Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 17
Association Between Non-HDL to HDL Cholesterol Ratio (NHHR) and Psoriasis in Adults: A Cross-Sectional Study Using 2009–2014 Data
Received 18 August 2024
Accepted for publication 30 October 2024
Published 9 November 2024 Volume 2024:17 Pages 2523—2531
DOI https://doi.org/10.2147/CCID.S492053
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Yizi Jiang,1 Min Jia2
1First Clinical Medical College, Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, People’s Republic of China; 2Department of Dermatology, The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, People’s Republic of China
Correspondence: Min Jia, Department of Dermatology, The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, People’s Republic of China, Tel +86 13985033118, Email [email protected]
Background: Because of its possible significance in metabolic diseases, the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) has garnered attention as a novel and trustworthy lipid biomarker. Psoriasis may be linked to metabolic problems and obesity, according earlier research. Uncertainty surrounds the relationship between NHHR and the onset of psoriasis, though. The primary aim of this investigation was to examine the relationship between NHHR and psoriasis.
Patients and methods: This cross-sectional analysis used data from the National Health and Nutrition Examination Survey (NHANES) conducted between 2009 and 2014. The association between psoriasis and NHHR was examined using multivariate logistic regression, and smoothed curve fitting was done to explore the non-linear relationship. Furthermore, Subgroup and sensitivity studies were performed in order to confirm the robustness of the findings.
Results: Psoriasis and NHHR were shown to be positively correlated in 15,951 adult individuals who were at least 20 years old. Psoriasis risk rose by 7% for each unit increase in NHHR [1.07 (1.01, 1.14)]. Individuals in the highest NHHR tertile were 39% more likely compared to those in the bottom tertile to have psoriasis [1.39 (1.09, 1.78)]. Across subgroups, this favorable connection remained consistent.
Conclusion: Elevated NHHR levels are positively correlated with an upsurge chance of psoriasis in the adult population in the United States. The significance of NHHR as an indication for early psoriasis risk assessment is shown by this study.
Keywords: non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio, psoriasis, lipids, NHANES, cross-sectional study
Introduction
Around the world, 2–3% of people suffer with psoriasis, a widespread chronic disorder.1 A typical sign of the illness is scaly or flaky erythema, which affects the entire skin surface as well as the extremities and may cause serious systemic disorders and impairments.2 This has a significant physical and psychological impact on the sufferer.
These days, it is believed that psoriasis is linked to several comorbidities, the most significant and prevalent of which is metabolic syndrome.3–8 Multiple inquiries have also proven that dyslipidemia may be a risk factor.9,10 The connection between psoriasis and the metabolic syndrome has come a long way, but little is known about how it interacts with cholesterol. Numerous studies have shown that patients with psoriasis have reduced levels of HDL and/or increased levels of low-density lipoprotein (LDL), very low-density lipoprotein (VLDL).11–13 Some studies have also shown that psoriasis alters HDL composition and cholesterol efflux capacity.14,15 Furthermore, it had no discernible correlation between psoriatic arthritis and serum TG, HDL-C, or LDL-C in several observational studies of psoriatic arthritis.16,17 There is considerable uncertainty regarding the association between psoriasis and plasma lipids because of indications of publication bias and environmental confounding variables.
Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) is a crucial statistic for assessing the lipid status in atherosclerosis.18 Numerous illnesses, such as depression, infertility, osteoporosis, and kidney stones, have been linked to it.19–21 It has a solid track record of accurately identifying insulin resistance and the metabolic syndrome.22
This study used NHANES data from 2009–2014 to further analyze the association between NHHR and psoriasis and to contribute to the understanding that NHHR is a significant indication for early psoriasis risk assessment.
Methods
Study Population
In this study, we used the NHANES database, which is a cross-sectional survey study of US population data conducted every two years by the NCHS. This database is available for free public access at https://www.cdc.gov/nchs/nhanes/. The sample selection flowchart is shown in Figure 1. This study used data from 2009 to 2014, with a total of 30,468 participants. We excluded 10,941 people with missing psoriasis data and 1,823 subjects with missing NHHR (HDL-Cholesterol and total cholesterol). In addition, individuals under the age of 20 were excluded. Finally, 15,951 participants were included.
 |
Figure 1 Flowchart of the sample selection from NHANES 2009–2014. |
Variables for Study
Evaluation of NHHR
Based on the participants’ cholesterol levels, the NHHR was computed. To calculate the NHHR, the non-HDL-C level was divided by the HDL-C level. To calculate the non-HDL-C level, HDL-C is subtracted from total cholesterol.  . NHHR was intended to be an exposure variable in our study and served as the independent variable.
