Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Association Between Metabolic and Obesity Phenotypes and Diabetes Risk in Children and Adolescents
Received 27 June 2024
Accepted for publication 16 November 2024
Published 26 November 2024 Volume 2024:17 Pages 4479—4487
DOI https://doi.org/10.2147/DMSO.S484639
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Huiling Hao, Yanhua Su, Mei Feng
Department of Endocrinology, Children’s Hospital of Shanxi, Taiyuan, Shanxi, People’s Republic of China
Correspondence: Huiling Hao, Email [email protected]
Introduction: Diabetes is a significant public health concern worldwide, having increased rapidly in recent decades among younger generations. The correlation between metabolic/obesity phenotypes and the development of pre-diabetes in children and adolescents remains unclear.
Methods: This study aimed to explore this association within a cohort of 1,524 subjects aged 7 to 18 years. Subjects were categorized into four groups based on their metabolic and obesity status: Metabolically Unhealthy with Normal Body Weight (MUNW), Metabolically Healthy Overweight/Obesity (MHO), Metabolic Healthy with Normal Body Weight (MHNW), and Metabolically Unhealthy Overweight/Obesity (MUO). Physical parameters such as body mass, as well as biochemical markers including blood pressure, fasting glucose, triglycerides, and high-density lipoprotein cholesterol, were measured.
Results: A total of 61.9% of children were within the normal range for both body weight and metabolism (MHNW), while 24.4% were classified as MUNW, 5.7% as MHO, and 8% as MUO. The risks of diabetes in the MUNW and MUO groups were 8.89 and 9.18 times higher than in the MHNW group for boys, and 8.15 and 11.24 times higher for girls (P< 0.05).
Conclusion: These findings suggest that abnormal metabolism, irrespective of body weight, significantly increases the risk of diabetes, while obesity alone does not predict pre-diabetes unless accompanied by metabolic dysregulation. Metabolic markers may serve as more sensitive indicators for assessing diabetes risk in this population.
Keywords: Abnormal metabolism, body mass, diabetes, children and adolescents
Introduction
In recent years, the prevalence of diabetes among children and adolescents has been rising rapidly worldwide. Epidemiological surveys indicate that the incidence of type 1 diabetes (T1DM) among children under 15 years is increasing by 3.4% annually, while the prevalence of type 2 diabetes mellitus (T2DM) is approximately 0.46% in children and adolescents.1,2 Specifically, in the Chinese population, approximately 0.74% of adolescents have been diagnosed with type 2 diabetes.3 Other risk factors for children and adolescents to develop diabetes include overweight and physical inactivity. Over time, diabetes that occurs in early life can cause inflammatory responses in the heart, kidneys, and eyes, overload the blood vessels, and eventually lead to chronic diseases later in life. To prevent the development of chronic diseases in multiple organs and to protect the quality of life, it is essential to identify and manage the risk factors associated with diabetes development as part of a medical plan for children with pre-diabetes.
Obesity in youths is frequently associated with abnormal metabolic factors and is widely considered a primary criterion for diagnosing metabolic diseases like diabetes.4 However, two other distinct metabolic/obesity phenotypes exist: Metabolically Unhealthy Normal Weight (MUNW) and Metabolically Healthy Overweight/Obesity (MHO). MUNW population exhibit metabolic abnormalities such as dyslipidemia, hyperglycemia, and insulin resistance despite maintaining normal body weight.5 Currently, no universal diagnostic standard for metabolic abnormalities (or metabolic syndrome) exists, but one widely used criterion includes a combination of Low-Density Lipoprotein Cholesterol (LDL-C), total cholesterol, and High-Density Lipoprotein (HDL-C), as outlined in the American Cholesterol Education Program Adult Treatment Panel III (ATPIII) guideline. Both the HDL-C and LDL-C can reflect insulin resistance, which is considered one of the diagnostic criteria of metabolic abnormalities. In MUNW children and adolescents, normal body weight often masks the risk of abnormal metabolism in LDL-C and HDL-C, making early identification of unhealthy metabolic states crucial for preventing diabetes. Although obesity is a common criterion for diagnosing pre-diabetes, MHO individuals exhibit healthy metabolic profiles, including high insulin sensitivity and normal lipid metabolism.6 MUO has been extensively studied in pediatric populations due to its severe impact on adult health,7,8 but research on pediatric MHO remains limited. No universal diagnostic criteria for MUNW or MHO exist worldwide, and the distribution of these populations varies across studies. MUNW prevalence ranges from 4.6% to 30% in boys and 6.2% to 21.1% in girls, while MHO prevalence ranges from 9.3% to 28% in boys and 7.2% to 28% in girls.9,10 Although the clinical distribution of MUNW and MHO has been reported,11,12 few studies have examined the connection between these metabolic phenotypes and diabetes development in youths.
