Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Association Between Triglyceride-Glucose Index and Lung Function Parameters in the General Population Undergoing Health Examinations
Authors Yang Y, Wang S, Jia B , Chen S
Received 19 July 2024
Accepted for publication 23 October 2024
Published 29 October 2024 Volume 2024:17 Pages 4031—4047
DOI https://doi.org/10.2147/DMSO.S487744
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Yu Yang,1,2 Shuqi Wang,1,3 Boying Jia,1,3 Shuchun Chen1,3
1Department of Internal Medicine, Hebei Medical University, Shijiazhuang, People’s Republic of China; 2Department of Pharmacy, The Second Hospital of Hebei Medical University, Shijiazhuang, People’s Republic of China; 3Department of Endocrinology, Hebei General Hospital, Shijiazhuang, People’s Republic of China
Correspondence: Shuchun Chen, Department of Endocrinology, Hebei General Hospital, 348 heping West Road, Shijiazhuang, Hebei, 050051, People’s Republic of China, Tel/Fax +86 311 85988406, Email [email protected]
Purpose: To investigate the relationship between the triglyceride-glucose (TyG) index and pulmonary function metrics among the general population undergoing health examinations.
Materials and Methods: The enrollment totaled 696 participants. Fasting triglycerides and glucose levels were used to calculate the TyG index. Participants were divided into two categories according to their median TyG: one with high TyG and the other with low TyG. A portable spirometer was used to assess lung function. Fundamental clinical features and lung function indicators were compared between the two groups, and the relationship between the TyG index and lung function parameters was explored.
Results: Compared with the low TyG group, the high TyG group exhibited significantly reduced levels of FEV1/FVC, FVC% pred, FEV1% pred, FEV3% pred, FEV3/FVC, FEF75, FEF75% pred, FEF25-75% pred, and MVV% pred, suggesting poor pulmonary function. The TyG index was significantly inversely correlated with multiple pulmonary function metrics, including FVC% pred, FEV1% pred, FEV3% pred, FEV1/FVC, FEV3/FVC, FEF75, FEF75% pred and FEF25-75% pred, which persisted even after accounting for confounding variables.
Conclusion: In summary, the present study establishes a correlation between the TyG index and some lung function indicators, offering a new indicator of metabolic abnormalities related to lung functionality.
Keywords: triglyceride-glucose index, lung function, insulin resistance, FEV1, FVC
Introduction
A decline in lung capacity may negatively impact health results and the quality of life. Accumulating evidence has shown that reduced pulmonary capacity is associated with mortality rates.1–3 Most individuals with reduced lung capacity, encompassing smokers and non-smokers, show no symptoms, indicating a greater risk of the disease’s pre-clinical phase with population aging. A multitude of research have reported a connection between diabetes and hyperglycemia and the emergence of several lung diseases.4,5 The deterioration of lung function is often seen as a significant complication associated with diabetes.6,7 The body produces abundant insulin to maintain steady blood sugar levels, resulting in hyperinsulinemia, closely linked to insulin resistance (IR). IR is a crucial contributor to obesity, type 2 diabetes (T2DM), metabolic syndrome (MetS), and non-alcoholic fatty liver disease (NAFLD).8,9 IR is strongly related to asthma, leading to reduced lung capacity, hastened deterioration of lung function, and less-than-ideal responses to bronchodilator and corticosteroid therapies.10 IR is also a major factor in the reduced lung capacity in children with asthma.11
The triglyceride-glucose (TyG) index quantifies metabolic impairment by tracking triglyceride (TG) and glucose levels in fasting blood.12 The TyG index is currently considered a more precise measure of IR. Earlier studies have confirmed a link between the TyG and various health issues, including NAFLD, diabetic nephropathy, cervical vascular dysfunction, coronary artery disease and non-small cell lung cancer.13–18 The TyG index even outperforms the homeostatic model assessment for insulin resistance (HOMA-IR) in forecasting conditions such as NAFLD and arterial stiffness.19,20
The link between the TyG and pulmonary performance remains ambiguous in the general population undergoing health examinations. This study investigated the association between the TyG and pulmonary performance, possibly offering new perspectives for the early identification, diagnosis, and treatment of pulmonary injury in the general populace.
