Back to Journals » Journal of Pain Research » Volume 18
Association Between Educational Attainment and Chronic Pain: A Mediation Mendelian Randomization Study
Authors Wang H, Liu M, Li H , Xu S
Received 6 January 2025
Accepted for publication 19 March 2025
Published 3 April 2025 Volume 2025:18 Pages 1793—1804
DOI https://doi.org/10.2147/JPR.S515921
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jonathan Greenberg
Hanqi Wang, Mingjuan Liu, Hongbo Li, Shijie Xu
Department of Anesthesiology and Pain Research Center, The First Hospital of Jiaxing University, Jiaxing, People’s Republic of China
Correspondence: Hanqi Wang, Department of Anesthesiology and Pain Research Center, The First Hospital of Jiaxing University, Jiaxing, People’s Republic of China, Tel +86 19517900056, Email [email protected]
Background: The underlying association between educational attainment (EA) and chronic pain (CP) risk is not clear. This study aimed to investigate the causal relationship of EA with CP using Mendelian randomization (MR).
Methods: Single nucleotide polymorphisms (SNPs) for EA were selected from the Social Science Genetic Association Consortium (SSGAC). Inverse-variance weighted (IVW), weighted median, penalized weighted median, maximum likelihood (ML), and MR-Egger methods were used to estimate causal effects. Two sample MR analyses were undertaken to assess whether EA has a causal effect on CP. We also performed mediation analyses to estimate the mediation effects.
Results: A genetically predicted higher EA was associated with a decreased risk of multisite chronic pain (MCP) (odds ratio [OR] = 0.772, 95% confidence interval [CI] 0.732– 0.816 per one standard deviation of longer education, P < 0.05), and the Genome-wide association studies (GWAS) data for chronic widespread pain (CWP) supported the result mentioned above. Potential mediators included body mass index (BMI) (OR = 1.176, 95% CI 1.091– 1.267, P < 0.05), smoking (OR = 1.054, 95% CI 1.028– 1.081, P < 0.05), and depression (OR = 1.201, 95% CI 1.147– 1.258, P < 0.05) have all been proven to be causally associated with MCP. The proportions of the effects of genetically predicted EA mediated through genetically predicted BMI, smoking, and depression were 17.1%, 23.6%, and 9.2%, respectively.
Conclusion: Genetically predicted higher educational attainment reduces multisite chronic pain risk, partially mediated by body mass index (17.1%), smoking (23.6%), and depression (9.2%), highlighting education’s protective role and its potential in chronic pain prevention strategies.
Keywords: educational attainment, chronic pain, Mendelian randomization, risk factors
Graphical Abstract:

Introduction
Chronic pain (CP) is commonly defined as pain that lasts >3 months.1 According to data from the National Health Interview Survey in 2016, about 20.4% of American adults reported having CP,2 a rate that is comparable to those of European ancestry.3 Chronic pain not only poses a major challenge to patients’ physical health but also seriously affects their mood, cognitive function, and quality of life.4,5 According to evidence from the Medical Expenditure Panel Survey (MEPS), the estimated national cost of CP ranged from $560 to $635 billion,6 which was still increasing. Therefore, chronic pain not only causes great distress to patients but also has a profound impact on the social health system and economic system, becoming an important public health issue worldwide. Currently, the treatment of chronic pain primarily employs a multimodal, integrated management strategy, including pharmacological treatments (such as nonsteroidal anti-inflammatory drugs, opioids, and adjunctive analgesics),7,8 physical therapy (such as exercise rehabilitation and neuromodulation techniques),9,10 psychological interventions (such as cognitive behavioral therapy),11,12 and interventional treatments (such as nerve blocks and spinal cord stimulation).13,14 However, existing treatments often face challenges such as significant individual variability in response, limited long-term effectiveness, and the risk of drug dependence.15,16 Against this backdrop, early prevention targeting high-risk populations has become an important focus in clinical research.17,18
Whereas the causal effects of some risk factors (obesity, psychological factors and unhealthy lifestyle, etc) are generally accepted and reflected in disease prevention strategies, substantial uncertainty still surrounds other potential factors. Many studies have indicated the relationship between educational attainment (EA) and chronic pain19,20 in the past few decades. Current evidence pointed out that individuals with lower levels of education are more likely to experience chronic pain compared to those with higher educational attainment.21 However, this association may not be due to a causal effect, but rather could be a result of the methodological constraints inherent in traditional observational studies. Meanwhile, increasing evidence suggests that various adverse health outcomes, including obesity, smoking, and depression, are co-morbid with chronic pain.22–26 These factors are believed to potentially play a role in the relationship between educational attainment and chronic pain. Therefore, clarifying the causal relationship between education level and chronic pain, while exploring potential mediating factors, is crucial for advancing our understanding of the underlying causes of chronic pain and for the development of novel population-based strategies for its prevention.
