Back to Journals » Nature and Science of Sleep » Volume 16
Association Between the Weight-Adjusted Waist Index and OSA Risk: Insights from the NHANES 2017–2020 and Mendelian Randomization Analyses
Authors Wang H , Yang B, Zeng X, Zhang S, Jiang Y, Wang L, Liao C
Received 14 August 2024
Accepted for publication 12 November 2024
Published 19 November 2024 Volume 2024:16 Pages 1779—1795
DOI https://doi.org/10.2147/NSS.S489433
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
HanYu Wang,1,* BoWen Yang,2,* XiaoYu Zeng,1,* ShiPeng Zhang,1 Yanjie Jiang,3 Lu Wang,1 Chao Liao1,4
1Clinical Medical College, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China; 2Dongguan Hospital, Guangzhou University of Chinese Medicine, Dongguan, Guangdong, People’s Republic of China; 3Department of Neurology, Nanjing Hospital of Chinese Medicine Affiliated to Nanjing University of Chinese Medicine, Nanjing, Jiangsu, People’s Republic of China; 4Department of Otorhinolaryngology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chao Liao, Department of Otorhinolaryngology, Hospital of Chengdu University of Traditional Chinese Medicine, No. 37 on the Street of Shi Er Bridge, City Chengdu, Province Sichuan, People’s Republic of China, Email [email protected]
Background: Obesity is a significant risk factor for obstructive sleep apnea (OSA). The weight-adjusted-waist index (WWI) reflects weight-independent centripetal obesity. Our study aims to evaluate the relationship between WWI and OSA.
Methods: The data used in the current cross-sectional investigation are from the National Health and Nutrition Examination Survey (NHANES), which was carried out between 2017 and 2020. We utilized weighted multivariable-adjusted logistic regression to evaluate the relationship between WWI and the risk of OSA. In addition, we applied various analytical methods, including subgroup analysis, smoothing curve fitting, threshold effect analysis and the receiver operating characteristic (ROC) curve. To further explore the relationship, we conducted a MR study using genome-wide association study (GWAS) summary statistics. We performed the main inverse variance weighting (IVW) method along with other supplementary MR methods. In addition, a meta-analysis was conducted to provide an overall evaluation.
Results: WWI was positively related to OSA with the full adjustment [odds ratio (OR)=1.14, 95% confidence interval (95% CI): 1.06– 1.23, P< 0.001]. After converting WWI to a categorical variable by quartiles (Q1-Q4), compared to Q1 the highest WWI quartile was linked to an obviously increased likelihood of OSA (OR=1.26, 95% CI: 1.06– 1.50. P=0.01). Subgroup analysis revealed the stability of the independent positive relationship between WWI and OSA. Smoothing curve fitting identified a saturation effect of WWI and OSA, with an inflection point of 11.62. In addition, WWI had the strongest prediction for OSA (AUC=0.745). Sensitivity analysis was performed to verify the significantly positive connection between WWI and stricter OSA (OR=1.18, 95% CI: 1.05– 1.32, P=0.005). MR meta-analysis further supported our results (OR=2.11, 95% CI: 1.94– 2.30, P< 0.001). Sensitivity analysis confirmed the robustness and reliability of these findings.
Conclusion: WWI was significantly associated with the risk of OSA, suggesting that WWI could potentially serve as a predictor for OSA.
Keywords: weight-adjusted waist circumference index, WWI, obstructive sleep apnea, OSA, National Health and Nutrition Examination Survey, NHANES, Mendelian randomization analysis, cross-sectional study
Introduction
Obstructive sleep apnea (OSA) is a clinical disorder characterized by recurrent episodes of complete upper airway obstruction (apneas) or partial upper airway obstruction (hypopneas). This condition leads to increased negative intrathoracic pressure, fragmented sleep, and intermittent hypoxia.1 Epidemiological studies indicate that OSA affects approximately 17% of women and 34% of men aged 30 to 70 in the United States.2 If left untreated, OSA can result in serious health complications such as hypertension,3 cardiovascular disease,4 and diabetes.5 Therefore, identifying novel and more accurate biomarkers is crucial for the early diagnosis of OSA.
Obesity has reached epidemic levels worldwide, affecting an increasing number of individuals.6,7 The rising prevalence of obesity significantly contributed to the growing incidence of OSA.8,9 The relationship between obesity and OSA is complex and interdependent.10 Excess abdominal fat, in particular, is a major risk factor for both the development and exacerbation of OSA. Previous studies have demonstrated a positive correlation between visceral fat mass and the incidence of OSA.11 Recent large observational trials strongly suggest that weight loss can either correct or significantly improve OSA symptoms.12 For instance, a longitudinal study conducted in Wisconsin found that a 10% increase in body weight (BW) was associated with a 32% rise in the Apnea-Hypopnea Index (AHI), while a 10% decrease in BW was linked to a 26% decrease in the AHI.13
Body mass index (BMI) is commonly used to assess obesity, but its limitations, including its inability to distinguish between fat and muscle mass and the existence of the obesity paradox, raise questions about its effectiveness.14,15 In contrast, the weight-adjusted waist circumference index (WWI) is a new obesity index that normalizes waist circumference (WC) by weight. This approach retains the benefits of WC measurement while reducing its association with BMI.16 WWI not only distinguishes between fat and muscle mass but also accounts for central obesity that is independent of overall weight.17,18 Previous studies have indicated that elevated WWI is strongly associated with various diseases, including stroke, erectile dysfunction, and heart failure.19–21 Despite WWI’s validation as an effective predictor for many diseases, its potential relationship with OSA has not yet been investigated.
In this study, we combined a large-scale observational study in the NHANES 2017–2020 and a two-sample MR analysis to comprehensively assess the relationship between WWI and OSA, and we assume that WWI make a strong predictive effect on OSA.
Methods
Study Samples in NHANES
The data for this analysis were sourced from the NHANES database (https://www.cdc.gov/nchs/nhanes/index.htm). The protocols of the NHANES study were authorized by the Research Ethics Review Board of NCHS. Informed consent was obtained from all the NHANES participants. The study was exempt from the approval of the institutional review board as it used de-identified, publicly available data. NHANES is a major national project conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention in the United States, designed to assess the health and nutritional status of the US population. All NHANES participants provided informed consent. As this study utilized anonymous, publicly available data, institutional review board (IRB) approval was not required. Between 2017 and 2020, a total of 15,560 participants were registered in NHANES. Following strict inclusion and exclusion criteria, this study identified a final sample size of 8048 US adults from the NHANES 2017–2020 cycle. Specifically, 2993 participants were excluded due to missing WWI data, 3659 participants were excluded due to missing OSA data, and 860 participants under the age of 20 were excluded from this study (Figure 1).