Materials and Methods
Study Population
A cross-sectional study was conducted based on outpatient data. From June 2023 to January 2024, 696 adults were enlisted from the Hebei Provincial Medical Examination Center. The Hebei General Hospital’s Ethics Committee sanctioned this research methodology in alignment with the Declaration of Helsinki’s tenets (No. 2024-LW-112). Individuals in the general populace who received a physical check-up were deemed prospective participants in the study. Exclusion criteria: (1) aged at most 18 years old; (2) individuals suffering from critical liver, lung, and kidney malfunctions, along with cancerous growths; (3) a history of lung-related illnesses; (4) those with various illnesses or drugs impacting pulmonary performance; (5) missing essential information; and (6) those utilizing of additional oxygen.
Data Collection and Laboratory Analysis
Patient baseline information was collected, including age, gender, smoking and drinking records, systolic blood pressure (SBP), diastolic blood pressure (DBP), height, and weight. Laboratory examinations included total cholesterol (TC), TG, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), fasting blood glucose (FBG), blood urea nitrogen (BUN), uric acid (UA), aspartate transaminase (AST), alanine transaminase (ALT), blood creatinine (Cr), γ-glutamyl transpeptidase (γ-GT), direct bilirubin (DBIL), indirect bilirubin (IBIL), and total bilirubin (TBIL). Blood routine indicators included neutrophil count (NEUT), hemoglobin (HGB), white blood count (WBC), red blood count (RBC), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), platelet count (PLT), plateletcrit (PCT), basophils (BA), eosinophils (EO), lymphocytes (LY), monocytes (MO), platelet-larger cell ratio (P-LCR), red cell distribution width-standard deviation (RDW-SD), red cell distribution width-standard deviation coefficient variation (RDW-CV), hematocrit (HCT), mean platelet volume (MPV), and platelet volume distribution width (PDW).
Lung Function Measures
Trained technicians conducted all lung function tests utilizing a portable spirometer III (Spiro-lab MIR, co. Ltd., Roma, Italy) as previously described.21 Participants were instructed to remain motionless, gripping the spirometer, before executing a compulsory exhalation. Each participant performed the procedure thrice and the peak reading was recorded. The examined lung functions included the forced expiratory volume in 1 second (FEV1), FEV1 to predicted value ratio (FEV1% pred), forced expiratory volume in 3 second (FEV3), FEV3 to predicted value ratio (FEV3% pred), forced vital capacity (FVC), FVC to predicted value ratio (FVC% pred), FEV1/FVC, peak expiratory flow (PEF), PEF to predicted value ratio (PEF% pred), maximal voluntary ventilation (MVV), MVV to predicted value ratio (MVV% pred), forced inspiratory vital capacity (FIVC), forced inspiratory volume in 1 second (FIV1), FIV1/FIVC, peak inspiratory flow (PIF), forced expiratory flow (FEF) at 25 and 75% of the pulmonary volume (FEF25-75), FEF25-75 to predicted value ratio (FEF25-75% pred), FEF25, FEF25 to predicted value ratio (FEF25% pred), FEF50, FEF50 to predicted value ratio (FEF50% pred), FEF75, FEF75 to predicted value ratio (FEF75% pred), and forced expiratory time (FET).
Calculation of Parameters
The TyG index was derived by multiplying TG and FBG levels using the following formula: Ln (fasting TG (mg/dl) × FBG (mg/dl)/2).14,17
Statistical Analysis
Every piece of data underwent analysis and visualization through the utilization of GraphPad Prism 10 and SPSS 27 software. Data following a normal distribution was presented as the average ± standard deviation and analyzed using the Student’s t-test. Data that did not follow a normal distribution were represented as the median values (25th and 75th percentiles) and analyzed through the Mann–Whitney U-test. The connection among variables was examined using Spearman or Pearson correlation analyses. Multiple linear regression was employed to investigate the independent relationships among variables. A P-value below 0.05 was deemed to hold statistical significance.
