Back to Journals » Journal of Pain Research » Volume 18
Association Between Spinopelvic Alignment and Reoperation Following Percutaneous Transforaminal Endoscopic Decompression: A Matched Case-Control Study
Authors Ge Y, Wang A, Song H, Fan N, Zang L
Received 9 November 2024
Accepted for publication 23 February 2025
Published 18 March 2025 Volume 2025:18 Pages 1351—1360
DOI https://doi.org/10.2147/JPR.S505372
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Krishnan Chakravarthy
Yang Ge, Aobo Wang, He Song, Ning Fan, Lei Zang
Department of Orthopedics, Beijing Chaoyang Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Lei Zang, Department of Orthopedics, Beijing Chaoyang Hospital, 5 JingYuan Road, Shijingshan District, Beijing, 100043, People’s Republic of China, Email [email protected]
Purpose: Percutaneous transforaminal endoscopic decompression (PTED) is widely used for treating lumbar spinal stenosis (LSS), yet predictors of reoperation remain unclear. This study aimed to explore the association between spinopelvic alignment and the reoperation following PTED.
Patients and Methods: A 1:2 matched case-control study was conducted, involving patients who underwent single-level PTED for LSS at our institution from May 2014 to August 2022. Cases comprised patients requiring reoperation after initial PTED, while controls were those without reoperation during the follow-up. Measured radiological parameters included pelvic tilt (PT), pelvic incidence (PI), sacral slope (SS), lumbar lordosis (LL), mismatch between pelvic incidence and lumbar lordosis (PI-LL), disc height (DH), Pfirrmann classification, and Modic changes (MCs). Univariate and multivariate logistic regression analyses were performed to identify predictors. Receiver operating characteristic (ROC) curves were generated to determine cut-off points.
Results: 76 cases and 152 controls were selected from 1967 enrolled patients. Both groups had an average age of 61 years, a male-to-female ratio of 43:33, and a mean BMI of 25.95 kg/m². No significant differences in baseline characteristics were found between groups. Multivariate analysis identified PT (OR = 1.061, P = 0.007), PI-LL (OR = 1.057, P = 0.021), and DH (OR = 1.194, P = 0.015) as independent risk factors for the reoperation. ROC analysis revealed PI-LL with an area under the curve (AUC) of 0.662 at a cut-off of 12.95° (95% CI = 0.582– 0.741), PT with an AUC of 0.685 at a cut-off of 21.98° (95% CI = 0.606– 0.763), and DH with an AUC of 0.602 at a cut-off of 8.22° (95% CI = 0.521– 0.683).
Conclusion: PI-LL ≥ 12.95°, PT ≥ 21.98°, and DH ≥ 8.22° are independent risk factors for reoperation following PTED.
Keywords: spinal stenosis, decompression, endoscopy, lordosis, lumbosacral region, case-control studies
Introduction
Lumbar spinal stenosis (LSS) is a leading cause of spinal operations in individuals over 65.1 It is characterized by the narrowing of the spinal canal due to degenerative changes, including osteophyte formation, disc bulging, hypertrophic facet joints, and thickening of the ligamentum flavum. These changes often result in the compression of neurovascular structures, leading to symptoms such as neurogenic claudication and radiculopathy.2 The management of LSS begins with conservative measures such as activity modification, physical therapy, and oral medications (eg, nonsteroidal anti-inflammatory drugs [NSAIDs]).3 In cases refractory to conservative therapies, open laminectomy has long been the standard surgical intervention. While generally efficacious, it can result in spinal instability and chronic pain by damaging the posterior midline structures.4 As a minimally invasive alternative, percutaneous transforaminal endoscopic decompression (PTED) integrates percutaneous endoscopic lumbar discectomy with foraminoplasty. By partially resecting the superior articular process (SAP), PTED enables access to deeper regions of the spinal canal, facilitating further decompression while preserving the posterior structures.5
PTED offers several advantages over open procedures, such as reduced operating time, fewer complications, less blood loss, and shorter hospitalization.6,7 It can be performed under local anesthesia, yielding satisfactory outcomes for elderly patients who are at risk under general anesthesia.8,9 However, the limited visibility and restricted working space can diminish the effectiveness of PTED in achieving decompression. Despite these advantages, studies report PTED reoperation rates ranging from 4.4% to 8.3%, raising concerns about patient suffering, increased risk of complications, and higher healthcare costs.10–12 Therefore, identifying the risk factors for reoperation after PTED is essential for enhancing patient outcomes.