Although randomized controlled trials (RCTs) are widely regarded as the “gold standard” for assessing causal relationships, their high costs, long research timelines, and the need for multidisciplinary collaboration often make their implementation challenging. Therefore, there is an urgent need for a new research method that can avoid the limitations of traditional observational studies and RCTs while providing more reliable evidence for causal inference.
Mendelian randomization (MR) is a method that is used to evaluate the causal relationship between exposures and outcomes as an adjuvant to traditional epidemiological methods. Genetic variants were randomly allocated from parents to offspring at conception, minimizing confounding and ruling out reverse causality.27 This method enables researchers to more accurately evaluate the causal relationship between exposures and outcomes. Particularly in situations where random assignment is not possible, MR offers a more reliable alternative. MR uses genetic variants to conduct causal inference, and the role of genetic variants in chronic pain susceptibility has been confirmed in multiple studies. For instance, the allele at the rs734784 locus in the KCNS1 gene has been associated with increased risk of various chronic pain conditions such as neuropathic pain and osteoarthritis pain, potentially involving abnormal potassium channel function leading to central nervous system sensitization.28 Similarly, the allele at the rs1042713 locus in the ADRB2 gene is linked to elevated risk of chronic widespread pain (CWP), possibly through influencing stress response and neuroendocrine regulation, thereby promoting chronic pain.29
This study used univariate MR analysis to determine whether exposure and outcome were causally associated. In addition, MR mediation analyses were performed to explore whether the phenotypes of risk factors could mediate the effect of education on the probability of developing chronic pain, so as to explore the early identification of mediating factors and provide clinicians with new evidence for personalized chronic pain intervention. At the same time, it aims to improve people’s awareness and provide support for health policy formulation, especially health promotion and intervention strategies for low-education groups. We hypothesize that there is a causal relationship between educational attainment and chronic pain, and risk factors mediate the association between them.
Methods
Because this MR study was performed based on publicly available Genome-wide association studies (GWAS) summary statistics, the Research Ethics Committee of the First Hospital of Jiaxing University deemed this work exempt from the Ethics Committee review. Considering that participant consent has been obtained by previously published studies included in this project, the Research Ethics Committee agreed to exempt the written informed consent. Data analyses started in February 2023 and was completed in May 2023.
Study Design
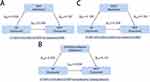
First, we performed a univariable MR analysis to determine whether there is a causal relationship between EA and CP. Based on the results of previous observational studies, we selected three possible risk factors to investigate the underlying mechanisms between EA and CP.22,23,25 The following phase involved a two-step MR analysis for those risk factors that may contribute to CP (Figure 1B). Specifically, we used univariable MR to describe the potential association between education and the factors we highlighted and to infer the causal relationship between three phenotypes of risk factors for CP. Two significant GWAS summary statistics were matched with each phenotype considering the repeatability of the results to confirm the robustness of the causal inference. Finally, mediation analyses determined how each risk factor mediated the relationship between EA and CP. Figure 1C shows the schematic diagram.
Data Sources of EA and CP
Okbay et al28 have conducted a large meta-analysis of GWAS for individuals of European descent over the age of 30. Educational attainment was measured in the analysis as the number of years of schooling completed (n = 293,723, mean = 14.3, sd. = 3.6). The genetic instruments of multisite chronic pain (MCP) were obtained from GWAS summary statistics performed in UK Biobank with a total of 387,649 individuals of European ancestry stratified by gender.29 MCP was regarded as a quasi-quantitative variable, and phenotypic values ranged from 0 to 7 due to the numbers of body sites. Age, sex, and genotyping array were adjusted by the original investigators. The GWAS summary statistics of CWP derived from UK biobank, when self-reported of pain all over the body lasting for more than 3 months; simultaneous pain that lasting for 3 months or more in the knee, shoulder, hip, and back, accompanied by fibromyalgia, can be defined as CWP.30 Table 1 lists characteristics of GWAS summary statistics included in this study.
 |
Table 1 Characteristics of GWAS Summary Statistics |
Selection of Genetic Variants
Genetic variants were retrieved using rigorous steps for GWAS summary statistics of EA to eliminate the bias and instability caused by weak instrumental bias. These fundamental assumptions must be met when using single nucleotide polymorphisms (SNPs) as instrumental variables (IVs) in MR analysis31 (Figure 1A): (1) IVs are strongly associated with the selected exposures; (2) IVs are associated with the outcome only through the exposure; and (3) IVs are not associated with confounders affecting the outcome. SNPs associated with EA and any other significant risk factor across the genome (P < 5e-08) were filtered out. A strict measure of clumping with a threshold of r2 < 0.001 and window size = 10,000 kb was also conducted to reduce the impact of linkage disequilibrium (LD). To investigate whether other confounders influenced genetic variants, we examined all the selected IVs in Phenoscanner.