Results
Medical Traits of Every Participant
The study encompassed 696 participants, of who 534 (76.72%) were males. Of the 696 participants, 327 (46.98%) and 282 (40.52%) were alcohol drinkers and smokers, respectively. The average age was 45 years, with a body mass index (BMI) of 25.10 kg/m2. The study participants were divided into groups according to their median TyG score (7.08), divided into the high TyG (n = 348) and low TyG (n = 348) clusters. The mean FBG, TC, TG, HDL-C, and LDL-C were 5.41, 4.97, 1.32, 1.29, and 3.14 mmol/L, respectively. The mean HGB, WBC, NEUT, PLT, and RBC were 152 g/L, 6.37 × 109/L, 3.73 × 109/L, 248 × 109/L, and 4.90 ± 0.47 × 1012/L, respectively. Table 1 displays the fundamental clinical attributes of each participant.
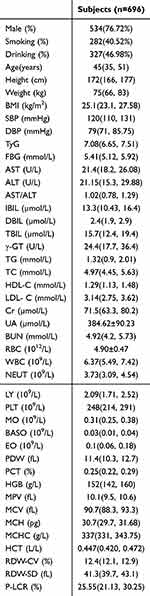 |
Table 1 Medical Traits of Every Participant |
Lung Function Parameters of Participants
The average FVC, FEV1, FEV1% pred, FEV3, PEF, FEF25% pred, FIVC, and MVV were 3.85 ± 0.77 L, 3.13 ± 0.62 L, 0.98 ± 0.11, 3.74 ± 0.74, 7.44 ± 1.59 L/s, 0.84 ± 0.16, 3.51 ± 0.76 L, and 109.67 ± 21.87 L/min, respectively. The mean FEF25, FEF50, FEF75, FEF25-75, FET, FIV1, and PIF were 6.28 L/s, 3.62 L/s, 1.25 L/s, 3.09 L/s, 4.08 s, 2.89 L, and 3.17 L/s, respectively. The mean FVC% pred, FEV3% pred, PEF% pred, FEF50% pred, FEF75% pred, FEF25-75% pred, and MVV% pred were 0.98, 1, 0.91, 0.81, 0.71, 0.93, and 0.86 respectively. The lung function metrics of participants are displayed in Table 2.
 |
Table 2 Lung Function Index of Participants |
Comparison of Clinical Traits Between High and Low TyG Groups
Furthermore, the high TyG group exhibited a greater percentage of males, alcohol consumers, and smokers. Participants in the high TyG group were older, with elevated BMI, and blood pressure compared with their counterparts in the low TyG group (Table 3) (Figure 1).
 |
Table 3 Comparison of Clinical Features Between High and Low TyG Groups |
An analysis of the biochemical markers revealed that the high TyG group exhibited significantly elevated TG, TC, LDL-C, FBG, Cr, and UA, along with a decrease in HDL-C, in contrast to the low TyG group (Figure 2). Additionally, the high TyG group had higher AST, ALT, and γ-GT and a lower AST/ALT than the low TyG group. There were no notable disparities in the levels of BUN, TBIL, and DBIL between the two groups (P > 0.05) (Table 3).
Regarding the blood routine index, individuals with elevated TyG indices exhibited increased levels of RBC, WBC, NEUT, LY, MO, BASO, EO, HGB, MCH, MCHC, and HCT and reduced RDW-SD compared with those with lower TyG indices. There were no notable disparities between the two groups regarding RDW-CV, MPV, PLT, PCT, MCV, and P-LCR (Figure 3) (Table 3).
Comparison of Functional Characteristics of Lungs Between High and Low TyG Groups
Compared with the low TyG group, the high TyG group exhibited significantly reduced levels of FVC% pred, FEV1% pred, FEV3% pred, FEV1/FVC, FEV3/FVC, FEF75, FEF75% pred, FEF25-75% pred, and MVV% pred, suggesting poor pulmonary function. The high TyG group exhibited markedly elevated levels of PEF, FEF25, and FET compared with the low TyG group. However, the TyG index had no significant impact on FIV1, FVC, FEV1, FEV3, PEF% pred, FEF25-75%, FEF25% pred, FEF50, FEF50% pred, FIVC, FIV1/FIVC, MVV, and PIF (Table 4) (Figure 4).
