Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Association Between Metabolic Syndrome and the Size of Renal Cysts: A Cross-Sectional Study
Authors Tao B, Gu D, Wang K, Li Y, Xu X, Chen J
Received 17 July 2024
Accepted for publication 9 October 2024
Published 16 October 2024 Volume 2024:17 Pages 3795—3802
DOI https://doi.org/10.2147/DMSO.S479665
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Biao Tao,* Dian Gu,* Kai Wang,* Yinan Li, Xianlin Xu, Jiexun Chen
Department of Urology, Sir Run Run Hospital, Nanjing Medical University, Nanjing, Jiangsu Province, 211100, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiexun Chen; Xianlin Xu, Department of Urology, Sir Run Run Hospital, Nanjing Medical University, 109 Longmian Road, Jiangning District, Nanjing, Jiangsu Province, 211100, People’s Republic of China, Email [email protected]; [email protected]
Background and Aims: Metabolic syndrome (MetS) is associated with the development of several diseases. However, the correlation between MetS and size of renal cysts remains unclear. This research aims to explore the potential connection between them, offering theoretical guidance for clinical prevention and treatment of renal cysts.
Methods: A total of 467 patients diagnosed with renal cysts and admitted to Sir Run Run Hospital, Nanjing Medical University from September 2019 to September 2020 were eventually included in this study. They were divided into the small cyst group (cyst volume≤ 1.5cm³) and the large cyst group (cyst volume> 1.5cm³) based on the median value of cyst volume. Multivariate logistic regression analysis was used to evaluate the association between MetS and size of renal cysts.
Results: Our results indicated that MetS (OR 1.941, 95% CI 1.286– 2.927, P=0.002) was positively associated with the size of renal cysts. Additionally, multiple renal cysts (OR 2.259, 95% CI 1.402– 3.640, P=0.001) and serum globulin (OR 0.945, 95% CI 0.905– 0.987, P=0.011) were positively and negatively related to size of renal cysts, respectively.
Conclusion: Our study reveals the association between MetS and the size of renal cysts. Patients with MetS are more likely to have larger renal cysts. The administration of MetS may help limit the development of renal cysts. Further prospective studies are needed to explore the causal relationship between MetS and renal cysts.
Keywords: size of renal cysts, metabolic syndrome, metabolic disease, renal cyst
Introduction
Simple Renal Cyst (SRC) is a common type of cystic renal disease, and is typically discovered during health screening programs. Clinically, its diagnosis mainly relies on abdominal imaging examinations. The prevalence of SRC is approximately 10% and rises with age.1,2 SRC has certain specific subtypes, such as parapelvic cysts.3 It is believed to be linked to various conditions, including Fabry disease. It is entirely different from other subtypes in terms of location, number, size, and origin.3 SRC does not exhibit characteristic clinical symptoms in general, while symptoms such as pain, hematuria, kidney function impairment, or even malignant transformations may gradually occur as the cyst size increases.4,5 Previous studies indicated that renal dysfunction, hypertension,6–8 age, male gender, obesity, smoking, and kidney stones were associated with the occurrence of SRC.9–12 However, there is limited research focusing on the factors related to the progression of SRC. In clinical practice, there is currently a lack of clinical research evidence regarding the factors influencing the progression of SRC and methods for prevention to guide patients.
MetS, also known as syndrome X or insulin resistance, is defined by the World Health Organization (WHO) as a pathological condition characterized by abdominal obesity, insulin resistance, hypertension, and hyperlipidemia. MetS constitutes a cluster of metabolic abnormalities and has been recognized as a key risk factor for cardiovascular diseases.13 MetS was originally more prevalent in Western societies, but due to the widespread adoption of Western lifestyles in the current era, it has become a global issue. MetS can impact our physical health in various ways and is associated with the development of diseases such as chronic kidney disease (CKD), type 2 diabetes, coronary heart disease, stroke, and other disabilities.13,14 Recently, research from Taiwan Region reported the potential mutual influence between the occurrence of SRC and MetS.15 This study provides support for the relationship between these two conditions. However, the influence of metabolic syndrome on the growth of SRC remains unknown.
The identification of risk factors for SRC progression is crucial for conservative treatment and clinical symptom prevention of SRC. This will also lighten the financial burden and improve the life quality for the patients with SRC. Thus, we conducted this cross-sectional study to investigate the relationship between MetS and size of renal cyst.