Spinopelvic alignment describes the anatomical relationship between the spine and pelvis, measured by indicators including pelvic incidence (PI), pelvic tilt (PT), sacral slope (SS), lumbar lordosis (LL), and the difference between PI and LL (PI-LL). These spinopelvic parameters have been utilized to predict the need for subsequent fusion and the risk of adjacent-segment disease following open decompression procedures.13,14 They have also shown predictive value for clinical outcomes following minimally invasive decompression procedures.15 Given the established correlation between spinopelvic parameters and the outcomes of other decompression operations, we hypothesized that reoperation after PTED is associated with spinopelvic malalignment.
While recent studies have found several risk factors for reoperation following PTED, including disc degeneration, facet joint deterioration, and muscle atrophy,16,17 the influence of spinopelvic alignment on the reoperation remains unexplored. Elucidating this relationship could deepen our understanding of PTED failures, refine preoperative planning, reduce the risk of reoperation, and guide rehabilitation protocols. Therefore, we conducted this 1:2 matched case-control study to investigate the association between spinopelvic alignment and reoperation following PTED.
Methods
Subjects
Patients with LSS (ICD-10: M48.06) who underwent single-level PTED at our hospital from May 2014 to August 2022 were retrospectively reviewed. The inclusion criteria were: (1) definitive LSS diagnosis confirmed through physical examination, clinical symptoms, and MRI/CT imaging; (2) both diagnostic evaluation and surgical procedures were performed by the same senior orthopedic surgeon; (3) age ≥18 years with independent ambulatory capacity; and (4) documented failure of conservative treatment for at least three months.
Patients were excluded based on the following criteria: (1) history of spinal surgery; (2) concomitant spinal conditions such as infections, fractures, tumors, or spondylolisthesis; (3) a Cobb angle greater than ten degrees; (4) follow-up duration of less than two years; (5) incomplete clinical or radiological data; (6) severe osteoporosis (T-score ≤ −2.5) or history of fragility fractures; (7) metabolic bone diseases such as Paget’s disease or hyperparathyroidism; and (8) neurological disorders affecting spinal stability, including Parkinson’s disease, cerebral palsy, or multiple sclerosis. This study adhered to the Declaration of Helsinki and obtained approval from the ethics committee of our institution.
Grouping and Matching
This 1:2 retrospective matched case-control study was conducted at our hospital from July 2024 to October 2024. Patients who underwent PTED were divided into two groups based on their need for reoperation. Cases comprised patients who received reoperation after at least one month of pain-free interval following the initial PTED. Subsequent interventions included an additional PTED, unilateral biportal endoscopy (UBE), and posterior lumbar interbody fusion (PLIF). All following reoperations were performed at the same disc level as the first surgery. Control patients, who did not require reoperation during the follow-up, were matched to the cases based on surgical level, gender, body mass index (BMI) classification, and age (within a five-year range). Follow-up for all participants was conducted through telephone consultations or outpatient visits.
Data Collection and Assessment
The demographic information of all patients, including gender, age, height, weight, and surgical level, was collected from the electronic medical records of our hospital. The measured radiological parameters included PT, PI, SS, LL, disc height (DH), Pfirrmann grading, and Modic changes (MCs). These parameters were obtained on the lumbar MRIs and lateral standing X-rays with the inclusion of the femoral heads, using DICOM (version 3.1) viewer software (Neusoft PACS/RIS). All pre-operative radiological examinations were conducted within three weeks before the initial PTED. An experienced surgeon repeated each measurement twice with a one-week interval. The average of the two measurements was used for analysis. The intra-rater reliability of radiological measurements was excellent, with intraclass correlation coefficients (ICC) above 0.9 for all variables.