MR Analysis
Prior to the performance of MR analyses, genetic variations from GWAS summary statistics for chronic pain were thought to be harmonized so that the effect allele of each SNP was consistent throughout the datasets mentioned above.
Inverse-variance weighted (IVW) is the primary method for estimating the causal relationship between exposure and outcome in two-sample MR analysis. The IVW method was essentially a meta-analysis technique that combined the effect of each SNP to obtain causal estimates.32 The odds ratios (ORs) and 95% confidence intervals (95% CIs) were computed using the estimates. However, to establish the heterogeneity of genetic variations based on the findings of the hypothesis test, we employed Cochran’s Q test to produce Q statistics. Subsequently, we then conducted several sensitivity analyses, including weighted median, MR-Egger, maximum likelihood (ML), and penalized weighted median to assess the robustness of the MR analysis results. In addition to IVW, we used a weighted median technique, which provided a steady estimate even when up to 50% of the genetic variations were ineffective IVs.33 Furthermore, the penalized weighted median method was less biased when a few genetic variants were invalid. A rough estimate of unbiased causal estimates can be obtained using MR-Egger regression. It was fairly credible as a tool to examine the robustness of the results of MR analyses when the instrument strength independent of the direct effect (InSIDE) assumption was satisfied.34 Another method that can estimate the parameters of a probability distribution by maximizing the likelihood function is ML.35 After excluding outliers with horizontal pleiotropy characteristics using MR-PRESSO analysis,36 we would reevaluate the causal effects in univariable analyses.
Additionally, we performed a leave-one-out analysis to determine whether the MR results were caused by SNP. Then, we eliminated each SNP one at a time to reevaluate the causal estimates. Complementary analyses, including scatter and forest plots, were used to visualize the heterogeneity in the causal effects of various genetic instruments.
Potential Risk Factors
Potential risk factors may affect the underlying mechanisms of EA on CP. Based on the results of previous observational studies, we further examined three potential risk factors for obesity (eg, BMI), lifestyle factors (eg, smoking), and mood disorders (eg, depression). Instrument variables for BMI were derived from the largest meta-analysis of GWASs, which included the summary statistics from the Genetic Investigation of ANthropometric Traits (GIANT) consortium and the dataset conducted in UK Biobank participants of European ancestry, reaching 681,275 individuals.37 The relationship findings for up to 339,224 individuals from 125 studies38 included in another meta-analysis for BMI were made public by the GIANT consortium. Liu et al39 reported the GWAS summary statistics on several smoking-related phenotypes in 1.2 million participants. Smoking initiation was a binary phenotype. Any participant reporting ever being a regular smoker in their life (current or former) were coded “2”, while any participant who reported never being a regular smoker in their life were coded “1”. A debilitating psychiatric illness known as major depressive disorder (MDD) is typically associated with low mood and anhedonia. Howard et al40 meta-analyzed data on 807,553 participants from the three largest depression-related GWAS. This GWAS dataset was adjusted for age, sex, genotyping array, and ancestry factors. A meta-analysis by Wray et al41 released by the psychiatric genomics consortium (PGC) provided further GWAS summary statistics for MDD.
Mediation Effects
We combined the total effect from the univariable MR analysis of education on CP with the causal estimates from the two-step MR to estimate the indirect effect of EA on CP via different risk factors.
As shown in Figure 1B, we assumed there was a relationship between EA, CP, and underlying risk factors. A univariate MR analysis indicated that EA had a causal association on CP, estimated to be β1, which was also considered the overall effect of exposure on the outcome. In contrast, the causal estimates of education on risk factors and those factors on CP were β2 and β3, respectively. Therefore, the product of β2 and β3 can be used to calculate the indirect effects of risk factors. The indirect effects were calculated using the coefficient product method, and the standard error (SE) and CI for the mediation effect were calculated using the Delta method.42
Statistical Analysis
R software (Version 4.0.5, RStudio Inc., USA) was used to conduct the statistical analysis. The R packages “TwoSampleMR” (version 0.5.6) and “MRPRESSO” (Version 1.0), which were loaded to conduct all MR analyses, were used for identifying the outliers. P < 0.05 was considered statistically significant. F statistics were calculated using the formula: F = β2/SE2 43,44 to evaluate the strength of IVs. The F value <10 may indicate that IVs were weak instruments.