 |
Table 4 Comparison of Functional Characteristics of Lungs Between High and Low TyG Groups |
Relationship Between the TyG Index and Pulmonary Functional Indicators
The TyG index was significantly inversely correlated with various pulmonary function metrics, including FEV1/FVC, FEV3/FVC, FVC% pred, FEV1% pred, FEF75, FEF75% pred, FEF25-75% pred, FEV3% pred, and MVV% pred. Additionally, a positive link exists between TyG and variables like PEF, FET, and FEF25. There was no notable link found between TyG and factors like FVC, FEV1, FEV3, PEF% pred, FEF25% pred, FEF50, FEF50% pred, FIV1, FIVC, FIV1/FIVC, PIF and MVV (Table 5) (Figure 5).
 |
Table 5 Relationship Between TyG and Pulmonary Functional Indicators |
Multivariate Linear Analysis of the Association Between the TyG Index and Lung Function Metrics
There was a positive correlation observed between the TyG index and PEF, FEF25, FET, FIV1, and FIVC across all subjects but negatively correlated with FVC% pred, FEV1% pred, FEV3% pred, FEV1/FVC, FEV3/FVC, FEF75, FEF75% pred and FEF25-75% pred in model 1 (unadjusted), model 2 (adjusted for age, DBP and SBP), and model 3 (adjusted for age, DBP, SBP, WBC, NEUT and LY) (Table 6).
 |
Table 6 Multivariate Linear Analysis of the Association Between TyG and Lung Function Metrics |
Discussion
An increase in smokers in developing countries, along with the growing number of seniors in advanced economies, is leading to reduced pulmonary capacity. The functionality of the lungs is a key factor in preventing and diagnosing respiratory illnesses.22 Numerous studies have reported a link between reduced lung capacity and subsequent risks of death, respiratory issues, and heart-related problems. Therefore, exploring other modifiable risk factors for lung function harm is of utmost importance. IR is closely linked to a heightened likelihood of obesity, MetS, NAFLD, and T2DM. Acknowledged for its comprehensive and invasive nature, the hyperinsulinemic-euglycemic clamp is the premier method for evaluating IR.23 Alternative laboratory methods, such as HOMA-IR, necessitate the direct measurement of insulin and thus are frequently unfeasible in epidemiological contexts. In addition, given that plasma insulin levels are typically gauged in diabetic individuals, these assessments are not appropriate for the general population. Given its focus on glucolipid metabolism, TyG is presently considered a more precise and reliable substitute indicator for IR.24,25 Despite established links between TyG and conditions such as NAFLD, diabetic nephropathy, cervical vascular dysfunction and coronary artery disease, the relationship between TyG and lung function remains largely unexplored. Thus, the present study explored the potential relationship between the TyG index and pulmonary function. As a result, this study explored the potential link between TyG and lung function in the general populace undergoing health examinations.
It was found that subjects in the high TyG group exhibited elevated blood pressure and BMI compared with those in the low TyG group. Moreover, individuals in the high TyG group were generally older and had a greater percentage of males, smokers, and drinkers. Given that the TyG index relies on TG and glucose levels, individuals with a high TyG index exhibited elevated FBG, TG, TC, and LDL-C and reduced HDL-C. A comparison of liver functionality revealed significantly higher AST and ALT levels and lower AST/ALT ratios in individuals with elevated TyG indices than in those with lower indices, consistent with findings from reports.14 An analysis comparing kidney performance revealed that subjects with a higher TyG index exhibited elevated Cr and UA.
The presence of total white blood cells, neutrophils, and lymphocytes is widespread, cost-effective, and widely used as indicators of inflammation. Multiple research have revealed a substantial link between MetS and a rise in total white blood cells, neutrophils, and lymphocytes.26–29 Further blood routine analysis revealed that individuals with a high TyG index had elevated levels of red and white blood cells, neutrophils, and lymphocytes in contrast to those exhibiting lower TyG.
