Back to Journals » Neuropsychiatric Disease and Treatment » Volume 21
Association Between Daytime Sleepiness and Quality of Life in Chinese Adolescents: A Moderated Mediation of Cognitive Dysfunction and Depressive Symptoms
Authors Li W, Hu N, Yang X, Zheng W, Tong J, Song J, Song Y, Gao X, Wang Z, Liu W, Wang L, Tan Y, Wang C, Deng H
Received 21 February 2025
Accepted for publication 20 May 2025
Published 3 June 2025 Volume 2025:21 Pages 1143—1159
DOI https://doi.org/10.2147/NDT.S524185
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Wei Li,1,* Na Hu,2,* Xingjie Yang,1,* Wenkai Zheng,3 Jinghui Tong,1 Jiaqi Song,1 Yanying Song,1 Xiaoxiao Gao,1 Zhiren Wang,1 Wenjie Liu,1 Leilei Wang,1 Yunlong Tan,1 Chundi Wang,4 Hu Deng1
1Peking University Huilongguan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, People’s Republic of China; 2Department of Psychiatry, Beijing Children’s Hospital, Capital Medical University, National Center for Children Healthy, Beijing, People’s Republic of China; 3School of Basic Medicine, Inner Mongolia Medical University, Hohhot, People’s Republic of China; 4Department of Psychology, School of Humanities and Social Sciences, Beihang University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chundi Wang, Department of Psychology, School of Humanities and Social Sciences, Beihang University, Beijing, People’s Republic of China, Email [email protected] Hu Deng, Peking University Huilongguan Clinical Medical School, Beijing Huilongguan Hospital, Beijing, 100096, People’s Republic of China, Email [email protected]
Background: Daytime sleepiness is prevalent among Chinese adolescents and has been associated with increased depressive symptoms, impaired cognitive function, and reduced quality of life. However, the interrelationships among these variables remain unclear, particularly regarding whether cognitive function moderates the association between daytime sleepiness and quality of life.
Methods: A large-scale cross-sectional survey was conducted among 52,964 students (grades 7– 12) across five geographically diverse regions of China. Data were collected on daytime sleepiness, depressive symptoms, cognitive dysfunction, and quality of life using standardized self-report measures.
Results: 1) Among the Chinese adolescents, excessive daytime sleepiness (13.87%) and poor quality of life were prevalent. 2) Quality of life was negatively correlated with daytime sleepiness (r = − 0.277), depressive symptoms (r = − 0.416), and cognitive dysfunction (r = − 0.217), all p-values < 0.001. 3) Depressive symptoms played a partially mediating role in the association between daytime sleepiness and quality of life (effect size = − 0.232), accounting for 82.86% of the total effect. 4) In the moderated mediation model of daytime sleepiness → depressive symptoms → quality of life, cognitive dysfunction plays a moderating role. Specifically, cognitive dysfunction significantly moderated the association between daytime sleepiness and depressive symptoms (a = 0.100, SE = 0.003, t = 34.618), the association between depressive symptoms and quality of life (b = − 0.014, SE = 0.005, t = − 2.929), and the direct effect of daytime sleepiness on quality of life (c’ = − 0.048, SE = 0.005, t = − 9.996), all p-values < 0.001.
Conclusion: Depressive symptoms partially mediate the relationship between daytime sleepiness and quality of life, while cognitive dysfunction plays a moderating role in both direct and indirect effects. Improving depressive symptoms and cognitive dysfunction may be potential strategies to mitigate the adverse effects of daytime sleepiness on adolescents’ quality of life.
Keywords: daytime sleepiness, quality of life, cognitive dysfunction, depressive symptoms, adolescents
Introduction
Excessive daytime sleepiness (EDS) is a prevalent symptom among children and adolescents, characterized by an increased propensity to fall asleep during daytime hours.1 Its consequences have been shown to affect the health, development, mood and cognitive function of children and adolescents,2 which would further contribute to the reduced academic performance and poor quality of life (QoL).1 However, the recommended sleep duration of 9–10 hours per night for school-aged children, particularly adolescents, is often not met in practice.3,4 This insufficient sleep and eveningness chronotype have the direct impact on daytime activity and may lead to EDS in children and adolescents.5,6 In recent years, growing evidence has indicated that reduced sleep duration may significantly impact cognitive function. For example, a study using accelerometer-measured sleep duration found that shorter sleep time was associated with decreased executive function in the Stroop task and revealed related neural activity changes through functional near-infrared spectroscopy (fNIRS).7 Furthermore, another study integrating population-based research and mouse experiments further explored the potential mechanisms between insufficient sleep and cognitive impairment, emphasizing the crucial role of inflammatory biomarkers and cellular signaling pathways.8 These findings suggest that insufficient sleep may affect individual cognitive ability through neurophysiological and immune-inflammatory mechanisms, further deepening our understanding of the relationship between sleep and brain health. Given the significant negative impact of EDS on mood, cognitive function and QoL,1 to clarify the psychological pathways through which daytime sleepiness causes poor QoL and manage modifiable risk factors would be valuable in improving the well-being of the adolescents.
Depression is among the most prevalent psychiatric disorders in adolescents globally and has been consistently linked to excessive daytime sleepiness (EDS).9,10 In China, a recent nationwide epidemiological survey reported a point prevalence of depression of 2.004% among children and adolescents.11 Individuals exhibiting depressive symptoms are more likely to experience EDS.12 Among patients with major depressive disorder, the prevalence of EDS can reach as high as 50.8%.13 Importantly, EDS has also been identified as a contributing risk factor for the development of depression.10,14 Conversely, baseline levels of daytime sleepiness have been shown to predict the subsequent onset of depressive symptoms.15–17 One study revealed a strong association between daytime sleepiness and depressive symptoms among rural Chinese adolescents.15 Collectively, these studies suggest a potentially complex and bidirectional relationship between EDS and depressive symptoms. Notably, depression is associated not only with EDS but also with impaired QoL. Both evidences from epidemiological and clinical studies found the significant association between severity of depression and poor QoL,18 and depression could significantly predict QoL outcomes.19–21 Recent studies have demonstrated this association across diverse populations.22–24 Therefore, given the strong associations between EDS and depression, and between depression and QoL, it is plausible that depression serves as a key psychological pathway linking EDS to QoL.
A growing body of evidence has established a robust association between EDS and cognitive dysfunction.25–28 Even one week of partial sleep deprivation in adolescents has been shown to impair various domains of cognitive function.29 Studies in older populations have consistently demonstrated that EDS is closely associated with cognitive decline and increased risk of dementia.30,31 Meanwhile, numerous studies have also revealed a significant relationship between depressive symptoms and cognitive dysfunction.32–34 Mehta et al found that individuals with depression are at a significantly higher risk of cognitive impairment.35 Another study reported that depression-related cognitive impairments may persist over extended periods.36 A meta-analysis showed that the effect size of cognitive dysfunction in patients with depression, compared to healthy controls, ranged from −0.34 to −0.65.37 MacKenzie et al provided compelling evidence of cognitive impairment in first-degree relatives of individuals with depression, suggesting a potential shared genetic vulnerability for both conditions.38 These findings collectively suggest that EDS is related to cognitive dysfunction, which, in turn, is strongly associated with depressive symptoms.
In addition to the established framework involving EDS, cognitive dysfunction, and depression, previous studies have also highlighted the interrelationship among depression, cognitive impairment, and QoL in older adults.39 Cognitive dysfunction has been used as a mediating variable to explore the link between emotional states and QoL, and has been shown to mediate the association between depressive symptoms and QoL.40 Moreover, Poletti et al demonstrated that cognitive dysfunction may interact with depressive symptoms in influencing QoL during the COVID-19 pandemic.41 From an intervention perspective, cognitive behavioral therapy (CBT) has been shown to significantly alleviate depressive symptoms and enhance QoL in individuals experiencing diabetes-related distress.42 According to existing evidence, including a systematic review by Gil-Gonzalez et al,43 depressive symptoms and cognitive dysfunction have consistently been identified as significant risk factors for reduced QoL. However, the precise interplay among these variables remains poorly understood. Therefore, further investigation is warranted to clarify the interrelationships among depressive symptoms, cognitive dysfunction, and QoL in adolescents.