Assessment of WWI
The WWI is a new obesity assessment indicator. A greater WWI score indicates a higher level of obesity. WWI is calculated using the following formulas, where WC is in cm and BW is in kg.16
The anthropometric assessments were performed by professional health technicians at the Mobile Examination Centers (MECs), with accuracy ensured through direct observation, data review, and expert examiner evaluation. The “body measurement” data on WC and BW are publicly available on the NHANES website. Generally, a higher WWI value indicates more severe obesity. In the analysis, respondents were divided into 4 groups (Q1-Q4) based on WWI quartiles for further analysis. WWI was set as both a continuous variable and a categorical variable and considered an exposure factor in our study.
Assessment of OSA
According to early research, a diagnosis of OSA can be made if a person answers “yes” to at least one of the following three NHANES questions:22 (1) feeling excessively sleepy during the day 16–30 times per month despite sleeping around 7 or more hours per night on weekdays or work nights; (2) gasping, snoring, or apnea occurring three or more times per week; and (3) snoring three or more times per week. In sensitivity analyses, we strictly redefined OSA as a participant who answered “yes” to two or more questions.
Other Covariates Used in NHANES
When selecting covariates, we referenced extensive research on obstructive sleep apnea (OSA) and factors related to weight-adjusted-waist indexes (WWIs) in the early stages. A meta-analysis has indicated that the incidence rate of OSA significantly increases with age, with the prevalence being notably higher among men compared to women.8 A research by Peter Debbaneh et al further revealed the differential incidence characteristics of OSA among various races.23 Another study revealed that elderly, untreated OSA patients with higher educational levels generally outperform those with lower educational attainment on cognitive function tests.24 Additionally, it is found that married OSA patients have a higher survival rate compared to those who are single.25 In terms of disease-related factors, OSA exhibits an independent and significant correlation with various chronic diseases such as diabetes, hypertension, and dyslipidemia.26–28 Drinking behavior has been identified as an independent risk factor for OSA, particularly for individuals with alcohol use disorders who face higher risks. Moreover, the frequency of alcohol consumption, the amount consumed each time, and the frequency of excessive drinking are all closely associated with an increased risk of OSA.29 Conversely, there is also a significant association between smoking behavior and OSA, especially for heavy smokers with a smoking history of more than 20 PYs, whose risk of developing OSA further increases.30 Therefore, to control for potential confounding effects, we adjusted for the following demographic characteristics: age, gender, race, education level, marital status, poverty-income ratio (PIR), smoking, alcohol consumption, BMI, diabetes, hypertension, total cholesterol (TC), high-density lipoprotein cholesterol (HDLC), low-density lipoprotein cholesterol (LDLC), and triglyceride (TG). Education level was divided into three groups: below high school, high school, and above high school. Marital status was categorized into two groups: married or cohabiting and living alone. The questionnaire included alcohol consumption, smoking, diabetes, and hypertension. Participants provided their medical history through self-report to assess chronic health conditions and indicate whether they had been informed of specific health issues by doctors or other healthcare professionals. Smoking status was classified based on whether participants had smoked 100 or more cigarettes. Ever have 4/5 or more drinks every day was the definition of alcohol consumption status.
Genome-Wide Association Study (GWAS) Sources and Single Nucleotide Polymorphisms (SNPs) Selection
Due to the lack of GWAS data related to the WWI, WC has been widely used as a substitute indicator for WWI in subsequent MR analysis. There is a strong correlation between WC and WWI, and since WC is a major component of WWI, it effectively captures most changes in WWI, making it a suitable proxy variable. Particularly in large-scale MR analyses, GWAS studies on WC are more prevalent and typically involve larger sample sizes compared to those on WWI. In contrast, GWAS studies on WWI remain underdeveloped, especially in certain datasets where WWI data may be entirely unavailable. Therefore, using WC as an alternative data source for MR analysis of WWI can enhance the robustness of the study. Although it is reasonable to use waist circumference (WC) as a substitute for WWI in Mendelian randomization (MR) analyses, the strong correlation between WC and WWI, along with the availability of genetic tools, has raised concerns about potential biases related to confounding, measurement errors, and pleiotropy. For example, WC may not accurately reflect central obesity like WWI, as it does not account for changes in weight, potentially leading to overestimation or underestimation of obesity levels. In addition, WC does not distinguish between fat and muscle tissue, potentially overlooking the impact of muscle mass on health when assessing obesity-related health risks. Individuals with higher muscle mass may have lower health risks, but using WC alone may not capture this distinction. Given the possible inconsistency in the correlation between WC and WWI, using WC as a substitute variable may lead to overestimation or underestimation of the impact of WWI on the results. For example, in some populations, WC may not be as sensitive as WWI and may fail to capture all health risks associated with central obesity. Additionally, in MR studies, there are unmeasured confounding factors that may affect WWI and the results, so using WC as a proxy variable may introduce bias. Although MR methods can mitigate some of this bias, they cannot completely eliminate it. Measurement errors in WC may also impact the interpretation of results. If the measurement of WC is not sufficiently accurate, using WC as a proxy for WWI may introduce additional random errors, affecting our estimation of the relationship between WWI and the outcomes. These biases may compromise the accuracy and reliability of causal estimation, so special caution should be exercised when interpreting the results. These biases may affect the accuracy and reliability of causal estimations, so special caution is warranted when interpreting the results. However, by employing one-by-one exclusion analysis and MR-Egger regression, it is possible to detect and correct for pleiotropy bias or weak instrument bias introduced by WC, thereby obtaining more reliable conclusions. We obtained GWAS data related to WC as exposure from the IEU Open GWAS database, which contains 462,166 individuals of European ancestry. In addition, we obtained summary data on the genetic associations with OSA from the FinnGen consortium, comprising 16,761 cases and 201,194 controls. We also incorporated WC-related exposure data from studies by Shungin et al and Mbatchou et al31,32 Finally, we included data on smoking and drinking from the GWAS and Sequencing Consortium of Alcohol and Nicotine use.33 These GWAS datasets are publicly available through the IEU Open GWAS database (https://gwas.mrcieu.ac.uk/). Detailed information on the data sources is provided in eTable 1. All studies were reviewed and approved by the local IRB, and informed consent was obtained from all participants.
The selection of instrumental variables (IVs) was based on three fundamental assumptions: (1) the IV must be strongly associated with the exposure, (2) the IV must be independent of confounding factors, and (3) the IV should be linked to the outcome exclusively through the exposure, with no direct association with the outcome.34 To identify genetic variations suitable for estimating the relationship between WC and OSA, we set the genome-wide significance threshold at P < 5×10−8 to screen for genetic variants that were closely associated with exposure. We also excluded SNPs with linkage disequilibrium (LD) (r2<0.001,10,000kb) where possible. We then eliminated SNPs with allelic discordance and SNPs with intermediate allele frequencies.35 Additionally, we applied the Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO) algorithm to detect and eliminate outliers with significant deviations.36 Finally, we calculated the F-statistic to assess the strength of the IVs and identified potential bias from weak instruments. An F-statistic greater than 10 is generally considered indicative of a strong correlation between the IV.37 The F-value was calculated using the formula F = Beta2/ Se2, where Beta represents the allele effect value and Se denotes the standard error of Beta.