To investigate the role of metabolic dysregulation and abnormal body mass in relation to diabetes, we recruited 1524 subjects and categorized them into four groups: MHNW, MUNW, MHO, and MUO, based on their physical body mass and biochemical markers, and analyzed their correlation with diabetes development.
Methods
Participant Recruitment and Eligibility Criteria
The current study has designed to complies with the Declaration of Helsinki, and the protocol has been reviewed and approved by the ethics committee of Shanxi Children’s Hospital (approval number: 2020-018). 1524 children and adolescents aged 7 to 18 were enrolled at the Endocrinology and Genetic Metabolism Department of Shanxi Children’s Hospital in Shanxi Province, China, between May 2020 and May 2023, with 786 boys and 738 girls. Given that this is a cross-sectional study, no follow-up was conducted after the initial data collection, apart from the diagnosis of diabetes, which is the one of the primary parameters of interest in this study. All participants were accompanied by their parents or guardians, who were provided with detailed information about the research project, Including the study design, objectives, methodology, potential risks, and benefits. Informed consent forms was signed by the parents or guardians before the start of the study. Given the limited size of the cohort, particularly the normal control group, and the fact that recruitment was not conducted blindly, morbidity cannot be accurately calculated based solely on the occurrence of diabetes. All subjects were screened based on the following eligibility criteria: (1) Age between 7 and 18 years; (2) A clear and complete clinical history, with no evidence of organic disease. Exclusion criteria were as follows: (1) subjects whose age did not meet the inclusion range; (2) missing follow-up data regarding diabetes diagnosis; (3) absence of key biochemical data such as lipid profile, blood pressure, blood glucose, or body mass index (BMI).
Study Design
This is considered a cross-sectional in which all participants underwent measurements of physical and biochemical parameters. All eligible subjects were assessed for height, weight, waist and hip circumference, and blood pressure as physical body parameters. Biochemical measurements, such as fasting blood glucose (FPG), triglycerides, and HDL-C levels, were determined using assay kits. The oral glucose tolerance test (OGTT) was performed simultaneously. Based on both the physical and bio-chemical test results, subjects were categorized into four groups: abnormal metabolism with normal body mass (MUNW), normal metabolic overweight/obesity (MHO), normal body mass with normal metabolism (MHNW), and metabolic abnormality overweight/obesity (MUO). The standards used for defining the groups were based on the age and gender-specific body mass index standards (BMI ≥95% threshold) established by the China Obesity Working Group13 and the standards defined by the International Diabetes Federation (IDF) for children and adolescents.14–17 The cutoff values for individual standards are shown in Table 1: BMI and waist circumference (WC) were used as the standards for obesity, and blood pressure was considered a factor indicative of metabolic dysregulation, along with the combination of biochemical markers listed below.
 |
Table 1 Boundary Values of Risk Factors Under Different Evaluation Criteria |
Diabetes in children and adolescents was diagnosed based on the 2018 ISPAD consensus guidelines on clinical practice:18 (1) Typical symptoms of diabetes or hyperglycemic crisis, plasma glucose concentration≥11.1 mmol/L (200 mg/dL); (2) FPG≥ 7.0 mmol/L (≥126 mg/dL); (3) plasma glucose concentration≥11.1 mmol/L (≥ 200 mg/dL) two hours after glucose load during OGTT. (4) Triglycerides >1.7 mmol; (5) HDL-C <1.2 mmol. The results from these measurements were cross-confirmed for the final diagnosis of diabetes.