Normal spirometry is characterized by an FEV1% forecast exceeding 80% and a FEV1/FVC ratio of 0.70 or more, whereas obstructive spirometry is identified by an FEV1/FVC ratio below 0.70.30 Diagnosing COPD hinges on the clinical signs and whether the post-bronchodilator FEV1/FVC ratio falls below 0.70.31 Preserved Ratio Impaired Spirometry, is characterized by an FEV1 less than 80% of the forecasted value and an FEV1/FVC ratio of 0.70 or more, indicative of a preclinical COPD condition.32,33 A correlation between FEV1 and FVC and death rates was reported in individuals without lung conditions from the general population.34 IR correlated with reduced FEV1% predicted, especially among the elderly.35 The current study found that levels of FVC% pred, FEV1% pred, FEV3% pred, FEV1/FVC, FEF75, FEF75% pred, FEF25-75% pred and MVV% pred were significantly reduced in subjects with elevated TyG indices compared with those with lower indices, suggesting potential pulmonary damage to a certain degree. After adjustment for possible interfering variables, the TyG index was associated with reduced FEV1/FVC, FVC% pred, FEV1% pred, FEV3% pred, FEF75, FEF75% pred, and FEF25-75% pred. These data imply that the TyG index could act as an epidemiological instrument to measure the impact of metabolic dysfunction, potentially offering predictive and diagnostic significance as an indicator of pulmonary health. In our study, some indicators appeared to be higher in the high TyG group compared to the low TyG group, such as FVC, FEV1, FET and PEF. The reason for this outcome, we consider, is that the BMI of the high TyG group is higher than that of the low TyG group, which means that the population in the high TyG group is more prone to obesity and being overweight. Research indicates elevated lung function metrics (FVC and FEV1) and a reduced FEV1/FVC ratio in obese adolescents compared to non-obese ones, aligning with our findings.36 Additionally, there is a greater male ratio in the high TyG group compared to the low TyG group, coupled with male participants exhibiting higher PEF and FET values than females.37,38
Diabetes, dyslipidemia, and MetS, a group of concurrent conditions supported by IR, are linked in various ways to the heightened occurrence, frequency, or intensity of COPD, asthma, and pulmonary fibrosis, sparking theories of their direct impact on the lungs.39–44 Diabetes and hyperglycemia heighten the risk and intensity of lung infections due to weakened immune responses in the host and increased virulence of infectious agents.45 Elevated insulin levels and resistance, often considered the root causes of dyslipidemia and diabetes, may trigger increased bronchial activity due to changes in parasympathetic signals and potentially lead to subepithelial fibrosis.46,47 IR plays a crucial role in pulmonary function. Presently, the TyG index is recognized for its greater precision in assessing IR, given its focus on glucolipid metabolism. As far as we are aware, this is a pioneering study to demonstrate the link between TyG and pulmonary performance in the general population undergoing health assessments. The TyG index may act as a dependable tool for assessing lung function damage. An elevated initial TyG index correlates with a reduction in pulmonary capacity among the healthy populace. Increased levels in the TyG are indicative of dyslipidemia and hyperglycemia, disorders impacting lung structure and functionality. TyG correlated with symptoms of breathing, persistent bronchitis, and a pattern of constricted spirometry.48 C-reactive protein and TyG index can mediate lung function and cognitive function in a widespread, mild inflammatory condition.49 Collectively, these results underscore the significant yet often overlooked importance of the TyG index in hastening the deterioration of lung function. While the precise mechanism by which the TyG index influences lung function remains unclear, it might be connected to IR. Insulin plays a direct role in airway malfunction by stimulating immune cells and structural cells in the airways, leading to inflammation and constriction.50,51 The current study has pinpointed TyG as a contributing factor to compromised pulmonary health. Therefore, determining measures to motivate the general public to maintain their TyG index within normal ranges may aid in lowering the occurrence of lung function damage.
Nonetheless, this research presents certain constraints. Initially, a direct cause-and-effect link between TyG and pulmonary function indicators was not established. Secondly, the association between TyG and pulmonary performance in individuals suffering from widespread chronic lung conditions warrants further investigation in the future. Third, the exclusion criteria do not mention whether the individual has had Corona Virus Disease 2019 (COVID-19).