Statistical Analysis
In the NHANES analysis, this research assessed the characteristics of participants based on quartiles of the WWI utilizing chi-square tests or t-tests. Multivariate logistic regression was used to examine the linear association between the WWI and OSA in three different models. Model 1 (unadjusted), Model 2 (adjusted for age, gender, and race), and Model 3 (adjusted for age, gender, race, education level, marital status, PIR, smoking, alcohol consumption, BMI, diabetes, hypertension, TC, HDLC, LDLC, and TG). The results are expressed as odds ratios (OR) with 95% confidence intervals (CI). Trend tests were conducted to examine the linear association between WWI and OSA after transforming WWI scores from continuous to categorical variables (quartiles). By conducting interaction tests, our goal was to explore the consistency of the association between WWI and OSA within different groups. To comprehensively analyze any potential nonlinear relationships between WWI and OSA, we also performed smooth curve fitting. The receiver operating characteristic (ROC) curve and the area under curve (AUC) were presented to assess the predictive power of WWI, BMI, and WC for OSA. Considering the complex, probabilistic clustering design of NHANES, weights were incorporated into the statistical analysis.
Before commencing the analysis, we first synchronized exposure and outcome data to ensure alignment of effect alleles with the positive strand, excluding intermediate allele frequency palindromes from further MR analysis.38 Inverse variance weighting (IVW) was regarded as the most effective method for detecting relationships in MR analysis.39 The IVW method, an extension of the Wald ratio estimation method based on meta-analysis principles,40 employs the inverse variance of SNPs as weights in meta-analysis to evaluate the combined effect. To further demonstrate the stability and directionality of the results, we utilized MR-Egger41 and the weighted median method42 for auxiliary assessments of the relationship.
To ensure the stability and reliability of our findings, we conducted sensitivity analyses and performed additional validations. Firstly, we used Cochrane’s Q-test to assess the impact of heterogeneity on the results. Heterogeneity within the IVW model was gauged using Cochran’s Q-test,43 with a P < 0.05 indicating significant heterogeneity. Notably, heterogeneity does not inherently compromise the IVW model’s validity, especially when employing a random-effects model in MR analysis. Considering the influence of unknown confounders on genetic diversity and causal effects, we used MR‒Egger regression to assess whether the included SNPs were potentially horizontally pleiotropic, and horizontally pleiotropic results (P < 0.05) were excluded.41 Additionally, we employed the MR-PRESSO algorithm to detect outliers with significant differences and evaluate adjusted causal effects after removing outliers.36 Although MR-Egger regression has its advantages, this method also has some limitations. The traditional MR-Egger regression method is based on the assumption that there is no measurement error in the association between single nucleotide polymorphisms (SNPs) and exposure (NOME assumption). Unfortunately, while MR-Egger enhances its robustness to pleiotropy, it does so at the expense of reducing stability, which violates the NOME assumption and leads to dilution bias in regression.44 Similarly, the MR-PRESSO method also has its limitations. In some situations, even with correction strategies such as outlier removal or covariate adjustment, the issue of horizontal pleiotropy revealed by the MR-PRESSO global test cannot be completely eliminated.36 Furthermore, we performed leave-one-out analysis to thoroughly evaluate the stability of the MR results.
In statistics, sample overlap refers to the presence of common samples in two or more datasets. In MR analysis, this situation may arise when using different genome-wide association study (GWAS) datasets from the same population or containing partially identical participants. Sample overlap may lead to estimation bias in the results, as it violates the assumption of independent samples. It should be noted that our summary statistics are based solely on the European population and cannot rule out the possibility of sample overlap. To minimize any errors that may be introduced due to the inability to directly determine the sample overlap rate, we used the MRlap function to correct the inverse variance weighted (IVW) results.45 If the disparity between the observed effect and the adjusted effect is not significant (p>0.05), it can be considered that the IVW-MR estimation is reliable. Conversely, if a significant difference (p<0.05) is found, we should rely more on the adjusted effect that is not affected by sample overlap. To further test the second core hypothesis of MR and mitigate the influence of variables that may distort the results of two-sample MR, we used multivariate Mendelian randomization (MVMR) to assess the relationship between exposure, confounding factors, and outcomes. Through literature review, we identified common risk factors associated with WC and OSA, including smoking30,46 and alcohol consumption.29,47
For the meta-analysis of the three sets of estimates derived from the MR analysis, we used a random effects model to calculate OR when heterogeneity was present, and a fixed effects model when no heterogeneity was detected. The I2 test was employed to test heterogeneity. Meta-analysis results were considered homogeneous if the I2 value was less than 50% and P>0.05.
P<0.05 was used as the statistical significance threshold. The statistical analysis was performed using R (version 4.3.1) and EmpowerStats (version 2.0).
Results
Participant Characteristics in NHANES
A total of 8048 subjects were included in this study, with a mean age of 50.63±17.39 years. The sample included 3932 males (48.86%) and 4116 females (51.14%). The prevalence of OSA was 49.85% among all subjects, with prevalence increasing across higher WWI quartiles. The mean WWI for all subjects was 11.12±0.86cm/√kg, with quartile ranges as follows: 8.44–10.54 cm/√kg for the first quartile, 10.55–11.12 cm/√kg for the second quartile, 11.13–11.71 cm/√kg for the third quartile, and 11.72–14.14 cm/√kg for the fourth quartile. Compared to individuals in the lowest WWI quartile, those in the highest quartile were more likely to be elderly, female, non-Hispanic white, less educated, and smokers, and they had a higher likelihood of having diabetes, hypertension, and OSA. Meanwhile, higher WWI was often associated with increased levels of BMI, WC, weight, TG, LDLC, and TC, but decreased levels of PIR and HDLC (Table 1).