Statistical Analysis
This study applied SPSS 22.0 for all statistical analyses. P<0.05 was considered statistically significant. The counting data are expressed as numbers and percentages, and the comparison between groups is evaluated by x2 test. Continuous data is represented by mean ± standard deviation (SD) and comparison between two groups using a Student’s t-test. Logistic regression was performed for correlation analysis with reference to the MHNW groups to calculate the p-value and fold change (OR) of the diabetes risk based on sex, adjustment of Age and family history of diabetes were applied and calculated separately as univariate analysis (without adjustment of Age and family diabetes history) and multivariate analysis (with adjustment of age and family diabetes history). Univariable and multivariable logistic regression analyses were conducted to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for the relationship between the four categories of metabolic obesity and the risk of diabetes. In addition, the variables included in the multivariate logistic regression for adjustment should refer to similar studies, such as age, sex, smoking status (never, former, or current smoker), alcohol consumption (never, former, or current drinker), physical activity (yes or no), family history of hypertension (yes or no), and family history of diabetes (yes or no).
Height, weight, and waist circumference (WC) were assessed by trained personnel using a metric scale and a vertical weighing scale in controlled conditions. Body weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively, with participants dressed in light clothing and barefoot. WC was recorded at the midpoint between the lower edge of the rib cage and the upper edge of the iliac crest, also to the nearest 0.1 cm, while participants stood and breathed gently. Each individual was measured twice, and the average of the two values was used. Blood pressure was recorded using an electronic sphygmomanometer after at least 5 minutes of rest in a seated position. The measurements were taken three times with a 30-second interval, and the average was calculated for analysis.
The automatic biochemical analyzer (Mindray BS-180 Analyzer) was utilized to determine total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TGs), and low-density lipoprotein cholesterol (LDL-C). HbA1c was measured using Bio-Rad reagents. Fasting blood glucose was assessed using the hexokinase enzymatic method. All participants underwent an oral glucose tolerance test (OGTT) following a 10-hour fasting period, after which morning fasting blood and spot urine samples were collected. These samples were utilized for routine biochemical analyses, including fasting blood glucose (FPG), lipid profile, glycated hemoglobin (HbA1c), and 2-hour post-glucose blood sugar (2-hPG). The diagnosis of diabetes was based on the 1999 WHO diagnostic criteria: the presence of typical symptoms of diabetes mellitus (such as polyphagia, polydipsia, polyuria, and unexplained weight loss), along with random blood glucose ≥ 11.1 mmol/L or fasting blood glucose (FBG) ≥ 7.0 mmol/L or 2-hour post-glucose blood sugar (2hPG) ≥ 11.1 mmol/L. For individuals without typical symptoms of diabetes, a follow-up OGTT was required on a different day.
Results
Baseline Characteristics
Based on the cutoff criteria (Table 1), 61.9% (944/1524) of children and adolescents in the Shanxi region of China were classified as having normal ranges for both body weight and metabolism (MHNW) according to the metabolic/body-size phenotypes (Table 2). There were no sex-dependent differences in group distribution, and no significant differences were observed in various parameters (blood pressure, fasting glycemia, triglycerides, and HDL-C) between boys and girls across different groups.
 |
Table 2 Comparison of Clinical Data and Biochemical Indicators Among Different Groups |
Notably, the blood pressure was higher in patients with abnormal metabolism, regardless of body weight being normal or obesity (Table 2). Significantly higher levels of fasting glycemia and triglycerides, along with lower levels of HDL-C, were observed in subjects identified as having abnormal metabolism, regardless of whether they had normal body weight or were overweight, compared to those in the MHNW or MHO group (Table 2). There were no significant differences in blood pressure, fasting glycemia, triglycerides, and HDL-C levels between MHNW and MHO groups (Table 2).
Analysis of the Prevalence of Diabetes and Correlations with Body mass and Metabolic Status
Among boys, 6.19% and 5.71% of subjects from the MHNW and MHO groups were identified as having diabetes, while 41.08% and 42.19% of subjects in the MUNW and MUO groups were diagnosed with diabetes. A similar pattern was observed in girls: with 4.14% and 5.88% of subjects in the MHNW and MHO groups, and 38.24% and 39.66% in the MUNW and MUO groups, respectively, diagnosed with diabetes. The rate of diabetes diagnosis was significantly higher in the MUNW and MUO groups compared to the MHNW group in both sexes, while no significant difference was observed between the MHO and MHNW groups (Table 3).