Materials and Methods
Study Population
In this cross-sectional study, a total of 514 patients who were hospitalized at Sir Run Run Hospital Nanjing Medical University from 15 September 2019 to 10 September 2020 and diagnosed with renal cysts were included. The medical records of them were retrospectively investigated from a computer-based databank. The study was approved by the Ethics Committee of Sir Run Run Hospital Nanjing Medical University (2021-SR-036). All patients participating in the study signed informed consent in advance and passed the review of the ethics committee. All procedures performed in the present study involving human participants followed the ethical standards of the institutional and national research committee.
The flow chart for patient selection in this study is shown in Figure 1. Of 514 patients, 31 patients were excluded due to missing key data including body mass index (BMI), blood glucose, blood pressure, and lipid profile. 3 patients may have Polycystic kidney disease and 13 patients who lacked imaging findings of renal cysts were also excluded. Consequently, this study included a final cohort of 467 patients. 210 patients were diagnosed by B-ultrasound while 257 patients were diagnosed by computerized tomography (CT) scans.
 |
Figure 1 Flow chart of the selection process. |
Details of the Study Design
We used a data collection checklist designed for this study to collect the baseline characteristics of enrolled patients. The information on the list covered general characteristics of the patients, including sociodemographic information (age and gender), anthropometric measurements [height, weight, and blood pressure (BP)], past medical history (hypertension, diabetes, and kidney stone), and laboratory examinations [fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, serum creatinine (Scr), blood urea nitrogen (BUN), and serum globulin]. Kidney stones were also identified by imaging examinations. Estimated glomerular filtration rate (eGFR) calculated according to the following formula:16  .
.
Assessment of Renal Cysts
The assessment of renal cysts relies on dependable imaging techniques such as abdominal ultrasonography, CT scans. The lesion on kidney ultrasound appears echolucent, with a smooth and clear boundary and a thin but distinct wall. It is a round or oval lesion with increased posterior echogenicity, easily penetrating the cyst. On CT scan, the lesion is round with a density similar to homogenized water. There is no change in the shadow concentration of the cyst after contrast medium injection, and the lesion has a clear boundary, closely resembling cystic parenchyma.17 The data of the length, width, and height of the biggest cyst, renal cysts number, as well as unilateral/bilateral were collected. The length, width, and thickness of the kidneys were also included in the collection. Renal parenchyma volume was calculated with the adjusted equation (length × width × thickness × 0.674).18 The volume of the biggest cyst was calculated according to the following formula: volume(cm3) = 3Π/4×(length/2)×(width/2)×(height/2). Drawing from pertinent literature and our dataset, patients were categorized into two groups based on the median of the biggest cyst volume: large cyst group and small cyst group, utilizing a cutoff value of 1.5 cm³.
Definitions of Metabolic Syndrome
According to the 2004 statement by the Chinese Diabetes Society on the definition of MetS, individuals who meet three or more conditions can be diagnosed with MetS.19 The diagnosed criteria are as follows: (1) Obesity: body mass index (BMI)≥ 25 kg/m2; (2) Hypertension: blood pressure (BP) ≥ 140/90 mmHg and/or has been confirmed and treated as hypertension; (3) Hyperglycemia: fasting blood glucose (FBG)≥ 6.1 mmol/L (110 mg/dl) and/or 2h plasma glucose(PG) ≥ 7.8 mmol/L (140 mg/dl), and/or have been diagnosed and treated for diabetes; (4) Dyslipidemia: high Triglycerides(TG) ≥ 1.7 mmol/L (150 mg/dl), and/or low high-density Lipoprotein(HDL) cholesterol < 0.9 mmol/L (35 mg/dl) in men or < 1.0 mmol/L (39 mg/dl) in women. The diagnostic criteria for diabetes and hypertension adhere to internationally recognized consensus standards.
