Back to Journals » Nature and Science of Sleep » Volume 16
Association Between Sleep Quality and Cirrhotic Cardiomyopathy: A Prospective Case-Control Study
Authors Liu F, Cao T, Liu Y, Huang D, Zhang J
Received 27 June 2024
Accepted for publication 1 December 2024
Published 6 December 2024 Volume 2024:16 Pages 1949—1958
DOI https://doi.org/10.2147/NSS.S482592
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Valentina Alfonsi
Fei Liu,1,* Tianqing Cao,1,* Yacong Liu,1 Dian Huang,2 Jingxin Zhang3
1Department of Cardiology, Northern Jiangsu People’s Hospital Affiliated to Yangzhou University, Yangzhou, Jiangsu, 225001, People’s Republic of China; 2Department of Radiology, Affiliated hospital of Jiangsu University, Zhenjiang, Jiangsu, 212001, People’s Republic of China; 3Department of General Surgery, People’s Hospital Affiliated to Jiangsu University, Zhenjiang, Jiangsu, 212002, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jingxin Zhang, Email [email protected]
Objective: The main purpose of this study is to evaluate the changes in sleep quality among patients with cirrhotic cardiomyopathy (CCM).
Methods: The study included liver cirrhosis patients aged 18– 75 from Northern Jiangsu People’s Hospital Affiliated to Yangzhou University and collected their clinical examination results to assess the clinical characteristics and related risk factors of patients with CCM.
Results: The study found that the onset of CCM was not related to the etiology of inducing cirrhosis. Pittsburgh Sleep Quality Index (PSQI) score (odds ratio (OR) = 13.476, 95% confidence interval (CI) = 1.514– 119.923, P = 0.020), absolute GLS (OR = 0.328, 95% CI = 0.210– 0.510, P < 0.001), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) (OR = 1.050, 95% CI = 1.025– 1.076, P < 0.001) were identified as independent risk factors for inducing CCM.
Conclusion: In patients with CCM, a decrease in sleep quality often occurs. When cirrhotic patients also have poor sleep quality, along with a decrease in absolute Global Left Ventricular Strain (GLS) levels and an increase in NT-proBNP levels, these factors may pose a higher risk for CCM development. However, further validation of these research findings is required in larger sample sizes.
Keywords: cardiomyopathy, liver cirrhosis, sleep quality, cirrhotic cardiomyopathy
Introduction
Cirrhosis is a terminal stage of chronic liver disease characterized by liver necrosis, fibrosis, nodules, and other manifestations.1 Decades ago, clinicians first observed circulatory dysfunction in patients with alcoholic cirrhosis.2 Subsequent research indicated that secondary cardiac functional abnormalities in patients may not be solely due to moderate alcohol consumption but could be primarily caused by the progression of cirrhosis itself. Lee introduced the concept of “cirrhotic cardiomyopathy (CCM)” to describe the symptom of circulatory dysfunction in cirrhotic patients without pre-existing cardiac conditions.3–5 While recent studies have elucidated the relationship between the liver and heart, the exact etiology of CCM remains uncertain.6,7
Cirrhotic cardiomyopathy (CCM) is a symptom of circulatory dysfunction in patients with cirrhosis, characterised by abnormal cardiac function in the absence of pre-existing cardiac disease. The pathogenesis of cirrhotic cardiomyopathy is multifactorial but consists of two broad pathways. The first is due to cirrhosis and synthetic liver failure, with structural and functional abnormalities of many substances, including proteins, lipids, hormones and carbohydrates (eg lectins). The second is due to portal hypertension, which always accompanies cirrhosis. Portal hypertension leads to leaky, congested gut, which leads to endotoxaemia and systemic inflammation. This inflammatory phenotype includes oxidative stress, apoptosis and inflammatory cell infiltration.8
Sleep is a necessary physiological activity for humans, which promotes physical recovery through regulating the variation of body temperature and metabolic processes between day and night, and influences memory, organizing information collected during the day, and controlling emotional fluctuations by regulating brain states.9–13
However, people are often troubled by insomnia due to physiological or psychological factors.14 According to related studies, half of the population has experienced insomnia in the past year, and even one-third of individuals are disturbed by insomnia every night.15–17 Furthermore, as age increases, the frequency and duration of awakenings during sleep also increase annually.18
Insomnia can also have a significant impact on various aspects of life. For example, insufficient sleep often leads to physical fatigue, poor mental state, irritability, anxiety, depression, and emotional instability, as well as cognitive decline.19–23 Over time, it can even affect immune function and increase the incidence of cardiovascular and cerebrovascular diseases.24–28 Research also suggests that a portion of the decreased quality of life in patients with chronic illnesses may be attributed to inadequate sleep quality.28,29