. NHHR was intended to be an exposure variable in our study and served as the independent variable.
Evaluation of Psoriasis
In our research, psoriasis served as the dependent variable. If a participant said “yes” when asked the query, “Have you ever been told by a doctor or other health care professional that you had psoriasis (sore-eye-asis)?” then psoriasis was diagnosed. Psoriasis history information was obtained from participant self-reports and verified by other studies that established the accuracy of self-reported psoriasis histories.23
Assessment of Covariates
Other known variables that may have an impact on the relationship between the NHHR and psoriasis were included in our study. Age (>20 years old), income to poverty ratio, weight, BMI, triglyceride, LDL- cholesterol, total cholesterol and HDL- cholesterol were continuous variables. Gender, race, education level, smoked at least 100 cigarettes, diabetes, marital status were categorical variables.
Statistical Analysis
A sufficient NHANES sample weight was used for the complicated sampling survey design in the research, and all statistical analyses were carried out in compliance with the guidelines established by the Centers for Disease Control and Prevention (CDC). Continuous variables were represented as mean plus or minus standard deviation in the investigation’s baseline table, while categorical data were given as percentages (%). The link between NHHR and psoriasis was examined using logistic regression; the results are shown as an odds ratio (OR) and 95% confidence interval (CI). Three models were used: Model 2 considered age, race, and gender, whereas Model 1 did not adjust for covariates. Lastly, based on Model 2, Model 3 also adjusted for diabetes, smoking at least 100 cigarettes per day, income to poverty ratio, marital status, and education level. After tertile classified NHHR as a categorical variable, we carried out further sensitivity analyses to evaluate the robustness of the data. Our use of smoothed curve fitting makes it easier to see the intrinsic link between psoriasis and NHHR data and to understand trends in the data. Because psoriasis is significantly affected by different gender, race, diabetes, and smoking populations, an interaction test was used in order to test whether the relationship between NHHR and psoriasis was stable in these populations. Statistics were regarded as significant when two-tailed P<0.05.
Results
Baseline Attributes of the Participants
This study included 15,951 participants with a mean age of 49.07±17.66 years from the NHANES data (2009–2014). Of them, 51.47% were female and 48.53% were male. Depending on whether or not they had psoriasis, we divided the individuals into two groups.
Table 1 demonstrated that the two teams differed significantly in the fields of age, race, weight, BMI, history of diabetes, smoking status, and NHHR (P<0.05). Those with psoriasis had a significantly higher NHHR (3.07 ±1.39 vs 2.93 ± 1.40, P<0.05), and those with psoriasis also tended to be older on average, had a greater number of non-Hispanic white people, weigh more, and have a higher BMI. They also had a greater incidence of diabetes and smoking.
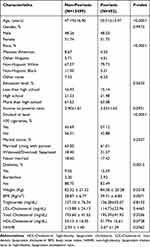 |
Table 1 Comparison of Characteristics of Psoriasis Patients and Non-Psoriasis Participants |
Associations Between Psoriasis and NHHR
The connections between NHHR and psoriasis are seen in Table 2. In unadjusted Model 1, each unit in crease in NHHR was associated with an 8% increased risk of psoriasis [1.08 (1.02, 1.14)]. Model 2 [1.08 (1.02, 1.15)] and Model 3 [1.07 (1.01, 1.14)] also showed this relationship. Sensitivity analyses were then carried out with NHHR treated as a categorical variable (tertiles). In Model 3, the likelihood of psoriasis was 39% higher in the highest NHHR tertile (T3) compared to the lowest tertile (T1). Figure 2 shows the smoothed curve fit, further supporting the positive relationship between NHHR and psoriasis.