Results
Extraction of Instrumental Variables
Summary information for SNPs is shown in Supplementary Table S1 following a rigorous genetic variants screening. The GWAS dataset was filtered for EA, leaving 75 independently related SNPs. A total of 489 SNPs were strongly associated with BMI,37 and 74 independent SNPs were found in another summary statistics for the repeatability test38 regarding risk factors. The smoking-related GWAS datasets extracted 21 and 80 SNPs for “Cigarettes smoked per day” and “smoking initiation.” Genetic variants associated with MDD were selected from two summary statistics published in 2019 and 2018 with 49 and 5 SNPs, respectively. We extracted the IVs using a more logical framework because the existence of proxies may cause deviation in causal inference. Additionally, Supplementary Table S2–S3 demonstrate the data extraction process and the data harmonization results. The F statistics of these genetic variants ranged from 32.439 to 74.600 (Supplementary Table S5), demonstrating the little possibility of weak instrument bias.
Causal Estimates of EA on CP
Univariable MR analysis revealed that individuals with lower EA had a higher risk of developing multisite MCP (OR=0.772, 95% CI 0.732 to 0.816, P=1.502E-20) and CWP (OR=0.979, 95% CI 0.968 to 0.989, P=8.929E-05). Four sensitivity analysis methods (Supplementary Table S5 and S9) were evaluated with similar results, including MR-Egger, weighted median, maximum likelihood, and penalized weighted median. The Cochran’s Q statistic allowed for the detection of heterogeneity in each univariable MR study, while the MR-Egger intercept provided no conclusive proof of the existence of horizontal pleiotropy. Significant outliers were identified using the MR-PRESSO method. We excluded IVs considered to have the trait of horizontal pleiotropy before investigating the causal effects of EA on CP (EA on MCP: rs111321694, rs11222416, rs12036042, rs4565697; EA on CWP: rs773107). Supplementary Table S4–S7 contains more detailed information. Supplementary Figure S1 displayed the results of visualization for the causal estimates of the genetically predicted EA on CP.
Causal Estimates of EA on Risk Factors
We also discovered evidence that a lower risk of BMI (BMI 2018: beta=−0.224, 95% CI −0.350 to −0.099, P=4.530E-04; BMI 2015: beta=−0.202, 95% CI −0.400 to −0.005, P=4.490E-02), smoking (Cigarettes smoked per day: beta=−0.357, 95% CI −0.496 to −0.219, P=4.391E-07; Smoking initiation: OR 0.669, 95% CI 0.592 to 0.755, P=8.899E-11), and depression (MDD 2019: OR 0.879, 95% CI 0.787 to 0.980, P=2.060E-02; MDD 2018: OR 0.814, 95% CI 0.666 to 0.994, P=4.400E-02) was genetically associated with EA. Supplementary Table S4-S7 and Supplementary Figure S2–S4 showed the results of sensitive analyses and visualization for EA on risk factors.
Causal Estimates of Risk Factors on CP
We calculated the causal effects of three potential risk factors for CP using univariable MR analyses. The findings indicated that a higher genetically predicted BMI (MCP: OR 1.217, 95% CI 1.189 to 1.246, P=5.003E-61; CWP: OR 1.023, 95% CI 1.019 to 1.027, P=1.286E-35), “smoking initiation” (MCP: OR 1.164, 95% CI 1.122 to 1.207, P=3.009E-16; CWP: OR 1.016, 95% CI 1.009 to 1.023, P=4.491E-06), and MDD (MCP: OR 1.201, 95% CI 1.147 to 1.258, P=1.071E-14; CWP: OR 1.021, 95% CI 1.013 to 1.029, P=2.742E-07) was associated with a higher risk of CP. The outcomings for other methods, such as weighted median (BMI on MCP: OR 1.182, 95% CI 1.149 to 1.216, P=3.425E-31; Smoking initiation on MCP: OR 1.167, 95% CI 1.120 to 1.215, P=2.069E-13; MDD on MCP: OR 1.139, 95% CI 1.084 to 1.196, P=2.376E-07) and MR-Egger (BMI on MCP: OR 1.176, 95% CI 1.091 to 1.267, P=2.589E-05; Smoking initiation on MCP: OR 1.198, 95% CI 1.000 to 1.436, P=5.371E-02; MDD on MCP: OR 1.482, 95% CI 1.155 to 1.901, P=3.589E-03), were qualitatively consistent with the causal relationship by IVW analysis. Table 2 and Supplementary Table S5 also contain lists of the outcomes of other MR methods. Additionally, we performed the two-sample MR analyses again using at least one independent summary statistic and obtained robust and reliable outcomes. According to Egger, intercept analysis failed to detect any pleiotropy, indicating that no horizontal pleiotropy was found in the causal estimates. We discovered no outliers in the univariable MR analysis of smoking initiation and MDD on CWP. Additionally, the limited number of SNPs included in the MR analysis prevented us from performing the identification process of outliers in CWP based on GWAS summary statistics of the validation dataset for MDD. No single SNP was found to be the key driver of the overall results in the primary MR analysis, according to leave-one-out analyses (Supplementary Figure S5-S10).
 |
Table 2 Causal Effects of Risk Factors on Chronic Pain in Univariable MR Analyses |
Mediation Effects
We selected the GWAS summary statistics with the largest sample sizes for the underlying risk factors and CP to estimate the mediation effects. Mediation analyses quantified the effects of educational attainment on chronic pain outcomes via three risk factors. As shown in Figure 2 and Supplementary Table S8, the mediation effects of EA on MCP mediated by BMI, smoking initiation, and MDD were −0.044 (−0.073 to −0.016), −0.061 (−0.110 to −0.012), and −0.024 (−0.040 to −0.007), respectively. The percentage of mediation effects of EA via BMI, smoking initiation, and MDD were approximately 17.104%, 23.586%, and 9.150%, respectively.