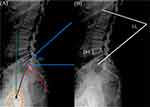
The radiological parameters assessed in our study are illustrated in Figure 1. PI is a morphological parameter that remains unchanged throughout adulthood. It is defined as the angle between a line perpendicular to the midpoint of the sacral plate and a line connecting this point to the femoral head axis. PT represents the pelvic rotation around the femoral heads, defined as the angle between a vertical line and a line drawn from the midpoint of the sacral plate to the femoral head axis. When the two femoral heads do not overlap on lateral radiographs, the axis is determined by the midpoint of a line connecting the centers of the two femoral heads. SS describes the orientation of the sacral plate relative to a horizontal line and is measured as the angle between the upper S1 endplate and this horizontal line. PT and SS reciprocally adapt, as illustrated by the equation: PI = PT + SS. LL is defined as the angle from the upper endplate of L1 to the lower endplate of S1. The PI-LL was calculated as the absolute difference between PI and LL, reflecting the alignment between lumbar curvature and the pelvis in maintaining spinopelvic balance. DH was determined by averaging the anterior and posterior heights of the disc at the surgical segment. MCs were categorized into three types based on variations in signal intensity observed in T1- and T2-weighted MRI images of the bony endplate beneath the cartilaginous endplate and adjacent bone marrow.18 Pfirrmann grading was classified into five levels by assessing the nucleus structure, distinction between nucleus and annulus, signal intensity of the nucleus, and disc height.19
Surgical Procedures
PTED was performed under local anesthesia (8–10 mL of 0.5% lidocaine) with the patient in the prone position on the operating table. The entry point was marked 10–14 cm lateral to the spinal midline at the index intervertebral level. Guided by fluoroscopy, an 18-gauge puncture needle was inserted at approximately a 40-degree angle in the craniocaudal direction towards the SAP. After injecting 15–20 mL of 0.5% lidocaine into the intervertebral foramen for anesthetic infiltration, the surgical pathway was then gradually established through serial hollow cannulas, ultimately accommodating an 8 mm working cannula. Through this portal, foraminoplasty was executed using a grinding drill or different burrs.
Following successful foraminoplasty, the endoscope and working cannula were introduced. Under fluoroscopic guidance, the cannula’s tip was confirmed at the posterior rim of the upper endplate of the distal vertebra in the lateral view and close to the medial pedicular line in the anteroposterior view. Decompression commenced under continuous irrigation via a pressure-regulated pump system delivering 0.9% normal saline. Pathological tissues including hypertrophic ligamentum flavum, ventral SAP, osteophytes, perineural fat, and degenerated disc tissue were removed as necessary to expose the traversing nerve root and dural sac. Hemostasis and annular modulation were achieved using a radiofrequency probe. Adequate decompression was confirmed by nerve root pulsations synchronous with heart rate, restored vascularity, and anatomic nerve root repositioning. The surgical field was inspected by rotating the cannula 360° to ensure no residual fragments or bleeding, followed by closure of the surgical wounds.
Statistical Analysis
The sample size was calculated based on the PI values reported by Pan et al.20 Using a two-sided α of 0.05, power of 0.90, and a 1:2 group ratio, PASS software (version 21.0.3; NCSS, LLC) estimated a minimum requirement of 60 case and 120 control participants.
SPSS statistical software (version 27.0; IBM Corp, USA) was used for data analysis. A P-value of <0.05 was deemed statistically significant. Baseline characteristics and clinical data were compared using Student’s t-test, Mann–Whitney U-test, and Fisher’s exact test. Odds ratios (ORs) for PTED reoperation and their corresponding 95% confidence intervals (CIs) were calculated using univariate logistic regression. Variables that were either statistically significant in the univariate regression or considered potentially relevant were included in the multivariate logistic regression. Statistically significant indicators from the multivariate regression were further assessed using receiver operating characteristic (ROC) analysis to determine cut-off points and their corresponding sensitivity and specificity.
Results
A total of 1967 patients diagnosed with LSS who underwent PTED at our hospital were retrospectively reviewed. Of these, 76 patients (3.9%) required reoperation. The interval between the first and second operations ranged from 31 days to 1906 days, with a median of 304 days. Among the reoperation cases, the surgical level was L4-L5 in 67 patients and L5-S1 in nine. Reoperations after PTED included 43 additional PTED, 24 PLIF, and nine UBE. For comparison, 152 patients were randomly selected as controls from the remaining 1891 patients, matched to the reoperation cases by surgical level, gender, BMI, and age.