LV remodelling (left ventricular remodelling) is an adaptive change in the heart in response to injury or stress loads, which may lead to changes in cardiac structure and function. In patients with CCM, LV remodelling is an important diagnostic basis, in which impaired systolic function may be one of the diagnostic criteria for CCM.30 NT-proBNP (N-terminal B-type natriuretic peptide precursor) is a biomarker released by cardiac myocytes in response to stress or injury, and is commonly used to assess cardiac function and the severity of heart failure. Studies have shown that serum levels of NT-proBNP are significantly elevated in patients with CCM, suggesting that it may serve as one of the diagnostic indicators of CCM and is associated with disease progression and poor patient prognosis.31 Although the direct causal relationship between insomnia and CCM has not been clearly established, some studies have suggested that decreased sleep quality may be associated with impaired cardiac function.32 We found that patients with poor sleep quality were often accompanied by elevated NT-proBNP levels, which may imply that poor sleep quality may exacerbate the burden on the heart.
Research has confirmed the impact of sleep disorders in patients with heart failure. Studies have found that 50–70% of heart failure patients also have sleep problems, and insomnia is suspected to be an independent risk factor for heart failure.33–35 However, so far, there have been no related studies published on the relationship between sleep status and clinical manifestations and outcomes in patients with chronic congestive myopathy. Therefore, the primary goal of this study is to innovatively evaluate the sleep quality of patients with chronic congestive myopathy and explore the relationship between sleep quality and factors assessing heart function status. The rationale for this study is that it aims to fill the knowledge gap on the relationship between cirrhotic cardiomyopathy and sleep quality. By prospectively assessing sleep quality in patients with CCM, we are able to better understand the potential link between cardiac functional status and sleep quality and provide important information for future treatment and intervention.
Materials and Methods
Inclusion and Exclusion Criteria
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and was approved by the Ethics Committee of Northern Jiangsu People’s Hospital Affiliated to Yangzhou University. Informed consent was obtained from all individual participants included in the study. The prospective analysis of this study included cirrhotic patients admitted to the Department of Gastroenterology from Jan 2022 to Jan 2024, excluding patients with other underlying diseases. In addition, patients who have undergone liver transplantation or transjugular intrahepatic portosystemic shunt procedures are also not the target population of this study. All patients participating in this study have signed informed consent.
Echocardiographic Evaluation
For the patients included in the study, we collected their basic information. After admission, we obtained a 12-lead electrocardiogram to evaluate the patients’ risk of myocardial ischemia and arrhythmias, and collected venous blood for laboratory tests of relevant parameters. We graded the liver function of the selected subjects using the Child-Pugh score and the Albumin-Bilirubin (ALBI) grade.
Doppler echocardiography was performed by experienced echocardiologists following the guidelines, and relevant parameters were collected.
Epworth Sleepiness Scale (ESS) Evaluation
We use the translated into Mandarin version of the Epworth Sleepiness Scale (ESS) to assess patients’ daytime sleepiness, which consists of 8 sections, each using a 4-point rating scale, with a total score of 24. A higher score indicates a higher tendency for daytime sleepiness.
PSQI Evaluation
Similarly, we also use the Mandarin version of a Pittsburgh Sleep Quality Index (PSQI) scale that includes 7 main sections subdivided into 18 items to assess patients’ sleep quality and sleep status.36,37
Statistical Analysis
The statistical analysis will be performed using IBM SPSS Statistics 26.0. Quantitative parameters will be presented as mean ± standard deviation. Group differences will be evaluated using either Student’s t-test or the Mann–Whitney nonparametric test. Categorical data will be expressed as percentages and compared using the chi-square test. Multiple regression analyses will be conducted to identify risk factors for the occurrence of CCM. Receiver Operating Characteristic (ROC) analysis will be utilized to determine sensitivity, specificity, and the area under the curve (AUC) for sleep quality and cardiac impairment indices. A p-value below 0.05 will be considered statistically significant.