Discussion
The innovation of this study lies in its pioneering use of the Mendelian randomization framework, which leverages genetic variations as instrumental variables to effectively mitigate confounding factors such as demographics, environmental influences, and other common confounders. This approach provides more reliable causal inferences. Furthermore, we employed multiple latest GWAS summary statistics to validate the stability of our results, further substantiating the causal impact of education attainment on chronic pain and the mediating effects of related risk factors. The results showed that low education level was significantly causally associated with the occurrence of chronic pain (EA on MCP: OR=0.772, 95% CI 0.732 to 0.816, P=1.502E-20; EA on CWP: OR=0.979, 95% CI 0.968 to 0.989, P=8.929E-05). The mediating percentages of BMI, “smoking initiation”, and MDD were approximately 17.104%, 23.586%, and 9.150%, respectively.
This finding not only aligns with the associations reported in previous studies but also further suggests a direct causal relationship. Several prior observational studies have reported similar results. Smith et al45 conducted a study in the Grampian region of Scotland to investigate the effects of CP on general health. After adjusting relevant variables, they discovered that individuals with secondary school certificates had a 0.5–1.2-fold higher risk of CP than those with higher education. In addition, the risk of CP increased by 1.0–1.6-fold in those with no qualifications. Macky et al46 also found that low education level was an independent risk factor for CP among 262 subjects.
There are several potential mechanisms that may explain why lower educational attainment increases susceptibility to chronic pain. Firstly, individuals with lower education levels typically have limited health literacy and lack resources for effective pain prevention and management. They may have insufficient knowledge of strategies to avoid or treat chronic pain, or engage in physically demanding or high-risk occupations, thereby increasing their risk of pain-related disorders. Additionally, individuals with lower educational attainment are more prone to pain catastrophizing—a cognitive tendency to perceive pain in an excessively negative way, which intensifies the sensation of pain.47 After controlling for factors such as depression, the association between lower education and pain significantly weakened, suggesting that emotional and cognitive factors (eg, depression) partially mediate the effect of education level on pain.47 In line with this, our Mendelian randomization analysis indicates that approximately 9% of the education–pain relationship can be explained by MDD. Depression not only reduces an individual’s pain tolerance but also impairs their ability to cope with pain, thereby exacerbating chronic pain symptoms.48,49
The lifestyle factors associated with education level also represent crucial pathways linking education to chronic pain. Lower educational attainment is often closely linked to unhealthy behaviors such as obesity, sedentary lifestyle, smoking, and alcohol consumption, all of which have been established as risk factors for chronic pain.50–52 Our study findings support this explanation; specifically, we found that approximately 17% of the total effect of low education on multisite chronic pain is mediated through higher BMI, while about 24% of the effect is mediated by an increased tendency to smoke. These findings indicate that lifestyle differences significantly contribute to the increased burden of chronic pain among lower educated groups. Obese individuals are more likely to experience a pro-inflammatory state characterized by a higher prevalence of CP.53,54 Briggs et al55 originally reported that low back pain (LBP) risk may be increased by high levels of C-reactive protein, particularly in obese individuals. Chronic inflammation may increase the sensitivity of neural pathways, thereby leading to persistent pain. Regardless of the specific mechanisms involved, our findings suggest that weight control could be an effective strategy for reducing the risk of chronic pain, particularly in populations with high obesity rates and low educational attainment.11
Interestingly, nicotine has an analgesic effect in experimental studies,56 but it appears paradoxical since epidemiological research found smoking to be a high-risk factor for CP. The results of our MR analysis confirmed the findings of observational studies that individuals who regularly smoked had a higher risk of developing CP than individuals who had never smoked (OR: 1.016–1.164). Still, whether individuals who underwent smoking cessation could benefit from experiencing CP is unclear. Shi et al24 have noted that although it has not been shown, recovering from the effects of long-term exposure to nicotine may alleviate CP. Another meta-analysis57 showed that there is currently insufficient evidence to conclude that smoking cessation has any clinically significant effects on individuals with CP. Nonetheless, given the many overall health benefits of smoking cessation and the established association between smoking and pain, it is recommended that smoking cessation measures be actively promoted among patients with chronic pain. In the long term, quitting smoking may contribute to improved overall health and potentially prevent the exacerbation of pain or the development of related pain disorders.24
This study has some limitations. First, we had to account for memory bias because the estimates of genetic associations such as CP and smoking were collected through self-reports or questionnaires. Second, the MR analysis was performed using data from individuals with European ancestry. We should be cautious with interpreting and generalizing other ethnicities because different races have distinct lifestyles and cultural backgrounds. Third, the non-collapsibility of OR58 may cause biased estimates for the proportions of mediation effects.