 |
Table 2 The Association Between Psoriasis and NHHR |
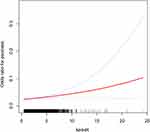 |
Figure 2 Detected Smooth Curve Fitting With the use of the generalized additive model, a positive connection between NHHR and psoriasis was found. |
Subgroup Examination
The stability of the relationship between psoriasis and NHHR across populations was evaluated through subgroup analyses; the results, as presented in Table 3, indicated that effect sizes were consistent across subgroups, with no significant differences by smoking status, gender, ethnicity, or diabetes history. Psoriasis and NHHR have a favorable correlation that remained constant across subgroups and was unaffected by smoking status, gender, race, or diabetes history.
 |
Table 3 Subgroup Analysis for the Association Between NHHR and Psoriasis |
Discussion
This nationally representative cross-sectional study included 15,951 participants to assess the relationship between NHHR and psoriasis. The connection between NHHR and psoriasis in adult US subjects was shown to be positive. Psoriasis and NHHR were shown to be correlated, even after controlling for variables. For every unit rise in NHHR, the prevalence of psoriasis increased by 7% [1.07 (1.01, 1.14)]. Across subgroups based on race, diabetes, smoking status, and sex, this association remained constant. According to the findings, NHHR is crucial for the early diagnosis of psoriasis in people who are at high risk.
Psoriasis is a systemic illness that has been associated with metabolic syndrome and a higher danger of cardiovascular disease.24–27 Various investigations have shown that, globally, people with psoriasis have a greater frequency of metabolic syndrome than people without the condition.28,29 Additionally, patients with psoriasis have been shown to have a number of altered lipid profiles, the most common of which being elevated serum concentrations of TC, LDL-C, and TG.27,30 Dyslipidaemia is the most common risk factor for cardiovascular morbidity. With its data on non-HDL vs HDL cholesterol, the NHHR is a valuable tool for assessing coronary heart disease.18
The mechanism between NHHR and psoriasis is unclear. According to some research, oxidative stress and low HDL levels are positively correlated,31 and oxidative stress elevation is connected to dyslipidemia of LDL.32 It has also been suggested that oxidative stress conditions might lead to the development of psoriasis.33–35 Furthermore, an investigation revealed that psoriasis patients had low amounts of lipocalin,36 which increases HDL by improving the breakdown of triglyceride-rich lipoproteins.37 As a result, psoriasis patients frequently experience hyperlipidemia with low HDL levels. Moreover, serum lipocalin levels and TNF-a and IL-6 levels are negatively correlated;38 that is, a drop in human lipocalin levels is associated with an increase in TNF-a and IL-6, which in turn raises the risk of psoriasis. Also, pro-inflammatory cells are linked to hyperlipidemia, diabetes mellitus, hypertension, and other illnesses. TH17 cells are known to generate many cytokines, such as IL-17, TNF-a, and IL-22,39 which can induce excessive proliferation and differentiation of keratinocytes.40,41 TH17 cells thus contribute significantly to the onset of psoriasis. Mehta found a more atherogenic lipoprotein profile and decreased HDL efflux capacity in 112 psoriasis patients when compared to controls.15 HDL isolated from 15 psoriatic patients revealed significant differences in the HDL-associated proteins.42 According to a large meta-analysis, psoriasis patients have been shown to have considerably higher levels of both VLDL and LDL.43
Strong clinical significance exists for the study’s findings, especially when it comes to patient care and illness prevention. The study’s identification of the relationship between NHHR and psoriasis offers clinicians a potential biomarker for identifying at-risk patients in the clinic and putting targeted preventative measures in place. Recognising that the NHHR is a predictive indicator, a proactive two-step approach to routine lipid screening is recommended: identification of potential psoriasis risk through abnormal NHHR levels and comprehensive skin health assessment. It also increases the frequency of monitoring these patients by routinely checking their skin conditions and lipid levels, which may result in the detection and intervention of problems early on. It is also advised that while managing patients with psoriasis, the connection between lipid metabolism and the condition be taken into account. These factors are especially crucial in avoiding the onset of psoriasis and lowering recurrence rates, which enhances patients’ quality of life.
This study is the first to evaluate the relationship between the NHHR and psoriasis using the extensive and comprehensive NHANES database, which is nationally representative. The robustness and interpretability of this study are further improved by accounting for potential influencing factors. Nevertheless, there are some limitations of this study: first, although the diagnosis of psoriasis comes from a medical professional or health care practitioner, reliance on self-reported psoriasis conditions can lead to reporting bias and lack of clinical validation. In addition to this, the small sample size of patients diagnosed with psoriasis may lead to biased results; second, because this study was cross-sectional, it was impossible to establish with accuracy the cause-and-effect link between psoriasis and the NHHR. Third, certain factors could still affect the study’s findings even after taking possible confounders into account. For instance, the daily medication and lifestyle choices of the individuals. Owing to these drawbacks, it is imperative that further research be done in order to clarify the connection between NHHR and psoriasis and to aid in the creation of more efficient preventative and treatment plans.