 |
Table 3 Prevalence of Diabetes in Different Metabolic Groups |
Logistic regression analysis revealed a 10.58- and 11.07- fold higher risk of diabetes in the MUNW and MUO groups, respectively, compared to the MHNW group, without adjusting for family history of diabetes, showing significance in boys. The MHO group showed a similar rate of diabetes occurrence as the MHNW group, without statistical significance in boys (Table 4). Similar results were observed in girls, with a 9.93- and 15.22-fold higher risk of developing diabetes in MUNW and MUO groups, respectively, compared to the MHNW group, without statistical significance in the MHO group (Table 4). After adjusting for family history of diabetes, an 8.89- and 9.18-fold higher risk of diabetes was found in the MUNW and MUO groups compared to the MHNW group, with significance in boys. No significant difference was observed between the MHO and MHNW groups in boys. Girls in the MUNW and MUO groups had an 8.15- and 11.24-fold higher risk of diabetes, respectively, compared to the MHNW group, with no significant difference observed between the MHO and MHNW groups (Table 4). When comparing metabolic markers between diabetic patients and non-diabetic subjects, all metabolic markers showed significant differences (Table 5). These findings are consistent with our regression analysis, which demonstrated that metabolic dysfunction is strongly correlated with diabetes development.
 |
Table 4 Evaluates the Risk Analysis of Diabetes in Each Group |
 |
Table 5 Comparison of Clinical Data and Biochemical Indicators Among None Diabetes and Diabetes |
Discussion
Based on our results, approximately 35.7% of boys and 23.04% of girls were classified as MUNW, while 4.46% of boys and 2.91% of girls were classified as MHO. These findings are consistent with previous studies, considering the varied standards for the diagnosis of MHO and MUNW across studies. The wide range of boys and girls in the MUNW group from different studies may be attributed to the varying application of metabolic standards and cut-off values utilized to categorize these groups. Most children and adolescents (61.7% in boys and 62.2% in girls) demonstrated a normal metabolic range. Among the 1524 patients, 372 were identified as metabolically unhealthy without a diagnosis of obesity, placing them at increased risk for developing diabetes. Incorporating the cutoff values of diabetes-associated metabolites into diagnostic standards is essential for accurately assessing the risk of pre-diabetes development, independent of body mass.
From our logistic regression analysis, we found that the risk of pre-diabetes development was 10.58 and 11.07 times higher in the MUNW and MUO groups, respectively, compared to MHNW in boys. Similarly, the risk was 9.93 and 15.22 times higher in girls in the MUNW and MUO groups. However, the risk of diabetes in the MHO group was not significantly increased in either sex. This reinforces our previous conclusion that being overweight may be an outcome of diabetes development, but should not be utilized as a diagnostic criterion. Dysregulation of metabolites is a more reliable and stable indicator for diagnosing diabetes. Clearly, metabolic abnormalities are more significant than obesity in elevating the risk of diabetes development.
Although the MHO population had a higher BMI, classifying them as obese, they exhibited normal metabolic profiles characterized by lower intra-abdominal fat content, lower blood lipid levels, and higher insulin sensitivity. The total fat in the MHO group may result from the accumulation of visceral fat surrounding the organs and within the abdominal cavity. While visceral fat is necessary for supporting and protecting the organs, it can also contribute to excessive weight due to a lack of physical activity and poor dietary habits. Importantly, visceral fat accumulation does not result from the development of unhealthy diseases or metabolic dysregulation. Although the MHO group showed no correlation of diabetes development, long-term accumulation of visceral fat may lead to metabolic disorders and increase the incidence of diabetes and cardiovascular diseases.19 Research by Neeland et al suggests that the accumulation of abdominal subcutaneous fat in obese patients has no independent effect on dyslipidemia, insulin resistance, and arteriosclerosis, while excessive visceral fat contributes to dyslipidemia and insulin resistance.20,21 Therefore, maintaining a healthy body weight and simultaneously monitoring metabolic health is crucial for protecting against diabetes development.
Although we have shown that obesity has a limited correlation with the development of diabetes in adolescents, childhood overweight can lead to obesity in adulthood, increasing the risk of diabetes development.22 Since this is a cross-sectional study rather than a longitude one, the risk of diabetes in adults related to childhood and adult obesity was not assessed. It has been established that increased daily physical activity, such as sports, can help mitigate the risk of type 2 diabetes development and reduce the prevalence of obesity. Given that our cohort focuses on adolescents, regular physical activity could benefit the obesity population, including those with dysregulated metabolism, by reducing the risk of type 2 diabetes.23
The results of this study specifically highlighted the diabetes risk among children and adolescents in the MUNW group. Although the subjects maintained a normal BMI, they exhibited dyslipidemia and insulin resistance. Meanwhile, the MUNW patients exhibited increased visceral fat, while abdominal obesity and insulin resistance were the indicative of pre-diabetes development in non-obese individuals. (PMID: 35615717) However, individuals in the MHO group do not exhibit a significantly increased risk of diabetes, which may be related to minimal visceral fat accumulation in these patients. Based on our results, individuals who are not obese but have abnormal metabolic profiles should be identified and informed of their risk of diabetes development as soon as possible in a clinical setting.
