, the analysis indicates that our sample size was just above the required threshold to detect the difference in peak troponin I levels with 80% power.
Anesthetic Management
Anesthetic management for all patients was performed by attending or associate chief physicians with over five years of experience in cardiovascular anesthesia. General anesthesia was induced with tracheal intubation, and analgesia in both groups was initiated with sufentanil and maintained intraoperatively with remifentanil. The specific DEX dosing regimen for the experimental group (Group D) included a loading dose of 1 µg/kg administered over 10 minutes, followed by a continuous infusion of 0.2 µg/kg/h, while the control group (Group S) received only propofol with an aim to maintain BIS values between 40 and 60 in both groups. Postoperatively, patients were transferred to the ICU, where they were managed by intensivists who had received specialized training.
Outcomes
The primary outcome of this study was to assess differences in the degree of acute myocardial injury between the two groups postoperatively. We used the peak values of troponin I and CK-MB measured within 48 hours postoperatively as the primary indicators for assessing PMD. Secondary outcomes included postoperative inflammation and cardiac function, assessed using IL-6, PCT, and Pro-BNP levels. Additionally, confounding factors such as age, preoperative diagnosis, and surgical duration were adjusted for to evaluate the correlation between DEX and PMI. Preoperative blood test results closest to the day of surgery were selected for inclusion in the study. Routine measurements of troponin I, CK-MB, IL-6, PCT, and Pro-BNP were performed postoperatively and on the morning of the first postoperative day, with testing extended to the second postoperative day if necessary. The peak values of these postoperative indicators were recorded for statistical analysis. Although the study was retrospective and not blinded, efforts were made to minimize bias. The primary outcomes (troponin I and CK-MB levels) were measured by laboratory technicians who were blinded to the group assignments. Data analysis was performed by researchers who were also blinded to the group allocations.
Statistical Analysis
Data was analyzed using SPSS 26.0 statistical software. A 1:2 propensity score matching (PSM) was used to balance the differences in baseline data. PSM is a statistical technique used in observational studies to reduce selection bias by creating comparable groups based on observed characteristics. PSM in our retrospective study helps simulate balanced groups by matching patients with similar baseline characteristics, thereby reducing the influence of confounding factors. Matching variables included age, sex, type of surgery, preoperative diagnosis, surgical duration, anesthesia duration, ASA classification, NYHA classification, and preoperative levels of troponin I, CK-MB, and BNP. Standardized mean differences (SMD) <0.1 indicated good balance between groups. Quantitative data conforming to a normal distribution were expressed as mean ± standard deviation (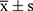 ), and comparisons between groups were made using independent sample t-tests. For data not conforming to a normal distribution, they were expressed as median (interquartile range) [M(Q1, Q3)], and comparisons between groups were made using Mann–Whitney U-tests. Categorical data were expressed as number (rate) and compared using χ2 tests. Any outliers or anomalous data points were identified using boxplot analysis and were addressed by exclusion from the statistical analysis if they significantly deviated from the expected range. The Differences-in-Differences (DID) method was used to explore the influencing factors of postoperative related indicators, which further adjusted for potential confounders by incorporating control variables such as gender, age, type of surgery, duration of surgery, duration of anesthesia, preoperative CK-MB, preoperative diagnosis, ASA classification, NYHA classification, and preoperative BNP. P<0.05 indicating statistically significant differences.
), and comparisons between groups were made using independent sample t-tests. For data not conforming to a normal distribution, they were expressed as median (interquartile range) [M(Q1, Q3)], and comparisons between groups were made using Mann–Whitney U-tests. Categorical data were expressed as number (rate) and compared using χ2 tests. Any outliers or anomalous data points were identified using boxplot analysis and were addressed by exclusion from the statistical analysis if they significantly deviated from the expected range. The Differences-in-Differences (DID) method was used to explore the influencing factors of postoperative related indicators, which further adjusted for potential confounders by incorporating control variables such as gender, age, type of surgery, duration of surgery, duration of anesthesia, preoperative CK-MB, preoperative diagnosis, ASA classification, NYHA classification, and preoperative BNP. P<0.05 indicating statistically significant differences.