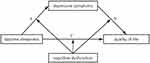
Despite growing evidence linking daytime sleepiness to depressive symptoms, cognitive dysfunction, and QoL,44 no studies to date have examined whether depressive symptoms mediate the association between daytime sleepiness and QoL, or whether cognitive dysfunction moderates the relationships between daytime sleepiness and depressive symptoms, and between depressive symptoms and QoL, particularly in Chinese adolescents. Therefore, this study first aimed to examine whether depressive symptoms mediate the relationship between daytime sleepiness and QoL in adolescents. In addition, it aimed to explore the moderating role of cognitive dysfunction in both the direct and indirect pathways linking daytime sleepiness and QoL via depressive symptoms (Figure 1). Based on a review of the literature and in light of the complex interrelations among these variables, we proposed two hypotheses: Hypothesis 1: Depressive symptoms mediate the relationship between daytime sleepiness and QoL. Hypothesis 2: Cognitive dysfunction moderates both the direct and indirect effects of daytime sleepiness on QoL, with depressive symptoms as a mediator.
Methods
Participants
This cross-sectional epidemiological study, conducted in China in January 2023, evaluated a sample of students selected from five independent regions, stratified by urbanization level and geographic location. All students enrolled in grades 7 through 12 in the five selected regions - Shandong Province, Inner Mongolia Autonomous Region, Hebei Province, Guangxi Zhuang Autonomous Region, and Xinjiang Uygur Autonomous Region - were eligible for inclusion. The sampling strategy was designed to ensure representativeness of the adolescent population in China. Inner Mongolia and Hebei were selected to represent northern China. Shandong, Xinjiang, and Guangxi were selected to represent eastern, western, and southern China, respectively. Inclusion criteria for participation were: (1) Chinese nationality; (2) aged between 12 and 18 years; and (3) provision of informed consent via an online platform. The study was approved by the Ethics Committee of Beijing Huilongguan Hospital. Informed consent was obtained from both the participants and their parents after they were fully informed about the purpose, procedures, and confidentiality of the study.
A total of 56696 questionnaires were collected for this study through the Wenjuanxing online platform (www.wjx.com). After excluding questionnaires that were not between the ages of 12–18, had missing answers, had the same answers for all items, or had a completion time of less than 270 seconds, we obtained a total of 50072 valid questionnaires with an effective response rate of 88.32%.
Measurement and Instruments
The Chinese Adolescent Daytime Sleepiness Scale (CADSS)
The Chinese Adolescent Daytime Sleepiness Scale (CADSS) was used to evaluate daytime sleepiness.45 While the Pediatric Daytime Sleepiness Scale (PDSS) remains the cornerstone of daytime sleepiness research,46 our choice of the CADSS was in line with the target population of this research, Chinese adolescents, a population with distinct cultural and environmental factors influencing sleep patterns. The CADSS was selected due to its cultural adaptation and validation for this demographic. The CADSS consists of seven questions that ask adolescents about their shared feelings of sleepiness and dozing in different situations during the day over the past month. All the seven items are rated on a 5-point scale from 1 = never, 2 = rarely (<1 times week), 3 = sometimes (1–2 times/week), 4 = often (3–5 times/week), to 5 = almost every day (6–7 times/week). The total score of these seven items equals the total CADSS score, which ranges from 7 to 35 points. The Cronbach’s alpha value of the CADSS was 0.89 and the test-retest reliability coefficient was 0.77. The higher the CADSS score, the greater the tendency to experience daytime sleepiness in the past month. A total score of 23 has been proposed as a cutoff value for identifying individuals with (or moderate or severe daytime sleepiness) over the past month.45,47
The Patient Health Questionnaire-9 (PHQ-9)
The scale was developed according to the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders.48 It is used to screen for depression in primary care and other medical Settings.49 We used the Chinese version of this scale. The scale consists of 9 items, each with a 0–3 scale. The severity of symptoms is determined by a total score of 5 to 9 mild, 10 to 14 moderate, 15 to 19 moderate and 20 to 27 severe. The Cronbach’s alpha value of PHQ-9 in the Chinese population was 0.86, and the retest reliability was 0.86, indicating that this scale has good reliability and validity.50
Screening for and Promotion of Health-Related QoL in Children and Adolescents-10 Items (KIDSCREEN-10)
The KIDSCREEN-10 was designed to be a cross-national tool for measuring Health-Related Quality of Life. This instrument was developed by Ravens-Sieberer et al in Germany.51 It is employed for the assessment of health-related QoL in children and adolescents between the ages of 8 and 18 years. And the Cronbach’s Alpha value of KIDSCREEN-10 in the Chinese population was 0.847, and the retest reliability was 0.842, indicating that the Chinese version of KIDSCREEN-10 has good reliability, and validity.52 All the seven items are rated on a 5-point scale from 1 = never, 2 = almost never, 3 = sometimes, 4 = almost always, to 5 = always. The total score of all the 10 items in Chinese version of KIDSCREEN-10 was calculated to assess the health-related QoL. A lower total score indicates a lower level of health-related QoL.
Perceived Deficits Questionnaire for Depression-5-Items (PDQ-5-D)
The Perceived Deficits Questionnaire for Depression-5-items scale (PDQ-5-D) was used to record the severity of self-reported cognitive dysfunction in the past 7 days.53 It consists of 5 questions and 4 subscales: Attention/concentration, prospective memory, planning/organization, and retrospective memory. The frequency of these symptoms was rated using a scale from 0 to 4 (0 = “not at all” to 4 = “very often, more than once a day”). The scale has a total score of 0–20, with higher scores indicating greater cognitive dysfunction. We used the Chinese version of PDQ-5-D. The Cronbach’s alpha value of Chinese version PDQ-5-D was 0.704,54 which was acceptable.55 And the test-retest reliability of Chinese version PDQ-5-D was good (intraclass correlation coefficient = 0.841).54
Covariates
The adolescents were asked to provide self-reported data on their age, place of residence (1 = urban/town, 2 = rural), gender (1 = male, 2 = female), grade (Grades 7–12), primary caregivers. Primary caregivers were categorized as parents, grandparents (father’s side), grandparents (mother’s side), and others.
Statistical Analyses
Continuous variables are expressed as mean and standard deviation and categorical variables are reported as frequency (percentage). Significance testing was conducted using either a t-test or analysis of variance (ANOVA). Pearson correlation analysis was performed to examine associations between daytime sleepiness, depressive symptoms, quality of life, and cognitive dysfunction. Mediation analysis was conducted using the PROCESS macro in SPSS.56 Hayes PROCESS estimates model coefficients and produces bias-corrected bootstrap confidence intervals for conditional indirect effects and tests of moderated or conditional mediation. The proportion mediated was calculated as the ratio of the indirect effect to the total effect, and statistical significance was determined using bootstrapped 95% confidence intervals. This robust method, widely applied in previous epidemiological studies, avoids assumptions of normality and provides reliable estimates of mediation effects.57,58 In our study, we used Model 4 (simple mediated model) and Model 59 (direct and indirect pathways mediated by one variable). First, we used model 4 to examine whether the association between daytime sleepiness and quality of life was mediated by depressive symptoms. Second, Model 59 was used to examine whether cognitive dysfunction moderated the direct (path c’: daytime sleepiness-quality of life) and indirect (path a: daytime sleepiness -depressive symptoms and path b: depressive symptoms-quality of life) effects of daytime sleepiness on quality of life. At the same time, demographic variables (gender, age, grade, place of residence, primary caregivers) were controlled for mediating and moderating effects. Simple slopes are then plotted to explore the significant interactions associated with low/high levels of cognitive dysfunction. Based on 5000 random samples, the bootstrap confidence interval (CI) determines whether the effect in model 4 and Model 59 is significant. In this study, all the data was standardized. All of the tests were two-tailed, and the significance level was set at p < 0.05. All of the analyses were performed using SPSS for Windows 24.0.