Conclusion
This Mendelian randomization analysis indicates a causal association between lower educational attainment and a higher risk of chronic pain, with BMI, smoking, and depression serving as mediators of the effect of education attainment on chronic pain. These findings underscore the need to focus on lifestyle and mental health in the prevention and management of chronic pain.
Abbreviations
EA, Educational attainment; CP, Chronic pain; MR, Mendelian randomization; SNPs, Single nucleotide polymorphisms; SSGAC, Social Science Genetic Association Consortium; IVW, Inverse-variance weighted; ML, Maximum likelihood; MCP, Multisite chronic pain; OR, Odds ratio; CI, Confidence interval; GWAS, Genome-wide association studies; CWP, Chronic widespread pain; BMI, Body mass index; MEPS, Medical Expenditure Panel Survey; RCTs, Randomized controlled trials; CWP, Chronic widespread pain; IVs, Instrumental variables; LD, Linkage disequilibrium; GIANT, Genetic Investigation of ANthropometric Traits; MDD, Major depressive disorder; PGC, Psychiatric genomics consortium; SE, Standard error; LBP, Low back pain.
Data Statement
Publicly available datasets were analyzed in this study. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to the SSGAC, UK Biobank, GIANT, GSCAN and PGC for making this research possible.
Funding
There is no funding to declare.
Disclosure
The authors declare no conflicts of interest.
References
1. Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. 2022;163(2):e328–e332. doi:10.1097/j.pain.0000000000002291
2. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults — United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–1006. doi:10.15585/mmwr.mm6736a2
3. Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur. J. Pain. 2006;10(4):287. doi:10.1016/j.ejpain.2005.06.009
4. Malfliet A, Coppieters I, Van Wilgen P, et al. Brain changes associated with cognitive and emotional factors in chronic pain: a systematic review. Eur. J. Pain. 2017;21(5):769–786. doi:10.1002/ejp.1003
5. Taylor AM, Phillips K, Patel KV, et al. Assessment of physical function and participation in chronic pain clinical trials: IMMPACT/OMERACT recommendations. Pain. 2016;157(9):1836–1850. doi:10.1097/j.pain.0000000000000577
6. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. doi:10.1016/j.jpain.2012.03.009
7. Sandbrink F, Murphy JL, Johansson M, et al. The use of opioids in the management of chronic pain: synopsis of the 2022 updated U.S. department of veterans affairs and U.S. department of defense clinical practice guideline. Ann Intern Med. 2023;176(3):388–397. doi:10.7326/M22-2917
8. Busse JW, Casassus R, Carrasco-Labra A, et al. Management of chronic pain associated with temporomandibular disorders: a clinical practice guideline. BMJ. 2023;383:e076227. doi:10.1136/bmj-2023-076227
9. McCrae CS, Williams J, Roditi D, et al. Cognitive behavioral treatments for insomnia and pain in adults with comorbid chronic insomnia and fibromyalgia: clinical outcomes from the SPIN randomized controlled trial. Sleep. 2019;42(3):zsy234. doi:10.1093/sleep/zsy234
10. Mg K, Y K, Cy Y, et al. Low-intensity transcranial focused ultrasound suppresses pain by modulating pain-processing brain circuits. Blood. 2024;144. doi:10.1182/blood.2023023718
11. Garland EL, Nakamura Y, Bryan CJ, et al. Mindfulness-oriented recovery enhancement for veterans and military personnel on long-term opioid therapy for chronic pain: a randomized clinical trial. Am J Psychiatry. 2024;181(2):125–134. doi:10.1176/appi.ajp.20230272
12. Burgess DJ, Calvert C, Hagel Campbell EM, et al. Telehealth mindfulness-based interventions for chronic pain: the LAMP randomized clinical trial. JAMA Intern Med. 2024;184(10):1163–1173. doi:10.1001/jamainternmed.2024.3940
13. Pers Y-M, Soler-Rich R, Vadalà G, et al. Allogenic bone marrow-derived mesenchymal stromal cell-based therapy for patients with chronic low back pain: a prospective, multicentre, randomised placebo controlled trial (RESPINE study). Ann Rheum Dis. 2024;83(11):1572–1583. doi:10.1136/ard-2024-225771
14. Busse JW, Genevay S, Agarwal A, et al. Commonly used interventional procedures for non-cancer chronic spine pain: a clinical practice guideline. BMJ. 2025;388:e079970. doi:10.1136/bmj-2024-079970
15. Wang Y, Aaron R, Attal N, et al. An update on non-pharmacological interventions for pain relief. Cell Rep Med. 2025;6(2):101940. doi:10.1016/j.xcrm.2025.101940
16. Martucci KT. Neuroimaging of opioid effects in humans across conditions of acute administration, chronic pain therapy, and opioid use disorder. Trends Neurosci. 2024;47(6):418–431. doi:10.1016/j.tins.2024.04.005