Conclusion
In summary, the present study establishes a correlation between the TyG index and some lung function indicators, offering a new indicator of metabolic abnormalities related to lung functionality.
Ethics Approval and Informed Consent
The study protocol was approved by the Ethics Committee of Hebei General Hospital in accordance with the principles of the Declaration of Helsinki (No. 2024-LW-112). Since this was a cross-sectional, retrospective, non-interventional study, and the patient’s information was anonymous and confidential, signed informed consent was waived.
Funding
This study was supported by Hebei Province Natural Science Foundation (H2022307026). This funding source did not influence the study outcomes, and the authors were free to interpret the data in accordance with a strict scientific rationale.
Disclosure
The authors declare no competing conflicts of interest in this work.
References
1. Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, Hawthorne VM. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ. 1996;313(7059):711–715. doi:10.1136/bmj.313.7059.711
2. Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. doi:10.1378/chest.127.6.1952
3. Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax. 2010;65(6):499–504. doi:10.1136/thx.2009.126052
4. Enomoto T, Usuki J, Azuma A, Nakagawa T, Kudoh S. Diabetes mellitus may increase risk for idiopathic pulmonary fibrosis. Chest. 2003;123(6):2007–2011. doi:10.1378/chest.123.6.2007
5. Perez MK, Piedimonte G. Metabolic asthma: is there a link between obesity, diabetes, and asthma? Immunol Allergy Clin. 2014;34(4):777–784. doi:10.1016/j.iac.2014.07.002
6. Klein O, Krishnan J, Glick S, Smith LJ. Systematic review of the association between lung function and Type 2 diabetes mellitus. Diabet Med. 2010;27(9):977–987. doi:10.1111/j.1464-5491.2010.03073.x
7. Yeh H-C, Punjabi NM, Wang N-Y, et al. Cross-sectional and prospective study of lung function in adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC. Study Diabetes Care. 2008;31(4):741–746. doi:10.2337/dc07-1464
8. Chen S, Chen Y, Liu X, et al. Insulin resistance and metabolic syndrome in normal-weight individuals. Endocrine. 2014;46(3):496–504. doi:10.1007/s12020-013-0079-8
9. Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48(3):792–798. doi:10.1002/hep.22429
10. Peters MC, Schiebler ML, Cardet JC, et al. The impact of insulin resistance on loss of lung function and response to treatment in asthma. Am J Respir Crit Care Med. 2022;206(9):1096–1106. doi:10.1164/rccm.202112-2745OC
11. Goyal JP, Kumar P, Thakur C, Khera D, Singh K, Sharma P. Effect of insulin resistance on lung function in asthmatic children. J Pediatr Endocrinol Metab. 2021;35(2):217–222. doi:10.1515/jpem-2021-0351
12. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6(4):299–304. doi:10.1089/met.2008.0034
13. Wang X, Xu W, Song Q, et al. Association between the triglyceride-glucose index and severity of coronary artery disease. Cardiovasc Diabetol. 2022;21(1):168. doi:10.1186/s12933-022-01606-5
14. Pan X, Yue L, Ren L, Ban J, Chen S. Association of triglyceride-glucose index and liver function parameters among healthy obese civil servants: a center-based study. Diabetes Metab Syndr Obes. 2022;15:3519–3531. doi:10.2147/DMSO.S392544
15. Lv L, Zhou Y, Chen X, et al. Relationship between the Tyg index and diabetic kidney disease in patients with type-2 diabetes mellitus. Diabetes Metab Syndr Obes. 2021;14:3299–3306. doi:10.2147/DMSO.S318255
16. Ye X, Li J, Wang H, Wu J. Pentraxin 3 and the TyG index as two novel markers to diagnose NAFLD in children. Dis Markers. 2021;2021:8833287. doi:10.1155/2021/8833287