Results
Characteristics of the Participants
The mean age of 50,072 participants included in the analysis was 15.69 ± 1.57 years, as presented in Table 1, approximately 46.9% (n = 23497) were males and 53.1% (n = 26575) were females. The majority of participants resided in urban or town areas (61.1%) and lived with their parents (92.1%). The EDS, depressive symptoms, quality of life, and cognitive dysfunction of the participants were significantly different among gender, grade, place of residence, and primary caregivers (Table 2).
 |
Table 1 Characteristics of Study Participants and Stratified by Daytime Sleepiness (N = 50,072) |
 |
Table 2 Scores on Various Scales of Study Participants (N = 50,072) |
Among the participants, the prevalence of EDS was 13.87%. For females, the prevalence rate of EDS was 8.50%, which was higher than that for males at 5.38%. The difference in EDS between males and females was statistically significant (χ 2 = 215.728, p < 0.001). Chi-square tests also revealed that the prevalence of EDS in high school (Grade 10–12) and middle school (Grade 7–9) was 9.43% and 4.45%, respectively. The significantly higher prevalence of EDS in high school students (χ 2 = 1549.233, p < 0.001) suggests an increased burden compared to middle school students. We also found that the prevalence of EDS in urban area and rural area was 8.44% and 5.44%, respectively. The majority of adolescents with EDS lived with their parents (12.54%), while 1.33% lived with grandparents or others (χ 2 = 45.852, p < 0.001). The score of KIDSCREEN-10 in Chinese adolescents was 35.01 ± 8.53, which is lower than the optimal cut points (from 40.26 to 41.93 for adolescents) suggested by the study of Hirschfeld et al.59 These results suggested that excessive daytime sleepiness and the poor quality of life are prevalent among adolescents.
Correlation Between Daytime Sleepiness, Depressive Symptoms, Quality of Life and Cognitive Dysfunction
Correlation analysis showed that daytime sleepiness was significantly positively correlated with depressive symptoms (r = 0.601, p < 0.001) and cognitive dysfunction (r = 0.539, p < 0.001), and significantly negatively correlated with QoL (r = −0.277, p < 0.001). Depressive symptoms were positively correlated with cognitive dysfunction (r = 0.569, p < 0.001) and negatively correlated with QoL (r = −0.416, p < 0.001). There was a significant negative correlation between and cognitive dysfunction and QoL (r = −0.217, p < 0.001) (Table 3). These results support further analysis of mediation effects.
 |
Table 3 Correlations for the Main Variables |
Mediating Effects of Depressive Symptoms in the Association Between Daytime Sleepiness and Quality of Life
As shown in Table 4, the total effect (c = −0.280, SE = 0.005, t = −62.382, p < 0.001) of daytime sleepiness on quality of life was found to be statistically significant. Additionally, daytime sleepiness significantly positively predicted depressive symptoms (a = 0.589, SE = 0.004, t = 157.806, p < 0.001). Daytime sleepiness and depressive symptoms entered simultaneously into the regression equation, with daytime sleepiness significantly negatively predicting quality of life (c′ = −0.048, SE = 0.005, t = −9.232, p < 0.001), and depressive symptoms negatively predicting quality of life (b = −0.394, SE = 0.005, t = −77.601, p < 0.001). Finally, to test the mediation model, the researchers employed the bias-corrected percentile bootstrap method. The present study generated 5,000 bootstrapping samples from the standardized data to estimate the indirect effects. The research findings indicate a significant mediating effect of depressive symptoms on the relationship between daytime sleepiness and quality of life (ab = −0.232, SE = 0.003, 95% CI: −0.239, −0.225). The mediating effect accounts for 82.86% of the total effect, while the direct effect size is −0.048, accounting for 17.14% of the total effect.
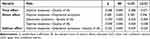 |
Table 4 The Mediating Role of Depressive Symptoms in the Relationship Between Daytime Sleepiness and Quality of Life |
Moderation Effect of Cognitive Dysfunction on the Relationship Between Daytime Sleepiness and Quality of Life as Mediated by Depressive Symptoms
Using Model 59, moderated mediation model tests showed that the interaction between daytime sleepiness and cognitive dysfunction was a significant positive predictor of depressive symptoms through path a (daytime sleepiness * cognitive dysfunction: β = 0.100, SE = 0.003, t = 34.618, p < 0.001). The interaction between depressive symptoms and cognitive dysfunction through path b was a significant negative predictor of quality of life (depressive symptoms * cognitive dysfunction: β = −0.014, SE = 0.005, t = −2.929, p < 0.001), and the interaction between daytime sleepiness and cognitive dysfunction through path c’ was also a significant negative predictor of quality of life (daytime sleepiness * cognitive dysfunction: β = −0.048, SE = 0.005, t = −9.996, p < 0.001). As shown in Figure 2 and Table 5, cognitive dysfunction modulates the effects of day time sleepiness on quality of life through pathways a, b, and c’.
 |
Table 5 The Moderated Mediating Effect of Daytime Sleepiness on Quality of Life by Depression Symptoms and Cognitive Dysfunctions |
 |
Figure 2 The moderated mediation model: cognitive deficits as moderators of the mediation model of depressive symptoms between daytime sleepiness and quality of life. (***p < 0.001). |
In addition, for path a, when cognitive dysfunction was low (M−1SD), daytime sleepiness positively predicted depressive symptoms (β = 0.270, SE = 0.006, t = 47.737, p < 0.001); when cognitive dysfunction was high (M+1SD), daytime sleepiness also positively predicted depressive symptoms (β = 0.470, SE = 0.004, t = 106.881, p < 0.001). As cognitive dysfunction worsens, the predictive effect of daytime sleepiness on depressive symptoms showed a gradual increasing trend (β = 0.100, SE = 0.003, t = 34.618, p < 0.001) (Figure 3A).
 |
Figure 3 The simple plot of path (A–C) indicating the relationship between daytime sleepiness, depressive symptoms and quality of life among different levels of cognitive dysfunction. |
For path b, when cognitive dysfunction was low (M−1SD), depressive symptoms negatively predicted quality of life (β = −0.377, SE = 0.009, t = −40.980, p < 0.001); when cognitive dysfunction was high (M+1SD), depressive symptoms negatively predicted quality of life (β = −0.405, SE = 0.006, t = −69.625, p < 0.001). As cognitive dysfunction worsens, the predictive effect of depressive symptoms on quality of life showed a gradual increasing trend (β = −0.014, SE = 0.005, t = −2.929, p < 0.001) (Figure 3B).
For path c, when cognitive dysfunction was low (M−1SD), the relationship between daytime sleepiness and quality of life was not statistically significant (β = −0.002, SE = 0.008, t = −0.295, p = 0.768); when cognitive dysfunction was high (M+1SD), daytime sleepiness negatively predicted quality of life (β = −0.098, SE = 0.006, t = −15.521, p < 0.001). As cognitive dysfunction worsens, the predictive effect of daytime sleepiness on quality of life showed a gradual increasing trend (β = −0.048, SE = 0.005, t = −9.996, p < 0.001) (Figure 3C).