Statistical Analysis
All data were analyzed by SPSS 22.0 software (IBM, New York, USA). Kolmogorov–Smirnov test was used to assess the normal distribution of continuous variables. Continuous variables with a normal distribution were presented as mean ± standard deviation (SD) and compared with Student’s t-test. Non-normally distributed variables were expressed as median (interquartile range 25–75%) and compared using the Mann–Whitney U-test. Categorical variables were expressed as percentages and compared by the Chi-square test. Logistic regression analysis was used to identify the relationship between MetS and the size of renal cysts. The odds ratio (OR) with 95% confidence intervals (CI) was calculated. In addition, subgroup analysis was conducted by the stratified multiple regression method according to gender (female or male), age (<60 or ≥60 years old), cyst location (unilateral or bilateral), and cyst number (single or multiple). Extensive sensitivity analysis was performed to validate the stability of our results. Firstly, to mitigate the potential impact of outliers, we constructed adjusted databases under the following conditions: (1) Exclusion of participants with extreme TG levels (beyond the range of mean±3×SD); (2) Exclusion of participants with extreme TC levels (beyond the range of mean±3×SD); (3) Exclusion of participants with extreme BMI levels (<15 or ≥40 kg/m2); (4) Exclusion of participants with extreme cyst size (beyond the range of mean±3×SD). Additionally, to further explore data within a manageable age range, participants aged <18 or ≥80 years were excluded. Finally, patients with normal FBG but a history of diabetes were excluded to further investigate the role of blood glucose manifestation. The multivariable logistic regression analysis for MetS in the newly constructed databases under these conditions was conducted successively. The sensitivity analysis results indicate that the findings of this study are stable.
Results
In this study, 467 patients with complete medical records, laboratory examinations, and imaging data were eventually included. They were categorized into two groups: the small cyst group (cyst volume≤1.5 cm³) and the large cyst group (cyst diameter>1.5 cm³). In the large cyst group, we observed higher levels of BMI, SCr, TC and TG compared to the small cyst group as shown in Table 1. Conversely, serum globulin levels were lower in the large cyst group compared to the small cyst group. Multiple renal cysts (cysts number ≥2) were more prevalent in the large cyst group (94/237, 39.7%) than in the small cyst group (43/230, 18.7%). Bilateral renal cysts were also more common in the large cyst group (107/237, 45.1%) compared to the small cyst group (62/230, 27.0%). Additionally, the proportion of patients with MetS in the large cyst group (97/237, 40.9%) was significantly higher than that in the small cyst group (58/230, 25.2%) (P<0.001).
 |
Table 1 Baseline Characteristics of the Study Population |
As shown in Table 2, MetS (OR 2.055, 95% CI 1.385–3.048, P<0.001), higher BMI (OR 1.078, 95% CI 1.026–1.132, P=0.003), higher TC level (OR 1.191, 95% CI 1.009–1.406, P=0.038), multiple renal cysts (OR 2.859, 95% CI 1.876–4.357, P<0.001), and bilateral renal cysts (OR 2.230, 95% CI 1.514–3.286, P<0.001) were associated with the larger cyst size. Moreover, low serum globulin level was negatively related to the larger cyst size. After adjusted with covariates, MetS (OR 1.941, 95% CI 1.286–2.927, P=0.002) and multiple renal cysts (OR 2.259, 95% CI 1.402–3.604, P=0.001) were still the independent risk factors for SRC development in multivariate logistic regression analysis. In the adjusted model, BMI and TC were excluded due to their collinearity with MetS. Additionally, serum globulin (OR 0.945, 95% CI 0.905–0.987, P=0.011) may be the independent protective factor for the growth of renal cysts. In the sensitivity analysis, multivariable logistic regression analysis for the relationship between MetS and renal cyst size in the newly constructed databases remained to show significant significance.
 |
Table 2 The Odds Ratio for the Association Between Metabolic Syndrome and Size of Renal Cyst |
A subgroup analysis model was employed to determine the effect size of certain factors on cyst size. We stratified by age, gender, cyst number, and cyst location in the subgroup analysis (Table 3). In the large cyst group, the individuals of males, with age ≥60 years old and bilateral renal cysts had modified effects on the association between MetS and the size of renal cysts. In addition, MetS significantly increased the risk of the expansion of renal cysts in both subgroups of single and multiple.
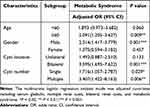 |
Table 3 Subgroup Analysis for the Association Between Metabolic Syndrome and Size of Renal Cyst |
Discussion
This study explored the association between MetS and renal cyst size based on the data from a single center. Our results suggested that renal cyst patients with MetS might tend to have a bigger size of renal cyst. Evaluation and treatment of MetS should be considered in the clinic for patients with renal cysts who do not meet operation indications.