17. Pan X, Yue L, Ren L, Ban J, Chen S. Triglyceride-glucose index and cervical vascular function: outpatient-based cohort study. BMC Endocr Disord. 2023;23(1):191. doi:10.1186/s12902-023-01449-5
18. Yan X, Gao Y, Tong J, Tian M, Dai J, Zhuang Y. Association between triglyceride glucose index and non-small cell lung cancer risk in Chinese population. Front Oncol. 2021;11:585388. doi:10.3389/fonc.2021.585388
19. Lee SB, Kim MK, Kang S, et al. Triglyceride glucose index is superior to the homeostasis model assessment of insulin resistance for predicting nonalcoholic fatty liver disease in Korean adults. Endocrinol Metab. 2019;34(2):179–186. doi:10.3803/EnM.2019.34.2.179
20. Lee SB, Ahn CW, Lee BK, et al. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17(1):41. doi:10.1186/s12933-018-0692-1
21. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
22. Fainardi V, Lombardi E. Lung function tests to monitor respiratory disease in preschool children. Acta Biomed. 2018;89(2):148–156. doi:10.23750/abm.v89i2.7155
23. Singh B, Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes. 2010;1(2):36–47. doi:10.4239/wjd.v1.i2.36
24. Wei A, Liu J, Wang L, Zheng S, Cong H. Correlation of triglyceride-glucose index and dyslipidaemia with premature coronary heart diseases and multivessel disease: a cross-sectional study in Tianjin, China. BMJ Open. 2022;12(9):e065780. doi:10.1136/bmjopen-2022-065780
25. Mohd Nor NS, Lee S, Bacha F, Tfayli H, Arslanian S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: comparison with the hyperinsulinemic-euglycemic clamp. Pediatr Diabetes. 2016;17(6):458–465. doi:10.1111/pedi.12303
26. Babio N, Ibarrola-Jurado N, Bulló M, et al. White blood cell counts as risk markers of developing metabolic syndrome and its components in the PREDIMED study. PLoS One. 2013;8(3):e58354. doi:10.1371/journal.pone.0058354
27. Yang H, Fu Y, Yang B, et al. Positive association between the metabolic syndrome and white blood cell counts in Chinese. Asia Pac J Clin Nutr. 2017;26(1):141–147. doi:10.6133/apjcn.102015.13
28. Meng G, Zhu Q, Shao J, et al. Comparing the diagnostic ability of inflammatory markers in metabolic syndrome. Clin Chim Acta. 2017;475:1–6. doi:10.1016/j.cca.2017.09.023
29. Sun S, Wu H, Zhang Q, et al. Subnormal peripheral blood leukocyte counts are related to the lowest prevalence and incidence of metabolic syndrome: Tianjin chronic low-grade systemic inflammation and health cohort study. Mediators Inflamm. 2014;2014:412386. doi:10.1155/2014/412386
30. Halpin DMG, Criner GJ, Papi A, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. the 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(1):24–36. doi:10.1164/rccm.202009-3533SO
31. Şerifoğlu İ, Ulubay G. The methods other than spirometry in the early diagnosis of COPD. Tuberk Toraks. 2019;67(1):63–70. doi:10.5578/tt.68162
32. Wan ES, Castaldi PJ, Cho MH, et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPD Gene. Respir Res. 2014;15(1):89. doi:10.1186/s12931-014-0089-y
33. Higbee DH, Granell R, Smith GD, Dodd JW. Prevalence, risk factors, and clinical implications of preserved ratio impaired spirometry: a UK Biobank cohort analysis. Lancet Respir Med. 2022;10(2):149–157. doi:10.1016/S2213-2600(21)00369-6
34. Magnussen C, Ojeda FM, Rzayeva N, et al. FEV1 and FVC predict all-cause mortality independent of cardiac function - results from the population-based Gutenberg health study. Int J Cardiol. 2017;234:64–68. doi:10.1016/j.ijcard.2017.02.012
35. Kim SH, Kim HS, Min HK, Lee SW. Association between insulin resistance and lung function trajectory over 4 years in South Korea: community-based prospective cohort. BMC Pulm Med. 2021;21(1):110. doi:10.1186/s12890-021-01478-7