Results
Study Population
Out of the 198 cirrhosis patients initially screened, 135 were included in the analysis after exclusions (see Figure 1). Using the 2020 CCM diagnostic criteria, the patients were divided into two groups: 45 patients in the CCM group and 90 patients in the non-CCM group. Table 1 presents a detailed overview of the baseline characteristics for each group.
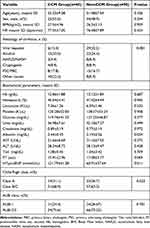 |
Table 1 Demographic Distribution of Groups |
 |
Figure 1 Patient enrollment flowchart. |
Comparison of Baseline Information
Alcohol (n = 37, 27.4%) was the most common etiology of cirrhosis among the patients, followed by viral hepatitis (n = 35, 25.9%). The prevalence of CCM was not associated with the cause of cirrhosis (P = 0.081). Despite patients with Child-B/C cirrhosis displaying an increased risk of CCM compared to Child-A patients, this difference did not reach statistical significance. Likewise, patients with ALBI grade 2/3 cirrhosis exhibited a higher likelihood of developing CCM than those with ALBI grade 1, although the difference was not statistically significant.
Serum laboratory indices, such as hemoglobin (P = 0.687), hematocrit (P = 0.945), leucocyte (P = 0.532), platelet (P = 0.958), glucose (P = 0.377), urea (P = 0.499), creatinine (P = 0.972), albumin (P = 0.054), AST (P = 0.375), ALT (P = 0.428), total bilirubin (P = 0.709), and prothrombin time (P = 0.069), showed no significant differences between the two groups. Nonetheless, NT-proBNP levels were significantly higher in patients with CCM compared to those without CCM (121.79 vs 60.91; P = 0.011).
Comparison of Echocardiographic Characteristics
In Table 2, echocardiographic parameters were compared between CCM patients and non-CCM patients. CCM patients showed a significantly higher E/e’ ratio (12.54 ± 0.82 vs 6.89 ± 0.56; P = 0.009) compared to non-CCM patients. Furthermore, the deceleration time was higher in CCM patients (219.44 ± 47.59) than in non-CCM patients (216.95 ± 34.87; P = 0.008). Left ventricle end diastolic volume was lower in CCM patients (45.15 ± 6.07) compared to non-CCM patients (46.02 ± 10.37; P = 0.001). Despite both groups having normal LVEF values (62.14 ± 6.93 vs 58.14 ± 6.44; P = 0.724), CCM patients had a significantly lower absolute GLS value (18.25 ± 1.86 vs 21.33 ± 1.30; P = 0.018). Isovolumic ventricular relaxation time showed no significant difference (P = 0.090) between the two groups, as did left ventricle end systolic volume (P = 0.054).
 |
Table 2 Comparison Echocardiographic Characteristics Between Groups |
Comparison of ESS and PSQI Scores
Patients with CCM had significantly higher PSQI scores, indicating poorer sleep quality compared to controls (p< 0.001), as shown in Table 3. Despite having a longer sleep duration, individuals with CCM reported longer sleep latency, more sleep disturbances, and daytime dysfunctions. Interestingly, there was no difference in daytime somnolence between patients with CCM and controls, reflected in similar scores on the Epworth Sleepiness Scale. Furthermore, an analysis within the study group revealed a potential weak association between markers of cardiac injury (Figure 2A), absolute GLS (Figure 2B), and the overall PSQI score.