Clinical characteristics are shown in Table 1. No significant differences were detected between the case and control groups in gender (P = 1.000), age (P = 0.971), BMI (P = 0.967), surgical level (P = 1.000), stenosis type (P = 0.597), or comorbidities.
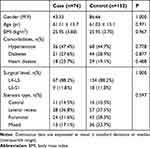 |
Table 1 Clinical Characteristics Between Case and Control Group |
Radiological parameters were compared between patients with and without reoperation after PTED (Table 2). All patients had disc degeneration with at least a Pfirrmann grade of three, with grade four being the most prevalent in both the case and control groups (81.6% vs 91.4%). Most patients in both groups did not show MCs (59.2% vs 67.1%), and type II MC was the most common among those identified (35.5% vs 27.0%). No statistical differences were found in Pfirrmann grades, MCs, PI, or LL. However, the case group exhibited higher PT (P < 0.001), lower SS (P = 0.005), greater PI-LL mismatch (P < 0.001), and larger DH (P = 0.046) compared to the control group.
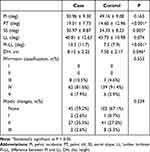 |
Table 2 Measured Parameters Between Case and Control Group |
Table 3 presents the results of univariate and multivariate logistic regression analyses. Crude ORs for all variables in Table 2 were calculated using univariate regression. The multivariate regression included PI, PT, PI-LL, DH, MCs, and Pfirrmann grades. SS was excluded from the model due to its linear relationship with PT, and because PT showed greater statistical significance than SS in univariate regression (P < 0.001 vs P = 0.006). As a result, PT (OR = 1.061, 95% CI = 1.016–1.108, P = 0.007), PI-LL (OR = 1.057, 95% CI = 1.008–1.108, P = 0.021), and DH (OR = 1.194, 95% CI = 1.035–1.377, P = 0.015) were identified as independent risk factors for reoperation after PTED.
 |
Table 3 Univariate and Multivariate Logistic Regression Analyses of the Association Between PTED Reoperation and Radiological Parameters |
A cut-off point for these independent risk factors was further explored through ROC analysis (Figure 2). The area under the curve (AUC) of the ROC analysis for PI-LL was 0.662 (95% CI = 0.582–0.741), with a sensitivity of 51.3% and a specificity of 81.6% when PI-LL was 12.95°. The ROC-AUC for PT was 0.685 (95% CI = 0.606–0.763), with a sensitivity of 48.7% and a specificity of 86.8% when PT was 21.98°. The ROC-AUC for DH was 0.602 (95% CI = 0.521–0.683), with a sensitivity of 59.2% and a specificity of 65.8% when DH was 8.22°.
Discussion
This study is the first to explore the association between spinopelvic alignment and reoperation following PTED. Our results indicate that PI-LL ≥ 12.95°, PT ≥ 21.98°, and DH ≥ 8.22 are independent risk factors for the reoperation.
Given the low incidence of reoperation after PTED and the variation in spinopelvic parameters by age, gender, and BMI,21,22 we adopted a matched case-control design to minimize confounders. In our study, reoperations predominantly occurred at the L4-L5 level (88.2%), with a few at L5-S1 (11.8%). This aligns with our expectations, as the L4-L5 segment is located in a transitional zone between the rigid sacrum and the more mobile lumbar spine, making it more susceptible to biomechanical stress and degeneration. MCs are associated with disc degeneration, infection, and inflammation.18 When disc degeneration leads to endplate fissures, the nucleus may trigger inflammatory responses and secondary changes within the vertebra. However, no significant difference in MCs has been observed between patients with LSS and healthy individuals,21 nor in our study between LSS patients with or without reoperation after PTED. These findings suggest that MCs may not play a significant role in the pathophysiological process of LSS.