There are some limitations in our study that prevent us from fully separating the subjects to identify the effects of sex-hormones during puberty. It has been noticed that girls have a relatively healthier metabolic phenotype than boys.24–27 This may be attributed to the differing ages of sexual maturity, as girls tend to exhibit less visceral and subcutaneous fat deposition, which may favor a reduced risk of metabolic dysregulation.28 However, our data showed that girls in the MUNW or MUO groups had a higher risk of diabetes development than boys, suggesting that girls who are metabolically unhealthy require close monitoring of diabetes development. Additionally, detailed measurements of visceral and subcutaneous fat could provide insight into why the MHO group showed no increased risk of diabetes. Furthermore, the overlap between this study and the COVID19 pandemic may have affected the results due to decreased activity levels among the younger generation, potentially increasing the obesity rates. Although our study suggests that obesity has a lesser correlation with the occurrence of diabetes, increased daily physical activity, such as sports, could help reduce the morbidity of type 2 diabetes.23
As far as we know, this study is the first time to compare the incidence rate of metabolic somatotypes among children and adolescents in the Shanxi region of China and to further explore the relationship between metabolic phenotype and the prevalence of diabetes (Figure 1). Our research findings provide valuable insights into intervention strategies aimed at improving the metabolic health and weight management of children and adolescents, as well as preventing diabetes.
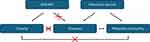 |
Figure 1 Diagram to describe the correlation between bodyweight/metabolism and diabetes occurrence. |
Conclusion
This study identified that metabolically unhealthy is more important than obesity or overweight status in predicting the development of diabetes. Both the MUNW and MUO groups showed a significant correlation with diabetes occurrence, while the MHO group did not differ significantly from the MHNW group. It is noteworthy that although obesity may serve as a marker for pre-diabetes evaluation, dysregulated metabolite levels should be prioritized to assess the risk of diabetes development. The risk of diabetes is higher in populations who are metabolically unhealthy with normal body mass than in those who are overweight but have a healthy metabolic profile.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Patterson CC, Karuranga S, Salpea P, et al. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabet Res Clin Pract. 2019;157:107842. doi:10.1016/j.diabres.2019.107842
2. Dündar İ, Akıncı A. Prevalence of type 2 diabetes mellitus, metabolic syndrome, and related morbidities in overweight and obese children. J Pediatr Endocrinol Metab. 2022;35:435–441. doi:10.1515/jpem-2021-0271
3. Wu H, Patterson CC, Zhang X, et al. Worldwide estimates of incidence of type 2 diabetes in children and adolescents in 2021. Diabetes Res Clin Pract. 2022;185:109785. doi:10.1016/j.diabres.2022.109785
4. Schulze MB. Metabolic health in normal-weight and obese individuals. Diabetologia. 2019;62:558–566. doi:10.1007/s00125-018-4787-8
5. Weihrauch-Blüher S, Schwarz P, Klusmann JH. Childhood obesity: increased risk for cardiometabolic disease and cancer in adulthood. Metabolism. 2019;92:147–152. doi:10.1016/j.metabol.2018.12.001
6. Chung YL, Rhie YJ. Severe obesity in children and adolescents: metabolic effects, assessment, and treatment. J Obes Metab Syndr. 2021;30:326–335. doi:10.7570/jomes21063
7. Wasniewska M, Pepe G, Aversa T, et al. Skeptical look at the clinical implication of metabolic syndrome in childhood obesity. Children. 2023;10:735. doi:10.3390/children10040735
8. Xi B, Cadenas-Sanchez C. Editorial: metabolically healthy and unhealthy obese children and adolescents, volume II. Front Endocrinol. 2022;13:1111060. doi:10.3389/fendo.2022.1111060