Discussion
In this cross-sectional epidemiological study of Chinese adolescents, the prevalence of EDS was approximately 13.87%, and poor quality of life was also widespread in this population. Our findings suggest that depressive symptoms play a crucial mediating role in the relationship between daytime sleepiness and QoL in adolescents. Additionally, cognitive dysfunction moderates the association between daytime sleepiness and QoL. Higher levels of cognitive dysfunction in adolescents amplify the impact of daytime sleepiness on QoL, strengthen the correlation between daytime sleepiness and depressive symptoms, and exacerbate the negative effects of depressive symptoms on QoL.
Our findings revealed that adolescents experienced a high prevalence of EDS, reaching 13.87%. Notably, excessive daytime sleepiness among adolescents has been increasingly recognized as a significant public health concern.60 This global issue is underscored by the high prevalence of daytime sleepiness among adolescents across various regions. International studies have reported that the prevalence of daytime sleepiness among adolescents ranges from 16% to 47%.6,61–63 The discrepancies between our findings and previous studies may be attributable to differences in sample size. Chronic sleep deprivation, which varies by grade level, gender, and other factors, is a primary contributor to daytime sleepiness among adolescents.64 Furthermore, our study demonstrated that the prevalence of daytime sleepiness significantly differed by grade, gender, and co-residency status. This finding aligns with previous research. A meta-analysis reported a decline in sleep duration among adolescents with increasing age, with gender also significantly influencing this trend.65 Reduced sleep duration among adolescents is associated with more pronounced levels of daytime sleepiness. Moreover, a longitudinal study revealed that enhanced sleep quality and diminished daytime sleepiness were linked to superior family supervision and parent-child relationships,66 suggesting that daytime sleepiness is influenced by different co-residents. In addition, we observed that the prevalence of EDS was higher among high school students compared to their middle school counterparts. In a follow-up study of 3736 adolescents from high schools in southern China, the 1-year persistence rate of excessive daytime sleepiness was 27.6% and the incidence rate was 9.3%,15 indicating that the problem of daytime sleepiness is also very serious among high school adolescents. Sleep disturbances and associated EDS in adolescents may adversely impact quality of life and contribute to mood disorders, depressive symptoms, cognitive impairments, decreased academic performance, and safety risks.3,67–69 These associations between daytime sleepiness, quality of life, depressive symptoms, and cognitive dysfunction underscore the importance of our investigation. Addressing excessive daytime sleepiness in adolescents is imperative, and developing effective intervention strategies should be prioritized.
In this study, we found that excessive daytime sleepiness (EDS) was negatively associated with quality of life (QoL) and positively correlated with depressive symptoms and cognitive dysfunction in adolescents. Further analysis indicated that all pairwise combinations among the four variables were significantly correlated, suggesting the presence of a potentially complex interrelationship. Although no prior studies have simultaneously examined the complex interactions among these four variables, existing research on pairwise relationships between daytime sleepiness and each of the other three variables supports our findings.3,70–72
In support of our first hypothesis, we found that the association between daytime sleepiness and QoL in adolescents is partially mediated by depressive symptoms. A previous study among Chinese college students reported a similar finding, indicating that sleep behaviors (including daytime sleepiness) indirectly affect physical and psychological health via negative emotions such as anxiety and depression.73 Several potential mechanisms may explain this mediating relationship. Genetics, dysfunction of the HPA axis and dysfunction of the melatonergic system are possible mechanisms for the mediating relationship among sleep-wake cycle, depression and quality of life.74–76 Multiple experimental studies have confirmed a strong association between daytime sleepiness and the neural mechanisms underlying depressive symptoms.77–79 A complex reciprocal relationship appears to exist between emotional processing on the preceding day, nighttime sleep, and emotional processing the following day.80 Most importantly, growing evidence suggests that sleep disorders, which contribute to daytime sleepiness, may share common pathophysiological mechanisms with mental illnesses such as depression, contributing to their onset and maintenance.81–83 From a biological perspective, brainstem cholinergic neurons are integral to the regulation of wakefulness, and dysfunction in these neurons can result in daytime sleepiness.84 Moreover, both the cholinergic system and wake-promoting neurotransmitters, such as norepinephrine, dopamine, and serotonin, have been implicated in the pathophysiology of depressive symptom.85–87 Therefore, the relationship between sleep behavior and depressive symptoms appears to be both complex and bidirectional.77 Adolescents experiencing daytime sleepiness are more likely to suffer from insufficient nocturnal sleep, which may contribute to both the development and persistence of depressive symptoms.
Consistent with previous studies,88,89 we also found that depressive symptoms were associated with poorer QoL. Previous research examining the relationship between psychological symptoms and QoL has identified depressive symptoms as the strongest predictor of diminished QoL.90 Depression is associated with a range of negative societal and individual outcomes, including increased healthcare costs and elevated risk for comorbid psychological disorders.91–93 These adverse effects consistently lead to reduced quality of life and diminished well-being.94–96 For example, a cross-sectional study reveals that people with depression have worse quality of life than those without depression,97 and some longitudinal studies confirm that depression at baseline predicts poorer quality of life at follow-up.98–100 Furthermore, a meta-analysis demonstrated that QoL was already reduced prior to the onset of depression and declined further during the course of the disorder.89 Taken together with our findings, the aforementioned studies suggest that the impact of daytime sleepiness on QoL is largely indirect, mediated through depressive symptoms. Therefore, comprehensive interventions targeting depressive symptoms in adolescents are warranted to improve QoL. And exercise therapy emerges as a particularly promising intervention for adolescent populations in depression prevention and management,101 owing to its cost-efficiency, minimal spatial demands, and inherently engaging nature. Its therapeutic value extends beyond physiological improvements (eg, neuroendocrine regulation, cardiovascular adaptation) to encompass psychosocial benefits such as resilience development, identity formation, and restoration of interpersonal connectivity.
In line with our second hypothesis, we found that daytime sleepiness indirectly affects QoL, with depressive symptoms acting as a mediator and cognitive dysfunction serving as a moderator. Higher levels of cognitive dysfunction significantly amplified the mediating effect of depressive symptoms on the relationship between daytime sleepiness and QoL in adolescents. Additionally, daytime sleepiness exerted a direct negative impact on QoL, and this relationship was moderated by cognitive dysfunction. These findings suggest that adolescents with more severe cognitive dysfunction may be at heightened risk for poor QoL. Previous research has indicated that excessive daytime sleepiness is related to cognitive dysfunction,25,26,102–104 and the relationship between cognitive dysfunction and depression has been confirmed.105–110 A growing body of evidence indicates that cognitive functioning is a critical determinant of QoL.111–113 Therefore, cognitive dysfunction may moderate the associations between daytime sleepiness and QoL, between daytime sleepiness and depressive symptoms, and between depressive symptoms and QoL. A study on obstructive sleep apnea/hypopnea syndrome (OSAHS) reported interactions among sleepiness, cognitive performance, and QoL in affected individuals,114 findings that partially align with those of the present study.
There is a lack of research examining cognitive dysfunction as a moderating factor in the associations between daytime sleepiness and QoL, or between depressive symptoms and QoL. However, our findings suggest that interventions targeting depressive symptoms and cognitive dysfunction may enhance QoL in adolescents experiencing daytime sleepiness. Notably, Drake et al systematically demonstrated the central therapeutic role of CBT-I,115 proposing that behavioral interventions can restore sleep homeostasis by modifying maladaptive cognitive patterns—a mechanism theoretically aligned with the cognitive regulatory pathways in adolescents revealed in this study. Interventions addressing both cognitive dysfunction and depressive symptoms may exert a particularly strong influence on adolescents’ quality of life and daily functioning. For instance, prior studies have shown that engagement in physical activity is a key strategy for enhancing adolescents’ mental health and cognitive functioning.116 These findings underscore the significance of our research and highlight the need for further investigation into the impact of cognitive therapies on QoL.