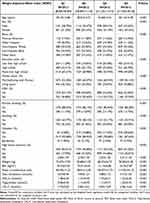 |
Table 1 Basic Characteristics of Participants by Weight-Adjusted Waist Index Quartile |
Association Between the WWI and OSA in NHANES
With the purpose of further investigating the correlation between WWI and OSA, three multiple regression models were constructed for analysis (Table 2). A significant positive relationship between WWI and OSA was observed across all models. In the fully adjusted model (Model 3), each unit increase in WWI was associated with a 14% increase in the risk of OSA (OR=1.14, 95% CI: 1.06–1.23, P<0.001). In addition, when continuous WWI variables were categorized into quartiles, this positive correlation remained stable (trend P values were all <0.05). In the fully adjusted model, individuals in the highest quartile (Q4) of the survey had a 26% higher risk of OSA compared to those in the lowest quartile (Q1) (OR=1.26, 95% CI: 1.06–1.50). To further visualize the correlation between WWI and OSA, smooth curve fitting based on Model 3 was conducted. Results in Figure 2 indicated a nonlinear relationship between WWI and OSA. Then, threshold effect analysis was performed to clarify their relationship. The inflection point for WWI was determined as 11.62 (log-likelihood ratio=0.004), indicating that when WWI levels were below 11.62, every one-unit increase in WWI showed a relationship to a 26% elevation in the risk of OSA. When LAP levels exceeded 11.62, the correlation between WWI and OSA risk disappeared, suggesting that further increases in WWI did not significantly elevate the risk of OSA.
 |
Table 2 The Association Between Weight-Adjusted Waist Index and OSA |
Subgroup Analyses
To evaluate the consistency of the association between WWI and OSA across different populations, we performed subgroup analyses and interaction tests based on age, gender, race, education level, marital status, alcohol drinking, smoking, diabetes, and hypertension. As depicted in Table 3, our results indicate that the relationship between WWI and OSA is independent of these factors (all interaction P-values > 0.05). Moreover, the positive association between WWI and OSA exhibited a similar trend across various subgroups, including gender, age, race, education level, diabetes, and hypertension. This suggests that the observed association may be applicable to diverse populations.
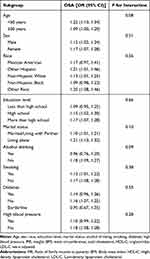 |
Table 3 Subgroup Analysis of the Association Between Weight-Adjusted Waist Index and OSA |
WWI as a Predictor for OSA
We utilized ROC analysis assess the predictive value of WWI, BMI and WC for OSA (eFigure 1). The area under the curve (AUC) for OSA indicated that WWI had a significantly larger AUC (AUC=0.647) compared to BMI and WC (eTable 2), indicating that WWI may be a better predictive index of OSA than BMI and WC.
Sensitivity Analysis
We designed the participants who answered the sleep questionnaire with two or three “yes” answers as individuals with more severe OSA for sensitivity analysis. As shown in Table 4, a significant positive relationship between WWI and OSA was observed across all models. In the fully adjusted model (Model 3), each unit increase in WWI was associated with a 18% increase in the risk of OSA (OR=1.18, 95% CI: 1.05–1.32, P=0.0047). In addition, when continuous WWI variables were categorized into quartiles, this positive correlation remained stable (trend P values were all <0.05). In the fully adjusted model, individuals in the highest quartile (Q4) of the survey had a 43% higher risk of OSA compared to those in the lowest quartile (Q1) (OR=1.43, 95% CI: 1.10–1.87). In addition, we explored the linear association between WWI and stricter OSA using a smooth curve fitting and generalized addition model (Figure 3), and WWI was still linearly positively related to stricter OSA.
 |
Table 4 Sensitivity Analysis for the Association Between WWI with Stricter OSA |
Two-Sample MR Analysis
There were 369 SNPs (Group 1), 327 SNPs (Group 2) and 41 SNPs (Group 3) with P < 5×10−8. Following the exclusion of intermediate allele frequency palindromes and unmatched SNPs, 351, 271, and 40 IVs respectively were retained for MR analysis. Additionally, the MR-PRESSO test identified outlier IVs as follows: for Group 1, rs13322435, rs56094641, rs7952436, rs1051613; for Group 2, rs10938398, rs56094641, rs72820274, rs7952436, rs13322435; and for Group 3, rs633715. These outlier IVs were removed and the analysis was conducted again. The F-statistic for each SNP is greater than 10 (eTable 3). In our single-factor MR study assessing the impact of WC on the risk of OSA, the IVW analysis revealed a significant correlation between WC and OSA: Group 1: OR=2.12, 95% CI: 1.88–2.39, P<0.001; Group 2: OR=2.09, 95% CI: 1.82–2.39, P<0.001; Group 3: OR=2.18, 95% CI: 1.71–2.78, P<0.001. These findings were consistent with the results from the weighted median analysis (Group 1: OR=2.05, 95% CI: 1.74–2.41, P<0.001; Group 2: OR=1.99, 95% CI: 1.67–2.37, P<0.001; Group 3: OR=1.84, 95% CI: 1.41–2.42, P<0.001). In addition, except for group 3, the MR Egger analysis results of the other two groups were also consistent with IVW (Group 1: OR=3.14, 95% CI: 2.22–4.43, P<0.001; Group 2: OR=3.31, 95% CI: 2.24–4.90, P<0.001; Group 3: OR=1.43, 95% CI: 0.60–3.44, P=0.43) (eTable 4). In the sensitivity analysis, although Cochran’s Q test indicated heterogeneity among the three groups (all P<0.05), this heterogeneity may distort the overall effect, increase uncertainty in result estimation, and weaken statistical significance and confidence. However, the estimations from the MR models showed consistency, with heterogeneity remaining within an acceptable range. Random effects inverse variance weighting (IVW) analysis continues to be the primary detection method.
The estimated effects sizes for gene predictions are illustrated in the scatterplots (Figure 4). MR-Egger intercept analysis confirmed the absence of pleiotropy (all P>0.05) (eTable 5). Furthermore, the leave-one-out sensitivity test demonstrated that the effect of any single SNP on the relationship between WC and OSA did not vary significantly. In addition, the funnel plot did not reveal any outlier IVs (eFigure 2), further affirming the robustness of our findings.
 |
Figure 4 Scatter plot of Mendelian randomization analyses from WC to OSA. Abbreviations: WC, waist circumference; OSA, Obstructive sleep apnea; SNP, Single Nucleotide Polymorphism. |
MRlap Analysis
The results of MRlap analysis are detailed in eTable 6. According to MRlap, the MR results for the association between WC and OSA were indeed influenced by sample overlap, leading to biased results. To address this issue, we employed calibrated data. Notably, the MRlap-corrected results aligned with the initial MR analysis results, further substantiating the robustness of the IVW method.
MVMR Analysis
MVMR is crucial for exploring the relationships between multiple exposures and a single outcome variable, especially when these associations are influenced by genetic tools. Additionally, MVMR has the potential to minimize bias caused by confounding factors. Considering the potential association between smoking and alcohol consumption in relation to WC and OSA, we performed an MVMR analysis to adjust for the genetic predictions of the effects of smoking and alcohol consumption. The analysis revealed that WC remained significantly associated with the risk of OSA (group 1: OR=2.27; 95% CI: 1.98–2.61; p<0.001; group 2: OR=2.22; 95% CI: 1.90–2.60; p<0.001; group 3: OR=1.96; 95% CI: 1.54–2.49; p<0.001). These findings further substantiate the robustness of our results (eTable 7).