The mean PI of 49.8°observed in our study is lower than previously reported.23,24 This difference may be attributed to the varied racial backgrounds of participants across studies. It also suggests a tendency for individuals with lower PI to develop LSS, as they have a reduced capacity to compensate for sagittal imbalance through pelvic retroversion.25 Our study also revealed a higher mean PT (16.6° vs 11.5°) and a lower mean LL (42.8° vs 55.4°) compared to the normative values reported by Hasegawa et al.24 These results are consistent with those of Zárate-Kalfópulos et al, suggesting that LSS patients typically exhibit a retroverted pelvis and flattened lumbar lordosis.26
The compensatory mechanisms in LSS have been well illustrated by Buckland et al through a comparison of patients with LSS and those with adult spinal deformity (ASD).27 In the early stage of LSS, patients tend to decompress neural elements through anterior truncal inclination, as flexion significantly increases the dimensions of the spinal canal and foramen.28 To maintain an upright posture while achieving neural decompression, pelvic shift is initially recruited for compensation, followed by pelvic retroversion (increased PT) and further flattening of LL (decreased LL). In elderly patients, paraspinal muscle degeneration may also contribute to this process, given its role in initiating pelvic retroversion and the progression from a compensated to a decompensated stage.29 As LSS progresses, the mismatch between PT and LL increases to malalignment, and a “permissive” deformity for positional decompression gradually becomes structural.25
In terms of PTED, it remains uncertain to what extent spinopelvic malalignment requires consideration of fusion or more invasive decompression procedures rather than solely focal treatment. Schwab et al proposed that a PI-LL < 10° and a PT < 20° signify harmonious alignment.30 They also suggested that a PT ≥ 22° and a PI-LL ≥ 11° can predict disability (Oswestry Disability Index > 40) in patients with ASD.23 In our study, we arrived at similar conclusions, finding that a PI-LL ≥ 12.95° and a PT ≥ 21.98° have predictive value for reoperation following PTED.
While we have clarified the association between spinopelvic malalignment and reoperation following PTED, the underlying mechanisms are yet to be understood. Lu et al have observed that inadequate decompression is the primary reason for repeat operations following percutaneous endoscopic procedures, consistent with our clinical observations.31 We hypothesize that spinopelvic malalignment in patients with LSS contributes to two main issues that result in inadequate decompression and thus necessitate reoperation: First, as Trenchfield et al suggested, severe central lumbar stenosis is associated with lower LL, lower SS, and higher PI-LL.32 When spinopelvic malalignment is present, the severity of stenosis may exceed the decompression capability of PTED, leading to inadequate decompression. Second, excessive biomechanical stresses from spinopelvic malalignment could promote the re-narrowing of the space initially expanded by PTED.
The potential biomechanical mechanisms are outlined as follows: Theoretically, the spinal column is subjected to forward forces (gravity, abdominal pressure) and forces from posterior spine muscles, collectively referred to as contact force (CF). When the lumbar spine is hypolordotic, the CF is primarily exerted on the anterior column, distributing forces perpendicularly to the discs and thereby increasing disc pressure.25 Bassani et al have validated this using a musculoskeletal model, demonstrating that changes in global sagittal alignment, lumbar typology, and sacral inclination can significantly affect intervertebral loads and muscle activation.33 Even after PTED, these forces may continue to promote disc degeneration, ligamentum flavum hypertrophy, and facet joint deterioration.34 Additionally, potential instability from SAP removal, coupled with post-operative scar formation, further compounds these issues, exacerbating re-stenosis following the procedure.
PTED does not address spinopelvic malalignment, and damaging the facet joints during the procedure can compromise segmental stability. Therefore, assessing spinopelvic alignment pre-operatively is crucial to prevent the revision. Although previous studies have observed increased LL and decreased PI-LL after decompression procedures without fusion,35,36 this likely occurs because patients no longer need to maintain a forward-bending posture for decompression. Yokoyama et al also demonstrated that those improvements in PI-LL and LL tended to deteriorate within five years.36 Recently, Asghar et al have performed short-segment fusions using 3D-printed personalized interbody fusion cages, which may serve as a complement to correcting spinopelvic malalignment.37 After fusion with personalized cages, the PI-LL mismatch was restored in nearly half of the patients (44.2%), showing a significantly higher restoration rate than with traditional stock implants.
Besides spinopelvic parameters, we also identified a DH value of ≥ 8.22 mm as a risk factor for reoperation following PTED. Similarly, Li et al reported that LSS patients typically exhibit higher DH, and their initial DH at L4-L5 was assessed to be taller before the onset of LSS.21 Note that most patients in our study manifested advanced disc degeneration with a Pfirrmann grade of four or five. We hypothesize that if a high DH remains in the presence of severe disc degeneration with a Pfirrmann grade of four or more, it may lead to increased sagittal mobility and reduced spinal stability, thereby facilitating re-stenosis after PTED.38,39 Conversely, while a loss of DH indicates disc degeneration, it also increases the stability between vertebrae.