 |
Table 3 ESS Scores, PSQI Components Scores and Global Scores for Both Groups |
 |
Figure 2 Correlation between global PSQI score and (A) NT-proBNP, (B) Absolute GLS. |
Multiple Logistic Regression Analysis of Factors Associated with the Occurrence of Cirrhotic Cardiomyopathy
After conducting stepwise multiple logistic regression analysis, independent predictors of CCM were identified as the PSQI score (odds ratio (OR) = 13.476, 95% confidence interval (CI) = 1.514–119.923, P = 0.020), absolute GLS (OR = 0.328, 95% CI = 0.210–0.510, P < 0.001), and NT-proBNP level (OR = 1.050, 95% CI = 1.025–1.076, P < 0.001) (refer to Table 4). The ROC curves, displayed in Figure 3, indicate the predictive performance of PSQI score, absolute GLS, and NT-proBNP values for CCM.
 |
Table 4 Logistic Regression Analysis of Factors Associated with Occurrence of Cirrhotic Cardiomyopathy |
 |
Figure 3 Comparison of amphiregulin with other indicators, such as PSQI score, absolute GLS and NT -proBNP for the diagnosis of CCM by areas under the receiver operating curves (AUROCs). |
Discussion
In 2005, experts proposed corresponding diagnostic criteria for CCM, a disease characterized by pathological physiological mechanisms of ventricular remodeling and clinical manifestations of cardiac dysfunction. Impaired systolic function may be one of the diagnostic criteria for CCM. However, due to the insidious onset of the disease, which often occurs in patients with advanced liver cirrhosis, diagnosis of CCM is easily overlooked.38–40 Current data remain very limited. It is worth noting that diastolic dysfunction is common in patients with cirrhosis and is associated with increased risk of mortality. The present study focused on the association between sleep quality and cirrhotic cardiomyopathy (CCM). It was found that the onset of CCM does not depend on the specific etiology leading to cirrhosis. Patients with CCM had significantly poorer sleep quality, as assessed by the Pittsburgh Sleep Quality Index (PSQI), and higher PSQI scores (p<0.001). Compared to controls, patients with CCM reported longer sleep latency, more sleep disturbances, and more severe daytime dysfunction. As CCM progresses, the increased cardiac burden may lead to repeated hospitalisations or limitations in daily activities, which may further affect their sleep quality and reduce their quality of life. Studies have suggested that poor sleep quality may be an important prognostic factor in patients with CCM, which is associated with not only poor sleep quality, but also reduced levels of left ventricular longitudinal strain (GLS) and increased levels of N-terminal B-type natriuretic peptide proteins (NT-proBNP), which collectively constitute a higher risk for the development of CCM.
Researchers in the field of myocardial injury have been exploring noninvasive cardiac markers for accurate diagnosis, some of which can trigger left ventricular changes that play a significant role in the development of dilated cardiomyopathy (CCM). NT-proBNP has emerged as a sensitive serum biomarker for myocardial injury, with elevated levels indicating a heightened risk of cardiovascular disease. In a study by Henriksen et al, it was discovered that high levels of serum NT-proBNP were linked to advanced cirrhosis, left ventricular systolic dysfunction, and poor patient outcomes.41 In the present study, echocardiography played an important role in assessing the functional status of the heart in patients with cirrhosis. The results showed that patients with CCM had a significantly higher E/A ratio, which reflects the impaired diastolic function of the heart. E/A ratio is an important indicator for assessing the diastolic function of the heart, and its elevation implies that the heart’s ability to fill up with blood during diastole is reduced, which may increase the risk of cardiovascular disease. In addition, the results found that serum NT-proBNP levels were significantly higher in patients with CCM than in non-CCM patients, suggesting that elevated serum NT-proBNP concentrations could be an important indicator for identifying those at risk for CCM. At the same time, the study also found that absolute GLS levels were decreased in patients with CCM. Although the exact mechanism is not fully understood, GLS is often considered a sensitive indicator of myocardial function. Its decline may reflect diminished myocardial contractility or strain, which is consistent with impaired cardiac function in patients with CCM.
Poor sleep quality has long been recognised as an important contributing factor to a wide range of diseases, including heart disease. Sleep deprivation or sleep disruption not only affects an individual’s daily functioning and quality of life, but is also closely associated with a range of health problems such as cardiovascular disease, metabolic disorders, and decreased immune system function. In this study, we found a significant correlation between poor sleep quality and the development of cirrhotic cardiomyopathy (CCM). Specifically, as assessed by the Pittsburgh Sleep Quality Index (PSQI), patients with CCM had significantly higher PSQI scores than non-CCM patients, suggesting that they had poorer sleep quality. This result implies that sleep quality may play a potentially important role in the pathogenesis of CCM and provides new insights into the relationship between sleep and heart health.