MR Meta-Analysis
Using IVW as the primary approach, we performed a meta-analysis to obtain the overall outcome. The results revealed no statistical heterogeneity (I2=0.0%, H=1.00, P=0.95). Consequently, we opted for the fixed-effect model to perform the results (OR=2.11, 95% CI: 1.94–2.30, P<0.001) (Figure 5). Meanwhile, sensitivity test demonstrated that Egger’s test (P = 0.61), Begg’s test (P = 0.33), and the visualization of funnel plots provided no indication of publication bias.
 |
Figure 5 Forest plot of results from Mendelian randomization analysis. |
MR analysis employs genetic variation as an instrumental variable to infer causal associations between exposure factors and outcomes. However, when exposure is represented as binary results, the limitations of MR analysis become more pronounced. In MR analysis, we generally assume that the association between genetic variation and exposure is causal and that genetic variation and confounding factors are independent of each other. Nonetheless, in the context of binary exposure, these assumptions may be harder to validate, as binary results are more susceptible to numerous unobserved confounding factors. Therefore, even when MR analysis provides robust evidence for the relationship between exposure and outcomes, these results should be interpreted as associations rather than definitive causal relationships. Particularly for binary results, the estimated values from MR analysis can be influenced by a combination of sample size, genetic variation effect size, and potential confounding factors, potentially leading to overestimation or underestimation of causal effects. Therefore, while MR analysis can reveal associations between exposure and outcomes, the interpretation of these relationships should avoid over-inference as true causal relationships.
Discussion
In this study, we integrated the observational study using the nationally representative NHANES 2017–2020 cohort and a two-sample MR analysis to investigate the relationship between WWI and OSA. Our results showed that an increase in WWI was closely related to a higher incidence rate of OSA. When converting WWI into categorical variables based on quartiles (Q1-Q4), this positive correlation remained significant. Subgroup analysis demonstrated that all stratified variables did not affect the stability of the relationship between WWI and OSA, and a positive correlation still existed. In addition, we used ROC curves to explore and compare the predictive effects of WWI, BMI, and WC on OSA, finding that WWI had better predictive ability for OSA than both BMI and WC. We also conducted a sensitivity analysis using a stricter definition of WWI and found that the impact of WWI on OSA was further enhanced. We determined a WWI saturation value of 11.62 for all participants, demonstrating a strong correlation between WWI levels and OSA within a specific range. This suggests that maintaining an ideal WWI level may have potential clinical significance for reducing the risk of OSA. At the same time, the MR meta-analysis further confirmed our results. It is noteworthy that, in this MR analysis, OSA appears as a binary result, and binary variables have limitations in capturing true causal relationships. Consequently, the causal estimation of binary exposure in MR studies lacks clear interpretability. In this scenario, a more cautious approach is to refrain from estimating causal effect parameters and to report only whether there is a correlation between variables and outcomes.48 Therefore, we only conclude that there is an association between WC and OSA, and further research is needed to verify whether a causal relationship exists between the two.
The association between obesity and OSA has received extensive research support. As early as 20 years ago, the relationship between visceral obesity and OSA was widely recognized. In an observational study, Vgontzas et al found that visceral fat mass was positively correlated with the incidence rate of OSA.11 Recent large-scale observational trials further indicate that weight loss and increased physical activity can effectively correct or at least improve the symptoms of OSA.12 As is well known, BMI is one of the most commonly used indicators for assessing obesity, and many studies have reported a positive correlation between BMI and OSA. Rezaie et al found that a higher BMI was closely related to a higher AHI index in patients with OSA.49 Additionally, Pancholi et al demonstrated that the BMI of OSA patients was statistically significantly higher than that of the non-OSA group.50 However, as research has advanced, some scholars have discovered the “obesity paradox”16,51 when using BMI as an indicator of obesity. Participants who are relatively obese may have a better prognosis than those with a normal BMI, especially in patients with coronary artery disease and acute coronary syndrome.14 This phenomenon underscores the importance of identifying an obesity index that can eliminate the obesity paradox. It is worth noting that a major limitation of BMI is that it cannot distinguish between body fat and lean body mass, nor can it assess the distribution of weight.52 For example, individuals with more muscle mass and less fat may be mistakenly considered to have a higher BMI, while individuals with increased fat mass and decreased lean body mass may be considered to have a normal BMI.20 WWI is a recently designed anthropometric index. Its simple method and ability to distinguish lean body mass from fat mass make WWI a reliable tool for measuring obesity, alongside BMI and WC.16 It is noteworthy that the obesity paradox observed in the relationship between BMI and mortality did not appear in the association between WWI and mortality.17 A recent study has further confirmed that WWI can effectively differentiate muscle mass from fat mass,53 making it an emerging indicator of obesity, closely related to multiple factors, especially cardiovascular disease (CVD). Zhao et al found a significant J-shaped relationship between higher WWI levels and CVD risk in patients with hypertension and OSA, especially when WWI exceeds 11.5cm/√kg.54 In addition, Li et al found that WWI was associated with a high incidence of hypertension in rural areas of China, and there was a statistically significant correlation between the two.51 OSA and CVD have been demonstrated to share many common risk factors, including but not limited to obesity, hypertension, and diabetes.55,56 Studies have shown that OSA is an independent predictor of CVD and provides crucial opportunities for prevention and treatment.57,58 Therefore, the relationship between CVD and OSA has been widely confirmed. Furthermore, there is robust evidence indicating a significant positive correlation between WWI and abdominal fat.59 Wang et al discovered that central adiposity assessed by a high waist-hip ratio (WHR) was highly associated with male OSA.60 Consequently, it can be inferred that WWI may be positively associated with the incidence of OSA. In our analysis, both the original model and the adjusted model demonstrated a positive correlation between WWI and elevated OSA. Additionally, sensitivity analysis using WWI as a quartile indicated a positive relationship between WWI and OSA. We observed a non-linear relationship between WWI and OSA, with a breakpoint at 11.62. On the left side of the breakpoint, there is a positive correlation between WWI and OSA, whereas there is no correlation on the right side, indicating a significant threshold effect between WWI and OSA. OSA has become a significant health concern, adversely impacting individuals’ quality of life. However, the diagnosis of OSA is often time-consuming, labor-intensive, and financially burdensome for patients. This highlights an urgent clinical need for a more convenient and effective diagnostic approach. WWI serves as a cost-effective and easily measurable marker that aligns well with these clinical needs. The findings from this study offer valuable guidance for healthcare providers to efficiently identify patients at risk of OSA. Given the limited research in sleep medicine on the association between WWI and OSA risk, this study presents important insights that may be particularly relevant for high-risk adult populations.