The limitations of this study are as follows: (1) This retrospective study relies on data extracted from electronic medical records in our hospital. Detailed reasons for reoperations, such as nerve entrapment by bone fragments, nerve injuries, or lumbar instability, were not fully recorded. Consequently, some reoperations may be unrelated to spinopelvic alignment. (2) Although we conducted a thorough investigation into reoperations by reviewing patient records and follow-up interviews, some patients who experienced recurrence may have opted for non-surgical treatments or undergone subsequent operations at other hospitals. This may underestimate our reported reoperation rates. (3) The median interval between the initial PTED and reoperation in this study was 304 days, with an interquartile range of 841.25 days. While patients in the control group were followed for a minimum of two years, this duration may not have been adequate to capture all reoperations, potentially biasing the data. (4) Due to the limited number of cases, this study included all types of spinal stenosis without conducting a stratified analysis. Spinopelvic malalignment may affect different types of stenosis to varying degrees, requiring distinct cut-off points for clinical significance. (5) As this is a retrospective study, intraoperative factors (eg, nerve root tension and real-time fluoroscopy adjustments) were not systematically recorded. This limited our capacity to analyze how dynamic surgical decisions might affect outcomes, potentially obscuring the relationship between spinopelvic alignment and the likelihood of reoperation. (6) The single-center design of this study may restrict the external validity of our results.
Conclusion
PI-LL ≥ 12.95°, PT ≥ 21.98°, and DH ≥ 8.22° are independent risk factors for reoperation following PTED. Assessing spinopelvic alignment before performing PTED is crucial to reduce the risk of revision surgery.
Abbreviations
PTED, percutaneous transforaminal endoscopic decompression; LSS, lumbar spinal stenosis; ASD, adult spinal deformity; PI, pelvic incidence; PT, pelvic tilt; SS, sacral slope; LL, lumbar lordosis; PI-LL, the difference between PI and LL; DH, disc height; SAP, superior articular process; UBE, unilateral biportal endoscopy; PLIF, posterior lumbar interbody fusion; BMI, body mass index; MCs, Modic changes; ORs, Odds ratios; CIs, confidence intervals; ROC, receiver operating characteristic; AUC, area under the curve; NSAIDs, nonsteroidal anti-inflammatory drugs; ICC, intraclass correlation coefficient.
Data Sharing Statement
The data generated and/or analyzed in this study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study adhered to the Declaration of Helsinki and obtained approval from the ethics committee of Beijing Chaoyang Hospital (LGH-2023-FEI-93). Informed consent was waived by the ethics committee of Beijing Chaoyang Hospital for participants, as this is a retrospective study. We confirm that all the data were maintained with confidentiality.
Disclosure
The authors declare that they have no competing interests.
References
1. Deyo RA. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259. doi:10.1001/jama.2010.338
2. Lurie J, Tomkins-Lane C. Management of lumbar spinal stenosis. BMJ. 2016;352:h6234. doi:10.1136/bmj.h6234
3. Katz JN, Zimmerman ZE, Mass H, Makhni MC. Diagnosis and management of lumbar spinal stenosis: a review. JAMA. 2022;327(17):1688–1699. doi:10.1001/jama.2022.5921
4. Victorio, Shen R, Nasution MN, Mahadewa TGB. Full endoscopic percutaneous stenoscopic lumbar decompression and discectomy: an outcome and efficacy analysis on 606 lumbar stenosis patients. J Craniovertebr Junction Spine. 2024;15(2):247–253. doi:10.4103/jcvjs.jcvjs_48_24