Further analyses revealed that PSQI scores correlated with a variety of CCM-related factors. These factors included, but were not limited to, cardiac damage markers, absolute left ventricular sphericity index (GLS), and serum N-terminal B-type natriuretic peptide proteins (NT-proBNP) levels. Cardiac injury markers and GLS are important parameters for assessing cardiac function and structure, whereas NT-proBNP levels are strongly associated with cardiac load and degree of heart failure. The association of PSQI scores with these factors found in this study may imply that poor sleep quality not only directly affects patients’ psychological status and daily functioning, but may also exacerbate the progression of CCM by affecting cardiac function and structure. Therefore, improving sleep quality may be a new way to prevent and treat CCM.
Reviewing previous studies, predictors of CCM mainly include severity of liver disease, cardiac function parameters, and serum markers.42 However, these predictors still have some limitations in comprehensively assessing the risk of CCM. In this study, a more comprehensive CCM prediction model was constructed by incorporating a new dimension of sleep quality. The model not only considered the traditional liver- and heart-related factors, but also incorporated PSQI scores, which resulted in a more accurate assessment of the risk of developing CCM. The strength of this model is that it provides a deeper understanding of the pathogenesis of CCM and offers new strategies for personalised treatment and risk management. However, it is worth noting that this study is a single-centre study with a limited sample size, and thus the constructed predictive model needs to be validated in further large-scale studies. In the future, with the accumulation of more data and in-depth studies, we expect to develop more accurate and reliable CCM prediction tools, which can provide more powerful support for patients’ health management and clinical treatment.
While the study had adequate power for data analysis, the classification of cirrhotic patients based on recent CCM criteria resulted in small subgroups, potentially affecting statistical relevance. Furthermore, being a single-center study with a limited sample size, there is a possibility of biased results. To better comprehend the current prevalence of CCM, future studies should involve larger multicenter prospective investigations. The study being solely cross-sectional, there was no subsequent follow-up of cirrhotic patients to explore CCM’s impact on the emergence of other cirrhosis complications. Additionally, the study utilized a sleep questionnaire to evaluate subjective sleep quality, although the complete elucidation of the new findings’ mechanisms remains incomplete.
Conclusion
In patients with CCM, a decrease in sleep quality often occurs. When cirrhotic patients also have poor sleep quality, along with a decrease in absolute GLS levels and an increase in NT-proBNP levels, these factors may pose a higher risk for CCM development. However, further validation of these research findings is required in larger sample sizes.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kalluru R, Gadde S, Chikatimalla R, Dasaradhan T, Koneti J, Cherukuri SP. Cirrhotic cardiomyopathy: the interplay between liver and heart. Cureus. 2022;14(8):e27969. doi:10.7759/cureus.27969
2. Kaur H, Premkumar M. Diagnosis and management of cirrhotic cardiomyopathy. J Clin Exp Hepatol. 2022;12(1):186–199. doi:10.1016/j.jceh.2021.08.016
3. Zhong H, Dong J, Zhu L, et al. Non-alcoholic fatty liver disease: pathogenesis and models. Am J Transl Res. 2024;16(2):387–399. doi:10.62347/KMSA5983
4. Anikhindi SA, Ranjan P, Kumar M, Mohan R. A prospective study of prevalence and predictors of cirrhotic cardiomyopathy and its role in development of hepatorenal syndrome. J Clin Exp Hepatol. 2022;12(3):853–860. doi:10.1016/j.jceh.2021.11.005
5. Srinivasamurthy BC, Saravanan SP, Marak FK, Manivel P, Bhat RV, Mathiyazhagan D. Morphological cardiac alterations in liver cirrhosis: an autopsy study. Heart Views. 2021;22(2):96–101. doi:10.4103/HEARTVIEWS.HEARTVIEWS_14_21
6. Scarpati G, De Robertis E, Esposito C, Piazza O. Hepatic encephalopathy and cirrhotic cardiomyopathy in intensive care unit. Minerva Anestesiol. 2018;84(8):970–979. doi:10.23736/S0375-9393.18.12343-1