The specific mechanism behind the association between increased WWI and a higher prevalence of OSA is not fully understood, but research suggests several potential mechanisms. Multiple factors and mechanisms are involved in the development of obesity and OSA. An important factor is the impact of obesity on respiratory function. Firstly, excessive fat accumulation in the neck of obese individuals can lead to increased neck circumference and directly contribute to upper airway stenosis. There is also a tendency for ectopic fat deposition within the upper airways, potentially leading to the development of pharyngeal pads. The accumulation of fat around the neck and upper airways can cause narrowing or obstruction of the airways during sleep, causing complete or partial cessation of breathing (apnea or hypopnea).61,62 Secondly, the distribution of body fat can affect the occurrence and development of OSA. A recent retrospective study indicated that visceral obesity increases the distensibility of the upper airway.63 Obesity leads to the enlargement of soft tissue structures in and around the airways, significantly contributing to the narrowing of the pharyngeal airway. In a study by Luciano et al, it was found that obesity also indirectly causes upper airway narrowing during sleep. Increased abdominal fat mass and a recumbent position result in significant reductions in lung volume. This reduction in lung volume may decrease longitudinal tracheal traction and pharyngeal wall tension, which may cause airway narrowing and promote the occurrence of OSA.4 Thirdly, obese individuals are more prone to upper airway collapse compared to non-obese individuals, and this collapse does not improve even when the mandible is advanced to expand the airway during swallowing. Additionally, obesity can also stimulate the release of various inflammatory and adipogenic factors in the body, leading to systemic inflammation and oxidative stress. These conditions can impair the function of upper respiratory muscles and promote the proliferation of adipose tissue around the upper respiratory tract, thereby increasing the risk of OSA.8,64 Obesity is often associated with a chronic low-grade inflammation state that contributes to metabolic disorders.65 Numerous studies have demonstrated that adipose tissue in obese individuals and animals commonly exhibits cell hypertrophy, inflammatory cell infiltration, and increased levels of inflammatory markers in the systemic circulation, such as C-reactive protein (CRP), interleukin-2, (IL-2), and IL-6.65–67 Many studies have shown that levels of CRP and IL-6 are also elevated in patients with OSA, indicating that macrophages in adipose tissue are targeted by intermittent hypoxia. Intermittent hypoxia activates the inflammatory pathway in adipose tissue, leading to the release of IL-2 and IL-6, which then stimulate the liver to produce CRP.68,69 Hypoxia also activates nuclear factor (NF)-κB in endothelial cells, which subsequently increases the release of inflammatory factors in adipocytes. Growing evidence suggests that hypoxia plays a crucial role in mediating the proinflammatory response of obese adipose tissue.70 As adipose tissue expands, angiogenesis increases to provide sufficient oxygen and nutrients. However, persistent hypertrophy of adipocytes leads to local tissue hypoxia, which activates hypoxia-inducible transcription factors.71
The main strength of this study lies in its combination of the observational analysis from NHANES 2017–2020 with the MR method. The comprehensive evaluation of various factors, coupled with a large sample size, enabled reliable adjustment for multiple confounders simultaneously in the multivariate regression model and provided sufficient statistical power to evaluate the relationship between WWI and OSA. Additionally, the MR method helps mitigate unmeasured confounding. Notably, the consistent results obtained from both methods enhance the reliability of the findings.
However, our study has several limitations. (1) This study utilized a cross-sectional design, which means we were unable to observe the dynamic changes in WWI indicators over time. As a result, there are limitations to the long-term trend analysis of health status and environmental factors, which may weaken the empirical strength of the association between exposure and outcomes; (2) Due to the lack of direct WWI data in the GWAS database, we only used WC as a substitute for WWI in MR analysis. This may lead to confusion between abdominal obesity and overall obesity, potentially introducing bias. Therefore, we look forward to using more detailed WWI data in the future to further validate our research findings; (3) Given the limitations of the GWAS dataset and the impact of sample overlap on MR results revealed by MRlap analysis, future studies are needed to further elucidate potential causal relationships and explore potential pathogenic mechanisms. This is crucial for the development of clinical intervention strategies. (4) The reliance on data from the US population may limit the generalizability of the findings to other populations; (5) Due to limitations in the NHANES database, we can only find some of the most typical OSA symptoms through sleep questionnaires, such as snoring, respiratory arrests, and daytime sleepiness. Because of this limitation, the respondents did not undergo polysomnography examinations, which may have resulted in overlooked cases of OSA and an underestimation of its actual prevalence. (6) Although we controlled for many covariates that were considered relevant confounders, it is possible that there was still residual and unmeasured confounding.
Conclusion
The results of this investigation indicated that a greater WWI was associated with an increased risk of OSA. This finding may provide new insights for the future of OSA prevention and treatment. However, higher-quality prospective studies are needed to corroborate our results.
Data Sharing Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Declarations
The ethical approval of NHANES was granted by the US National Center for Health Statistics Research Ethics Review Board (Protocol No. 98 − 12, Protocol No. 2011-17, Continuation of Protocol No. 2011-17, Protocol No. 2018-01) (available at: https://www.cdc.gov/nchs/nhanes/irba98.htm). The NHANES is a publicly available dataset. The analysis in this study is a secondary analysis of NHANES data; thus, ethical approval is exempted under the US Health and Human Services (HHS) regulations at 45 CFR 46.104 (available at: https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/common-rule-subpart-A-46104/index.html). Written informed consent was acquired before household interview and health examinations; the participants were assured that the data collected will be used only for stated purposes and will not be disclosed or released to others without the consent (available at: https://www.cdc.gov/nchs/nhanes/genetics/genetic_participants.htm).