Results
A total of 175 patients who underwent TAVI were initially included in the study. After excluding 19 patients who either died within 24 hours postoperatively (n=10), changed surgical methods (n=2), required postoperative ECMO treatment (n=2), or were lost to follow-up (n=5), a final cohort of 156 patients was analyzed (Figure 1).
The study included 156 patients with a median age of 73 years (IQR 67–79), comprising 72 females (46.15%) and 84 males (53.85%) (Table 1). Of these, 47.44% had aortic regurgitation (AR), 36.54% had aortic stenosis (AS), and 16.03% had combined aortic stenosis and regurgitation (AS-R). The duration of surgery (median 160 minutes; IQR 129.25–185.00) and anesthesia (median 205.50 minutes; IQR 185.00–250.00) were similar between groups (p = 0.706 and p = 0.500, respectively). Preoperative troponin I levels were significantly higher in Group D (0.02; IQR 0.01–0.07) compared to Group S (0.02; IQR 0.01–0.04; p = 0.026), while preoperative CK-MB levels were comparable (Group S: 12.00; IQR 9.00–15.00; Group D: 11.85; IQR 10.00–13.50; p = 0.980). Emergency operations were more frequent in Group D (23.08%) compared to Group S (6.84%; p = 0.012). Preoperative BNP levels >500 pg/mL were also higher in Group D (56.41%) versus Group S (39.32%), though this difference was not statistically significant (p = 0.062). Other factors, including gender distribution, diagnosis type, ASA, and NYHA classifications, did not show significant differences between the groups.
 |
Table 1 Demographic Data and Anesthesia Related Data |
Following 1:2 propensity score matching (PSM), Group D consisted of 36 patients and Group S of 66 patients, totaling 102 patients. Before PSM, differences in preoperative troponin I levels (p = 0.026) and surgical types (p = 0.012) were significant between the groups. After PSM, univariate analyses revealed no statistically significant differences in age, surgical duration, anesthesia duration, preoperative troponin I, and CK-MB levels (all p > 0.05). Specifically, the median age in Group S was 71 years (IQR 66–81) compared to 74 years (IQR 69–79) in Group D (p = 0.501). Surgical duration was 150 minutes (IQR 120.5–170.75) in Group S and 157.5 minutes (IQR 131.5–195.0) in Group D (p = 0.326). Anesthesia duration was 200 minutes (IQR 180.0–235.0) for Group S and 205 minutes (IQR 188.75–266.25) for Group D (p = 0.517). Preoperative troponin I levels were 0.02 ng/mL (IQR 0.01–0.03) in Group S and 0.02 ng/mL (IQR 0.01–0.06) in Group D (p = 0.077). Preoperative CK-MB levels were 11.15 ng/mL (IQR 9.00–14.75) in Group S and 11.43 ng/mL (IQR 10.00–14.00) in Group D (p = 0.969). Standardized mean differences (SMDs) for age, surgical type, and preoperative CK-MB were between 0.1 and 0.2, with other baseline variables showing SMDs less than 0.1, indicating a well-balanced comparison between the groups (Table 2).
 |
Table 2 Demographic Data and Anaesthesia Related Data after PSM |
Postoperative data from matched patients revealed that Group D had significantly lower peak values for both troponin I and CK-MB compared to Group S. Specifically, the median peak troponin I was 0.53 ng/mL (IQR 0.34–0.89) in Group D versus 1.10 ng/mL (IQR 0.51–2.44) in Group S (p < 0.001), and median peak CK-MB was 19.00 U/L (IQR 15.25–25.75) in Group D versus 27.50 U/L (IQR 19.75–38.00) in Group S (p < 0.001). Conversely, there were no significant differences between the groups for IL-6, with levels of 70.42 pg/mL (IQR 19.97–118.83) in Group D versus 70.26 pg/mL (IQR 32.71–158.15) in Group S (p = 0.46), and for PCT, with levels of 0.38 ng/mL (IQR 0.12–0.94) in Group D versus 0.34 ng/mL (IQR 0.09–0.98) in Group S (p = 0.845). The proportion of patients with NT-proBNP levels >1800 pg/mL was not significantly different between the groups, with 30 (45.45%) in Group S and 22 (61.11%) in Group D (p = 0.131) (Table 3).