36. Engwa GA, Anye C, Nkeh-Chungag BN. Association between obesity and lung function in South African adolescents of African Ancestry. BMC Pediatr. 2022;22(1):109. doi:10.1186/s12887-022-03164-x
37. Ijaz A, Bashir I, Ikhlaq A, Ijaz F, Aftab RK, Zia R. Correlation between peak expiratory flow rate, markers of adiposity, and anthropometric measures in medical students in Pakistan. Cureus. 2020;12(12):e12408. doi:10.7759/cureus.12408
38. Skloot GS, O’Connor-Chapman KL, Schechter CB, Markley DJ, Bates JHT. Forced expiratory time: a composite of airway narrowing and airway closure. J Appl Physiol. 2021;130(1):80–86. doi:10.1152/japplphysiol.00556.2020
39. Lipovec NC, Beijers RJ, van den Borst B, Doehner W, Lainscak M, Schols AMWJ. The prevalence of metabolic syndrome in chronic obstructive pulmonary disease: a systematic review. COPD. 2016;13(3):399–406. doi:10.3109/15412555.2016.1140732
40. Serafino-Agrusa L, Spatafora M, Scichilone N. Asthma and metabolic syndrome: current knowledge and future perspectives. World J Clin Cases. 2015;3(3):285–292. doi:10.12998/wjcc.v3.i3.285
41. Thuesen BH, Husemoen LLN, Hersoug LG, Pisinger C, Linneberg A. Insulin resistance as a predictor of incident asthma-like symptoms in adults. Clin Exp Allergy. 2009;39(5):700–707. doi:10.1111/j.1365-2222.2008.03197.x
42. Ehrlich SF, Quesenberry CP, Van Den Eeden SK, Shan J, Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33(1):55–60. doi:10.2337/dc09-0880
43. Breyer MK, Spruit MA, Hanson CK, et al. Prevalence of metabolic syndrome in COPD patients and its consequences. PLoS One. 2014;Vol. 9(6):e98013. doi:10.1371/journal.pone.0098013
44. Wang D, Ma Y, Tong X, Zhang Y, Fan H. Diabetes mellitus contributes to idiopathic pulmonary fibrosis: a review from clinical appearance to possible pathogenesis. Front Public Health. 2020;8:196. doi:10.3389/fpubh.2020.00196
45. Klekotka RB, Mizgała E, Król W. The etiology of lower respiratory tract infections in people with diabetes. Pneumonol Alergol Pol. 2015;83(5):401–408. doi:10.5603/PiAP.2015.0065
46. Nie Z, Jacoby DB, Fryer AD. Hyperinsulinemia potentiates airway responsiveness to parasympathetic nerve stimulation in obese rats. Am J Respir Cell Mol Biol. 2014;51(2):251–261. doi:10.1165/rcmb.2013-0452OC
47. Lee H, Kim SR, Oh Y, Cho SH, Schleimer RP, Lee YC. Targeting insulin-like growth factor-I and insulin-like growth factor-binding protein-3 signaling pathways. A novel therapeutic approach for asthma. Am J Respir Cell Mol Biol. 2014;50(4):667–677. doi:10.1165/rcmb.2013-0397TR
48. Wu TD, Fawzy A, Brigham E, et al. Association of triglyceride-glucose index and lung health: a population-based study. Chest. 2021;160(3):1026–1034. doi:10.1016/j.chest.2021.03.056
49. Chen C, Lu Z, Wang X, Zhang J, Zhang D, Li S. The chain mediating role of C-reactive protein and triglyceride-glucose index between lung function and cognitive function in a systemic low-grade inflammation state. J Psychiatr Res. 2022;155:380–386. doi:10.1016/j.jpsychires.2022.09.004
50. Singh S, Bodas M, Bhatraju NK, et al. Hyperinsulinemia adversely affects lung structure and function. Am J Physiol Lung Cell Mol Physiol. 2016;310(9):L837–45. doi:10.1152/ajplung.00091.2015
51. Park YH, Oh EY, Han H, et al. Insulin resistance mediates high-fat diet-induced pulmonary fibrosis and airway hyperresponsiveness through the TGF-β1 pathway. Exp Mol Med. 2019;51(5):1–12. doi:10.1038/s12276-019-0258-7
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Association Between Lung Function of Children and Their Socioeconomic Conditions: A Systematic Review
Alzayed A
International Journal of General Medicine 2024, 17:2265-2278
Published Date: 18 May 2024