Acknowledgments
We sincerely thank all the projects (NHANES, IEU Open GWAS database, UKB, and FinnGen) who participated in this study, and thank all investigators for making available their data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Sichuan Administration of Traditional Chinese Medicine “Systematic Exploration and Inheritance and Promotion of the Source and Development of Otolaryngology in Sichuan” (CKY2022082).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. doi:10.1093/sleep/22.5.667
2. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi:10.1093/aje/kws342
3. Brown J, Yazdi F, Jodari-Karimi M, Owen JG, Reisin E. Obstructive Sleep Apnea and Hypertension: updates to a Critical Relationship. Curr Hypertens Rep. 2022;24(6):173–184. doi:10.1007/s11906-022-01181-w
4. Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569–576. doi:10.1016/j.jacc.2013.05.045
5. Reutrakul S, Mokhlesi B. Obstructive Sleep Apnea and Diabetes: a State of the Art Review. Chest. 2017;152(5):1070–1086. doi:10.1016/j.chest.2017.05.009
6. Yanovski JA. Obesity: trends in underweight and obesity - scale of the problem. Nat Rev Endocrinol. 2018;14(1):5–6. doi:10.1038/nrendo.2017.157
7. L Abarca-Gómez, ZA Abdeen, ZA Hamid. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642. doi:10.1016/s0140-6736(17)32129-3
8. Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi:10.1016/j.smrv.2016.07.002
9. Lyons MM, Bhatt NY, Pack AI, Magalang UJ. Global burden of sleep-disordered breathing and its implications. Respirology. 2020;25(7):690–702. doi:10.1111/resp.13838
10. Deng H, Duan X, Huang J, et al. Association of adiposity with risk of obstructive sleep apnea: a population-based study. BMC Public Health. 2023;23(1):1835. doi:10.1186/s12889-023-16695-4
11. Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85(3):1151–1158. doi:10.1210/jcem.85.3.6484
12. AlBishi L, Alkhuraisi LS, Alqahtani MM, et al. Obstructive Sleep Apnea Among Obese Children in Tabuk City, Saudi Arabia. Cureus. 2024;16(4):e58714. doi:10.7759/cureus.58714
13. Korcarz CE, Peppard PE, Young TB, et al. Effects of Obstructive Sleep Apnea and Obesity on Cardiac Remodeling: the Wisconsin Sleep Cohort Study. Sleep. 2016;39(6):1187–1195. doi:10.5665/sleep.5828
14. Antonopoulos AS, Oikonomou EK, Antoniades C, Tousoulis D. From the BMI paradox to the obesity paradox: the obesity-mortality association in coronary heart disease. Obes Rev. 2016;17(10):989–1000. doi:10.1111/obr.12440
15. Hainer V, Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes Care. 2013;36(Suppl 2):S276–81. doi:10.2337/dcS13-2023
16. Park Y, Kim NH, Kwon TY, Kim SG. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep. 2018;8(1):16753. doi:10.1038/s41598-018-35073-4
17. Kim NH, Park Y, Kim NH, Kim SG. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing. 2021;50(3):780–786. doi:10.1093/ageing/afaa208
18. Qin Z, Chang K, Yang Q, Yu Q, Liao R, Su B. The association between weight-adjusted-waist index and increased urinary albumin excretion in adults: a population-based study. Front Nutr. 2022;9:941926. doi:10.3389/fnut.2022.941926
19. Ye J, Hu Y, Chen X, et al. Association between the weight-adjusted waist index and stroke: a cross-sectional study. BMC Public Health. 2023;23(1):1689. doi:10.1186/s12889-023-16621-8
20. Cao S, Hu X, Shao Y, et al. Relationship between weight-adjusted-waist index and erectile dysfunction in the United State: results from NHANES 2001-2004. Front Endocrinol (Lausanne). 2023;14:1128076. doi:10.3389/fendo.2023.1128076
21. Zhang D, Shi W, Ding Z, Park J, Wu S, Zhang J. Association between weight-adjusted-waist index and heart failure: results from National Health and Nutrition Examination Survey 1999-2018. Front Cardiovasc Med. 2022;9:1069146. doi:10.3389/fcvm.2022.1069146
22. Tao X, Niu R, Lu W, Zeng X, Sun X, Liu C. Obstructive sleep apnea (OSA) is associated with increased risk of early-onset sarcopenia and sarcopenic obesity: results from NHANES 2015-2018. Int J Obes (Lond). 2024;48(6):891–899. doi:10.1038/s41366-024-01493-8
23. Debbaneh P, Ramirez K, Block-Wheeler N, Durr M. Representation of Race and Sex in Sleep Surgery Studies. Otolaryngol Head Neck Surg. 2022;166(6):1204–1210. doi:10.1177/01945998221088759
24. Hlaing EE, Dollinger SMC, Brown TM. The role of education in cognitive functions among middle-age and older patients with untreated obstructive sleep apnea. Sleep Sci. 2021;14(4):319–329. doi:10.5935/1984-0063.20200099
25. Goosmann M, Williams AM, Springer K, Yaremchuk KL. The Impact of Marital Status and Race in Obstructive Sleep Apnea. Ear Nose Throat J. 2022;1455613221120068. doi:10.1177/01455613221120068
26. Torrella M, Castells I, Gimenez-Perez G, et al. Intermittent hypoxia is an independent marker of poorer glycaemic control in patients with uncontrolled type 2 diabetes. Diabetes Metab. 2015;41(4):312–318. doi:10.1016/j.diabet.2015.01.002
27. Tkacova R, McNicholas WT, Javorsky M, et al. Nocturnal intermittent hypoxia predicts prevalent hypertension in the European Sleep Apnoea Database cohort study. Eur Respir J. 2014;44(4):931–941. doi:10.1183/09031936.00225113
28. Trzepizur W, Le Vaillant M, Meslier N, et al. Independent association between nocturnal intermittent hypoxemia and metabolic dyslipidemia. Chest. 2013;143(6):1584–1589. doi:10.1378/chest.12-1652
29. Ko J, Lim JH, Kim DB, et al. Association between alcohol use disorder and risk of obstructive sleep apnea. J Sleep Res. 2024;33(4):e14128. doi:10.1111/jsr.14128
30. Zeng X, Ren Y, Wu K, et al. Association Between Smoking Behavior and Obstructive Sleep Apnea: a Systematic Review and Meta-Analysis. Nicotine Tob Res. 2023;25(3):364–371. doi:10.1093/ntr/ntac126
31. Shungin D, Winkler TW, Croteau-Chonka DC, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–196. doi:10.1038/nature14132
32. Mbatchou J, Barnard L, Backman J, et al. Computationally efficient whole-genome regression for quantitative and binary traits. Nat Genet. 2021;53(7):1097–1103. doi:10.1038/s41588-021-00870-7
33. Liu M, Jiang Y, Wedow R, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51(2):237–244. doi:10.1038/s41588-018-0307-5
34. Lawlor DA. Commentary: two-sample Mendelian randomization: opportunities and challenges. Int J Epidemiol. 2016;45(3):908–915. doi:10.1093/ije/dyw127
35. Yin KJ, Huang JX, Wang P, et al. No Genetic Causal Association Between Periodontitis and Arthritis: a Bidirectional Two-Sample Mendelian Randomization Analysis. Front Immunol. 2022;13:808832. doi:10.3389/fimmu.2022.808832
36. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(5):693–698. doi:10.1038/s41588-018-0099-7
37. Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740–752. doi:10.1093/ije/dyq151