 |
Table 3 Postoperative Indexes Before and After PSM Matching |
In the regression analysis using the DID model, DEX use was assessed for its impact on postoperative biomarkers, troponin I and CK-MB. The analysis included control variables such as gender, age, type of surgery, duration of surgery, duration of anesthesia, preoperative CK-MB, preoperative diagnosis, ASA classification, NYHA classification, and preoperative BNP. The results demonstrated that DEX use was significantly associated with reduced levels of troponin I and CK-MB. Specifically, the coefficient for DEX use on troponin I was −0.396 (β = −0.396, SE = 3.982, t = −0.099, 95% CI: −8.251 to 7.459), and for CK-MB, it was −2.493 (β = −2.493, SE = 0.966, t = −2.58, 95% CI: −4.399 to −0.587). Both associations were statistically significant (p < 0.001 for both troponin I and CK-MB), indicating that DEX use was a significant negative predictor of PMD following TAVI, supporting its role as a favorable factor in mitigating PMD incidence (Table 4).
 |
Table 4 OLS Regression of Postoperative CK-MB and Troponin I Based on the DID Model |
Discussion
The findings of this study demonstrate a statistically significant reduction in myocardial injury markers, such as troponin I and CK-MB, in Transcatheter Aortic Valve Implantation (TAVI) patients administered Dexmedetomidine (DEX). This reduction in myocardial injury is consistent with previous studies, such as those reported by Forrest et al12 and O’Hair et al13 which highlighted reductions in myocardial injury markers like CK-MB and troponin I. This study extends these observations to the context of TAVI, a minimally invasive alternative for high-risk patients traditionally considered unsuitable for open-heart surgery. By showing that DEX significantly lowers postoperative CK-MB and troponin I levels in TAVI patients, our results suggest its potential benefits in reducing myocardial injury and improving outcomes for this specific patient group.
The cardioprotective effects of DEX are attributed to its anti-inflammatory and antioxidant properties.14 DEX reduces the inflammatory response associated with myocardial damage by inhibiting the release of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α).15 Additionally, DEX combats oxidative stress by scavenging free radicals and reducing lipid peroxidation, which helps protect myocardial cells from injury.16 These mechanisms help mitigate myocardial ischemia-reperfusion injury, which is a significant concern in TAVI procedures. The reduction in myocardial injury markers observed in this study supports the hypothesis that DEX exerts its protective effects through these mechanisms. Numerous studies have demonstrated that DEX provides effective myocardial protection in both cardiopulmonary bypass surgeries and non-cardiac surgeries in patients with cardiac conditions. Elgebaly17 et al reported that the use of DEX in cardiopulmonary bypass surgery significantly reduced troponin and CK-MB levels, as well as decreased short-term mortality. As an α2-adrenoceptor agonist, DEX produces varying clinical effects depending on the dosage18 Low doses (0.2–1 mcg/kg) reduce heart rate and peripheral resistance. In our center, DEX was administered at 0.5–1 mcg/kg/h, based on the anesthesiologist’s comprehensive evaluation of each patient. DEX has a favorable safety profile in most patients, but potential side effects, such as hypotension and bradycardia, should be considered, especially in high-risk TAVI patients. Higher doses (1–4 mcg/kg) can elevate blood pressure and reflexively slow heart rate, effects that are not favorable for patients undergoing TAVI surgery.19 In our study, DEX was administered at a dosage of 0.5–1 mcg/kg/h, which is within the recommended range and has been shown to be safe in previous studies. However, careful monitoring of hemodynamic parameters is essential to manage any potential side effects. For instance, in patients with pre-existing hypotension or bradycardia, the use of DEX should be approached with caution, and alternative anesthetic strategies may need to be considered. Additionally, the potential for drug interactions, particularly with other sedatives or analgesics, should be carefully evaluated to avoid adverse effects.