This cross-sectional epidemiological study presents several noteworthy strengths. To our knowledge, this is among the first studies to elucidate the mediating role of depressive symptoms and the moderating role of cognitive dysfunction in the relationship between daytime sleepiness and QoL among Chinese adolescents. Previous studies have primarily examined pairwise associations among these variables. Furthermore, the articulation of the moderated mediation model provides a concise and integrated framework capturing the complex interrelationships among daytime sleepiness, depressive symptoms, cognitive dysfunction, and QoL. Evidence on the psychological pathways linking daytime sleepiness and QoL may support the early identification of mental health concerns and inform timely interventions to mitigate poor QoL.
Limitations
It should be noted that the present study is not without limitations. Firstly, our study was based on a cross-sectional design and lacked a dynamic monitoring system to track changes, making it difficult to fully elucidate the temporal sequence among daytime sleepiness, depressive symptoms, cognitive dysfunction, and quality of life in adolescents. Also, the study’s narrow timeframe precludes generalization to other seasonal or academic contexts. Seasonal variations in daylight exposure and school schedules (eg, exam periods, winter holidays) could affect sleep patterns, mood and cognitive function and could potentially influence the results, which warrants investigation in longitudinal designs. Secondly, we cannot rule out the possibility of the reverse relationship that cognitive dysfunction may act as the role of a mediator and depressive symptoms may act as the role of a moderator. Indeed, establishing a causal link between cognitive dysfunction and depressive symptoms is often challenging.
Thirdly, all variables in our study, including excessive daytime sleepiness, depressive symptoms, and cognitive dysfunction, were measured using self-reported questionnaires. Although these instruments have been validated and widely used in Chinese populations, self-reported data are inherently susceptible to recall bias and subjective interpretation. Moreover, considering the cultural diversity across different regions of China, participants may underreport or overreport symptoms due to social desirability bias or limited awareness of their own psychological or cognitive states. Additionally, the use of self-administered surveys limited our ability to monitor the response process, and there remains a possibility of careless or perfunctory answers, which could influence the accuracy and reliability of the findings. Future studies are recommended to incorporate clinical assessments or objective indicators to enhance the accuracy of the findings.
Fourthly, although we adjusted for several demographic variables (eg, age, gender, grade, residence, and primary caregiver), we acknowledge that other potential confounding factors, such as anxiety, socioeconomic status, academic stress, diet, physical activity, family dynamics, and undiagnosed medical conditions related to sleep (eg, insomnia or sleep apnea), were not measured or controlled for in our analyses. These factors may influence both sleep quality and psychological well-being, potentially biasing the associations observed in our study. In particular, it should be noted that these psychological and environmental factors—such as anxiety, academic workload, family dynamics, and sleep hygiene—may serve as critical mediators and moderators in shaping the relationship between EDS and QoL. Future research should consider incorporating a broader range of covariates to better isolate the effects of excessive daytime sleepiness and enhance the interpretability and robustness of the findings.
Conclusions
In conclusion, this study is the first to comprehensively examine the relationships among daytime sleepiness, depressive symptoms, cognitive dysfunction, and quality of life in Chinese adolescents. Our findings demonstrate that daytime sleepiness, a growing issue among adolescents, is significantly associated with poorer quality of life, highlighting the urgency of addressing this public health concern. Importantly, depressive symptoms were found to partially mediate the association between daytime sleepiness and quality of life, while cognitive dysfunction moderated this indirect effect, further exacerbating the impact. These results underscore the importance of early screening and psychological support for adolescents experiencing excessive daytime sleepiness. From a practical perspective, school-based mental health programs, exercise therapy, cognitive training interventions, and public health policies aimed at improving sleep hygiene and emotional well-being may serve as effective strategies to mitigate the adverse effects of daytime sleepiness on adolescents’ quality of life. This study contributes novel evidence by integrating both mediation and moderation mechanisms into the analysis of sleep-related quality of life in adolescents, an area that has been largely underexplored in previous Chinese studies. By doing so, it fills a critical gap in understanding the complex psychological pathways linking sleepiness to well-being in this vulnerable population.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. The study was approved by the Ethics Committee of Beijing Huilongguan Hospital. Informed consent was obtained from both the participants and their parents after they were fully informed about the purpose, procedures, and confidentiality of the study.
Acknowledgments
The authors would like to thank all the subjects who participated in this study. The authors thank all the researchers and scientific advisors for their contribution to the design of this study.
Funding
This work was supported by Beijing Municipal Science & Technology Commission, Z221100007422047; Beijing Municipal Administration of Hospitals Incubating Program, PX2023071; the National Natural Science Foundation of China, 82301693 & 31900751, the Capital’s Funds for Health Improvement and Research, CFH2024-4-2132 & CFH2024-4-2134, the BeijingMunicipal Administration of Hospitals’ Youth Program QML20232003 & QML20232006.
Disclosure
All authors declare no competing financial interest.
References
1. Fallone G, Owens JA, Deane J. Sleepiness in children and adolescents: clinical implications. Sleep Med Rev. 2002;6(4):287–306. doi:10.1053/smrv.2001.0192
2. Willis TA, Gregory AM. Anxiety disorders and sleep in children and adolescents. Sleep Med Clin. 2015;10(2):125–131. doi:10.1016/j.jsmc.2015.02.002
3. Gustafsson ML, Laaksonen C, Aromaa M, et al. Association between amount of sleep, daytime sleepiness and health-related quality of life in schoolchildren. J Adv Nurs. 2016;72(6):1263–1272. doi:10.1111/jan.12911
4. Matricciani L, Blunden S, Rigney G, Williams MT, Olds TS. Children’s sleep needs: is there sufficient evidence to recommend optimal sleep for children?. Sleep. 2013;36(4):527–534. doi:10.5665/sleep.2538
5. Beebe DW. Cognitive, behavioral, and functional consequences of inadequate sleep in children and adolescents. Pediatr Clin North Am. 2011;58(3):649–665. doi:10.1016/j.pcl.2011.03.002
6. Liu Y, Zhang J, Li SX, et al. Excessive daytime sleepiness among children and adolescents: prevalence, correlates, and pubertal effects. Sleep Med. 2019;53:1–8. doi:10.1016/j.sleep.2018.08.028
7. You Y, Liu J, Li X, Wang P, Liu R, Ma X. Relationship between accelerometer-measured sleep duration and Stroop performance: a functional near-infrared spectroscopy study among young adults. PeerJ. 2024; 12:e17057.