7. Elleuch N, Mrabet S, Ben Slama A, et al. Cirrhotic cardiomyopathy. Tunis Med. 2020;98(3):206–210.
8. Liu H, Hwang S-Y, Lee SS. Role of galectin in cardiovascular conditions including cirrhotic cardiomyopathy. Pharmaceuticals. 2023;16(7):978. doi:10.3390/ph16070978
9. Morin CM, Bei B, Bjorvatn B, et al. World sleep society international sleep medicine guidelines position statement endorsement of ”behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of sleep medicine clinical practice guidelines”. Sleep Med. 2023;109:164–169. doi:10.1016/j.sleep.2023.07.001
10. Baranwal N, Yu PK, Siegel NS. Sleep physiology, pathophysiology, and sleep hygiene. Prog Cardiovasc Dis. 2023;77:59–69. doi:10.1016/j.pcad.2023.02.005
11. Douglas NJ. Clinicians’ guide to sleep medicine. London;Arnold:2002:xi,251p.
12. Empson J, Wang MB. Sleep and Dreaming.
13. Chaput JP, McHill AW, Cox RC, et al. The role of insufficient sleep and circadian misalignment in obesity. Nat Rev Endocrinol. 2023;19(2):82–97. doi:10.1038/s41574-022-00747-7
14. Foundation NS. “Sleep in America” poll. 2002;44.
15. Liu S, Wang X, Zheng Q, Gao L, Sun Q. Sleep deprivation and central appetite regulation. Nutrients. 2022;14(24):5196. doi:10.3390/nu14245196
16. Schreck KA, Richdale AL. Sleep problems, behavior, and psychopathology in autism: inter-relationships across the lifespan. Curr Opin Psychol. 2020;34:105–111. doi:10.1016/j.copsyc.2019.12.003
17. Di H, Guo Y, Daghlas I, et al. Evaluation of sleep habits and disturbances among US adults, 2017-2020. JAMA Network Open. 2022;5(11):e2240788. doi:10.1001/jamanetworkopen.2022.40788
18. Liang X, Haegele JA, Healy S, et al. Age-related differences in accelerometer-assessed physical activity and sleep parameters among children and adolescents with and without autism spectrum disorder: a meta-analysis. JAMA Network Open. 2023;6(10):e2336129. doi:10.1001/jamanetworkopen.2023.36129
19. Gandhi KD, Mansukhani MP, Silber MH, Kolla BP. Excessive daytime sleepiness: a clinical review. Mayo Clin Proc. 2021;96(5):1288–1301. doi:10.1016/j.mayocp.2020.08.033
20. Giannoni A, Gentile F, Sciarrone P, et al. Upright Cheyne-stokes respiration in patients with heart failure. J Am Coll Cardiol. 2020;75(23):2934–2946. doi:10.1016/j.jacc.2020.04.033
21. Akkaoui MA, Palagini L, Geoffroy PA. Sleep immune cross talk and insomnia. Adv Exp Med Biol. 2023;1411:263–273. doi:10.1007/978-981-19-7376-5_12
22. Garbarino S, Lanteri P, Bragazzi NL, Magnavita N, Scoditti E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun Biol. 2021;4(1):1304. doi:10.1038/s42003-021-02825-4
23. Hudgens S, Phillips-Beyer A, Newton L, Seboek Kinter D, Benes H. Development and Validation of the Insomnia Daytime Symptoms and Impacts Questionnaire (IDSIQ). Patient. 2021;14(2):249–268. doi:10.1007/s40271-020-00474-z
24. Zhao M, Tuo H, Wang S, Zhao L. The effects of dietary nutrition on sleep and sleep disorders. Mediators Inflamm. 2020;2020:3142874. doi:10.1155/2020/3142874
25. Choi K, Lee YJ, Park S, Je NK, Suh HS. Efficacy of melatonin for chronic insomnia: systematic reviews and meta-analyses. Sleep Med Rev. 2022;66:101692. doi:10.1016/j.smrv.2022.101692
26. Pearson O, Uglik-Marucha N, Miskowiak KW, et al. The relationship between sleep disturbance and cognitive impairment in mood disorders: a systematic review. J Affect Disord. 2023;327:207–216. doi:10.1016/j.jad.2023.01.114