There are differing perspectives on how DEX exerts its myocardial protective effects during surgery. Some preclinical studies suggest that DEX may provide myocardial protection through anti-inflammatory mechanisms, antioxidative stress, and improved microcirculation.20,21 In our study, we included postoperative IL-6 levels in the statistical analysis, but the results showed no significant difference between the two groups. This contrasts with the findings of Ji et al20 likely because TAVI procedures are shorter than SAVR surgeries, leading to less severe cardiac ischemia-reperfusion injury. Furthermore, the overall trauma from TAVI is minimal and does not trigger a significant systemic inflammatory response. Additionally, as this is a retrospective cohort study and IL-6 is not routinely measured preoperatively, we were unable to assess dynamic changes in IL-6 levels. PCT, another acute-phase protein included in the study, reflects the severity of inflammation. Our results indicated no significant difference between Group D and Group S in PCT levels. Compared to IL-6, PCT is more commonly used for the early diagnosis of severe bacterial infections. Myocardial cell injury alone may not cause an increase in PCT, as elevated PCT is more often associated with myocardial injury complicated by infection or systemic organ failure.22,23
CK-MB and troponin I are well-established biomarkers for assessing PMD, each with high sensitivity and specificity.24 In clinical practice, both markers are frequently measured together to enhance diagnostic accuracy and provide a comprehensive assessment of myocardial injury. The reduction in myocardial injury markers observed in this study not only provides evidence for the cardioprotective effects of DEX but also suggests the potential for improved clinical outcomes in TAVI patients. Previous research has shown that elevated levels of cardiac troponin I and CK-MB are associated with adverse outcomes, including increased complication rates and prolonged recovery times.25,26 By significantly lowering these markers, DEX may contribute to a decreased risk of major adverse postoperative events, such as myocardial infarction, heart failure, and arrhythmias. This could lead to a reduction in the overall complication rate and improved patient safety. The reduction in myocardial injury markers observed in this study is clinically relevant, as elevated levels of these biomarkers are associated with adverse outcomes and increased mortality in cardiac conditions. Although the study’s follow-up period was limited to 48 hours postoperatively, the reduction in myocardial injury markers suggests that DEX may contribute to improved short-term outcomes. Future research should extend the follow-up period to assess the long-term prognostic implications of DEX administration on cardiac outcomes and overall survival.
Moreover, the protective effects of DEX on myocardial tissue may translate into shorter recovery times and faster postoperative rehabilitation. Patients with reduced myocardial injury are likely to experience less postoperative discomfort and have a quicker return to normal activities.27 This is particularly important in the context of TAVI, where patients are often elderly and have comorbidities that can complicate recovery. By mitigating myocardial damage, DEX may help optimize the perioperative management of these patients, leading to better overall outcomes.
In evaluating the efficacy of DEX in reducing postoperative myocardial injury, it is crucial to consider potential confounding factors that may influence our results. One significant aspect is the variability in patients’ underlying conditions, such as comorbidities like diabetes, hypertension, or pre-existing heart disease, which can affect myocardial susceptibility to injury and overall recovery. Additionally, differences in surgical techniques or procedural approaches, such as variations in valve implantation methods or differences in anesthetic management practices, might contribute to the observed outcomes. For instance, while our study controlled for standard procedural variables, the potential influence of less controlled factors, such as operator experience and procedural complexity, cannot be fully excluded. Moreover, patient management in the intensive care unit postoperatively, including differences in fluid management and use of adjunctive therapies, could also impact myocardial recovery and complicate the interpretation of DEX’s effects. To address these issues, future research should aim to control for a broader range of confounders, ideally through well-designed randomized controlled trials or prospective cohort studies that can account for and adjust these variables more comprehensively.