Conclusions
The results indicate the 7% jump in risk per unit increase in NHHR, suggesting a connection between an elevated NHHR and a higher chance of developing psoriasis. However, longitudinal studies are still needed to validate the reliability and validity of the NHHR as a predictive biomarker, thus providing a solid scientific basis for psoriasis treatment and prevention.
Abbreviations
BMI, Body mass index; NCHS, National Center for Health Statistics; NHANES, National Health and Nutrition Examination Survey; NHHR, Non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio; HDL-C, High-density lipoprotein cholesterol; Non-HDL-C, Non-high-density lipoprotein cholesterol.
Data Sharing Statement
Publicly available datasets were examined for this investigation. The information is available at https://www.cdc.gov/nchs/nhanes/.
Ethics Statement
According to item 1 and 2 of Article 32 of “the Measures for Ethical Review of Life Science and Medical Research Involving Human Subjects”, this study is exempt from ethical review and approval.
Acknowledgments
The NHANES database is gratefully acknowledged for providing these valuable data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors state that there were no financial or commercial connections that would have given rise to a conflict of interest in relation to the study.
References
1. Scher JU, Ogdie A, Merola JF, Ritchlin C. Preventing psoriatic arthritis: focusing on patients with psoriasis at increased risk of transition. Nat Rev Rheumatol. 2019;15(3):153–166. doi:10.1038/s41584-019-0175-0
2. AlQassimi S, AlBrashdi S, Galadari H, Hashim MJ. Global burden of psoriasis - comparison of regional and global epidemiology, 1990 to 2017. Int J Dermatol. 2020;59(5):566–571. doi:10.1111/ijd.14864
3. Hao Y, Zhu YJ, Zou S, et al. Metabolic syndrome and psoriasis: mechanisms and future directions. Front Immunol. 2021;12:711060. doi:10.3389/fimmu.2021.711060
4. Souza CS, de Castro CCS, Carneiro FRO, et al. Metabolic syndrome and psoriatic arthritis among patients with psoriasis vulgaris: quality of life and prevalence. J Dermatol. 2019;46(1):3–10. doi:10.1111/1346-8138.14706
5. Ferdinando LB, Fukumoto PK, Sanches S, Fabricio LHZ, Skare TL. Metabolic syndrome and psoriasis: a study in 97 patients. Revista da Associacao Medica Brasileira. 2018;64(4):368–373. doi:10.1590/1806-9282.64.04.368
6. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377–390. doi:10.1016/j.jaad.2016.07.064
7. Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9(1):48. doi:10.1186/1741-7015-9-48
8. Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68(4):654–662. doi:10.1016/j.jaad.2012.08.015
9. Wu S, Li WQ, Han J, Sun Q, Qureshi AA. Hypercholesterolemia and risk of incident psoriasis and psoriatic arthritis in US women. Arthritis Rheumatol. 2014;66(2):304–310. doi:10.1002/art.38227
10. Mallbris L, Granath F, Hamsten A, Ståhle M. Psoriasis is associated with lipid abnormalities at the onset of skin disease. J Am Acad Dermatol. 2006;54(4):614–621. doi:10.1016/j.jaad.2005.11.1079
11. Ma C, Harskamp CT, Armstrong EJ, Armstrong AW. The association between psoriasis and dyslipidaemia: a systematic review. Br J Dermatol. 2013;168(3):486–495. doi:10.1111/bjd.12101
12. Pietrzak A, Michalak-Stoma A, Chodorowska G, Szepietowski JC. Lipid disturbances in psoriasis: an update. Mediators Inflammation. 2010;2010:1–13. doi:10.1155/2010/535612