27. Nyhuis CC, Fernandez-Mendoza J. Insomnia nosology: a systematic review and critical appraisal of historical diagnostic categories and current phenotypes. J Sleep Res. 2023;32(6):e13910. doi:10.1111/jsr.13910
28. Leger D, Scheuermaier K, Philip P, Paillard M, Guilleminault C. SF-36: evaluation of quality of life in severe and mild insomniacs compared with good sleepers. Psychosom Med. 2001;63(1):49–55. doi:10.1097/00006842-200101000-00006
29. Wong AK, Wang D, Marco D, Le B, Prevalence PJ. Severity, and predictors of insomnia in advanced colorectal cancer. J Pain Symptom Manage. 2023;66(3):e335–e342. doi:10.1016/j.jpainsymman.2023.05.020
30. Groen RA, Ajmone Marsan N, Jukema JW, Coenraad MJ. Assessment of myocardial dysfunction in cirrhotic patients: should we look at the left atrium rather than at the left ventricle? Liver Int. 2023;43(12):2586–2588. doi:10.1111/liv.15755
31. Echeverría LE, Rojas LZ, Villamizar MC, et al. Echocardiographic parameters, speckle tracking, and brain natriuretic peptide levels as indicators of progression of indeterminate stage to Chagas cardiomyopathy. Echocardiography. 2020;37(3):429–438. doi:10.1111/echo.14603
32. Türoff A, Thiem U, Fox H, et al. Sleep duration and quality in heart failure patients. Sleep Breathing. 2017;21(4):919–927. doi:10.1007/s11325-017-1501-x
33. Locke BW, Lee JJ, Sundar KM. OSA and chronic respiratory disease: mechanisms and epidemiology. Int J Environ Res Public Health. 2022;19(9):5473. doi:10.3390/ijerph19095473
34. Riemann D, Benz F, Dressle RJ, et al. Insomnia disorder: state of the science and challenges for the future. J Sleep Res. 2022;31(4):e13604. doi:10.1111/jsr.13604
35. Andrade CCF, Silva RT, Brunherotti MAA. Effects of inspiratory muscle training in patients with class III and IV heart failure. Curr Probl Cardiol. 2022;47(10):101307. doi:10.1016/j.cpcardiol.2022.101307
36. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
37. Tsai PS, Wang SY, Wang MY, et al. Psychometric evaluation of the Chinese version of the Pittsburgh sleep quality index (CPSQI) in primary insomnia and control subjects. Qual Life Res. 2005;14(8):1943–1952. doi:10.1007/s11136-005-4346-x
38. Douschan P, Kovacs G, Sassmann T, et al. Pulmonary vascular disease and exercise hemodynamics in chronic liver disease. Respir Med. 2022;202:106987. doi:10.1016/j.rmed.2022.106987
39. Gojowy D, Urbaniec-Stompor J, Adamusik J, et al. Cardiovascular risk factors in patients before and after successful liver transplantation. Ann Transplant. 2022;27:e935656. doi:10.12659/AOT.935656
40. Simic S, Svagusa T, Grgurevic I, Mustapic S, Zarak M, Prkacin I. Markers of cardiac injury in patients with liver cirrhosis. Croat Med J. 2023;64(5):362–373. doi:10.3325/cmj.2023.64.362
41. Henriksen JH, Gotze JP, Fuglsang S, Christensen E, Bendtsen F, Moller S. Increased circulating pro-brain natriuretic peptide (proBNP) and brain natriuretic peptide (BNP) in patients with cirrhosis: relation to cardiovascular dysfunction and severity of disease. Gut. 2003;52(10):1511–1517. doi:10.1136/gut.52.10.1511
42. Vergaro G, Echeverría LE, Rojas LZ, et al. Cardiovascular biomarkers as predictors of adverse outcomes in chronic Chagas cardiomyopathy. PLoS One. 2021;16(10). doi:10.1371/journal.pone.0258622
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