Limitations
Although this study provides compelling evidence for the cardioprotective effects of DEX in AVI, several issues require further validation. Firstly, due to the retrospective nature of our study and the limited study period, it was not feasible to include long-term follow-up. This short follow-up period may limit the ability to observe long-term outcomes, which are crucial for evaluating the sustained effects of Dexmedetomidine (DEX) on myocardial recovery and overall patient prognosis. Future research should extend the follow-up period to assess the long-term prognostic implications of DEX administration on cardiac outcomes and overall survival. Additionally, the study’s inclusion criteria were restricted to patients who underwent uneventful surgical procedures, exhibited stable vital signs intraoperatively, and did not require ECMO support postoperatively. The efficacy of DEX in patients who altered their surgical approach during the procedure or underwent TAVI via alternative routes remains to be determined. Furthermore, the retrospective design of this study limits the evidence available to elucidate the mechanisms by which DEX prevents myocardial injury, such as the absence of preoperative IL-6 and PCT levels. Additionally, despite the use of PSM, there is a risk of residual confounding. While PSM helps to balance the baseline characteristics between groups, it cannot account for all potential confounding factors. Unmeasured variables or biases in data collection could influence the results. For example, factors such as patient comorbidities, surgical techniques, and anesthetic management practices might affect the observed outcomes. The study had sufficient statistical power to detect a significant difference in the primary outcomes, as confirmed by a post-hoc power analysis. However, the generalizability of the findings may be limited by the retrospective nature of the study and the specific patient population. Future research should employ randomized controlled trials or prospective cohort studies to further validate the efficacy of DEX in a broader patient population.
Conclusion
In the context of TAVI, a continuous infusion of dexmedetomidine at a dosage of 0.5–1 mcg/kg/h during surgery, relative to a placebo, was associated with significantly reduced postoperative levels of CK-MB and troponin I, suggesting a reduction in myocardial injury. However, no correlation was observed between dexmedetomidine administration and changes in PCT or IL-6 levels during TAVI.
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the First Affiliated Hospital of Wannan Medical College, and informed consent was obtained from all participants.
Disclosure
All of the authors had no any personal, financial, commercial, or academic conflicts of interest separately.
References
1. Cribier A. Invention and uptake of TAVI over the first 20 years. Nat Rev Cardiol. 2022;19(7):427–428. doi:10.1038/s41569-022-00721-w
2. Mazhar K, Warwick R, Balacumaraswami L. Transcatheter or surgical treatment of aortic-valve stenosis. N Engl J Med. 2024;391(6):574.
3. Devereaux PJ, Lamy A, Chan MTV, et al. High-sensitivity troponin i after cardiac surgery and 30-day mortality. N Engl J Med. 2022;386(9):827–836. doi:10.1056/NEJMoa2000803
4. Stolte T, Lopez-Ayala P, Reichl J, et al. The impact of myocardial injury on outcomes in TAVI patients. Clin Res Cardiol. 2025;114(3):385–394. doi:10.1007/s00392-024-02585-1
5. de Sá Marchi MF, Calomeni P, Gauza MM, et al. Impact of periprocedural myocardial injury after transcatheter aortic valve implantation on long-term mortality: a meta-analysis of Kaplan-Meier derived individual patient data. Front Cardiovasc Med. 2023;10:1228305. doi:10.3389/fcvm.2023.1228305
6. Nara Y, Watanabe Y, Kataoka A, et al. Incidence, predictors, and midterm clinical outcomes of myocardial injury after transcatheter aortic-valve implantation. Int Heart J. 2018;59(6):1296–1302. doi:10.1536/ihj.17-645
7. Altınkaya ÇM, Gökbulut Bektaş S, Turan S. Comparison of clinical safety and efficacy of dexmedetomidine, remifentanil, and propofol in patients who cannot tolerate non-invasive mechanical ventilation: a prospective, randomized, cohort study. Front Med Lausanne. 2022;9:995799. doi:10.3389/fmed.2022.995799