8. You Y, Li J, Zhang Y, Li X, Li X, Ma X. Exploring the potential relationship between short sleep risks and cognitive function from the perspective of inflammatory biomarkers and cellular pathways: insights from population-based and mice studies. CNS Neurosci Ther. 2024;30(5):e14783. doi:10.1111/cns.14783
9. Huang Y, Wang Y, Wang H, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiat. 2019;6(3):211–224. doi:10.1016/S2215-0366(18)30511-X
10. Chellappa SL, Schroder C, Cajochen C. Chronobiology, excessive daytime sleepiness and depression: is there a link?. Sleep Med. 2009;10(5):505–514. doi:10.1016/j.sleep.2008.05.010
11. Deng H, Wen F, Xu H, et al. Prevalence of affective disorders in Chinese school-attending children and adolescents aged 6-16 based on a national survey by MINI-Kid. J Affect Disord. 2023;331:192–199. doi:10.1016/j.jad.2023.03.060
12. Chellappa SL, Araujo JF. Excessive daytime sleepiness in patients with depressive disorder. Braz J Psychiatry. 2006;28(2):126–129. doi:10.1590/S1516-44462006000200010
13. Hein M, Lanquart JP, Loas G, Hubain P, Linkowski P. Prevalence and risk factors of excessive daytime sleepiness in major depression: a study with 703 individuals referred for polysomnography. J Affect Disord. 2019;243:23–32. doi:10.1016/j.jad.2018.09.016
14. Soni N, Jaiswal S, Kumar S, Malik S, Rani S. The association between depression, daytime sleepiness, chronotype and fatigue among the students. Asian J Med Health. 2024;22(7):204–213. doi:10.9734/ajmah/2024/v22i71061
15. Luo C, Zhang J, Chen W, Lu W, Pan J. Course, risk factors, and mental health outcomes of excessive daytime sleepiness in rural Chinese adolescents: a one-year prospective study. J Affect Disord. 2018;231:15–20. doi:10.1016/j.jad.2018.01.016
16. Chen SJ, Zhang JH, Li SX, et al. The trajectories and associations of eveningness and insomnia with daytime sleepiness, depression and suicidal ideation in adolescents: a 3-year longitudinal study. J Affect Disord. 2021;294:533–542. doi:10.1016/j.jad.2021.07.033
17. Goldstone A, Javitz HS, Claudatos SA, et al. Sleep disturbance predicts depression symptoms in early adolescence: initial findings from the adolescent brain cognitive development study. J Adolesc Health. 2020;66(5):567–574. doi:10.1016/j.jadohealth.2019.12.005
18. Sivertsen H, Bjorklof GH, Engedal K, Selbaek G, Helvik AS. Depression and quality of life in older persons: a review. Dement Geriatr Cognit Disord. 2015;40(5–6):311–339. doi:10.1159/000437299
19. Rubio JM, Olfson M, Perez-Fuentes G, Garcia-Toro M, Wang S, Blanco C. Effect of first episode axis I disorders on quality of life. J Nerv Ment Dis. 2014;202(4):271–274. doi:10.1097/NMD.0000000000000117
20. Rubio JM, Olfson M, Villegas L, Perez-Fuentes G, Wang S, Blanco C. Quality of life following remission of mental disorders: findings from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2013;74(5):e445–450. doi:10.4088/JCP.12m08269
21. Stevanovic D, Jancic J, Lakic A. The impact of depression and anxiety disorder symptoms on the health-related quality of life of children and adolescents with epilepsy. Epilepsia. 2011;52(8):e75–78. doi:10.1111/j.1528-1167.2011.03133.x
22. Reich M, Lesur A, Perdrizet-Chevallier C. Depression, quality of life and breast cancer: a review of the literature. Breast Cancer Res Treat. 2008;110(1):9–17. doi:10.1007/s10549-007-9706-5
23. Bota AV, Bogdan I, Razvan DV, et al. A three-year cross-sectional analysis of depression, anxiety, and quality of life in patients with post-COVID-19 syndrome. Int J Gen Med. 2024;17:751–762. doi:10.2147/IJGM.S453247
24. Obeid S, Lahoud N, Haddad C, et al. Factors associated with depression among the Lebanese population: results of a cross-sectional study. Perspect Psychiatr Care. 2020;56(4):956–967. doi:10.1111/ppc.12518
25. Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162(2):201–208. doi:10.1001/archinte.162.2.201
26. Zhou J, Camacho M, Tang X, Kushida CA. A review of neurocognitive function and obstructive sleep apnea with or without daytime sleepiness. Sleep Med. 2016;23:99–108. doi:10.1016/j.sleep.2016.02.008
27. Bonanni E, Maestri M, Tognoni G, et al. Daytime sleepiness in mild and moderate Alzheimer’s disease and its relationship with cognitive impairment. J Sleep Res. 2005;14(3):311–317. doi:10.1111/j.1365-2869.2005.00462.x
28. Jaussent I, Bouyer J, Ancelin ML, et al. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. 2012;35(9):1201–1207. doi:10.5665/sleep.2070
29. Lo JC, Ong JL, Leong RL, Gooley JJ, Chee MW. Cognitive performance, sleepiness, and mood in partially sleep deprived adolescents: the need for sleep study. Sleep. 2016;39(3):687–698. doi:10.5665/sleep.5552
30. Tsapanou A, Gu Y, O’Shea D, et al. Daytime somnolence as an early sign of cognitive decline in a community-based study of older people. Int J Geriatr Psychiatry. 2016;31(3):247–255. doi:10.1002/gps.4318
31. Smagula SF, Jia Y, Chang CH, Cohen A, Ganguli M. Trajectories of daytime sleepiness and their associations with dementia incidence. J Sleep Res. 2020;29(6):e12952. doi:10.1111/jsr.12952
32. Jorm AF. Is depression a risk factor for dementia or cognitive decline? A review. Gerontology. 2000;46(4):219–227. doi:10.1159/000022163
33. Bierman EJ, Comijs HC, Jonker C, Beekman AT. Symptoms of anxiety and depression in the course of cognitive decline. Dement Geriatr Cognit Disord. 2007;24(3):213–219. doi:10.1159/000107083
34. Botto R, Callai N, Cermelli A, Causarano L, Rainero I. Anxiety and depression in Alzheimer’s disease: a systematic review of pathogenetic mechanisms and relation to cognitive decline. Neurol Sci. 2022;43(7):4107–4124. doi:10.1007/s10072-022-06068-x
35. Mehta K, Thandavan SP, Mohebbi M, et al. Depression and bone loss as risk factors for cognitive decline: a systematic review and meta-analysis. Ageing Res Rev. 2022;76:101575. doi:10.1016/j.arr.2022.101575
36. Bortolato B, Miskowiak KW, Kohler CA, et al. Cognitive remission: a novel objective for the treatment of major depression?. BMC Med. 2016;14(1):9. doi:10.1186/s12916-016-0560-3
37. Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029–2040. doi:10.1017/S0033291713002535
38. MacKenzie LE, Uher R, Pavlova B. Cognitive performance in first-degree relatives of individuals with vs without major depressive disorder: a meta-analysis. JAMA Psychiatry. 2019;76(3):297–305. doi:10.1001/jamapsychiatry.2018.3672
39. Wang W, Si H, Yu R, et al. Effects of reversible cognitive frailty on disability, quality of life, depression, and hospitalization: a prospective cohort study. Aging Mental Health. 2022;26(10):2031–2038. doi:10.1080/13607863.2021.2011835
40. Zhang H, Xing Y, Zhang Y, et al. Association between depression and quality of life in older adults with type 2 diabetes: a moderated mediation of cognitive impairment and sleep quality. J Affect Disord. 2023;340:17–24. doi:10.1016/j.jad.2023.07.105
41. Poletti S, Palladini M, Mazza MG, et al. Long-term consequences of COVID-19 on cognitive functioning up to 6 months after discharge: role of depression and impact on quality of life. Eur Arch Psychiatry Clin Neurosci. 2022;272(5):773–782. doi:10.1007/s00406-021-01346-9
42. Abbas Q, Latif S, Ayaz Habib H, et al. Cognitive behavior therapy for diabetes distress, depression, health anxiety, quality of life and treatment adherence among patients with type-II diabetes mellitus: a randomized control trial. BMC Psychiatry. 2023;23(1):86. doi:10.1186/s12888-023-04546-w
43. Gil-Gonzalez I, Martin-Rodriguez A, Conrad R, Perez-San-Gregorio MA. Quality of life in adults with multiple sclerosis: a systematic review. BMJ Open. 2020;10(11):e041249. doi:10.1136/bmjopen-2020-041249
44. Roth T. Effects of excessive daytime sleepiness and fatigue on overall health and cognitive function. J Clin Psychiatry. 2015;76(9):e1145. doi:10.4088/JCP.14019tx1c
45. Xianchen Liu YY, Liu -Z-Z, Chen H, Fan F, Jia C-X, Jia C-X. Psychometric assessment of the Chinese adolescent daytime sleepiness scale (CADSS). Sleep Biol Rhythms. 2017;15(3):207–216. doi:10.1007/s41105-017-0106-x
46. Drake C, Nickel C, Burduvali E, Roth T, Jefferson C, Pietro B. The pediatric daytime sleepiness scale (PDSS): sleep habits and school outcomes in middle-school children. Sleep. 2003;26(4):455–458.