We observed that there were no significant differences in age and sex between the small cyst group and the large cyst group. The probability of kidney cysts in elderly men was significantly higher than that in young women, which aligns with the consensus of various previous studies. Could it be possible that renal cysts in middle-aged and older men with MetS increase in magnitude and rate more than those in younger women? This aspect has not been systematically discussed in another article. However, based solely on the research results, it appears certain. MetS is a collection of various diseases, so we cannot view each component in isolation, nor should we consider them solely as a unified whole. We should conduct a comprehensive analysis to explore the underlying mechanisms.
There was no difference in hypertension between the two samples; however, we cannot overlook the role of hypertension in renal cysts. In China, with a significantly aging population, systolic hypertension is one of the most common diseases.20 Long-term hypertension can lead to a decline in renal vascular function, which may be one of the reasons for a reduction in nephron number. The reduction of nephron number increases the workload on renal tubules, potentially leading to tubule cell hypertrophy and proliferation, which can contribute to the formation of renal cysts.21 Under the influence of hypertension, existing renal cysts experience increased hydrostatic pressure, leading to their expansion. Nevertheless, the exact mechanisms remain unclear and may be related to the regulation of the Renin-Angiotensin-Aldosterone system (RAAS).22 The production of hypertension also involves the RAAS, and its related mechanisms may provide an explanation. The mutual promotion mechanism between hypertension and renal cysts requires further research and explanation.
The difference in the prevalence of diabetes between the large cyst group and the small cyst group was not particularly significant, which may be attributed to the sample size not being sufficiently large. Therefore, it is necessary to continue increasing the sample size. Additionally, a single random blood glucose test is not sufficient to diagnose diabetes, which can lead to diagnostic inaccuracies for some patients. This does not mean that diabetes does not affect the size of renal cysts. On the contrary, studies have shown that glucose concentration strongly influences the growth of renal tubular cell cysts. Cyst growth is driven by epithelial chloride ion secretion, dependent on intracellular levels of cAMP and calcium. Glucose can enhance the expression of this aspect.23 This indicates that good blood sugar control may effectively restrain the growth of epithelial cells on the renal cyst wall, thereby achieving the goal of controlling the growth of renal cysts.
The lipid profile includes TC, TG, and some lipid components such as phospholipids, among which TC and TG are clinically significant. In this study, differences in TC and TG levels between the large cyst group and the small cyst group were observed. Studies have found that lipid presence is quite common, and lipid deposition can frequently be observed in renal biopsy pathology, a phenomenon that can be mediated by macrophages or directly present in the nephron.24 Although many of these disorders suggest a potential link between alterations in lipid metabolism and the onset and progression of kidney disease, further experimental studies are needed to determine whether and how changes in glomerular lipid metabolism contribute to the progression of kidney cysts.25 It has been speculated that after a disorder in lipid metabolism, lipoproteins are more susceptible to oxidation, forming lipid peroxides and other secondary oxides, which can stimulate macrophages and other effector cells to produce catalytic factors that accelerate local inflammatory responses.26 Some have proposed similar theories: disturbances in lipid metabolism led to elevated blood TC and TG levels, activating pathways involved in fat synthesis from TC and TG, increasing oxidative stress responses, inducing mitochondrial division in renal tubule cells, and subsequently activating the apoptosis pathway, resulting in damage to renal tubule cells.27,28 Damage to the renal tubules can directly lead to the formation and progression of renal cysts. Therefore, maintaining optimal lipid levels in the body may also benefit the stability of renal cysts.
Obesity is regarded as a state of low-grade systemic inflammation due to adipose tissue being the primary source of inflammatory mediators.29 This further strengthens the notion that lipid metabolism disorders may contribute to kidney disease. Obese individuals often experience hypertension, hyperglycemia, hyperlipidemia, and other metabolic conditions, all of which may potentially change the size of kidney cysts through the aforementioned mechanisms.