38. Hartwig FP, Davies NM, Hemani G, Davey Smith G. Two-sample Mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. 2016;45(6):1717–1726. doi:10.1093/ije/dyx028
39. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985–1998. doi:10.1093/ije/dyx102
40. Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30(7):543–552. doi:10.1007/s10654-015-0011-z
41. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. Apr. 2015;44(2):512–525. doi:10.1093/ije/dyv080
42. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40(4):304–314. doi:10.1002/gepi.21965
43. Kulinskaya E, Dollinger MB. An accurate test for homogeneity of odds ratios based on Cochran’s Q-statistic. BMC Med Res Methodol. 2015;15(1):49. doi:10.1186/s12874-015-0034-x
44. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45(6):1961–1974. doi:10.1093/ije/dyw220
45. Mounier N, Kutalik Z. Bias correction for inverse variance weighting Mendelian randomization. Genet Epidemiol. 2023;47(4):314–331. doi:10.1002/gepi.22522
46. Mizuno O, Okamoto K, Sawada M, Mimura M, Watanabe T, Morishita T. Obesity and smoking: relationship with waist circumference and obesity-related disorders in men undergoing a health screening. J Atheroscler Thromb. 2005;12(4):199–204. doi:10.5551/jat.12.199
47. Vadstrup ES, Petersen L, Sørensen TI, Grønbaek M. Waist circumference in relation to history of amount and type of alcohol: results from the Copenhagen City Heart Study. Int J Obes Relat Metab Disord. 2003;27(2):238–246. doi:10.1038/sj.ijo.802203
48. Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. 2018;33(10):947–952. doi:10.1007/s10654-018-0424-6
49. Rezaie L, Maazinezhad S, Fogelberg DJ, Khazaie H, Sadeghi-Bahmani D, Brand S. Compared to Individuals with Mild to Moderate Obstructive Sleep Apnea (OSA), Individuals with Severe OSA Had Higher BMI and Respiratory-Disturbance Scores. Life. 2021;11(5):368. doi:10.3390/life11050368
50. Pancholi C, Chaudhary SC, Gupta KK, et al. Obstructive sleep apnea in hypothyroidism. Ann Afr Med. 2022;21(4):403–409. doi:10.4103/aam.aam_134_21
51. Li Q, Qie R, Qin P, et al. Association of weight-adjusted-waist index with incident hypertension: the Rural Chinese Cohort Study. Nutr Metab Cardiovasc Dis. 2020;30(10):1732–1741. doi:10.1016/j.numecd.2020.05.033
52. Wang X, Yang S, He G, Xie L. The association between weight-adjusted-waist index and total bone mineral density in adolescents: NHANES 2011-2018. Front Endocrinol (Lausanne). 2023;14:1191501. doi:10.3389/fendo.2023.1191501
53. Kim JE, Choi J, Kim M, Won CW. Assessment of existing anthropometric indices for screening sarcopenic obesity in older adults. Br J Nutr. 2023;129(5):875–887. doi:10.1017/s0007114522001817
54. Zhao J, Cai X, Hu J, et al. J-Shaped Relationship Between Weight-Adjusted-Waist Index and Cardiovascular Disease Risk in Hypertensive Patients with Obstructive Sleep Apnea: a Cohort Study. Diabetes Metab Syndr Obes. 2024;17:2671–2681. doi:10.2147/dmso.S469376
55. Cai X, Song S, Hu J, et al. Body roundness index improves the predictive value of cardiovascular disease risk in hypertensive patients with obstructive sleep apnea: a cohort study. Clin Exp Hypertens. 2023;45(1):2259132. doi:10.1080/10641963.2023.2259132
56. Hu J, Cai X, Li N, et al. Association Between Triglyceride Glucose Index-Waist Circumference and Risk of First Myocardial Infarction in Chinese Hypertensive Patients with Obstructive Sleep Apnoea: an Observational Cohort Study. Nat Sci Sleep. 2022;14:969–980. doi:10.2147/nss.S362101
57. Redline S, Azarbarzin A, Peker Y. Obstructive sleep apnoea heterogeneity and cardiovascular disease. Nat Rev Cardiol. 2023;20(8):560–573. doi:10.1038/s41569-023-00846-6
58. Jehan S, Farag M, Zizi F, et al. Obstructive sleep apnea and stroke. Sleep Med Disord. 2018;2(5):120–125.
59. Kim JY, Choi J, Vella CA, Criqui MH, Allison MA, Kim NH. Associations between Weight-Adjusted Waist Index and Abdominal Fat and Muscle Mass: multi-Ethnic Study of Atherosclerosis. Diabetes Metab J. 2022;46(5):747–755. doi:10.4093/dmj.2021.0294
60. Wang Y, Mao L, Zhang X. Waist-Hip ratio is an independent predictor of moderate-to-severe OSA in nonobese males: a cross-sectional study. BMC Pulm Med. 2022;22(1):151. doi:10.1186/s12890-022-01886-3
61. Abourjeili J, Salameh E, Noureddine M, Bou Khalil P, Eid AA. Obstructive sleep apnea: beyond the dogma of obesity! Respir Med. 2024;222:107512. doi:10.1016/j.rmed.2023.107512
62. Kainulainen S, Suni A, Lipponen JA, et al. Morbid obesity influences the nocturnal electrocardiogram wave and interval durations among suspected sleep apnea patients. Ann Noninvasive Electrocardiol. 2024;29(1):e13101. doi:10.1111/anec.13101
63. Grewal G, Joshi GP. Obesity and Obstructive Sleep Apnea in the Ambulatory Patient. Anesthesiol Clin. 2019;37(2):215–224. doi:10.1016/j.anclin.2019.01.001
64. Veasey SC, Rosen IM. Obstructive Sleep Apnea in Adults. N Engl J Med. 2019;380(15):1442–1449. doi:10.1056/NEJMcp1816152
65. Das UN. Is obesity an inflammatory condition? Nutrition. 2001;17(11–12):953–966. doi:10.1016/s0899-9007(01)00672-4
66. Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69(1):29–35. doi:10.1016/j.diabres.2004.11.007
67. Ramos EJ, Xu Y, Romanova I, et al. Is obesity an inflammatory disease? Surgery. 2003;134(2):329–335. doi:10.1067/msy.2003.267
68. Bleau C, Karelis AD, St-Pierre DH, Lamontagne L. Crosstalk between intestinal microbiota, adipose tissue and skeletal muscle as an early event in systemic low-grade inflammation and the development of obesity and diabetes. Diabetes Metab Res Rev. 2015;31(6):545–561. doi:10.1002/dmrr.2617
69. Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep Apnea: types, Mechanisms, and Clinical Cardiovascular Consequences. J Am Coll Cardiol. 2017;69(7):841–858. doi:10.1016/j.jacc.2016.11.069
70. Trayhurn P, Wang B, Wood IS. Hypoxia and the endocrine and signalling role of white adipose tissue. Arch Physiol Biochem. 2008;114(4):267–276. doi:10.1080/13813450802306602
71. Wood IS, de Heredia FP, Wang B, Trayhurn P, de Heredia FP. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc. 2009;68(4):370–377. doi:10.1017/s0029665109990206
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Link Between Obstructive Sleep Apnea and Kidney Stones: NHANES 2015–2018 and Mendelian Randomization
Liu Y, Wang L, Bao EH, Wang JH, Yang L, Wang L, Xia L, Wang B, Zhu PY
Nature and Science of Sleep 2024, 16:1557-1568
Published Date: 3 October 2024
Association Between Obstructive Sleep Apnea and Non-Alcoholic Fatty Liver Disease: Epidemiological Cross-Sectional Study and Mendelian Randomization Analysis
Yu T, Zhou Y, Wu X, Fang Z, Liu C
Nature and Science of Sleep 2025, 17:1361-1376
Published Date: 17 June 2025