47. Wang ZY, Liu ZZ, Jia CX, Liu X. Age at menarche, menstrual problems, and daytime sleepiness in Chinese adolescent girls. Sleep. 2019;42(6). doi:10.1093/sleep/zsz061
48. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
49. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study primary care evaluation of mental disorders patient health questionnaire. JAMA. 1999;282(18):1737–1744.
50. Wang W, Bian Q, Zhao Y, et al. Reliability and validity of the Chinese version of the patient health questionnaire (PHQ-9) in the general population. Gen Hosp Psychiatry. 2014;36(5):539–544. doi:10.1016/j.genhosppsych.2014.05.021
51. Ravens-Sieberer U, Gosch A, Rajmil L, et al. KIDSCREEN-52 quality-of-life measure for children and adolescents. Expert Rev Pharmacoecon Outcomes Res. 2005;5(3):353–364. doi:10.1586/14737167.5.3.353
52. Li J, Zhu Y, Zhu G, et al. Measuring health-related quality of life in a Chinese Mainland adolescent population: psychometric properties of the Mandarin Chinese self-reported KIDSCREEN-27 and KIDSCREEN-10 index. BMC Psychol. 2024;12(1):600. doi:10.1186/s40359-024-01876-6
53. Lam RW, Lamy FX, Danchenko N, et al. Psychometric validation of the Perceived Deficits Questionnaire-Depression (PDQ-D) instrument in US and UK respondents with major depressive disorder. Neuropsychiatr Dis Treat. 2018;14:2861–2877. doi:10.2147/NDT.S175188
54. Hou Y, Yao S, Hu S, et al. PSYCHOMETRIC properties of the Chinese version of the THINC-it tool for cognitive symptoms in patients with major depressive disorder. J Affect Disord. 2020;273:586–591. doi:10.1016/j.jad.2020.03.146
55. Harrison JE, Barry H, Baune BT, et al. Stability, reliability, and validity of the THINC-it screening tool for cognitive impairment in depression: a psychometric exploration in healthy volunteers. Int J Methods Psychiatr Res. 2018;27(3):e1736. doi:10.1002/mpr.1736
56. Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol. 2014;67(3):451–470. doi:10.1111/bmsp.12028
57. You Y, Ding H, Tang M, et al. Dose-response relationship between leisure-time physical activity and metabolic syndrome in short sleep US adults: evidence from a nationwide investigation. Appl Physiol Nutr Metab. 2025;50:1–10. doi:10.1139/apnm-2024-0347
58. You Y, Ablitip A, Chen Y, et al. Saturation effects of the relationship between physical exercise and systemic immune inflammation index in the short-sleep population: a cross-sectional study. BMC Public Health. 2024;24(1):1920. doi:10.1186/s12889-024-19432-7
59. Hirschfeld G, von Brachel R, Thiele C. Screening for health-related quality of life in children and adolescents: optimal cut points for the KIDSCREEN-10 for epidemiological studies. Qual Life Res. 2020;29(2):529–536. doi:10.1007/s11136-019-02324-4
60. Merdad RA, Akil H, Wali SO. Sleepiness in adolescents. Sleep Med Clin. 2017;12(3):415–428. doi:10.1016/j.jsmc.2017.03.014
61. Chung KF, Cheung MM. Sleep-wake patterns and sleep disturbance among Hong Kong Chinese adolescents. Sleep. 2008;31(2):185–194. doi:10.1093/sleep/31.2.185
62. Hysing M, Pallesen S, Stormark KM, Lundervold AJ, Sivertsen B. Sleep patterns and insomnia among adolescents: a population-based study. J Sleep Res. 2013;22(5):549–556. doi:10.1111/jsr.12055
63. Joo S, Shin C, Kim J, et al. Prevalence and correlates of excessive daytime sleepiness in high school students in Korea. Psychiatry Clin Neurosci. 2005;59(4):433–440. doi:10.1111/j.1440-1819.2005.01396.x
64. Owens J, Au R, Carskadon M. Adolescent sleep working g, committee on a. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics. 2014;134(3):e921–932. doi:10.1542/peds.2014-1696
65. Olds T, Blunden S, Petkov J, Forchino F. The relationships between sex, age, geography and time in bed in adolescents: a meta-analysis of data from 23 countries. Sleep Med Rev. 2010;14(6):371–378. doi:10.1016/j.smrv.2009.12.002
66. Meijer AM, Reitz E, Dekovic M. Parenting matters: a longitudinal study into parenting and adolescent sleep. J Sleep Res. 2016;25(5):556–564. doi:10.1111/jsr.12406
67. Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–161. doi:10.2147/NSS.S134864
68. Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002;73(2):405–417. doi:10.1111/1467-8624.00414
69. Shochat T, Cohen-Zion M, Tzischinsky O. Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep Med Rev. 2014;18(1):75–87. doi:10.1016/j.smrv.2013.03.005
70. Fernandez-Mendoza J, Vgontzas AN, Kritikou I, Calhoun SL, Liao D, Bixler EO. Natural history of excessive daytime sleepiness: role of obesity, weight loss, depression, and sleep propensity. Sleep. 2015;38(3):351–360. doi:10.5665/sleep.4488
71. Thomas JH, Burgers DE. Sleep is an eye-opener: behavioral causes and consequences of hypersomnolence in children. Paediatr Respir Rev. 2018;25:3–8. doi:10.1016/j.prrv.2016.11.004
72. Keage HA, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly?. Sleep Med. 2012;13(7):886–892. doi:10.1016/j.sleep.2012.02.003
73. Wong ML, Lau EY, Wan JH, Cheung SF, Hui CH, Mok DS. The interplay between sleep and mood in predicting academic functioning, physical health and psychological health: a longitudinal study. J Psychosom Res. 2013;74(4):271–277. doi:10.1016/j.jpsychores.2012.08.014
74. Yin J, Wang H, Li S, et al. Nonlinear relationship between sleep midpoint and depression symptoms: a cross-sectional study of US adults. BMC Psychiatry. 2023;23(1):671. doi:10.1186/s12888-023-05130-y
75. Asarnow LD. Depression and sleep: what has the treatment research revealed and could the HPA axis be a potential mechanism?. Curr Opin Psychol. 2020;34:112–116. doi:10.1016/j.copsyc.2019.12.002
76. Cardinali DP, Srinivasan V, Brzezinski A, Brown GM. Melatonin and its analogs in insomnia and depression. J Pineal Res. 2012;52(4):365–375. doi:10.1111/j.1600-079X.2011.00962.x
77. Walker MP, van der Helm E. Overnight therapy? The role of sleep in emotional brain processing. Psychol Bull. 2009;135(5):731–748. doi:10.1037/a0016570
78. Regestein Q, Natarajan V, Pavlova M, Kawasaki S, Gleason R, Koff E. Sleep debt and depression in female college students. Psychiatry Res. 2010;176(1):34–39. doi:10.1016/j.psychres.2008.11.006
79. Lessov-Schlaggar CN, Bliwise DL, Krasnow RE, Swan GE, Reed T. Genetic association of daytime sleepiness and depressive symptoms in elderly men. Sleep. 2008;31(8):1111–1117.