9. Chen F, Liu J, Yan Y, Mi J. Abnormal metabolic phenotypes among urban Chinese children: epidemiology and the Impact of DXA-measured body composition. Obesity. 2019;27:837–844. doi:10.1002/oby.22426
10. Zhou J, Bai L, Dong Y, Cai R, Ding W. The association between a metabolically healthy overweight/obesity phenotype and markers of inflammation among Chinese children and adolescents aged 10–18 years. J Pediatr Endocrinol Metab. 2022;35:109–114. doi:10.1515/jpem-2021-0224
11. Yang C, Liu X, Dang Y, et al. Obesity metabolic phenotype, changes in time and risk of diabetes mellitus in an observational prospective study on general population. Int J Public Health. 2022;67:1604986. doi:10.3389/ijph.2022.1604986
12. Zhu X, Hu J, Guo H, et al. Effect of metabolic health and obesity phenotype on risk of diabetes mellitus: a population-based longitudinal study. Diabetes Metab Syndr Obes. 2021;14:3485–3498. doi:10.2147/DMSO.S317739
13. Liu J, Ma T, Chen M, et al. Prevalence and associated factors of metabolic body size phenotype in children and adolescents: a national cross-sectional analysis in China. Front Endocrinol. 2022;13:952825. doi:10.3389/fendo.2022.952825
14. Zimmet P, Alberti KG, Kaufman F, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi:10.1111/j.1399-5448.2007.00271.x
15. Cook S, Weitzman M, Auinger P, Nguyen M, Dietz WH. Prevalence of a metabolic syndrome phenotype in adolescents: findings from the third National Health and Nutrition Examination Survey, 1988–1994. Arch Pediatr Adolesc Med. 2003;157:821–827. doi:10.1001/archpedi.157.8.821
16. Damanhoury S, Newton AS, Rashid M, et al. Defining metabolically healthy obesity in children: a scoping review. Obes Rev. 2018;19:1476–1491. doi:10.1111/obr.12721
17. Chiesa C, Pacifico L, Xi B, Cadenas-Sanchez C. Editorial: metabolically healthy and unhealthy obese children and adolescents. Front Endocrinol. 2020;11:613703. doi:10.3389/fendo.2020.613703
18. Mayer-Davis EJ, Kahkoska AR, Jefferies C, et al. ISPAD clinical practice consensus guidelines 2018: definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2018;19(Suppl 27):7–19. doi:10.1111/pedi.12773
19. Jung SH, Ha KH, Kim DJ. Visceral fat mass has stronger associations with diabetes and prediabetes than other anthropometric obesity indicators among Korean adults. Yonsei Med J. 2016;57:674–680. doi:10.3349/ymj.2016.57.3.674
20. Kącka A, Charemska A, Jarocka-Cyrta E, Głowińska-Olszewska B. Comparison of novel markers of metabolic complications and cardiovascular risk factors between obese non-diabetic and obese type 1 diabetic children and young adults. Front Endocrinol. 2022;13:1036109. doi:10.3389/fendo.2022.1036109
21. Blüher M. Metabolically healthy obesity. Endocr Rev. 2020;41. doi:10.1210/endrev/bnaa004
22. Carrasquilla GD, Ängquist L, Sørensen TIA, Kilpeläinen TO, Loos RJF. Child-to-adult body size change and risk of type 2 diabetes and cardiovascular disease. Diabetologia. 2024;67:864–873. doi:10.1007/s00125-023-06058-4
23. Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147–e167. doi:10.2337/dc10-9990
24. Aldhoon-Hainerová I, Hainer V, Zamrazilová H. Impact of dietary intake, lifestyle and biochemical factors on metabolic health in obese adolescents. Nutr Metab Cardiovasc Dis. 2017;27:703–710. doi:10.1016/j.numecd.2017.05.002
25. Vukovic R, Dos Santos TJ, Ybarra M, Atar M. Children with metabolically healthy obesity: a review. Front Endocrinol. 2019;10:865. doi:10.3389/fendo.2019.00865
26. Ooi DSQ, Ong SG, Lee OMH, et al. Prevalence and predictors of metabolically healthy obesity in severely obese Asian children. Pediatr Res. 2022;92:1374–1380. doi:10.1038/s41390-022-01941-z
27. Mutie PM, Pomares-Milan H, Atabaki-Pasdar N, et al. Investigating the causal relationships between excess adiposity and cardiometabolic health in men and women. Diabetologia. 2023;66:321–335. doi:10.1007/s00125-022-05811-5
28. Tramunt B, Smati S, Grandgeorge N, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453–461. doi:10.1007/s00125-019-05040-3
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