13. Farshchian M, Zamanian A, Farshchian M, Monsef AR, Mahjub H. Serum lipid level in Iranian patients with psoriasis. J Eur Acad Dermatol Venereol. 2007;21(6):802–805. doi:10.1111/j.1468-3083.2006.02099.x
14. Paiva-Lopes MJ, Delgado Alves J. Psoriasis-associated vascular disease: the role of HDL. J Biomed Sci. 2017;24(1):73. doi:10.1186/s12929-017-0382-4
15. Mehta NN, Li R, Krishnamoorthy P, et al. Abnormal lipoprotein particles and cholesterol efflux capacity in patients with psoriasis. Atherosclerosis. 2012;224(1):218–221. doi:10.1016/j.atherosclerosis.2012.06.068
16. Caso F, Del Puente A, Oliviero F, et al. Metabolic syndrome in psoriatic arthritis: the interplay with cutaneous involvement. Evidences from literature and a recent cross-sectional study. Clin Rheumatol. 2018;37(3):579–586. doi:10.1007/s10067-017-3975-0
17. Wang Y, Ding L, Chen J, et al. Risk factors for progression from subclinical to clinical phase of psoriatic arthritis: a case-control study. Rheumatol ther. 2021;8(1):585–597. doi:10.1007/s40744-021-00295-y
18. Zhu L, Lu Z, Zhu L, et al. Lipoprotein ratios are better than conventional lipid parameters in predicting coronary heart disease in Chinese Han people. Kardiol Pol. 2015;73(10):931–938. doi:10.5603/KP.a2015.0086
19. Wang J, Li S, Pu H, He J. The association between the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and the risk of osteoporosis among U.S. adults: analysis of NHANES data. Lipids Health Dis. 2024;23(1):161. doi:10.1186/s12944-024-02152-7
20. Du YZ, Dong QX, Hu HJ, et al. A cross-sectional analysis of the relationship between the non-high density to high density lipoprotein cholesterol ratio (NHHR) and kidney stone risk in American adults. Lipids Health Dis. 2024;23(1):158. doi:10.1186/s12944-024-02150-9
21. Yang Q, Tao J, Xin X, Zhang J, Fan Z. Association between depression and infertility risk among American women aged 18–45 years: the mediating effect of the NHHR. Lipids Health Dis. 2024;23(1):178. doi:10.1186/s12944-024-02164-3
22. Kim SW, Jee JH, Kim HJ, et al. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int J Cardiol. 2013;168(3):2678–2683. doi:10.1016/j.ijcard.2013.03.027
23. Phan C, Ezzedine K, Lai C, et al. Agreement between self-reported inflammatory skin disorders and dermatologists’ diagnosis: a Cross-sectional Diagnostic Study. Acta Derm Venereol. 2017;97(10):1243–1244. doi:10.2340/00015555-2749
24. Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F. Dislipidemia and oxidative stress in mild and in severe psoriasis as a risk for cardiovascular disease. Int j Clin Chem. 2001;303(1–2):33–39. doi:10.1016/S0009-8981(00)00358-2
25. Kaur S, Kingo K, Zilmer M. Psoriasis and cardiovascular risk-do promising new biomarkers have clinical impact? Mediators Inflammation. 2017;2017:7279818. doi:10.1155/2017/7279818
26. Vanaclocha F, Belinchón I, Sánchez-Carazo JL, et al. Cardiovascular risk factors and cardiovascular diseases in patients with moderate to severe psoriasis under systemic treatment. PSO-RISK, descriptive study. Eur j dermatol. 2014;24(6):662–669. doi:10.1684/ejd.2014.2440
27. Pietrzak A, Chodorowska G, Szepietowski J, Zalewska-Janowska A, Krasowska D, Hercogová J. Psoriasis and serum lipid abnormalities. Dermatologic Therapy. 2010;23(2):160–173.