5. Sairyo K, Chikawa T, Nagamachi A. State-of-the-art transforaminal percutaneous endoscopic lumbar surgery under local anesthesia: discectomy, foraminoplasty, and ventral facetectomy. J Orthop Sci. 2018;23(2):229–236. doi:10.1016/j.jos.2017.10.015
6. Wei F-L, Zhou C-P, Liu R, et al. Management for lumbar spinal stenosis: a network meta-analysis and systematic review. Int J Surg. 2021;85:19–28. doi:10.1016/j.ijsu.2020.11.014
7. Song Q, Zhu B, Zhao W, Liang C, Hai B, Liu X. Full-endoscopic lumbar decompression versus open decompression and fusion surgery for the lumbar spinal stenosis: a 3-year follow-up study. J Pain Res. 2021;14:1331–1338. doi:10.2147/JPR.S309693
8. Wang L, Wang T, Fan N, et al. Clinical outcome of percutaneous endoscopic lumbar decompression in treatment of elderly patients with lumbar spinal stenosis: a matched retrospective study. Int Orthop. 2023;48(1):201–209. doi:10.1007/s00264-023-05947-y
9. Xie P, Feng F, Chen Z, et al. Percutaneous transforaminal full endoscopic decompression for the treatment of lumbar spinal stenosis. BMC Musculoskelet Disord. 2020;21(1):546. doi:10.1186/s12891-020-03566-x
10. Ahn Y, Lee SH, Park WM, Lee HY. Posterolateral percutaneous endoscopic lumbar foraminotomy for L5-S1 foraminal or lateral exit zone stenosis. Technical note. J Neurosurg. 2003;99(3 Suppl):320–323. doi:10.3171/spi.2003.99.3.0320
11. Yeung A, Roberts A, Zhu L, Qi L, Zhang J, Lewandrowski KU. Treatment of soft tissue and bony spinal stenosis by a visualized endoscopic transforaminal technique under local anesthesia. Neurospine. 2019;16(1):52–62. doi:10.14245/ns.1938038.019
12. Giordan E, Billeci D, Del Verme J, Varrassi G, Coluzzi F. Endoscopic transforaminal lumbar foraminotomy: a systematic review and meta-analysis. Pain Therapy. 2021;10(2):1481–1495.
13. Lambrechts MJ, Heard JC, D’Antonio ND, et al. Preoperative radiographic predictors of subsequent fusion after lumbar decompression surgery. Spine. 2024;49:1598–1606. doi:10.1097/BRS.0000000000005109
14. Matsumoto T, Okuda S, Maeno T, et al. Spinopelvic sagittal imbalance as a risk factor for adjacent-segment disease after single-segment posterior lumbar interbody fusion. J Neurosurg Spine. 2017;26(4):435–440. doi:10.3171/2016.9.SPINE16232
15. Toyoda H, Terai H, Yamada K, et al. A decision tree analysis to predict clinical outcome of minimally invasive lumbar decompression surgery for lumbar spinal stenosis with and without coexisting spondylolisthesis and scoliosis. Spine J. 2023;23(7):973–981. doi:10.1016/j.spinee.2023.01.023
16. Wang A, Wang T, Zang L, et al. Identification of preoperative radiological risk factors for reoperation following percutaneous endoscopic lumbar decompression to treat degenerative lumbar spinal stenosis. Front Surg. 2022;9:1054760.
17. Yuan S, Wang A, Fan N, et al. Recompression after percutaneous transforaminal endoscopic decompression for degenerative lumbar spinal stenosis: risk factors and outcomes of two different reoperation procedures. Front Surg. 2024;11:1392215.
18. Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193–199. doi:10.1148/radiology.166.1.3336678
19. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–1878. doi:10.1097/00007632-200109010-00011
20. Pan YH, Wan D, Wang Q, et al. Association of spinal-pelvic parameters with recurrence of lumbar disc herniation after endoscopic surgery: a retrospective case-control study. Eur Spine J. 2024;33:444–452. doi:10.1007/s00586-023-08073-w
21. Li D, Wang L, Wang Z, et al. Age-related radiographic parameters difference between the degenerative lumbar spinal stenosis patients and healthy people and correlation analysis. J Orthop Surg Res. 2022;17(1). doi:10.1186/s13018-022-03374-0
22. Caprariu R, Oprea M, Popa I, Andrei D, Birsasteanu F, Poenaru VD. Cohort study on the relationship between morphologic parameters of paravertebral muscles, BMI and lumbar lordosis on the severity of lumbar stenosis. Eur J Orthop Surg Traumatol. 2022;33(6):2435–2443.