17. Finneran JJ, Ilfeld BM. Role of peripheral nerve stimulation and percutaneous cryoneurolysis in preventing chronic postsurgical pain. Reg Anesth Pain Med. 2025;50(2):168–174. doi:10.1136/rapm-2024-105605
18. Elsharkawy H, Clark JD, El-Boghdadly K. Evidence for regional anesthesia in preventing chronic postsurgical pain. Reg Anesth Pain Med. 2025;50(2):153–159. doi:10.1136/rapm-2024-105611
19. Elzahaf RA, Tashani OA, Unsworth BA, et al. The prevalence of chronic pain with an analysis of countries with a Human Development Index less than 0.9: a systematic review without meta-analysis. Curr. Med. Res. Opin. 2012;28(7):1221–1229. doi:10.1185/03007995.2012.703132
20. Allen SA, Dal Grande E, Abernethy AP, et al. Two colliding epidemics – obesity is independently associated with chronic pain interfering with activities of daily living in adults 18 years and over; a cross-sectional, population-based study. BMC Public Health. 2016;16(1):1034. doi:10.1186/s12889-016-3696-3
21. Zajacova A, Rogers RG, Grodsky E, et al. The relationship between education and pain among adults aged 30–49 in the United States. J Pain. 2020;21(11–12):1270–1280. doi:10.1016/j.jpain.2020.03.005
22. Holmes A, Christelis N, Arnold C. Depression and chronic pain. Med J Aust. 2012;1(4):17–20. doi:10.5694/mjao12.10589
23. Ditre JW, Brandon TH, Zale EL, et al. Pain, nicotine, and smoking: research findings and mechanistic considerations. Psychol Bull. 2011;137(6):1065–1093. doi:10.1037/a0025544
24. Shi Y, Weingarten TN, Mantilla CB, et al. Smoking and Pain: pathophysiology and Clinical Implications. Anesthesiology. 2010;113(4):977–992. doi:10.1097/ALN.0b013e3181ebdaf9
25. Narouze S, Souzdalnitski D. Obesity and chronic pain: systematic review of prevalence and implications for pain practice. Reg Anesth Pain Med. 2015;40(2):91–111. doi:10.1097/AAP.0000000000000218
26. Bonilla-Jaime H, Sánchez-Salcedo JA, Estevez-Cabrera MM, et al. Depression and Pain: use of Antidepressants. CN. 2022;20(2):384–402. doi:10.2174/1570159X19666210609161447
27. Freuer D, Linseisen J, Meisinger C. Impact of body composition on COVID-19 susceptibility and severity: a two-sample multivariable Mendelian randomization study. Metabolism. 2021;118:154732. doi:10.1016/j.metabol.2021.154732
28. Okbay A, Beauchamp JP, Fontana MA, et al. Genome-wide association study identifies 74 loci associated with educational attainment. Nature. 2016;533(7604):539–542. doi:10.1038/nature17671
29. Johnston KJA, Ward J, Ray PR, et al. Sex-stratified genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet. 2021;17(4):e1009428. doi:10.1371/journal.pgen.1009428
30. Rahman MS, Winsvold BS, Chavez Chavez SO, et al. Genome-wide association study identifies RNF123 locus as associated with chronic widespread musculoskeletal pain. Ann Rheumatic Dis. 2021;80(9):1227–1235. doi:10.1136/annrheumdis-2020-219624
31. Boef AGC, Dekkers OM, Le Cessie S. Mendelian randomization studies: a review of the approaches used and the quality of reporting. Int J Epidemiol. 2015;44(2):496–511. doi:10.1093/ije/dyv071
32. Luo G, Yao Y, Tao J, et al. Causal association of sleep disturbances and low back pain: a bidirectional two-sample Mendelian randomization study. Front Neurosci. 2022;16:1074605. doi:10.3389/fnins.2022.1074605
33. Tang B, Meng W, Hägg S, et al. Reciprocal interaction between depression and pain: results from a comprehensive bidirectional Mendelian randomization study and functional annotation analysis. Pain. 2022;163(1):e40–e48. doi:10.1097/j.pain.0000000000002305
34. Bowden J, Davey Smith G, Haycock PC, et al. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genetic Epidemiol. 2016;40(4):304–314. doi:10.1002/gepi.21965
35. Xu J, Zhang S, Tian Y, et al. Genetic causal association between iron status and osteoarthritis: a two-sample Mendelian randomization. Nutrients. 2022;14(18):3683. doi:10.3390/nu14183683
36. Verbanck M, Chen C-Y, Neale B, et al. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi:10.1038/s41588-018-0099-7
37. Yengo L, Sidorenko J, Kemper KE, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Human Molecular Genetics. 2018;27(20):3641–3649. doi:10.1093/hmg/ddy271
38. Locke AE, Kahali B, Berndt SI, The LifeLines Cohort Study, The ADIPOGen Consortium, The AGEN-BMI Working Group. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. doi:10.1038/nature14177
39. Liu M, Jiang Y, Wedow R, 23andMe Research Team, HUNT All-In Psychiatry. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51(2):237–244. doi:10.1038/s41588-018-0307-5
40. Howard DM, Adams MJ, Clarke T-K, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. 2019. DOI: 10.1038/s41593-018-0326-7.