28. Liu L, Cai XC, Sun XY, et al. Global prevalence of metabolic syndrome in patients with psoriasis in the past two decades: current evidence. J Eur Acad Dermatol Venereol. 2022;36(11):1969–1979. doi:10.1111/jdv.18296
29. Sigit FS, Tahapary DL, Trompet S, et al. The prevalence of metabolic syndrome and its association with body fat distribution in middle-aged individuals from Indonesia and the Netherlands: a cross-sectional analysis of two population-based studies. Diabetol Metab Syndr. 2020;12:2. doi:10.1186/s13098-019-0503-1
30. Jones SM, Harris CP, Lloyd J, Stirling CA, Reckless JP, McHugh NJ. Lipoproteins and their subfractions in psoriatic arthritis: identification of an atherogenic profile with active joint disease. Ann Rheumatic Dis. 2000;59(11):904–909. doi:10.1136/ard.59.11.904
31. Hofmanis J, Hofmane D, Svirskis S, et al. HDL-C role in acquired aortic valve stenosis patients and its relationship with oxidative stress. Medicina. 2019;55(8):416. doi:10.3390/medicina55080416
32. Bastard JP, Couffignal C, Fellahi S, et al. Diabetes and dyslipidaemia are associated with oxidative stress independently of inflammation in long-term antiretroviral-treated HIV-infected patients. Diabetes Metabolism. 2019;45(6):573–581. doi:10.1016/j.diabet.2019.02.008
33. Pleńkowska J, Gabig-Cimińska M, Mozolewski P. Oxidative stress as an important contributor to the pathogenesis of psoriasis. Int J Mol Sci. 2020;21(17):6206. doi:10.3390/ijms21176206
34. Oszukowska M, Kozłowska M, Kaszuba A. Paraoxonase-1 and other factors related to oxidative stress in psoriasis. Postepy dermatologii i alergologii. 2020;37(1):92–96. doi:10.5114/ada.2020.93386
35. Nemati H, Khodarahmi R, Sadeghi M, Ebrahimi A, Rezaei M, Vaisi-Raygani A. Antioxidant status in patients with psoriasis. Cell Biochem. Funct. 2014;32(3):268–273. doi:10.1002/cbf.3011
36. Bai F, Zheng W, Dong Y, et al. Serum levels of adipokines and cytokines in psoriasis patients: a systematic review and meta-analysis. Oncotarget. 2018;9(1):1266–1278. doi:10.18632/oncotarget.22260
37. Yanai H, Yoshida H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression. Mech Perspect. 2019;20(5):1190.
38. Sereflican B, Goksugur N, Bugdayci G, Polat M, Haydar Parlak A. Serum visfatin, adiponectin, and Tumor Necrosis Factor Alpha (TNF-α) levels in patients with psoriasis and their correlation with disease severity. Acta Dermatovenerol Croat. 2016;24(1):13–19.
39. Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–994. doi:10.1016/S0140-6736(14)61909-7
40. Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science. 2013;339(6116):172–177. doi:10.1126/science.1230721
41. Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13(6):465–476. doi:10.1038/nrd4275
42. Holzer M, Wolf P, Curcic S, et al. Psoriasis alters HDL composition and cholesterol efflux capacity. J Lipid Res. 2012;53(8):1618–1624. doi:10.1194/jlr.M027367
43. Nowowiejska J, Baran A, Flisiak I. Aberrations in lipid expression and metabolism in psoriasis. Int J Mol Sci. 2021;22(12). doi:10.3390/ijms22126561
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Fibrinogen-Like Protein 1 as a Novel Biomarker of Psoriasis Severity
Sun X, Liu L, Chen S, Wang J, Cai X, Song J, Zhou M, Guo D, Kuai L, Ding X, Li B, Li X
Journal of Inflammation Research 2022, 15:4637-4647
Published Date: 15 August 2022
The SELP, CD93, IL2RG, and VAV1 Genes Associated with Atherosclerosis May Be Potential Diagnostic Biomarkers for Psoriasis
Liu S, Liu F, Zhang Z, Zhuang Z, Yuan X, Chen Y
Journal of Inflammation Research 2023, 16:827-843
Published Date: 27 February 2023
Metabolomics Reveals Molecular Signatures for Psoriasis Biomarkers and Drug Targets Discovery
Song Q, Chen Y, Ma J, Zhou W, Song J, Wu C, Liu J
Clinical, Cosmetic and Investigational Dermatology 2023, 16:3181-3191
Published Date: 4 November 2023
Association of Complete Blood Cell Count-Derived Inflammatory Biomarkers with Psoriasis and Mortality
Zhao Y, Yang XT, Bai YP, Li LF
Clinical, Cosmetic and Investigational Dermatology 2023, 16:3267-3278
Published Date: 13 November 2023
Serum Direct Bilirubin as a Biomarker for Breast Cancer
Hu J, Cai Y, Chen Y, Zhu X
Breast Cancer: Targets and Therapy 2024, 16:735-743
Published Date: 7 November 2024