Chapman et al, speculated that multiple renal cysts, especially in patients with a large number of them, could potentially enhance the activation of the RAAS.30 A higher number of renal cysts increases the likelihood of compressing surrounding tissues, leading to kidney ischemia, and then stimulates the release of renin. RAAS activation can intensify sodium and water reabsorption, which results in fluid build-up within the cysts, thereby contributing to their growth. The activation of RAAS also can promote the enlargement of kidney cysts by increasing intrarenal hydrostatic pressure via renal blood vessels constriction. Furthermore, we unexpectedly found that slightly higher serum globulin levels are a protective factor. Given the role of globulin in inhibiting inflammatory factors, we speculate that this protective effect may be exerted by reducing renal oxidative stress and inflammatory responses.31 Further specific experiments are needed to clarify the underlying mechanism.
Our study also had some limitations. Firstly, the stringent criteria used for disease diagnosis and data collection resulted in the exclusion of a significant amount of data that could have been included in the analysis, potentially influencing the overall findings. Secondly, this study was conducted at a single center, and future research should aim to involve multiple centers for broader validation. Thirdly, the sample size in this study was relatively small, and a larger sample size is needed to further strengthen the evidence. Fourthly, the diagnosis of renal cysts relied primarily on imaging examinations, lacking pathological evidence, which might affect the diagnostic rigor. Finally, this article is a cross-sectional study, it only could infer a correlation between MetS and the size of renal cysts, not causality.
Conclusion
In this study, we observed a significant association between MetS and the size of renal cysts, indicating that patients with MetS may be more likely to develop larger renal cysts. Effective administration of MetS might be helpful to control the renal cysts progression. More well-designed prospective studies are required to confirm these findings.
Abbreviations
MetS, Metabolic syndrome; BP, blood pressure; BMI, body mass index; SCr, serum creatinine; FBG, fasting blood glucose; TC, total cholesterol; TG, total triglycerides; HDL, high-density lipoprotein; LDL, low-density lipoprotein; OR, odds ratio; CI, confidence interval; RAAS, Renin-Angiotensin-Aldosterone System; eGFR, Estimated glomerular filtration rate.
Ethics and Consent Statements
This study was conducted in strict adherence to the Helsinki Declaration of Principles and received approval from the Ethics Committee of Sir Run Run Hospital, Nanjing Medical University. All patients participating in the study signed informed consent in advance and passed the review of the Ethics Committee.
Acknowledgments
Thank you to all the teachers and students who have contributed to this article in technical help, writing assistance and general support.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Ravine D, Gibson RN, Donlan J, Sheffield LJ. An ultrasound renal cyst prevalence survey: specificity data for inherited renal cystic diseases. Am J Kidney Dis. 1993;22(6):803–807. doi:10.1016/S0272-6386(12)70338-4
2. Tada S, Yamagishi J, Kobayashi H, Hata Y, Kobari T. The incidence of simple renal cyst by computed tomography. Clin Radiol. 1983;34(4):437–439. doi:10.1016/S0009-9260(83)80238-4
3. Capuano I, Buonanno P, Riccio E, Crocetto F, Pisani A. Parapelvic cysts: an imaging marker of kidney disease potentially leading to the diagnosis of treatable rare genetic disorders? Narrat Rev Literature J Nephrol. 2022;35(8):2035–2046.
4. Nicolau C, Antunes N, Pano B, Sebastia C. Imaging Characterization of Renal Masses. Medicina. 2021;57(1):51. doi:10.3390/medicina57010051
5. Terada N, Arai Y, Kinukawa N, Terai A. The 10-year natural history of simple renal cysts. J Urol. 2006;175(4):18. doi:10.1016/S0022-5347(18)32320-6
6. Hong S, Lim JH, Jeong IG, Choe J, Kim CS, Hong JH. What association exists between hypertension and simple renal cyst in a screened population? J Hum Hypertens. 2013;27(9):539–544. doi:10.1038/jhh.2013.12