41. Wray NR, Ripke S, Mattheisen M; eQTLGen. 23andMe, the Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–681. doi:10.1038/s41588-018-0090-3
42. Chen L, Peters JE, Prins B, et al. Systematic Mendelian randomization using the human plasma proteome to discover potential therapeutic targets for stroke. Nat Commun. 2022;13(1):6143. doi:10.1038/s41467-022-33675-1
43. Bowden J, Del Greco MF, Minelli C, et al. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol;2016. dyw220. doi:10.1093/ije/dyw220
44. Niu -P-P, Wang X, Xu Y-M. Higher circulating vitamin D levels are associated with decreased migraine risk: a Mendelian randomization study. Front Nutr. 2022;9:907789. doi:10.3389/fnut.2022.907789
45. Smith BH, Elliott AM, Chambers WA, et al. The impact of chronic pain in the community. Family Pract. 2001;18(3):292–299. doi:10.1093/fampra/18.3.292
46. Mackey LM, Blake C, Squiers L, et al. An investigation of healthcare utilization and its association with levels of health literacy in individuals with chronic pain. Musculoskeletal Care. 2019;17(2):174–182. doi:10.1002/msc.1386
47. Edwards RR, Goble L, Kwan A, et al. Catastrophizing, pain, and social adjustment in scleroderma: relationships with educational level. Clin J Pain. 2006;22(7):639–646. doi:10.1097/01.ajp.0000210918.26159.94
48. Meeus M, Nijs J. Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin Rheumatol. 2007;26(4):465–473. doi:10.1007/s10067-006-0433-9
49. Sariyildiz A, Coskun Benlidayi I, Turk I, et al. Biopsychosocial factors should be considered when evaluating central sensitization in axial spondyloarthritis. Rheumatol Int. 2023;43(5):923–932. doi:10.1007/s00296-023-05317-2
50. Urbán R, Kugler G, Oláh A, et al. Smoking and education: do psychosocial variables explain the relationship between education and smoking behavior? Nicotine & Tobacco Res. 2006;8(4):565–573. doi:10.1080/14622200600789940
51. Sulander TT, Uutela AK. Obesity and education: recent trends and disparities among 65- to 84-year-old men and women in Finland. Preventive Med. 2007;45(2–3):153–156. doi:10.1016/j.ypmed.2007.02.008
52. Tomioka K, Kurumatani N, Saeki K. The association between education and smoking prevalence, independent of occupation: a nationally representative survey in Japan. J Epidemiol. 2020;30(3):136–142. doi:10.2188/jea.JE20180195
53. Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. doi:10.1038/nri2921
54. Arranz L-I, Rafecas M, Alegre C. Effects of Obesity on Function and Quality of Life in Chronic Pain Conditions. Curr Rheumatol Rep. 2014;16(1):390. doi:10.1007/s11926-013-0390-7
55. Briggs MS, Givens DL, Schmitt LC, et al. Relations of C-reactive protein and obesity to the prevalence and the odds of reporting low back pain. Arch Phys Med Rehabil. 2013;94(4):745–752. doi:10.1016/j.apmr.2012.11.026
56. Hirotsu C, Pedroni MN, Berro LF, et al. Nicotine and sleep deprivation: impact on pain sensitivity and immune modulation in rats. Sci Rep. 2018;8(1):13837. doi:10.1038/s41598-018-32276-7
57. Saragiotto BT, Kamper SJ, Hodder R, et al. Interventions targeting smoking cessation for patients with chronic pain: an evidence synthesis. Nicotine Tobacco Res. 2018. doi:10.1093/ntr/nty255
58. Carter AR, Sanderson E, Hammerton G, et al. Mendelian randomisation for mediation analysis: current methods and challenges for implementation. Eur J Epidemiol. 2021;36(5):465–478. doi:10.1007/s10654-021-00757-1
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.