8. Yuan B, Huang X, Wen J, Peng M. Dexmedetomidine pretreatment confers myocardial protection and reduces mechanical ventilation duration for patients undergoing cardiac valve replacement under cardiopulmonary bypass. Ann Thorac Cardiovasc Surg. 2024;30(1):23–00210. doi:10.5761/atcs.oa.23-00210
9. Takahashi K, Yoshikawa Y, Kanda M, et al. Dexmedetomidine as a cardioprotective drug: a narrative review. J Anesth. 2023;37(6):961–970. doi:10.1007/s00540-023-03261-w
10. Wang C, Yuan W, Hu A. et al. Dexmedetomidine alleviated sepsis‑induced myocardial ferroptosis and septic heart injury. Mol Med Rep. 2020;22(1):175–184. doi:10.3892/mmr.2020.11114
11. Sun M, Wang R, Xia RX, et al. Amelioration of myocardial ischemia/reperfusion injury in diabetes: a narrative review of the mechanisms and clinical applications of dexmedetomidine. Front Pharmacol. 2022;13:949754. doi:10.3389/fphar.2022.949754
12. Forrest JK, Deeb GM, Yakubov SJ, et al. Low risk trial investigators. 3-year outcomes after transcatheter or surgical aortic valve replacement in low-risk patients with aortic stenosis. J Am Coll Cardiol. 2023;81(17):1663–1674. doi:10.1016/j.jacc.2023.02.017
13. O’Hair D, Yakubov SJ, Grubb KJ, et al. Structural valve deterioration after self-expanding transcatheter or surgical aortic valve implantation in patients at intermediate or high risk. JAMA Cardiol. 2023;8(2):111–119. doi:10.1001/jamacardio.2022.4627
14. Borger M, von Haefen C, Bührer C, Endesfelder S. Cardioprotective effects of dexmedetomidine in an oxidative-stress in vitro model of neonatal rat cardiomyocytes. Antioxidants. 2023;12(6):1206. doi:10.3390/antiox12061206
15. Wang Z, Yao M, Jiang L, et al. Dexmedetomidine attenuates myocardial ischemia/reperfusion-induced ferroptosis via AMPK/GSK-3β/Nrf2 axis. Biomed Pharmacother. 2022;154:113572. doi:10.1016/j.biopha.2022.113572
16. Cai S, Liu Y, Cheng Y, Yuan J, Fang J. Dexmedetomidine protects cardiomyocytes against hypoxia/reoxygenation injury via multiple mechanisms. J Clin Lab Anal. 2022;36(7):e24119. doi:10.1002/jcla.24119
17. Elgebaly AS, Fathy SM, Sallam AA, et al. Cardioprotective effects of propofol-dexmedetomidine in open-heart surgery: a prospective double-blind study. Ann Card Anaesth. 2020;23(2):134–141. doi:10.4103/aca.ACA_168_18
18. Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59(2):263–268. (). doi:10.2165/00003495-200059020-00012
19. Madamsetty VS, Mohammadinejad R, Uzieliene I, et al. Dexamethasone: insights into PHARMACOLOGICAL aspects, therapeutic mechanisms, and delivery systems. ACS Biomater Sci Eng. 2022;8(5):1763–1790. doi:10.1021/acsbiomaterials.2c00026
20. Ji F, Li Z, Nguyen H, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation. 2013;127(15):1576–1584. doi:10.1161/CIRCULATIONAHA.112.000936
21. Reindl M, Tiller C, Holzknecht M, et al. Association of myocardial injury with serum procalcitonin levels in patients with ST-elevation myocardial infarction. JAMA Network Open. 2020;3(6):e207030. doi:10.1001/jamanetworkopen.2020.7030
22. AlRawahi AN, AlHinai FA, Doig CJ, et al. The prognostic value of serum procalcitonin measurements in critically injured patients: a systematic review. Crit Care. 2019;23(1):390. doi:10.1186/s13054-019-2669-1
23. Landesberg G, Shatz V, Akopnik I, et al. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol. 2003;42(9):1547–1554.
24. Kilger E, Pichler B, Weis F, et al. Markers of myocardial ischemia after minimally invasive and conventional coronary operation. Ann Thorac Surg. 2000;70(6):2023–2028. doi:10.1016/s0003-4975(00)01848-8
25. Domanski MJ, Mahaffey K, Hasselblad V, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA. 2011;305(6):585–591. doi:10.1001/jama.2011.99
26. Chi X, Liao M, Chen X, et al. Dexmedetomidine attenuates myocardial injury in off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2016;30(1):44–50. doi:10.1053/j.jvca.2015.06.026
27. She H, Hu Y, Zhao G, et al. Dexmedetomidine ameliorates myocardial ischemia-reperfusion injury by inhibiting MDH2 lactylation via regulating metabolic reprogramming. Adv Sci. 2024;11(48). doi:10.1002/advs.202409499
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.