80. Walker MP, Harvey AG. Obligate symbiosis: sleep and affect. Sleep Med Rev. 2010;14(4):215–217. doi:10.1016/j.smrv.2010.02.003
81. National Institutes of H. National institutes of health state of the science conference statement on manifestations and management of chronic insomnia in adults. Sleep. 2005;28(9):1049–1057. doi:10.1093/sleep/28.9.1049
82. Fang H, Tu S, Sheng J, Shao A. Depression in sleep disturbance: a review on a bidirectional relationship, mechanisms and treatment. J Cell Mol Med. 2019;23(4):2324–2332. doi:10.1111/jcmm.14170
83. Thase ME. Depression and sleep: pathophysiology and treatment. Dialogues Clin Neurosci. 2006;8(2):217–226. doi:10.31887/DCNS.2006.8.2/mthase
84. Yang J, Zhang L, Hou Y, et al. Excessive daytime sleepiness in idiopathic blepharospasm. Parkinsonism Relat Disord. 2021;89:134–138. doi:10.1016/j.parkreldis.2021.07.005
85. Szabo ST, Thorpy MJ, Mayer G, Peever JH, Kilduff TS. Neurobiological and immunogenetic aspects of narcolepsy: implications for pharmacotherapy. Sleep Med Rev. 2019;43:23–36. doi:10.1016/j.smrv.2018.09.006
86. Dagyte G, Den Boer JA, Trentani A. The cholinergic system and depression. Behav Brain Res. 2011;221(2):574–582. doi:10.1016/j.bbr.2010.02.023
87. Pomara N, Bruno D, Plaska CR, et al. Evidence of upregulation of the cholinergic anti-inflammatory pathway in late-life depression. J Affect Disord. 2021;286:275–281. doi:10.1016/j.jad.2021.03.012
88. Malhi GS, Mann JJ. Depression. Lancet. 2018;392(10161):2299–2312. doi:10.1016/S0140-6736(18)31948-2
89. Hohls JK, Konig HH, Quirke E, Hajek A. Anxiety, depression and quality of life-a systematic review of evidence from longitudinal observational studies. Int J Environ Res Public Health. 2021;18(22):12022. doi:10.3390/ijerph182212022
90. Tang AL, Thomas SJ. Relationships between depressive symptoms, other psychological symptoms, and quality of life. Psychiatry Res. 2020;289:113049. doi:10.1016/j.psychres.2020.113049
91. Gold SM, Kohler-Forsberg O, Moss-Morris R, et al. Comorbid depression in medical diseases. Nat Rev Dis Primers. 2020;6(1):69. doi:10.1038/s41572-020-0200-2
92. Konig H, Konig HH, Konnopka A. The excess costs of depression: a systematic review and meta-analysis. Epidemiol Psychiatr Sci. 2019;29:e30.
93. Shorey S, Ng ED, Wong CHJ. Global prevalence of depression and elevated depressive symptoms among adolescents: a systematic review and meta-analysis. Br J Clin Psychol. 2022;61(2):287–305. doi:10.1111/bjc.12333
94. Nierenberg AA, Rapaport MH, Schettler PJ, et al. Deficits in psychological well-being and quality-of-life in minor depression: implications for DSM-V. CNS Neurosci Ther. 2010;16(4):208–216. doi:10.1111/j.1755-5949.2009.00108.x
95. Kim JY, Oh DJ, Yoon TY, Choi JM, Choe BK. The impacts of obesity on psychological well-being: a cross-sectional study about depressive mood and quality of life. J Prev Med Public Health. 2007;40(2):191–195. doi:10.3961/jpmph.2007.40.2.191
96. Micoulaud-Franchi JA, Bartolomei F, Duncan R, McGonigal A. Evaluating quality of life in epilepsy: the role of screening for adverse drug effects, depression, and anxiety. Epilepsy Behav. 2017;75:18–24. doi:10.1016/j.yebeh.2017.07.016
97. Chan SW, Chien WT, Thompson DR, Chiu HF, Lam L. Quality of life measures for depressed and non-depressed Chinese older people. Int J Geriatr Psychiatry. 2006;21(11):1086–1092. doi:10.1002/gps.1611
98. Shrira A. The effect of lifetime cumulative adversity on change and chronicity in depressive symptoms and quality of life in older adults. Int Psychogeriatr. 2012;24(12):1988–1997. doi:10.1017/S1041610212001123
99. Shmuely Y, Baumgarten M, Rovner B, Berlin J. Predictors of improvement in health-related quality of life among elderly patients with depression. Int Psychogeriatr. 2001;13(1):63–73. doi:10.1017/S1041610201007463
100. Enkvist A, Ekstrom H, Elmstahl S. What factors affect life satisfaction (LS) among the oldest-old? Arch Gerontol Geriatr. 2012;54(1):140–145. doi:10.1016/j.archger.2011.03.013
101. You Y, Wang D, Wang Y, Li Z, Ma X. A bird’s-eye view of exercise intervention in treating depression among teenagers in the last 20 years: a bibliometric study and visualization analysis. Frontiers in Psychiatry. 2021;12:661108. doi:10.3389/fpsyt.2021.661108
102. Merlino G, Piani A, Gigli GL, et al. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: a population-based study. Sleep Med. 2010;11(4):372–377. doi:10.1016/j.sleep.2009.07.018
103. Werli KS, Otuyama LJ, Bertolucci PH, et al. Neurocognitive function in patients with residual excessive sleepiness from obstructive sleep apnea: a prospective, controlled study. Sleep Med. 2016;26:6–11. doi:10.1016/j.sleep.2016.06.028
104. Cohen AD, Jia Y, Smagula S, et al. Cognitive functions predict trajectories of sleepiness over 10 years: a population-based study. J Gerontol a Biol Sci Med Sci. 2021;76(3):520–527. doi:10.1093/gerona/glaa120
105. Hayden KM, Anderson A, Spira AP, et al. Daytime sleepiness is associated with lower cognitive scores: the look AHEAD study. JAR Life. 2023;12:46–55. doi:10.14283/jarlife.2023.9
106. Roca M, Vives M, Lopez-Navarro E, Garcia-Campayo J, Gili M. Cognitive impairments and depression: a critical review. Actas Esp Psiquiatr. 2015;43(5):187–193.
107. Muhammad T, Meher T. Association of late-life depression with cognitive impairment: evidence from a cross-sectional study among older adults in India. BMC Geriatr. 2021;21(1):364. doi:10.1186/s12877-021-02314-7
108. Prevot T, Sibille E. Altered GABA-mediated information processing and cognitive dysfunctions in depression and other brain disorders. Mol Psychiatry. 2021;26(1):151–167. doi:10.1038/s41380-020-0727-3
109. Aajami Z, Kazazi L, Toroski M, Bahrami M, Borhaninejad V. Relationship between depression and cognitive impairment among elderly: a cross-sectional study. J Caring Sci. 2020;9(3):148–153. doi:10.34172/jcs.2020.022
110. Halahakoon DC, Lewis G, Roiser JP. Cognitive impairment and depression-cause, consequence, or coincidence? JAMA Psychiatry. 2019;76(3):239–240. doi:10.1001/jamapsychiatry.2018.3631
111. Cumming TB, Churilov L, Collier J, et al. Early mobilization and quality of life after stroke: findings from AVERT. Neurology. 2019;93(7):e717–e728. doi:10.1212/WNL.0000000000007937
112. Chang EJ, Woo HJ, Jeong KH. Mediating effect of cognitive function on the relationship between geriatric Oral health and quality of life among Korean seniors. J Prev Med Public Health. 2022;55(1):106–113. doi:10.3961/jpmph.21.536
113. Buttner M, Krogh D, Siggelkow H, Singer S. What are predictors of impaired quality of life in patients with hypoparathyroidism? Clin Endocrinol. 2022;97(3):268–275. doi:10.1111/cen.14701
114. Engleman HM, Douglas NJ. Sleep. 4: sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59(7):618–622. doi:10.1136/thx.2003.015867
115. Drake CL, Roehrs T, Roth T. Insomnia causes, consequences, and therapeutics: an overview. Depress Anxiety. 2003;18(4):163–176. doi:10.1002/da.10151
116. Lubans D, Richards J, Hillman C, et al. Physical activity for cognitive and mental health in youth: a systematic review of mechanisms. Pediatrics. 2016;138(3). doi:10.1542/peds.2016-1642
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.