7. Lee YJ, Kim MS, Cho S, Kim SR. Association between simple renal cysts and development of hypertension in healthy middle-aged men. J Hypertens. 2012;30(4):700–704. doi:10.1097/HJH.0b013e32835050e8
8. Zerem E, Imamovic G, Omerovic S. Simple renal cysts and arterial hypertension: does their evacuation decrease the blood pressure? J Hypertens. 2009;27(10):2074–2078. doi:10.1097/HJH.0b013e32832f1458
9. Terada N, Ichioka K, Matsuta Y, Okubo K, Yoshimura K, Arai Y. The natural history of simple renal cysts. J Urol. 2002;167(1):21–23. doi:10.1016/S0022-5347(05)65373-6
10. Simms RJ, Ong ACM. How simple are ‘simple renal cysts’? Nephrol Dial Transplant. 2014;29:106–112. doi:10.1093/ndt/gfu106
11. Mosharafa AA. Prevalence of renal cysts in a Middle-Eastern population: an evaluation of characteristics and risk factors. BJU Int. 2008;101(6):736–738. doi:10.1111/j.1464-410X.2007.07234.x
12. Chang -C-C, Kuo J-Y, Chan W-L, Chen -K-K, Chang LS. Prevalence and clinical characteristics of simple renal cyst. J Chin Med Assoc. 2007;70(11):486–491. doi:10.1016/S1726-4901(08)70046-7
13. Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20(2):12. doi:10.1007/s11906-018-0812-z
14. Scurt FG, Ganz MJ, Herzog C, Bose K, Mertens PR, Chatzikyrkou C. Association of metabolic syndrome and chronic kidney disease. Obes Rev. 2024;25(1):e13649. doi:10.1111/obr.13649
15. Shen WC, Sun ZJ, Chou CY, et al. Association of simple renal cysts with metabolic syndrome in adults. Front Public Health. 2022;10:951638. doi:10.3389/fpubh.2022.951638
16. Musso CG, Alvarez-Gregori J, Jauregui J, Macias-Nunez JF. Glomerular filtration rate equations: a comprehensive review. Int Urol Nephrol. 2016;48(7):1105–1110. doi:10.1007/s11255-016-1276-1
17. Park H, Kim CS. Natural 10-year history of simple renal cysts. Korean J Urol. 2015;56(5):351–356. doi:10.4111/kju.2015.56.5.351
18. Janki S, Kimenai H, Dijkshoorn ML, Looman CWN, Dwarkasing RS, IJzermans JN. Validation of ultrasonographic kidney volume measurements: a reliable imaging modality. Exp Clin Transplant. 2018;16(1):16–22. doi:10.6002/ect.2016.0272
19. Xiang K, Ji L, Xiang H, et al. Diagnosis and management of the metabolic syndrome: a Chinese Diabetes Society Scientific Statement. Chin J Diabetes. 2004;12:156–161.
20. Strandberg TE, Pitkala K. What is the most important component of blood pressure: systolic, diastolic or pulse pressure? Curr Opin Nephrol Hypertens. 2003;12(3):293–297. doi:10.1097/00041552-200305000-00011
21. Grantham JJ. Acquired cystic kidney disease. Kidney Int. 1991;40(1):143–152. doi:10.1038/ki.1991.192
22. Chin HJ, Ro H, Lee HJ, Na KY, Chae DW. The clinical significances of simple renal cyst: is it related to hypertension or renal dysfunction? Kidney Int. 2006;70(8):1468–1473. doi:10.1038/sj.ki.5001784
23. Kraus A, Schley G, Kunzelmann K, et al. Glucose promotes secretion-dependent renal cyst growth. J Mol Med. 2016;94(1):107–117. doi:10.1007/s00109-015-1337-4
24. Suzaki K, Kobori S, Ueno S, et al. Effects of plasmapheresis on familial type III hyperlipoproteinemia associated with glomerular lipidosis, nephrotic syndrome and diabetes mellitus. Atherosclerosis. 1990;80(3):181–189. doi:10.1016/0021-9150(90)90025-E
25. Wahl P, Ducasa GM, Fornoni A. Systemic and renal lipids in kidney disease development and progression. Am J Physiol Renal Physiol. 2016;310(6):F433–445. doi:10.1152/ajprenal.00375.2015
26. Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104(4):503–516. doi:10.1016/S0092-8674(01)00238-0
27. Sun Y, Ge X, Li X, et al. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 2020;11(10):914. doi:10.1038/s41419-020-03122-4
28. Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115(5):911–919. doi:10.1016/j.jaci.2005.02.023
29. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi:10.1146/annurev-immunol-031210-101322
30. Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N Engl J Med. 1990;323(16):1091–1096. doi:10.1056/NEJM199010183231602
31. Hou YB, Chang S, Chen S, Zhang WJ. Intravenous immunoglobulin in kidney transplantation: mechanisms of action, clinical applications, adverse effects, and hyperimmune globulin. Clin Immunol. 2023;256:109782. doi:10.1016/j.clim.2023.109782
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