23. Schwab FJ, Blondel B, Bess S, et al. Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: a prospective multicenter analysis. Spine. 2013;38(13):E803–12. doi:10.1097/BRS.0b013e318292b7b9
24. Hasegawa K, Okamoto M, Hatsushikano S, Shimoda H, Ono M, Watanabe K. Normative values of spino-pelvic sagittal alignment, balance, age, and health-related quality of life in a cohort of healthy adult subjects. Eur Spine J. 2016;25(11):3675–3686. doi:10.1007/s00586-016-4702-2
25. Roussouly P, Pinheiro-Franco JL. Biomechanical analysis of the spino-pelvic organization and adaptation in pathology. Eur Spine J. 2011;20(Suppl 5):609–618. doi:10.1007/s00586-011-1928-x
26. Zárate-Kalfópulos B, Reyes-Tarrago F, Navarro-Aceves LA, et al. Characteristics of spinopelvic sagittal alignment in lumbar degenerative disease. World Neurosurg. 2019;126:e417–e421. doi:10.1016/j.wneu.2019.02.067
27. Buckland AJ, Vira S, Oren JH, et al. When is compensation for lumbar spinal stenosis a clinical sagittal plane deformity? Spine J. 2016;16(8):971–981.
28. Inufusa A, An HS, Lim TH, Hasegawa T, Haughton VM, Nowicki BH. Anatomic changes of the spinal canal and intervertebral foramen associated with flexion-extension movement. Spine. 1996;21(21):2412–2420. doi:10.1097/00007632-199611010-00002
29. Han G, Zhou S, Qiu W, et al. Role of the paraspinal muscles in the sagittal imbalance cascade: the effects of their endurance and of their morphology on sagittal spinopelvic alignment. J Bone Joint Surg Am. 2023;105(24):1954–1961. doi:10.2106/JBJS.22.01175
30. Schwab F, Ungar B, Blondel B, et al. Scoliosis research society—Schwab adult spinal deformity classification. Spine. 2012;37(12):1077–1082. doi:10.1097/BRS.0b013e31823e15e2
31. Lu L, Yuan Z, Li H, Sun S. Intraoperative changes of surgical approach and a second surgery after percutaneous endoscopic surgery for lumbar spinal stenosis. Clinics. 2024;79:100498.
32. Trenchfield D, Lee Y, Lambrechts M, et al. Correction of spinal sagittal alignment after posterior lumbar decompression: does severity of central canal stenosis matter? Asian Spine J. 2023;17(6):1089–1097. doi:10.31616/asj.2023.0075
33. Bassani T, Casaroli G, Galbusera F. Dependence of lumbar loads on spinopelvic sagittal alignment: an evaluation based on musculoskeletal modeling. PLoS One. 2019;14(3):e0207997. doi:10.1371/journal.pone.0207997
34. Soydan Z, Bayramoglu E, Altas O. The impact of spinopelvic alignment on the facet joint degeneration. Global Spine J. 2023;9(2023):21925682231162813.
35. Bouknaitir JB, Carreon LY, Brorson S, Andersen M. Change in sagittal alignment after decompression alone in patients with lumbar spinal stenosis without significant deformity: a prospective cohort study. J Neurosurg Spine. 2022;37(1):57–63. doi:10.3171/2021.10.SPINE21445
36. Yokoyama K, Ikeda N, Tanaka H, et al. Long-term changes in sagittal balance after microsurgical decompression of lumbar spinal canal stenosis in elderly patients: a follow-up study for 5-years after surgery. World Neurosurg. 2023;176:e384–e390. doi:10.1016/j.wneu.2023.05.069
37. Asghar J, Patel AI, Osorio JA, et al. Mismatch between pelvic incidence and lumbar lordosis after personalized interbody fusion: the importance of preoperative planning and alignment in degenerative spine diseases. Int J Spine Surg. 2024;18(S1):S24–S31. doi:10.14444/8638
38. Muriuki MG, Havey RM, Voronov LI, et al. Effects of motion segment level, Pfirrmann intervertebral disc degeneration grade and gender on lumbar spine kinematics. J Orthop Res. 2016;34(8):1389–1398. doi:10.1002/jor.23232
39. Sawa AGU, Lehrman JN, Crawford NR, Kelly BP. Variations among human lumbar spine segments and their relationships to in vitro biomechanics: a retrospective analysis of 281 motion segments from 85 cadaveric spines. Int J Spine Surg. 2020;14(2):140–150. doi:10.14444/7021
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.